Abstract
Introduction
Kidney disease secondary to mercury poisoning has not been well documented and is often misdiagnosed and mistreated.
Methods
We performed a retrospective analysis of patients diagnosed with having mercury poisoning over a 6-year period between July 2013 and June 2019. Demographics, clinical measures, renal pathologic examinations, treatments, and outcomes were compared between patients with kidney disease and those without kidney disease.
Results
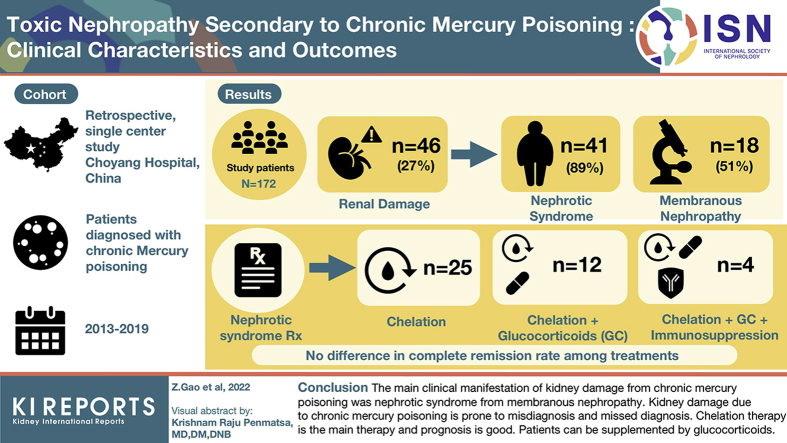
Of the 172 patients with mercury poisoning, 46 (26.74%) had renal damage. Among the 46 patients, 41 (89.13%) presented nephrotic syndrome, and 5 (10.87%) showed proteinuria alone. The pathologic abnormality associated with kidney disease caused by mercury poisoning was mainly membranous nephropathy (18 of 35 patients, 51.43%). Among 41 patients with nephrotic syndrome, 25 were treated with chelation therapy alone and 12 with mercury chelation therapy and glucocorticoids. The remaining 4 patients were treated with chelation therapy, glucocorticoids, and immunosuppressive therapies. The overall effective rate was 97.5% (40 patients). There was no significant difference in complete remission rate among the 3 treatment methods (P < 0.05).
Conclusion
The main clinical manifestation of kidney disease secondary to chronic mercury poisoning was nephrotic syndrome, which was reflected in pathologic examinations as membranous nephropathy. Kidney disease to chronic mercury poisoning is prone to misdiagnosis and missed diagnosis. Chelation therapy is the main treatment, and the prognosis is good. Patients with severe condition can be supplemented with glucocorticoid.
Keywords: clinical features, diagnosis and treatment, kidney disease, mercury poisoning
Graphical abstract
See Commentary on Page 1157
Mercury is a ubiquitous heavy metal with potential toxic impacts on human and environmental health.1,2 Mercury poisoning is common in China and is mainly caused by exposure to mercury vapor or mercury compounds in dust. Both occupational poisoning and nonoccupational poisoning occur. The presence of mercury compounds in cosmetics, which are illegally used to inhibit melanin production, whiten the skin, and remove facial plaque, is another source of poisoning. Misuse of Chinese traditional medicine is also a common source of mercury poisoning.3, 4, 5
When absorbed through the respiratory tract, the digestive tract, or the skin, mercury compounds dissociate to yield mercury ions after entering the blood. Subsequently, mercury ions accumulates in the brain and kidney and causes multiple organ damage in the renal, nervous, cardiovascular, and musculoskeletal systems.6, 7, 8 Because of the strong affinity of mercury for the kidney tissue, the kidney is one of the most susceptible organs to mercury poisoning. When mercury accumulates in the body to a certain degree, kidney disease occurs and is reflected by proteinuria and toxic encephalopathy. Clinically, patients with renal damage secondary to mercury poisoning typically show edema, urine volume changes, proteinuria, and/or nephrotic syndrome in addition to the typical clinical characteristics of mercury poisoning, such as excitability, gingivitis, and tremor.9 However, some patients have atypical or asymptomatic mercury poisoning. Thus, chronic mercury poisoning-associated renal damage is often misdiagnosed as primary nephritis or nephrotic syndrome and treated with glucocorticoids or immunosuppressants, such as ciclosporin A. Misdiagnosis and inappropriate treatment result in a variety of injuries to, and burdens upon, patients.10,11
Here, we summarized 46 patients with toxic nephropathy secondary to chronic mercury poisoning. Clinical manifestations, renal pathology, treatments, and prognosis were analyzed to enhance our understanding of the disease, thereby enabling early diagnosis and treatment of secondary renal damage. Moreover, we investigated whether glucocorticoids or immunosuppressants were essential for the treatment of toxic nephropathy secondary to chronic mercury poisoning.
