Abstract
Introduction
Cell therapy with regulatory T cells (Tregs) in solid organ transplantation is a promising approach for the prevention of graft rejection and induction of immunologic tolerance. Previous clinical studies have demonstrated the safety of Tregs in renal transplant recipients. Antigen-specific Tregs, such as chimeric antigen receptor (CAR)-Tregs, are expected to be more efficacious than polyclonal Tregs in homing to the target antigen. We have developed an autologous cell therapy (TX200-TR101) where a human leukocyte antigen (HLA) class I molecule A∗02 (HLA-A∗02)-CAR is introduced into autologous naive Tregs from a patient with HLA-A∗02-negative end-stage renal disease (ESRD) awaiting an HLA-A∗02-positive donor kidney.
Methods
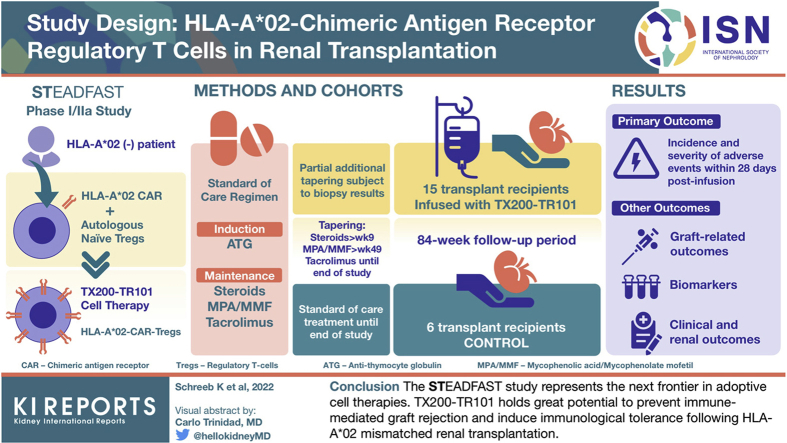
This article describes the design of the STEADFAST study, a first-in-human, phase I/IIa, multicenter, open-label, single-ascending dose, dose-ranging study to assess TX200-TR101 in living-donor renal transplant recipients. Up to 15 transplant recipients will receive TX200-TR101 and will be followed up for a total of 84 weeks post-transplant, alongside a control cohort of up to 6 transplant recipients. All transplant recipients will receive a standard of care immunosuppressive regimen, with the intent of intensified tapering of the regimen in the TX200-TR101 cohort.
Results
The primary end point is the incidence and severity of treatment-emergent adverse events (AEs) within 28 days post–TX200-TR101 infusion. Other end points include additional safety parameters, clinical and renal outcome parameters, and the evaluation of biomarkers.
Conclusion
The STEADFAST study represents the next frontier in adoptive cell therapies. TX200-TR101 holds great potential to prevent immune-mediated graft rejection and induce immunologic tolerance after HLA-A∗02-mismatched renal transplantation.
Keywords: acute and chronic kidney rejection, CAR-Tregs, HLA-A∗02 mismatch, end-stage renal disease, immunotherapy, living-donor kidney transplantation
Graphical abstract
See Commentary on Page 1149
Application of Tregs in Renal Transplantation
Renal transplantation is a life-saving therapy for patients with ESRD, but it requires lifelong immunosuppressive treatment to prevent rejection of the graft.1,2 In addition to the risks of transplant rejection and dysfunction, immunosuppressant drug-related side effects, such as nephrotoxicity, infections, and de novo malignancies, contribute to an increased morbidity and mortality in transplant recipients.1,2 Spontaneous development of operational tolerance to renal transplants to enable the withdrawal of immunosuppressive treatments has been documented in only a few very rare cases.3 Therefore, the creation of a tolerogenic environment, enabling reduction of immunosuppressive therapy while maintaining allograft acceptance, is an important goal in transplantation medicine.
Scientific advances in the past decade have paved the way to explore the use of cell therapy with Tregs for the induction of a tolerogenic environment and prevention of transplant rejection.4, 5, 6 Tregs are key mediators of immune tolerance and maintain immune homeostasis.7 After antigenic stimulation by their target antigen in the allograft, antigen-specific Tregs are recruited to the allograft and activated to exert their immunosuppressive function, including suppression of effector T cell (Teff) expansion and activity.8,9 This involves a variety of mechanisms, including the secretion of anti-inflammatory cytokines such as transforming growth factor-β and interleukin-35, and induction of Teff apoptosis among others.6,7 Importantly, Tregs are postulated to have the potential to create immunologic tolerance by not only dampening inflammation but also creating a tissue microenvironment that fosters the recruitment of additional immunosuppressive cell populations, thereby amplifying and prolonging the immunosuppressive effects of antigen-specific Tregs beyond just the duration of their survival after infusion.8,9 Accordingly, Tregs are thought to have the potential to generate long-term tolerance to transplanted organs.8,9
