Abstract
Introduction: Primary dyslipidemias are inherited disorders in plasma lipoprotein metabolism that lead to serious cardiovascular and other complications. The Japanese Ministry of Health, Labor and Welfare (MHLW) covers medical expenses, under the Research Program on Rare and Intractable Diseases, for homozygous familial hypercholesterolemia (FH), familial chylomicronemia, sitosterolemia, cerebrotendinous xanthomatosis, lecithin:cholesterol acyltransferase deficiency, Tangier disease, and abetalipoproteinemia. Apolipoprotein A1 deficiency, heterozygous FH, and type III hyperlipoproteinemia are covered by the MHLW Pediatric Chronic Disease Program. Heterozygous FH and type III hyperlipoproteinemia are also important for their relatively common prevalence and, accordingly, high impact on Japanese public health by significant contribution to the overall prevalence of cardiovascular diseases. Therefore, a systemic survey of these diseases is mandatory to estimate their actual situation, such as prevalence, clinical manifestations, and prognoses among the Japanese population. The impact of these rare and intractable diseases on cardiovascular and other complications will likely be higher among Japanese people than other ethnicities because the general Japanese population has many cardioprotective aspects. The current study intends to conduct a multicenter registry of these diseases to assess their demographics and clinical features comprehensively.
Methods and Analysis: The Prospective Registry Study of Primary Dyslipidemia is a registry-based prospective, observational, multicenter cohort study in Japan, enrolling patients who fulfill the Japanese clinical criteria of the primary dyslipidemias listed above, from 26 participating institutes from August 2015 to March 2023. A total of 1,000 patients will be enrolled in the study and followed for 10 years. Clinical parameters are collected, including physical and laboratory findings, genetic analysis, drugs, lifestyle management, and clinical events, especially cardiovascular events. The primary endpoint of this study is the new onset of cardiovascular disease and acute pancreatitis, and the secondary endpoint is death from any causes.
Ethics and Dissemination: This study complies with the Declaration of Helsinki, the Ethical Guidelines for Medical and Health Research Involving Human Subjects, and all other applicable laws and guidelines in Japan. The institutional review boards have approved this study protocol at all participating institutes. The final results are to be published at appropriate international conferences and in peer-reviewed journals.
Keywords: Intractable disease, Dyslipidemia, Genetics, Registry
Trial registration: This study was registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN ID: UMIN000042782).
Introduction
Molecular and genetic bases have been clarified for many primary dyslipidemias and monogenic inherited disorders of lipid and lipoprotein metabolism during the past decades. There have also been extensive efforts to improve their morbidity and mortality, including attempted gene therapies. However, many of them remain intractable, leaving patients to suffer from poor prognoses by their complications. Under these circumstances, the Japanese Ministry of Health, Labor and Welfare (MHLW) specified seven primary dyslipidemias were “rare and intractable diseases” to cover the cost of their medical care and alleviate patients’ economic burden, including homozygous familial hypercholesterolemia (HoFH), sitosterolemia, cerebrotendinous xanthomatosis (CTX), lecithin cholesterol acyltransferase (LCAT) deficiency, Tangier disease, primary chylomicronemia, and abetalipoproteinemia. In addition, APOA1 deficiency, heterozygous FH (HeFH), and type III hyperlipoproteinemia are covered under the MHLW program for Pediatric Chronic Diseases. Heterozygous FH and type III hyperlipoproteinemia are also important because of their relatively high prevalence among the general population and their substantial impact on public health in Japan due to their risk for cardiovascular complications. Both of the disorders are treatable to reduce cardiovascular risk and therefore should be paid more attention to from the public health viewpoint.
Since saving patients with rare and intractable diseases by clarifying the disease causes will surely lead to saving the general dyslipidemias caused by common genetic variations, it is important to investigate the real situation of these diseases across general populations. Some cross-sectional studies estimated the prevalence, clinical manifestations, and complications in Japanese patients with FH or CTX 1 , 2) . However, these studies were underpowered because of their relatively small sample size, and cross-sectional nature of study design limits the power of assessments for their clinical events. In that sense, few longitudinal cohort studies have been performed in a large scale to find the current status, clinical features, and prognosis of the primary dyslipidemias in particular. A Prospective Registry Study of Primary Dyslipidemia (PROLIPID) was launched in 2015 to circumvent these issues, aiming to recruit at least 1,000 patients with the primary dyslipidemias specified above.
Familial hypercholesterolemia (FH) is an autosomal dominant inherited disorder caused by mutations in the genes related to the LDL receptor pathway to clear plasma LDL. The prevalence of heterozygous FH (HeFH) has been estimated as 1 in 300 among general populations worldwide 3 , 4) . Hyper-LDL-cholesterolemia, systemic xanthomas characterize heFH, and premature coronary artery disease, accounting for one of the major causes of premature CVD 5) . HyperLDLemia in HeFH is mostly treatable by lifestyle management and/or drug therapy to reduce the CVD risk to a similar level to the general population if initiated early 6 , 7) . The other common primary dyslipidemia is type III hyperlipoproteinemia. This disease is mainly caused by E2/E2 genotype in apolipoprotein E (APOE) as 1 for 1,600 in the general Japanese population under certain nutritional conditions 8) . It is known to be associated with elevated CVD risk mainly due to the elevation in remnant lipoprotein cholesterol 9) . The risk of this type of dyslipidemia is also manageable by lifestyle control and drug regimens.
Pathogenic mutations in two alleles cause homozygous FH (HoFH), either in LDL receptor (LDLR), apolipoprotein B (APOB), proprotein convertase subtilisin/kexin type 9 (PCSK9), or LDLR adaptor protein 1 (LDLRAP1) or by the mutations combined in those genes and exhibits much severer clinical manifestation than HeFH. The prevalence is estimated as 1 in 300,000 10 , 11) . Timely diagnosis and commencement of LDL-lowering therapies would significantly reduce cardiovascular complication risk in FH patients 12 , 13) . Similar to HoFH, sitosterolemia is caused by double pathogenic mutations in ATP-binding cassette sub-family G member 5 (ABCG5) or ATP-binding cassette sub-family G member 8 (ABCG8) to exhibit systemic xanthomas and premature cardiovascular disease (CVD) associated with elevated LDL cholesterol and sitosterol 14 , 15) . Double pathogenic mutations cause CTX in CYP27A to show very similar clinical manifestations that can be difficult to differentiate from HoFH and sitosterolemia. CTX is characterized by progressive cerebellar ataxia after puberty along with juvenile cataracts, chronic diarrhea of juvenile or infantile-onset, childhood neurological deficit, and tendinous or tuberous xanthomas 16) . In contrast, abetalipoproteinemia is characterized by extremely low LDL cholesterol and triglycerides, caused by double deleterious mutations in microsomal triglyceride transfer protein. Patients with this disorder typically suffer from complications such as spinocerebellar degeneration and pigmentary degeneration of the retina due to fat-soluble vitamin deficiencies 17) .