Methods
Subjects
Retrospective analysis of 458 patients with chronic mercury poisoning in Beijing Chaoyang Hospital, Capital Medical University, from June 2013 to June 2019. Patient selection criteria: clear history of mercury exposure; definitely diagnosed as having mercury poisoning; no history of primary or secondary renal disease and abnormal urine examination; no history of drug allergy; and no history of infection, immune disease, or tumor before onset. After excluding the patients with repeated hospitalization (252 patients) and incomplete clinical data (34 patients), 172 patients with chronic mercury poisoning were enrolled in this study. The study underwent ethical review by the Capital Medical University. Experimental group: 46 patients with chronic mercury poisoning and renal injury. Control group: 126 patients of chronic mercury poisoning without renal injury.
Diagnostic Standard and Laboratory Examination
Diagnosis of chronic mercury poisoning was made according to the Diagnostic Criteria for Occupational Mercury Poisoning (GBZ089-2007)12 (nonoccupational mercury poisoning does not consider occupational history). All patients with elevated urinary mercury concentrations (normal reference, 0–2.25 mmol/mol creatinine [Cr]) together with clinical manifestations of chronic mercury poisoning, such as irritability, fatigue, dizziness, headache, tremor, gingivitis, proteinuria, nephrotic syndrome, or toxic encephalopathy, were included.
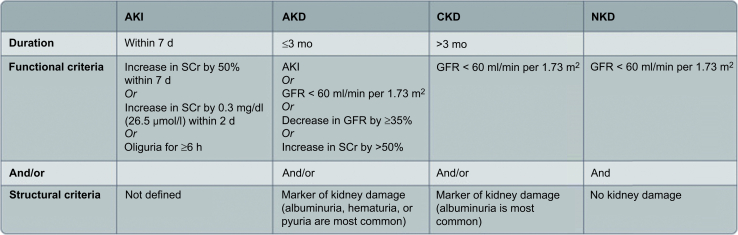
Defining “kidney diseases and disorders” (KD): The Kidney Disease: Improving Global Outcomes guidelines define KD as functional and/or structural abnormalities of the kidneys with implications for health13 (Figure 1).
Figure 1.
Functional and structural criteria for kidney diseases and disorders. AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; GFR, glomerular filtration rate; NKD, no kidney disease; SCr, serum creatinine.
Demographic and epidemiologic information was collected, including age, sex, duration of mercury exposure, mercury exposure type, and route of mercury exposure. Clinical data included clinical manifestations; severity of poisoning; laboratory tests (blood urea nitrogen, serum Cr, total cholesterol, urinary total protein); renal biopsies; and pathologic examinations, treatments, and outcomes.
Urinary mercury concentrations were determined by atomic fluorescence spectrometry14 (ASF-9800) and were adjusted for urinary Cr.
Treatments
All patients with chronic mercury poisoning received chelation therapy and symptomatic treatment. The sodium salt of 2,3-dimercaptopropane-1-sulfonic acid (DMPS, 0.25 g) was administered i.m. once a day for 3 days and then intermittently for 4 days. Following chelation therapy, 24-hour urinary samples were collected in clean containers to monitor urinary mercury levels. When the 24-hour urinary mercury level was <45.0 μg/d (normal reference <45.0 μg/d), chelation therapy with DMPS was terminated.12 Other treatments included reduced glutathione, trace elements, calcium supplementation, diuresis, anticoagulation, cholesterol-reducing medications, and symptomatic treatment to improve clinical symptoms.
For patients who had received glucocorticoids before admission to our hospital (e.g., prednisone 40–60 mg/d), slow dose reduction was recommended. For patients treated with immunosuppressive agents (cyclophosphamide, methotrexate, or cyclosporine A) outside of our hospital, treatment was discontinued on admission and cessation was recommended. Patients in the control group (no kidney disease) also received chelation therapy and symptomatic treatment.
Follow-Up and Outcomes
Definitions of clinical responses in patients with nephrotic syndrome were as follows15: (i) complete remission, 24-hour urinary protein <0.3 g/l; (ii) partial remission, 24-hour urinary protein >0.3 g/l but <3.5 g/l with stable glomerular filtration rate (decline of <15%); (iii) no remission, 24-hour urinary protein >3.5 g/l; and (iv) relapse, recurrence of 24-hour urinary protein >3.5 g/l after remission has been achieved. Complete and partial remissions of proteinuria are valid as surrogate markers of good outcomes, therapeutic goals, and end points for therapeutic trials.16, 17, 18 Patients were followed up for 6 months in our outpatient clinic to evaluate the effects of treatment and prognosis.
Statistical Analysis
Statistical analysis was performed using SPSS 23.0 software. Patients' characteristics were expressed as counts and percentages (%); differences between groups were assessed using the χ2 test. Normally distributed variables were expressed as means ± SDs; differences between groups were assessed using the Student t test. Non-normally distributed variables were expressed as medians and interquartile ranges (P25–P75); differences between groups were assessed using the Mann–Whitney U test. Correlation analysis was performed by calculating Pearson correlation coefficients between 2 variables. Values of P < 0.05 were considered statistically significant.
