Abstract
Aims: Familial hypercholesterolemia (FH) is characterized by high low-density lipoprotein (LDL) cholesterol levels, xanthomas including Achilles tendon thickening, and premature coronary artery disease (CAD). Carotid intima-media thickness (IMT) is a well-established surrogate marker for CAD in FH and Achilles tendon thickening is a specific physical finding in patients with FH. The objective of the present study was to identify factors associated with carotid IMT and Achilles tendon thickness in FH heterozygotes on lipid-lowering therapy. This study also aimed to examine the follow-up changes in carotid IMT and Achilles tendon thickness among them in the current real-world FH practice.
Methods: The current study is a subanalysis of the Familial Hypercholesterolemia Expert Forum (FAME) Study. The severity of carotid atherosclerosis was assessed with the maximal and mean IMT using ultrasonography, and Achilles tendon thickness was measured using X-rays. The present study used 571 patients under medical treatment for heterozygous FH who had baseline measurements for maximal IMT (n=511), mean IMT (n=459), or Achilles tendon thickness (n=486). The IMT was measured annually, and Achilles tendon thickness was evaluated every two years.
Results: Higher LDL cholesterol (LDL-C) level and lower HDL cholesterol (HDL-C) level were associated with greater maximal and mean IMT as well as greater Achilles tendon thickness. Achilles tendon thickness tended to be greater in patients who had a smoking history than in never-smokers. Maximal IMT and Achilles tendon thickness were significantly greater in patients with CAD than in those without. Additionally, lower HDL-C level and hypertension were associated with higher values of maximal and mean IMT, suggesting the importance of comprehensive risk management including reduced HDL-C and blood pressure control in FH care. In longitudinal observations, percentage changes in maximal IMT and mean IMT gradually increased during the observation period. In contrast, percentage changes in Achilles tendon thickness became progressively thinner throughout the observation period.
Conclusions: We found a positive association between LDL-C levels and severity of carotid atherosclerosis in heterozygous FH patients on treatment. This observation suggests the insufficiency of lipid-lowering therapy and the presence of therapeutic inertia among clinicians in the real-world FH practice.
Keywords: Familial hypercholesterolemia, Carotid intima-media thickness, Achilles tendon thickness, LDL-cholesterol, Drug treatment
Introduction
Familial hypercholesterolemia (FH) is characterized by high levels of low-density lipoprotein (LDL) cholesterol (LDL-C), tendon xanthomas including Achilles tendon thickening, and premature coronary artery disease (CAD) due to pathogenic mutations in the LDL receptor and its related genes 1) . The frequency of heterozygous FH is estimated to be 1 in 200 to 300 in a Japanese population 2) . Since there are several reports that FH patients account for 5-10% of patients with acute coronary syndromes in Japan 3 , 4) prevention of cardiovascular events in FH patients is an important public health issue.
Carotid intima-media thickness (IMT) is a well-established surrogate marker for CAD in both FH 5) and non-FH patients 6) . Although carotid IMT itself is a non-specific parameter in FH, it has been reported that carotid atherosclerosis develops more progressively in FH patients than in non-FH patients 5 , 7) . In addition, lipid-lowering therapy could delay the progression of carotid IMT even in FH patients 8 - 10) . However, factors associated with the degree of carotid IMT in FH patients receiving lipid-lowering therapy have not been fully elucidated. Moreover, the longitudinal changes in carotid IMT in these patients under the real-world lipid-lowering treatment are unknown.
Achilles tendon thickening is a specific physical finding in patients with FH. Most diagnostic criteria for FH such as developed in the Dutch Lipid Clinic Network 11) , the Simon Broome Register Group 12) and the Japan Atherosclerosis Society 13) – include tendon xanthomas. In Japan, X-ray radiography has been used to measure Achilles tendon thickness 13 , 14) , and ultrasonography has also been used as an imaging modality for its evaluation 15 - 17) . Importantly, Achilles tendon thickness is not only one of the diagnostic criteria for FH, but has also been reported to be associated with the severity of atherosclerosis 16) . However, factors associated with Achilles tendon thickness and its longitudinal changes in FH patients under lipid-lowering treatment are not clear.
Therefore, the aim of the current study was to identify factors associated with carotid IMT and Achilles tendon thickness in FH heterozygotes on lipid-lowering therapy. The present study also aimed to examine the follow-up changes in carotid IMT and Achilles tendon thickness among them.
1. Subjects and Methods
The present study is a subanalysis of the Familial Hypercholesterolemia Expert Forum (FAME) Study, a multicenter prospective observational study to investigate the current real-world lipid-lowering therapies for patients with FH. This study was registered with UMIN (UMIN000003211). Diagnosis of FH, the inclusion, exclusion and discontinuation criteria for the study subjects, enrollment period and observation period, clinical information such as basic characteristics, laboratory parameters, and lipid-lowering treatment details obtained from patients in this study have been reported previously 18) .
The present study used 571 patients under medical treatment for heterozygous FH who had baseline measurements for maximal IMT (n=511), mean IMT (n=459), or Achilles tendon thickness (n=486). The severity of carotid atherosclerosis was assessed with the IMT using ultrasonography as previously reported 18) . The IMT on both sides was averaged. The thickness of Achilles tendon on both sides was measured using X-rays and was averaged. The carotid IMT was measured annually, and Achilles tendon thickness was evaluated every two years.
The study protocol was initially reviewed and approved by the Institutional Review Board (IRB) of Osaka University Hospital, and thereafter by the IRBs of the participating institutions. Investigators obtained IRB approval and permission from the head of each institution before conducting the study. Written informed consent was obtained from each FH patient who participated in the current study. If patients were under 16 years old, a written informed consent was taken from their legally authorized representatives. If patients were 16 to 19 years old, a written informed consent was taken from both patients and their legally authorized representatives.
Proportions and means with standard deviation (SD) were calculated for categorical variables and for continuous variables, respectively, as descriptive statistics. Covariates of interest in relation to maximal IMT, mean IMT, and Achilles tendon thickness were serum LDL-C, HDL-C, triglycerides (TG), remnant lipoprotein cholesterol (RLP-C) by direct or immunoadsorption method, lipoprotein (a) (Lp(a)), relative to front (Rf) on polyacrylamide gel disc electrophoresis, CAD, cerebral infarction, hypertension, diabetes mellitus, and smoking status (classified into never, past, and current smoking) as well as sex and age. The cross-sectional associations were assessed by using regression analysis. Firstly, the associations of sex and age with outcome measurements were examined by univariate regression analysis. Secondly, the associations with the covariates other than sex and age were examined in models each including sex, age, and a covariate of interest. Variables showing a statistically significant association with adjustment for sex and age were finally assessed by the multiple regression analysis. In the regression analysis, natural logarithm of serum TG, RLP-C, and Lp(a) were used because these variables showed an extreme skewness to the right side. Variance in the regression analysis was always estimated by the robust method because preliminary analyses indicated heteroskedastic variance: i.e., residuals were not constant over predictors, especially with regard to age ( Supplementary Fig.1 ) . Relative importance of the covariates was assessed in terms with standardized regression coefficients, which were regression coefficients multiplied by SD of a covariate of interest.
Supplementary Fig.1.
Residuals from predicted values versus age for maximal (A) and mean (B) IMT and Achilles tendon thickness (C) in each regression with age
Follow-up changes in maximal IMT, mean IMT, and Achilles tendon thickness were examined by using means and 95% confidence intervals of percent changes from the baseline according to follow-up years.
Statistical significance was declared if two-sided P value was less than 0.05. Statistical analyses and graphical presentation were performed by using Stata version 13 (StataCorp, College Station, TX).
2. Results
2.1 Baseline Characteristics of Heterozygous FH Patients
The clinical characteristics of heterozygous FH who had baseline measurements for maximal IMT, mean IMT, or Achilles tendon thickness in each sex as well as in both sexes combined are summarized in Table 1 . The mean age at registration of the 571 patients was 56.1 years in total, 53.8 years in 231 males and 57.6 years in 340 females. The mean FH prevalence period from the diagnosis was nearly 10 years and mean LDL-C level under treatment was 141 mg/dL. Ever smokers (current smokers and past smokers) accounted for 47.1% in males and 14.0% in females. Maximal IMT, mean IMT, and Achilles tendon thickness differed by sex and markedly varied with age. Men had higher values than women with respect to maximal IMT (mean difference 0.15 mm, P=0.05), mean IMT (mean difference 0.10 mm, P=0.002), and Achilles tendon thickness (mean difference 1.67 mm, P<10−3. The mean changes per 10-year increase in age were 0.28 mm (P<10−29) for maximal IMT, 0.11 mm (P<10−29) for mean IMT, and 0.56 mm (P<10−4). Hypertension and diabetes mellitus were observed in 30.8% and 17.3%, respectively. CAD was twice more frequent in men (34.2%) than in women (15.9%).