Pathogenic mutations cause familial chylomicronemia in lipoprotein lipase (LPL), or its regulators, apolipoprotein CII (APOC2), apolipoprotein A5 (APOA5), glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1), and lipase maturation factor 1 (LMF1). The patients have severe hypertriglyceridemia and fasting chylomicronemia, and this phenotype predisposes the affected individuals to acute pancreatitis 18) .
The other group of intractable dyslipidemia is a disorder of HDL metabolism; Tangier disease, lecithin cholesterol acyltransferase (LCAT) deficiency, and apolipoprotein A1 (APOA1) deficiency. Tangier disease is caused by double pathogenic mutations in ATP-binding cassette transporter A1 (ABCA1) and appears with an extremely low level of plasma HDL cholesterol. Patients with this disease develop massive cholesterol ester depositions in various organs, including tonsils, liver, and spleen. In addition, these patients often develop premature atherosclerosis caused by fatty deposits in the arteries 19) . LCAT deficiency is caused by double pathogenic mutations in the LCAT gene and appears in two different forms. Familial LCAT deficiency is caused by complete loss of the LCAT activity, while in fish-eye disease, LCAT activity is partially deficient. LCAT is the enzyme that transfers acyl chain from the 2-position of lecithin to cholesterol on HDL to generate cholesterol acylester and leads HDL to “maturation.” Clinical manifestations of this disease include diffuse corneal opacity, “target cell” hemolytic anemia, renal failure, atherosclerosis, hepatosplenomegaly, and enlarged lymph nodes, due to accumulated unesterified cholesterol 20) . APOA1 deficiency also exhibits extremely low levels of HDL cholesterol, caused by double pathogenic mutations in APOA1 gene. The manifestations of this disease include xanthomas, corneal opacity, and CVD 21 , 22) .
Due to their rarity, the accumulation of the data of these intractable diseases is not adequate for their natural history, current clinical situations, and factors associated with prognosis. However, several large scale registry studies have clarified some useful clinical information and genetic backgrounds 23 - 29) . Therefore, it is essential to understand the unique profiles of these diseases in Japan for frequency, phenotype, and genotype and their clinical prognosis, hopefully, based on the registration of all the patients, to help useful feedback to the current guidelines for their diagnosis and management. In addition, it is also important to investigate the real fact in Japan on heterozygous FH and type III hyperlipoproteinemia from the viewpoint of public health for their phenotype and genotype and clinical prognosis, in order to shape up the current guidelines and criteria to evaluate the socioeconomic value of treatment of these disorders.
Under these conditions, a nationwide, multicenter registry was organized by the Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the Ministry of Health, Labor and Welfare of Japan to comprehensively assess their phenotypes and genotypes. This should lead to a better understanding of these genetic disorders, not only for Japanese patients but also for patients elsewhere in the world.
Methods
Study Design
This nationwide, registry-based prospective, observational, multicenter cohort study starts from August 2015 to March 2023. Enrolled patients have primary dyslipidemias, including FH (homozygous or heterozygous), type III hyperlipoproteinemia, familial chylomicronemia, sitosterolemia, CTX, LCAT deficiency, Tangier disease, APOA1 deficiency, and abetalipoproteinemia who fulfill the clinical criteria of each disorder 9 - 11 , 15 - 21) from 26 participating institutes across Japan (Fig.1) . The patients are to be followed for 10 years. A total of 1,000 patients will be enrolled. This is based on the numbers of registered patients of intractable diseases in 2019 open to the public by MHLW of Japan 30) , and those with other diseases who can register in this study. Fig.1 shows the participating institutes. Participating institutes where the members and supporters of the Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the MHLW of Japan are working have been selected. Table 1 shows a schedule of the assessments and evaluations of this study. The primary outcome is the new onset of CVD events or acute pancreatitis. The secondary outcome is all causes of death.
Fig.1. Participating study centers, including 26 institutes across Japan.
Blue circles indicate the institutes participating in this study.
Table 1. Assessments of this study.
| Units | Baseline | 1 yr | 2-4 yr | 5 yr | 6-9 yr | 10 yr |
|---|---|---|---|---|---|---|
| Entry | X | |||||
| Demographics | X | |||||
| Untreated lipids | X | |||||
| Blood tests | X | |||||
| Physical and imaging | X | |||||
| Family history | X | |||||
| Medical history | X | |||||
| Medicine | X | X | X | |||
| Lifestyle habits | X | |||||
| DNA | X | |||||
| Events (CVD) | X | X | X | X | X | |
| Events (Stroke) | X | X | X | X | X | |
| Events (valvular and aortic disease) | X | X | X | X | X | |
| Events (acute pancreatitis) | X | X | X | X | X | |
| Events (adverse events) | X | X | X | X | X |
The protocol of this study (version 9.0, dated 7 Feb 2020) has been approved by the Institutional Review Board at National Cerebral and Cardiovascular Center and all institutes participating in this study. In addition, this study complies with the Declaration of Helsinki, the Ethical Guidelines for Medical and Health Research Involving Human Subjects, and all other applicable laws and guidelines in Japan. It was registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000042782).
Study Participants
Patients clinically diagnosed with each intractable disease are recruited from August 2015 to March 2023 and followed up over 10 years. The participants fulfilling both inclusion criteria participate in this study ( Table 2 ) . The participants with any of the exclusion criteria are excluded ( Table 3 ) . Written informed consents are obtained from all the participants in a form approved by the IRB.
Table 2. Inclusion criteria.
|
|
Table 3. Exclusion criteria.
|
|
Collection of Data
The doctors in charge of each patient assess clinical data. Each doctor will input data into the Research Electronic Data Capture (REDcap), and then we will refer to those data, which is the browser-based electronic data capture (EDC) system. Table 1 shows the timing of data collection following patient enrollment. We will collect many phenotypes, including clinical, demographic, laboratory data, and events. Details are described in Supplemental Material. Events include new onset of general events, CVD, stroke, valvular/aortic, acute pancreatitis, and adverse events. In addition, we monitor clinical events specific to each intractable disease.