Results
General Characteristics of Patients
A total of 458 patients with chronic mercury poisoning were enrolled from June 2013 to June 2019. After excluding those with repeated hospitalizations and incomplete clinical data, 172 patients with chronic mercury poisoning were included in this study. A total of 46 patients with kidney disease were identified, and 126 patients without kidney disease were selected as controls. According to the criteria for diagnosis and classification of chronic mercury poisoning, 41 of 46 patients with kidney disease (89.13%) had moderate chronic mercury poisoning and nephrotic syndrome, whereas 5 patients (10.87%) had mild chronic mercury poisoning with proteinuria (Table 1). Among the 46 patients with kidney disease, 36 were females (78.26%) and 8 were males (21.74%). The average age of the patients was 39.8 ± 11.1 years old, mainly young and middle-aged. Mercury exposure time was 5 (3.6) months. Mercury poisoning was caused by industrial exposure in 2 patients (4.35%), cosmetics in 33 (71.74%), application of folk prescription in 10 (21.74%), and mercury pollution in the living environment in 1 (2.17%). Mercury exposure pathway: 37 (80.43%) were exposed to mercury in the skin, 3 (6.52%) in the respiratory tract, and 6 (13.04%) in the digestive tract. Contact mercury species: mercury compounds in 36 cases (97.83%) and metallic mercury in 1 case (2.17%). Sex, form of mercury exposure, mercury type, and route of mercury exposure were significantly different in the patients with kidney disease compared with the controls (P < 0.001), Age and duration of mercury exposure were similar in patients with and without kidney disease (P > 0.05). Compared with the controls without kidney disease, the proportion of women among the patients with kidney disease was higher (78.26% vs. 21.74%). In the patients with kidney disease, the main cause of poisoning was nonoccupational mercury poisoning, and the skin contact was mainly through the contact route, particularly through ionic mercury. Urinary mercury concentrations pretreatment were significantly different in the control patients (median 16.03 μmol/mmol Cr; interquartile range 7.32–39.56 μmol/mmol Cr) and the patients with kidney disease (median 33.06 μmol/mmol Cr; interquartile range 11.01–57.65 μmol/mmol Cr) (P < 0.05).
Table 1.
The general characteristics of patients with chronic mercury poisoning
| Variables | No kidney disease (n = 126) | Kidney disease (n = 46) | χ2/F | P |
|---|---|---|---|---|
| Sex, n (%) | 11.02 | 0.001 | ||
| Male | 63 (50) | 10 (21.74) | ||
| Female | 63 (50) | 36 (78.26) | ||
| Age (y) | ||||
| (x ± SD) | 40.48 (13.17) | 39.91 (10.76) | 0.264 | 0.8 |
| Kinds of exposed mercury, n (%) | 37.402 | <0.001 | ||
| Cosmetics | 31 (24.60) | 33 (71.74) | ||
| Folk prescription | 32 (25.40) | 10 (21.74) | ||
| Industrial exposure | 58 (46.03) | 2 (4.35) | ||
| Others | 5 (3.97) | 1 (2.17) | ||
| Mercury exposure pathway, n (%) | 129.77 | <0.001 | ||
| Skin | 55 (43.65) | 37 (80.43) | ||
| Respiratory | 57 (45.24) | 3 (6.52) | ||
| Digestive tract | 14 (11.11) | 6 (13.04) | ||
| Mercury species, n (%) | 28.762 | <0.001 | ||
| Metallic mercury | 58 (46.03) | 1 (2.17) | ||
| Mercury compounds | 68 (53.97) | 45 (97.83) | ||
| Duration of exposure time (M) | ||||
| M (P25–P75) | 12 (2–42) | 5 (3–6.5) | −1.496 | 0.1 |
| Urinary mercury concentration before treatment (μmol/mmol Cr) | ||||
| M (P25–P75) | 16.03 (7.32–39.56) | 33.06 (11.01–57.65) | −2.11 | 0.04 |
Cr, creatinine; M, median.
Clinical Features of Patients
The main clinical manifestations of mercury poisoning were nervous system and kidney diseases as shown in Table 2. Some patients were asymptomatic and showed only proteinuria and elevated urinary mercury concentrations. In all 46 patients with mercury poisoning and kidney disease, urea nitrogen levels and blood Cr levels were normal. Plasma albumin in the 41 patients with nephrotic syndrome was decreased, and significant proteinuria was evident. B-ultrasound examination of the kidneys revealed no obvious abnormalities in kidney size and morphology for all patients.
Table 2.
