Abstract
Social isolation during the juvenile and adolescent stages (peri-adolescent social isolation) can have long-term consequences for behavioral and neural development. Most of this research, however, has relied on data from males, and very few studies have included both sexes. The present study investigated the impact of peri-adolescent social isolation on social preference, anxiety-like behavior, and vasopressin neural circuitry of male and female Long Evans rats. Rats were either housed alone for 3 weeks beginning at weaning (Isolated) or in groups (Group-housed). In adulthood, rats were tested in social preference, open field, marble burying, and light/dark box tests, and brains were processed for vasopressin immunohistochemistry. Isolated males exhibited a lower social preference score and spent more time in the light zone of the light/dark box than their group-housed counterparts. Isolated and Group-housed females did not differ in these measures. Peri-adolescent social isolation did not alter vasopressin fiber density in target areas known to influence social and anxiety-like behaviors (the lateral septum or lateral habenula), but increased fiber density in an output pathway of the circadian pacemaker (projections to the paraventricular nucleus of the thalamus); an effect detected across both sexes. A previously unreported sex difference was also detected for vasopressin fiber density in the paraventricular nucleus of the thalamus (females > males). These findings demonstrate long-term consequences of peri-adolescent social isolation on social preference, anxiety-like behavior, and the circadian vasopressin pathway and suggest that socio-affective development of males is more vulnerable to social stressors during the juvenile and adolescent stages.
Introduction
Childhood and adolescence are crucial stages for cognitive, social, and emotional development. During this period, individuals display increased sensitivity to stressful and rewarding stimuli (Eiland & Romeo, 2013; Walker et al., 2017), and adverse experiences can have lasting impacts on the brain, behavior, and mental health (Gutman & Nemeroff, 2003; Lukkes, Watt, et al., 2009; Walker et al., 2019). Adversity increases the risk of many physical, psychological, and behavioral disorders including anxiety, depression, post-traumatic stress disorder, and substance use disorder (McFarlane et al., 2005; Espejo et al., 2007; Heim et al., 2008; Childhood Welfare Information Gateway, 2019) all of which display stark sex differences in their emergence and presentation (Wittchen et al., 1998; Merikangas et al., 2010; Avenevoli et al., 2015; Gogos et al., 2019). Social interactions during childhood and adolescence influence the development of maladaptive behaviors in adulthood (Patterson et al., 1992; Hankin et al., 1998) and negative social interactions during these life stages are associated with increased rates of depression (Thapar et al., 2012). Hence, understanding the impacts of the juvenile and adolescent social environment on neurobiology and behavior of both sexes is critical to our understanding of mental health disorders.
Socially isolating rodents at, or shortly after, weaning (post-weaning social isolation) has long-term consequences for neural and behavioral development and is often used as a preclinical model to study the neurobiology underlying childhood and adolescent adversity (Fone & Porkess, 2008; Burke et al., 2017). Post-weaning social isolation protocols can vary markedly across studies, with animals isolated at different ages and for different durations (for a detailed review of these protocols see Lukkes, Watt, et al., 2009). Broadly speaking, there are two main categories of post-weaning social isolation protocols. In most studies, isolated animals remain single-housed for the remainder of the experiment and are tested while still in isolation. This protocol has recently been referred to as isolation housing (Burke et al., 2017). In other studies, animals are only isolated during the juvenile and adolescent periods then re-housed in groups for several weeks before testing. To emphasize the transient nature of the isolation period, we refer to this second protocol as peri-adolescent social isolation (PASI), meaning that isolation occurs around, but is not solely restricted to, adolescence. Distinguishing between isolation housing and PASI is important, and results can differ depending on which protocol is used. Behavior tests of isolation housing protocols can be affected by the ongoing isolation stress at the time of testing, whereas those of PASI protocols can be affected by the re-socialization period occurring after re-housing (Lukkes, Watt, et al., 2009). In the present study, we used the PASI protocol, because unlike isolation housing, it can assess long-term effects of post-weaning social isolation that persist after the cessation of the social stressor.
PASI has a long-term impact on social development, but most of this research has relied on data from males. PASI has been repeatedly shown to decrease prosocial behaviors of adult male rats in the social interaction test (e.g., social approach, social contact/investigation; Hol et al., 1999; Van den Berg et al., 1999; Van Den Berg et al., 1999; Ferdman et al., 2007; Lukkes, Mokin, et al., 2009; Lukkes, Vuong, et al., 2009). To our knowledge, only one study has tested the impact of PASI on social behavior of female rats, and this study did not detect an effect of PASI in the social interaction test (Lukkes et al., 2012). Hence, whether there are long-term consequences of PASI in female rats remains an open question.
How PASI impacts social behavior of males (and possibly females) is not understood. PASI may impact social behavior by altering preference for social stimuli. Social preference can be assessed by providing an experimental animal the opportunity to interact with a novel object and novel conspecific, either simultaneously or sequentially. The novel conspecific is typically restrained in a cage that allows limited social contact (e.g., wire-mesh cage or plastic cage with small openings) to ensure that interactions are initiated by the experimental animal. The time spent investigating the novel object and novel conspecific are compared to assess the animal’s “preference” for social versus non-social stimuli. In these tests, rodents typically prefer social over non-social stimuli (e.g., Lukas et al., 2011; Toth & Neumann, 2013). Effects of PASI on social preference of rats have not been reported in either sex.
Social deficits from PASI could also be the result of increased anxiety during social interactions (Lukkes, Vuong, et al., 2009). Male rats subjected to PASI exhibit increased endocrine responses and fear during social or aggressive interactions (Van den Berg et al., 1999; Lukkes, Mokin, et al., 2009). This may reflect a more general effect on anxiety-like behavior, as male rats exposed to PASI can exhibit increased anxiety-like behavior in tests that do not include a social component (e.g., open field test and elevated plus maze; Wright et al., 1991; Lukkes, Mokin, et al., 2009; but see Weintraub et al., 2010). Data in females are scarce, but two studies have found little to no effect of PASI on anxiety-like behavior in the open field test (tested in the dark) and the elevated plus maze (Weintraub et al., 2010; Lukkes et al., 2012).
The vasopressin (VP) pathway emerging from cells in the bed nucleus of the stria terminalis (BNST) and medial amygdala (MeA) is a prime candidate for mediating the effects of PASI on social behavior. This pathway regulates a variety of social and anxiety-like behaviors (Landgraf et al., 1995; Liebsch et al., 1996; Beiderbeck et al., 2007; Veenema et al., 2012, 2013; Bredewold et al., 2014; Caldwell & Albers, 2016). Males have a greater number of VP cells in the BNST and MeA than females as well as more dense fiber projections to target areas such as the lateral septum (LS) and lateral habenula (LHb; reviewed in De Vries & Panzica, 2006; De Vries et al., 2014). Consequently, pharmacological and genetic manipulations of this pathway often impact social and affective behaviors in a sex-specific manner (Albers, 2015; Bredewold & Veenema, 2018; Rigney et al., 2019; Whylings et al., 2020). The BNST/MeA VP pathway continues to develop across the juvenile and adolescent periods (De Vries et al., 1981; Szot & Dorsa, 1993), raising the possibility that adverse environments during these life stages could derail its development. Only one study has assessed the effects of post-weaning social isolation on the BNST/MeA VP pathway, which assessed vasopressin 1a receptor (V1aR) binding after isolation housing. Oliveira et al. (2019) found that isolation housing reversed the sex difference in V1aR binding in the BNST but did not alter receptor binding in the dorsal lateral septum of male or female rats. Potential long-term effects on this pathway using the PASI protocol have not been tested.
In the present experiment, we tested the hypothesis that post-weaning social isolation has long-term consequences for social preference of male and female rats. We predicted that PASI would decrease social preference in male rats. Due to the dearth of information on the effects of PASI on female social behavior, we tested whether PASI affects social preference in females, but remained agnostic to the presence or direction of the effect. We further tested the hypotheses that PASI-induced deficits in social preference, if present, would be accompanied by increased anxiety-like behavior and altered density of BNST/MeA VP fiber projections. To determine whether effects of PASI on VP-ir fibers, if present, were specific to the BNST/MeA VP pathway, we also assessed VP-ir fibers in the paraventricular nucleus of the thalamus (PVT), which originate from cells located in the suprachiasmatic nucleus of the hypothalamus (SCN; Hoorneman & Buijs, 1982; Rood et al., 2013). The PVT is located at the same rostro-caudal level as the LHb, and fibers from these brain areas can be quantified from the same tissue sections within a single image. Hence, comparison of PVT and LHb vasopressin-ir staining provides a strong test of the hypothesis that group differences would manifest in one brain area, but not the other.
