Abstract
Cryparin is a cell-surface-associated hydrophobin of the filamentous ascomycete Cryphonectria parasitica. This protein contains a signal peptide that directs it to the vesicle-mediated secretory pathway. We detected a glycosylated form of cryparin in a secretory vesicle fraction, but secreted forms of this protein are not glycosylated. This glycosylation occurred in the proprotein region, which is cleaved during maturation by a Kex2-like serine protease, leaving a mature form of cryparin that could be isolated from both the cell wall and culture medium. Pulse-chase labeling experiments showed that cryparin was secreted through the cell wall, without being bound, into the culture medium. The secreted protein then binds to the cell walls of C. parasitica, where it remains. Binding of cryparin to the cell wall occurred in submerged culture, presumably because of the lectin-like properties unique to this hydrophobin. Thus, the binding of this hydrophobin to the cell wall is different from that of other hydrophobins which are reported to require a hydrophobic-hydrophilic interface for assembly.
Filamentous fungi can degrade most naturally occurring complex polymers by secreting large amounts of enzymes and other proteins into their environment. Many of these secreted proteins have industrial applications and are being commercially produced through fermentation. The filamentous plant pathogenic ascomycete Cryphonectria parasitica, which produces endothiapepsin, an aspartic protease used in milk clotting (13), is a “generally recognized as safe” (GRAS) organism. Protein secretion is key to the success of fungi as primary degraders of polymers, as pathogens, and as commercial organisms, but the study of protein secretion by filamentous fungi has been very limited and protein secretion remains poorly understood (9). Yeast and mammalian systems have been studied in more depth, and protein secretion in yeast serves as an excellent model for some aspects of protein secretion in the filamentous fungi. However, it is likely that there also are important differences between these organisms. Protein secretion must play a key role in the foraging of filamentous fungi and in the organization of hyphae into complex structures during fungal development.
The most abundantly produced protein of C. parasitica is the hydrophobin cryparin. Hydrophobins are small cell surface hydrophobic proteins which are ubiquitous in filamentous fungi and confer a hydrophobic property to various surfaces of fungal tissues and structures; their functional and structural conservation suggests that they are very important to fungi (for recent reviews, see references 6 and 14). Cryparin is found on the surface of aerial hyphae and in fruiting bodies of cultures grown on agar, in fruiting bodies of the fungus when grown on natural substrates, and as a free protein when the fungus is grown in liquid media (1). The protein has been purified and the N terminus sequenced; comparison of this sequence with that of the cloned cDNA indicates that the protein undergoes posttranslational processing, with the mature protein being cleaved by a Kex2-like serine protease. Cryparin is the most abundant mRNA during the late growth phase (17), and more than 1% of the dry weight of the fungus grown in liquid culture consists of the secreted protein. Antibody localization studies suggest that this protein is found only in fruiting bodies when the fungus grows in wood, its natural substrate. The involvement of Kex2 in the processing and secretion of this protein makes study of the secretion of this protein particularly interesting, since this secretory pathway is conserved in most eukaryotes, including mammals, plants, and yeast (11).
We also are interested in studying the transport of cryparin, because when this fungus is infected by hypovirus CHV1, cryparin secretion is impaired. The hypothesis that the virus disrupts protein transport is consistent with the finding that viral replication occurs on small fungal vesicles that appear to be transport vesicles (2–4). Understanding transport and processing of cryparin will also provide insight into the function of cryparin. Cryparin has lectin-like properties and binds to the cell walls of the fungus as well as being secreted into the media. Most lectins are glycoproteins, so we wanted to learn if the cryparin that binds to the cell wall is glycosylated during secretion and later processed to a nonglycosylated form before release into the culture medium. Our objectives in this study were (i) to determine if binding of cryparin to cell walls preceded secretion into the culture fluid and (ii) to determine if cryparin is glycosylated during the secretion process.
MATERIALS AND METHODS
Fungal strains and culture conditions.
