Abstract
Introduction
Depressive symptoms, even without a clinical diagnosis of depression, are common in kidney failure patients and may be a barrier to completing the complex process of kidney transplant (KT) evaluation. We assessed depressive symptom burden and association between depressive symptoms and access to KT waitlist by age.
Methods
In a prospective cohort of 3728 KT patients (aged 18–88 years), the Center for Epidemiologic Studies—Depression (CES-D) scale was used to measure depressive symptoms at evaluation. Depressive symptom severity was defined as follows: none: 0; minimal: 1 to 15; mild: 16 to 20; moderate: 21 to 25; severe: 26 to 60. Hazard ratios (HRs) of active listing within 1 year after evaluation were estimated using Cox proportional hazards models, adjusted for clinical and social factors.
Results
At evaluation, 85.8% of the patients reported at least minimal depressive symptoms; the proportion was lower among older patients: 18 to 29 years = 92.0%; 30 to 39 years = 88.3%; 40 to 49 years = 87.2%; 50 to 59 years = 87.0%; 60 to 69 years = 83.4%; and ≥70 years = 82.0%. Chance of active listing decreased with more severe depressive symptoms (log-rank, P < 0.001). After adjustment, every 5-point higher CES-D score (more depressive symptoms) was associated with a 13% lower chance of listing (HR = 0.87, 95% CI: 0.85–0.90); the strongest association was found among patients aged ≥70 years (adjusted HR [aHR] = 0.73, 95% CI: 0.62–0.86). Furthermore, minimal (HR = 0.69, 95% CI: 0.60–0.79), mild (HR = 0.57, 95% CI: 0.44–0.72), moderate (HR = 0.53, 95% CI: 0.39–0.71), and severe (HR = 0.44, 95% CI: 0.34–0.57) depressive symptoms were all associated with a lower chance of listing.
Conclusion
Older candidates were less likely to report depressive symptoms at KT evaluation. Regardless of age, candidates who did report depressive symptoms, and even minimal symptoms, had a lower chance of listing. Transplant centers should routinely screen patients for depressive symptoms and refer the affected patients to mental health services to improve access to KT.
Keywords: age, depressive symptoms, end-stage kidney disease, kidney transplantation, waitlisting
Graphical abstract
See Commentary on Page 1153
Kidney failure patients are at high risk of depressive symptoms, because of the increased burden of self-care and physical symptoms related to kidney disease, comorbidities, and reliance on dialysis.1, 2, 3 Approximately 40% of kidney failure patients undergoing dialysis are affected by subclinical depressive symptoms.4 Presence of depressive symptoms, even without a clinical diagnosis of depression, is an important predictor of adverse outcomes among patients with kidney failure: dialysis patients with depressive symptoms have higher likelihood of medication noncompliance,5 dialysis withdrawal,6,7 and dietary indiscretion,8 and thus, are more likely to suffer from cardiovascular events and mortality.8,9
Although extensive research sheds light on the high burden and poor clinical outcomes of depressive symptoms in dialysis population, it is unclear whether kidney failure patients who present depressive symptoms have obstacles in seeking KT. The KT candidacy evaluation is a complex process, requiring a comprehensive assessment of each patient’s medical, surgical, and psychosocial histories.10 Such a process may be demanding for kidney failure patients, especially for those with depressive symptoms, and may introduce various stressors that potentially increase vulnerability to psychological disorders and further hinder access to the KT waitlist. Depressive symptoms affect treatment adherence,11 which is crucial throughout the evaluation process, and may impact patient selection by health providers.12 In addition, although the severity of depressive symptoms likely varies by age among KT candidates, as has been observed in community-dwelling populations,13 it is unclear whether the presence of depressive symptoms affects the access to KT regardless of candidate’s age.
The goals of this study included the following: (i) to evaluate the severity of depressive symptoms by age, (ii) to identify risk factors of depressive symptoms, and (iii) to estimate the association of depressive symptom severity with KT listing among adults of all ages being evaluated for KT.
Methods
Study Design
We leveraged a prospective cohort study of 3879 adult kidney failure patients being evaluated for KT at the Johns Hopkins Hospital (January 2009 to March 2020). Eligible participants in this study were English speaking and aged ≥18 years at the time of evaluation. During the first evaluation visit at the transplant center, the participants were asked to complete the CES-D scale to assess depressive symptoms. Depressive symptoms were solely measured for research purposes and not considered during the listing meeting. In analysis, we excluded participants who did not complete the CES-D scale (n = 151, 3.9%). The excluded and included participants did not differ by age, sex, or race/ethnicity (P > 0.09).
Characteristics at evaluation were self-reported or calculated, including age, sex, race/ethnicity, body mass index, type of dialysis, years on dialysis, smoking history, education, marital status, and employment. On the basis of the Charlson Comorbidity Index (CCI) adapted for patients with kidney failure,14,15 we ascertained comorbidities from self-report and supplemented the data with electronic medical chart abstraction. Physical functional status of the participants at evaluation was also assessed, including frailty (≥3 of the 5 components from the Fried physical frailty phenotype),16 lower extremity impairment (Short Physical Performance Battery score ≤ 10),17 activities of daily living (ADL) dependence (≥1 activity),18 and instrumental ADL (IADL) dependence (≥1 activity).19 We obtained the patient urban/rural residence type from self-reported zip code combined with data from the United States Census Bureau.20 The percentage of population below poverty level was obtained by linking with the American Community Survey 2014 data.21
All clinical and research activities being reported are consistent with the Declaration of Helsinki and the Declaration of Istanbul. The study protocol was approved by the Johns Hopkins University Institutional Review Board. All participants provided written informed consent.
Depressive Symptoms
Depressive symptoms were assessed using the 20-item CES-D scale, which queries about the frequencies of 20 symptoms during the prior week.22 There are 4 possible responses to each question which include the following: “rarely or none of the time” (<1 day), “some or a little of the time” (1–2 days), “occasionally or a moderate amount of time” (3–4 days), and “most or all of the time” (5–7 days). Each question is assigned a score of 0 to 3, and when summed, it creates a total score ranging from 0 to 60; a higher score represents greater severity of depressive symptoms. On the basis of previously published cutoffs for symptom severity,23 we categorized the severity of depressive symptoms as follows: none (0 point), minimal (1–15 points), mild (16–20 points), moderate (21–25 points), and severe (26–60 points).
Outcomes
The participants were followed until their first listing, administrative censoring at March 2, 2021, or 1 year after evaluation. The listing status was ascertained by linking the cohort to the Scientific Registry of Transplant Recipients, which includes data on all waitlisted candidates in the United States. The Scientific Registry of Transplant Recipients data set provided exact dates of active and/or inactive listing. The events of interest were any listing (inactive/active) and active listing. Time to event was defined as the period from evaluation to the event date or end of follow-up.
Descriptive Statistics
We examined distributions of characteristics overall and by depressive symptom severity, using mean with SD or median with interquartile range (IQR) for continuous variables and proportion for categorical variables. Differences by depressive symptom severity were tested using analysis of variance tests, Kruskal–Wallis tests, or Fisher exact tests, where appropriate. We used restricted cubic splines to estimate the proportion of patients reporting mild or more severe depressive symptoms by continuous age with 4 knots placed at percentiles based on previously recommended approaches.24 We also compared depressive symptom severity by age group (18–29, 30–39, 40–49, 50–59, 60–69, ≥70 years) using stacked bar chart.