Methods
Animals and Housing Conditions
Fifty-nine offspring from 7 Long Evans rat breeding pairs from our colony were used as subjects. Litters were not culled or cross-fostered; offspring from each litter were distributed across groups. All rats (Group-housed and Isolated) were housed in plastic cages (44cm X 22.5cm X 20.5cm) with corn-cob bedding (Envigo, Indianapolis, IN). Room lights were set to a 12 h light/12 h dark cycle with lights off at 6:00 PM EST, and ambient temperature was maintained at 23°C. Food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University at Buffalo and were in accordance with the Guide for the Care and Use of Laboratory Animals.
Experimental Timeline
Figure 1 illustrates the timeline of the housing manipulations, testing, and sacrifice. Rats were weaned at 21 days of age and housed either alone (Isolated; n=14 females, 11 males) or in groups of 2–3 rats/cage (Group-housed; n=19 females, 15 males). All rats were housed in the same room as our colony, and hence, Isolated rats could smell and hear other rats in the room. After 3 weeks of isolation, Isolated rats were group-housed (2–3 rats/cage) for the remainder of the experiment. Four weeks later, all rats were tested in a combined open field and social preference test (at P70-71), the marble burying test (at P72-73), and the light/dark box test (at P74-75), with 1 day between behavioral tests. Rats were sacrificed 1–2 days after the light/dark box test, and brains were removed (at P75-77). A subset of brains (n=36, 9 per group) was processed for vasopressin immunohistochemistry.
Figure 1. Experimental Timeline.
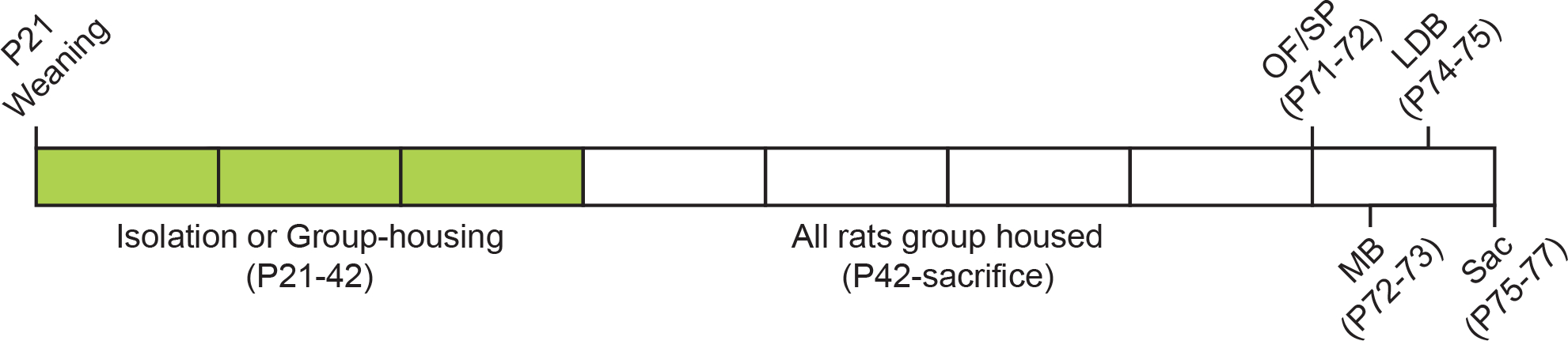
Abbreviations: OF/SP = combined open field / social preference test; MB = marble burying test; LDB = light/dark box test; Sac = sacrifice.
Behavioral Testing
Combined Open Field and Social Preference Test.
Open field and social preference tests were conducted on the same day between zeitgeber time (ZT)5.5 and ZT10.5 under white light; lights on = ZT0 and lights off = ZT12. In the open field phase of the test, a rat was placed in an open arena (73.7cm X 73.7cm X 47.3cm) for 20 min. This phase served as an open field test, but also allowed the animal to acclimate to the testing arena for subsequent phases of the social preference test. The rat was then briefly removed while a novel empty wire-mesh cage was placed along a wall of the arena and secured to the wall with suction cups. The rat was reintroduced along the wall opposite the empty cage and allowed to explore the arena and cage for 10 min (novel object phase). The rat was again removed briefly while the empty cage was replaced with a cage containing a novel, sex-matched adult stimulus rat. The experimental rat was reintroduced again to the arena along the opposite wall of the cage and allowed to investigate the arena and the caged stimulus animal for an additional 10 min (novel animal phase). All stimulus rats were acclimated to the arena and wire-mesh cage for 10 minutes per day for 5 days before the start of the first test. Behaviors were recorded using a camera mounted above the arena. For the open field phase, time spent in the center 25% of the arena and distance travelled were assessed. For the novel object and novel animal phases, time spent in the investigation zone surrounding the novel cage or novel animal was assessed. A social preference score was calculated as (time spent investigating the novel animal − time spent investigating the novel cage) / (time spent investigating the novel animal + time spent investigating the novel cage). All measures were scored automatically using EthoVision software (Noldus Information Technology Inc., Wageningen, The Netherlands).
Marble Burying Test.
Marble burying tests were conducted between ZT3.5 and ZT6 under white light. Each rat was placed in a fresh housing cage (plastic, 44cm X 22.5cm X 20.5cm) containing 5 cm of corn-cobb bedding and 12 large multi-colored marbles (diameter = 2.5cm) arranged in a 3 × 4 array on the surface of the bedding. Rats were allowed to freely move about and investigate the cage and marbles for 30 min. The number of marbles buried in the bedding by 2/3 or more was recorded at the end of the testing session.
Light/Dark Box Test.
Light/dark box tests were conducted between ZT8.5 and ZT11. The rat was placed inside a dark box (38.9cm X 12.7cm X 15.2cm) with a single entrance to an illuminated open arena (40.0cm X 39.9cm X 31.2cm). The rat was allowed to explore the light and dark zones of the apparatus for 10 min. Activity in the light zone was recorded by a camera mounted above the arena. Time spent in the light zone was scored manually by a researcher unaware of group assignments using Observer software (Noldus Information Technology Inc., Wageningen, The Netherlands).
Tissue Preparation, Immunohistochemistry, and Quantification
Tissue Preparation.
At sacrifice, rats were sacrificed by CO2 inhalation. Brains were removed and drop-fixed in 5% acrolein overnight, then immersed in 30% sucrose until microtome sectioning at a thickness of 40μm. Every fourth section was processed by immunohistochemistry for vasopressin.
Vasopressin Immunohistochemistry.
Sections were rinsed in phosphate-buffered saline (PBS), then incubated for 30 minutes in 0.05M sodium citrate (at 70°C), 0.1M glycine (at room temperature), and 10% normal goat serum, 0.4% Triton-X, and 1% hydrogen peroxide (at room temperature), with PBS rinses between each incubation. Sections were then incubated overnight with an anti-vasopressin antibody (1:20,000 dilution, T-4563, rabbit, Bachem, Torrance, CA) at room temperature. The following day, sections were incubated in secondary antibody (60 min; biotinylated goat anti-rabbit, BA-1000, Vector Laboratories, Burlingame, CA), ABC-Elite standard kit (60 min; Vector Laboratories), and Peroxidase DAB substrate kit (30 min; Vector Laboratories, SK-4100). The proportions of solutions of the DAB kit were adjusted to prolong the DAB reaction, thereby providing more consistent results between wells: 160μl Buffer, 320μl DAB, 2μl hydrogen peroxide, and 160μl nickel per 10ml of water. PBS rinses were conducted before and after each incubation. Sections were mounted onto gelatin-coated slides, air-dried, then coverslipped for microscope analysis.
This immunohistochemistry protocol was optimized for the detection of parvocellular vasopressin fibers. In double-label immunofluorescent pilot experiments, we found that the dilution of the primary antibody in this protocol faintly co-labels magnocellular oxytocin cells of the paraventricular and supraoptic nuclei of the hypothalamus. To be certain that measures of the present experiment reflect vasopressin staining, we restricted our analysis to brain areas where there are no oxytocin fibers (LHb and PVT) or where they are very rarely detected (LS).
Quantification of Vasopressin-ir Fibers and Cell Bodies.
VP staining was visualized on a Nikon Eclipse Ni-U microscope (Nikon Instruments, Melville, NY). For most animals, 3 images were taken of the LS, LHb, and PVT – one from the rostral, mid, and caudal aspects of each brain area. The density of VP fibers was quantified by a researcher unaware of group assignments as the integrated density of staining above background using the threshold feature of ImageJ software (NIH, Bethesda, MD). For the LS and PVT, fiber density was quantified for both the left and right hemispheres within a single measure. For the LHb, fiber density was measured separately for the left and right hemispheres and then summed. Hence, rostral, mid, and caudal measures represented fiber density of both hemispheres. The integrated density across the rostral, mid, and caudal images was then averaged to provide a mean integrated density measure of each brain area for each animal. In some sections, tissue damage/folding or staining artifacts made accurate quantification impossible. Animals were included in statistical analyses as long as 2 of the 3 images could be quantified. The number of animals where 2 images could not be quantified, and therefore excluded, was: 2 for the LS, 0 for the LHb, and 0 for the PVT.