Clinical characteristics of chronic mercury poisoning with kidney disease
| Variables | Clinical findings | No. (case) | Percentage, % |
|---|---|---|---|
| Nervous system | Hypodynamia | 15 | 26.32 |
| Insomnia | 4 | 5.26 | |
| Dizziness/headache | 4 | 7.89 | |
| Hypomnesis | 2 | 5.26 | |
| Tremor | 5 | 7.89 | |
| Change of temper character | 1 | 2.63 | |
| Numbness of limbs | 1 | 2.63 | |
| Urinary system | Proteinuria | 46 | 100 |
| Edema | 41 | 89.47 | |
| Oliguria | 3 | 7.89 | |
| Urine increased | 1 | 2.63 | |
| Digestive system | Abdominal pain | 3 | 7.89 |
| Musculoskeletal | Myalgia | 4 | 5.26 |
| Joint pain | 1 | 2.63 | |
| Oral stomatitis | Oral gingivitis | 3 | 5.26 |
Mercury levels in a sample of a whitening cosmetic used by a patient with nephrotic syndrome were measured. The mercury concentration was 4.6 × 103 mg/kg (national standard <1 mg/kg), placing the product in a high-risk category.
Pathologic Examinations of Patients With Nephrotic Syndrome
Among the 41 patients with nephrotic syndrome, 35 underwent percutaneous renal biopsies. Pathologic examinations identified 18 cases of membranous nephropathy (51.43%), 13 cases of minimal-change disease (37.14%), 3 cases of mesangial proliferative glomerulonephritis (8.57%), and 1 case of mesangial proliferative IgA nephropathy (2.86%). The following are the pathologic results of 2 patients with nephrotic syndrome:
-
1.
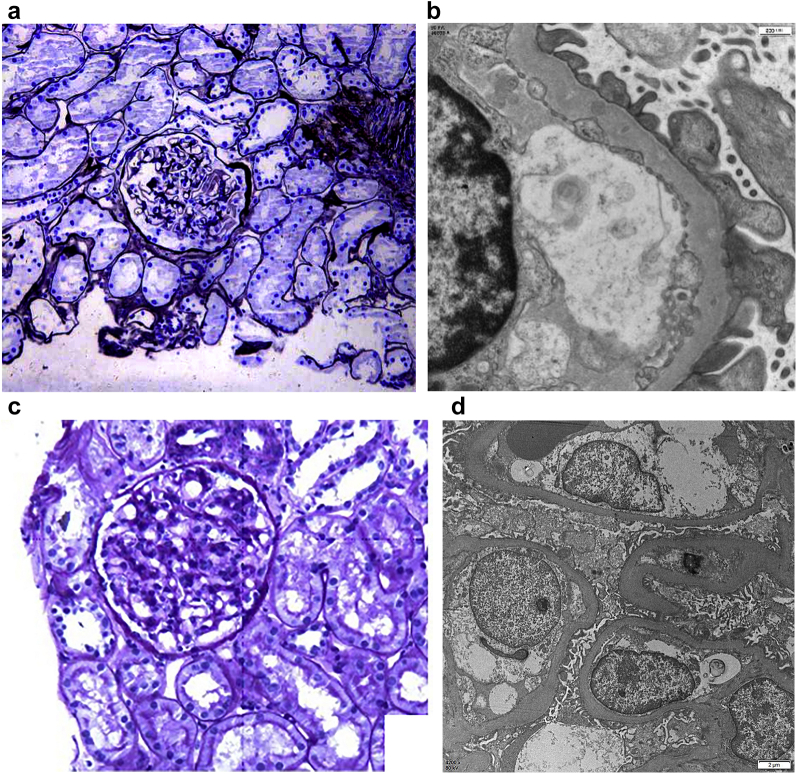
A 50-year-old man with chronic mercury poisoning and nephrotic syndrome. His urinary mercury level was 84.401 μmol/mol Cr (normal reference <2.25 μmol/mol Cr). His pathologic examination result shows a type of membranous nephropathy (Figure 2a and b).
-
2.
A 25-year-old woman with nephrotic syndrome secondary to chronic mercury poisoning because of cosmetics misuse. Her urinary mercury level was 46.25 μmol/mol Cr. Her pathologic examination result showed a minimal-change disease type (Figure 2c and d).
Figure 2.
Representative images from a case of membranous nephropathy (a and b) and minimal-change disease (c and d) from patients with chronic mercury poisoning. (a) Glomerulus with thickened capillary loops. (b) Small subepithelial immune complex deposits. (c) Unremarkable glomerulus. (d) Global podocyte foot process effacement.
Correlation Between Chelation Therapy and 24-Hour Urinary Total Protein Levels
All patients with toxic nephrotic syndrome received chelation therapy. After 1 to 8 courses of DMPS therapy, 24-hour urinary mercury concentrations in all 41 patients returned to normal (<45.0 μg/d). Urinary mercury concentrations peaked 24 hours after the first course of chelation therapy and then gradually decreased. After the second course of treatment, 24-hour urinary total protein levels were higher than pretreatment levels (P < 0.05), implying DMPS may cause transiently elevated urinary protein levels. With the gradual decline of urinary mercury concentrations, 24-hour urinary total protein level declined (Table 3) and plasma albumin level increased (Table 4).