Cell therapy with Tregs continues to be explored not only in the transplantation setting but also in autoimmune and inflammatory conditions.5,8, 9, 10, 11 Feasibility, tolerability, and safety of Treg therapy have been demonstrated in early phase clinical studies in transplantation and autoimmune disease,5,8,11 including studies with polyclonal, autologous Tregs in renal transplantation.12,13 First evidence of potential efficacy has also been provided, albeit limited by the small size and early stage of the studies.5,8,11 The ONE study consortium published data in 2020 from a series of studies performed in renal transplant recipients treated with regulatory cell therapies, including polyclonal and donor-reactive Tregs, comparing against a reference group of renal transplant recipients on standard immunosuppressive therapy.14 Transplant recipients treated with Treg therapies achieved a reduction of immunosuppressive regimen compared with the reference group, without an increased risk of rejection.14 The cell therapy arm also had fewer infectious complications in the first year postinfusion, and immunophenotyping showed restoration of the immune cell composition toward a homeostatic phenotype, whereas no safety concerns emerged compared with the reference group.14 The 2 individual studies included in the ONE study that treated with polyclonal, autologous Tregs observed 100% allograft survival 3 and 4 years after transplantation in renal transplant recipients, along with substantial tapering of immunosuppressive treatments.15,16
Although most Treg clinical studies performed to date have used polyclonal cells expanded ex vivo, antigen-specific Tregs are expected to be more effective in migrating to and persisting in the target tissue, delivering local immunosuppression, and preventing graft rejection,5,9,10 based on preclinical studies.17, 18, 19, 20 It has been postulated that the local immunosuppressive function of antigen-specific Tregs will limit unwanted nonspecific immunosuppression.5
Antigen-specific Tregs can be obtained by the introduction of engineered T cell receptors or CARs into isolated Tregs.10,11 CARs are synthetic receptors comprising an intracellular T cell signaling domain combined with a transmembrane domain and an extracellular antigen-binding domain. This antigen-binding domain is usually derived from an antibody single-chain variable fragment that confers antigen specificity and allows directing of the CAR-Tregs to a target of choice. In recent years, there has been considerable progress in the optimization of the individual structural components of CARs and improvements in isolation and purification protocols for Tregs to be used in cell therapy.10,11
Successful preclinical proof-of-concept has been demonstrated with CAR-Tregs in various murine preclinical models, including colitis and transplant rejection.21 HLAs are promising targets for the CAR-Tregs to be used in transplantation settings.5 The HLA-A∗02 is a common HLA allele group, often leading to mismatches in organ transplantation, with reports of 21.1% to 27.7% of renal transplant recipients in Europe and the United States receiving an HLA-A∗02-mismatched renal transplant (i.e., HLA-A∗02-negative transplant recipients receiving an HLA-A∗02-positive renal transplant).22, 23, 24, 25, 26 Therefore, HLA-A∗02 has been in the center of several preclinical studies that have shown effectiveness of HLA-A∗02-CAR-Tregs in mouse models of graft-versus-host disease and skin transplantation.27, 28, 29, 30, 31, 32 Recently, an HLA-A∗02-CAR has been optimized, and proof-of-concept has been demonstrated in mouse models, where HLA-A∗02-CAR-Tregs migrated to HLA-A∗02–positive human skin allografts, persisted in the target tissue, and were efficacious at preventing allograft rejection.29
Clinical Study Approach and Proposed Mechanism of Action
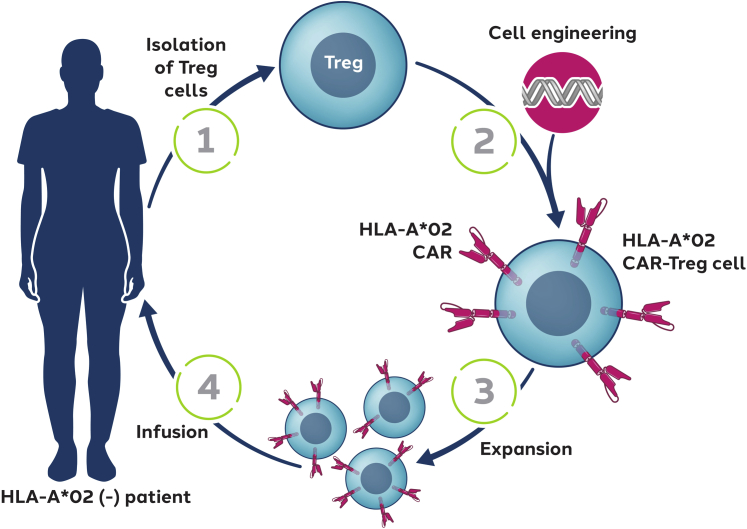
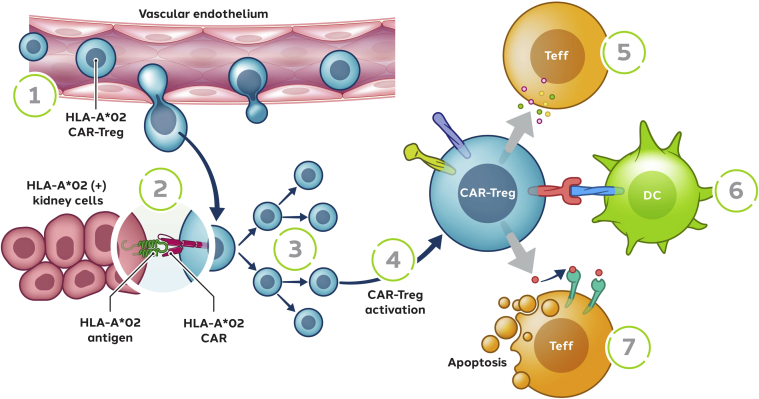
We have developed an autologous cell therapy (designated TX200-TR101) based on naive Tregs (cluster of differentiation [CD]4+/CD45RA+/CD25+/CD127low/neg) isolated from an HLA-A∗02–negative renal transplant recipient designated to receive a mismatched HLA-A∗02–positive allograft (Figure 1). The isolated Tregs are genetically modified using a third-generation, self-inactivating lentiviral vector to express an HLA-A∗02-CAR and expanded ex vivo before infusion to the transplant recipient after a defined period after receipt of their allograft. After i.v. infusion, the HLA-A∗02-CAR-Tregs are anticipated to migrate into the HLA-A∗02–positive allograft tissue, where they are expected to activate on binding to the HLA-A∗02 antigen expressed within the allograft (Figure 2). On activation, the HLA-A∗02-CAR-Tregs are expected to proliferate further within the allograft and acquire their full immunomodulatory capacities. Within the renal allograft, the aim is to create an environment of immune homeostasis, potentially enabling establishment of immunologic tolerance. It is hypothesized that HLA-A∗02-CAR-Treg therapy might allow the reduction of systemic immunosuppressive therapy, thus reducing the risk of infections and the long-term risks of malignancy.33 Moreover, immunosuppressive therapy is also known to cause nephrotoxicity and a few metabolic abnormalities, such as new-onset type 2 diabetes, hypercholesterolemia, and hypertension, all of which increase the risk of cardiovascular events.2,33
Figure 1.