In this study, an independent staff member conducts data monitoring. The trial institution is monitored after patients’ initial enrollment and every 6 months until each participant has a case report on file. The independent staff member monitors and reviews the trial database, and data queries and cleaning will be raised if necessary.
Outcomes
The primary endpoint of this study is new CVD events, including myocardial infarction or angina pectoris, or acute pancreatitis. The secondary endpoint is death from any causes. Details are described in the Supplemental Material. The information of death and its cause are colleced to local authorities.
Schedule of the Study
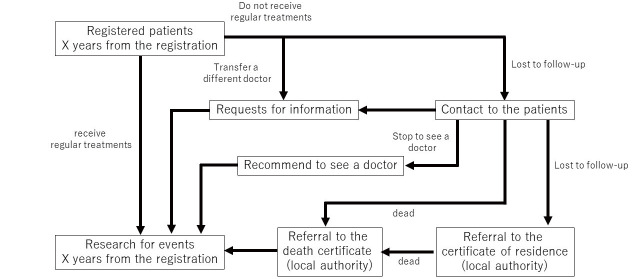
Table 1 shows the schedule of this study. Follow-up visits are anticipated at each participated institute based on the conditions of the patients. Drop-out patients are to be traced by direct approach to the patients first and the maximum effort for further tracking ( Fig.2 ) . Nevertheless, we will collect those clinical variables at least once a year through the EDC system. Independent staff members monitor data at a certain interval (almost once a year). Whenever necessary, data queries will be sent to the investigators to check the data collection status and ensure the accuracy of the dataset.
Fig.2.
Tracking of patients
Statistical Analysis
Mean values of continuous variables will be compared using Student’s t-test for independent data variables, and median values will be compared using nonparametric Wilcoxon Mann–Whitney rank-sum test. Chi-square or Fisher’s post-hoc tests will be used for categorical variables. A multivariable logistic regression model will be used to assess events and variables. Cox proportional hazard model will be used to assess relationships between all variables and events. Cumulative Kaplan–Meier survival curves starting at baseline will be constructed to compare times to the first events to find the meaningful variable. The statistical analysis was conducted using R statistics (https://www.r-project.org). P-values <0.05 were considered statistically significant.
Data Availability Statement
Dataset will not be available to public.
Discussion
The PROLIPID registry, organized by the Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the MHLW of Japan, is a nationwide, registry-based prospective, observational, multicenter cohort intended to understand clinical characteristics, genetic backgrounds, treatments, and prognosis of patients with primary dyslipidemias, including intractable diseases in Japan. This is a unique attempt, especially the registry, which will include rare lipid-related intractable diseases. This registry accepts all the patients with these intractable diseases treated in participating centers, trying to avoid selection bias, leading to illuminate a more detailed information of those disorders. We set the primary endpoint as new CVD events, and the secondary endpoint is all causes of death. Patients with HeFH, HoFH, type III hyperlipoproteinemia, sitosterolemia, LCAT deficiency, Tangier disease, and APOA1 deficiency are at increased risk for CVD because of their lifelong elevation of LDL cholesterol and/or extremely low HDL cholesterol. On the other hand, the patients with abetalipoproteinemia is quite cardioprotective while they suffer from various complications associated with deficiency of soluble vitamin. FH-associated mutation status is associated with an increased risk for CVD 31) . Moreover, classical risk factors for CVD, including hypertension, smoking, or diabetes, have been associated with worsening the phenotype of HeFH although it is known as primary dyslipidemia 32) . Given these facts, we try to assess those factors as well as genetic factors as well in order to understand the factors contributing to affect their phenotype fully. In addition, we set no exclusion criteria to include as many patients as possible, including those at younger ages, enabling us to know the age of onset of the clinical manifestations and complications in each disorder.
We emphasize that the inclusion of genetic information for all patients is one of our major strengths. Genetic testing can provide us an accurate diagnosis of these intractable diseases and risk stratifications for future CVD risk/other complications and help us support decision-making and facilitate cascade screening of those patients. Furthermore, this study provides us a useful insight into improving the current clinical diagnostic criteria of those diseases. Results of the genetic testing will be informed to the patients when necessary.
We hypothesize that patients with pathogenic mutations and/or other classical risk factors for CVD/other complications are associated with an increased risk of CVD/other complications. We also expect that interventions manipulate their increased risk via genetic factors and/or classical risk factors according to treatment initiation and/or the intensiveness of the treatments. We hope to see more and more patients with these diseases are diagnosed accurately through this registry of these lipid-associated intractable diseases.
This study has several limitations. First, the final number of cases registered should vary by each disease, and that it is very difficult to assume the number of patients who will be enrolled. However, we set 1,000 as a target of the total number. Meanwhile, we will try to recruit consecutive patients with these disorders and enroll as many patients as possible. Second, blood testing will not be collected at follow-up, while medications will be followed for 5 years and 10 years. However, analyses will be performed based on baseline characteristics as well as medications as variables. Third, genotyping for all of the rare and intractable diseases included in this study is not covered by the public health insurance of Japan as of September 2021. In that sense, all the genotypes and its assessments will be left to each investigator’s discretion. Fourth, there is no clear rationale for the duration of follow-up in this study because there is no data that we can estimate how many events will be occurred due to the rarity of these diseases. In that sense, we wanted to follow-up as much as possible, while the longest follow-up period the IRB can allow us to set is 10 years. Fifth, we do not use the universal definition of events, like an ICD 10 code. However, we will collect many information, such as date of onset, number of diseased arteries, change of electrocardiogram, elevation of cardiac enzymes, revascularization procedure, and coronary imaging in CVD. We believe that this information will make sure that the assessments of CVD are reliable.
Acknowledgements
We would like to thank the participants in this study.
Competing Interests Statement
Dr. Masatsune Ogura has received lecture fees from Amgen, Astellas Pharma Inc, and Kowa. Dr. Hidenori Arai has received lecture fees from MSD, Kowa, Daiichi Sankyo, Takeda, Pfizer, UCB, Sanofi, Astelas, and Otsuka. Dr. Mariko Harada-Shiba holds shares of Liid Pharma, has received lecture fees from Amgen, Astellas, and Sanofi, has received scholarship grants from Aegerion, Recordati, and Kaneka. Dr. Shun Ishibashi has received lecture fees from Kowa, and has received research grants from Ono Pharmaceutical Co.