Risk Factors of Depressive Symptoms
Risk factors of depressive symptoms were identified from literature and conceptual framework, including clinical factors (age, sex, race, time on dialysis, body mass index, smoking, CCI), social factors (education, marital status, employment, neighborhood poverty level), and physical functional status (frailty, lower extremity impairment, ADL dependence, IADL dependence). To assess the associations between depressive symptoms and risk factors, we used modified Poisson regressions to estimate adjusted prevalence ratio (aPR) of every 1 point increase in CES-D score and aPR of mild/moderate/severe depressive symptoms (CES-D ≥ 16) by each risk factor. In addition, we used proportional Venn diagrams to estimate joint prevalence of depressive symptoms (mild/moderate/severe symptoms), frailty, and other functional disabilities (lower extremity impairment, ADL dependence, or IADL dependence) at evaluation, owing to their high co-occurrences in community-dwelling older adults.25, 26, 27
Depressive Symptoms and Chance of Listing
Unadjusted cumulative incidence of any listing for KT within 1 year after evaluation was estimated using the Kaplan–Meier method; log-rank test was used to compare the curves by depressive symptom severity. After examining proportional hazards assumptions by visually inspecting log-log plots, we fitted the following 3 sets of Cox models to estimate crude HRs and aHRs of any listing for KT by binary depressive symptoms (mild/moderate/severe vs. none/minimal symptoms), symptom severity, and every 5-point higher CES-D score, respectively: (i) crude models; (ii) clinical factor models adjusted for age group, sex, Black, years on dialysis, body mass index, smoking, and CCI; and (iii) clinical and social factor models further adjusted for education, marital status, employment, and neighborhood poverty level. We further examined the association between chance of listing and CES-D score by age group by including an interaction term between age group and continuous CES-D score; Wald test was used to examine the differences between age groups. To account for patient listing status, parallel analyses were performed for active listing within 1 year as the outcome event.
Statistical Analyses
All analyses were performed using Stata version 15 (StataCorp, College Station, TX). Two-sided P < 0.05 was considered statistically significant.
Missing Data
Covariates were missing for <0.6% of the cohort, with a few exceptions: years on dialysis was missing in 4.8% (n = 179), CCI was missing in 14.2% (n = 529), neighborhood poverty level was missing in 14.3% (n = 532), and marital status was missing in 1.4% (n = 52). In addition, physical functional status was not assessed in all participants: frailty was missing in 4.0% (n = 149) of the cohort, Short Physical Performance Battery was missing in 15.8% (n = 588), ADL was missing in 1.6% (n = 60), and IADL was missing in 1.8% (n = 68). We used complete cases (n = 3037) for primary analysis.
Sensitivity Analyses
To confirm the robustness of our inferences, we included several sensitivity analyses: (i) we examined the risk factors of depressive symptoms using CES-D ≥ 18 as the binary cutoff, which was previously proposed in hemodialysis patients28; (ii) we imputed the missing values for all covariates using multiple imputation method with 10 iterations for all regression models; and (iii) we included frailty, lower extremity impairment, ADL dependence, and IADL dependence in the fully adjusted Cox models to further estimate aHRs independent of physical functional status.
Results
Participant Characteristics
Among 3728 patients evaluated, median age was 56 years (IQR = 45–65), 40.7% were female, 46.7% were Black, and 70.6% received dialysis, with a median time on dialysis of 0.8 year (IQR = 0.0–3.0). At evaluation, 14.2% of the patients reported no depressive symptoms, 67.4% minimal, 6.6% mild, 4.6% moderate, and 7.2% severe symptoms (Table 1).
Table 1.
Characteristics of kidney transplant evaluation patients overall and by severity of depressive symptoms (N = 3728)
| Characteristics at evaluation | Overall (N = 3728) |
Severity of depressive symptoms |
|||||
|---|---|---|---|---|---|---|---|
| None (n = 529) |
Minimal (n = 2513) |
Mild (n = 246) |
Moderate (n = 173) |
Severe (n = 267) |
P value | ||
| Age, median (IQR) | 56.0 (45.0–65.0) | 58.0 (48.0–67.0) | 56.0 (45.0–65.0) | 55.0 (46.0–63.0) | 54.0 (43.0–62.0) | 51.0 (39.0–58.0) | <0.001 |
| Female, % | 40.7 | 35.5 | 39.8 | 46.3 | 43.4 | 52.8 | <0.001 |
| Race/ethnicity, % | |||||||
| White | 44.4 | 45.7 | 44.0 | 47.6 | 49.7 | 39.3 | 0.20 |
| Black | 46.7 | 47.3 | 47.1 | 42.7 | 38.2 | 50.6 | |
| Hispanic | 3.2 | 2.1 | 3.2 | 4.9 | 4.0 | 3.4 | |
| Other | 5.7 | 4.9 | 5.6 | 4.9 | 8.1 | 6.7 | |
| High school or less, % | 44.3 | 42.1 | 44.2 | 49.2 | 41.6 | 47.2 | 0.32 |
| Marital status, % | |||||||
| Single | 24.5 | 18.7 | 23.8 | 26.8 | 26.7 | 38.9 | <0.001 |
| Married/cohabitating | 58.3 | 66.1 | 59.8 | 51.5 | 50.6 | 39.6 | |