Statistical Analyses
Behavioral measures were assessed using a 2 X 2 ANOVA with housing (Isolated versus Group-housed) and sex as independent variables. When main effects or interactions were significant, post hoc comparisons were conducted using the Tukey test. Significance was assumed when P<0.05. Social preference was assessed in two ways. First, a social preference score was calculated (see above for equation) and differences were assessed using a 2 X 2 ANOVA with housing and sex as independent variables. We then tested whether the social preference scores of each group were statistically different from chance (i.e., no preference). Negative social preference scores indicate a preference for the novel object, whereas positive social preference scores indicate a preference for the novel animal. Equal investigation of both stimuli results in a 0 social preference score. Therefore, a One-Sample t-Test was conducted on each group to determine whether their social preference scores were statistically different from 0. All measures were assessed for outliers using the Box and Whiskers plot in SPSS that defines outliers as greater than 1.5 times the interquartile range, and outliers were removed from subsequent statistical analyses (Table 1). Potential effects of group-housing density (2/cage versus 3/cage) were assessed in Group-housed rats for each measure using a 2 X 2 ANOVA with housing density and sex as independent variables. Housing density did not alter social preference, open field, or vasopressin-ir measures, and hence, statistical analyses proceeded with both group-housing densities included as a single group for these measures. The number of marbles buried in the marble burying test and the time spent in the light zone of the light/dark box test, however, were impacted by group-housing density. Because of insufficient sample size in females housed 3/cage (there was only a single cage of 3 females), conclusions regarding effects of this housing density were considered unreliable, and rats housed at 3/cage (both male and female) were removed from marble burying and light/dark box analyses. Hence, the Group-housing variable for these tests consisted only of animals housed at 2/cage. Note that this led to a marked reduction in the sample size of Group-house males to n=6. Final group sample sizes for each analysis are denoted in the bars of each graph.
Table 1.
Number of outliers within each group.
| Measure | Group-housed Females | Group-housed Males | Isolated Females | Isolated Males |
|---|---|---|---|---|
| Social Preference Score | 2 | 0 | 2 | 1 |
| OF Time in Center | 0 | 0 | 0 | 1 |
| OF Distance Travelled | 1 | 1 | 2 | 0 |
| MB Marbles Buried | 0 | 0 | 1 | 0 |
| LDB Time in Light | 0 | 0 | 0 | 0 |
| AVP LS | 0 | 1 | 0 | 0 |
| AVP LHb | 0 | 0 | 0 | 1 |
| AVP PVT | 0 | 1 | 0 | 0 |
Results
Peri-adolescent social isolation decreases social preference in male rats
Isolated rats had a lower social preference score than Group-housed rats (Fig. 2A; F(1,50)=7.19, P=0.01, ηp2=0.13, main effect of housing, ANOVA), and there was a trend toward a significant interaction between housing and sex (P=0.078, ANOVA). Group-housed females, Group-housed males, and Isolated females each exhibited a significant preference for the novel animal over the novel cage (Group-housed females: P=0.002, Cohen’s d=0.91, One-Sample t-Test; Group-housed males: P=0.042, Cohen’s d=0.58, One-Sample t-Test; Isolated females: P=0.0008, Cohen’s d=1.33, One-sample t-Test). Isolated males, however, did not exhibit a significant preference for either stimulus (P=0.24, One-sample t-Test). Because the One-Sample t-Test suggested that PASI only impacted male rats and the ANOVA housing X sex interaction approached significance (P=0.078), we conducted the Tukey post hoc test as a second assessment of whether the main effect of housing in the ANOVA statistical approach was significant for both sexes. In post hoc comparisons, the effect of PASI was significant in males (P=0.019, Cohen’s d=0.99, Isolated males versus Group-housed males, Tukey test), but not in females (P=0.92, Isolated females versus Group-housed females, Tukey test).
Figure 2. Peri-adolescent social isolation decreases social preference, but not locomotor activity of male rats.
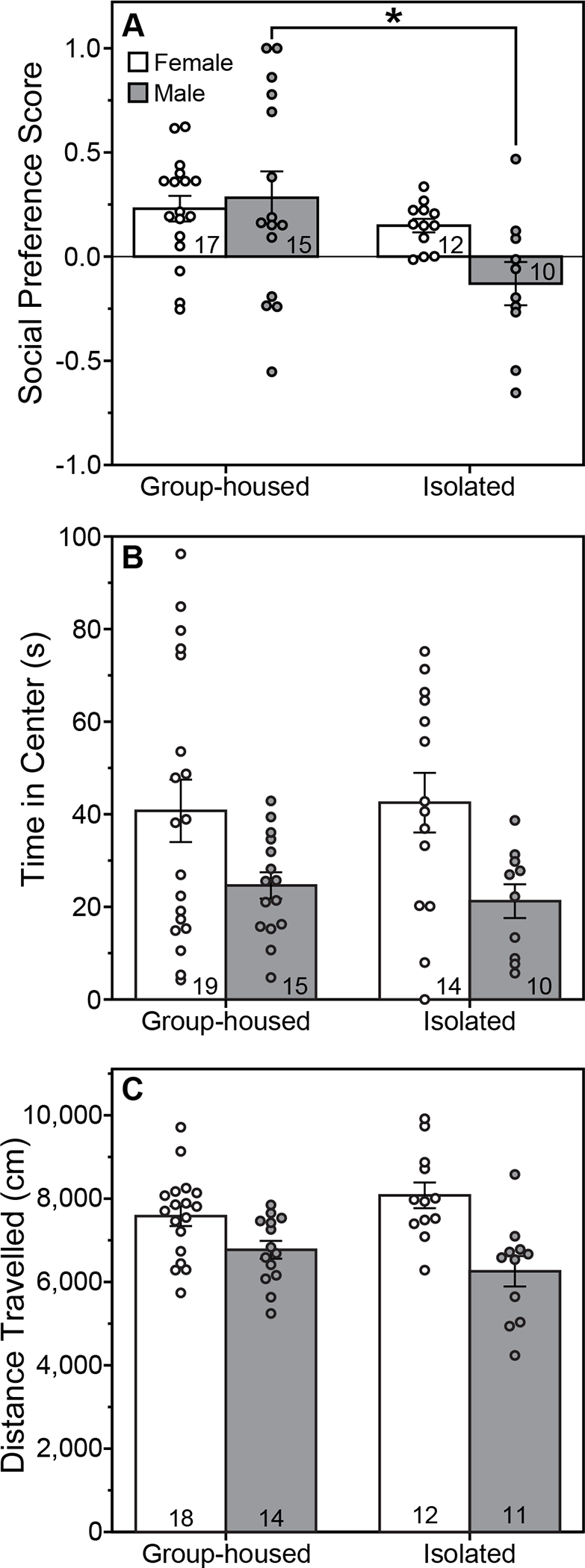
Performance on the social preference test (A) and open field test (B,C) of male and female rats group-housed or isolated from P21-P42 and tested between P70-71. (A) Mean (±s.e.) social preference score during the social preference test. (B) Mean (±s.e.) time spent in the center zone and (C) mean (±s.e.) distance travelled during an open field test. *Indicates significant difference between Group-housed and Isolated male rats (P<0.05, Tukey test). Females spent more time in the center and travelled a greater distance than males in the open field test (P<0.05, main effect of sex, ANOVA). Sample size indicated within bars.
PASI did not alter the time spent in the center of the open field (Fig. 2B; F(1,54)=0.02, P=0.89 main effect of housing, ANOVA; F(1,54)=0.19, P=0.66, housing X sex interaction, ANOVA) or the distance travelled in the arena (Fig. 2C; F(1,51)=0.001, P=0.97, main effect of housing, ANOVA; F(1,51)=3.29, P=0.08, housing X sex interaction, ANOVA). Females spent more time in the center (F(1,54)=9.98, P=0.003, ηp2=0.16, main effect of sex, ANOVA) and travelled a greater distance (F(1,51)=22.13, P=0.00002, ηp2=0.30, main effect of sex, ANOVA) than males.
Peri-adolescent social isolation alters anxiety-like behavior in the light/dark box test, but not the marble burying test
There was a significant interaction between housing and sex for the number of marbles buried in the marble burying test (Fig. 3; F(1,42)=5.66, P=0.02, ηp2=0.12, housing X sex interaction, ANOVA). Isolated males tended to bury more marbles than isolated females, but this difference fell just short of significance (P=0.05, Tukey Test); Group-housed males and females did not differ on this measure (P=0.82, Tukey Test). PASI did not significantly impact the number of marbles buried in either males (P=0.19, Tukey Test) or females (P=0.59, Tukey Test).