Table 1. Baseline Characteristics of the Study Patients.
| Variable | Both sexes | Male | Female | |||
|---|---|---|---|---|---|---|
| N* | Value | N* | Value | N* | Value | |
| Mean (SD) | ||||||
| Age (year) | 571 | 56.1 (15.2) | 231 | 53.8 (15.3) | 340 | 57.6 (15.0) |
| Heterozygous FH score | 571 | 13.5 (5.2) | 231 | 14.4 (5.5) | 340 | 12.9 (4.8) |
| Years from FH diagnosis | 557 | 9.9 (8.9) | 227 | 10.0 (9.4) | 330 | 9.8 (8.6) |
| Height (cm) | 557 | 160 (9) | 227 | 168 (6) | 330 | 155 (6) |
| Body weight (kg) | 561 | 60 (12) | 229 | 69 (11) | 332 | 54 (8) |
| Body mass index (kg/m2) | 550 | 23.3 (3.5) | 227 | 24.4 (3.5) | 323 | 22.6 (3.4) |
| LDL cholesterol (mg/dL) | 553 | 141 (41) | 221 | 134 (39) | 332 | 145 (42) |
| HDL cholesterol (mg/dL) | 569 | 55 (17) | 231 | 49 (15) | 338 | 59 (17) |
| Triglycerides (mg/dL) | 570 | 106 (67) | 231 | 110 (66) | 339 | 103 (67) |
| RLP cholesterol (mg/dL) | 322 | 5.2 (4.3) | 133 | 5.3 (4.2) | 189 | 5.2 (4.4) |
| Lp(a) (mg/dL) | 388 | 31.3 (34.1) | 151 | 29.7 (27.8) | 237 | 32.4 (37.6) |
| Rf | 158 | 0.34 (0.04) | 65 | 0.34 (0.04) | 93 | 0.34 (0.05) |
| Maximal IMT (mm) | 511 | 1.57 (0.85) | 202 | 1.66 (0.87) | 309 | 1.51 (0.83) |
| Mean IMT (mm) | 459 | 0.89 (0.33) | 183 | 0.95 (0.34) | 276 | 0.85 (0.33) |
| Achilles tendon thickness (mm) | 486 | 11.4 (4.1) | 198 | 12.3 (4.8) | 288 | 10.8 (3.5) |
| Number (%) | ||||||
| Coronary artery disease (CAD); | 571 | 133 (23.3) | 231 | 79 (34.2) | 340 | 54 (15.9) |
| Cerebral infarction | 571 | 18 (3.2) | 231 | 11 (4.8) | 340 | 7 (2.1) |
| Hypertension | 571 | 176 (30.8) | 231 | 86 (37.2) | 340 | 90 (26.5) |
| Diabetes mellitus | 571 | 99 (17.3) | 231 | 48 (20.8) | 340 | 51 (15.0) |
| Smoking | 559 | 223 | 336 | |||
| Never | 407 (72.8) | 118 (52.9) | 289 (86.0) | |||
| Past | 104 (18.6) | 76 (34.1) | 28 (8.3) | |||
| Current | 48 (8.6) | 29 (13.0) | 19 (5.7) | |||
*Denominators were not uniform because of missing information.
2.2 Factors Associated with Maximal IMT and Mean IMT, and Achilles Tendon Thickness in Heterozygous FH Patients at Baseline
Table 2 summarizes the associations of the covariates under study with the outcome measurements after adjustment for sex and age. LDL-C was significantly associated with increased levels of maximal and mean IMT and Achilles tendon thickness. Inverse associations of HDL-C were highly significant for all of the three outcome measurements. TG, RLPC, Lp(a), Rf were not related to maximal IMT, mean IMT, or Achilles tendon thickness with exceptions of a negative association between TG and mean IMT (P=0.02) and a positive association between Rf and Achilles tendon thickness (P=0.04). CAD was significantly positively associated with each of the outcome variables. Hypertension was also significantly associated with maximal and mean IMT, but not with Achilles tendon thickness. Body mass index and diabetes mellitus as well as cerebral infarction were unrelated to maximal and mean IMT and Achilles tendon thickness.
Table 2. Regression analyses on maximal and mean intima-media thickness (IMT) of the carotid arteries and Achilles tendon thickness (mm) in relation to lipid and clinical parameters at baseline.
| Parameter | Unit in change or comparison | n | Regression coefficient* | Robust SE | P-value | Standardized regression coefficient† |
|---|---|---|---|---|---|---|
| Maximal IMT (mm) | ||||||
| LDL cholesterol | Per 10 mg/dL | 495 | 0.015 | 0.007 | 0.04 | 0.072 |
| HDL cholesterol | Per 10 mg/dL | 510 | -0.098 | 0.024 | <10-4 | -0.200 |
| HDL cholesterol‡ | Per 10 mg/dL | 412 | -0.045 | 0.027 | 0.10 | -0.090 |
| Triglycerides | Per ln (mg/dL) | 511 | -0.059 | 0.060 | 0.32 | -0.037 |
| RLP-C | Per ln (mg/dL) | 304 | 0.037 | 0.052 | 0.48 | 0.028 |
| Lp(a) | Per ln (mg/dL) | 359 | 0.035 | 0.038 | 0.36 | 0.037 |
| Rf | Per 0.10 | 151 | 0.074 | 0.146 | 0.61 | 0.043 |
| Body mass index | Per kg/m2 | 491 | -0.011 | 0.009 | 0.18 | -0.049 |
| CAD | (+) vs. (−) | 115/396 | 0.345 | 0.093 | <10-3 | 0.170 |
| Cerebral infarction | (+) vs. (−) | 17/494 | 0.263 | 0.185 | 0.16 | 0.056 |
| Hypertension | (+) vs. (−) | 153/358 | 0.250 | 0.083 | 0.003 | 0.135 |
| Diabetes mellitus | (+) vs. (−) | 91/420 | 0.123 | 0.093 | 0.19 | 0.055 |
| Smoking | Past vs. never | 95/363 | 0.134 | 0.091 | 0.14 | 0.062 |
| Current vs. never | 41/363 | 0.226 | 0.121 | 0.06 | 0.074 | |
| Mean IMT (mm) | ||||||
| LDL cholesterol | Per 10 mg/dL | 444 | 0.009 | 0.003 | 0.002 | 0.114 |
| HDL cholesterol | Per 10 mg/dL | 458 | -0.040 | 0.008 | <10-6 | -0.212 |
| HDL cholesterol‡ | Per 10 mg/dL | 370 | -0.029 | 0.009 | 0.001 | -0.156 |
| Triglycerides | Per ln (mg/dL) | 459 | -0.056 | 0.023 | 0.02 | -0.090 |
| RLP-C | Per ln (mg/dL) | 271 | -0.005 | 0.020 | 0.82 | -0.010 |
| Lp(a) | Per ln (mg/dL) | 324 | 0.020 | 0.017 | 0.24 | 0.056 |
| Rf | Per 0.10 | 128 | 0.052 | 0.053 | 0.32 | 0.073 |
| Body mass index | Per kg/m2 | 445 | 0.002 | 0.003 | 0.54 | 0.021 |
| CAD | (+) vs. (−) | 102/357 | 0.125 | 0.039 | 0.001 | 0.155 |
| Cerebral infarction | (+) vs. (−) | 14/445 | 0.078 | 0.072 | 0.28 | 0.040 |
| Hypertension | (+) vs. (−) | 135/324 | 0.076 | 0.035 | 0.03 | 0.103 |
| Diabetes mellitus | (+) vs. (−) | 79/380 | 0.017 | 0.037 | 0.65 | 0.019 |
| Smoking | Past vs. never | 89/325 | 0.048 | 0.038 | 0.20 | 0.059 |
| Current vs. never | 37/325 | 0.037 | 0.045 | 0.41 | 0.031 | |
| Achilles tendon thickness (mm) | ||||||
| LDL cholesterol | Per 10 mg/dL | 470 | 0.134 | 0.046 | 0.004 | 0.139 |
| HDL cholesterol | Per 10 mg/dL | 484 | -0.386 | 0.101 | <10-3 | -0.163 |
| HDL cholesterol‡ | Per 10 mg/dL | 388 | -0.372 | 0.119 | 0.002 | -0.145 |
| Triglycerides | Per ln (mg/dL) | 485 | -0.223 | 0.429 | 0.60 | -0.028 |
| RLP-C | Per ln (mg/dL) | 296 | -0.137 | 0.394 | 0.73 | -0.021 |
| Lp(a) | Per ln (mg/dL) | 347 | 0.262 | 0.222 | 0.24 | 0.060 |
| Rf | per 0.10 | 142 | 2.233 | 1.067 | 0.04 | 0.236 |
| Body mass index | Per kg/m2 | 466 | -0.015 | 0.050 | 0.76 | -0.013 |
| CAD | (+) vs. (−) | 112/374 | 1.893 | 0.498 | <10-3 | 0.193 |
| Cerebral infarction | (+) vs. (−) | 14/472 | 0.222 | 0.949 | 0.81 | 0.009 |
| Hypertension | (+) vs. (−) | 153/333 | 0.310 | 0.451 | 0.49 | 0.035 |
| Diabetes mellitus | (+) vs. (−) | 86/400 | 0.682 | 0.554 | 0.22 | 0.063 |
| Smoking | Past vs. never | 95/342 | 1.306 | 0.551 | 0.02 | 0.127 |
| Current vs. never | 38/342 | 0.827 | 0.773 | 0.29 | 0.055 |
*Adjusted for sex and age. †Regression coefficient divided by standard deviation of the parameter of study.
‡Excluding probucol users.
