Abstract
Aims: In this study, we integrated two randomized control trials, PROSPECTIVE and IMPACT, to address the effect of probucol on cerebrocardiovascular events and carotid intima-media thickness (IMT) in Japanese, Korean, and Chinese patients with coronary artery disease (CAD).
Methods: A total of 1,025 patients from the PROSPECTIVE and IMPACT studies were enrolled. The time to the first major adverse cerebrocardiovascular event, in addition to carotid IMT and lipid levels, was compared between the control and probucol groups.
Results: In the integrated analysis, the adjusted hazard ratio (HR) and 95% confidence interval (CI) were 0.67 and 0.44–1.03, respectively, indicating a tendency to show the effect of probucol on cerebrocardiovascular events in secondary prevention. We also found no significant differences between the control and probucol groups in the mean IMT of the carotid arteries and its changes. However, we found a significant decrease in cerebrocardiovascular events in patients with reduced levels of HDL cholesterol (HDL-C) (≥ 6.25 mg/dL) compared with those with levels <6.25 mg/dL (p=0.024), without any increase in adverse events such as severe ventricular arrhythmias.
Conclusion: We demonstrated a marginal effect of probucol on cerebrocardiovascular events in Asian patients with CAD, with reasonable safety profiles. A larger study may be needed to support the effect of probucol for cardiovascular prevention.
Keywords: Atherosclerotic cardiovascular disease, Probucol, Secondary prevention, Integrated analysis
Introduction
Lipid-lowering therapy has been a cornerstone of treatment in both the primary and secondary prevention of atherosclerotic cardiovascular disease. Previous mega-trials testing the effects of statins 1) , ezetimibe 2 , 3) , and PCSK9 inhibitors 4 , 5) have clearly demonstrated that aggressive LDL cholesterol (LDL-C)-lowering therapies significantly reduce the incidence of atherosclerotic cardiovascular events. However, cardiovascular events are not completely prevented even if serum LDL-C levels are lowered to <50 mg/dL. Thus, residual risks, such as high serum triglyceride (TG) and/or low HDL cholesterol (HDL-C) levels, have been the targets of pharmacological interventions 6) . However, studies using fibrates to improve high serum TG and/or low HDL-C levels did not show positive results in inhibiting atherosclerotic cardiovascular events in high-risk patients, such as those with type 2 diabetes mellitus 7 , 8) . However, a meta-analysis of fibrates demonstrated their favorable effect on reducing the coronary event rate, but not mortality 9) . Omega-3 polyunsaturated fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have also been used to reduce serum TG levels. Although the JELIS trial showed the benefit of adding EPA in addition to statins in Japanese patients, study results on omega-3 polyunsaturated fatty acids, such as EPA and DHA, have been controversial 10 - 12) .
The inhibitors of plasma cholesteryl ester transfer protein (CETP), such as torcetrapib, dalcetrapib, evacetrapib, and anacetrapib, were developed with the expectation that they would increase HDL-C and prevent atherosclerosis. These CETP inhibitors were used in addition to statins but failed to reduce cardiovascular events 13 - 15) or attenuate atherosclerosis of the coronary and carotid arteries 16 - 18) (except for anacetrapib 19) ). The REVEAL study 19) , which used anacetrapib in addition to atorvastatin, demonstrated a positive result in reducing cardiovascular events; however, its clinical development was terminated because of its long-term accumulation in adipose tissues 20) . Therefore, additional drug therapies in addition to statins are still needed to mitigate residual risks.
Probucol is a potent antioxidant and was developed for lowering LDL-C levels in patients with hypercholesterolemia, including those with familial hypercholesterolemia (FH) who are deficient in LDL receptor and exhibit xanthomas on the Achilles tendon 21) . Probucol showed a unique benefit in attenuating skin and tendon xanthomas despite a marked reduction in serum HDL-C. In the Probucol Quantitative Regression Swedish Trial (PQRST) 22) , a randomized controlled trial, the administration of probucol for 3 years to patients with hypercholesterolemia lowered LDL-C levels but did not increase the lumen volume of femoral arteries compared with controls as evaluated by angiography. Thereafter, probucol disappeared from the market worldwide except in Japan, probably because of the failure of the PQRST trial, the reduction in serum HDL-C, and possible QT interval prolongation. Nevertheless, probucol has been used by Japanese clinicians, especially lipid specialists.
Previous clinical studies have demonstrated marked beneficial effects of probucol on xanthoma regression, restenosis after percutaneous coronary intervention (PCI), and atherosclerosis, as reviewed by Yamashita et al. 23 - 27) . The retrospective Probucol Observational Study Illuminating Therapeutic Impact on Vascular Events (POSITIVE) study showed that long-term treatment with probucol decreased secondary cardiovascular events in very high-risk patients with heterozygous FH 28) , in which the primary outcome was the time to first cardiovascular event involving hospitalization. Multivariate Cox regression analysis showed that the hazard ratio (HR) of probucol use was 0.13 (95% confidence interval (CI), 0.05–0.34, P<0.001) in patients for secondary prevention, suggesting that long-term probucol treatment prevents secondary cardiovascular events in patients with heterozygous FH. Furthermore, the effects of probucol therapy on long-term survival after complete revascularization with PCI and/or coronary aorta bypass graft were reported in 1,694 patients following a propensity score matching analysis 29) . In addition, probucol administration was associated with a significant decrease in all-cause death in the propensity score-adjusted model (HR, 0.57, p=0.008). In post-matched patients, all-cause mortality was markedly reduced in the probucol group compared with the non-probucol group (HR, 0.45, p=0.002). Cardiac death tended to be lower in the probucol group; however, noncardiac death was significantly reduced in the probucol group compared with the non-probucol group. These data suggest that probucol can significantly reduce all-cause mortality and noncardiac death in patients with coronary artery disease (CAD) following complete revascularization.