| Divorced/separated | 12.9 | 10.6 | 12.3 | 17.2 | 16.3 | 17.7 | |
| Widowed | 4.3 | 4.6 | 4.1 | 4.6 | 6.4 | 3.8 | |
| Employed, % | 35.1 | 39.5 | 35.6 | 33.8 | 32.4 | 24.2% | <0.001 |
| Urban, % | 97.3 | 97.4 | 97.2 | 97.3 | 96.0 | 98. | 0.53 |
| Neighborhood poverty level %, median (IQR) | 9.6 (6.0–15.1) | 9.4 (6.0–14.4) | 9.6 (5.9–15.0) | 10.4 (6.1–17.8) | 9.1 (5.9–14.4) | 10.4 (7.1–19.3) | 0.01 |
| Smoking history, % | |||||||
| Nonsmoker | 68.4 | 80.7 | 68.8 | 60.0 | 55.8 | 55.8 | <0.001 |
| Previous smoker | 23.4 | 14.4 | 23.3 | 32.2 | 31.4 | 29.2 | |
| Current smoker | 8.2 | 4.9 | 7.9 | 7.8 | 12.8 | 15.0 | |
| BMI (kg/m2), mean (SD) | 28.7 (6.2) | 28.4 (5.6) | 28.7 (6.1) | 29.0 (6.4) | 28.8 (6.2) | 28.6 (7.2) | 0.71 |
| Dialysis type, % | |||||||
| No dialysis | 29.4 | 27.4 | 29.3 | 34.6 | 28.7 | 29.4 | 0.43 |
| Hemodialysis | 57.9 | 61.7 | 57.7 | 54.7 | 57.3 | 55.7 | |
| Peritoneal dialysis | 12.7 | 11.0 | 13.0 | 10.7 | 14.0 | 14.9 | |
| Years on dialysis, median (IQR) | 0.8 (0.0–3.0) | 0.9 (0.0–3.5) | 0.8 (0.0–3.0) | 0.5 (0.0–2.4) | 0.7 (0.0–2.4) | 0.6 (0.0–2.7) | 0.05 |
| Frailty, % | 20.7 | 8.3 | 19.7 | 32.2 | 28.7 | 41.0 | <0.001 |
| Lower extremity impairment, % | 54.4 | 46.1 | 53.6 | 59.7 | 60.7 | 68.4 | <0.001 |
| ADL dependence, % | 8.2 | 3.1 | 8.0 | 11.7 | 13.4 | 13.5 | <0.001 |
| IADL dependence, % | 20.6 | 9.7 | 19.0 | 31.8 | 29.7 | 41.6 | <0.001 |
| Charlson Comorbidity Index, % | |||||||
| 0 | 37.3 | 43.5 | 37.4 | 29.4 | 32.2 | 36.2 | 0.10 |
| 1 | 7.3 | 6.7 | 7.3 | 9.0 | 6.7 | 6.6 | |
| 2 | 16.7 | 13.4 | 17.3 | 15.4 | 19.5 | 15.6 | |
| 3 | 18.5 | 17.9 | 18.5 | 22.2 | 15.4 | 18.5 | |
| ≥4 | 20.2 | 18.4 | 19.5 | 24.0 | 26.2 | 23.0 | |
| Comorbidities, % | |||||||
| Myocardial infarction | 9.3 | 9.6 | 9.0 | 10.0 | 10.1 | 10.7 | 0.86 |
| Peripheral vascular disease | 6.3 | 6.9 | 5.7 | 10.0 | 9.4 | 5.3 | 0.05 |
| Cerebral vascular disease | 6.0 | 2.6 | 5.8 | 6.3 | 7.4 | 11.5 | <0.001 |
| Dementia | 0.4 | 0.2 | 0.3 | 0.9 | 0.7 | 0.8 | 0.39 |
| Chronic lung disease | 6.0 | 4.5 | 6.4 | 7.3 | 4.1 | 4.5 | 0.31 |
| Rheumatological disease | 6.9 | 4.5 | 7.1 | 9.1 | 4.0 | 9.6 | 0.04 |
| Peptic ulcer disease | 3.4 | 3.3 | 3.2 | 4.6 | 6.0 | 2.1 | 0.22 |
| Diabetes | 42.3 | 39.9 | 42.3 | 47.5 | 43.2 | 41.2 | 0.46 |
| Diabetes with complication | 34.4 | 40.0 | 34.6 | 34.1 | 28.7 | 30.0 | 0.12 |
| Moderate/severe liver disease | 3.6 | 1.4 | 3.4 | 6.3 | 6.7 | 4.6 | 0.004 |
| Metastatic cancer | 1.0 | 1.0 | 1.1 | 0.9 | 0.7 | 0.8 | 0.99 |
| Leukemia | 0.3 | 0.5 | 0.3 | 0.0 | 0.0 | 0.0 | 0.65 |
| Lymphoma | 0.8 | 0.7 | 0.9 | 0.5 | 2.0 | 0.0 | 0.27 |
| HIV | 3.3 | 2.2 | 3.2 | 3.2 | 3.4 | 5.3 | 0.29 |
| Congestive heart failure | 14.0 | 11.5 | 13.5 | 17.8 | 21.5 | 16.7 | 0.04 |
ADL, activities of daily living; BMI, body mass index; CES-D, Center for Epidemiologic Studies—Depression; IADL, instrumental activities of daily living; IQR, interquartile range.
The CES-D score ranges from 0 to 60. Depressive symptom severity was categorized as follows: none (0 point), minimal (1–15 points), mild (16–20 points), moderate (21–25 points), and severe (26–60 points). Neighborhood poverty level was defined as the percentage of population below poverty level in the neighborhood. Percentages are presented unless otherwise indicated. Differences that are statistically significant at P < 0.05 are in bold.
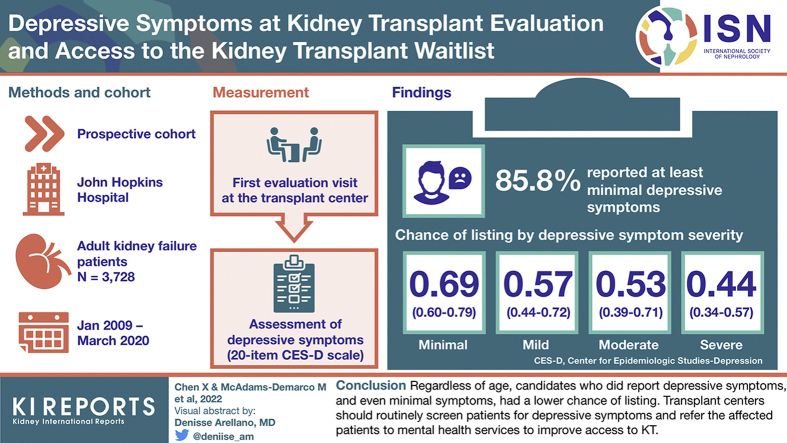
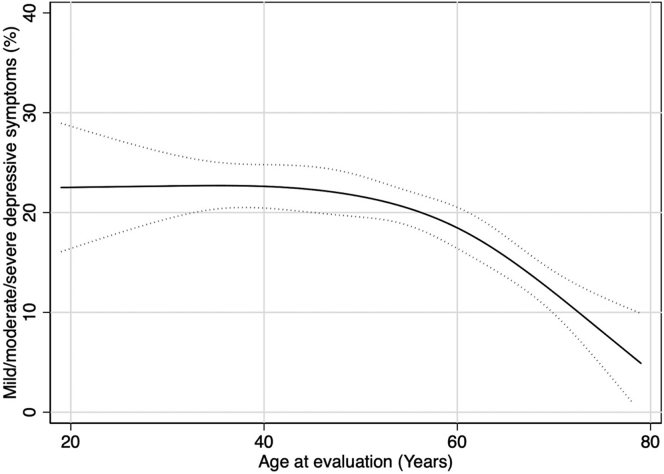
Overall, the proportion of patients reporting mild or more severe depressive symptoms decreased with age such that it remained >20% until age 55 years and decreased steadily afterward (Figure 1). At evaluation, the proportion of patients with any depressive symptoms was lower among older patients: 92.0% of the patients aged 18 to 29 years; 88.3% of the patients aged 30 to 39 years; 87.2% of those aged 40 to 49 years; 87.0% of those aged 50 to 59 years; 83.4% of those aged 60 to 69 years; and 82.0% of those aged ≥70 years reported minimal or more depressive symptoms. Furthermore, the severity of depressive symptoms was greater among younger age groups; in particular, severe symptoms were reported in 10.2% of the patients aged 18 to 29 years, 13.0% of those aged 30 to 39 years, 8.2% of those aged 40 to 49 years, 8.9% of those aged 50 to 59 years, 4.2% of those aged 60 to 69 years, and 2.2% of those aged ≥70 years (Figure 2).
Figure 1.
Proportion of patients with mild or more severe depressive symptoms (CES-D ≥ 16) by age at kidney transplant evaluation (N = 3728). The CES-D score ranges from 0 to 60, with the higher score representing more severe depressive symptoms. Depressive symptom severity was categorized as follows: none (0 point), minimal (1–15 points), mild (16–20 points), moderate (21–25 points), and severe (26–60 points). Age was treated as a continuous variable at evaluation. Restricted cubic splines were used, and 95% CIs are depicted as the region between the dotted curves. CES-D, Center for Epidemiologic Studies—Depression.
