Abstract
Ki-67 assessment is a key step in the diagnosis of neuroendocrine neoplasms (NENs) from all anatomic locations. Several challenges exist related to quantifying the Ki-67 proliferation index due to lack of method standardization and inter-reader variability. The application of digital pathology coupled with machine learning has been shown to be highly accurate and reproducible for the evaluation of Ki-67 in NENs. We systematically reviewed all published studies on the subject of Ki-67 assessment in pancreatic NENs (PanNENs) employing digital image analysis (DIA). The most common advantages of DIA were improvement in the standardization and reliability of Ki-67 evaluation, as well as its speed and practicality, compared to the current gold standard approach of manual counts from captured images, which is cumbersome and time consuming. The main limitations were attributed to higher costs, lack of widespread availability (as of yet), operator qualification and training issues (if it is not done by pathologists), and most importantly, the drawback of image algorithms counting contaminating non-neoplastic cells and other signals like hemosiderin. However, solutions are rapidly developing for all of these challenging issues. A comparative meta-analysis for DIA versus manual counting shows very high concordance (global coefficient of concordance: 0.94, 95% CI: 0.83–0.98) between these two modalities. These findings support the widespread adoption of validated DIA methods for Ki-67 assessment in PanNENs, provided that measures are in place to ensure counting of only tumor cells either by software modifications or education of non-pathologist operators, as well as selection of standard regions of interest for analysis. NENs, being cellular and monotonous neoplasms, are naturally more amenable to Ki-67 assessment. However, lessons of this review may be applicable to other neoplasms where proliferation activity has become an integral part of theranostic evaluation including breast, brain, and hematolymphoid neoplasms.
Subject terms: Endocrine cancer, Endocrine cancer
Introduction
The world health organization (WHO) released in 2019 a consensus document entitled “Recommendations on digital interventions for health system strengthening”, acknowledging that artificial intelligence (AI) and digital technologies can offer limitless possibilities to advance health management and achievements (https://apps.who.int/iris/handle/10665/311980, last access 10/30/2021). Indeed, AI-based technologies are emerging in every medical field, especially in radiology and pathology1–3. In pathology, AI-based systems utilize machine- and/or deep- learning models to assist pathologists in analyzing digital images to perform different tasks, including screening for rare events, quantification, diagnosing lesions, and prognostication1,4,5. Digital pathology, which includes the digitizing of glass slides to generate whole slide images, facilitates the application of AI in pathology6–8. A key benefit of employing AI-based systems in pathology is to provide reliable, objective and reproducible results, thereby reducing inter- and intra-pathologist variability and enabling automation to augment routine practice1,6–8.
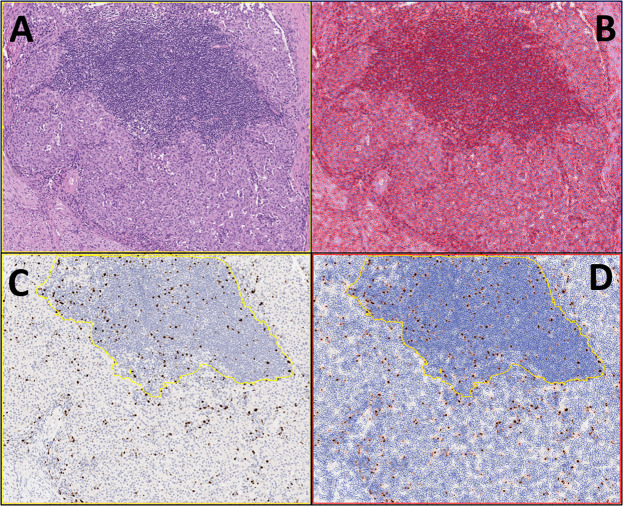
In this context, digital image analysis (DIA) has been utilized to evaluate neuroendocrine neoplasms (NENs). Among well-differentiated neuroendocrine tumors (NETs), grading is based on the assessment of mitotic rate and the proliferation index determined by Ki-67 immunostaining9–11. Currently, the WHO classification of some NENs specifies that Ki-67 should be assessed by manual counting on a printed image including at least 500 neoplastic cells from the regions of highest labeling (hotspots)9,10. Recently, different DIA-based systems have been developed to assist pathologists with this important task (Fig. 1), which has implications for the clinical management of patients with NENs. To date, the majority of studies on this topic were performed on NENs of the gastro-entero-pancreatic system.
Fig. 1. An example of the use of a digitalized system for assessing Ki-67 in pancreatic neuroendocrine neoplasms is shown here.
This is a particularly illustrative case due to the presence of a lymphocytic infiltrate at the tumor periphery, which represents a potential source of bias for Ki67 assessment with digital systems. A A pancreatic neuroendocrine tumor, G2, is shown. (Hematoxylin-eosin, 10x original magnification); B the digitalized system can count all cells present in a specific field, also on hematoxylin-eosin slides; C, D modern systems can select a specific area for the Ki-67 count: in this example, the field with lymphocytes has been excluded from the count, reducing potential important biases in tumor grading (Ki67 immunohistochemistry, 10x original magnification).
The aim of our study was to systematically review all published studies that compared manual Ki-67 assessment in pancreatic NENs (PanNENs) with DIA-based determination, highlighting the benefits and drawbacks of each approach. A comparative meta-analysis is also undertaken of manual counting versus DIA for PanNENs.
Materials and methods
This systematic review adhered to the MOOSE guidelines12 and PRISMA statement13. Studies were considered eligible for inclusion if they reported original data on DIA-based assessment of Ki-67 in PanNENs. Both neuroendocrine tumors (PanNETs) and carcinomas (PanNECs) were included. For the comparative meta-analysis of manual counting vs. DIA, we considered all manuscripts reporting an analytical comparison between these two modalities used in the assessment of Ki-67. In the case of duplicate cohorts, the largest and then most recent was selected. Exclusion criteria included no definitive histological diagnosis of PanNEN, and in vitro or animal studies.