Autologous Tregs for generation of antigen-specific CAR-Tregs. Naive Tregs (CD4+/CD45RA+/CD25+/CD127low/neg) will be isolated from HLA-A∗02–negative renal transplant recipients designated to receive mismatched HLA-A∗02–positive organs (1). The isolated Tregs will be transduced with a lentiviral vector encoding the HLA-A∗02-CAR (2) and expanded ex vivo before cryopreservation (3). After renal transplantation, transplant recipients will receive an intravenous infusion of the autologous HLA-A∗02-CAR-Tregs (4). CAR, chimeric antigen receptor; CD, cluster of differentiation; HLA-A∗02, human leukocyte antigen class I molecule A∗02; Tregs, regulatory T cells.
Figure 2.
Proposed mechanism of action of TX200-TR101. HLA-A∗02-CAR-Tregs are expected to migrate into the HLA-A∗02-positive allograft tissue (1), where they will interact with their target antigen HLA-A∗02 (2), leading to their activation and proliferation (3, 4). On the basis of the known mechanism of action of Tregs,6 HLA-A∗02-CAR-Tregs are then expected to exert a variety of immunomodulatory functions to dampen effector and cytotoxic T cell activation (5, 7) responsible for transplant rejection. Through the production of immunomodulatory cytokines, HLA-A∗02-CAR-Tregs are expected to modulate the activation of Teff (5) and antigen-presenting cells. Moreover, the interaction of activated HLA-A∗02-CAR-Tregs with antigen-presenting cells should lead to the creation of an unfavorable microenvironment for the Teff, leading to their apoptosis (6). HLA-A∗02-CAR-Tregs might also have a cytolytic activity and induce the apoptosis of Teff (7). Moreover, the HLA-A∗02-CAR-Tregs may suppress B-cell activation and differentiation into antibody-producing cells, reducing the risk of antibody-dependent cellular cytotoxicity directed against the transplanted tissue (not depicted). CAR, chimeric antigen receptor; DC, dendritic cell; HLA-A∗02, human leukocyte antigen class I molecule A∗02; Teff, effector T cell; Tregs, regulatory T cells.
We have completed a series of preclinical studies to confirm quality and in vivo efficacy and safety of the cell product and to establish release criteria; the release criteria will ensure that the cell product contains >90% of naive Tregs.
Moreover, we have successfully confirmed the feasibility of obtaining naive Tregs from the target population of patients with ESRD and the subsequent manufacturing process for production of TX200-TR101 in a manufacturing feasibility study.
This article describes the design of the STEADFAST study (registered as NCT04817774 and EudraCT 2019-001730-34), a phase I/IIa study to assess TX200-TR101 in living-donor renal transplant recipients. We anticipate that this will be the first time that CAR-Tregs are administered to humans.
As of February 2022, recruitment into the study has started and 4 sites have been initiated in Belgium, The Netherlands, and United Kingdom.
The study is funded by Sangamo Therapeutics France. It is performed in accordance with the Declaration of Helsinki and International Council for Harmonization Good Clinical Practice after approval by the relevant ethics committees at the clinical sites.
Methods
Study Sites and Participants
The study is conducted in at least 4 renal transplant centers, with sites activated to date in Belgium, The Netherlands, and United Kingdom. Up to 21 patients with ESRD aged 18 to 70 years awaiting a renal transplant from a living, related, or unrelated donor will be recruited; up to 15 subjects will receive TX200-TR101 and up to 6 subjects will be assigned to a control cohort. Transplant recipients receiving TX200-TR101 must be HLA-A∗02 negative and assigned to receive a kidney from an HLA-A∗02–positive donor. Transplant recipients in the control cohort will be HLA mismatched, though mismatch for HLA-A∗02 specifically is optional. Moreover, the subjects in the control cohort should have a similar immunologic risk as the TX200-TR101 cohort. Subjects with clinically active infection or significant unstable or poorly controlled diseases will not be eligible. The key inclusion and exclusion criteria for transplant recipients and donors are provided in Table 1.
Table 1.