Figure 2.
Depressive symptom severity by age group among kidney transplant evaluation patients (N = 3728). The CES-D score ranges from 0 to 60. Depressive symptom severity was categorized as follows: none (0 point), minimal (1–15 points), mild (16–20 points), moderate (21–25 points), and severe (26–60 points). CES-D, Center for Epidemiologic Studies—Depression.
Risk Factors of Depressive Symptoms
After adjustment, proportion of patients reporting mild/moderate/severe depressive symptoms was 1.59-fold (aPR = 1.59, 95% CI: 1.13–2.25) higher among patients aged 18–29 years, but 44% lower (aPR = 0.56, 95% CI: 0.39–0.79) among those aged ≥70 years, compared with their counterparts aged 60 to 69 years. In addition, male sex (aPR = 0.76, 95% CI: 0.66–0.88), Black race (aPR = 0.76, 95% CI: 0.65–0.89), being married/cohabitating (aPR = 0.70, 95% CI: 0.60–0.81), and employment (aPR = 0.73, 95% CI: 0.62–0.87) were associated with fewer depressive symptoms, whereas previous smoker (aPR = 1.56, 95% CI: 1.33–1.83), current smoker (aPR = 1.53, 95% CI: 1.21–1.93), and a CCI score ≥ 4 (aPR = 1.53, 95% CI: 1.21–1.93) were associated with more depressive symptoms (Table 2).
Table 2.
Correlates of CES-D score and mild/moderate/severe depressive symptoms (CES-D ≥ 16) among kidney transplant evaluation patients using complete case analysis
| Factor | CES-D score, per 1 point increase aPR (95% CI) |
Mild/moderate/severe depressive symptoms aPR (95% CI) |
|---|---|---|
| Age (vs. 60–69 yr), yr | ||
| 18–29 | 1.51 (1.26–1.81) | 1.59 (1.13–2.25) |
| 30–39 | 1.49 (1.31–1.71) | 1.55 (1.20–2.01) |
| 40–49 | 1.45 (1.30–1.62) | 1.70 (1.36–2.13) |
| 50–59 | 1.32 (1.20–1.45) | 1.39 (1.14–1.70) |
| ≥70 | 0.74 (0.66–0.84) | 0.56 (0.39–0.79) |
| Male | 0.84 (0.78–0.90) | 0.76 (0.66–0.88) |
| Black | 0.90 (0.84–0.98) | 0.76 (0.65–0.89) |
| Years on dialysis | 0.99 (0.98–1.00) | 0.98 (0.96–1.00) |
| BMI, per 5 kg/m2 | 1.02 (0.99–1.05) | 1.01 (0.95–1.08) |
| Smoking (vs. nonsmoker) | ||
| Previous smoker | 1.36 (1.26–1.48) | 1.56 (1.33–1.83) |
| Current smoker | 1.45 (1.29–1.64) | 1.53 (1.21–1.93) |
| CCI score (vs. 0) | ||
| 1 | 1.03 (0.89–1.19) | 1.04 (0.77–1.40) |
| 2 | 1.13 (1.02–1.25) | 1.15 (0.92–1.44) |
| 3 | 1.13 (1.01–1.25) | 1.23 (0.99–1.52) |
| ≥4 | 1.23 (1.11–1.36) | 1.38 (1.13–1.70) |
| High school or below | 0.95 (0.89–1.03) | 0.97 (0.83–1.12) |
| Married/cohabitating | 0.82 (0.76–0.89) | 0.70 (0.60–0.81) |
| Employed | 0.81 (0.75–0.88) | 0.73 (0.62–0.87) |
| Neighborhood poverty level, per 10% | 1.03 (0.99–1.08) | 1.06 (0.97–1.15) |
| Frailty | 1.61 (1.48–1.74) | 2.00 (1.71–2.33) |
| Lower extremity impairment | 1.28 (1.18–1.40) | 1.35 (1.13–1.60) |
| ADL dependence | 1.42 (1.26–1.59) | 1.74 (1.41–2.14) |
| IADL dependence | 1.56 (1.44–1.69) | 2.12 (1.82–2.47) |
ADL, activities of daily living; aPR, adjusted prevalence ratio; BMI, body mass index; CCI, Charlson Comorbidity Index; CES-D, Center for Epidemiologic Studies—Depression; IADL, instrumental activities of daily living.
The CES-D score ranges from 0 to 60, with the higher score representing more severe depressive symptoms. aPRs with 95% CIs are presented from modified Poisson models. All models adjusted for all clinical and social factors, including age group, sex, Black race, years on dialysis, BMI, smoking status, CCI, education, marital status, employment, and neighborhood poverty level. The aPRs for clinical and social factors were estimated among patients with all clinical and social factors nonmissing (n = 3037); the aPRs for physical functional measures were estimated among patients with both clinical and social factors and corresponding functional measure nonmissing (frailty, n = 2894; lower extremity impairment, n = 2665; ADL dependence, n = 2981; IADL dependence, n = 2971). Associations that are statistically significant at P < 0.05 are in bold.
Frailty, lower extremity impairment, ADL dependence, and IADL dependence were all independently associated with depressive symptoms. At evaluation, 30.1% of frail patients, 21.7% of patients with lower extremity impairment, 28.1% of patients with ADL dependence, and 30.2% of patients with IADL dependence reported mild or more severe depressive symptoms at the same time (Supplementary Figure S1). After adjustment, there was significantly higher proportion of patients reporting mild/moderate/severe depressive symptoms among those who were frail (aPR = 2.00, 95% CI: 1.71–2.33) and had lower extremity impairment (aPR = 1.35, 95% CI: 1.13–1.60), ADL dependence (aPR = 1.74, 95% CI: 1.41–2.14), and IADL dependence (aPR = 2.12, 95% CI: 1.82–2.47) (Table 2).