Data sources and literature search strategy
Two investigators (CL, PA) independently searched PubMed, Embase and SCOPUS databases up until 30/06/2021. The search strategy included combinations of the following keywords: #1 “digital”[Title/Abstract] AND “pathology”[Title/Abstract]; #2 “image”[Title/Abstract] AND “analysis”[Title/Abstract]; #3 “artificial intelligence”[Title/Abstract] OR “AI”[Title/Abstract] OR “machine learning”[Title/Abstract] OR “deep learning”[Title/Abstract] OR “automated”[Title/Abstract] OR “semiautomated”[Title/Abstract] OR “algorithm*“[Title/Abstract] OR “neural network”[Title/Abstract] OR “computer-aid”[Title/Abstract] OR “computer-aided”[Title/Abstract] OR “image analysis”[Title/Abstract] OR “digital pathology”[Title/Abstract] OR “WSI”[Title/Abstract] OR “whole slide”[Title/Abstract] OR “digital”[Title/Abstract]; #4 #1 OR #2 OR #3; #5 “neuroendocrine”[Title/Abstract] OR “carcinoid”[Title/Abstract] OR “medullary”[Title/Abstract]; #6 #4 AND #5; #7 “artificial intelligence”[MeSH Terms]; #8 “Neuroendocrine Tumors”[MeSH Terms] OR “carcinoma, neuroendocrine”[MeSH Terms] OR “Gastro-enteropancreatic neuroendocrine tumor” [Supplementary Concept] OR “Carcinoid Tumor”[MeSH Terms]; #9 #7 AND #8; #10 #6 OR #9.
Study selection and data extraction
Following the aforementioned search strategy, duplicates were removed and then two reviewers (CL, PA) independently screened titles and abstracts of all potentially eligible articles. These two authors applied eligibility criteria and reviewed the full texts of included studies. A final list of articles was subsequently established for both the systematic review and comparative meta-analysis by consensus with a third independent author (AE). Two authors were involved in extracting data in a preset Excel database: one (CL) extracted data from the selected articles; the other (AS) independently validated the extracted data. For each article, we extracted the following information: authors; year of publication; country study originated from; number of cases; patient demographics; type of analyzed material; tumor grading; as well as methods for manual counting and DIA. For the comparative meta-analysis, the primary outcome was the coefficient of agreement between manual counting vs. DIA in the assessment of Ki-67 in PanNENs.
Data synthesis, quality, and publication bias assessment
The comparative meta-analysis was conducted using Comprehensive Meta-Analysis v2 software (Biostat; Englewood, NJ, USA). Furthermore, the Newcastle–Ottawa Scale (NOS) was used to assess study quality, following existing guidelines14,15. Finally, we investigated publication bias by visual inspection of funnel plots and with the Egger bias test16.
Results
Search results
The search yielded a total of 4286 potential eligible studies. Following in-depth screening based on title/abstract, only 56 (1.3%) of these studies were retrieved for further analysis. Of them, 22 were considered eligible for the systematic review17–38, and 4 for the correlation meta-analysis (Supplementary Fig. 1)25,27,28,34.
Study and patient characteristics
The most important features from the extracted data are summarized in Table 1. Overall, the selected studies reported data on a total of 752 PanNENs. The majority of the investigated cohorts (59.1%) were from the USA, with the remaining composed of European patients (27.3%) and mixed cohorts including Asian patients (13.6%). There was an almost equal distribution of male (50.5%) and female (49.5%) patients. Regarding tumor grading (G), the majority of cases were G1 (55.3%), followed by G2 (40.6%) and G3 (4.1%). The type of specimen material analyzed varied, including surgical resection specimens, biopsies, and cytology cell blocks. The majority of the studies (54.5%) did not report specific data on the type of specimens analyzed. The reported procedures used for manual counting and the specific DIA technologies adopted in the assessment of Ki-67 in PanNENs are summarized in Table 1.
Table 1.
Summary of studies about AI-based systems used for Ki-67 assessment in PanNENs.
| AUTHOR, YEAR | COUNTRY | N° OF CASES | GENDER | MATERIAL | GRADING | MANUAL COUNT | DIA |
|---|---|---|---|---|---|---|---|
| Bagci, 2012 | USA-Japan | 21 | N/A | SRS | WD | EE, ECM, CC/PI | N/S |
| Remes, 2012 | Finland | 31a | N/A | N/S | WD | ECM of at least 2000 cells (hotspots) | Publicly available ImmunoRatio software, capturing five different image fields (minimum of 400 tumor cells per picture, altogether 2000 cells) |
| Fung, 2012 | USA | 16b | N/A | CB | WD | N/S | Automated Cellular Imaging System III (ACIS, Dako, Carpinteria, CA, USA) at 20x objective in 3 tumor “hotspots” |
| Goodell, 2012 | USA | 45 | 22 M, 22FΩ | SRS | WD | ECM | VIAS (Ventana): count in 1 hotspot; count in 10 consecutive random fields |
| Tang, 2012 | USA | 12c | N/A | N/S | WD |
1. ECM of >2000 cells 2. EE |
Aperio immunohistochemistry nuclear quantitative image analysis (QIA) algorithm analyzing representative images scanned at 20x magnification |
| Cimic, 2014 | USA | 28 | 10 M, 18 F | SRS | WD | EE | Free software available online (Immunoratio.com) |
| van Velthuysen, 2014 | The Netherlands | 6d | N/A | N/S | N/A | EE at x20 | ImageJ freeware at different magnifications (20x and 40x). |
| Reid, 2015 | USA, Turkey, Japan, Korea | 68 | 33 M, 35 F | N/S | 26 G1, 39 G2, 3 G3 |
1. EE at intermediate power (x10 objective) 2. ECM on the x20 objective 3. CC/PI 4. Careful, extensive, and exhaustive analysis by an expert. |