When past and current smoking were combined as ever-smoking, maximal IMT was statistically significantly greater in ever-smokers as compared with never-smokers (mean difference 0.164 mm, SE 0.079, P=0.04) while mean IMT was not significantly higher in ever-smokers (mean difference 0.045 mm, SE 0.031, P=0.15). Achilles tendon thickness was 1.154 mm (SE 0.482 mm) greater in ever-smokers than in never-smokers (P=0.02).
Probucol is known to decrease HDL-C and may have affected the associations with HDL-C. Probucol was used by 109 of the 571 study patients. When the patients using probucol were excluded, the associations of HDL-C with maximal and mean IMT were substantially attenuated while the association between HDL-C and Achilles tendon thickness was almost the same. The regression coefficients were −0.045 (SE 0.027) for maximal IMT (P=0.10), −0.029 (SE 0.009) for mean IMT (P=0.001), and −0.372 (SE 0.119) for Achilles tendon thickness (P=0.002).
2.3 Multiple Regression Analyses on Maximal IMT and Mean IMT, and Achilles Tendon Thickness in Heterozygous FH Patients at Baseline
Table 3 presents the results from the multiple regression analyses on maximal IMT and mean IMT, and Achilles tendon thickness. The covariates of study were LDL-C, HDL-C, CAD, hypertensin and ever-smoking as well as sex and age. Hypertension was unrelated to Achilles tendon thickness in the above analysis, but included in the model for a comparative purpose. All of the covariates except for sex and smoking were significantly associated with both maximal and mean IMT; LDL-C, CAD, and hypertension were positively related to the IMT measurements, and HDL-C was negatively related to the IMT measurements. LDL-C, HDL-C, and CAD were also important covariates of Achilles tendon thickness. Ever-smoking was positively associated with Achilles tendon thickness, but marginally failed to reach the statistical significance (P=0.0548).
Table 3. Multiple regression analyses on maximal and mean intima-media thickness (IMT) and Achilles tendon thickening in relation to lipid and clinical parameters at baseline.
| Parameter | Unit in change | Regression coefficient | Robust SE | P-value | Standardized regression coefficient* |
|---|---|---|---|---|---|
| Maximal IMT (n = 483) | |||||
| Sex | Male vs. female | 0.063 | 0.075 | 0.40 | 0.036 |
| Age | Per 10 years | 0.238 | 0.024 | <10-19 | 0.429 |
| LDL cholesterol | Per 10 mg/dL | 0.018 | 0.007 | 0.01 | 0.085 |
| HDL cholesterol | Per 10 mg/dL | -0.075 | 0.025 | 0.003 | -0.156 |
| CAD | (+) vs. (−) | 0.256 | 0.092 | 0.006 | 0.127 |
| Hypertension | (+) vs. (−) | 0.170 | 0.079 | 0.03 | 0.092 |
| Ever-smoking | (+) vs. (−) | 0.098 | 0.078 | 0.21 | 0.052 |
| Mean IMT (n = 436) | |||||
| Sex | Male vs. female | 0.096 | 0.031 | 0.002 | 0.141 |
| Age | Per 10 years | 0.108 | 0.010 | <10-23 | 0.479 |
| LDL cholesterol | Per 10 mg/dL | 0.009 | 0.003 | 0.003 | 0.114 |
| HDL cholesterol | Per 10 mg/dL | -0.031 | 0.008 | <10-4 | -0.168 |
| CAD | (+) vs. (−) | 0.075 | 0.038 | 0.051 | 0.094 |
| Hypertension | (+) vs. (−) | 0.058 | 0.034 | 0.09 | 0.080 |
| Ever-smoking | (+) vs. (−) | 0.023 | 0.031 | 0.47 | 0.030 |
| Achilles tendon thickness (n = 459) | |||||
| Sex | Male vs. female | 0.484 | 0.434 | 0.27 | 0.059 |
| Age | Per 10 years | 0.474 | 0.137 | 0.001 | 0.183 |
| LDL cholesterol | Per 10 mg/dL | 0.120 | 0.043 | 0.005 | 0.122 |
| HDL cholesterol | Per 10 mg/dL | -0.269 | 0.100 | 0.007 | -0.120 |
| CAD | (+) vs. (−) | 1.893 | 0.516 | <10-3 | 0.200 |
| Hypertension | (+) vs. (−) | 0.015 | 0.444 | 0.97 | 0.002 |
| Ever-smoking | (+) vs. (−) | 0.912 | 0.474 | 0.055 | 0.102 |
*Regression coefficient divided by standard deviation of the parameter of study.
Fig.1 shows maximal and mean IMT and Achilles tendon thickness according to LDL-C ( Fig.1, A-C ) and HDL-C levels ( Fig.1, D-F ) . Adjusted means of IMT and Achilles tendon thickness derived from the final multiple regression model were depicted over a range of 50–250 mg/dL of LDL-C and over a range of 20–100 mg/dL of HDL-C. Positive associations with LDL-C and negative associations with HDL-C were clearly visible. The associations were stronger but less stable for mean IMT than for maximal IMT.
Fig.1. Maximal and mean IMT and Achilles tendon thickness according to LDL-C (A-C) and HDL-C (D-F) levels.
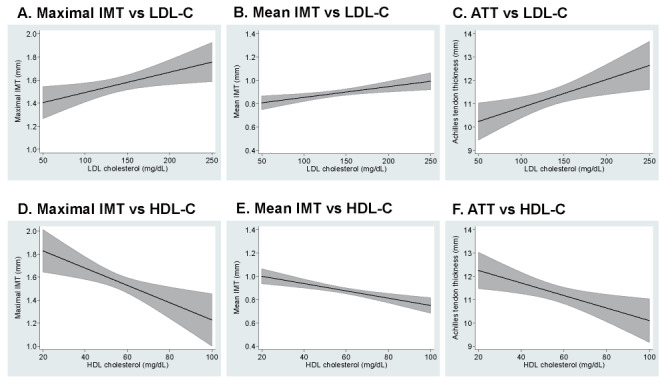
Solid lines represent predicted means in the multiple regression analyses, shaded areas showing 95% confidence intervals.
IMT; intima-media thickness, LDL-C; low-density lipoprotein cholesterol, ATT; Achilles tendon thickness, and HDL-C; high-density lipoprotein cholesterol
Fig.2 shows the adjusted means of maximal and mean IMT and Achilles tendon thickness according to CAD (A-C) and hypertension (D-F) status. Maximal IMT was significantly greater in patients with prevalent CAD than those without, but the difference in mean IMT between the two groups was much less (P=0.051). Patients with CAD had approximately 2 mm greater Achilles tendon thickness than those without. Hypertension was associated with higher values of maximal IMT, but the difference between patients with hypertension and those without was almost negligible for mean IMT and null for Achilles tendon thickness.
Fig.2. Maximal and mean IMT and Achilles tendon thickness according to prevalent CAD (A-C) and hypertension (HT) (D-F).
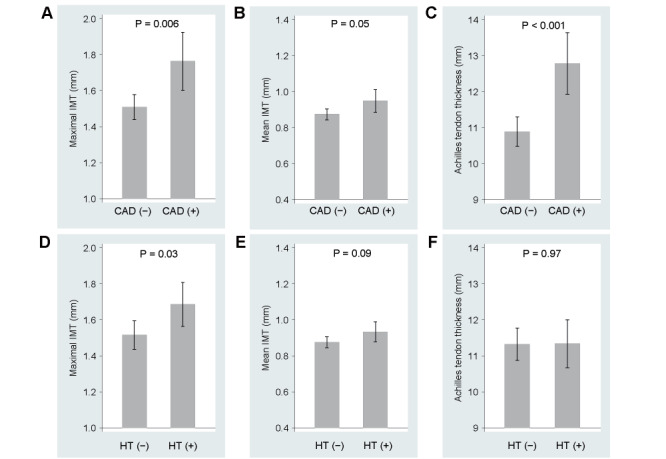
Presented are adjusted means (shaded bars) and 95% confidence intervals (vertical bars) derived from the multiple regression analyses.
The multiple regression analyses were repeated after exclusion of patients with probucol use ( Table 4 ) . As noted above, the inverse association of HDL-C with maximal IMT was almost nullified, and the association between HDL-C and mean IMT was much weakened although the association remained statistically significant. The associations with the covariates other than HDL-C with maximal and mean IMT did not change after exclusion of probucol users. The results on Achilles tendon thickness were almost the same as those observed for the whole patients. The association between smoking and Achilles tendon thickness was again statistically significant.