Figure 3. Peri-adolescent social isolation does not alter anxiety-like behavior in the marble burying test.
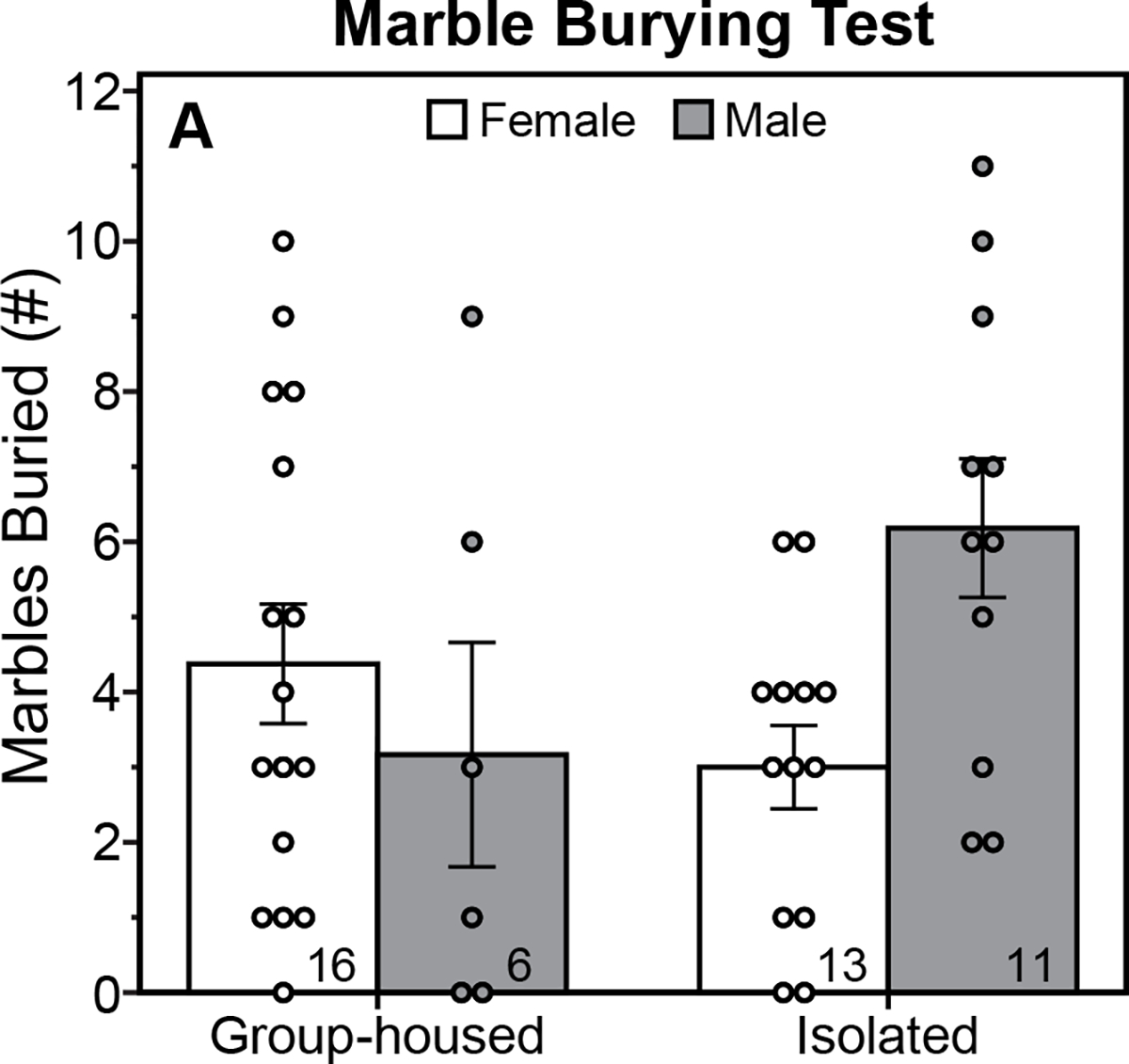
Mean (±s.e.) number of marbles buried by male and female rats, group-housed or isolated from P21-P42 and tested between P72-73. Sample size indicated within bars.
PASI affected the amount of time spent in the light zone of the light/dark box test (Fig. 4; F(1,41)=19.78, P=0.00007, ηp2=0.33, main effect of housing, ANOVA), but in a sex-dependent manner (F(1,41)=4.55, P=0.04, ηp2=0.10, housing X sex interaction, ANOVA). PASI increased the amount of time male rats spent in the light zone of the light/dark box (P=0.001, Cohen’s d=2.39, Isolated males vs. Group-housed males, Tukey test), but did not alter this measure in females (P=0.23, Isolated females vs. Group-housed females, Tukey test). Although Group-housed females appeared to spend more time in the light zone than Group-housed males, this difference was not significant after post-hoc correction (P=0.12, Tukey Test).
Figure 4. Peri-adolescent social isolation decreases anxiety-like behavior of male rats in the light/dark box test.
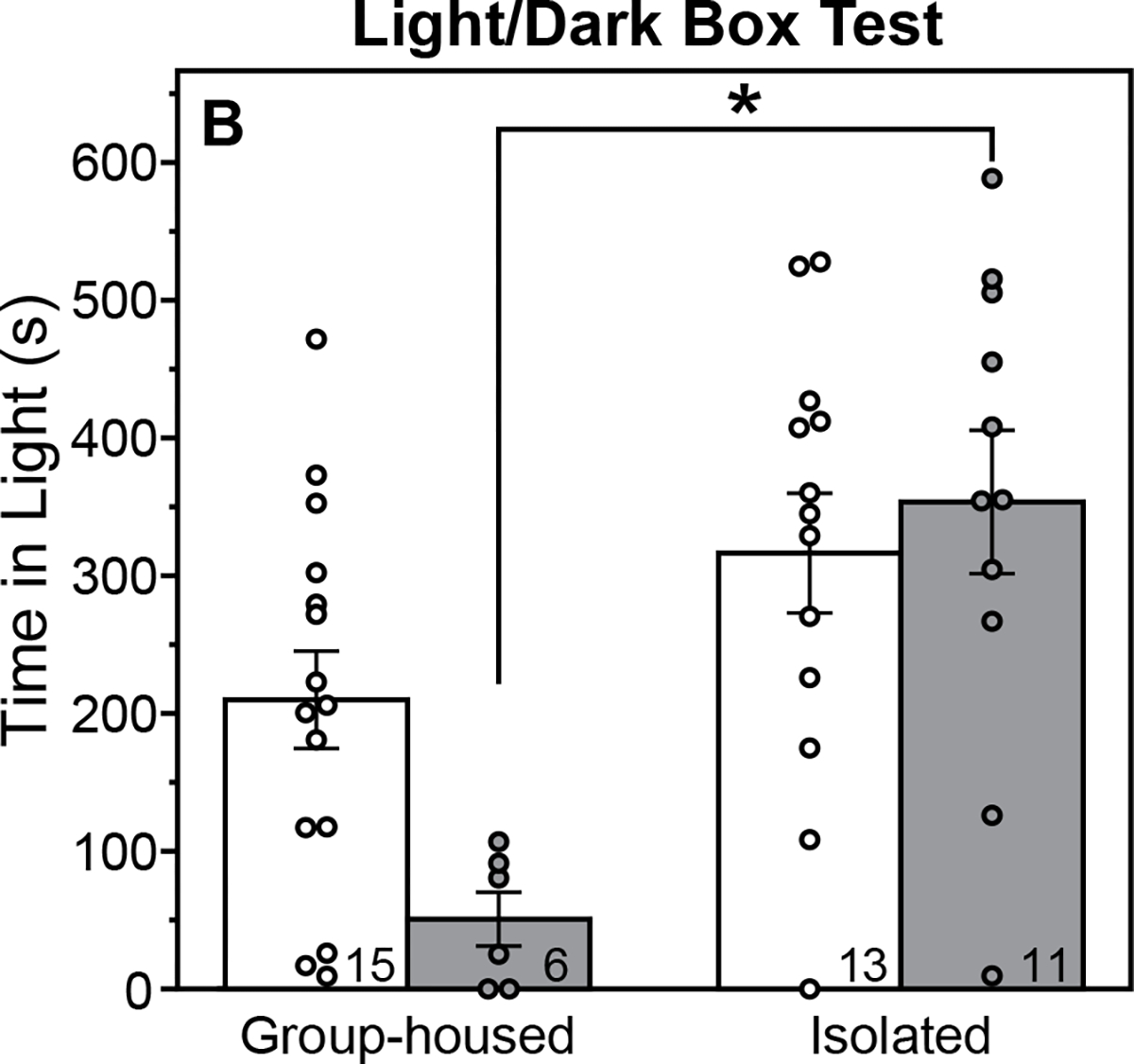
Mean (±s.e.) time spent in the light zone of the light/dark box test of male and female rats, group-housed or isolated from P21-P42 and tested between P74-75. *Indicates significant difference between Group-housed and Isolated male rats (P<0.05, Tukey test). Sample size indicated within bars.