C. parasitica EP155 and the isogenic cryparin deletion mutant (Δ119) were used in these studies. The strains were grown in EP complete liquid medium (10) at pH 5.6. Inoculum for liquid culture was grown at 25°C on PDAmb, which consists of potato dextrose agar (Difco Laboratories, Detroit, Mich.) supplemented with 100 mg of l-methionine and 1 mg of biotin per liter (Sigma Chemical Company, St. Louis, Mo.). The cryparin deletion mutant was created by site-directed recombination. The coding region of the cryparin gene was replaced with a hygromycin B resistance cassette, and mutants were screened for hygromycin resistance and loss of cryparin. The deletion mutant strain produces neither cryparin mRNA nor protein. The only detectable phenotype change in culture is loss of surface hydrophobicity, i.e., a wettable phenotype (5a).
Growth determination.
Strain EP155 was grown for 7 days on PDAmb and cut into six segments, and each segment was homogenized separately with a VirTis handheld homogenizer (The VirTis Company, Gardiner, N.Y.). The resultant slurry was used to inoculate a 250-ml baffled flask containing 100 ml of EP complete medium. Flasks were incubated at 25°C with shaking (120 rpm). Contents of the flasks were filtered through a Buchner funnel, and cells were collected on Miracloth (Calbiochem-Novabiochem Corporation, La Jolla, Calif.), frozen, and lyophilized, and the mycelial dry weight was determined.
The concentration of cryparin associated with growth was measured by using [35S]cysteine. Cryparin is one of the few C. parasitica proteins that is soluble in 60% ethanol and was the only one that readily incorporated radiolabeled cysteine. A separate 100-ml culture was used for each time point. [35S]cysteine at 50 μCi (1,000 Ci/mmol) was added to the growing culture, which was then incubated for 5 h at 25°C with shaking (120 rpm). Cells were collected and lyophilized as described above. Cryparin was extracted from cells as described below, and the relative amounts of cryparin were quantified by using a phosphorimager (Fuji Medical Systems USA, Inc., Stamford, Conn.). The density of cryparin per gram of mycelia was calculated with the Quant program on the phosphorimager. Confirmation of the protein as cryparin was based on its size and a specific reaction with anticryparin antibody.
Cryparin binding studies.
C. parasitica EP155 and Δ119 were grown for 7 days on PDAmb. One mycellium-covered plate (90 mm in diameter) was homogenized in a Waring blender (Waring Products Division, Dynamics Corporation of America, New Hartford, Conn.), and the slurry was used to inoculate a Fernbach flask containing 1 liter of EP complete medium. Flasks were incubated and cells were collected as described above. The culture medium was collected in an empty sterile Fernbach flask. The cells from Δ119 were inoculated into media in which EP155 had been growing for 3 days and incubated for 4 h at 25°C with shaking (120 rpm). The flask was removed from the shaker, and the contents were filtered as described above. Following collection on Miracloth, cells were lyophilized and cryparin was extracted from them by the method of Carpenter et al. (1).
Pulse-chase analysis.
Mycelia grown on PDAmb media for 7 days were used as the inoculum. One-sixth of a plate (90 mm in diameter) was ground in a VirTis handheld homogenizer, inoculated into 100 ml of EP complete liquid medium in 250-ml baffled flasks, and grown for 3 days at 25°C with shaking, at which time, 50 μCi (1,000 Ci/mmol) of [35S]cysteine (Amersham International, Arlington Heights, Ill.) was added to the medium. Cells were transferred to fresh medium containing no radiolabel 30 min after the addition of [35S]cysteine. At various times following transfer into fresh medium, 10 ml of the mixture of cells and culture medium was removed and filtered through a Buchner funnel, and cells were collected on Miracloth. To measure cryparin in cell walls and culture fluid, the cells and medium were separately frozen, lyophilized, and ground with a VirTis homogenizer. The relative amount of cryparin in the cytoplasm was negligible at all time points compared to that in the cell wall, so all experiments reporting the cryparin content of cell walls used whole cells for the analysis. Samples were resuspended in 60% ethanol and solubilized in sodium dodecyl sulfate (SDS) loading buffer, and proteins were separated on 12% polyacrylamide gel electrophoresis (PAGE) gels. The gels were dried and exposed to a phosphorimager bioimaging screen.