Table 4. Multiple regression analyses on maximal and mean intima-media thickness (IMT) and Achilles tendon thickening in relation to lipid and clinical parameters at baseline after exclusion of probucol users probucol users.
| Parameter | Unit in change | Regression coefficient | Robust SE | P-value | Standardized regression coefficient* |
|---|---|---|---|---|---|
| Maximal IMT (n = 391) | |||||
| Sex | Male vs. female | 0.099 | 0.085 | 0.25 | 0.062 |
| Age | Per 10 years | 0.217 | 0.026 | <10-14 | 0.430 |
| LDL cholesterol | Per 10 mg/dL | 0.019 | 0.008 | 0.02 | 0.103 |
| HDL cholesterol | Per 10 mg/dL | -0.012 | 0.028 | 0.68 | -0.024 |
| CAD | (+) vs. (−) | 0.249 | 0.102 | 0.01 | 0.130 |
| Hypertension | (+) vs. (−) | 0.205 | 0.084 | 0.02 | 0.119 |
| Ever-smoking | (+) vs. (−) | 0.152 | 0.088 | 0.09 | 0.085 |
| Mean IMT (n = 352) | |||||
| Sex | Male vs. female | 0.070 | 0.034 | 0.04 | 0.113 |
| Age | Per 10 years | 0.095 | 0.010 | <10-19 | 0.479 |
| LDL cholesterol | Per 10 mg/dL | 0.007 | 0.003 | 0.03 | 0.095 |
| HDL cholesterol | Per 10 mg/dL | -0.017 | 0.008 | 0.04 | -0.094 |
| CAD | (+) vs. (−) | 0.097 | 0.040 | 0.01 | 0.131 |
| Hypertension | (+) vs. (−) | 0.096 | 0.035 | 0.007 | 0.145 |
| Ever-smoking | (+) vs. (−) | 0.027 | 0.033 | 0.41 | 0.040 |
| Achilles tendon thickness (n = 367) | |||||
| Sex | Male vs. female | 0.508 | 0.483 | 0.29 | 0.063 |
| Age | Per 10 years | 0.454 | 0.148 | 0.002 | 0.184 |
| LDL cholesterol | Per 10 mg/dL | 0.118 | 0.045 | 0.01 | 0.127 |
| HDL cholesterol | Per 10 mg/dL | -0.270 | 0.117 | 0.02 | -0.112 |
| CAD | (+) vs. (−) | 1.774 | 0.591 | 0.003 | 0.184 |
| Hypertension | (+) vs. (−) | -0.320 | 0.487 | 0.51 | -0.038 |
| Ever-smoking | (+) vs. (−) | 1.182 | 0.534 | 0.03 | 0.133 |
*Regression coefficient divided by standard deviation of the parameter of study.
2.4 Changes in Maximal and Mean IMT and Achilles Tendon Thickness during Follow-up
Percentage changes in maximal and mean IMT gradually increased with advancing years in follow-up. Increases from the baseline in maximal IMT at 4 years and in mean IMT at 2, 3, and 4 years were statistically significant. On the contrary, percentage changes in Achilles tendon thickness progressively decreased with years ( Supplementary Fig.2 ) .
Supplementary Fig.2. The percentage changes of maximal (A) and mean (B) IMT and Achilles tendon thickness (C) according to follow-up years.
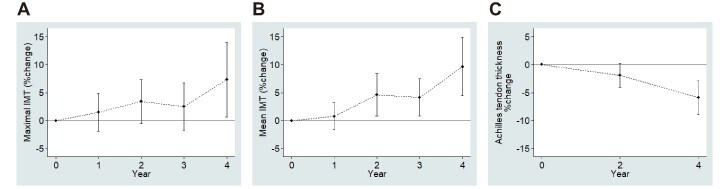
Number of patients were: 511 at baseline, 347 at 1 year, 327 at 2 years, 264 at 3 years, and 185 at 4 years for maximal IMT; 459 at baseline, 321 at 1 year, 299 at 2 years, 241 at 3 years, and 170 at 4 years for mean IMT; and 486 at baseline, 199 at 2 years, and 71 at 4 years for Achilles tendon thickness.
Multiple regression analyses were performed regarding changes in maximal and mean IMT and Achilles tendon thickness at 2 and 4 years of follow-up in relation to sex, age, changes of LDL-C and HDL-C from baseline, CAD, hypertension, and ever-smoking ( Supplementary Tables 1 and 2 ) . No measurable associations were found with respect to the covariates under study except for sex (for maximal IMT at 2 years) and age (for mean IMT at 4 years).
Supplementary Table 1. Multiple regression analyses on changes in maximal and mean intima-media thickness (IMT) and Achilles tendon thickness at 2 years of follow-up in relation to lipid and clinical parameters.
| Parameter | Unit in change | Regression coefficient | Robust SE | P-value | Standardized regression coefficient* |
|---|---|---|---|---|---|
| Maximal IMT (n = 306) | |||||
| Sex | Male vs. female | 0.192 | 0.080 | 0.02 | 0.153 |
| Age | Per 10 years | 0.001 | 0.032 | 0.98 | 0.002 |
| LDL cholesterol† | Per 10 mg/dL | -0.011 | 0.010 | 0.26 | -0.058 |
| HDL cholesterol† | Per 10 mg/dL | 0.009 | 0.033 | 0.78 | 0.013 |
| CAD | (+) vs. (−) | -0.143 | 0.099 | 0.15 | -0.100 |
| Hypertension | (+) vs. (−) | 0.001 | 0.088 | 0.99 | 0.001 |
| Ever-smoking | (+) vs. (−) | 0.001 | 0.091 | 0.99 | 0.001 |
| Mean IMT (n = 281) | |||||
| Sex | Male vs. female | -0.002 | 0.044 | 0.96 | -0.004 |
| Age | Per 10 years | 0.009 | 0.014 | 0.52 | 0.044 |
| LDL cholesterol† | Per 10 mg/dL | -0.003 | 0.005 | 0.57 | -0.034 |
| HDL cholesterol† | Per 10 mg/dL | -0.017 | 0.014 | 0.23 | -0.048 |
| CAD | (+) vs. (−) | 0.011 | 0.049 | 0.83 | 0.015 |
| Hypertension | (+) vs. (−) | 0.017 | 0.045 | 0.71 | 0.027 |
| Ever-smoking | (+) vs. (−) | -0.015 | 0.045 | 0.73 | -0.024 |
| Achilles tendon thickness (n = 187) | |||||
| Sex | Male vs. female | 0.118 | 0.299 | 0.69 | 0.034 |
| Age | Per 10 years | 0.035 | 0.100 | 0.72 | 0.029 |
| LDL cholesterol† | Per 10 mg/dL | 0.036 | 0.040 | 0.37 | 0.070 |
| HDL cholesterol† | Per 10 mg/dL | 0.166 | 0.125 | 0.19 | 0.078 |
| CAD | (+) vs. (−) | 0.238 | 0.406 | 0.56 | 0.062 |
| Hypertension | (+) vs. (−) | 0.372 | 0.332 | 0.26 | 0.100 |
| Ever-smoking | (+) vs. (−) | 0.061 | 0.322 | 0.85 | 0.016 |
*Regression coefficient divided by standard deviation of the parameter of study.
†Change from baseline at 2 years of follow-up.
Supplementary Table 2. Multiple regression analyses on changes in maximal and mean intima-media thickness (IMT) and Achilles tendon thickness at 4 years of follow-up in relation to lipid and clinical parameters.
| Parameter | Unit in change | Regression coefficient | Robust SE | P-value | Standardized regression coefficient* |
|---|---|---|---|---|---|
| Maximal IMT (n = 174) | |||||
| Sex | Male vs. female | 0.193 | 0.113 | 0.09 | 0.140 |
| Age | Per 10 years | 0.034 | 0.042 | 0.42 | 0.070 |
| LDL cholesterol† | Per 10 mg/dL | -0.035 | 0.020 | 0.08 | -0.155 |
| HDL cholesterol† | Per 10 mg/dL | -0.048 | 0.047 | 0.30 | -0.064 |
| CAD | (+) vs. (−) | -0.164 | 0.144 | 0.26 | -0.110 |
| Hypertension | (+) vs. (−) | 0.066 | 0.135 | 0.63 | 0.045 |
| Ever-smoking | (+) vs. (−) | -0.110 | 0.125 | 0.38 | -0.075 |
| Mean IMT (n = 161) | |||||
| Sex | Male vs. female | 0.017 | 0.076 | 0.83 | 0.024 |
| Age | Per 10 years | 0.049 | 0.020 | 0.02 | 0.200 |
| LDL cholesterol† | Per 10 mg/dL | -0.007 | 0.010 | 0.47 | -0.063 |
| HDL cholesterol† | Per 10 mg/dL | 0.008 | 0.025 | 0.74 | 0.021 |
| CAD | (+) vs. (−) | -0.006 | 0.083 | 0.94 | -0.008 |
| Hypertension | (+) vs. (−) | 0.143 | 0.084 | 0.09 | 0.195 |
| Ever-smoking | (+) vs. (−) | -0.052 | 0.071 | 0.47 | -0.072 |
| Achilles tendon thickness (n = 64) | |||||
| Sex | Male vs. female | -0.286 | 0.404 | 0.48 | -0.091 |
| Age | Per 10 years | -0.116 | 0.196 | 0.56 | -0.094 |
| LDL cholesterol† | Per 10 mg/dL | 0.019 | 0.088 | 0.83 | 0.030 |
| HDL cholesterol† | Per 10 mg/dL | 0.047 | 0.220 | 0.83 | 0.029 |
| CAD | (+) vs. (−) | 0.293 | 0.556 | 0.60 | 0.088 |
| Hypertension | (+) vs. (−) | 0.414 | 0.555 | 0.46 | 0.109 |
| Ever-smoking | (+) vs. (−) | 0.391 | 0.468 | 0.41 | 0.112 |
*Regression coefficient divided by standard deviation of the parameter of study.
†Change from baseline at 4 years of follow-up.