Peri-adolescent social isolation impacts the circadian, but not BNST/MeA, VP pathway
Representative vasopressin staining in the LHb and PVT is illustrated in figure 5. Males had a greater density of VP-ir fibers in the LS and LHb compared to females (Fig. 6A&B; main effects of sex, ANOVA, F(1,29)=55.38, P=0.00000003, ηp2=0.66 for the LS; F(1,31)= 61.73, P=0.000000007, ηp2=0.67 for the LHb). PASI, however did not alter VP-ir fiber density in either brain area (F(1,29)=0.72, P=0.40, for the LS, F(1,31)=1.75, P=0.20, for the LHb, main effects of housing, ANOVA; F(1,29)=0.11, P=0.74, for the LS, F(1,31)=1.12, P=0.30, for the LHb, housing X sex interactions, ANOVA). For the PVT, females had a greater density of VP-ir fibers than males (Fig. 6C; F(1,31)=15.11, P=0.0005, ηp2=0.33, main effect of sex, ANOVA). PASI increased fiber density in the PVT (F(1,31)=5.8, P=0.02, ηp2=0.16, main effect of housing, ANOVA); the interaction was not significant (F(1,31)=0.22, P=0.64, ANOVA).
Figure 5. Vasopressin immunohistochemstry.
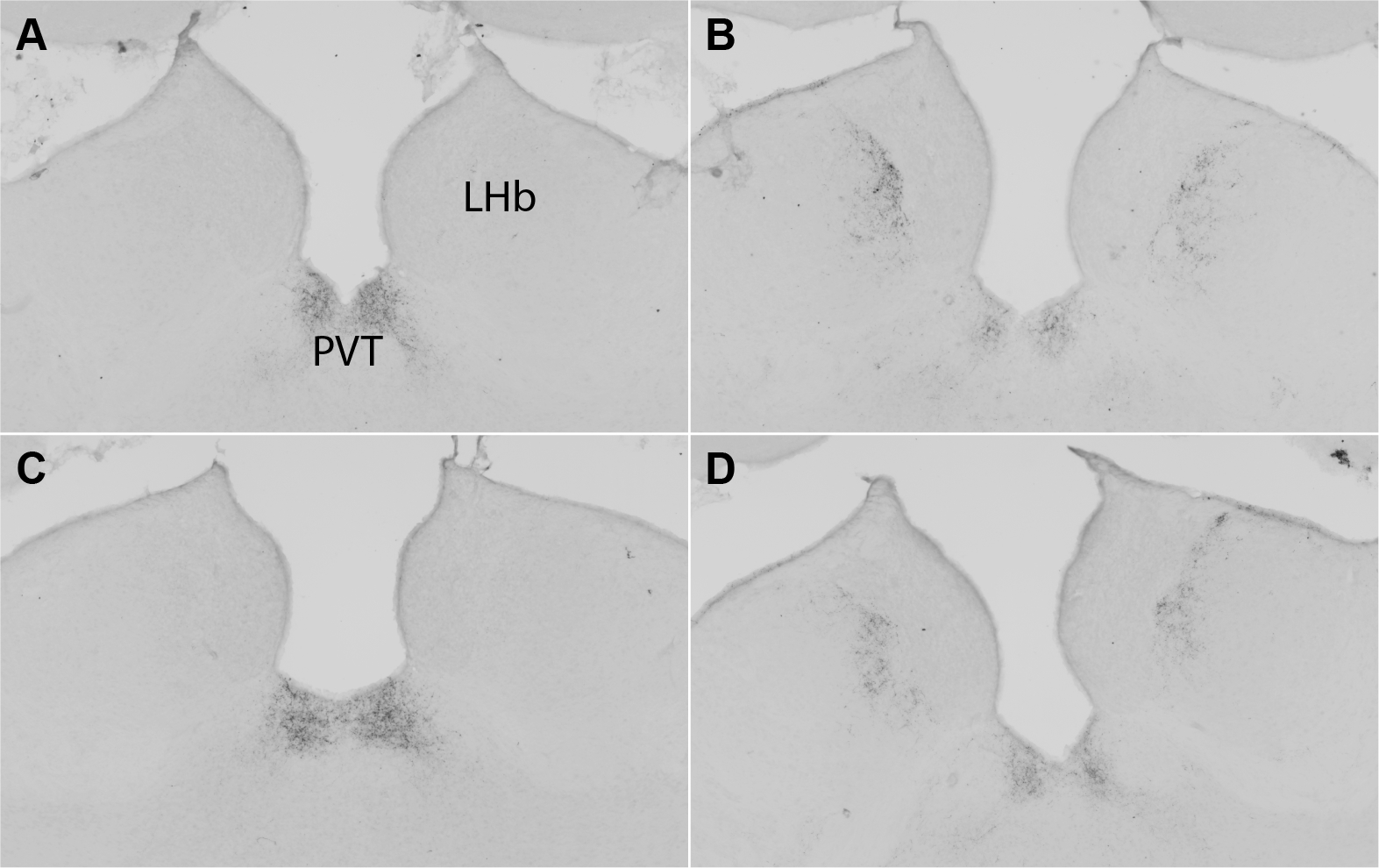
Representative vasopressin staining in the lateral habenula (LHb) and paraventricular nucleus of the thalamus (PVT) in a Group-housed female (A), Group-housed male (B), Isolated female (C), and Isolated male (D).
Figure 6. Peri-adolescent social isolation increases vasopressin fiber density in the paraventriulcar nucleus of the thalamus.
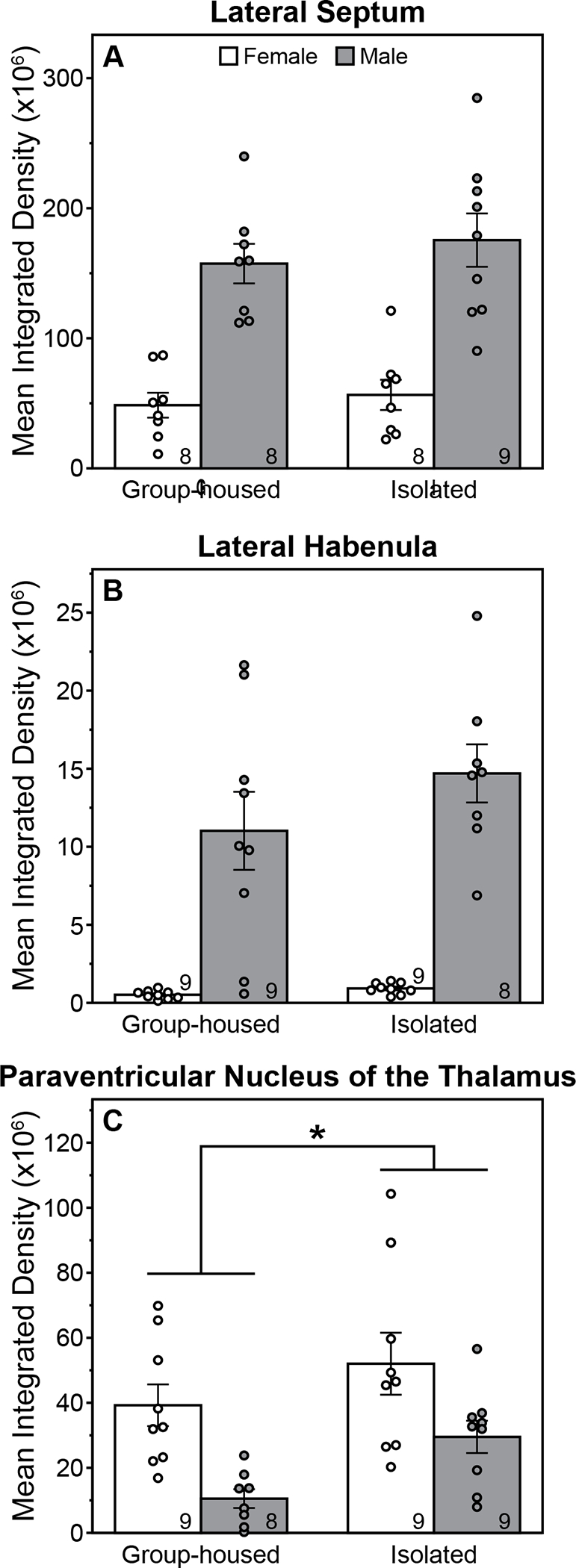
Mean (±s.e.) integrated density of vasopressin fibers in the lateral septum (A), lateral habenula (B), and paraventricular nucleus of the thalamus (C) of male and female rats, group-housed or isolated from P21-P42 and sacrificed between P75-77. *Indicates significant main effect of housing condition (P<0.05, ANOVA). Main effect of sex was also significant for all brain areas (P<0.05, ANOVA). Sample size indicated within bars.