To monitor cryparin transport within cells, 20 ml of the growing culture was removed at each sampling time. Following filtration, cells were immediately added to a tube containing 0.5 g of glass beads and 1 ml of TMD buffer (50 mM Tris [pH 8.0], 10 mM MgCl2, 5 mM dithiothreitol) on ice. Cells were broken with a Mini-Bead-Beater (Biospec Products, Bartlesville, Okla.), and the cell debris was removed by centrifugation for 10 min at 12,800 × g. The supernatant was lyophilized overnight, and cryparin was extracted as described above.
Isolation of vesicles.
Fungal vesicles were isolated by the method of Fahima et al. (2) with the following modifications. Mycelial walls were disrupted by blending three times for 1 min each in a Waring blender, and the slurry was transferred to a bead beater containing 210 g of 0.5-mm-diameter glass beads and beaten six times for 30 s each to complete cell wall breakage.
Western immunoblot analysis.
Total vesicle proteins were solubilized in SDS loading buffer and separated by 12% PAGE (Protogel; National Diagnostics, Atlanta, Ga.). The proteins were transferred to nitrocellulose (Trans-Blot transfer medium; Bio-Rad Laboratories, Hercules, Calif.) and incubated for 2 h with antibody to the cryparin protein (1). Following incubation with the secondary antibody, bound antibody was detected by using the ECL enhanced chemiluminescence kit (Amersham International) or by using alkaline phosphatase colorimetric detection (Bio-Rad Laboratories).
Glycosylation.
Glycosylated forms of cryparin were detected with the Glycotrack carbohydrate detection kit (Oxford Glycosciences, Abingdon, United Kingdom), and the deglycosylation reaction was carried out with the Glycofree kit (Oxford Glycosciences).
RESULTS
Cryparin secretion.
A pulse-chase procedure was used to measure the relative amount of cryparin per 10 ml of liquid culture at given times following the chase. All labeled cryparin left the cytoplasm within 10 min of the chase (data not shown).
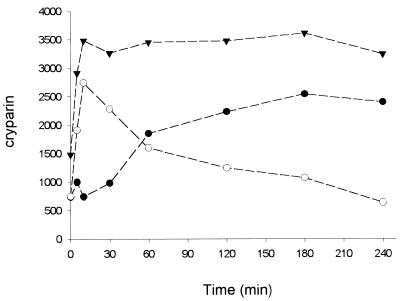
Growing cultures were labeled for 30 min with [35S]cysteine and then transferred to fresh medium and chased for up to 5 h (Fig. 1A and 2). The amount of newly synthesized cryparin in the fungal cell wall was low during the first 30 min of the chase. After 30 min, the amount of labeled cryparin associated with the cell wall started to increase, and this increase continued for the duration of the experiment. Analysis of the culture medium showed the opposite trend (Fig. 1B and 2). The amount of labeled cryparin increased during the first 30 min of the chase and then decreased from 60 min onwards, until it was barely detectable. The total amount of labeled cryparin detected remained the same throughout the assay (Fig. 2). Cryparin in the growth medium decreased as the amount of the protein associated with the cell wall fraction increased (Fig. 2).
FIG. 1.
Pulse-chase analysis of C. parasitica EP155. A 30-min pulse of [35S]cysteine was added to a 3-day old liquid culture of the fungus. Samples were taken at the indicated time points (in minutes) following removal of the radiolabel from the culture medium. Cryparin was extracted from the lyophilized cell walls or culture fluid and electrophoresed on 12% polyacrylamide SDS-PAGE gel. The gel was dried and exposed to a phosphorimager screen for 24 h. (A) Labeled cryparin in cells. (B) Labeled cryparin in the culture medium.
FIG. 2.