Table 3.
The 24-hour urinary total mercury and 24-hour urinary total protein in patients with chronic mercury-induced nephrotic syndrome during chelation therapy
| Variables | Case number | 24-h urinary mercury (μg/d) | Total urine protein (mg/d) |
|---|---|---|---|
| 1 | 41 | 963.6 (353.1–1716.8) | 3809 (585–5578) |
| 2 | 37 | 148.2 (67.9–355.2) | 4065 (681–5446) |
| 3 | 30 | 104.8 (47.16–159.7) | 2246 (478–3456) |
| 4 | 21 | 54.6 (41–113.6.0) | 446 (126–3359) |
| 5 | 11 | 56.8 (44.5–105.8) | 468 (246–679) |
| 6 | 8 | 64.8 (54.5–67.7) | 149 (89–467) |
| 7 | 2 | 36.4–55.4a | 571–469a |
| 8 | 1 | 32.6a | — |
—, no such data; M, median.
Only 1 or 2 patients could not be counted as M (P25–P75).
Table 4.
Analysis of laboratory examination indexes of patients with chronic mercury poisoning renal damage pre- and post-treatment (x ± SD)
| Group | Total urine protein (g/d) | Serum creatinine (μmol/l) | Urea nitrogen (mmol/l) | Serum albumin (g/l) | Cholesterol (mmol/l) |
|---|---|---|---|---|---|
| Normal reference range | Negative | 44–106 | 2.2–7.2 | 35–55 | 0–5.17 |
| Pretreatment | 5.7 ± 5.4 | 59.5 ± 9.7 | 3.9 ± 1 | 29.6 ± 11.4 | 5.9 ± 3.6 |
| Post-treatment | 2.4 ± 1.9 | 56.0 ± 9.0 | 3.1 ± 1.3 | 35.2 ± 8.8 | 4.4 ± 1.9 |
| T | 3.276 | 1.32 | 0.35 | 3.42 | 3.34 |
| P | 0.001 | 0.3 | 0.9 | 0.01 | 0.02 |
We analyzed correlations between the urinary mercury concentrations and urinary total protein, serum albumin, and serum total cholesterol levels on admission. We found a weak positive correlation between urinary mercury concentrations and urine total protein levels (r = 0.35, P < 0.05) but no significant correlation between urinary mercury concentrations and serum albumin or serum total cholesterol levels (P > 0.05).
Treatments and Outcomes of Chronic Mercury-Induced Renal Injury
All 46 patients received chelation therapy and symptomatic treatment. In the 5 patients with proteinuria only, proteinuria disappeared soon after chelation therapy. Clinical responses of the 41 patients with chronic mercury poisoning-induced nephrotic syndrome are shown in Table 5. Among the 25 patients treated with chelation therapy alone, 22 achieved complete remission and 3 achieved partial remission while in hospital. In addition, 4 patients were treated with chelation and a glucocorticoid, oral prednisone tablets 40 to 60 mg, once a day, every morning, until urine protein level gradually reduced. Complete remission was achieved after 2 to 6 courses of treatment. There was 1 patient who was exposed to mercury again after remission and had a relapse after 1 month. There were 12 patients who had no definite diagnosis of mercury poisoning initially and were only treated as having primary nephrotic syndrome. Furthermore, 8 patients were treated with glucocorticoid, in whom 3 achieved complete relief after 2 months of treatment and had a relapse after 1 month; 5 patients achieved partial remission after glucocorticoid treatment for 2 to 3 months. These 8 patients were treated with chelation after the diagnosis of mercury poisoning, in whom 7 achieved complete remission and 1 achieved partial remission. There were 4 patients who had partial relief after 2 to 6 months of glucocorticoid and immunosuppressive therapy before diagnosis of mercury poisoning. After diagnosis of mercury poisoning, immunosuppressive therapy was discontinued, and complete remission was achieved after 2 to 4 courses of chelation therapy. Patients with complete remission were followed for 6 months without relapse. The overall effective rate was 97.5% (40 patients).
Table 5.
Outcomes of different treatments for chronic mercury-induced nephrotic syndrome
| Treatments | Outcomes |
Total | Effective rate, % | |||
|---|---|---|---|---|---|---|
| Complete remission | Partial remission | Nonremission | Relapse | |||
| Chelation therapy | 22 | 3 | 0 | 0 | 25 | 100 |
| Chelation therapy + corticosteroids | 10 | 1 | 0 | 1 | 12 | 91.67 |
| Chelation therapy + corticosteroids + Immunosuppressive therapy | 4 | 0 | 0 | 0 | 4 | 100 |
| Total | 36 | 4 | 0 | 1 | 41 | |
Because of the small sample size, Fisher’s exact test was used to compare the efficacy of the different treatment methods. There was no significant difference in complete remission rate among the 3 treatment methods (P < 0.05). Among the 18 patients with membranous nephropathy, 10 (55.55%) received only chelation therapy, and among the 13 patients with minimal-change disease, 8 (61.54%) received only chelation therapy. Chelation therapy was 100% in these patients.