Discussion
This is the first study to determine the factors associated with the severity of carotid atherosclerosis as well as Achilles tendon thickness and to investigate the longitudinal changes in these indices in the real-world FH practice. Besides our original report of the FAME study 18) , another prospective observational study has reported data on lipid levels, medication use, and cardiovascular events, but not carotid IMT nor Achilles tendon thickness, at baseline and last follow-up in 339 Canadian heterozygous FH patients 19) . In Japan, Achilles tendon thickness has been quantified using X-ray. Therefore, one of the strengths of our study is that we were able to use Achilles tendon thickness as an objective parameter to clarify the relationship with atherosclerosis and its risk factors, as well as the changes over time.
It is noteworthy that LDL-C levels under lipid-lowering treatment were positively associated with both IMT and Achilles tendon thickness in multiple regression analyses ( Tables 3 and 4 ) . These findings suggest that strict control of LDL-C levels is important even though the analyses were not adjusted for the effects of untreated LDL-C levels and untreated duration. In addition, the progression of carotid atherosclerosis in FH has been reported to be faster than that in non-FH, and LDL-C accumulation levels may be related to the severity of carotid atherosclerosis 7 , 20) . Actually, it has been reported that lipid-lowering treatment with statins suppresses the progression of carotid atherosclerosis in Japanese non-FH patients 21 , 22) . Therefore, early diagnosis and strict lipid management are important for the treatment of FH. However, several studies, including our original report of the FAME Study 18) , have demonstrated inadequate control of LDL-C in FH patients 23 - 25) . It is true that many FH patients do not reach their target LDL-C levels even with aggressive lipid-lowering therapy. We still need to discuss and resolve clinical inertia in FH care.
Another interesting finding in the current study was that HDL-C levels under lipid-lowering treatment were negatively associated with both IMT and Achilles tendon thickness ( Tables 3 and 4 ) . However, it should be noted that probucol affected the associations of HDL-C with maximal and mean IMT. In the analyses excluding patients taking probucol, the negative association between HDL-C level and maximal IMT almost disappeared ( Table 4 ) . While probucol markedly lowers HDL-C levels, probucol tends to be prescribed to patients with more severe atherosclerotic lesions as it is expected to regress atherosclerosis 26 , 27) as well as skin and Achilles tendon xanthomas 28) . It was considered that the association between HDL-C and IMT may have been more pronounced in the analysis including patients taking probucol probably because probucol was also used in high-risk patients in this study. In fact, HDL-C was 18 mg/dL lower in probucol users than in nonusers after adjustment for sex and age (P<10−27). Probucol users had 0.44 mm greater maximal IMT (P<10−5) and 0.18 mm greater mean IMT (P<10−5) in terms of sex-and age-adjusted mean while there was no material association between probucol use and Achilles tendon thickness (P=0.41).
The inverse association between plasma HDL-C levels and IMT has been reported in previous studies 29 , 30) , and the results of the present study support them. Since this negative association has been reported even in populations without high LDL-C levels 31) , there may be a mechanism independent of the effect of high LDL-C levels on IMT. Similarly, the negative association between plasma HDL-C levels and Achilles tendon thickness is consistent with a previous report 30) . Oosterveer et al. reported that gene variants of reverse cholesterol transport pathway as well as LDL oxidation pathway were associated with the presence of xanthomas in FH patients 32) . In the future, it may be necessary to clarify which of the functions of HDL particles, such as reverse cholesterol transport, antioxidant capacity and anti-inflammatory effects, are responsible for the attenuation of Achilles tendon thickening in FH patients. A previous study reported the association between cholesterol efflux capacity and atherosclerotic cardiovascular diseases in patients with FH 30) .
It is interesting to note that Achilles tendon thickness was higher in the group of patients with a history of smoking ( Tables 3 and 4 ) . A previous cross-sectional study has also reported that smoking history is associated with increased Achilles tendon thickness 16) . Artieda et al. 33) reported that tendon xanthoma formation was associated with higher intracellular lipid content, and a stronger inflammatory response of macrophages to oxidized LDL. Additionally, oxidatively modified LDL epitopes are immunochemically detected intracellularly in macrophages of tendon xanthomas from FH patients 34) . Accordingly, we speculate that oxidatively modified LDL 35) and decreased HDL functionalities 36) caused by smoking play significant roles in the formation of Achilles tendon xanthomas. Based upon our data, it goes without saying that smoking cessation is extremely important in FH practice. Whether smoking cessation suppresses the growth of Achilles tendon xanthomas or not is an issue for future studies.
Maximal IMT was significantly greater in patients with CAD than in those without, but the difference in mean IMT was not significant ( Tables 3 and 4 ) . In patients with type 2 diabetes, maximal IMT has been reported to be more important in relation to the presence of CAD compared to mean IMT 37 , 38) . The results of the present study show that maximal IMT is also more strongly associated with CAD than mean IMT in FH patients. Furthermore, Achilles tendon was significantly thicker in patients with CAD than in those without ( Tables 3 and 4 ) . Achilles tendon thickness seemed to be more strongly related to preexisting CAD than carotid IMT as indicated by standardized regression coefficients. A previous study has also reported that ultrasonographic Achilles tendon thickness was associated with carotid IMT and previous history of CAD in FH patients, but not in non-FH patients 16) . Achilles tendon thickness was at most modestly correlated with maximal IMT (r=0.30) and mean IMT (r=0.31) while the IMT parameters were strongly correlated with each other (r=0.64). Achilles tendon thickness may have a mechanistic implication different from the IMT parameters in atherosclerosis. Since it has been reported that FH patients with xanthomas have a higher cardiovascular risk than those without 39 , 40) , there may be a common mechanism for coronary atherosclerosis and xanthoma formation.
Hypertension was associated with higher values of maximal IMT, but the difference between patients with hypertension and those without was almost negligible for mean IMT ( Tables 3 and 4 ) . A large prospective follow-up study in Japan reported that maximal IMT was associated with the incidence of stroke 41) . Since hypertension is the greatest risk factor for stroke, it is presumed that there is a strong association between hypertension and maximal IMT even in FH patients. These findings suggest the importance of comprehensive risk management including blood pressure control in the treatment of FH patients. Diabetes mellitus was not associated with carotid IMT in this study. It seems that dyslipidemia and hypertension are more important factors for carotid atherosclerosis than hyperglycemia in FH patients.
There have been few reports regarding the longitudinal changes of IMT and Achilles tendon thickness in patients with heterozygous FH. The percent changes in maximal IMT and mean IMT gradually increased during the observation period. In contrast, the percent change in the thickness of Achilles tendon became progressively thinner throughout the observation period ( Supplementary Fig.2 ) as reported in previous studies 10 , 42) . We speculate that this difference is due to the fact that age and lipid levels are the only factors that contribute to Achilles tendon thickness, while other risk factors for atherosclerosis, such as blood pressure, are also involved in the development of carotid atherosclerosis. In addition, the LDL-C levels at 2 years and at 4 years did not change from the value at baseline as our original report of the FAME study reported 18) . In the current study, means of LDL-C at 2 and 4 years of follow-up were both 133 mg/dL (SD 41 mg/dL). Since aggressive lipid-lowering therapy has been reported to regress carotid atherosclerosis 42) , we believe that the control of LDL-C levels may still be inadequate in the real-world FH practices. Furthermore, it is assumed that there are differences in the mechanisms of lipid efflux or regression between the Achilles tendon xanthoma and carotid atherosclerotic plaque.
There are several limitations in the present study. The diagnosis of FH was based on the scoring system of the Annual Report of the Research Committee on Primary Hyperlipidemia of the Ministry of Health and Welfare of Japan reported by Harada-Shiba et al. 43) . Therefore, there may be differences in FH diagnosis and clinical indices when compared to the guidelines we currently use 13) . Additionally, the measurement of carotid IMT was performed manually in each participating institution based on the guidelines published by the Japan Academy of Neurosonology in 2006 44) . Therefore, variations due to differences in measurement devices and examiners, as well as misalignment of measurement positions at the follow-up, may have existed. Furthermore, the analyses were unable to take into account the effects of untreated LDL-C levels and untreated duration. Finally, in the longitudinal analysis, the number of subjects for whom data were available decreased markedly with advancing years, and we were not able to follow up the same subjects.
Conclusion
We found a positive association between LDL-C levels and severity of carotid atherosclerosis as well as a negative association between HDL-C levels and carotid IMT even in heterozygous FH patients under treatment. Since there is still no clear evidence that drug therapy to increase HDL-C levels reduces the risk of CAD 45) , this observation suggests the insufficiency of LDL-C lowering therapy in the real-world FH practice. Guidelines have clearly stated control targets for LDL-C levels 13) , and we have powerful lipid-lowering drugs today. One possible reason for the insufficiency of lipid-lowering therapy may be the presence of clinical inertia among clinicians treating FH patients. Therefore, the undertreatment of FH patients is an unresolved issue comparable to its underdiagnosis.