Time course of relative distribution of pulse-labeled cryparin. Samples from the pulse-chase analysis (Fig. 1) were quantified with a phosphorimager. Units are arbitrary phosphorimager units. ●, 35S-cryparin isolated from cell walls at different times following the chase with unlabeled cysteine; ○, 35S-cryparin isolated from culture medium at these times; ▾, total 35S detected in cryparin in cell walls and culture fluid at each time. The data shown are representative of multiple experiments. These could not all be shown, because the quantitative counts are different for each experiment, but the pattern of labeling is the same in all cases.
Binding of cryparin in culture fluid to the fungal cell wall was confirmed independently by using a C. parasitica strain (Δ119) from which the cryparin gene had been deleted and which produces no cryparin (Fig. 3). Incubation of Δ119 for 4 h in the culture medium in which EP155 had grown and into which it had secreted cryparin resulted in binding of the cryparin produced by EP155 to the cell walls of Δ119 (Fig. 3).
FIG. 3.
Binding of cryparin to the cell surface of a C. parasitica strain from which cryparin had been genetically deleted. Cryparin was extracted from the cell walls of C. parasitica strains and detected by PAGE. Lanes: M, protein size standards; 1, cryparin extracted from 3-day-old mycelia of EP155; 2, cryparin extracted from 3-day-old mycelia of the cryparin deletion strain Δ119, which had been incubated with the culture fluid of EP155 for 4 h followed by two washes with water.
Comparison of cryparin production with growth of C. parasitica.
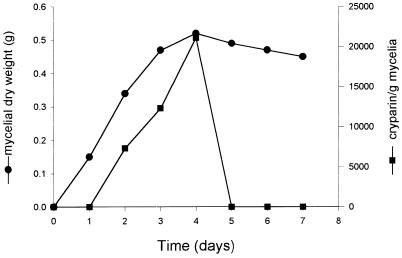
Comparison of cryparin secretion with fungal growth showed that most cryparin was secreted during the log phase of C. parasitica growth (Fig. 4). Cryparin was secreted during the stationary phase, and secretion stopped when fungal growth stopped.
FIG. 4.
Growth rate of C. parasitica EP155 and incremental cryparin accumulation in liquid culture. ●, dry weight of mycelia (grams) in 100 ml of EP complete liquid medium. ■, amount of cryparin produced per gram of mycelia in the first 5 h of a 24-h period. For each day, a pulse of [35S]cysteine was added to a new flask of 100 ml of EP complete liquid culture of the fungus that had been growing for the indicated time. Samples were taken 5 h after addition of the radiolabel. Cryparin was extracted from the lyophilized mycelium and separated by 12% polyacrylamide SDS-PAGE. The gel was dried and exposed to a phosphorimager screen for 24 h, and the total amount of cryparin was measured.
Location of cryparin during secretion.
To determine if cryparin was associated with a previously described fraction of putative secretory vesicles (2), these vesicles were isolated by differential centrifugation and polyethylene glycol precipitation. Vesicle proteins were separated by SDS-PAGE followed by Western blotting with antibody to cryparin. Cryparin was detected in association with this vesicle fraction (Fig. 5). Most of the cryparin detected with this vesicle fraction was the mature (24 kDa) form of cryparin, suggesting that the prepro sequence had been removed. There also was a small amount of a higher-molecular-mass protein (36 kDa) that reacted with the antibody which may be the pro form of cryparin. When vesicle proteins separated by PAGE were detected with silver stain, the 36-kDa protein was not visible, but this protein was bound by antibody to cryparin. This high antigenicity in conjunction with a specific reaction with antibody to cryparin suggested that the 36-kDa protein was a glycosylated form of cryparin (Fig. 5).
FIG. 5.
Presence of cryparin in a vesicle fraction. Vesicles were isolated by polyethylene glycol precipitation and differential centrifugation from strain EP155 and the isogenic strain Δ119 from which the cryparin gene had been deleted (Δcrp). (A) Silver-stained gel of vesicle proteins. (B) Corresponding Western blot with antibody against cryparin.