Discussion
In this study, we found that chronic mercury poisoning-induced renal damage was more common in women and was clinically manifested as nephrotic syndrome in most patients. The major pathologic type was membranous nephropathy. The prognosis of secondary kidney disease following chronic mercury poisoning was good with DMPS therapy. Recovery occurred slowly following chelation therapy, and corticosteroids were indicated only under specific conditions, such as lack of efficacy or severe nephrotic syndrome. Urinary mercury concentrations and urine total protein levels were weakly correlated, implying mercury-induced renal damage may be the combined result of immune mechanisms and direct toxicity of mercury rather than any single factor.
Nephrotic syndrome is usually caused by a variety of factors, including increased glomerular basement membrane permeability, and is characterized by a significant proteinuria, hypoproteinemia, edema, and hyperlipidemia. There have been sporadic pathologic reports on changes in human kidneys secondary to mercury poisoning. These reports showed that the pathologic features associated with mercury poisoning included membranous nephropathy and minimal-change disease, focal segmental glomerulosclerosis, mesangial proliferative IgA nephropathy, and renal tubular damage in varying degrees.19, 20, 21 Among the 35 patients in this study who underwent renal puncture biopsy, 48.6% (17 of 35) had membranous nephropathy and 37.1% (13 of 35) had minimal-change disease. These results were consistent with the literature.18,19
The pathogenesis of mercury-induced kidney disease has not yet been fully elucidated. It is believed that immune mechanisms play important roles in glomerular diseases. Mercury combines with proteins to form haptens, which produce antigen-antibody complexes through immune reactions; subsequently, these complexes penetrate the glomerular membrane leading to formation of glomerular lesions.22,23 Mercury itself has negative impacts on the immune system and can promote autoantibody production, inhibit T lymphocyte function, and induce autoimmune diseases.24,25 Mercury ions have strong renal tubular toxicity, resulting in vacuolation, degeneration, and necrosis of the tubular epithelial cells. Together, these effects result in kidney disease. Furthermore, mercury binds sulfhydryl groups in vivo, resulting in decreased levels of sulfhydryl-containing enzymes, decreased activity of membrane sulfhydryl-containing proteins (such as sodium ion-potassium ion adenosine triphosphatases), and changes in the structure and function of the cell membrane. Levels of active free radicals outside the cell increase, whereas the activities of free radical scavenging systems decrease, resulting in oxidative stress injury.26
In this study, pretreatment urinary mercury levels in patients with kidney disease were significantly higher than those in control patients without kidney disease. Urinary mercury concentrations and urine total protein levels were only weakly correlated (r = 0.35), indicating higher urinary mercury levels are more likely to lead to kidney disease. Animal studies have shown a dose-effect relationship between renal cell apoptosis and mercury level.27,28 With increasing doses of exposure, levels of renal cell apoptosis increased gradually. In our study, we found a large range of pretreatment urinary mercury concentrations in patients with kidney disease (range: 4.36 μmol/mol Cr–128.32 μmol/mol Cr; normal reference <2.25 μmol/mol Cr). Some patients with low levels of urinary mercury had severe nephrotic syndrome, implying kidney disease caused by mercury poisoning could be a combined result of direct injury and immune mechanisms.
It remains unclear whether glucocorticoids or immunosuppressants are essential for patients with mercury poisoning and toxic nephropathy. In our study, treatment with DMPS alone was effective in the 25 patients (61%) with mercury-induced nephrotic syndrome. No significant differences in the complete remission rate were observed compared with patients receiving DMPS with glucocorticoids or immunosuppressants. Following chelation therapy, the symptoms of mercury poisoning gradually disappeared, edema and elevated urinary protein levels subsided, and nephrotic syndrome reached clinical cure standards. Thus, the clinical course of chronic mercury poisoning is significantly different from primary and recurrent nephrotic syndrome.
The above-mentioned results show that chelation therapy alone can achieve good results in patients with chronic mercury poisoning-associated nephrotic syndrome. Glucocorticoids are not recommended for first-line therapy. In our opinion, glucocorticoids should only be used in patients in whom chelation therapy is ineffective in ameliorating secondary severe nephrotic syndrome or refractory nephrotic syndrome, or in patients with severe clinical characteristics, such as hypoproteinemia, edema, and progressive hyperlipidemia. Furthermore, immunosuppressants are not recommended in this patient group because damage to the kidney is secondary.