Study Organization and Their Roles
Principal Investigator:
Shizuya Yamashita (Professor, Department of Community Medicine, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, Osaka, Japan) (Present address: Department of Cardiology, Rinku General Medical Center, Izumisano, Osaka, Japan)
Executive Committee:
Hidenori Arai (Department of Human Health Sciences, Kyoto University Graduate School of Medicine, Kyoto; present address: The National Center for Geriatrics and Gerontology, Obu, Aichi, Japan)
Hideaki Bujo (Department of Clinical Laboratory and Experimental Research Medicine, Toho University Sakura Medical Center, Sakura, Chiba, Japan)
Hiroyuki Daida (Department of Cardiovascular Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan)
Mariko Harada-Shiba (Department of Molecular Innovation in Lipidology, National Cerebral and Cardiovascular Center Research Institute, Suita, Osaka; present address: Department of Molecular Pathogenesis, National Cerebral and Cardiovascular Center Research Institute, Suita, Osaka, Japan)
Shun Ishibashi (Division of Endocrinology and Metabolism, Department of Medicine, Jichi Medical University, Shimotsuke, Tochigi, Japan)
Nobuhiko Koga (Department of Cardiology, Cardiovascular Center, Shin-Koga Hospital, Kurume, Japan)
Shinichi Oikawa (Department of Endocrinology, Diabetes and Metabolism, Graduate School of Medicine, Nippon Medical School, Tokyo; present address: Diabetes and Lifestyle Disease Center, Fukujyuji Hospital, Kiyose, Tokyo, Japan)
Shizuya Yamashita (Professor, Department of Community Medicine, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, Osaka, Japan) (Present address: Department of Cardiology, Rinku General Medical Center, Izumisano, Osaka, Japan)
Project Director:
Daisaku Masuda (Assistant Professor, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, Osaka, Japan; Present address: Department of Cardiology, Rinku General Medical Center, Izumisano, Osaka, Japan)
Data Center and Analysis:
Suminori Kono (Professor, Department of Preventive Medicine, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; present address: MedStat Corporation, Fukuoka)
Efficacy and Safety Evaluation Committee:
Yuichi Ishikawa (Professor, Kobe University Graduate School of Health Sciences, Kobe, Japan)
Shinsuke Nanto (Professor, Department of Advanced Cardiovascular Therapeutics, Osaka University Graduate School of Medicine, Suita, Japan)
Yoshiyuki Nagai (Vice-director, Rinku General Medical Center, Izumisano, Osaka, Japan)
Participating Faculties:
The names of institutions of participating faculties are listed as those at the time of the initiation of the current study. Tetsuji Miura (Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine, Sapporo, Hokkaido), Masahiro Tsuji (Health Sciences University of Hokkaido Hospital, Sapporo, Hokkaido), Ichiro Sakuma (Cardiovascular Medicine, Hokko Memorial Clinic, Sapporo, Hokkaido), Yoshio Kurihara (Kurihara Clinic, Sapporo, Hokkaido), Shigeo Nakajima (Nakajima Cardiovascular Mental Clinic, Hakodate, Hokkaido), Naoki Tamasawa (Department of Endocrinology and Metabolism, Hirosaki University Graduate School of Medicine, Hirosaki, Aomori), Masahiko Igarashi (Internal Medicine, Miyukikai Hospital, Kaminoyamashi, Yamagata), Yashushi Ishigaki (Division of Molecular Metabolism and Diabetes, Tohoku University Graduate School of Medicine, Sendai, Miyagi), Hidetoshi Kotake (Tsurugaya Clinic, Sendai, Miyagi), Hiroaki Sato (Third Department of Internal Medicine, Fukushima Medical University, Fukushima, Fukushima), Toshiyuki Ishibashi (Department of Cardiovascular Medicine, Ohara General Hospital Medical Center, Fukushima, Fukushima), Hitoshi Shimano (Department of Internal Medicine (Endocrinology and Metabolism), Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki), Kunihiro Suzuki (Department of Endocrinology and Metabolism, Dokkyo Medical University Hospital, Shimotsugagun, Tochigi), Katsunori Ikewaki (Division of Anti-aging and Vascular Medicine, National Defense Medical College, Tokorozawa, Saitama), Jun Tashiro (Department of Internal Medicine, Matsudo Municipal Hospital, Matsudo, Chiba), Koji Shirai (Department of Internal Medicine, Sakura Hospital, Toho University, Sakura, Chiba), Masaki Shinomiya (Nishifuna Clinic, Funabashi, Chiba), Kazuhisa Tsukamoto (Department of Metabolism, Diabetes and Nephrology, Aizu Medical Center, Fukushima Medical University, Fukushima), Jun-ichi Osuga (Division of Endocrinology and Metabolism, Department of Medicine, Jichi Medical University, Shimotsuke, Tochigi), Ikuo Inoue (Department of Endocrinology and Diabetes, School of Medicine, Saitama Medical University), Shinichi Momomura (Division of Cardiovascular Medicine, Saitama Medical Center, Jichi Medical University, Saitama, Saitama), Hiroaki Okazaki (Department of Diabetes and Metabolic Diseases, Graduate School of Medicine, The University of Tokyo, Tokyo), Masayuki Yoshida (Division of Medical Genetics, Medical Hospital of Tokyo Medical and Dental University, Bunkyo-ku, Tokyo), Hiroyuki Daida (Department of Cardiovascular Medicine, Juntendo University School of Medicine, Bunkyo-ku, Tokyo), Kazunori Shimada (Department of Cardiovascular Medicine, Juntendo University School of Medicine, Bunkyo-ku, Tokyo), Takashi Miida (Department of Laboratory Medicine, Juntendo University Graduate School of Medicine, Bunkyo-ku, Tokyo), Tomoo Okada (Department of Pediatrics and Child Health, Nihon University School of Medicine, Itabashi-ku, Tokyo), Takafumi Hiro (Division of Cardiology, Department of Medicine, Nihon University School of Medicine, Itabashi-ku, Tokyo), Gen Yoshino (Division of Diabetes, Metabolism and Endocrinology, Department of Internal Medicine, Toho University Omori Medical Center, Ota-ku, Tokyo), Tsutomu Hirano (Department of Medicine, Division of Diabetes, Metabolism, and Endocrinology, Showa University School of Medicine, Shinagawa-ku, Tokyo), Shinji Koba (Division of Cardiology, Department of Medicine, Showa University School of Medicine, Shinagawa-ku, Tokyo), Nobuyoshi Hirose (Center for Supercentenarian Medical Research, Keio University School of Medicine, Shinjuku-ku, Tokyo), Makoto Kinoshita (Department of Internal Medicine, Teikyo University School of Medicine, Itabashi-ku, Tokyo), Mitsuo Ohni (Department of Geriatric Medicine, Kyorin University School of Medicine, Mitaka, Tokyo), Noriaki Nakaya (Nakaya Clinic, Nakano-ku, Tokyo), Yuichi Nakamura (Department of Cardiovascular Medicine, Nagaoka Chuo General Hospital Medical Center, Nagaoka, Niigata), Masako Waki (Department of Internal Medicine, Shizuoka City Shizuoka Hospital, Shizuoka), Shinji Yokoyama (Department of Biochemistry, Nagoya City University, Nagoya), Toshio Hayashi (School of Health Sciences, Nagoya University Graduate School of Medicine, Nagoya), Koji Kajinami (Department of Cardiology, Kanazawa Medical University, Kahoku-gun, Ishikawa), Sadao Takahashi (Third Department of Internal Medicine, Faculty of Medical Sciences, University of Fukui, Eiheiji-cho, Fukui), Tatsuaki Murakami (Division of Cardiovascular Medicine, Fukui Cardiovascular Center, Fukui), Hiroshi Maegawa (Division of Diabetology, Endocrinology and Department of Medicine, and Nephrology, Shiga University of Medical Science, Otsu, Shiga), Koichi Ikenoue (Ikenoue Clinic, Otsu, Shiga), Masayuki Yokode (Department of Clinical Innovative Medicine, Kyoto University Graduate School of Medicine, Kyoto, Kyoto), Tohru Funahashi (Department of Metabolic Medicine, Suita, Osaka), Shinji Kihara (Department of Biomedical Informatics, Division of Health Sciences, Osaka University Graduate School of Medicine, Suita, Osaka), Tetsuo Shoji (Department of Vascular Medicine, Osaka City University Graduate School of Medicine, Osaka, Osaka), Koji Yanagi (Kenporen Osaka Central Hospital, Osaka, Osaka), Yukihiko Ueda (Hirakatakohsai Hospital, Hirakata, Osaka), Hideki Hidaka (Medical and Health Care Center, Sanyo Electric Group Health Insurance Association, Moriguchi, Osaka), Masaharu Kubo (Kubo Clinic, Suita, Osaka), Masakazu Menju (Menju Clinic, Osaka), Akira Ohno (Division of Diabetes and Endocrinology, Department of Internal Medicine, Rinku General Medical Center, Izumisano, Osaka), Tatsuro Ishida (Division of Cardiovascular Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Hyogo), Gen Yoshino (Shinzuma General Hospital, Kobe, Hyogo), Tetsuya Kawashima (Jinkeikai Ishii Hospital, Akashi, Hyogo), Kenji Kaihotsu (Division of Cardiology, Department of Internal Medicine, Kakogawa East City Hospital, Kakogawa, Hyogo), Kazufumi Nakamura (Department of Cardiovascular Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Okayama), Kohki Takada (Hishokai Kanda Clinic, Hiroshima, Hiroshima), Tetsuji Shingu (Yamasaki Hospital, Hiroshima, Hiroshima), Genshi Egusa (Egusa Genshi Clinic, Hiroshima, Hiroshima), Seiji Umemoto (Center for Clinical Research, Yamaguchi University Hospital, Ube, Yamaguchi), Yoshitaka Kumon (Department of Laboratory Medicine, Kochi Medical School, Kochi, Kochi), Tadashi Suehiro (Department of Diabetes, Kochi Takasu Hospital, Kochi, Kochi), Hiromi Tasaki (Department of Cardiovascular Medicine, Kitakyushu Municipal Yahata Hospital, Kitakyushu, Fukuoka), Nobuhiko Koga (Department of Cardiology, Shin-Koga Hospital, Kurume, Fukuoka), Jun Sasaki* (International University of Health and Welfare, Graduate School of Pharmaceutical Medicine, Fukuoka, Fukuoka), Kimitoshi Nakamura (Department of Pediatrics, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Kumamoto), Akira Matsunaga (Department of Laboratory Medicine, Faculty of Medicine, Fukuoka University, Fukuoka, Fukuoka), Mieko Takada (Takada-chuo Hospital, Takada, Oita), Masaaki Miyata (Department of Cardiovascular Medicine and Hypertension, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Kagoshima), Sadatoshi Biro (Tsukasa Health Care Hospital, Kagoshima, Kagoshima), Mitsuo Shimabukuro (The Second Department of Internal Medicine, University of the Ryukyus, School of Medicine, Nishihara-cho, Okinawa), Yusuke Ohya (The Third Department of Internal Medicine, University of the Ryukyus, School of Medicine, Nishihara-cho, Okinawa).