Funding Statement
Ministry of Health, Labor and Welfare Research Grant for Research on Rare and Intractable Diseases of Japan supports this study.
Supplemental Material
| Unit | Items | choice/units |
| Entry | Research ID | |
| Date of registration | ||
| Confirmation if the patient is diagnosed as either of target diseases | Yes/No | |
| Homozygous FH | Yes/No | |
| Heterozygous FH | Yes/No | |
| Type III hyperlipoproteinemia | Yes/No | |
| Familial chylomicronemia | Yes/No | |
| Sitosterolemia | Yes/No | |
| Cerebrotendinous xanthomatosis (CTX) | Yes/No | |
| Lecithin cholesterol acyltransferase (LCAT) deficiency | Yes/No | |
| Tangier disease | Yes/No | |
| Apolipoprotein A1 (APOA1) deficiency | Yes/No | |
| Abetalipoproteinemia | Yes/No | |
| Date of diagnosis | ||
| Use the National aid for intractable diseases |
1. Applications are accepted, 2. Applications are denied, 3. Applications are evaluated, 4. not apply |
|
| Registered in FAME study | Yes/No | |
| Informed consent | 1. Reject, 2. written informed consent (baseline analysis and follow-up), 3. written informed consent (only baseline analysis), 4. Provide the chance to opt out | |
| Date of consent | ||
| Gender | ||
| Menstruation | ||
| The age of menopause | ||
| The year of birth | ||
| The month of birth | ||
| Age (at registration) | ||
| Demographics | Date of measurements | |
| Height | cm | |
| Body weight | kg | |
| Waist circumference | cm | |
| Vision test | Yes/No | |
| Vision | ||
| Systolic blood pressure (First time) | mmHg | |
| Diastolic blood pressure (First time) | mmHg | |
| Systolic blood pressure (Second time) | mmHg | |
| Diastolic blood pressure (Second time) | mmHg | |
| Physical findings | 1. Achilles tendon thickness, 2. xanthoma tuberosum, 3. xanthoma planum, 4. xanthoma striata palmaris, 5. eruptive xanthoma, 6. xanthelasma palpebrarum, 7. other tendinous xanthomas, 8. arcus cornea, 9. other | |
| Physical findings (sitosterolemia) | 1. splenomegaly | |
| Specific physical findings and symptoms (CTX) | 1. jaundice, 2. neurologic manifestation | |
| Details of neurologic manifestation | 1. cognitive impairment, 2. psychological symptom, 3. cerebellar symptom, 4. pyramidal sign, 5. extrapyramidal sign, 6. seizures, 7. spinalsensory disturbance, 8. peripheral neuropathy, 9. other neuropathy | |
| Specific physical findings and symptoms (LCAT deficiency) | 1. opacity corneae, 2. edema | |
| Specific physical findings and symptoms (Tangier disease, and APOA1 deficiency) | 1. opacity corneae, 2. Enlarged tonsils (orange-color), 3. hepatomegaly, 4. splenomegaly, 5. peripheral neuropathy | |
| Specific symptoms (abetalipoproteinemia) digestive symptom | Yes/No | |
| Details of digestive symptom | 1. vomiting, 2. abdominal distension, 3. impaired development, 4. chronic diarrhea, 5. steatorrhea, 6. others | |
| Specific symptoms (abetalipoproteinemia) eye symptom | Yes/No | |
| Details of eye symptom | 1. night blindness, 2. color blindness, 3. low vision, 4. blindness, 5. visual field defect, 6. retinal pigment degeneration, 7. other | |
| Specific symptoms (abetalipoproteinemia) neuropathy | Yes/No | |
| Details of neuropathy |
1. reduced deep tendon reflex, 2. disturbance of vibration sense, 3. disturbance of position sense, 4. Romberg’s test positive, 5. dysmetria, 6. ataxia, 7. ataxic gait, 8. dysbasia, 9. spastic paralysis, 10. contracture of skeletal muscle, 11. dysphemia, 12. other spinocerebellar symptoms, 13. autonomic symptom, 14. peripheral neuropathy, 15. other neuropathy, 16. other symptoms related to muscles |
|
| Untreated lipids | Total cholesterol | mg/dl |
| LDL cholesterol | mg/dl | |
| HDL cholesterol | mg/dl | |
| Triglycerides | mg/dl | |
| Nutrition (infant, one year or under) | 1. mother’s milk, 2. formula milk, 3. both, 4. other | |
| LDL cholesterol (the highest value in life) | mg/dl | |
| LDL cholesterol (the lowest value in life) | mg/dl | |
| HDL cholesterol (the highest value in life) | mg/dl | |
| HDL cholesterol (the lowest value in life) | mg/dl | |
| Triglycerides (the highest value in life) | mg/dl | |
| Triglycerides (the lowest value in life) | mg/dl | |
| Blood tests | Date of blood tests | |
| Condition of blood tests | 1. Fasting (≥ 10 hours), 2. other, 3. Unknown | |
| Total cholesterol (TC) | mg/dl | |
| LDL cholesterol | mg/dl | |
| HDL cholesterol | mg/dl | |
| Triglycerides | mg/dl | |
| Blood glucose | mg/dl | |
| Insulin | µIU/mL | |
| 75g OGTT | Yes/No | |
| Insulin (0 min) | µIU/mL | |
| Insulin (30 min) | µIU/mL | |
| Insulin (60 min) | µIU/mL | |
| Insulin (120 min) | µIU/mL | |
| Blood glucose (0 min) | mg/dl | |
| Blood glucose (30 min) | mg/dl | |
| Blood glucose (60 min) | mg/dl | |
| Blood glucose (120 min) | mg/dl | |
| HOMA-IR | ||
| Inslulinogenic index | ||
| BUN | mg/dl | |
| Cr | mg/dl | |
| eGFR | mL/min/1.73m2 | |
| WBC | /µL | |
| RBC | /µL | |
| Ht | % | |
| Plt | /µL | |
| Total bilirubin | mg/dl | |
| Direct bilirubin | mg/dl | |
| ALT | IU/L | |
| AST | IU/L | |
| γ-GTP | IU/L | |
| Alb | g/dl | |
| HbAc1 | % | |
| Hb | g/dl | |
| MCV | fl | |
| MCHC | % | |
| Erythrocyte morphology abnormality | Yes/No | |
| Erythrocyte morphology abnormality detail | 1. target erythrocyte, 2. difference in size, 3. erythrocyte malformation, 4. stomatocyte | |
| Reticulocytes | ||
| Acanthocyte | Yes/No | |
| LCAT activity | nmol/ml/h/37℃ | |