Discussion
The present study was the first to test whether social isolation of male and female rats during the juvenile and adolescent periods has long-term consequences for social preference and vasopressin neural circuitry that persist beyond the isolation period. We further assessed the impact of PASI on anxiety-like behavior in 3 behavioral tests to test the hypothesis that deficits in social preference, if present, would be accompanied by increased anxiety-like behavior. Overall, we found that PASI decreased social preference, largely in males, but this effect was not accompanied by increased anxiety-like behavior or large structural changes in vasopressin pathways emanating from the BNST and MeA. Instead, PASI increased vasopressin fiber density in the paraventricular nucleus of the thalamus in both sexes, a projection site of the SCN.
PASI decreased social preference. Although the sex X housing interaction of the ANOVA fell short of significance, the One-Sample t-Test suggested the effect was restricted to males: Group-housed males, Group-housed females, and Isolated females exhibited a significant preference for the social stimulus, whereas Isolated males did not. Hence, we conducted post-hoc tests on the ANOVA as a second assessment of whether the significant main effect of housing was due to effects in both sexes. As with the One-Sample t-Tests, Tukey post-hoc tests indicated that the effect of PASI was only significant in males: Isolated males differed from Group-housed males, whereas Isolated females did not differ from Group-housed females. Collectively, these analyses suggest that the effects of PASI on social preference are more robust in males than in females. Previous studies have reported deficits in other prosocial behaviors after PASI, including decreased social approach and social contact of male rats (Hol et al., 1999; Van den Berg et al., 1999; Van Den Berg et al., 1999; Ferdman et al., 2007; Lukkes, Mokin, et al., 2009; Lukkes, Vuong, et al., 2009). The present finding suggests that decreased preference for social stimuli may contribute to PASI-induced deficits in prosocial behaviors of male rats.
In contrast to the present findings, Oliveira et al. (2019) did not detect an effect of isolation housing on social preference of male rats. Other studies have also reported differing effects of PASI and isolation housing on social behavior: e.g., isolation housing increases aggression of male rats in the resident-intruder test (Tóth et al., 2008; Toth et al., 2011), whereas PASI does not (Potegal & Einon, 1989). It is tempting to speculate that it is the social reintegration with novel, previously isolated cage mates, rather than the period of isolation itself, that leads to deficits in social preference after PASI. Alternatively, ongoing isolation at the time of social preference testing may maintain the salience of conspecific stimuli, thereby preventing a decrease in time spent investigating the stimulus animal during testing. For example, isolation-housed rats may continue to approach and investigate the stimulus animal due to elevated aggression. Testing during the inactive phase under white light also could have affected performance in the social preference test of the present experiment; Oliveira et al. (2019) tested animals in the dark phase under dim red light. White light can decrease investigation time in social preference tests (Rein et al., 2020). Importantly, rats in the present experiment spent a significant proportion of time investigating both the social and non-social stimuli, indicating that the presence of white light did not prevent exploratory behavior (mean novel object investigation time across groups = 250–311 s; mean social investigation time across groups = 285–443 s; out of a possible 600 s). Previous studies have conducted social preference tests in the dark phase under dim red light (e.g., Oliveira et al., 2019) and during the light phase under white light (e.g., Lukas et al., 2011; Smith et al., 2015). Several other methodological differences between the two studies could also contribute to the divergent findings, including strain of rat tested (Wistar versus Long Evans), behavioral testing history (prior resident-Intruder and elevated plus maze testing versus no prior testing), habituation to the arena (30 s versus 20 min), and duration of the social preference test (4 min versus 10 min).
The absence of a significant effect of PASI on social preference of females is largely consistent with the limited number of post-weaning social isolation studies conducted to date. Isolation housing does not impact the social preference of female rats (Oliveira et al., 2019), and neither PASI nor isolation housing alter prosocial behaviors of female rats in the social interaction test (Ferdman et al., 2007; Lukkes et al., 2012). Hermes et al. (2011) found that isolation housing coupled with early rearing decreases social interactions of female rats. Given the negative findings discussed above, it seems likely that the social deficits were due to, or at least contingent on, early weaning. Not all social behaviors of female rats are resilient to post-weaning social isolation. Isolation housing disrupts social discrimination and social recognition in female (and male) rats (Tanaka et al., 2010; Oliveira et al., 2019); whether similar effects are seen after PASI is not known. Future studies using a variety of social behavior assays are needed in order to fully understand the effects of isolation housing and PASI on female social behavior and to further test the hypothesis of greater vulnerability in males.
Our second prediction was that PASI-induced deficits in social preference would be accompanied by a general increase in anxiety-like behavior. Counter to our prediction, PASI increased exploratory behavior of male rats in the light/dark box test and did not affect performance on the open field and marble burying tests. These findings do not support the hypothesis that PASI-induced social deficits are due to increased anxiety. Consistent with previous PASI studies (Weintraub et al., 2010; Lukkes et al., 2012), we failed to detect an effect of PASI on anxiety-like behavior of females on all 3 anxiety-like behavior tests employed. Most studies using the isolation housing protocol also report limited effects on anxiety-like behavior of females in the elevated plus maze (Weiss et al., 2004; Jahng et al., 2012; Butler et al., 2014; but see Chmelova et al., 2019), but this may depend upon the behavioral test employed (Einon & Morgan, 1977; Arakawa, 2007).
Inconsistent anxiety-like behavioral responses after post-weaning social isolation are common in the literature, including across behavioral assays within individual studies (Weiss et al., 2004; McCool & Chappell, 2009; Skelly et al., 2015). It is important to note that factors other than anxiety can affect performance on these behavioral assays (Hascoët & Bourin, 1998; Prut & Belzung, 2003; de Brouwer et al., 2019). In the present experiment, PASI increased the time males spent in the light zone of the light/dark box test, but did not alter performance in open field or marble burying tests, raising the question as to whether PASI impacted anxiety or some other internal drive/behavioral state that could affect performance on the light/dark box test. Increased time in the light zone could be due to increased general locomotor activity (Hascoët & Bourin, 1998). However, locomotor activity in the open field, as measured by distance travelled, was not affected by PASI in the present experiment. Other studies also do not report increased locomotor activity after PASI (Lukkes, Mokin, et al., 2009; Lukkes, Vuong, et al., 2009). The light/dark box test is also impacted by rodents’ natural drive to explore novelty (Bourin & Hascoët, 2003). PASI increases contact time with novel objects in an open field (Einon & Morgan, 1977; Einon & Potegal, 1991). Hence, increased time in the light zone of the light/dark box test of isolated males could reflect increased drive to explore a novel illuminated environment. However, Isolated males did not exhibit significantly greater investigation of the novel stimulus cage during the social preference test (mean ± s.e.m. = 264.7 ± 50.6 and 296.5 ± 52.2 seconds for Group-housed and Isolated males, respectively; P=0.96, Tukey test). We did not set out to test the hypothesis that PASI impacts novelty seeking. Hence, future studies are required before definitive conclusions can be drawn.
Caution is warranted in the interpretation of the marble burying and light/dark box findings of the present experiment. Sample sizes of Group-housed rats, particularly those of Group-housed males, were markedly reduced, because preliminary analyses indicated that behavioral performance on these tests differed between animals housed 2/cage and those housed 3/cage (see Statistical Analyses section in the Methods). Hence, group-housing densities could not be analyzed as a single group in the marble burying and light/dark box tests. While this was a limitation of the present study, it raises the interesting possibility that the number of group-housed animals per cage can impact anxiety-like behavior. This could underlie some of the conflicting findings in the post-weaning social isolation literature, in which the number of animals housed per cage in the group-housed control often differs. Future studies are needed to directly test this hypothesis.
We failed to find support for our third prediction that PASI would alter VP-ir fiber density in the projections of the BNST/MeA VP pathway. As reported previously (De Vries et al., 1981; Rood et al., 2013), males had greater VP-ir fiber density than females in both the LS and LHb. PASI, however, did not alter VP-ir fiber density in either sex. Immunohistochemistry can only detect large structural changes. Hence, it remains possible that PASI impacts the microstructure or functioning of this pathway. Isolation rearing also did not alter V1aR binding in the LS but did reverse the sex difference in V1aR binding in the BNST (Oliveira et al., 2019).