Cryparin glycosylation.
The cellular fraction of C. parasitica contained a 36-kDa protein which was not present in the culture medium (Fig. 6A). This 36-kDa form was the same form of cryparin found in the secretory vesicles. The 36-kDa protein was present in much lower quantities than the mature cryparin and could not be identified without the use of antibody. Most of the cryparin purified was from the cell wall; however, during this process, some cryparin was still in transit through the secretory pathway, so a very small amount of the glycosylated cryparin was purified. The 36-kDa form of the protein is more highly antigenic than the 24-kDa form, but is present in smaller amounts relative to the vesicle fraction (Fig. 5). Carbohydrate was detected with the Glycotrack carbohydrate detection kit (Fig. 6B). Carbohydrate was associated only with the 36-kDa form of cryparin, not the 24-kDa protein. We confirmed that cryparin was glycosylated by using a chemical deglycosylation method (Glycofree kit) that nonspecifically removes carbohydrate associated with the protein, returning it to approximately 24 kDa (data not shown).
FIG. 6.
Evidence for a high-molecular-mass glycosylated intermediate form of cryparin in cells. (A) Western blot of cryparin purified from culture medium (M) and C. parasitica whole cells (i.e., cell wall and cytoplasm [C]). Cryparin was detected with a polyclonal antibody to cryparin. (B) Detection of glycosylated cryparin by using the Glycotrack carbohydrate detection kit. Only the cryparin extracted from mycelium was shown to possess carbohydrate residues.
DISCUSSION
Fungal hydrophobins such as cryparin are well suited for use to study protein secretion, because they are ubiquitous and large amounts are produced and secreted during growth. During secretion, the procryparin sequence appears to undergo posttranslational glycosylation. We assume that the glycosylation occurs in the pro region because it is transient and because glycosylated cryparin was detected only in secretory vesicles, never in the mature protein that had been secreted into the culture medium. Serine or threonine residues of the proprotein are presumed to be glycosylated in an O-linked conformation, as seen in the SC3 hydrophobin of Schizophyllum commune (12). Sacchoromyces cerevisiae α-factor, like cryparin, is glycosylated in the pro region during secretion, but following Kex2 cleavage, the glycosylated portion is removed, leaving a mature secreted protein that is not glycosylated (5).
Secretion of cryparin is relatively rapid, and the proprotein is transient and was never detected by pulse-labeling. Due to the antigenic nature of carbohydrate, however, the transient glycosylated form of cryparin could be detected in purified vesicles with a polyclonal antibody which had been raised against cellular cryparin (1). Cryparin isolated from the vesicles showed a processed pattern similar to post-Golgi secretory mutants in yeast when α-factor secretion is monitored (8). This similarity suggests that the vesicles purified from C. parasitica are also post-Golgi.
The pulse-label studies of the secretion of cryparin have provided some insights into the binding of cryparin to the fungal cell wall. Past studies have demonstrated clearly that cryparin is present in both the culture fluid and the cell wall (1). Since protein secretion is assumed to occur through the cell wall, the easiest conceptual model to explain the presence of cryparin in both the cell wall and culture fluid is that cryparin binds to the cell wall as it is secreted and that saturation of the binding sites within the wall results in the release of excess cryparin into the culture fluid. The results of the pulse-label study do not support this model, since the bulk of labeled cryparin was first found to be free in the culture fluid and only later to be bound to the cell wall (Fig. 2). Cryparin binding to the cell wall was rapid and increased with time. During the 6-h duration of the assay, there was no saturation of labeled cryparin and no equilibrium was reached (Fig. 2). All of the available free labeled cryparin was quickly bound to the cell wall and remained there throughout the period of the assay. Because cryparin has lectin-like properties and most lectins are glycoproteins, it would be expected that the cryparin found in the cell wall is glycosylated; however, addition of crude unglycosylated cryparin from the culture medium to cells that lack cryparin also showed rapid and stable binding of cryparin to these cell walls, and within a 4-h period, almost as much cryparin could be detected associated with the walls of the strain from which cryparin was genetically deleted as with the wild-type cryparin-producing strain (Fig. 3).