Unadjusted Cumulative Incidence of Listing by Depressive Symptoms
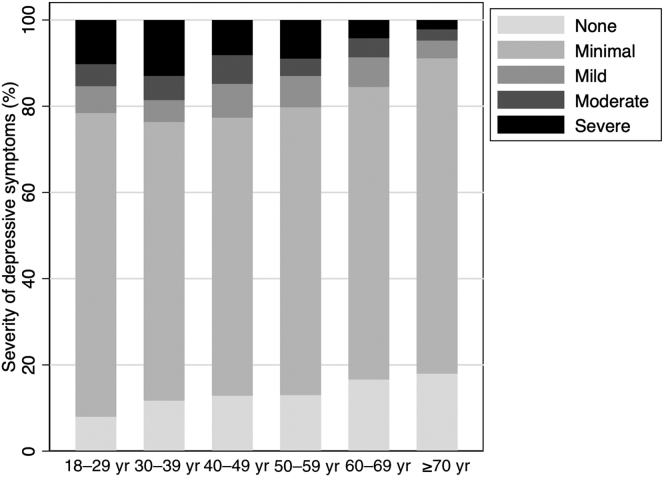
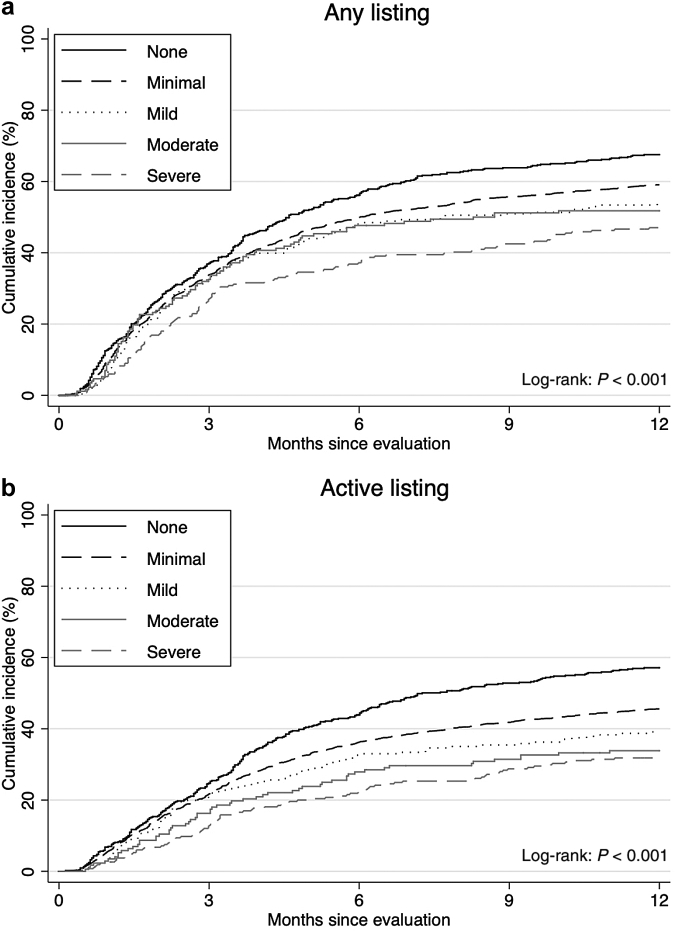
Among 3728 patients being evaluated, 2180 patients (58.5%) were listed, and 1673 (44.9%) were actively listed within 1 year after evaluation. The median follow-up time to listing was 6.0 months (IQR = 2.1–12.0), and median follow-up time to active listing was 12.0 months (IQR = 3.4–12.0). The cumulative incidence of any listing was lower among patients with more severe depressive symptoms (log-rank, P < 0.001); at 1 year after evaluation, 67.5% of patients with no symptoms, 59.1% of patients with minimal symptoms, 53.8% of patients with mild symptoms, 51.8% of patients with moderate symptoms, and 47.0% of patients with severe symptoms were listed (log-rank, P < 0.001) (Figure 3a). Similar trends were noted for active listing (log-rank, P < 0.001) (Figure 3b).
Figure 3.
Unadjusted cumulative incidence of (a) any listing and (b) active listing for kidney transplant within 1 year after evaluation by depressive symptom severity among evaluation patients (N = 3728). The CES-D score ranges from 0 to 60. Depressive symptom severity was categorized as follows: none (0 point), minimal (1–15 points), mild (16–20 points), moderate (21–25 points), and severe (26–60 points). CES-D, Center for Epidemiologic Studies—Depression.
Adjusted Chance of Listing by Depressive Symptoms
After adjustment, mild/moderate/severe depressive symptoms were associated with lower chances of any listing (aHR = 0.75, 95% CI: 0.66–0.85) and active listing (aHR = 0.69, 95% CI: 0.60–0.80). Even patients with minimal symptoms showed lower chances of any listing (aHR = 0.73, 95% CI: 0.65–0.83) and active listing (aHR = 0.69, 95% CI: 0.60–0.79) compared with their counterparts with no symptoms (Table 3). The chances of any listing and active listing decreased with higher depressive symptom scores; every 5-point higher (worse) CES-D score was associated with 9% lower likelihood of being listed (aHR = 0.91, 95% CI: 0.88–0.93) and 13% lower likelihood of being actively listed (aHR = 0.87, 95% CI: 0.85–0.90) (Table 3).
Table 3.
Chance of listing for kidney transplant within 1 year after evaluation by depressive symptoms among transplant evaluation patients using complete case analysis
| Outcomes | Crude model (n = 3728) cHR (95% CI) |
Clinical factor adjusted model (n = 3112) aHR (95% CI) |
Clinical and social factor adjusted model (n = 3037) aHR (95% CI) |
|---|---|---|---|
| Any listing | |||
| Mild/moderate/severe symptoms | 0.78 (0.69–0.87) | 0.71 (0.63–0.81) | 0.75 (0.66–0.85) |
| Severity of depressive symptoms | |||
| No symptoms | Reference | Reference | Reference |
| Minimal symptoms | 0.82 (0.73–0.92) | 0.71 (0.63–0.80) | 0.73 (0.65–0.83) |
| Mild symptoms | 0.72 (0.59–0.88) | 0.56 (0.45–0.69) | 0.59 (0.48–0.73) |
| Moderate symptoms | 0.70 (0.55–0.88) | 0.62 (0.49–0.79) | 0.67 (0.52–0.85) |
| Severe symptoms | 0.58 (0.47–0.71) | 0.47 (0.38–0.58) | 0.51 (0.41–0.63) |
| Continuous score (5 points worse) | 0.92 (0.90–0.94) | 0.90 (0.87–0.92) | 0.91 (0.88–0.93) |
| Active listing | |||
| Mild/moderate/severe symptoms | 0.67 (0.58–0.77) | 0.65 (0.57–0.75) | 0.69 (0.60–0.80) |
| Severity of depressive symptoms | |||
| No symptoms | Reference | Reference | Reference |
| Minimal symptoms | 0.74 (0.65–0.84) | 0.67 (0.59–0.77) | 0.69 (0.60–0.79) |
| Mild symptoms | 0.61 (0.49–0.77) | 0.54 (0.42–0.68) | 0.57 (0.44–0.72) |
| Moderate symptoms | 0.51 (0.38–0.67) | 0.49 (0.36–0.65) | 0.53 (0.39–0.71) |
| Severe symptoms | 0.46 (0.36–0.58) | 0.40 (0.31–0.51) | 0.44 (0.34–0.57) |
| Continuous score (5 points worse) | 0.87 (0.85–0.90) | 0.86 (0.84–0.89) | 0.87 (0.85–0.90) |
aHR, adjusted hazard ratio; cHR, crude hazard ratio.
The CES-D score ranges from 0 to 60, with the higher score representing more severe depressive symptoms. Depressive symptom severity was categorized as follows: none (0 point), minimal (1–15 points), mild (16–20 points), moderate (21–25 points), and severe (26–60 points). cHRs and aHRs with 95% CIs were presented from adjusted Cox proportional hazards models. Clinical factor adjusted model adjusted for age group, sex, Black race, years on dialysis, body mass index, smoking history, and Charlson Comorbidity Index; clinical and social factor adjusted model further adjusted for education, marital status, employment, and neighborhood poverty level. Associations that are statistically significant at P < 0.05 are in bold.