Other VP pathways are altered by post-weaning social isolation and could contribute to the behavioral consequences of this early life stressor. Parvocellular VP cells in the paraventricular nucleus of the hypothalamus (PVN) regulate neuroendocrine and autonomic stress responses (Swanson & Sawchenko, 1980; Palkovits, 1999; Aguilera et al., 2008). Hence, changes in these cells would likely impact anxiety-like behavior and perhaps social behavior. Isolation housing decreases the number of VP-ir cells in the parvocellular division of the PVN in male, but not female rats (Tanaka et al., 2010), whereas PASI increases VP mRNA in the parvocellular division of the PVN of female, but not male rats (Weintraub et al., 2010). Isolation housing also decreases V1aR binding in the lateral hypothalamus and dentate gyrus of male and female rats (Oliveira et al., 2019). At present, however, the functional consequences of these effects are difficult to discern.
PASI increased VP-ir fiber density in the PVT of male and female rats. These fibers originate from the suprachiasmatic nucleus of the hypothalamus (SCN) and are thought to influence circadian rhythms (Hoorneman & Buijs, 1982; De Vries & Miller, 1998; Rood et al., 2013). Behavioral rhythms change during adolescence in several mammalian species, suggesting that the development of the circadian system continues across this life stage (Hagenauer & Lee, 2012). In rats, both behavioral (e.g., circadian period, phase, and chronotype) and anatomical (e.g., size of nucleus and nucleoli of cells in the SCN) changes across adolescence have been reported (Morishita et al., 1974, 1978; Anderson, 1981; Hagenauer et al., 2011). The present findings demonstrate that PASI disrupts the development of at least one circadian pathway and perhaps circadian regulation of behavior and/or physiology.
The present study uncovered a previously unknown sex difference – females had greater VP-ir fiber density in the PVT than males. To our knowledge, only one study has quantified VP fibers in the PVT of both sexes, but this study did not detect a sex difference in mice (Rood et al., 2013). Hence, the sex difference in VP PVT may be species-dependent. Greater VP fiber density in females has been reported for other circadian outputs, including the periventricular, retrochiasmatic, and dorsomedial nuclei of the hypothalamus (Rood et al., 2013). VP projections to the anteroventral periventricular nucleus of the hypothalamus regulate the circadian timing of the LH surge, and consequently ovulation, in females (Williams et al., 2011; Smarr et al., 2013; Bittman, 2019). The functional significance of sex differences in other VP outputs of the SCN is not known. Nevertheless, a role for VP in sex differences in behavioral timing is consistent with a recent finding that VP deficiency impacts circadian locomotor rhythms differently in male and female mice (Rohr et al., 2021).
The present study found sex differences in the long-term effects of PASI on the social and anxiety-like behavior of rats, with males being more robustly affected than females. The present findings do not support the hypothesis that increased anxiety-like behavior contributes to social deficits seen after PASI, although 2 of the 3 anxiety-like behavior assays were underpowered in the present study. Contradictory outcomes across anxiety-like behavior assays in this study and in the literature suggest that other affective states should be considered. PASI likely impacts multiple facets of affective behavior, which could explain conflicting findings across behavioral tests that vary in their sensitivity to different affective behaviors, e.g., anxiety-like behavior versus exploration/novelty seeking. Nevertheless, behavioral findings of the present experiment are consistent with the hypothesis that the social, and potentially affective, development of males is more vulnerable to the effects of juvenile and adolescent social isolation. PASI effects on the macrostructure (i.e., fiber density) of VP pathways were limited to the circadian output pathway to the PVT, raising the possibility that PASI impacts circadian regulation of behavior and/or physiology. Future studies into this possibility should include both males and females as several VP output pathways of the SCN are sexually dimorphic, with greater fiber density in females. The present study adds to this list with the discovery of a sex difference in VP fiber density in the PVT. Collectively, these findings highlight the importance of considering sex as a biological variable in post-weaning social isolation studies and the far-reaching consequences of juvenile and adolescent social stress.
Acknowledgements
The authors thank Kelcie Schatz, Nina Ventura, and Akua Ayoluwa for technical assistance and the University at Buffalo Laboratory Animal Facility personnel for providing excellent care to the animals used in these studies. This work was supported by the University at Buffalo Research Foundation (UBF) and the National Science Foundation (NSF; IOS-1754878). UBF and NSF had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations
- BNST
bed nucleus of the stria terminalis
- ir
immunoreactive
- LHb
lateral habenula
- LS
lateral septum
- MeA
medial amygdala
- PASI
peri-adolescent social isolation
- PBS
phosphate-buffered saline
- PVN
paraventricular nucleus of the hypothalamus
- PVT
paraventricular nucleus of the thalamus
- SCN
suprachiasmatic nucleus of the hypothalamus
- V1aR
vasopressin 1a receptor
- VP
vasopressin
- ZT
zeitgeber time
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Data Accessibility Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author (MJP) on request.
References
- Aguilera G, Subburaju S, Young S, & Chen J (2008) The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog. Brain Res, 170, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE (2015) Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocrinol, 36, 49–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CH (1981) Nucleolus: changes at puberty in neurons of the suprachiasmatic nucleus and the preoptic area. Exp. Neurol, 74, 780–786. [DOI] [PubMed] [Google Scholar]
- Arakawa H (2007) Ontogenetic interaction between social relationships and defensive burying behavior in the rat. Physiol. Behav, 90, 751–759. [DOI] [PubMed] [Google Scholar]
- Avenevoli S, Swendsen J, He J-P, Burstein M, & Merikangas KR (2015) Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J. Am. Acad. Child Adolesc. Psychiatry, 54, 37–44.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiderbeck DI, Neumann ID, & Veenema AH (2007) Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur. J. Neurosci, 26, 3597–3605. [DOI] [PubMed] [Google Scholar]
- Bittman EL (2019) Circadian function in multiple cell types is necessary for proper timing of the preovulatory LH surge. J. Biol. Rhythms, 34, 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M & Hascoët M (2003) The mouse light/dark box test. Eur. J. Pharmacol, 463, 55–65. [DOI] [PubMed] [Google Scholar]
- Bredewold R, Smith CJW, Dumais KM, & Veenema AH (2014) Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front. Behav. Neurosci, 8, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredewold R & Veenema AH (2018) Sex differences in the regulation of social and anxiety-related behaviors: insights from vasopressin and oxytocin brain systems. Curr. Opin. Neurobiol, 49, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, McCormick CM, Pellis SM, & Lukkes JL (2017) Impact of adolescent social experiences on behavior and neural circuits implicated in mental illnesses. Neurosci. Biobehav. Rev, 76, 280–300. [DOI] [PubMed] [Google Scholar]
- Butler TR, Carter E, & Weiner JL (2014) Adolescent social isolation does not lead to persistent increases in anxiety- like behavior or ethanol intake in female long-evans rats. Alcohol. Clin. Exp. Res, 38, 2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK & Albers HE (2016) Oxytocin, Vasopressin, and the Motivational Forces that Drive Social Behaviors. Curr. Top. Behav. Neurosci, 27, 51–103. [DOI] [PubMed] [Google Scholar]
- Childhood Welfare Information Gateway (2019) Long-Term Consequences of Child Abuse and Neglect. U.S. Department of Health and Human Services, Administration for Children and Families, Children’s Bureau, Washington, D.C., p. 9. [Google Scholar]
- Chmelova M, Balagova L, Marko M, Vrankova S, Cebova M, Jezova D, Riecansky I, & Hlavacova N (2019) Behavioral alterations induced by post-weaning isolation rearing of rats are accompanied by reduced VGF/BDNF/TrkB signaling in the hippocampus. Neurochem. Int, 129, 104473. [DOI] [PubMed] [Google Scholar]
- de Brouwer G, Fick A, Harvey BH, & Wolmarans DW (2019) A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive-compulsive disorder: Mapping the way forward. Cogn. Affect. Behav. Neurosci, 19, 1–39. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, & Swaab DF (1981) Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res, 218, 67–78. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Fields CT, Peters NV, Whylings J, & Paul MJ (2014) Sensitive periods for hormonal programming of the brain. Curr. Top. Behav. Neurosci, 16, 79–108. [DOI] [PubMed] [Google Scholar]
- De Vries GJ & Miller MA (1998) Anatomy and function of extrahypothalamic vasopressin systems in the brain. Prog. Brain Res, 119, 3–20. [DOI] [PubMed] [Google Scholar]
- De Vries GJ & Panzica GC (2006) Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience, 138, 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L & Romeo RD (2013) Stress and the developing adolescent brain. Neuroscience, 249, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon D & Potegal M (1991) Enhanced defense in adult rats deprived of playfighting experience as juveniles. Aggress. Behav, 17, 27–40. [DOI] [PubMed] [Google Scholar]
- Einon DF & Morgan MJ (1977) A critical period for social isolation in the rat. Dev. Psychobiol, 10, 123–132. [DOI] [PubMed] [Google Scholar]
- Espejo EP, Hammen CL, Connolly NP, Brennan PA, Najman JM, & Bor W (2007) Stress sensitization and adolescent depressive severity as a function of childhood adversity: a link to anxiety disorders. J. Abnorm. Child Psychol, 35, 287–299. [DOI] [PubMed] [Google Scholar]
- Ferdman N, Murmu RP, Bock J, Braun K, & Leshem M (2007) Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav. Brain Res, 180, 174–182. [DOI] [PubMed] [Google Scholar]