Pulse-chase labeling has also allowed us to gain some insight into where cryparin is secreted. Protein secretion in filamentous fungi commonly occurs at the growing hyphal tip (7, 16). Our results are consistent with such a model. This pattern would explain the lack of initial binding of cryparin to the cell wall during secretion, because the targets for cryparin binding are not yet present in the developing wall of the hyphal tip. Once the targets are present and cryparin is in the culture fluid, binding to the cell wall follows rapidly. It also is possible that cryparin is secreted through portions of the mycelium in which the cell wall is already saturated with cryparin, thus explaining the lack of initial binding of cryparin to the cell wall. Because cryparin is found under field conditions only in fruiting bodies (1), secretion through cryparin-saturated cell walls may be the simplest explanation. Under our culture conditions, the older hyphae, which may be saturated with cryparin, would be the most analogous tissue to the older tissues in fungal stromata that produce the fruiting bodies.
It is postulated (15) that hydrophobins are secreted from hyphae and then form a stable layer on the surface of hyphae when they are in contact with a hydrophobic/hydrophilic interface. This model explains why hydrophobins are found only on aerial hyphae and conidia in most fungi. This proposed mechanism of interaction of hydrophobins with hyphal cell walls is clearly not how cryparin binds to cell walls. Cryparin is found in culture fluids and binds to cell walls of submerged hyphae under conditions lacking a hydrophobic-hydrophilic interface. It is likely that the lectin-like properties of cryparin are responsible for this binding to the cell wall. Previous studies have shown that cell walls of C. parasitica can inhibit the lectin-like binding of cryparin to specific erythrocyte types (1). While SC3 must be treated with trifluoroacetic acid to release it from the fungal cell wall, cryparin can easily be extracted from the cell walls into 60% ethanol. Lectin-like properties have not been reported for other type II hydrophobins, but similar, easily disrupted bonding to cell walls could explain some properties of these hydrophobins. Our pulse-chase experiments clearly demonstrate that the binding of cryparin to the cell walls is very efficient, since within a 4-h period, all detectable labeled cryparin was scavenged by the cell walls. There was no evidence for cryparin turnover in these experiments; all free cryparin remained bound.
Hydrophobins are known to be secreted proteins due to a signal peptide in their protein sequence and their presence in liquid growth media. Our current work provides experimental evidence for the secretion of cryparin, via vesicles, through the cell wall and into the culture medium. The protein undergoes extensive posttranslational modifications during its transport through the cell. First the signal peptide is removed, presumably in the endoplasmic reticulum, followed by glycosylation of the proregion, which is subsequently removed by a Kex2-like serine protease. Serine protease cleavage results in the mature form of cryparin, which can be purified from both the cell wall and culture fluid. Cryparin is unique in that it is secreted first into the culture media during submerged liquid growth and then rapidly associates with the mycelial cell wall, where it remains.
ACKNOWLEDGMENTS
This work was supported by grants from the USDA National Research Initiative (96-35303-3401) and the National Science Foundation (MCB-9205818).
We thank Joanna Mirabile and Darrah Rippy for technical assistance.