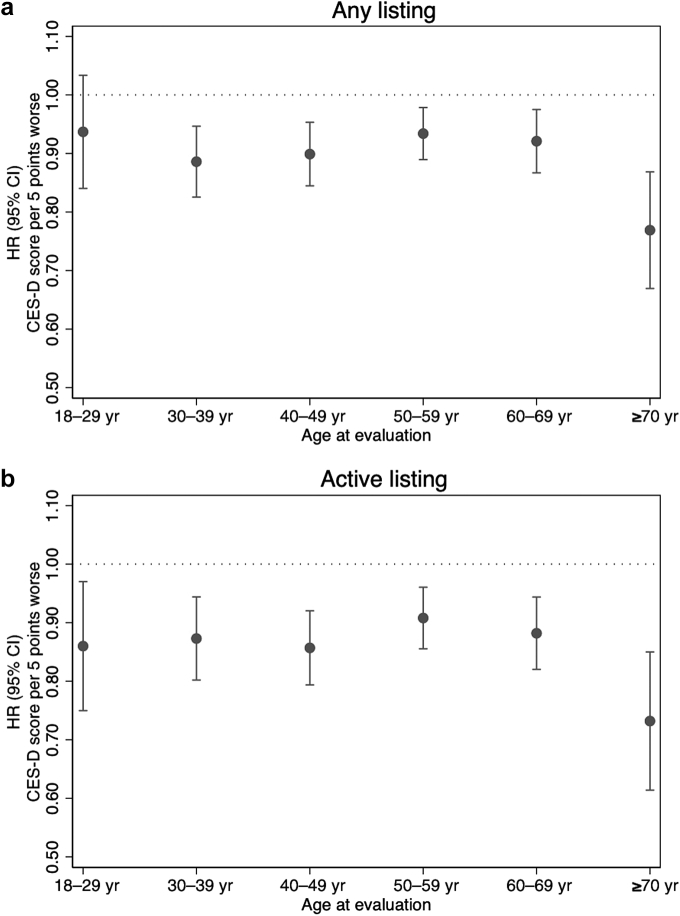
The association of continuous CES-D score with any listing remained significant within age groups, except for the 18 to 29 years group (P = 0.21). In addition, the association was greater among patients aged ≥70 years than those aged 18 to 29 years (P for interaction = 0.039); every 5-point higher CES-D score was associated with a 23% lower chance of any listing (aHR = 0.77, 95% CI: 0.68–0.88) among patients aged ≥70 years (Figure 4a). Meanwhile, the association between higher CES-D score and active listing was similar across age groups (all P for interaction > 0.05), whereas the greatest magnitude of association was also found among patients aged ≥70 years (aHR = 0.73, 95% CI: 0.62–0.86) (Figure 4b).
Figure 4.
Impact of depressive symptoms on (a) chance of any listing and (b) chance of active listing for kidney transplant within 1 year after evaluation by age group using complete case analysis. The CES-D score ranges from 0 to 60, with the higher score representing more severe depressive symptoms. Adjusted HRs with 95% CIs were presented from adjusted Cox proportional hazards models adjusted for age group, sex, Black race, years on dialysis, BMI, smoking history, Charlson Comorbidity Index, education, marital status, employment, and neighborhood poverty level. BMI, body mass index; CES-D, Center for Epidemiologic Studies—Depression; HR, hazard ratio.
Sensitivity Analyses
First, risk factors for depressive symptoms were similar using the cutoff of CES-D score ≥ 18 (Supplementary Table S1). Second, after multiple imputations to account for missing data, risk factors of depressive symptoms remained robust, whereas the association between years on dialysis and binary depressive symptoms became significant (aPR = 0.98, 95% CI: 0.96–1.00) (Supplementary Table S2); the associations of binary depressive symptoms, symptom severity, and CES-D score with any listing and active listing were similar (Supplementary Table S3). Third, impact of depressive symptoms on any listing and active listing remained robust in both significance and magnitude after accounting for frailty, lower extremity impairment, ADL dependence, or IADL dependence (Supplementary Table S4).
Discussion
In this prospective cohort of 3728 kidney failure patients, 85.8% of the patients suffered at least minimal depressive symptoms at KT evaluation. The burden and severity were particularly high among patients younger than 55 years. Patients reporting mild or more severe depressive symptoms had 25% lower chance of being listed for KT and 31% lower chance of being actively listed within 1 year after evaluation, adjusted for clinical and social factors; even those reporting minimal depressive symptoms showed 27% lower chance of any listing and 31% lower chance of active listing. More specifically, every 5-point higher (worse) CES-D score was associated with 9% lower chance of any listing and 13% lower chance of active listing within 1 year after evaluation; worse CES-D scores were more likely to affect listing among candidates aged ≥70 years.
Our findings suggest that there is a high burden of depressive symptoms among kidney failure patients at evaluation, although most patients experience minimal symptoms. The high burden of depressive symptoms among patients with chronic kidney disease and dialysis patients has been previously observed.4 Our finding that 18.4% of patients at evaluation had depressive symptoms was slightly lower than the previously reported estimates of 23.1% among a cohort of US adult patients undergoing hemodialysis using the same CES-D threshold.29 Despite the higher proportions of Black (61.8% vs. 45.0%) and low education (52.8% vs. 44.3%) in the prior study population,29 the differences suggested that the burden of depressive symptoms might be lower in kidney failure patients being evaluated for KT, which included 30% candidates who are preemptive recipients. These differences may be attributable to the relatively better health status among patients being referred for KT evaluation. Moreover, there might be social desirability bias. Even though we stated that depressive symptoms were only being assessed for research purposes, patients may respond to the questions in a way that they think would present themselves as better candidates for KT. Thus, it is possible that we underestimated the true burden of depressive symptoms among patients undergoing KT evaluation, and the potentially higher burden of depressive symptoms requires particular attention.
Our study confirmed substantial differences in depressive symptom severity across the age spectrum among KT evaluation patients: depressive symptoms are more prevalent and more severe among younger patients. This pattern is consistent with the findings from the general population.13 Apart from age disparities, our findings that KT evaluation patients who were female, non-Black, smoking, living alone, unemployed, and had more comorbidities were more likely to have depressive symptoms are consistent with the findings from chronic kidney disease and dialysis patients.30, 31, 32 This finding highlights the subgroups who bear a higher burden of depressive symptoms and may need mental health services. Furthermore, we extended previous findings that frailty and functional disabilities are related to depressive symptoms among general older adults33, 34, 35, 36 and dialysis patients29 into evaluation patients regardless of age or dialysis status. More importantly, we identified the prevalent co-occurrence of depressive symptoms and frailty or other functional disabilities among evaluation patients. It has been found in previous studies that frailty,37,38 physical impairment,39 and functional dependence40 are all associated with decreased chance of KT listing, elevated risk of waitlist mortality, and worse KT access. Thus, KT candidates with both more depressive symptoms and frailty or functional disabilities are likely more vulnerable to adverse outcomes while waiting for a KT.
Although existing studies have demonstrated that kidney failure patients with depressive symptoms are at high risk of developing poor clinical outcomes including mortality,41, 42, 43, 44 we sought to understand the impact of depressive symptoms on patients’ access to KT listing. Our findings suggest that kidney failure patients with more depressive symptoms have lower chance of being listed for KT within 1 year after evaluation, independent of clinical and social factors and physical functional status; even among patients with minimal symptoms, approximately 30% lower chance of listing was observed. Our results are consistent with a cross-sectional study by Szeifert et al.,12 in which hemodialysis patients aged 18 to 65 years with more depressive symptoms were less likely to be on the waitlist. However, we used a prospective study design that allowed us to assess the temporality of this association, and we extended the findings to patients aged >65 years. In contrast, Harhay et al.45 suggested that having fewer depressive symptoms was associated with lower waitlisting rates among patients with stages 4 to 5 chronic kidney disease (estimated glomerular filtration rate ≤ 30 ml/min per 1.73 m2) in the Chronic Renal Insufficiency Cohort, but such association was not observed in patients younger than 65 years. Nonetheless, this chronic kidney disease population was substantially different from our KT evaluation cohort, which may explain the inconsistent findings. The difference in time to listing calculation may be another reason: the Chronic Renal Insufficiency Cohort participants contributed time to listing from the index date of estimated glomerular filtration rate eligibility without accounting for the timing of referral for KT, whereas time to listing was calculated from evaluation date in our study.