Key inclusion and exclusion criteria of transplant recipients and transplant donors in the STEADFAST study
| Inclusion criteria | Exclusion criteria |
|---|---|
| Transplant recipients | |
| Aged between 18 and 70 yrs (inclusive) | HLA identical to the prospective organ donor |
| Diagnosis of ESRD and currently waiting for a new kidney from an identified live donor | Known hypersensitivity or contraindications for IS |
| Single-organ recipients (kidney) | Prior organ transplant |
| Normal or nonclinically significantly abnormal electrocardiogram | Evidence of HIV, syphilis, EBV, HBV, or HCV infection or prespecified hepatic and hematologic laboratory abnormalities |
| HLA-A∗02 negativea | Positive flow-cytometric crossmatch using donor lymphocytes and recipient serum |
| HLA-A∗69 negativea,b | PRA > 20% recent or historic |
| Adequate venous access for leukapheresis, and no other contraindications for leukapheresisa | Current, recent, or historical donor-specific antibodies |
| Previous treatment with any desensitization procedure | |
| Underlying renal disease with a high risk of disease reoccurrence in the transplanted kidney | |
| Concomitant clinically active local or systemic infection | |
| Systemic immunosuppressive agents administered for other indications | |
| Significant unstable or poorly controlled acute or chronic diseases or laboratory abnormality (except ESRD) which, in the opinion of the investigator, could confound the results of the study or put the subject at undue risk, including high risk of renal thrombotic event | |
| Current or previous history of malignancy (within the last 5 yr) | |
| Transplant donors | |
| Age ≥18 yr | Evidence of HBV or HCV infection |
| ABO blood type compatible with the organ recipient | |
| Negative serology result for HIV and syphilis | |
| HLA-A∗02 positivec |
CAR, chimeric antigen receptor; EBV, Epstein-Barr virus; ESRD, end-stage renal disease; HBV, hepatitis B virus; HCV, hepatitis C virus; HLA, human leukocyte antigen; HLA-A∗02/69, human leukocyte antigen class I molecule A∗02/69; IS, immunosuppressive; PRA, panel-reactive antibody; ScFv, single-chain variable fragment.
Inclusion criterion only for transplant recipients in the TX200-TR101 cohort.
Transplant recipients in the TX200-TR101 cohort must be HLA-A∗69 negative because in vitro studies using an HLA-A∗02-CAR constructed from the same humanized ScFv used to manufacture TX200-TR101 showed some cross-reactivity between the HLA-A∗02-CAR and the HLA-A∗69 allele.
Inclusion criterion only for transplant donors for the TX200-TR101 cohort.
Study Objectives and End Points
The primary objective of the study is to evaluate the short-term safety and tolerability of TX200-TR101 over 28 days after infusion, as reflected in the primary end point, which is the incidence and severity of treatment-emergent AEs occurring within 28 days after TX200-TR101 infusion. Other objectives include clinical evaluation of the long-term effects of TX200-TR101 on acute and chronic graft-related outcomes, further safety outcomes, reduction of immunosuppressive therapy, and assessment of biomarkers. Further clinical and safety end points will be assessed to week 84 post-transplant, including acute rejection, assessed as biopsy-confirmed acute rejection, and chronic graft dysfunction, assessed based on histopathology (Banff 2019 criteria) and on estimated glomerular filtration rate. Moreover, graft loss due to rejection, occurrence of de novo donor-specific antibodies, and the extent of required immunosuppressive therapy will be analyzed. End points regarding graft dysfunction or rejection will be based on established diagnostic methods. Allograft biopsies will be analyzed according to the most recent Banff criteria, including grading of antibody-mediated changes, T cell-mediated rejection, and polyomavirus nephropathy, based on Banff lesion scores.34
Biomarker assessments in blood and urine will include flow cytometric analyses and assessment of chemokines/cytokines. Biomarker assessments will be predominantly exploratory and have been developed for the STEADFAST study to assess immunologic responses and the potential to achieve immunologic tolerance to the allograft. The biomarker panel will determine the localization and kinetics of TX200-TR101 in the graft and the effects on graft and peripheral immune responses. The localization of TX200-TR101 will be assessed based on CD4-positive cells in the renal transplant biopsy that are also positive for the HLA-A∗02-CAR. In addition, biomarker levels and changes from baseline related to the presence of HLA-A∗02-CAR-Tregs will be assessed, including changes from baseline in donor/recipient mixed lymphocyte reaction assays. The assessment of TX200-TR101 in allograft tissue will enable the confirmation of homing of TX200-TR101 to the allograft where the target antigen HLA-A∗02 is expressed and will provide valuable data on local persistence of the HLA-A∗02-CAR-Tregs.
Study Design, Intervention, and Assessments
The STEADFAST study is a first-in-human, phase I/IIa, multicenter, open-label, single-ascending dose, dose-ranging study with a control cohort.
Up to 15 patients with HLA-A∗02–negative ESRD designated to receive living-donor renal transplants from HLA-A∗02–positive donors assigned to one of the TX200-TR101 cohorts will undergo leukapheresis up to 24 weeks before planned transplant surgery for the collection of white blood cells. These will be sorted to select naive Tregs for manufacture of HLA-A∗02-CAR-Tregs (TX200-TR101). Manufacture involves transduction with a CAR comprising a humanized HLA-A∗02 single-chain variable fragment fused to a transmembrane domain and a signaling domain composed of the intracellular domains of human CD28 and CD3ζ, followed by cell expansion. After Good Manufacturing Practice-compliant manufacture at a central manufacturing site, TX200-TR101 will be shipped to the sites in cryovials at a fixed cell concentration for bedside dose titration on site on the day of infusion. A control cohort of up to 6 living-donor renal transplant recipients with a low immunologic risk similar to that of the subjects in the TX200-TR101 cohorts will also be recruited.