| Free cholesterol (FC) | mg/dl | |
| Cholesteryl ester (CE) | mg/dl | |
| FC/CE ratio | ||
| CE/TC ratio | ||
| Phospholipid | mg/dl | |
| Amylase | IU/L | |
| Pancreatic amylase | IU/L | |
| Lipase | IU/L | |
| Uric acid | mg/dl | |
| CRP | mg/dl | |
| PT-INR | ||
| Sedimentation rate | mm | |
| Ca | mg/dl | |
| IP | mg/dl | |
| Fe | µg/dL | |
| Folic acid | ng/mL | |
| Vitamin B6 | pg/mL | |
| Vitamin B12 | pg/mL | |
| Vitamin A | µg/dL | |
| 25-OH-VitaminD | ng/mL | |
| Vitamin E (α-tocopherol) | µmol/L | |
| Vitamin E | µmol/L | |
| Vitamin K1 | ng/mL | |
| Vitamin K2 | ng/mL | |
| TSH | mIU/L | |
| Free T3 | ng/dL | |
| Free T4 | ng/dL | |
| ApoB | mg/dl | |
| ApoC-II | mg/dl | |
| ApoC-III | mg/dl | |
| APOE | mg/dl | |
| APOA-I | mg/dl | |
| APOA-II | mg/dl | |
| Lp(a) | mg/dl | |
| Lipoprotein electrophoresis (PAG) HDL | mg/dl | |
| Lipoprotein electrophoresis (PAG) LDL | mg/dl | |
| Lipoprotein electrophoresis (PAG) VLDL | mg/dl | |
| Lipoprotein electrophoresis (PAG) IDL | mg/dl | |
| Lp-X | Yes/No | |
| TG-rich large LDL | mg/dl | |
| RLP-C | mg/dl | |
| Broad-β pattern | Yes/No | |
| LDL-MI | mg/dl | |
| LPL (pre-heparin) | ng/ml | |
| LPL (post-heparin) | ng/ml | |
| heparin unit | unit/kg | |
| Timing of blood test after heparin injection | min | |
| LPL activity | µmol/mL/hour | |
| HL activity | µmol/mL/hour | |
| Timing of blood test after heparin injection | min | |
| EPA | µg/mL | |
| AA | µg/mL | |
| EPA/AA ratio | ||
| HPLC-HDL | mg/dl | |
| HPLC-LDL | mg/dl | |
| HPLC-IDL | mg/dl | |
| HPLC-VLDL | mg/dl | |
| HPLC-other | mg/dl | |
| HPLC-total cholesterol | mg/dl | |
| sitosterol | µg/ml | |
| cholestanol | µg/ml | |
| lathosterol | µg/ml | |
| campesterol | µg/ml | |
| X-ray of Achilles tendon | Yes/No | |
| Physical and imaging | Achilles tendon thickness (right) | Mm |
| Achilles tendon thickness (left) | Mm | |
| ABI | Yes/No | |
| ABI (right) | ||
| ABI (left) | ||
| ECG | Yes/No | |
| Abnormality of ECG | Yes/No | |
| Details of abnormality of ECG | ||
| Carotid ultrasound | Yes/No | |
| ≥ 70% stenosis (NASCET) | Yes/No | |
| UCG | Yes/No | |
| findings of UCG | 1. valvular disease, 2. other | |
| Abdominal ultrasound | Yes/No | |
| Fatty liver | Yes/No | |
| Cirrhosis | Yes/No | |
| Other findings | Yes/No | |
| Details of other findings of abdominal ultrasound | ||
| Ophthalmoscopy | Yes/No | |
| retinal pigment degeneration | Yes/No | |
| Other findings | Yes/No | |
| Details of other findings | ||
| FMD | % | |
| Visceral fat area | cm2 | |
| Splenomegaly (CT or ultrasound) | Yes/No | |
| Head MRI | Yes/No | |
| atrophy | Yes/No | |
| location of atrophy | ||
| T2-FLAIR high intensity | Yes/No | |
| location of T2-FLAIR high intensity | ||
| Spinal MRI | Yes/No | |
| atrophy | Yes/No | |
| location of atrophy | ||
| T2 high intensity | Yes/No | |
| location of T2 high intensity | ||
| Electroencephalography | Yes/No | |
| epilepsy | Yes/No | |
| slow wave | Yes/No | |
| Nerve conduction study | Yes/No | |
| axon dysfunction | Yes/No | |
| Demyelinisation | Yes/No | |
| Bone density test | Yes/No | |
| lumbar vertebra | ||
| thigh bone | ||
| Urine protein/Cr ratio | ||
| Urine alb /Cr ratio | ||
| Urine protein (24 hours) | g | |
| Gastrointestinal endoscopy | Yes/No | |
| snow white duodenum | Yes/No | |
|
Family history of coronary artery disease (<55 yr male, <65 yr female) |
1. Yes, 2. No, 3. Unknown, 4. No investigation | |
| Family history | relationship | 1. parent, 2. child, 3. sibling, 4. grandparent, 5. grandchild |
| Family history of FH | 1. Yes, 2. No, 3. Unknown, 4. No investigation | |
| relationship | 1. parent, 2. child, 3. sibling, 4. grandparent, 5. grandchild | |
| Family history of hypertriglyceridemia | 1. Yes, 2. No, 3. Unknown, 4. No investigation | |
| relationship | 1. parent, 2. child, 3. sibling, 4. grandparent, 5. grandchild | |
| Consanguineous marriage | 1. Yes, 2. No, 3. Unknown, 4. No investigation | |
| Family history of sitosterolemia | 1. Yes, 2. No, 3. Unknown, 4. No investigation | |
| relationship | 1. parent, 2. child, 3. sibling, 4. grandparent, 5. grandchild | |
| Family history of CTX | 1. Yes, 2. No, 3. Unknown, 4. No investigation | |
| relationship | 1. parent, 2. child, 3. sibling, 4. grandparent, 5. grandchild | |
| Family history of low HDL-C | 1. Yes, 2. No, 3. Unknown, 4. No investigation | |
| relationship | 1. parent, 2. child, 3. sibling, 4. grandparent, 5. grandchild | |
| Family history of low LDL-C | 1. Yes, 2. No, 3. Unknown, 4. No investigation | |
| relationship | 1. parent, 2. child, 3. sibling, 4. grandparent, 5. grandchild | |
| lipids in parent | 1. TC <120 mg/dl or LDL-C <70 mg/dl, 2. TC <50 mg/dl or LDL-C <15 mg/dl, 3. Unknown | |
| lipids in child | 1. TC <120 mg/dl or LDL-C <70 mg/dl, 2. TC <50 mg/dl or LDL-C <15 mg/dl, 3. Unknown | |
| lipids in sibling | 1. TC <120 mg/dl or LDL-C <70 mg/dl, 2. TC <50 mg/dl or LDL-C <15 mg/dl, 3. Unknown | |
| lipids in grandparent | 1. TC <120 mg/dl or LDL-C <70 mg/dl, 2. TC <50 mg/dl or LDL-C <15 mg/dl, 3. Unknown | |
| lipids in grandchild | 1. TC <120 mg/dl or LDL-C <70 mg/dl, 2. TC <50 mg/dl or LDL-C <15 mg/dl, 3. Unknown | |
| Glucose intolerance | Yes/No | |
| Medical history | Diabetes | Yes/No |
| type | 1. type 1, 2. type 2, 3. other | |
| diabetic retinopathy (right) | A1~B5, or No | |
| other eye complications (right) | Yes/No | |