Automated cellular image cytometer (ACISs III, Dako) scanned the entire slide at x4 and 3 hotspots were selected |
| Kroneman, 2015 | USA | 97 | 51 M, 46 F | N/S | N/A |
1. EE 2. ECM of at least 500 tumor cells |
Automated Cellular Imaging System (ACIS) (Dako) to select 8 to 10 hotspots within the hottest staining region(s) of the tumor present on the slide |
| Mejias, 2015 | USA | 21 | N/A | N/S | 7 G1, 14 G2 | N/S | Ventana Image-VIAS |
| Neely, 2016 | USA | 24 | N/A | CB | N/A | CC/PI, selection of 3 hotspots | Calculation of PI on 3 hotspots with a DIA software algorithm |
| Burdette, 2016 | USA | 57 | N/A | N/S | WD | CC/PI, selection of 6 hotspots | Whole slide scanning with Aperio ImageScope, manual revision and selection of 6 hotspots, Aperio immunohistochemistry nuclear quantitative analysis algorithm |
| Jin, 2016 | USA | 58 | 33 M, 25 F | CB and SRS | 31 G1, 23 G2, 4 G3 | CC/PI of at least 500 tumor cells. For cases where TTCN was less than 500 on the entire slide, all tumor cells were counted. | Publicly available ImmunoRatio software. Basic mode was used for analysis |
| Conemans, 2017 | The Netherlands | 69 | N/A | SRS | 57 G1, 11 G2, 1 G3 | ECM 2000 cells (hotspot) | Digital quantification of Ki67 LI (PACS, Sectra AB, Linköping, Sweden) on manually selected hotspots |
| Niazi, 2018 | USA | 33 | N/A | Biopsy | WD | N/S | Deep learning method to automatically differentiate between NET and non-tumor regions based on images of Ki67 stained biopsies |
| Dere, 2019 | Turkey | 8e | N/S | N/S | N/A | ECM of 500 to 2000 tumor cells | Software designed by Technology Faculty of the institution |
| Sajjan, 2019 | USA | 50f | N/S | N/S | N/A | N/S | Ki67-stained whole slide images were captured and the tumor area with the greatest mitotic activity was manually identified. The Ki67-positive cells were counted in 0.5 mm2 using Ventana Virtuoso software |
| Owens, 2020 | UK | 42 | N/A | N/S | G1 and G2, NOS | CC/PI, 1 hotspot | Open-source image analysis program QuPath version 0.1.34 analyzing the same hotspot regions used for the manual Ki67 assessments. Each hotspot was classified into tumor and stromal compartments using a detection classifier based on training regions |
| Saadeh, 2020 | Jordan | 3g | N/S | N/S | WD | CC/PI of at least 1000 tumor cells | ImageJ |
| Satturwar, 2020 | USA | 39h | N/S | CB | N/A |
1. EE 2. CC/PI of up to 3 hotspot at ×20 magnification |
Aperio immunohistochemistry color convo-luted, nuclearV9 quantitative image analysis algorithm (Leica Biosystems) |
| Lea, 2021 | Norway | 21i | N/S | SRS and biopsy | N/A | ECM of 500 to 2000 tumor cells |
Visiopharm image analysis software (Hoersholm, Denmark) measured Ki67and PHH3 on IHC slides including 500 to 2000 tumor cells |
| Boukhar, 2021 | USA | 3j | 1 M, 2 F | N/S | 2 G2, 1 G3 | CC/PI of hotspot images | Two DIA platforms: QuantCenter and HALO |
| TOTAL | 13/22 USA, 6/22 Europe, 3/22 Asia and mixed | 752 | 50.5% M, 49.5% F | 12 N/S; 4 SRS, 3 CB, 1 biopsy, 2 other | 55.3% G1, 40.6% G2, 4.1% G3, NOS | – | – |
Abbreviations: AI Artificial intelligence; MC Manual count; DIA Digital image analysis; PanNENs Pancreatic neuroendocrine neoplasms; CB Cell blocks; SRS Surgical resection specimens; NET Neuroendocrine tumor; N/A Not available; EE Eyeball estimation; ECM Eye-counting with microscope; CC/PI Camera captured/printed image; N/S Not specified; WD Well-differentiated; M Male; F Female; PI Proliferation index; PHH3 Phosphohistone H3; IHC Immunohistochemistry, NOS Not otherwise specified.
Notes: aThis study investigated a total of 51 cases, 31 with pancreatic origin and 20 with ileal origin; bThis study investigated a total of 22 cases, 16 with pancreatic origin (including 3 liver metastases) and 6 with gastro-intestinal origin (including 4 liver metastases); cThis study investigated a total of 27 cases, 12 with pancreatic origin, 12 originated from small bowel and 3 with rectal origin; dThis study investigated a total of 73 cases, 2 with gastric origin, 18 originated from small bowel, 8 with colonic origin, 18 with pulmonary origin, and 6 with pancreatic origin and 21 liver metastases; eThis study investigated a total of 50 cases, 26 with gastric origin, 10 with appendiceal origin, 3 with colorectal origin, 3 with ileal origin and 8 with pancreatic origin; fThis study investigated a total of 134 cases, 6 with gastric origin, 64 originated from small bowel, 6 originated from large bowl, 7 with appendiceal origin, 31 with mesenterial origin and 50 with pancreatic origin; gThis study investigated a total of 20 cases, 3 with pancreatic origin, 2 with gastric origin, 2 with duodenal and ampullary origin, 7 with jejunal and ileal origin, 2 with appendiceal origin and 2 with colonic origin; hThis study investigated 50 cases, 39 with pancreatic origin and 11 liver metastases; iThis study investigated a total of 159 cases, 2 with esophageal origin, 9 with gastric origin, 54 originated from small bowel, 1 originated form Meckel’s diverticulum, 31 with appendiceal origin, 21 with pancreatic origin, 15 with colonic origin, 14 with rectal origin and 7 liver metastases and metastases with unknown primary tumor; jThis study investigated a total of 25 cases, 3 with pancreatic origin, 5 with ileal origin, 5 with duodenal origin, 2 with gastric origin, 3 nodal metastases, 1 ileal metastasis, 5 liver metastases and 1 diaphragmatic metastasis; Ωthis study reported data on a total of 45 cases but the total number of patients was 44: there were 22 females (one had two tumors, for a total of 23 tumors) and 22 males.