All transplant recipients will undergo transplant surgery on day 0, and their immunosuppressive regimen will be initiated. The immunosuppressive regimen is based on standard of care and will comprise antithymocyte globulin induction therapy, together with a triple maintenance regimen consisting of corticosteroids, mycophenolic acid/mycophenolate mofetil, and tacrolimus. Corticosteroids will be dosed and tapered post-transplant as per standard of care for all transplant recipients. Tapering and cessation of mycophenolic acid/mycophenolate mofetil will be attempted for transplant recipients in the TX200-TR101 cohort with dose reduction starting 2 weeks post-transplantation and the aim of cessation from week 49, leading ultimately to tacrolimus monotherapy at a low-dose trough level. For control subjects, tapering of mycophenolic acid/mycophenolate mofetil will be performed with dose reduction starting 2 weeks after transplantation and further tapering from week 13 as per the clinical site’s usual standard of care. The dose of tacrolimus will be reduced at week 16 post-transplantation. Alternative tapering regimens are permitted where required in the clinical management of individual subjects at the discretion of the treating investigator. Information to inform on tapering of the immunosuppression therapy will be derived from renal function monitoring and from biopsies taken at weeks 16 and 36 and assessed per Banff criteria.
Antiviral and pneumocystis pneumonia prophylaxes in line with the standard of care will be provided for 200 days for all transplant recipients. Antibacterial and antifungal prophylaxes will also be administered according to standard of care.
Transplant recipients will receive a single i.v. infusion of TX200-TR101 at a prespecified time point between 2 and 3 months after transplant surgery. Administration of TX200-TR101 will be withheld if transplant recipients have an acute systemic or local infection on the day of the planned infusion or treatment with parenteral antibiotics within 14 days of the planned infusion, or acute graft rejection, unstable graft function, treatment for acute rejection, or other major clinical events within 28 days of the planned infusion.
If clinically feasible, mandatory allograft biopsies of the transplanted kidney will be taken on the day of transplantation (day 0), at week 16, and at week 36; the latter being optional for the control cohort. All biopsies will be assessed by a local and a central pathologist according to Banff criteria.34 Additional biopsies will be taken if transplant rejection is suspected. To assess safety and clinical study end points, subjects will undergo physical, clinical, and laboratory assessments, including collection of AEs and a set of biomarker assessments.
The follow-up time after transplant surgery will be 84 weeks post-transplant for both cohorts. The TX200-TR101 cohort will be invited to join a subsequent long-term follow-up study, providing a total of 15 years of observation for each subject.
Assessments and precautions during the coronavirus 2019 pandemic are specified in the protocol and will include polymerase chain reaction testing according to local guidance, and at a minimum before transplantation, the leukapheresis procedure, and TX200-TR101 administration.
Dose Cohorts, Staggered Dose Escalation, and Safety Monitoring
A Safety Monitoring Committee will review relevant safety data and discuss continuation, termination, dosing considerations, or other modifications of the study with the sponsor.
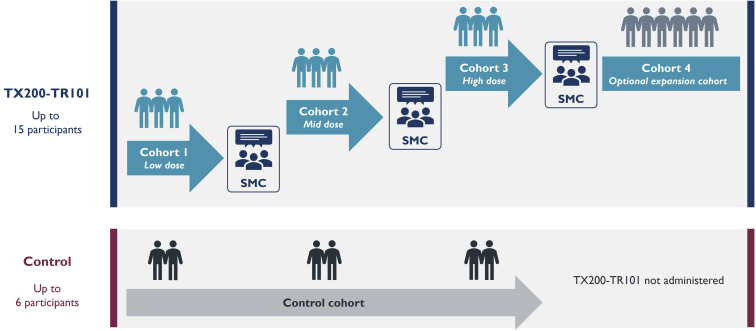
Three doses of TX200-TR101 in the range of 104 to 109 cells/kg body weight will be assessed in 3 single-ascending dose cohorts, with 3 evaluable subjects per dose level (Figure 3). An additional 6 transplant recipients may be recruited at the recommended dose level for expansion after consultation of the sponsor and the Safety Monitoring Committee, resulting in a total of up to 15 subjects administered TX200-TR101. It is aimed to enroll at least 1 control subject per dose cohort during the dose-escalation phase of the study.
Figure 3.
STEADFAST study scheme. SMC, Safety Monitoring Committee.
Transplantation and subsequent TX200-TR101 administration will be staggered, and sentinel dosing will be applied within and between dose groups. The first and second subjects in each dose cohort will be observed for safety surveillance for a minimum of 28 and 14 days after infusion, respectively, before the next subject may be dosed. A review of the data by the Safety Monitoring Committee will be required before starting a new dose cohort.
After infusion of TX200-TR101, subjects will be monitored closely for 24 to 48 hours in an inpatient clinical setting. This includes assessment of vital signs, clinical status, inflammatory markers, and cytokines immediately before, during, and after TX200-TR101 infusion. After discharge, additional outpatient monitoring will be in place for the subsequent 12 to 13 days postinfusion.
All AEs, including serious AEs, will be documented and reported throughout the study, from the time of informed consent (serious AEs) or first invasive procedure (nonserious AEs) to the end of study participation (week 84), according to Good Clinical Practice. Severity will be graded according to Common Terminology Criteria for Adverse Events.
Statistical Considerations and Sample Size
No formal statistical hypotheses will be tested, and no formal sample size calculation was performed for this first-in-human study. A total of 3 subjects per dose cohort were considered sufficient to evaluate the safety and tolerability of TX200-TR101 before dose escalation. All statistical summaries will be descriptive.