| complications (right) | 1. macular disease, 2. retinal detachment, 3. rubeotic glaucoma, 4. ischemic optic neuropathy, 5. photocoagulation, 6. vitreous surgery | |
| diabetic retinopathy (left) | A1~B5, or No | |
| other eye complications (left) | Yes/No | |
| complications (left) | 1. macular disease, 2. retinal detachment, 3. rubeotic glaucoma, 4. ischemic optic neuropathy, 5. photocoagulation, 6. vitreous surgery | |
| CKD | Yes/No | |
| PAD | Yes/No | |
| Hypertension | Yes/No | |
| TIA | Yes/No | |
| Stroke | Yes/No | |
| Ischemic stroke | Yes/No | |
| Cerebral hemorrhage | Yes/No | |
| Coronary artery disease | Yes/No | |
| age of onset | ||
| intervention | 1. PCI, 2. CABG | |
| AS | Yes/No | |
| Supravalvular aortic stenosis | Yes/No | |
| Dissecting aneurysm of aorta | Yes/No | |
| Thoracic aortic aneurysm | Yes/No | |
| max diameter of aorta (CT) | mm | |
| Abdominal aortic aneurysm | Yes/No | |
| max diameter of aorta (CT) | mm | |
| Hypothyroidism | Yes/No | |
| Other endocrine disease | Yes/No | |
| Acute pancreatitis | Yes/No | |
| Gallstone | Yes/No | |
| Diagnostic ERCP | Yes/No | |
| Therapeutic ERCP | Yes/No | |
| Chronic pancreatitis | Yes/No | |
| Malfusion of pancreaticobiliary ducts | Yes/No | |
| Pancreas divisum | Yes/No | |
| Pancreatic tumor | Yes/No | |
| Hepatomegaly | Yes/No | |
| Splenomegaly | Yes/No | |
| Lipemia retinalis | Yes/No | |
| Blood disorder | Yes/No | |
| Details of blood disorder | ||
| Cataract | Yes/No | |
| Chronic diarrhea | Yes/No | |
| Osteoporosis | Yes/No | |
| Prolonged Neonatal Jaundice | Yes/No | |
| Neuropathy | Yes/No | |
| Details of neuropathy | 1. cognitive impairment, 2. psychological symptom, 3. cerebellar symptom, 4. pyramidal sign, 5. extrapyramidal sign, 6. seizures, 7. Spinal sensory disturbance, 8. peripheral neuropathy, 9. other neuropathy | |
| Arthritis | Yes/No | |
| Hemorrhagic diathesis | Yes/No | |
| Loss of vision | Yes/No | |
| Blindness | Yes/No | |
| Dialysis | Yes/No | |
| Nephrotic syndrome | Yes/No | |
| Cardiomyopathy | Yes/No | |
| Fatty liver | Yes/No | |
| Cirrhosis | Yes/No | |
| Hepatocellular carcinoma | Yes/No | |
| Other liver disease | Yes/No | |
| Details of other liver disease | ||
| Hyperthyroidism | Yes/No | |
| Cancer | Yes/No | |
| Details of cancer | ||
| Secondary causes of hypolipidemia | Yes/No | |
| Details of secondary causes of hypolipidemia | ||
| Other specific disease | Yes/No | |
| Details of other specific disease | ||
| Antihypertensive drug | Yes/No | |
| Medicine | class of antihypertensive drug | |
| Antidiabetic drug (oral) | Yes/No | |
| antidiabetic drug (injection) | Yes/No | |
| class of antidiabetic drug (injection) | 1. insulin, 2. GLP1 | |
| Antithrombotic drug | Yes/No | |
| Immunosuppressive agent | Yes/No | |
| class of immunosuppressive agent | 1. azathioprine, 2. cyclosporine, 3. other | |
| details of immunosuppressive agent | ||
| Anticancer agent | Yes/No | |
| class of anticancer agent | ||
| Antiinfective agent | Yes/No | |
| class of antiinfective agent | ||
| Hormone preparations | Yes/No | |
| class of hormone preparations | ||
| Treatments for neuropathy | Yes/No | |
| class of treatments for neuropathy | ||
| Treatments fof acne vulgaris | Yes/No | |
| Medications associated with pancreatitis | Yes/No | |
| details of medications associated with pancreatitis | ||
| Medications associated with hypertriglyceridemia | Yes/No | |
| details of medications associated with hypertriglyceridemia | ||
| Statin | Yes/No | |
| details of statin (class and dose) | ||
| Other lipid modifying medications | Yes/No | |
| details of other lipid modifying medications (class and dose) | ||
| Smoking habits | 1. current smoker, 2. former smoker, 3. never | |
| Lifestyle habits | peaces of smoking (daily) | |
| duration of smoking | ||
| The age of quit smoking | ||
| Drinking | 1. daily, 2. quit, 3. never | |
| days of drinking (week) | ||
| The age of starting drinking | ||
| The age of quit drinking | ||
| duration of drinking | ||
| Types and amount of alcohol | ||
| FH pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| DNA | LDLR pathogenic mutation | 1. Yes, 2. No, 3. No investigation |
| PCSK9 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| LDLRAP1 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| APOE genetic test | Yes/No | |
| APOE genotype-1 | ||
| APOE genotype-2 | ||
| APOE phenotype test | Yes/No | |
| APOE phenotype-1 | ||
| APOE phenotype-2 | ||
| LPL pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| APOC2 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| GPIHBP1 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| LMF1 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| APOA5 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| ABCG5 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| ABCG8 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| CYP27A1 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| LCAT pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| ABCA1 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| APOA1 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| ANGPTL3 pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| SAR1B pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| Other pathogenic mutation | 1. Yes, 2. No, 3. No investigation | |
| details of other pathogenic mutation | ||
| Date of investigation | ||