Advantages and limitations of DIA-based systems in the assessment of Ki-67
The key advantages and limitations of DIA-based systems in the assessment of Ki-67 in PanNENs are summarized in Table 2. The most commonly described advantages of DIA were improved reproducibility and reliability, as well as reduced time required for Ki-67 assessment. The most common limitations of DIA were counting non-neoplastic (“contaminants”) cells (e.g., lymphocytes), the higher cost compared with manual counting, and the potential delay in diagnosis, which was dependent on some procedures or technician availability.
Table 2.
Summary of reported advantages and limitations when utilizing DIA systems to assess Ki-67 for PanNENs.
| AUTHOR, YEAR | ADVANTAGES | LIMITATIONS |
|---|---|---|
| Bagci, 2012 | NR | Highest impact on turnaround time, depending on technician availability; low practicality and moderate accuracy |
| Remes, 2012 | Quick, precise and reliable; not influenced by changes in cell size or growth patterns | NR |
| Goodell, 2012 | Efficient method | Can be influenced by counting hotspot vs. randomly selected fields; low reproducibility if standardized thresholds are lacking |
| Tang, 2012 | Ki67 quantification by MC and DIA demonstrate comparable accuracy | Inability to evaluate each tumor cell |
| Cimic, 2014 | Reproducible | NR |
| van Velthuysen, 2014 | Reproducible | NR |
| Reid, 2015 | Pathologist independent | Dependent upon laboratory technician availability and instrument accessibility; high cost |
| Kroneman, 2015 | Almost perfect correlation between MC and DIA | Difficulty with cell counting due to inability to separate individual cells because of indistinct cell borders |
| Mejias, 2015 | NR | Inability to distinguish infiltrating lymphocytes and other non-neoplastic cells |
| Neely, 2016 | Accurate for cytology | Risk of counting non-tumor contaminants (lymphocytes, pigmented macrophages) |
| Burdette, 2016 | Accuracy | NR |
| Jin, 2016 | NR | Non-tumor cell contamination and insufficient sampling |
| Dere, 2019 | Reduction of time for Ki67 evaluation | Expensive |
| Saadeh, 2020 | Accurate, efficient, reliable and reproducible | Inability to evaluate each tumor cell |
| Satturwar, 2020 | Excellent reliability | NR |
| Lea, 2021 | Improved reliability and reproducibility of grading | NR |
| Boukhar, 2021 | Non-inferiority and substantial time savings | Expert morphologic assessment required for quantitative evaluation |
Abbreviations: PanNENs Pancreatic neuroendocrine neoplasms; NR Not reported; MC Manual count; DIA Digital image analysis.
Comparative meta-analysis, quality and publication bias assessment
Overall, for the comparative meta-analysis of 4 studies including 238 patients with PanNEN were selected25,27,28,34. The pooled correlation estimate was 0.94 (95%CI: 0.83–0.98; I2 = 24.15%), indicating a high correlation between manual (reference value) and digital count. The heterogeneity was low (i.e., I2 < 50%), reinforcing the reliability of these results. The quality of the studies did not represent risk of bias (mean score of the Newcastle–Ottawa Scale: 8). Furthermore, no publication bias emerged (Egger’s test = 1.42; p = 0.90). The fail-safe number was 660, a value that indicates strong statistical significance of our results based on existing guidelines15,16.
Discussion
The Ki-67 proliferative index is critical in the pathologic assessment of PanNEN, and has important clinical implications for patient management. The adoption of international recommendations released by the WHO classification of tumors and the European neuroendocrine tumor society (ENETS) for assessing Ki-67 has improved the standardization of methodologies for this task9,39. However, given the persistence of interlaboratory and interobserver discrepancies, as well as potential inconsistencies with different scoring systems, accurately grading PanNENs remains a challenge for pathologists, especially in the G1-G2 and G2-G3 transition areas for PanNETs. Multiple factors affecting the interpretation of the Ki-67 index include the use of different antibody clones and staining protocols, tissue section thickness, tumor cell density, and difficulty distinguishing tumor from non-tumor cells. According to Adsay, “to count or not to count is not the question, but rather how to count”40. Manually counting camera captured or printed images is generally favored over eyeballing. Further, more recently DIA has proven to be an acceptable method for Ki-67 assessment. In this study, we reviewed all published investigations that employed DIA for Ki-67 determination in PanNENs, highlighting some of the advantages and limitations of utilizing this technology. Furthermore, by comparing the coefficient of correlation between manual counting and DIA by means of a comparative meta-analysis, we demonstrated a high value of consistency (0.94, 95%CI: 0.83–0.98) between these two approaches.
The advantages derived from utilizing DIA include more reproducible results, higher accuracy, and reduced time to evaluate Ki-67 in PanNENs1,6–8. Current guidelines for assessing Ki-67 recommended manual counting from a printed image that includes at least 500 neoplastic cells from tumor hotspots. While still time consuming, this manual method does promote standardization that helps reduce interobserver variability24. However, for grade transitions between G1 and G2 (3% of Ki-67) and between G2 and G3 (20% of Ki-67), there were still discrepancies with manual counting from a printed image. The use of DIA for Ki-67 determination resulted in greater consistency in grading of all PanNEN cases, particularly for those cases belonging to the aforementioned gray transition areas G1-G2 and G2-G3. However, it should be noted that even when using DIA one can obtain different results depending on the selection of hotspots and by altering the number of cells counted. Access to DIA allows rapid counting of more cells, and that alone can push a tumor from G2 to G1 or G3 to G2, whereas counting fewer cells in the same hotspot can achieve the reverse41.