Discussion
Study Design
The STEADFAST study is the first study to our knowledge to initiate the testing of CAR-Tregs in humans and has been designed based on preclinical studies with CAR-Tregs and clinical studies using polyclonal Tregs. Clinical studies to date show that autologous polyclonal Tregs are safe over a wide dose range.8 Ongoing and completed trials with polyclonal Tregs in a renal transplant setting use doses ranging from 0.5 to 10 × 106 cells/kg.8 As antigen-specific Tregs provide targeted therapy, and thereby are expected to increase potency, efficacious cell doses needed to induce immunologic tolerance will likely be lower than doses for polyclonal Tregs. Starting dose calculations for this study are based on a number of factors, including the desired ratio of Tregs versus Teff for the regulation of alloimmune responses.
The timing of the TX200-TR101 administration at a defined time point post-transplantation was chosen based on clinical data and considering the immunosuppressive regimen. Several groups have demonstrated an initial decline of polyclonal Tregs in peripheral blood immediately after transplantation.35,36 Thus, administration of TX200-TR101 at approximately 2 to 3 months post-transplant is expected to boost the number of Tregs that interact with the overall Teff population.
This study aims to evaluate the short-term safety and tolerability of TX200-TR101. Assessment of TX200-TR101 in allografts will evaluate homing and local persistence of the HLA-A∗02-CAR-Tregs in the kidney. Preclinical data demonstrating the homing of TX200-TR101 Tregs to their target antigen in a mouse model are the subject of a separate article (currently under revision). An exploratory biomarker panel will be used to assess immunologic responses and the potential to achieve immunologic tolerance to the allograft. Previous clinical studies deploying the use of exploratory biomarkers have demonstrated restoration of the immune cell composition toward the homeostatic phenotype of healthy controls in renal transplant recipients treated with regulatory cell therapies.14
Immunosuppressive Regimen and Tregs
Induction treatment in this study will be performed and immunosuppressant treatment started according to standard of care. In this study, if clinically feasible, it is planned to taper mycophenolic acid/mycophenolate mofetil to achieve tacrolimus monotherapy, in line with clinical practice. Moreover, tacrolimus is known to be efficacious in reducing the activity of Teff,37 which should support the establishment of a tolerogenic environment by the Tregs.
Safety Considerations
The TX200-TR101 cell product consists of highly purified, homogeneous Tregs, as confirmed by flow-cytometric T-cell phenotyping and as ensured by the established release criteria, both ensuring the high purity of the product and minimizing the risk of any contaminant cells. Therefore, the potential risk of allograft rejection caused by any trace levels of transduced Teff is minimal. In addition, it is well described that the immunomodulatory properties of Tregs include the ability to suppress CD8+ and CD4+ T cell activation and proliferation.38, 39, 40, 41 Lymphopenic mouse models suggest that a Treg to Teff ratio of 1:1 to 1:2 is required to regulate graft rejection by establishing a dominant tolerogenic environment.42,43 This ratio is substantially exceeded in the TX200-TR101 cell product. Therefore, in the event that occasional transduced Teff would be administered to a subject, the favorable balance of CAR-Tregs to Teff within the allograft would enable the suppression of cytotoxic T cell activation and proliferation, preventing graft rejection.
The transformation of Tregs into proinflammatory T cells has been described in the literature.44 However, naive Tregs similar to those used to produce TX200-TR101 appear to be resilient to such transformation.45, 46, 47 Phenotypic stability, shown by persistent hypomethylation of the FOXP3 Treg-specific demethylated region, was found over 60 weeks in patients receiving cell therapy in the ONE study,14 and in preclinical studies using HLA-A∗02-CAR-Tregs,27,32 which are in line with our internal preclinical data. Moreover, our data from an immunodeficient humanized mouse model show that there is no switch of TX200-TR101 to a proinflammatory phenotype over a 3-month duration, based on flow-cytometric immunophenotyping and cytokine assessments. In this mouse model, no TX200-TR101 engraftment-related toxicity or abnormal tissue findings were observed; administration of TX200-TR101 was well tolerated and did not result in the onset of xenogeneic graft-versus-host disease. Therefore, the risk of transformation of TX200-TR101 cells to proinflammatory cytokine-producing cells is considered to be very low.
Cytokine release syndrome is an acute and potentially life-threatening side effect of CAR-Teff therapies that are used in oncology settings.48 This risk is expected to be very low with CAR-Tregs as Tregs do not produce proinflammatory cytokines,10 and, as outlined previously, a conversion to proinflammatory T cells is unlikely based on the literature and our own preclinical data, combined with the fact that the TX200-TR101 release criteria ensure a high-purity cell product in line with regulatory requirements. Nevertheless, clinical status, vital signs, inflammatory markers, and cytokines will be monitored at frequent intervals during and after the TX200-TR101 infusion.
Cell therapy with polyclonal Tregs has a theoretical potential to suppress the immune response against infections and tumors due to the large number of Tregs with broad, undefined specificity.9 However, to date, clinical trials with polyclonal Tregs have in fact shown a trend toward a reduced rate of infections.14 Given that TX200-TR101 is specific for the HLA-A∗02 antigen, this risk is expected to be even lower than for polyclonal Treg therapy. Moreover, antiviral and other anti-infectious prophylaxes will be provided.