*Deceased.
Acknowledgement
The authors are grateful to Dr. Suminori Kono (MedStat Corporation, Fukuoka), Professor Emeritus at Kyushu University, for his technical support in data management and statistical analysis. La Neuvelle Place (Tokyo) supported the registration of patients.
Conflict of Interest
Hidenori Arai has received honoraria from Sanofi, Daiichi-Sankyo Co., Ltd., MSD K.K., Kowa Pharmaceutical Co., Ltd., and Pfizer Co., Ltd. Hideaki Bujo has nothing to disclose. Hiroyuki Daida has received honoraria from Amgen Inc., Daiichi-Sankyo Co., Ltd., Kowa Pharmaceutical Co., Ltd., and MSD K.K., and received clinical research funding from Canon Medical Systems Corporation, Philips Japan, Ltd., Toho Holdings Co., Ltd., Asahi Kasei Corporation, and Inter Reha Co., Ltd. HD has also received scholarship grants from Nippon Boehringer Ingelheim Co., Ltd., Otsuka Pharmaceutical Company, Ltd., Sanofi K.K., MSD K.K., Daiichi-Sankyo Co., Ltd., Pfizer Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Teijin Pharma, Ltd., Shionogi & Co., Ltd., Actelion Pharmaceuticals, Ltd., Actelion Ltd., Kowa Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd. HD has also courses endowed by companies, including Philips Japan, Ltd., ResMed, Fukuda Denshi Co., Ltd., and Paramount Bed Co., Ltd. Mariko Harada-Shiba has received stock holdings or options from Liid Pharma, honoraria from Amgen Inc., Astellas Pharma Inc., Sanofi K.K., and scholarship grants from Aegerion Pharmaceuticals, Inc., Recordati Rare Diseases Japan, and Kaneka Cooperation. Shun Ishibashi has received honoraria from Kowa Pharmaceutical Co., Ltd., and a scholarship grant from Ono Pharmaceutical Co., Ltd. Nobuhiko Koga has nothing to disclose. Daisaku Masuda has received clinical research funding from MSD K.K., Takeda Pharmaceutical Co., Ltd., Daiichi-Sankyo Co., Ltd., Kowa Company, Ltd., Otsuka Pharmaceutical Co., Ltd., and scholarship grants from Skylight Biotec, Inc., Pfizer Japan Inc., Amgen Astellas Biopharma K.K., and Sanofi K.K. Masatsune Ogura has received honoraria from Amgen and Astellas Pharma Inc. Shinichi Oikawa has nothing to disclose. Shizuya Yamashita has received honoraria from Amgen Astellas BioPharma K.K., Kowa Pharmaceutical Co. Ltd., Sanofi K.K., MSD K.K., Bayer Yakuhin, Ltd., clinical research fundings from Ono Pharmaceutical Co., Ltd., Hitachi Chemical Diagnostics Systems Co., Ltd., Takeda Pharmaceutical Company Ltd, Mitsubishi Tanabe Pharma Corporation, Rohto Pharmaceutical Co., Ltd., Astellas Pharma Inc., Nippon Boehringer Ingelheim Co., Ltd., MSD K.K., Bayer Yakuhin, Ltd., scholarship grants from Astellas Pharma Inc., Nippon Boehringer Ingelheim Co., Ltd., MSD K.K., Bayer Yakuhin, Ltd., and Courses endowed by Izumisano City.
References
- 1).Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, Wierzbicki AS: Familial hypercholesterolaemia. Nat Rev Dis Primers, 2017; 3: 17093 [DOI] [PubMed] [Google Scholar]
- 2).Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, Nakanishi C, Mori M, Yamagishi M, Inazu A, Koizumi J, Hokuriku FHSG: Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis, 2011; 214: 404-407 [DOI] [PubMed] [Google Scholar]
- 3).Ohmura H, Fukushima Y, Mizuno A, Niwa K, Kobayashi Y, Ebina T, Kimura K, Ishibashi S, Daida H, Research Committee on Primary Hyperlipidemia of the Ministry of Health and Welfare of Japan: Estimated prevalence of heterozygous familial hypercholesterolemia in patients with acute coronary syndrome. Int Heart J, 2017; 58: 88-94 [DOI] [PubMed] [Google Scholar]
- 4).Harada-Shiba M, Ako J, Arai H, Hirayama A, Murakami Y, Nohara A, Ozaki A, Uno K, Nakamura M: Prevalence of familial hypercholesterolemia in patients with acute coronary syndrome in Japan: Results of the EXPLORE-J study. Atherosclerosis, 2018; 277: 362-368 [DOI] [PubMed] [Google Scholar]
- 5).Wendelhag I, Wiklund O, Wikstrand J: Arterial wall thickness in familial hypercholesterolemia. Ultrasound measurement of intima-media thickness in the common carotid artery. Arterioscler Thromb, 1992; 12: 70-77 [DOI] [PubMed] [Google Scholar]
- 6).O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr: Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med, 1999; 340: 14-22 [DOI] [PubMed] [Google Scholar]
- 7).Wiegman A, de Groot E, Hutten BA, Rodenburg J, Gort J, Bakker HD, Sijbrands EJ, Kastelein JJ: Arterial intima-media thickness in children heterozygous for familial hypercholesterolaemia. Lancet, 2004; 363: 369-370 [DOI] [PubMed] [Google Scholar]
- 8).Rodenburg J, Vissers MN, Wiegman A, van Trotsenburg AS, van der Graaf A, de Groot E, Wijburg FA, Kastelein JJ, Hutten BA: Statin treatment in children with familial hypercholesterolemia: the younger, the better. Circulation, 2007; 116: 664-668 [DOI] [PubMed] [Google Scholar]
- 9).van Wissen S, Smilde TJ, Trip MD, Stalenhoef AF, Kastelein JJ: Long-term safety and efficacy of high-dose atorvastatin treatment in patients with familial hypercholesterolemia. Am J Cardiol, 2005; 95: 264-266 [DOI] [PubMed] [Google Scholar]
- 10).Tsouli SG, Xydis V, Argyropoulou MI, Tselepis AD, Elisaf M, Kiortsis DN: Regression of Achilles tendon thickness after statin treatment in patients with familial hypercholesterolemia: an ultrasonographic study. Atherosclerosis, 2009; 205: 151-155 [DOI] [PubMed] [Google Scholar]
- 11).Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg-Hansen A: Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. Eur Heart J, 2013; 34: 3478-3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ, 1991; 303: 893-896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K, Working Group by Japan Atherosclerosis Society for Making Guidance of Familial Hypercholesterolemia: Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Mabuchi H, Ito S, Haba T, Ueda K, Ueda R: Discrimination of familial hypercholesterolemia and secondary hypercholesterolemia by Achilles' tendon thickness. Atherosclerosis, 1977; 28: 61-68 [DOI] [PubMed] [Google Scholar]
- 15).Tsouli SG, Kiortsis DN, Argyropoulou MI, Mikhailidis DP, Elisaf MS: Pathogenesis, detection and treatment of Achilles tendon xanthomas. Eur J Clin Invest, 2005; 35: 236-244 [DOI] [PubMed] [Google Scholar]
- 16).Michikura M, Ogura M, Yamamoto M, Sekimoto M, Fuke C, Hori M, Arai K, Kihara S, Hosoda K, Yanagi K, Harada-Shiba M: Achilles tendon ultrasonography for diagnosis of familial hypercholesterolemia among Japanese subjects. Circ J, 2017; 81: 1879-1885 [DOI] [PubMed] [Google Scholar]
- 17).Sugisawa T, Okamura T, Makino H, Watanabe M, Kishimoto I, Miyamoto Y, Iwamoto N, Yamamoto A, Yokoyama S, Harada-Shiba M: Defining patients at extremely high risk for coronary artery disease in heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2012; 19: 369-375 [DOI] [PubMed] [Google Scholar]
- 18).Yamashita S, Masuda D, Harada-Shiba M, Arai H, Bujo H, Ishibashi S, Daida H, Koga N, Oikawa S, on Behalf of the FAME Study Group: Effectiveness and safety of lipid-lowering drug treatments in Japanese patients with familial hypercholesterolemia: Familial Hypercholesterolemia Expert Forum (FAME) Study. J Atheroscler Thromb, 2022; 29: 608-638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Brunham LR, Cermakova L, Lee T, Priecelova I, Alloul K, de Chantal M, Francis GA, Frohlich J: Contemporary trends in the management and outcomes of patients with familial hypercholesterolemia in Canada: a prospective observational study. Can J Cardiol, 2017; 33: 385-392 [DOI] [PubMed] [Google Scholar]