DIA assistance with grading PanNEN is of particular benefit in fine needle aspiration (FNA) cytology samples. Guidelines established using histological samples have been extrapolated to grading PanNENs in cytology material (e.g., cell blocks) procured by FNA. Several studies have demonstrated that Ki-67 assessment by manual counting of tumor cells in cell blocks can result in under-grading of these neoplasms when compared with matched surgical resection specimens36,42, with discrepancies more often observed in G2 cases20,29. Intriguingly, Abi-Raad and colleagues demonstrated that counting hotspots instead of the complete cell block can provide a higher concordance with surgical specimens, especially for FNA samples that contain ≥ 1000 cells43. A different perspective was provided by Satturwar and colleagues who investigated the potential role of augmented reality microscopy (ARM) for Ki-67 assessment in cytology specimens36. ARM, which is basically a modified microscope associated with an attached computer unit, enables real-time image analysis using a traditional light microscope and glass slides, without the need to first photograph or digitize slides36,44,45. If coupled with image analysis software, ARM allows quantifying immunohistochemical stains including Ki-67, and can also be combined with elaborate AI-based algorithms to perform more complex tasks44–46. Like other DIA methods, ARM has significant potential for improving PanNEN grading on cell block material36.
Currently, DIA for Ki-67 assessment has some limitations that may need to be addressed if counting in practice is to be performed with this approach. The most commonly reported drawback is the risk of counting dividing non-neoplastic “contaminating” cells (e.g., endothelial cells, lymphocytes), thereby erroneously increasing the overall tumor grade. Other brown-pigmented signals (hemosiderin and hematoidin) also cause this over-counting phenomenon. Such issues are enhanced in NEC, especially due to the effect of artefacts (e.g., smeared chromatin material, nuclear molding in small cell NEC) on DIA. However, these problems can be overcome by having pathologists directly annotate regions of interest to be scored, with the intent of excluding contaminating cells from being counted. Further studies that specifically address these challenges in PanNEC are needed. This issue becomes particularly important if non-pathologist personnel such as trainees and technicians are used as key operators. Of note, more sophisticated AI-algorithms are being developed that only count neoplastic cells47–49 and become more operator-independent. One potential solution that also has been employed is to utilize double-stained slides (e.g., Ki-67 and synaptophysin) with deep learning algorithms to improve the accuracy of Ki-67 index quantification50–53. More recently, some investigators have shown that they are able to predict Ki-67 positive cells directly from H&E images using AI-based methods51. Another important pending issue that needs to be addressed for improving Ki-67 assessment in PanNENs is related to standardizing hotspot size and number to be evaluated41,54. Hotspots are defined as tumor areas with higher Ki-67 nuclear staining. It has been shown that the greater the hotspot size, the lower the Ki-67 count, highlighting the importance of standardizing this parameter for reliable evaluation34,41. Furthermore, not only is the size of the hotspot difficult to define, but so is the shape55. Most pathologists and algorithms define a hotspot as a circular shape; however, there is no biological evidence to support this notion. Another important factor to consider is the number of hotspots when determining the Ki-67 index. Training operators not to select a geographic region that may lead to hyper-selection of positive cells in a given hotspot region is also important, which erroneously creates higher “percentage” positivity. However, all of the above shortcomings are relatively easy to address with proper training and application of improved AI software.
Despite the clear advantages of DIA for determination of the Ki-67 labeling index, scoring with this digital modality is still subject to the fundamental limitation that applies to any cut-off in a continuous variable: it can be changed randomly, as it was for PanNETs in 2017 when it was moved from 2 to 3 for Grade 19. Moreover, any cut-off of a continuous variable can be shown to have value, but the actual grading is inherently arbitrary41. Indeed, how best to employ K-i67 as a reliable prognosticator of PanNETs has been a study in progress. For example, in 2017 the cut-off was clarified such that cases with an index less than 3.0 (including 2.99), which were previously unclear as to which grade this belonged, now clearly included Grade 19. Naturally, as in any grading and staging system that assesses a continuous variable, the Ki-67 index-based system is imperfect54. For example, it can be expected that cases with 2.99 (now in G1) and 3.0 (now in G2) will be similar in biological behavior. Nevertheless, DIA will help standardize the process, not only offering more reproducible results in daily clinical practice, but also allowing for better comparison between studies that aim to fine-tune this grading system. For example, there have been proposals to move the G1/G2 cut-off to 5%; but it is difficult to verify the results of these proposals due to variation in counting methods. Fundamentally, the reality is that even with more accurate analysis provided by DIA, a G2 tumor with a Ki67 of 4% will still be more likely to behave in an indolent fashion than a G2 tumor with a Ki67 of 19%. Thus, the issue of a continuous variable, which is a complex concept involving statistical and biological sciences56, enhances the need for accurate Ki-67 quantification and may ultimately be more important than the actual grade score. Finally, a significant limitation of DIA for widespread adoption has been the accessibility of this technology due to cost and maintenance. However, as whole slide scanners and digital cameras (and related software) become more widely available, the adaptation of facilities to perform DIA for Ki-67 counting is becoming increasingly feasible and amenable to employ57. Another issue to be considered is the need to better integrate Ki-67 counting by DIA into routine workflow24,58.
In this review, we chose to focus on PanNENs. However, the topic of manual vs. digital pathology scoring of Ki-67 is also certainly of importance for NENs at many other anatomic sites54, as well as for other neoplasms in which DIA-based systems are being leveraged to assess biomarkers. In 2015, Joseph et al. studying a cohort of 48 lung carcinoids, demonstrated an overall similarity of manual counting vs. DIA; although Ki-67 estimation resulted in slightly higher results than manual counting59. Of note, a more recent analysis by Swarts et al. comparing the use of manual analysis vs. DIA (in-house Leica Qwin program) in a cohort of 201 lung tumors, described a substantial equivalence of both methodologies60. It is also worth noting that Ki-67 assessment may be of importance in other tumor types. For example, in 2020 Hida et al. compared the use of manual analysis vs. DIA (Visiopharm software) for proliferative index evaluation in a total of 413 cases of breast cancer, showing a high value of correlation (coefficient of correlation = 0.82, p < 0.0001) between both methods61. Alataki et al. corroborated these findings, demonstrating a high correlation in Ki-67 assessment between manual and DIA in both surgical breast resections and biopsies62.