- Fone KCF & Porkess MV (2008) Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev, 32, 1087–1102. [DOI] [PubMed] [Google Scholar]
- Gogos A, Ney LJ, Seymour N, Van Rheenen TE, & Felmingham KL (2019) Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: Are gonadal hormones the link? Br. J. Pharmacol, 176, 4119–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA & Nemeroff CB (2003) Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol. Behav, 79, 471–478. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, King AF, Possidente B, McGinnis MY, Lumia AR, Peckham EM, & Lee TM (2011) Changes in circadian rhythms during puberty in Rattus norvegicus: developmental time course and gonadal dependency. Horm. Behav, 60, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH & Lee TM (2012) The neuroendocrine control of the circadian system: adolescent chronotype. Front. Neuroendocrinol, 33, 211–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, & Angell KE (1998) Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J. Abnorm. Psychol., 107, 128–140. [DOI] [PubMed] [Google Scholar]
- Hascoët M & Bourin M (1998) A new approach to the light/dark test procedure in mice. Pharmacol. Biochem. Behav, 60, 645–653. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, & Nemeroff CB (2008) The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology, 33, 693–710. [DOI] [PubMed] [Google Scholar]
- Hermes G, Li N, Duman C, & Duman R (2011) Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol. Behav, 104, 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol T, Van den Berg CL, Van Ree JM, & Spruijt BM (1999) Isolation during the play period in infancy decreases adult social interactions in rats. Behav. Brain Res, 100, 91–97. [DOI] [PubMed] [Google Scholar]
- Hoorneman EM & Buijs RM (1982) Vasopressin fiber pathways in the rat brain following suprachiasmatic nucleus lesioning. Brain Res, 243, 235–241. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Yoo SB, Ryu V, & Lee J-H (2012) Hyperphagia and depression-like behavior by adolescence social isolation in female rats. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci., 30, 47–53. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, & Engelmann M (1995) V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J. Neurosci. Off. J. Soc. Neurosci, 15, 4250–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch G, Wotjak CT, Landgraf R, & Engelmann M (1996) Septal vasopressin modulates anxiety-related behaviour in rats. Neurosci. Lett, 217, 101–104. [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, & Neumann ID (2011) The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol, 36, 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J, Vuong S, Scholl J, Oliver H, & Forster G (2009) Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J. Neurosci. Off. J. Soc. Neurosci, 29, 9955–9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Engelman GH, Zelin NS, Hale MW, & Lowry CA (2012) Post-weaning social isolation of female rats, anxiety-related behavior, and serotonergic systems. Brain Res, 1443, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, & Forster GL (2009) Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm. Behav, 55, 248–256. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, & Forster GL (2009) Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front. Behav. Neurosci, 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA & Chappell AM (2009) Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol. Clin. Exp. Res, 33, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane A, Clark CR, Bryant RA, Williams LM, Niaura R, Paul RH, Hitsman BL, Stroud L, Alexander DM, & Gordon E (2005) The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects. J. Integr. Neurosci, 4, 27–40. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, & Swendsen J (2010) Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry, 49, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Kawamoto M, Masuda Y, Higuchi K, & Tomioka M (1974) Quantitative histological changes in the hypothalamic nuclei in the prepuberal, puberal and postpuberal female rat. Brain Res, 76, 41–47. [DOI] [PubMed] [Google Scholar]
- Morishita H, Nagamachi N, Kawamoto M, Tomioka M, Higuchi K, Hashimoto T, Tanaka T, Kuroiwa S, Nakago K, Mitani H, Miyauchi Y, Ozasa T, & Adachi H (1978) The effect of prepuberal castration on the development of the nuclear sizes of the neurons in the hypothalamic nuclei of female rats. Brain Res, 146, 388–391. [DOI] [PubMed] [Google Scholar]
- Oliveira V.E. de M., Neumann ID, & de Jong TR (2019) Post-weaning social isolation exacerbates aggression in both sexes and affects the vasopressin and oxytocin system in a sex-specific manner. Neuropharmacology, 156, 107504. [DOI] [PubMed] [Google Scholar]
- Palkovits M (1999) Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Geoffrey Harris Memorial Lecture, Kitakyushu, Japan, October 1998. Front. Neuroendocrinol, 20, 270–295. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Smith LW, Smith TL, Yager J, & Grant I (1992) Symptoms of illness in late adulthood are related to childhood social deprivation and misfortune in men but not in women. J. Behav. Med, 15, 113–125. [DOI] [PubMed] [Google Scholar]
- Potegal M & Einon D (1989) Aggressive behaviors in adult rats deprived of playfighting experience as juveniles. Dev. Psychobiol, 22, 159–172. [DOI] [PubMed] [Google Scholar]
- Prut L & Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol, 463, 3–33. [DOI] [PubMed] [Google Scholar]
- Rein B, Ma K, & Yan Z (2020) A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc, 15, 3464–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigney N, Whylings J, Mieda M, de Vries G, & Petrulis A (2019) Sexually dimorphic vasopressin cells modulate social investigation and communication in sex-specific ways. eNeuro, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr KE, Telega A, Savaglio A, & Evans JA (2021) Vasopressin regulates daily rhythms and circadian clock circuits in a manner influenced by sex. Horm. Behav, 127, 104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJW, Woodbury ME, & De Vries GJ (2013) Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J. Comp. Neurol, 521, 2321–2358. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E, & Weiner JL (2015) Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology, 97, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr BL, Gile JJ, & de la Iglesia HO (2013) Oestrogen-independent circadian clock gene expression in the anteroventral periventricular nucleus in female rats: possible role as an integrator for circadian and ovarian signals timing the luteinising hormone surge. J. Neuroendocrinol, 25, 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJW, Wilkins KB, Mogavero JN, & Veenema AH (2015) Social Novelty Investigation in the Juvenile Rat: Modulation by the μ-Opioid System. J. Neuroendocrinol, 27, 752–764. [DOI] [PubMed] [Google Scholar]
- Swanson LW & Sawchenko PE (1980) Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology, 31, 410–417. [DOI] [PubMed] [Google Scholar]
- Szot P & Dorsa DM (1993) Differential timing and sexual dimorphism in the expression of the vasopressin gene in the developing rat brain. Brain Res. Dev. Brain Res, 73, 177–183. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Osako Y, & Yuri K (2010) Juvenile social experience regulates central neuropeptides relevant to emotional and social behaviors. Neuroscience, 166, 1036–1042. [DOI] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS, & Thapar AK (2012) Depression in adolescence. Lancet Lond. Engl, 379, 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I & Neumann ID (2013) Animal models of social avoidance and social fear. Cell Tissue Res, 354, 107–118. [DOI] [PubMed] [Google Scholar]
- Tóth M, Halász J, Mikics E, Barsy B, & Haller J (2008) Early social deprivation induces disturbed social communication and violent aggression in adulthood. Behav. Neurosci, 122, 849–854. [DOI] [PubMed] [Google Scholar]
- Toth M, Mikics E, Tulogdi A, Aliczki M, & Haller J (2011) Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Horm. Behav, 60, 28–36. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, & Koolhaas JM (1999) Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol, 34, 129–138. [PubMed] [Google Scholar]
- Van Den Berg CL, Van Ree JM, & Spruijt BM (1999) Sequential analysis of juvenile isolation-induced decreased social behavior in the adult rat. Physiol. Behav, 67, 483–488. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, & De Vries GJ (2012) Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav, 61, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, & De Vries GJ (2013) Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology, 38, 2554–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Bell MR, Flores C, Gulley JM, Willing J, & Paul MJ (2017) Adolescence and reward: making sense of neural and behavioral changes amid the chaos. J. Neurosci. Off. J. Soc. Neurosci, 37, 10855–10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Cunningham AM, Gregory JK, & Nestler EJ (2019) Long-Term Behavioral Effects of Post-weaning Social Isolation in Males and Females. Front. Behav. Neurosci, 13, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, & Bhatnagar S (2010) Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res, 1343, 83–92. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, & Feldon J (2004) Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res, 152, 279–295. [DOI] [PubMed] [Google Scholar]
- Whylings J, Rigney N, Peters NV, de Vries GJ, & Petrulis A (2020) Sexually dimorphic role of BNST vasopressin cells in sickness and social behavior in male and female mice. Brain. Behav. Immun, 83, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WP, Jarjisian SG, Mikkelsen JD, & Kriegsfeld LJ (2011) Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology, 152, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Nelson CB, & Lachner G (1998) Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol. Med, 28, 109–126. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, & Marsden CA (1991) Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol. Behav, 50, 1129–1132. [DOI] [PubMed] [Google Scholar]