Our findings indicate that despite the relatively lower burden of depressive symptoms, older kidney failure patients with higher CES-D scores may be more vulnerable to limited access to KT. One potential explanation for such association is that depressive symptoms might have exposed patients to higher risks of increased comorbidities and disease severity43 and further affected their qualification for transplant, which is more likely in older patients. In addition, considering that patients may be asked to make many decisions and adhere to various required treatments across the complex evaluation process, depressive symptoms may be a barrier to cope with these requirements. Therefore, there is a need to routinely screen for depressive symptoms among kidney failure patients to improve their access to KT.
To our knowledge, our study is the largest prospective study of depressive symptoms in a cohort of adult kidney failure patients across the age spectrum undergoing KT evaluation. In addition, with the linkage to Scientific Registry of Transplant Recipients, we were able to obtain the complete data on time of listing and active status for KT candidates. Nevertheless, our study is not without limitations. First, our results are limited by the transplant center included. However, our study sample comprises similar population distributions to all candidates listed at the Johns Hopkins during 2009 to 2021 in the national registry, so the inferences are likely reflective of centers similar to the study site. Second, there were potential misclassifications regarding depressive symptom severity because of the data unavailability of pharmacologic or nonpharmacologic treatment for depression. In fact, multiple studies have found that depression is undertreated among dialysis patients, with <30% of patients with severe depressive symptoms or physician-diagnosed depression receiving treatment.30,46 Therefore, we expected that very few patients with depressive symptoms might have received pharmacologic or nonpharmacologic treatment in our cohort. It should also be noted that the misclassification of the severity of depressive symptoms might be differential by age. It has been reported that older patients tend to minimize their depressive symptoms than younger patients.47 Health providers should be aware that the severity of depressive symptoms among older patients could be underestimated if the assessment solely relies on self-reports.
In summary, 85.8% of KT candidates reported at least minimal depressive symptoms at KT evaluation, and the burden was higher among younger patients. Candidates with depressive symptoms, even those with minimal symptoms, had a lower chance of being listed. Furthermore, the impact of higher CES-D scores (more depressive symptoms) on access to KT was most pronounced among older candidates. To improve access to KT, specifically in those with depressive symptoms, transplant centers and providers should routinely screen for depressive symptoms and refer patients with even mild depressive symptoms to a provider with appropriate mental health expertise in a timely manner. Even though depressive symptoms are not contraindicated in transplant, this study demonstrated that KT candidates with depression are at a disadvantage in being listed for a life-saving transplant. As such, increased efforts to identify and then support these patients in their mental health are vital to providing equitable care. These patients would benefit from coordination between their transplant teams and outpatient clinics to best identify mental health concerns and, in turn, provide treatment to improve access to KT. For those patients receiving dialysis, the dialysis centers play a vital role in identifying mental health concerns, including depression, given the close and frequent contact with patients. Although centers are required to screen for depression, more frequent and in-depth screening by a mental health professional should be considered for patients who are at a higher risk for developing depression.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute on Aging: grant numbers K01AG064040 (principal investigator: NMC), R01AG055781 (principal investigator: MAMD), R01DK114074 (principal investigator: MAMD), and K24AI144954 (principal investigator: DLS).
Footnotes
Figure S1. Joint prevalence of mild/moderate/severe depressive symptoms (CES-D ≥ 16), frailty, and disabilities (lower extremity impairment, ADL dependence, and IADL dependence) among kidney transplant evaluation patients.
Table S1. Correlates of binary depressive symptoms (CES-D ≥ 18) among kidney transplant evaluation patients using complete case analysis.
Table S2. Correlates of CES-D score and binary depressive symptoms among kidney transplant evaluation patients using multiple imputations for missing data (n = 3728).
Table S3. Chance of listing for kidney transplant within 1 year after evaluation by depressive symptoms among transplant evaluation patients using multiple imputations for missing data (n = 3728).
Table S4. Chance of listing for kidney transplant within 1 year after evaluation by depressive symptoms adjusted for physical functional status among evaluation patients using complete case analysis.
Supplementary Material
Figure S1. Joint prevalence of mild/moderate/severe depressive symptoms (CES-D ≥ 16), frailty, and disabilities (lower extremity impairment, ADL dependence, and IADL dependence) among kidney transplant evaluation patients.
Table S1. Correlates of binary depressive symptoms (CES-D ≥ 18) among kidney transplant evaluation patients using complete case analysis.
Table S2. Correlates of CES-D score and binary depressive symptoms among kidney transplant evaluation patients using multiple imputations for missing data (n = 3728).
Table S3. Chance of listing for kidney transplant within 1 year after evaluation by depressive symptoms among transplant evaluation patients using multiple imputations for missing data (n = 3728).
Table S4. Chance of listing for kidney transplant within 1 year after evaluation by depressive symptoms adjusted for physical functional status among evaluation patients using complete case analysis.
References
- 1.Katon W.J. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 2.Song M.K., Ward S.E., Hladik G.A., Bridgman J.C., Gilet C.A. Depressive symptom severity, contributing factors, and self-management among chronic dialysis patients. Hemodial Int. 2016;20:286–292. doi: 10.1111/hdi.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Lullo L., Rivera R., Barbera V., et al. Sudden cardiac death and chronic kidney disease: from pathophysiology to treatment strategies. Int J Cardiol. 2016;217:16–27. doi: 10.1016/j.ijcard.2016.04.170. [DOI] [PubMed] [Google Scholar]
- 4.Palmer S., Vecchio M., Craig J.C., et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84:179–191. doi: 10.1038/ki.2013.77. [DOI] [PubMed] [Google Scholar]
- 5.Cukor D., Rosenthal D.S., Jindal R.M., Brown C.D., Kimmel P.L. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int. 2009;75:1223–1229. doi: 10.1038/ki.2009.51. [DOI] [PubMed] [Google Scholar]
- 6.McDade-Montez E.A., Christensen A.J., Cvengros J.A., Lawton W.J. The role of depression symptoms in dialysis withdrawal. Health Psychol. 2006;25:198–204. doi: 10.1037/0278-6133.25.2.198. [DOI] [PubMed] [Google Scholar]
- 7.Weisbord S.D., Mor M.K., Sevick M.A., et al. Associations of depressive symptoms and pain with dialysis adherence, health resource utilization, and mortality in patients receiving chronic hemodialysis. Clin J Am Soc Nephrol. 2014;9:1594–1602. doi: 10.2215/CJN.00220114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedayati S.S., Finkelstein F.O. Epidemiology, diagnosis, and management of depression in patients with CKD. Am J Kidney Dis. 2009;54:741–752. doi: 10.1053/j.ajkd.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko G.J., Kim M.G., Yu Y.M., et al. Association between depression symptoms with inflammation and cardiovascular risk factors in patients undergoing peritoneal dialysis. Nephron Clin Pract. 2010;116:c29–c35. doi: 10.1159/000314548. [DOI] [PubMed] [Google Scholar]
- 10.Pham P.T., Pham P.A., Pham P.C., Parikh S., Danovitch G. Evaluation of adult kidney transplant candidates. Semin Dial. 2010;23:595–605. doi: 10.1111/j.1525-139X.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 11.Akman B., Uyar M., Afsar B., Sezer S., Ozdemir F.N., Haberal M. Adherence, depression and quality of life in patients on a renal transplantation waiting list. Transpl Int. 2007;20:682–687. doi: 10.1111/j.1432-2277.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- 12.Szeifert L., Bragg-Gresham J.L., Thumma J., et al. Psychosocial variables are associated with being wait-listed, but not with receiving a kidney transplant in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2012;27:2107–2113. doi: 10.1093/ndt/gfr568. [DOI] [PubMed] [Google Scholar]
- 13.Fiske A., Wetherell J.L., Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 15.Hemmelgarn B.R., Manns B.J., Quan H., Ghali W.A. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis. 2003;42:125–132. doi: 10.1016/s0272-6386(03)00415-3. [DOI] [PubMed] [Google Scholar]
- 16.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 18.Katz S., Akpom C.A. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 19.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 20.United States Census Bureau TIGER/line Shapefiles: 2010. United States Census Bureau. https://www.census.gov/geographies/mapping-files/time-series/geo/tiger-line-file.html Updated December 16, 2021.