- 20).de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, Kastelein JP: Measurement of arterial thickness as a surrogate marker for atherosclerosis. Circulation, 2004; 109: III33-38 [DOI] [PubMed] [Google Scholar]
- 21).Nohara R, Daida H, Hata M, Kaku K, Kawamori R, Kishimoto J, Kurabayashi M, Masuda I, Sakuma I, Yamazaki T, Yokoi H, Yoshida M: Effect of intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness in Japanese patients – Justification for Atherosclerosis Regression Treatment (JART) Study –. Circ J, 2012; 76: 221-229 [DOI] [PubMed] [Google Scholar]
- 22).Koga M, Toyoda K, Minematsu K, Yasaka M, Nagai Y, Aoki S, Nezu T, Hosomi N, Kaginuma T, Origasa H, Kamiyama K, Suzuki R, Ohtsuki T, Maruyama H, Kitagawa K, Uchiyama S, Matsumoto M: Long term effect of pravastatin on carotid intima-media thickness, The J-STARS Echo Study (Japan Statin Treatment against Recurrent Stroke). Stroke, 2018; 49: 107-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Pijlman AH, Huijgen R, Verhagen SN, Imholz BP, Liem AH, Kastelein JJ, Abbink EJ, Stalenhoef AF, Visseren FL: Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in the Netherlands. Atherosclerosis, 2010; 209: 189-194 [DOI] [PubMed] [Google Scholar]
- 24).Ogura M: PCSK9 inhibition in the management of familial hypercholesterolemia. J Cardiol, 2017; 71: 1-7 [DOI] [PubMed] [Google Scholar]
- 25).Perez de Isla L, Alonso R, Watts GF, Mata N, Saltijeral Cerezo A, Muniz O, Fuentes F, Diaz-Diaz JL, de Andres R, Zambon D, Rubio-Marin P, Barba-Romero MA, Saenz P, Sanchez Munoz-Torrero JF, Martinez-Faedo C, Miramontes-Gonzalez JP, Badimon L, Mata P, SAFEHEART Investigators: Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART Registry follow-up. J Am Coll Cardiol, 2016; 67: 1278-1285 [DOI] [PubMed] [Google Scholar]
- 26).Yamashita S, Bujo H, Arai H, Harada-Shiba M, Matsui S, Fukushima M, Saito Y, Kita T, Matsuzawa Y: Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb, 2008; 15: 292-303 [DOI] [PubMed] [Google Scholar]
- 27).Yamashita S, Arai H, Bujo H, Masuda D, Ohama T, Ishibashi T, Yanagi K, Doi Y, Nakagawa S, Yamashiro K, Tanabe K, Kita T, Matsuzaki M, Saito Y, Fukushima M, Matsuzawa Y, PROSPECTIVE Study Group: Probucol trial for secondary prevention of atherosclerotic events in patients with coronary heart disease (PROSPECTIVE). J Atheroscler Thromb, 2021; 28: 103-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Yamamoto A, Matsuzawa Y, Yokoyama S, Funahashi T, Yamamura T, Kishino B: Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol, 1986; 57: 29H-35H [DOI] [PubMed] [Google Scholar]
- 29).Junyent M, Cofan M, Nunez I, Gilabert R, Zambon D, Ros E: Influence of HDL cholesterol on preclinical carotid atherosclerosis in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol, 2006; 26: 1107-1113 [DOI] [PubMed] [Google Scholar]
- 30).Ogura M, Hori M, Harada-Shiba M: Association between cholesterol efflux capacity and atherosclerotic cardiovascular disease in patients with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol, 2016; 36: 181-188 [DOI] [PubMed] [Google Scholar]
- 31).Wilt TJ, Rubins HB, Robins SJ, Riley WA, Collins D, Elam M, Rutan G, Anderson JW: Carotid atherosclerosis in men with low levels of HDL cholesterol. Stroke, 1997; 28: 1919-1925 [DOI] [PubMed] [Google Scholar]
- 32).Oosterveer DM, Versmissen J, Yazdanpanah M, Defesche JC, Kastelein JJ, Sijbrands EJ: The risk of tendon xanthomas in familial hypercholesterolaemia is influenced by variation in genes of the reverse cholesterol transport pathway and the low-density lipoprotein oxidation pathway. Eur Heart J, 31: 1007-1012 [DOI] [PubMed] [Google Scholar]
- 33).Artieda M, Cenarro A, Junquera C, Lasierra P, Martinez-Lorenzo MJ, Pocovi M, Civeira F: Tendon xanthomas in familial hypercholesterolemia are associated with a differential inflammatory response of macrophages to oxidized LDL. FEBS Lett, 2005; 579: 4503-4512 [DOI] [PubMed] [Google Scholar]
- 34).Sugiyama N, Marcovina S, Gown AM, Seftel H, Joffe B, Chait A: Immunohistochemical distribution of lipoprotein epitopes in xanthomata from patients with familial hypercholesterolemia. Am J Pathol, 1992; 141: 99-106 [PMC free article] [PubMed] [Google Scholar]
- 35).Scheffler E, Wiest E, Woehrle J, Otto I, Schulz I, Huber L, Ziegler R, Dresel HA: Smoking influences the atherogenic potential of low-density lipoprotein. Clin Investig, 1992; 70: 263-268 [DOI] [PubMed] [Google Scholar]
- 36).Takata K, Imaizumi S, Kawachi E, Suematsu Y, Shimizu T, Abe S, Matsuo Y, Tsukahara H, Noda K, Yahiro E, Zhang B, Uehara Y, Miura S, Saku K: Impact of cigarette smoking cessation on high-density lipoprotein functionality. Circ J, 2014; 78: 2955-2962 [DOI] [PubMed] [Google Scholar]
- 37).Irie Y, Katakami N, Kaneto H, Kasami R, Sumitsuji S, Yamasaki K, Tachibana K, Kuroda T, Sakamoto K, Umayahara Y, Ueda Y, Kosugi K, Shimomura I: Maximum carotid intima-media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Atherosclerosis, 2012; 221: 438-444 [DOI] [PubMed] [Google Scholar]
- 38).Fujihara K, Suzuki H, Sato A, Ishizu T, Kodama S, Heianza Y, Saito K, Iwasaki H, Kobayashi K, Yatoh S, Takahashi A, Yahagi N, Sone H, Shimano H: Comparison of the Framingham risk score, UK Prospective Diabetes Study (UKPDS) Risk Engine, Japanese Atherosclerosis Longitudinal Study-Existing Cohorts Combine (JALS-ECC) and maximum carotid intima-media thickness for predicting coronary artery stenosis in patients with asymptomatic type 2 diabetes. J Atheroscler Thromb, 2014; 21: 799-815 [DOI] [PubMed] [Google Scholar]
- 39).Oosterveer DM, Versmissen J, Yazdanpanah M, Hamza TH, Sijbrands EJ: Differences in characteristics and risk of cardiovascular disease in familial hypercholesterolemia patients with and without tendon xanthomas: a systematic review and meta-analysis. Atherosclerosis, 2009; 207: 311-317 [DOI] [PubMed] [Google Scholar]
- 40).Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M: Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J, 2017; 38: 1573-1579 [DOI] [PubMed] [Google Scholar]
- 41).Kitamura A, Iso H, Imano H, Ohira T, Okada T, Sato S, Kiyama M, Tanigawa T, Yamagishi K, Shimamoto T: Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke, 2004; 35: 2788-2794 [DOI] [PubMed] [Google Scholar]
- 42).de Sauvage Nolting PR, de Groot E, Zwinderman AH, Buirma RJ, Trip MD, Kastelein JJ: Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia: treatment with simvastatin. Arch Intern Med, 2003; 163: 1837-1841 [DOI] [PubMed] [Google Scholar]
- 43).Harada-Shiba M: Evaluation of clinical management guidelines for familial hypercholesterolemia. Research Committee of Primary Hyperlipidemia supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Health, Labor and Welfare. Study Report in 2008, 2009: 50-55 (in Japanese) [Google Scholar]
- 44).The Joint Committee of the Japan Academy of Neurosonology and the Japan Society of Embolus Detection and Treatment on Guideline for Neurosonology: Carotid Ultrasound Examination. Neurosonology, 2006; 19: 49-67 (in Japanese) [Google Scholar]
- 45).Keene D, Price C, Shun-Shin MJ, Francis DP: Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ, 2014; 349: g4379 [DOI] [PMC free article] [PubMed] [Google Scholar]