Mercury is mainly excreted via the kidney, and urinary mercury concentrations reflect recent mercury exposure and the efficacy of mercury excretion. Our study showed that urinary mercury concentrations peaked after the first course of chelation therapy and then gradually decreased. The decline was fastest initially and slowed, consistent with the literature.29 The 24-hour urinary total protein level is an early indicator of renal injury and can also provide a reference for prognosis and recovery. In this study, we found that urinary albumin level increased significantly between the first and second courses of chelation therapy and then decreased. A potential explanation of these data is that DMPS has high affinity for mercury and can replace the mercury ion to form a mercury complex that is rapidly discharged in the urine. This process increases the burden on the kidney and leads to increased 24-hour urinary total protein levels. However, with urinary excretion of mercury, urine protein levels showed a downward trend. Therefore, attention should be paid to the patient's tolerance during mercury expulsion, and intermittent use of chelating agents may be required to avoid excessive mercury expulsion and aggravation to renal injury. This strategy is consistent with the conclusions of a previous report.30
Clinically, it is worth noting that renal damage caused by mercury poisoning is mainly manifested as typical primary nephrotic syndrome. Most patients have no obvious specific features, including neurologic symptoms, and some were asymptomatic. Clinicians should be vigilant and pay close attention to potential history of toxic exposure to avoid misdiagnosis (Supplementary Table S1). Furthermore, mercury-induced renal damage was more common among women and primarily resulted from application of cosmetics. No confirmed evidence has suggested sex as a risk factor for chronic kidney disease, though some studies showed that the mercury excretion rate in female was lower than that in male animals.31 However, in animal models of mercury-induced nephrotoxicity, the degree of damage to renal tubules in female rats was lower than in male rats.32
Conclusion
Our study showed that the major pathologic type of kidney disease secondary to mercury poisoning was membranous nephropathy. Prognosis secondary to chronic mercury poisoning following chelation therapy was good. Patients with kidney disease recovered slowly with chelation therapy, and supplemental corticosteroids can be administered in patients in whom chelation therapy is ineffective or patients with severe nephrotic syndrome. Nephropathy secondary to mercury poisoning often leads to misdiagnosis and delays in administration of appropriate treatments. Thus, the possibility of mercury poisoning should be excluded in the diagnosis and treatment of idiopathic nephropathy in patients with a history of toxic exposure.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The present study and production to this article were funded by the National Natural Science Foundation of China (grants 81773373, 81172614, and 81441089). The authors thank Dr. Xiaoli Zhu of Department of Occupational Medicine and Clinical Toxicology, Beijing Chaoyang Hospital, for helping with data collection.
Footnotes
Table S1. Characteristic table of chronic mercury poisoning.
Supplementary Material
Table S1. Characteristic table of chronic mercury poisoning.
References
- 1.Budnik L.T., Casteleyn L. Mercury pollution in modern times and its socio-medical consequences. Sci Total Environ. 2019;654:720–734. doi: 10.1016/j.scitotenv.2018.10.408. [DOI] [PubMed] [Google Scholar]
- 2.Obrist D., Kirk J.L., Zhang L., Sunderland E.M., Jiskra M., Selin N.E. A review of global environmental mercury processes in response to human and natural perturbations: changes of emissions, climate, and land use. Ambio. 2018;47:116–140. doi: 10.1007/s13280-017-1004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamath S.U., Pemiah B., Sekar R.K., Krishnaswamy S., Sethuraman S., Krishnan U.M. Mercury-based traditional herbo-metallic preparations: a toxicological perspective. Arch Toxicol. 2012;86:831–838. doi: 10.1007/s00204-012-0826-2. [DOI] [PubMed] [Google Scholar]
- 4.Mo T., Sun S., Wang Y., Luo D., Peng B., Xia Y. Mercury poisoning caused by Chinese folk prescription (CFP): a case report and analysis of both CFP and quackery. Medicine. 2016;95:e5162. doi: 10.1097/MD.0000000000005162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu M.L., Deng J.F., Lin K.P., Tsai W.J. Lead, mercury, and arsenic poisoning due to topical use of traditional Chinese medicines. Am J Med. 2013;126:451–454. doi: 10.1016/j.amjmed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Chan T.Y. Inorganic mercury poisoning associated with skin-lightening cosmetic products. Clin Toxicol (Phila) 2011;49:886–891. doi: 10.3109/15563650.2011.626425. [DOI] [PubMed] [Google Scholar]
- 7.Kuehn B. Mercury poisoning from skin cream. JAMA. 2020;323:500. doi: 10.1001/jama.2020.0292. [DOI] [PubMed] [Google Scholar]
- 8.Liu X.L., Wang H.B., Sun C.W., et al. The clinical analysis of mercury poisoning in 92 cases. Chin J Intern Med. 2011;50:687–689. [PubMed] [Google Scholar]