- 21.United States Census Bureau. American community survey poverty status in the past 12 months. United States Census Bureau. Accessed March 28, 2022. https://data.census.gov/cedsci/table?q=S1701&tid=ACSST5Y2020.S1701
- 22.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23.Kim J., Chung H., Askew R.L., et al. Translating CESD-20 and PHQ-9 scores to PROMIS depression. Assessment. 2017;24:300–307. doi: 10.1177/1073191115607042. [DOI] [PubMed] [Google Scholar]
- 24.Harrell F. Springer; 2001. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. [Google Scholar]
- 25.Buigues C., Padilla-Sánchez C., Garrido J.F., Navarro-Martínez R., Ruiz-Ros V., Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging Ment Health. 2015;19:762–772. doi: 10.1080/13607863.2014.967174. [DOI] [PubMed] [Google Scholar]
- 26.Barry L.C., Allore H.G., Bruce M.L., Gill T.M. Longitudinal association between depressive symptoms and disability burden among older persons. J Gerontol A Biol Sci Med Sci. 2009;64A:1325–1332. doi: 10.1093/gerona/glp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong D., Solomon P., Dong X. Depressive symptoms and onset of functional disability over 2 years: a prospective cohort study. J Am Geriatr Soc. 2019;67:S538–S544. doi: 10.1111/jgs.15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedayati S.S., Bosworth H.B., Kuchibhatla M., et al. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69:1662–1668. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 29.Sy J., McCulloch C.E., Johansen K.L. Depressive symptoms, frailty, and mortality among dialysis patients. Hemodial Int. 2019;23:239–246. doi: 10.1111/hdi.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes A.A., Albert J.M., Young E.W., et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS [published correction appears in Kidney Int. 2004;66:2486] Kidney Int. 2004;66:2047–2053. doi: 10.1111/j.1523-1755.2004.00977.x. [DOI] [PubMed] [Google Scholar]
- 31.Fischer M.J., Kimmel P.L., Greene T., et al. Sociodemographic factors contribute to the depressive affect among African Americans with chronic kidney disease. Kidney Int. 2010;77:1010–1019. doi: 10.1038/ki.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedayati S.S., Grambow S.C., Szczech L.A., Stechuchak K.M., Allen A.S., Bosworth H.B. Physician-diagnosed depression as a correlate of hospitalizations in patients receiving long-term hemodialysis. Am J Kidney Dis. 2005;46:642–649. doi: 10.1053/j.ajkd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Noh J.-W., Kwon Y.D., Park J., Oh I.H., Kim J. Relationship between physical disability and depression by gender: a panel regression model. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotarou E.S., Sakellariou D. Depressive symptoms in people with disabilities; secondary analysis of cross-sectional data from the United Kingdom and Greece. Disabil Health J. 2018;11:367–373. doi: 10.1016/j.dhjo.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Tsai H.J. Nutrition risk, functional dependence, and co-morbidities affect depressive symptoms in Taiwanese aged 53 years and over: a population-based longitudinal study. J Psychosom Res. 2013;75:173–177. doi: 10.1016/j.jpsychores.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T., Michikawa T., Imamura H., et al. Relationship between depressive symptoms and activity of daily living dependence in older Japanese: the Kurabuchi study. J Am Geriatr Soc. 2017;65:2639–2645. doi: 10.1111/jgs.15107. [DOI] [PubMed] [Google Scholar]
- 37.Haugen C.E., Chu N.M., Ying H., et al. Frailty and access to kidney transplantation. Clin J Am Soc Nephrol. 2019;14:576–582. doi: 10.2215/CJN.12921118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAdams-DeMarco M.A., Ying H., Thomas A.G., et al. Frailty, inflammatory markers, and Waitlist mortality among patients with end-stage renal disease in a prospective cohort study. Transplantation. 2018;102:1740–1746. doi: 10.1097/TP.0000000000002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haugen C.E., Agoons D., Chu N.M., et al. Physical impairment and access to kidney transplantation. Transplantation. 2020;104:367–373. doi: 10.1097/TP.0000000000002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu N.M., Sison S., Muzaale A.D., et al. Functional independence, access to kidney transplantation and waitlist mortality. Nephrol Dial Transplant. 2020;35:870–877. doi: 10.1093/ndt/gfz265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noohi S., Khaghani-Zadeh M., Javadipour M., et al. Anxiety and depression are correlated with higher morbidity after kidney transplantation. Transplant Proc. 2007;39:1074–1078. doi: 10.1016/j.transproceed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Chilcot J., Spencer B.W., Maple H., Mamode N. Depression and kidney transplantation. Transplantation. 2014;97:717–721. doi: 10.1097/01.TP.0000438212.72960.ae. [DOI] [PubMed] [Google Scholar]
- 43.Goh Z.S., Griva K. Anxiety and depression in patients with end-stage renal disease: impact and management challenges - a narrative review. Int J Nephrol Renovasc Dis. 2018;11:93–102. doi: 10.2147/IJNRD.S126615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farrokhi F., Abedi N., Beyene J., Kurdyak P., Jassal S.V. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63:623–635. doi: 10.1053/j.ajkd.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 45.Harhay M.N., Yang W., Sha D., et al. Health-related quality of life, depressive symptoms, and kidney transplant access in advanced CKD: findings from the chronic renal insufficiency cohort (CRIC) study. Kidney Med. 2020;2:600–609.e1. doi: 10.1016/j.xkme.2020.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watnick S., Kirwin P., Mahnensmith R., Concato J. The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis. 2003;41:105–110. doi: 10.1053/ajkd.2003.50029. [DOI] [PubMed] [Google Scholar]
- 47.Carter J.D., Frampton C.M., Mulder R.T., Luty S.E., Joyce P.R. The relationship of demographic, clinical, cognitive and personality variables to the discrepancy between self and clinician rated depression. J Affect Disord. 2010;124:202–206. doi: 10.1016/j.jad.2009.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.