An important question is whether the comparison of Ki-67 assessment between manual vs. DIA-based systems influences clinical management and prognostication. Among all selected manuscripts, only four provided data on this specific topic20,23,25,30. Goodell et al. demonstrated significant reliability in predicting nodal and distant metastasis of PanNETs with the ventana image analysis system (VIAS), with the highest specificity (94% in their cohort) demonstrated when analyzing 10 consecutive and randomly selected fields20. Similarly, van Velthuysen et al. investigating the performance of manual vs. digital (ImageJ) Ki-67 scoring in a cohort of 73 PanNENs, showed that tumor grading correlated with survival irrespective of the way Ki-67 was assessed23. Similar results were replicated by Kroneman et al.25. and Conemans et al.30, showing substantial similarities in terms of prognostication between manual vs. DIA scoring of Ki-67. It is important to note that only four studies in the literature provided data on this fundamental topic. Moreover, all of these studies were conducted prior to the introduction of the 2017 grading system. Thus, further studies on larger cohorts and based on current grading methods are needed. We advocate that DIA-based systems could provide a more standardized method, guaranteeing a more reliable basis for prognostic stratification.
In summary, this systematic review and comparative meta-analysis demonstrates that the advantages outweigh the limitations of using DIA to assess Ki-67 in PanNENs. We advocate that the next logical step for more broadly adopting DIA in pathology practice would be to further explore the relationship between hotspot parameters (number, size, and shape) and the Ki-67 index with patient outcome. Currently, most studies use manual counting from captured images as the gold standard; however, the ultimate validation will naturally come from prognostic correlation. Based upon current evidence provided by peer-reviewed literature, DIA appears to offer pathologists higher reliability and reproducibility than manual counting for grading PanNENs. The overall findings of this review, therefore, support widespread adoption of carefully optimized and validated DIA-based methods for this important diagnostic task in clinical practice. Lessons learned from the application of DIA to the PanNEN model can also be extrapolated to different tumors in other organ systems, such as breast carcinoma in which Ki-67 quantification is increasingly becoming a key driver for patient management.
Supplementary information
Acknowledgements
The authors thank Dr. Enrico Cavallo for his support.
Author contributions
C.L., L.P., V.A., S.L.A., P.A., I.G., A.N., N.V., M.K.N., M.N.G., A.E., I.A.C., A.S.: study conception and design; C.L., L.P., P.A., I.G., A.N., N.V., A.E., I.A.C., A.S.: systematic review and meta-analysis; all authors: data interpretation and discussion; C.L., L.P., V.A., S.L.A., P.A., M.K.N., M.N.G., A.E., I.A.C., A.S.: paper writing; all authors: final editing and approval of the present version.
Funding
This study is supported by Associazione Italiana Ricerca sul Cancro (AIRC IG n. 26343); Fondazione Cariverona: Oncology Biobank Project “Antonio Schiavi” (prot. 203885/2017); Fondazione Italiana Malattie Pancreas (FIMP-Ministero Salute J38D19000690001)
Data availability
All data/information are available in the manuscript and in the supplementary material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Claudio Luchini, Email: claudio.luchini@univr.it.
Aldo Scarpa, Email: aldo.scarpa@univr.it.
Supplementary information
The online version contains supplementary material available at 10.1038/s41379-022-01055-1.
References
- 1.Luchini C., Pea A., Scarpa A. Artificial intelligence in oncology: current applications and future perspectives. Br. J. Cancer10.1038/s41416-021-01633-1 (2021). [DOI] [PMC free article] [PubMed]
- 2.Benzekry S. Artificial Intelligence and Mechanistic Modeling for Clinical Decision Making in Oncology. Clin. Pharm. Ther. 2020;108:471–486. doi: 10.1002/cpt.1951. [DOI] [PubMed] [Google Scholar]
- 3.Huynh E, et al. Artificial intelligence in radiation oncology. Nat. Rev. Clin. Oncol. 2020;17:771–781. doi: 10.1038/s41571-020-0417-8. [DOI] [PubMed] [Google Scholar]
- 4.Niazi MKK, Parwani AV, Gurcan MN. Digital pathology and artificial intelligence. Lancet. Oncol. 2019;20:e253–e261. doi: 10.1016/S1470-2045(19)30154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison JH, et al. Introduction to artificial intelligence and machine learning for pathology. Arch. Pathol. Lab. Med. 2021;145:1228–1254. doi: 10.5858/arpa.2020-0541-CP. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Levenson R, Pantanowitz L. Artificial intelligence in pathology. Am. J. Pathol. 2021;191:1670–1672. doi: 10.1016/j.ajpath.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JY, Abel JT, Ugj B, McClintock DS, Pantanowitz L. Challenges in the development, deployment, and regulation of artificial intelligence in anatomic pathology. Am. J. Pathol. 2021;191:1684–1692. doi: 10.1016/j.ajpath.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Tizhoosh HR, I Pantanowitz L. Artificial intelligence and digital pathology: challenges and opportunities. J. Pathol. Inf. 2018;14:9. doi: 10.4103/jpi.jpi_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd R. V., Osamura R. Y., Kloppel G., Rosai J. WHO Classification of Tumours of Endocrine Organs 4th edn. Lyon, IARC Press, 2017.
- 10.WHO Classification of Tumours Editorial Board. Digestive System Tumours 5th edn. IARC Press, Lyon, 2019.