Last, there is a potential risk that HLA-A∗02-CAR-Tregs could bind to the HLA-A∗02 antigen within the vascular endothelium in the transplanted kidney, activate, proliferate, and induce hypercoagulability, leading to thrombus formation. This risk is considered to be very low based on our knowledge and understanding of the mechanism of action of TX200-TR101, our internal preclinical studies, and considering the relatively low HLA-A expression levels within the human vasculature compared with other cell types throughout the kidney.49,50 Moreover, subjects considered to be at a high risk of a renal thrombotic event according to investigator judgment will be excluded per the exclusion criteria.
In conclusion, the STEADFAST study represents the next frontier in adoptive cell therapies, being the first clinical study to our knowledge to administer a CAR-Treg therapy in humans. TX200-TR101 holds great promise in potentially inducing immunologic tolerance and preventing immune-mediated rejection after HLA-A∗02-mismatched renal transplantation and potentially in other solid organ transplants with the HLA-A∗02 mismatch. This could provide the opportunity to reduce long-term pharmacologic immunosuppression, thereby reducing immunosuppressant drug-related side effects, including infections, diabetes, and malignancies.2 CAR-Treg technology has the potential to provide a new modality of treatment not only in the transplant setting but also in a number of autoimmune diseases in the future.
Disclosure
The STEADFAST study is sponsored by Sangamo Therapeutics. KS, ECS, CD, GA, and BH are full-time employees of Sangamo Therapeutics. ER is a former full-time employee of Sangamo Therapeutics. PR is the coordinating investigator of the STEADFAST Study.
Acknowledgments
This study was funded and materials were provided by Sangamo Therapeutics. The authors thank the STEADFAST investigators and the patient advocacy group of kidney transplant patients for reviewing the study design. The authors also thank the University of British Columbia and adMare Bioinnovations (formerly known as the Center for Drug Research and Development) for their collaboration on the development of the HLA-A∗02-CAR. Medical writing assistance was provided by Dr. Mike F Mueller (Scinopsis, France). The STEADFAST study is registered on clinicaltrials.gov as NCT04817774 and on EudraCT as 2019-001730-34.
References
- 1.Birnbaum L.M., Lipman M., Paraskevas S., et al. Management of chronic allograft nephropathy: a systematic review. Clin J Am Soc Nephrol. 2009;4:860–865. doi: 10.2215/CJN.05271008. [DOI] [PubMed] [Google Scholar]
- 2.Katabathina V., Menias C.O., Pickhardt P., et al. Complications of immunosuppressive therapy in solid organ transplantation. Radiol Clin North Am. 2016;54:303–319. doi: 10.1016/j.rcl.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Newell K.A., Adams A.B., Turka L.A. Biomarkers of operational tolerance following kidney transplantation—the immune tolerance network studies of spontaneously tolerant kidney transplant recipients. Hum Immunol. 2018;79:380–387. doi: 10.1016/j.humimm.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluestone J.A., Anderson M. Tolerance in the age of immunotherapy. N Engl J Med. 2020;383:1156–1166. doi: 10.1056/NEJMra1911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira L.M.R., Muller Y.D., Bluestone J.A., Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. 2019;18:749–769. doi: 10.1038/s41573-019-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaikunthanathan T., Safinia N., Boardman D., Lechler R.I., Lombardi G. Regulatory T cells: tolerance induction in solid organ transplantation. Clin Exp Immunol. 2017;189:197–210. doi: 10.1111/cei.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Duggleby R., Danby R.D., Madrigal J.A., Saudemont A. Clinical grade regulatory CD4+ T cells (Tregs): moving toward cellular-based immunomodulatory therapies. Front Immunol. 2018;9:252. doi: 10.3389/fimmu.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bluestone J.A., Tang Q. Treg cells—the next frontier of cell therapy. Science. 2018;362:154–155. doi: 10.1126/science.aau2688. [DOI] [PubMed] [Google Scholar]
- 10.Raffin C., Vo L.T., Bluestone J.A. Treg cell-based therapies: challenges and perspectives. Nat Rev Immunol. 2020;20:158–172. doi: 10.1038/s41577-019-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rana J., Biswas M. Regulatory T cell therapy: current and future design perspectives. Cell Immunol. 2020;356:104193. doi: 10.1016/j.cellimm.2020.104193. [DOI] [PubMed] [Google Scholar]
- 12.Chandran S., Tang Q., Sarwal M., et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am J Transplant. 2017;17:2945–2954. doi: 10.1111/ajt.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew J.M., H-Voss J., LeFever A., et al. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep. 2018;8:7428. doi: 10.1038/s41598-018-25574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawitzki B., Harden P.N., Reinke P., et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials [published correction appears in Lancet. 2020;395:1972] Lancet. 2020;395:1627–1639. doi: 10.1016/S0140-6736(20)30167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harden P.N., Game D.S., Sawitzki B., et al. Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients. Am J Transplant. 2021;21:1603–1611. doi: 10.1111/ajt.16395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roemhild A., Otto N.M., Moll G., et al. Regulatory T cells for minimising immune suppression in kidney transplantation: phase I/IIa clinical trial. BMJ. 2020;371:m3734. doi: 10.1136/bmj.m3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putnam A.L., Safinia N., Medvec A., et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant. 2013;13:3010–3020. doi: 10.1111/ajt.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trenado A., Charlotte F., Fisson S., et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagoo P., Ali N., Garg G., Nestle F.O., Lechler R.I., Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golshayan D., Jiang S., Tsang J., et al. In vitro–expanded donor alloantigen–specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109:827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 21.Dawson N.A.J., Vent-Schmidt J., Levings M.K. Engineered tolerance: tailoring development, function, and antigen-specificity of regulatory T cells. Front Immunol. 2017;8:1460. doi: 10.3389/fimmu.2017.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton D., Hamilton P., Doherty C.C., Douglas J.F., McGeown M.G. Mismatching for HLA-A, -B antigens and renal graft survival. Clin Nephrol. 1985;23:26–27. [PubMed] [Google Scholar]