| Events (CVD) | Location of infarction | 1. anteroseptal, 2. lateral, inferior/posterior, 4. Unclassified |
| Type of angina pectoris | 1. stable, 2. unstable, 3. coronary spasm angina, 4. other | |
| Date of onset | ||
| Number of diseased arteries | ||
| Change of ECG | Yes/No | |
| details of ECG change | ||
| Elevation of CPK or TnT | Yes/No | |
| PCI or CABG | Yes/No | |
| coronary CT or MRI | Yes/No | |
| Events (stroke) | Type of ischemic stroke | 1. lacunar, 2. atherothrombotic, 3. cardioembolic, 4. unclassified |
| Date of onset | ||
| Symptom | Yes/No | |
| Details of symptom | ||
| Imaging | Yes/No | |
| type of imaging | 1. CT, 2. MRI, 3. other | |
| results of imaging | ||
| Af | Yes/No | |
| Thrombus in left atrium | Yes/No | |
| Type | 1. aortic valve, 3. mitral valve, 3. tricuspid valve | |
| Events (valvular disease) | Details of valvular disease | |
| TAVI | Yes/No | |
| Date of TAVI | ||
| Surgical treatment | Yes/No | |
| Date of surgical treatment | ||
| Aortic disease | Yes/No | |
| type of aortic disease | ||
| Intervention | Yes/No | |
| Type of PAD | ||
| Imaging of PAD | 1. CT, 2. MRI, 3. other | |
| Pancreatitis | Yes/No | |
| Events (pancreatitis) | Date of onset | |
| Symptom | ||
| Cause of pancreatitis | ||
| Type |
1. diabetes (new onset or worsening, 2. rhabdomyolysis, 3. dementia, 4. venous thrombosis, 5. gallstone, 6. other |
|
| Events (adverse) |
References
- 1).Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, Nakanishi C, Mori M, Yamagishi M, Inazu A, Koizumi J; Hokuriku FH Study Group. Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis, 2011; 214: 404-407 [DOI] [PubMed] [Google Scholar]
- 2).Sekijima Y, Koyama S, Yoshinaga T, Koinuma M, Inaba Y. Nationwide survey on cerebrotendinous xanthomatosis in Japan. J Hum Genet, 2018; 63: 271-280 [DOI] [PubMed] [Google Scholar]
- 3).Hu P, Dharmayat KI, Stevens CAT, Sharabiani MTA, Jones RS, Watts GF, Genest J, Ray KK, Vallejo-Vaz AJ. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Circulation, 2020; 141: 1742-1759 [DOI] [PubMed] [Google Scholar]
- 4).Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J Am Coll Cardiol, 2020; 75: 2553-2566 [DOI] [PubMed] [Google Scholar]
- 5).Mabuchi H. Half a century tales of familial hypercholesterolemia (FH) in Japan. J Atheroscler Thromb, 2017; 24: 189-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, Kastelein JJP, Hutten BA. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N Engl J Med, 2019; 381: 1547-1556 [DOI] [PubMed] [Google Scholar]
- 7).Tada H, Okada H, Nomura A, Nohara A, Yamagishi M, Takamura M, Kawashiri MA. Prognostic impact of cascade screening for familial hypercholesterolemia on cardiovascular events. J Clin Lipidol, 2021; 15: 358-365 [DOI] [PubMed] [Google Scholar]
- 8).Yamamura T. Apolipoprotein E and Atherosclerosis. Doumyakukouka (Japanese), 1998; 25: 415-420 [Google Scholar]
- 9).Hopkins PN, Brinton EA, Nanjee MN. Hyperlipoproteinemia type 3: the forgotten phenotype. Curr Atheroscler Rep, 2014; 16: 440 [DOI] [PubMed] [Google Scholar]
- 10).Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K and Working Group by Japan Atherosclerosis Society for Making Guidance of Familial H. Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Nohara A, Tada H, Ogura M, Okazaki S, Ono K, Shimano H, Daida H, Dobashi K, Hayashi T, Hori M, Matsuki K, Minamino T, Yokoyama S, Harada-Shiba M; Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the Ministry of Health, Labour and Welfare of Japan. Homozygous familial hypercholesterolemia. J Atheroscler Thromb, 2021; 28: 665-678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, Kastelein JJP, Hutten BA. 20-year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med, 2019; 381: 1547-1556 [DOI] [PubMed] [Google Scholar]
- 13).Tada H, Okada H, Nomura A, Nohara A, Yamagishi M, Takamura M, Kawashiri MA. Prognostic impact of cascade screening for familial hypercholesterolemia on cardiovascular events. J Clin Lipidol, 2021 in press [DOI] [PubMed] [Google Scholar]
- 14).Tada H, Nohara A, Inazu A, Sakuma N, Mabuchi H, Kawashiri MA. Sitosterolemia, hypercholesterolemia, and coronary artery disease. J Atheroscler Thromb, 2018; 25: 783-789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Tada H, Nomura A, Ogura M, Ikewaki K, Ishigaki Y, Inagaki K, Tsukamoto K, Dobashi K, Nakamura K, Hori M, Matsuki K, Yamashita S, Yokoyama S, Kawashiri MA, Harada-Shiba M; Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Desease of the Ministry of Health, Labour and Welfare of Japan. Diagnosis and management of sitosterolemia 2021. J Atheroscler Thromb, 2021; 28: 791-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Koyama S, Sekijima Y, Ogura M, Hori M, Matsuki K, Miida T, Harada-Shiba M, Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the Ministry of Health, Labor and Welfare of Japan. Cerebrotendinous xanthomatosis: molecular pathogenesis, clinical spectrum, diagnosis, and disease-modifying treatments. J Atheroscler Thromb, 2021; 28: 905-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Takahashi M, Okazaki H, Ohashi K, Ogura M, Ishibashi S, Okazaki S, Hirayama S, Hori M, Matsuki K, Yokoyama S, Harada-Shiba M, Committee on Primary Dyslipidemia under the Research Program on R, Intractable Disease of the Ministry of Health, Labor and Welfare of Japan. Current Diagnosis and Management of