REFERENCES
- 1.Carpenter C E, Mueller R J, Kazmierczak P, Zhang L, Villalon D K, Van Alfen N K. Effect of a virus on accumulation of a tissue-specific cells-surface protein of the fungus Cryphonectria (Endothia) parasitica. Mol Plant-Microbe Interact. 1992;4:55–61. doi: 10.1094/mpmi-5-055. [DOI] [PubMed] [Google Scholar]
- 2.Fahima T, Kazmierczak P, Hansen D R, Pfeiffer P, Van Alfen N K. Membrane-associated replication of an unencapsidated double-stranded RNA of the fungus Cryphonectria parasitica. Virology. 1993;195:81–89. doi: 10.1006/viro.1993.1348. [DOI] [PubMed] [Google Scholar]
- 3.Fahima T, Wu Y, Zhang L, Van Alfen N K. Identification of the putative RNA polymerase of Cryphonectria hypovirus in a solubilized replication complex. J Virol. 1994;68:6116–6119. doi: 10.1128/jvi.68.9.6116-6119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen D R, Van Alfen N K, Gilles K, Powell W A. Naked dsRNA association with hypovirulence of Endothia parasitica is packaged in fungal vesicles. J Gen Virol. 1985;66:2605–2614. [Google Scholar]
- 5.Julius D, Schekman R, Thorner J. Glycosylation and processing of prepro-α-factor through the yeast secretory pathway. Cell. 1984;36:309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- 5a.Kazmierczak, P., D.-H. Kim, L. Zhang, and N. K. Van Alfen. Unpublished observations.
- 6.Kershaw M J, Talbot N J. Hydrophobins and repellents: proteins with fundamental roles in fungal morphogenisis. Fungal Genet Biol. 1998;23:18–33. doi: 10.1006/fgbi.1997.1022. [DOI] [PubMed] [Google Scholar]
- 7.Mouka S M, Wosten H A B, Asther M, Wessels J G H. In situ localization of the secretion of lignin peroxidases in colonies of Phanerochete chrysosporium using sandwiched mode of culture. J Gen Microbiol. 1993;139:969–978. doi: 10.1099/00221287-139-5-969. [DOI] [PubMed] [Google Scholar]
- 8.Novick P, Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peberdy J F. Protein secretion in filamentous fungi—trying to understand a highly productive black box. Trends Biotechnol. 1994;12:50–57. doi: 10.1016/0167-7799(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 10.Puhalla J E, Anagnostakis S L. Genetics and nutritional requirements of Endothia parasitica. Phytopathology. 1971;61:169–173. [Google Scholar]
- 11.Punt P J, Veldhuisen G, Van den Hondel C A M J J. Protein targeting and secretion in filamentous fungi. Antonie Leeuwenhoek. 1994;65:211–216. doi: 10.1007/BF00871949. [DOI] [PubMed] [Google Scholar]
- 12.Schuren F H J, Wessels J G H. Two genes specifically expressed in fruiting dikaryons of Schizophyllum commune: homologies with a gene not regulated by mating type genes. Gene. 1990;90:199–205. doi: 10.1016/0378-1119(90)90180-y. [DOI] [PubMed] [Google Scholar]
- 13.Valverde V, Delmas P, Kaghad M, Loison G, Jara P. Secretion and maturation study of endothiapepsin in Saccharomyces cerevisiae. J Biol Chem. 1995;270:15821–15826. doi: 10.1074/jbc.270.26.15821. [DOI] [PubMed] [Google Scholar]
- 14.Wessels J G H. Hydrophobins: proteins that change the nature of the fungal surface. Adv Microb Physiol. 1997;38:1–45. doi: 10.1016/s0065-2911(08)60154-x. [DOI] [PubMed] [Google Scholar]
- 15.Wosten H A B, Asgeirsdottir S A, Kroole J H, Drenth J H H, Wessels J G H. The fungal hydrophobin Sc3p self-assembles at the surface of aerial hyphae as a protein membrane constituting the hydrophobic rodlet layer. Eur J Cell Biol. 1994;63:122–129. [PubMed] [Google Scholar]
- 16.Wosten H A B, Moukha S M, Sietsma J H, Wessels J G H. Localization of growth and secretion of proteins in Aspergillus niger. J Gen Microbiol. 1991;137:2017–2023. doi: 10.1099/00221287-137-8-2017. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Vilallon D, Sun Y, Kazmierczak P, Van Alfen N K. Virus-associated down-regulation of the gene encoding cryparin, an abundant cell-surface protein from the chestnut blight fungus, Cryphonectria parasitica. Gene. 1994;139:59–64. doi: 10.1016/0378-1119(94)90523-1. [DOI] [PubMed] [Google Scholar]