- 11.van Velthuysen ML, et al. Grading of neuroendocrine neoplasms: mitoses and ki-67 are both essentials. Neuroendocrinology. 2014;100:221–227. doi: 10.1159/000369275. [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa scale. World J. Meta-Anal. 2017;5:80–84. doi: 10.13105/wjma.v5.i4.80. [DOI] [Google Scholar]
- 15.Luchini C, et al. Assessing the quality of studies in meta-research: Review/guidelines on the most important quality assessment tools. Pharm. Stat. 2021;20:185–195. doi: 10.1002/pst.2068. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagci P, et al. Comparative analysis of different counting methodologies for Ki-67 in pancreatic neuroendocrine tumors. Lab. Invest. 2012;92:441A. [Google Scholar]
- 18.Remes SM, Tuominen VJ, Helin H, Isola J, Arola J. Grading of neuroendocrine tumors with Ki-67 requires high-quality assessment practices. Am. J. Surg. Pathol. 2012;36:1359–1363. doi: 10.1097/PAS.0b013e3182632038. [DOI] [PubMed] [Google Scholar]
- 19.Fung A, Cohen C, Kavuri S, Gao X, Reid M. Measurement of interobserver variability in calculating MIB1 labeling index by counting tumor cells in well differentiated neuroendocrine tumors (WDNETs) of the pancreas and gastrointestinal tract: a cytologic analysis of 22 cases. J. Am. Soc. Cytopathol. 2012;1:S95. doi: 10.1016/j.jasc.2012.08.208. [DOI] [Google Scholar]
- 20.Goodell PP, Krasinskas AM, Davison JM, Hartman DJ. Comparison of methods for proliferative index analysis for grading pancreatic well-differentiated neuroendocrine tumors. Am. J. Clin. Pathol. 2012;137:576–582. doi: 10.1309/AJCP92UCXPJMMSDU. [DOI] [PubMed] [Google Scholar]
- 21.Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am. J. Surg. Pathol. 2012;36:1761–1770. doi: 10.1097/PAS.0b013e318263207c. [DOI] [PubMed] [Google Scholar]
- 22.Cimic A, Johnsen AE, Harrison W, Sirintrapun JS, Mott RT. KI67 by image analysis and phosphohistone H3 are objective methods in grading pancreatic neuroendocrine tumors. Lab. Invest. 2013;94:397A. [Google Scholar]
- 23.van Velthuysen M, et al. Reliability of proliferation assessment by Ki-67 expression in neuroendocrine neoplasms: eyeballing or image analysis? Neuroendocrinology. 2014;100:288–292. doi: 10.1159/000367713. [DOI] [PubMed] [Google Scholar]
- 24.Reid MD, et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod. Pathol. 2015;28:686–694. doi: 10.1038/modpathol.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroneman TN, et al. Comparison of three Ki-67 index quantification methods and clinical significance in pancreatic neuroendocrine tumors. Endocr. Pathol. 2015;26:255–262. doi: 10.1007/s12022-015-9379-2. [DOI] [PubMed] [Google Scholar]
- 26.Mejias L, Bhalla A, Salem N, Thomas S, Shidham V. Evaluation of KI-67 (MIB-1) labeling index with dual-color immunocytochemistry (KI-67 with LCA) for grading of pancreatic neuroendocrine tumors. Lab. Invest. 2015;95:520A. [Google Scholar]
- 27.Neely C, et al. A comparison of automated digital image analysis (DIA) and manual count of camera-captured images in calculating Ki67 proliferation index (PI) in cytologic samples from pancreatic neuroendocrine neoplasms (PanNENs) Lab. Invest. 2015;96:111A. [Google Scholar]
- 28.Burdette E, et al. A comparison of manual counting with camera captured images and digital image analysis for KI-67 proliferative index assessment in pancreatic neuroendocrine tumors. Lab. Invest. 2015;96:510A. [Google Scholar]
- 29.Jin M, et al. Grading pancreatic neuroendocrine neoplasms by Ki-67 staining on cytology cell blocks: manual count and digital image analysis of 58 cases. J. Am. Soc. Cytopathol. 2016;5:286–295. doi: 10.1016/j.jasc.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Conemans E, et al. Prognostic value of WHO grade in pancreatic neuro-endocrine tumors in Multiple Endocrine Neoplasia type 1: Results from the DutchMEN1 Study Group. Pancreatology. 2017;17:766–772. doi: 10.1016/j.pan.2017.07.196. [DOI] [PubMed] [Google Scholar]
- 31.Niazi MKK, et al. Identifying tumor in pancreatic neuroendocrine neoplasms from Ki67 images using transfer learning. PLoS One. 2018;13:e0195621. doi: 10.1371/journal.pone.0195621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dere Y, Ozkaraca O, Cetin G, Dere O. Evaluation of an image-based automated detection system in detecting Ki67 proliferation index and correlation with the traditional eye-ball method in gastroenteropancreatic neuroendocrine tumors. J. Coll. Physicians. Surg. Pak. 2019;29:137–140. doi: 10.29271/jcpsp.2019.02.137. [DOI] [PubMed] [Google Scholar]
- 33.Sajjan S, Yang Y, Mansoor N, Lee L. Low incidence mitotic activity best detected by manual count as compared to whole slide imaging digital computer assessed counting: Lessons learned. Mod. Pathol. 2019;32:3. [Google Scholar]
- 34.Owens R, et al. Comparison of different anti-Ki67 antibody clones and hot-spot sizes for assessing proliferative index and grading in pancreatic neuroendocrine tumours using manual and image analysis. Histopathology. 2020;77:646–658. doi: 10.1111/his.14200. [DOI] [PubMed] [Google Scholar]
- 35.Saadeh H, Abdullah N, Erashdi M, Sughayer M, Al-Kadi O. Histopathologist-level quantification of Ki-67 immunoexpression in gastroenteropancreatic neuroendocrine tumors using semiautomated method. J. Med. Imag. 2020;7:012704. doi: 10.1117/1.JMI.7.1.012704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satturwar SP, et al. Ki-67 proliferation index in neuroendocrine tumors: Can augmented reality microscopy with image analysis improve scoring? Cancer Cytopathol. 2020;128:535–544. doi: 10.1002/cncy.22272. [DOI] [PubMed] [Google Scholar]