Abetalipoproteinemia. J Atheroscler Thromb, 2021; 28: 1009-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Okazaki H, Gotoda T, Ogura M, Ishibashi S, Inagaki K, Daida H, Hayashi T, Hori M, Masuda D, Matsuki K, Yokoyama S, Harada-Shiba M, Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the Ministry of Health, Labor and Welfare of Japan. Current diagnosis and management of primary chylomicronemia. J Atheroscler Thromb, 2021; 28: 883-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Koseki M, Yamashita S, Ogura M, Ishigaki Y, Ono K, Tsukamoto K, Hori M, Matsuki K, Yokoyama S, Harada-Shiba M, Committee on Primary Dyslipidemia under the Research Program on R, Intractable Disease of the Ministry of Health, Labor and Welfare of Japan. Current diagnosis and management of Tangier disease. J Atheroscler Thromb, 2021; 28: 802-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Kuroda M, Bujo H, Yokote K, Murano T, Yamaguchi T, Ogura M, Ikewaki K, Koseki M, Takeuchi Y, Nakatsuka A, Hori M, Matsuki K, Miida T, Yokoyama S, Wada J, Harada-Shiba M; Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the Ministry of Health, Labour and Welfare of Japan. Current status of familial LCAT deficiency in Japan. J Atheroscler Thromb, 2021; 28: 679-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Moriyama K, Sasaki J, Takada Y, Matsunaga A, Fukui J, Albers JJ, Arakawa K. A cysteine-containing truncated apo A-I variant associated with HDL deficiency. Arterioscler Thromb Vasc Biol, 1996; 16: 1416-1423 [DOI] [PubMed] [Google Scholar]
- 22).Huang W, Sasaki J, Matsunaga A, Nanimatsu H, Moriyama K, Han H, Kugi M, Koga T, Yamaguchi K, Arakawa K. A novel homozygous missense mutation in the apo A-I gene with apo A-I deficiency. Arterioscler Thromb Vasc Biol, 1998; 18: 389-396 [DOI] [PubMed] [Google Scholar]
- 23).Bujo H, Takahashi K, Saito Y, Maruyama T, Yamashita S, Matsuzawa Y, Ishibashi S, Shionoiri F, Yamada N, Kita T; Research Committeon Primary Hyperlipidemia of the Ministry of Health, Labour, and Welfare of Japan. Clinical features of familial hypercholesterolemia in Japan in a database from 1996-1998 by the research committee of the ministry of health, labour and welfare of Japan. J Atheroscler Thromb, 2004; 11: 146-151 [DOI] [PubMed] [Google Scholar]
- 24).Maruyama T, Yamashita S, Matsuzawa Y, Bujo H, Takahashi K, Saito Y, Ishibashi S, Ohashi K, Shionoiri F, Gotoda T, Yamada N, Kita T; Research Committee on Primary Hyperlipidemia of the Ministry of Health and Welfare of Japan. Mutations in Japanese subjects with primary hyperlipidemia--results from the Research Committee of the Ministry of Health and Welfare of Japan since 1996--. J Atheroscler Thromb, 2004; 11: 131-145 [DOI] [PubMed] [Google Scholar]
- 25).Pérez de Isla L, Alonso R, Mata N, Fernández-Pérez C, Muñiz O, Díaz-Díaz JL, Saltijeral A, Fuentes-Jiménez F, de Andrés R, Zambón D, Piedecausa M, Cepeda JM, Mauri M, Galiana J, Brea Á, Sanchez Muñoz-Torrero JF, Padró T, Argueso R, Miramontes-González JP, Badimón L, Santos RD, Watts GF, Mata P. Predicting cardiovascular events in familial hypercholesterolemia: The SAFEHEART Registry (Spanish Familial Hypercholesterolemia Cohort Study). Circulation, 2017; 135: 2133-2144 [DOI] [PubMed] [Google Scholar]
- 26).Humphries SE, Cooper JA, Seed M, Capps N, Durrington PN, Jones B, McDowell IFW, Soran H, Neil HAW; Simon Broome Familial Hyperlipidaemia Register Group. Coronary heart disease mortality in treated familial hypercholesterolaemia: Update of the UK Simon Broome FH register. Atherosclerosis, 2018; 274: 41-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Yamashita S, Masuda D, Harada-Shiba M, Arai H, Bujo H, Ishibashi S, Daida H, Koga N, Oikawa S and Group FS. Effectiveness and safety of lipid-lowering drug treatments in Japanese patients with familial hypercholesterolemia: Familial Hypercholesterolemia Expert Forum (FAME) Study. J Atheroscler Thromb, 2022; 29: 608-638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Naito R, Daida H, Masuda D, Harada-Shiba M, Arai H, Bujo H, Ishibashi S, Koga N, Oikawa S, Yamashita S. Relation of Serum Lipoprotein(a) Levels to Lipoprotein and Apolipoprotein Profiles and Atherosclerotic Diseases in Japanese Patients with Heterozygous Familial Hypercholesterolemia: Familial Hypercholesterolemia Expert Forum (FAME) Study. J Atheroscler Thromb, 2021 in press. doi: http: //doi.org/10.5551/jat.63019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Ogura M, Harada-Shiba M, Masuda D, Arai H, Bujo H, Ishibashi S, Daida H, Koga N, Oikawa S, Yamashita S; FAME Study Group. Factors Associated with Carotid Atherosclerosis and Achilles Tendon Thickness in Japanese Patients with Familial Hypercholesterolemia: A Subanalysis of the Familial Hypercholesterolemia Expert Forum (FAME) Study. J Atheroscler Thromb, 2022; 29: 906-922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).https: //www.nanbyou.or.jp/entry/5354 (Last accessed at July 21, 2021) [Google Scholar]
- 31).Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J, 2017; 38: 1573-1579 [DOI] [PubMed] [Google Scholar]
- 32).Tada H, Kawashiri MA, Nohara A, Sakata K, Inazu A, Mabuchi H, Yamagishi M, Hayashi K. Remnant-like particles and coronary artery disease in familial hypercholesterolemia. Clin Chim Acta, 2018; 482: 120-123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dataset will not be available to public.