- 9.Bensefa-Colas L., Andujar P., Descatha A. Mercury poisoning. Rev Med Intern. 2011;32:416–424. doi: 10.1016/j.revmed.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Voitzuk A., Greco V., Caputo D., Alvarez E. Toxic nephropathy secondary to occupational exposure to metallic mercury. Medicina B (Aires) 2014;74:397–399. [PubMed] [Google Scholar]
- 11.Zhang L., Liu F., Peng Y., Sun L., Chen C. Nephrotic syndrome of minimal change disease following exposure to mercury-containing skin-lightening cream. Ann Saudi Med. 2014;34:257–261. doi: 10.5144/0256-4947.2014.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diagnostic criteria of occupational mercury poisoning (GBZ89-2007). National Occupational Health Standards, PRC. http://www.csres.com/detail/189710.html
- 13.Lameire N.H., Levin A., Kellum J.A., et al. Harmonizing acute and chronic kidney disease definition and classification: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021;100:516–526. doi: 10.1016/j.kint.2021.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F.G., Zhao W., Li C.L. Atomic fluorescence spectrometry for determination of Hg in blood and urine samples. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2008;26:319–320. [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 16.De Vriese A.S., Glassock R.J., Nath K.A., Sethi S., Fervenza F.C. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–430. doi: 10.1681/ASN.2016070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattran D., Brenchley P. Membranous nephropathy: thinking through the therapeutic options. Nephrol Dial Transplant. 2017;32:i22–i29. doi: 10.1093/ndt/gfw404. [DOI] [PubMed] [Google Scholar]
- 18.Barbour S., Reich H., Cattran D. Short-term complications of membranous nephropathy. Contrib Nephrol. 2013;181:143–151. doi: 10.1159/000349976. [DOI] [PubMed] [Google Scholar]
- 19.Dias D., Bessa J., Guimarães S., Soares M.E., Bastos MdL., Teixeira H.M. Inorganic mercury intoxication: a case report. Forensic Sci Int. 2016;259:20–24. doi: 10.1016/j.forsciint.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Onwuzuligbo O., Hendricks A.R., Hassler J., Domanski K., Goto C., Wolf M.T.F. Mercury intoxication as a rare cause of membranous nephropathy in a child. Am J Kidney Dis. 2018;72:601–605. doi: 10.1053/j.ajkd.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Li P., Du B., Chan H.M., Feng X. Human inorganic mercury exposure, renal effects and possible pathways in Wanshan mercury mining area, China. Environ Res. 2015;140:198–204. doi: 10.1016/j.envres.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Pollard K.M., Cauvi D.M., Toomey C.B., Hultman P., Kono D.H. Mercury-induced inflammation and autoimmunity. Biochim Biophys Acta Gen Subj. 2019;1863:129299. doi: 10.1016/j.bbagen.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin A.B., Zhou F.D., Zhao M.H. Advances in diagnosis and treatment of patients with mercury poisoning-associated glomerulonephropathy. Zhonghua Nei Ke Za Zhi. 2016;55:973–975. doi: 10.3760/cma.j.issn.0578-1426.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson T.W., Magos L., Myers G.J. The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y., Li Y., Rao J., Liu Z., Chen Q. Effects of inorganic mercury exposure on histological structure, antioxidant status and immune response of immune organs in yellow catfish (Pelteobagrus fulvidraco) J Appl Toxicol. 2018;38:843–854. doi: 10.1002/jat.3592. [DOI] [PubMed] [Google Scholar]
- 26.Rana M.N., Tangpong J., Rahman M.M. Toxicodynamics of lead, cadmium, mercury and arsenic-induced kidney toxicity and treatment strategy: a mini review. Toxicol Rep. 2018;5:704–713. doi: 10.1016/j.toxrep.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Wang D., Wu J., et al. Cinnabar-induced subchronic renal injury is associated with increased apoptosis in rats. Biomed Res Int. 2015;2015:278931. doi: 10.1155/2015/278931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L., Li X., Shang L., Zheng Y.J. Mechanism of renal injury and apoptosis in rats with nephrotic syndrome induced by mercury. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020;38:13–19. doi: 10.3760/cma.j.issn.1001-9391.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J.P., Ruha A.M., Curry S.C., et al. Plasma and urine dimercaptopropanesulfonate concentrations after dermal application of transdermal DMPS (TD-DMPS) J Med Toxicol. 2013;9:9–15. doi: 10.1007/s13181-012-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qasim S.F., Baloch M. Lead toxicity in battery workers. J Coll Physicians Surg Pak. 2014;24(suppl 3):S284–S286. [PubMed] [Google Scholar]
- 31.Akerstrom M., Barregard L., Lundh T., Sallsten G. Relationship between mercury in kidney, blood, and urine in environmentally exposed individuals, and implications for biomonitoring. Toxicol Appl Pharmacol. 2017;320:17–25. doi: 10.1016/j.taap.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Hazelhoff M.H., Bulacio R.P., Torres A.M. Gender related differences in kidney injury induced by mercury. Int J Mol Sci. 2012;13:10523–10536. doi: 10.3390/ijms130810523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.