- 37.Lea D, et al. Digital image analysis of the proliferation markers Ki67 and phosphohistone H3 in gastroenteropancreatic neuroendocrine neoplasms: accuracy of grading compared with routine manual hot spot evaluation of the Ki67 index. Appl. Immunohistochem. Mol. Morphol. 2021;29:499–505. doi: 10.1097/PAI.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boukhar S, Gosse M, Bellizzi A, Rajan KDA. Ki-67 proliferation index assessment in gastroenteropancreatic neuroendocrine tumors by digital image analysis with stringent case and hotspot level concordance requirements. Am. J. Clin. Pathol. 2021;156:607–619. doi: 10.1093/ajcp/aqaa275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Toole D, Kianmanesh R, Enets CM. Consensus guidelines for the management of patients with digestive neuroendocrine tumors: an update. Neuroendocrinology. 2016;103:117–118. doi: 10.1159/000443169. [DOI] [PubMed] [Google Scholar]
- 40.Adsay V. Ki67 labeling index in neuroendocrine tumors of the gastrointestinal and pancreatobiliary tract: to count or not to count is not the question, but rather how to count. Am. J. Surg. Pathol. 2012;36:1743–1746. doi: 10.1097/PAS.0b013e318272ff77. [DOI] [PubMed] [Google Scholar]
- 41.Volynskaya Z, Mete O, Pakbaz S, Al-Ghamdi D, Asa SL. Ki67 quantitative interpretation: insights using image analysis. J. Pathol. Inf. 2019;10:8. doi: 10.4103/jpi.jpi_76_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrell JM, Pang JC, Kim GE, Tabatabai ZL. Pancreatic neuroendocrine tumors: accurate grading with Ki-67 index on fine-needle aspiration specimens using the WHO 2010/ENETS criteria. Cancer Cytopathol. 2014;122:770–778. doi: 10.1002/cncy.21457. [DOI] [PubMed] [Google Scholar]
- 43.Abi-Raad R, et al. Grading pancreatic neuroendocrine tumors by Ki-67 index evaluated on fine-needle aspiration cell block material. Am. J. Clin. Pathol. 2020;153:74–81. doi: 10.1093/ajcp/aqz110. [DOI] [PubMed] [Google Scholar]
- 44.Chen PC, Gadepalli K, MacDonald R. An augmented reality microscope with real-time artificial intelligence integration for cancer diagnosis. Nat. Med. 2019;25:1453–1457. doi: 10.1038/s41591-019-0539-7. [DOI] [PubMed] [Google Scholar]
- 45.Razavian N. Augmented reality microscopes for cancer histopathology. Nat. Med. 2019;25:1334–1336. doi: 10.1038/s41591-019-0574-4. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh A, et al. The potential of artificial intelligence to detect lymphovascular invasion in testicular cancer. Cancers. 2021;16:13. doi: 10.3390/cancers13061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dov D., et al. Hybrid human-machine learning approach for screening prostate biopsies can improve clinical efficiency without compromising diagnostic accuracy. Arch. Pathol. Lab. Med.10.5858/arpa.2020-0850-OA (2021). [DOI] [PubMed]
- 48.D’Alfonso TM, et al. Multi-magnification-based machine learning as an ancillary tool for the pathologic assessment of shaved margins for breast carcinoma lumpectomy specimens. Mod. Pathol. 2021;34:1487–1494. doi: 10.1038/s41379-021-00807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen S, et al. Clinical use of a machine learning histopathological image signature in diagnosis and survival prediction of clear cell renal cell carcinoma. Int. J. Cancer. 2021;148:780–790. doi: 10.1002/ijc.33288. [DOI] [PubMed] [Google Scholar]
- 50.Govind D, et al. Improving the accuracy of gastrointestinal neuroendocrine tumor grading with deep learning. Sci. Rep. 2020;10:11064. doi: 10.1038/s41598-020-67880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, et al. Predict Ki-67 positive cells in H&E-stained images using deep learning independently from IHC-stained images. Front. Mol. Biosci. 2020;7:183. doi: 10.3389/fmolb.2020.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsukuma K, et al. Synaptophysin-Ki67 double stain: a novel technique that improves interobserver agreement in the grading of well-differentiated gastrointestinal neuroendocrine tumors. Mod. Pathol. 2017;30:620–629. doi: 10.1038/modpathol.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hacking SM, et al. Potential pitfalls in diagnostic digital image analysis: experience with Ki-67 and PHH3 in gastrointestinal neuroendocrine tumors. Pathol. Res. Pr. 2020;216:152753. doi: 10.1016/j.prp.2019.152753. [DOI] [PubMed] [Google Scholar]
- 54.Cree IA, et al. Counting mitoses: SI(ze) matters! Mod. Pathol. 2021;34:1651–1657. doi: 10.1038/s41379-021-00825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan Niazi MK, Yearsley MM, Zhou X, Frankel WL, Gurcan MN. Perceptual clustering for automatic hotspot detection from Ki-67-stained neuroendocrine tumour images. J. Microsc. 2014;256:213–225. doi: 10.1111/jmi.12176. [DOI] [PubMed] [Google Scholar]
- 56.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snead DR, et al. Validation of digital pathology imaging for primary histopathological diagnosis. Histopathology. 2016;68:1063–1072. doi: 10.1111/his.12879. [DOI] [PubMed] [Google Scholar]
- 58.Volynskaya Z, et al. Integrated pathology informatics enables high-quality personalized and precision medicine: digital pathology and beyond. Arch. Pathol. Lab. Med. 2018;142:369–382. doi: 10.5858/arpa.2017-0139-OA. [DOI] [PubMed] [Google Scholar]
- 59.Joseph MG, et al. Usefulness of Ki-67, mitoses, and tumor size for predicting metastasis in carcinoid tumors of the lung: a study of 48 cases at a tertiary care centre in Canada. Lung. Cancer Int. 2015;2015:545601. doi: 10.1155/2015/545601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swarts DR, et al. Limited additive value of the Ki-67 proliferative index on patient survival in World Health Organization-classified pulmonary carcinoids. Histopathology. 2017;70:412–422. doi: 10.1111/his.13096. [DOI] [PubMed] [Google Scholar]
- 61.Hida AI, et al. Automated assessment of Ki-67 in breast cancer: the utility of digital image analysis using virtual triple staining and whole slide imaging. Histopathology. 2020;77:471–480. doi: 10.1111/his.14140. [DOI] [PubMed] [Google Scholar]
- 62.Alataki A, Zabaglo L, Tovey H, Dodson A, Dowsett M. A simple digital image analysis system for automated Ki67 assessment in primary breast cancer. Histopathology. 2021;79:200–209. doi: 10.1111/his.14355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data/information are available in the manuscript and in the supplementary material.