- 23.Barocci S., Santori G., Fiordoro S., et al. Detection and analysis of HLA class I specific alloantibodies in the sera of sensitised dialysis recipients waiting for kidney retransplantation. Riv Ital Med Lab. 2007;3:189–195. [Google Scholar]
- 24.Barocci S., Valente U., Nocera A. Detection and analysis of HLA class I and class II specific alloantibodies in the sera of dialysis recipients waiting for a renal retransplantation. Clin Transpl. 2007;21:47–56. doi: 10.1111/j.1399-0012.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 25.Marrari M., Duquesnoy R.J. Detection of donor-specific HLA antibodies before and after removal of a rejected kidney transplant. Transpl Immunol. 2010;22:105–109. doi: 10.1016/j.trim.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Schnitzler M.A., Woodward R.S., Brennan D.C., et al. Cytomegalovirus and HLA-A, B, and DR locus interactions: impact on renal transplant graft survival. Am J Kidney Dis. 1997;30:766–771. doi: 10.1016/s0272-6386(97)90080-9. [DOI] [PubMed] [Google Scholar]
- 27.Noyan F., Zimmermann K., Hardtke-Wolenski M., et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am J Transplant. 2017;17:917–930. doi: 10.1111/ajt.14175. [DOI] [PubMed] [Google Scholar]
- 28.Boardman D.A., Philippeos C., Fruhwirth G.O., et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am J Transplant. 2017;17:931–943. doi: 10.1111/ajt.14185. [DOI] [PubMed] [Google Scholar]
- 29.Dawson N.A., Lamarche C., Hoeppli R.E., et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bézie S., Charreau B., Vimond N., et al. Human CD8+ Tregs expressing a MHC-specific CAR display enhanced suppression of human skin rejection and GVHD in NSG mice. Blood Adv. 2019;3:3522–3538. doi: 10.1182/bloodadvances.2019000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner J.C., Tang Q. CAR-Tregs as a strategy for inducing graft tolerance. Curr Transplant Rep. 2020;7:205–214. doi: 10.1007/s40472-020-00285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald K.G., Hoeppli R.E., Huang Q., et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girlanda R. In: Regenerative Medicine and Tissue Engineering. Andrades J., editor. IntechOpen; 2013. Complications of post-transplant immunosuppression. [Google Scholar]
- 34.Loupy A., Haas M., Roufosse C., et al. The Banff 2019 kidney meeting report:(I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. doi: 10.1111/ajt.15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libetta C., Esposito P., Gregorini M., et al. Sirolimus vs cyclosporine after induction with basiliximab does not promote regulatory T cell expansion in de novo kidney transplantation: results from a single-center randomized trial. Transpl Immunol. 2015;33:117–124. doi: 10.1016/j.trim.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 36.San Segundo D., Fernández-Fresnedo G., Ruiz J.C., et al. Two-year follow-up of a prospective study of circulating regulatory T cells in renal transplant patients. Clin Transplant. 2010;24:386–393. doi: 10.1111/j.1399-0012.2009.01086.x. [DOI] [PubMed] [Google Scholar]
- 37.Kogina K., Shoda H., Yamaguchi Y., et al. Tacrolimus differentially regulates the proliferation of conventional and regulatory CD4(+) T cells. Mol Cells. 2009;28:125–130. doi: 10.1007/s10059-009-0114-z. [DOI] [PubMed] [Google Scholar]
- 38.de Goër de Herve M.G., Jaafoura S., Vallée M., Taoufik Y. FoxP3+ regulatory CD4 T cells control the generation of functional CD8 memory. Nat Commun. 2012;3:986. doi: 10.1038/ncomms1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okeke E.B., Uzonna J.E. The pivotal role of regulatory T cells in the regulation of innate immune cells. Front Immunol. 2019;10:680. doi: 10.3389/fimmu.2019.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohr A., Atif M., Balderas R., Gorochov G., Miyara M. The role of FOXP3+ regulatory T cells in human autoimmune and inflammatory diseases. Clin Exp Immunol. 2019;197:24–35. doi: 10.1111/cei.13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romano M., Fanelli G., Albany C.J., Giganti G., Lombardi G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43. doi: 10.3389/fimmu.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hara M., Kingsley C.I., Niimi M., et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 43.Graca L., Thompson S., Lin C.Y., Adams E., Cobbold S.P., Waldmann H. Both CD4(+)CD25(+) and CD4(+)CD25(−) regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002;168:5558–5565. doi: 10.4049/jimmunol.168.11.5558. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X., Bailey-Bucktrout S.L., Jeker L.T., et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann P., Eder R., Boeld T.J., et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann P., Boeld T.J., Eder R., et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 47.Schmidl C., Hansmann L., Andreesen R., Edinger M., Hoffmann P., Rehli M. Epigenetic reprogramming of the RORC locus during in vitro expansion is a distinctive feature of human memory but not naïve Treg. Eur J Immunol. 2011;41:1491–1498. doi: 10.1002/eji.201041067. [DOI] [PubMed] [Google Scholar]
- 48.June C.H., O’Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 49.Uhlén M., Fagerberg L., Hallström B.M., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 50.The Human Protein Atlas. https://www.proteinatlas.org Accessed August 2021.