Based on the evidence of probucol, we 30) and Kang et al. 31) conducted randomized control trials (PROSPECTIVE and IMPACT) to address the effect of probucol on high-risk patients, i.e., patients with CAD taking statins. Although these studies had a similar but different study design and showed a marginal benefit of probucol, we aimed to conduct an integrated analysis of these two studies to determine the effect of probucol in a larger sample size. This integrated analysis was a prespecified analysis when we planned these clinical studies in a collaborative manner.
To test the hypothesis that the addition of probucol to other lipid-lowering drugs prevents cerebrovascular and cardiovascular events in secondary prevention patients, the PROSPECTIVE study was conducted in Japan 30) , and the IMPACT study was conducted in South Korea and China 31) . In this study, we examined the efficacy and safety of probucol for cardiovascular events in Japan, Korea, and China by integrating the results of cardiovascular events in these study participants.
Subjects and Methods
Integrated Analysis
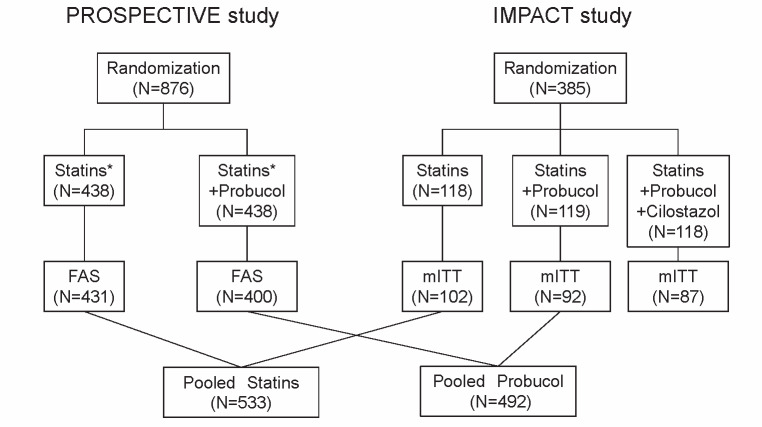
See the Appendix for the details of the PROSPECTIVE and IMPACT studies. In this integrated study, we evaluated the effect of probucol in addition to conventional lipid-lowering therapy in Asian (Chinese, Korean, and Japanese) patients with a history of CAD. Overall, 831 subjects (FAS) from the PROSPECTIVE study and 194 subjects (mITT) from the IMPACT study were enrolled. The conventional LLT group from the PROSPECTIVE study (n=431) and IMPACT study (n=102) were defined as the “control group” (n=533), and the test group from the PROSPECTIVE study (n=400) and the probucol group from the IMPACT study (n=92) were defined as the “probucol group” (n=492). In addition to protocol similarities, the validity of the integrated analysis of the PROSPECTIVE and IMPACT studies was confirmed by comparing the demographics/characteristics and baseline variables that were believed to be related to the occurrence of cerebrocardiovascular events and by comparing the relation with time to MACCEs between the probucol and control groups in each study. The demographics and baseline variables of patients in the probucol and control groups were compared using the Fisher’s exact test or the two-sample Wilcoxon test. Lipid levels and hs-CRP level were also compared at 3 months and 3 years after registration. The mean IMT level at registration and its change at 1, 2, and 3 years from registration were compared among the groups.
The time to the first MACCE was compared between the control and probucol groups. Event-free survival curves were estimated using the Kaplan–Meier method. The adjusted HR was calculated using a stratified proportional hazards model (with baseline LDL-C, the presence or absence of diabetes, and the presence or absence of hypertension as covariates and the study as a stratified factor). The comparison between groups was tested using the stratified log-rank test (stratified by the study, baseline LDL-C, the presence or absence of diabetes, and the presence or absence of hypertension).
Additionally, a cutoff value for a reduced concentration of HDL-C was obtained from the ROC curve analysis of the probucol group. The event-free survival rate and the HR divided by two according to the cutoff value were estimated using a proportional hazards model. The two-tailed significance level in the statistical test was 0.05. All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patients
For the present study, 831 subjects (FAS) from the PROSPECTIVE study and 194 subjects (mITT) from the IMPACT study were enrolled ( Fig.1 ) . The mean age of the patients was 69 years, and 24.7% of the patients were women; 34.3% of the patients had a history of myocardial infarction, 59.9% of angina pectoris, 6.1% of cerebrovascular disease, and 2.9% of peripheral artery disease. Most of the patients (96.4%) qualified for study enrollment, with an LDL-C level of <140 mg/dL. Of the patients, 77.9% had hypertension, and 41.7% had diabetes mellitus ( Tables 1 and 2 ) .
Fig.1.
Study flow of the integrated analysis of the PROSPECTIVE and IMPACT studies
Table 1. Baseline demographics and other characteristics (qualitative variables).
| Characteristic | PROSPECTIVE | IMPACT | PROSPECTIVE+IMPACT | |||||
|---|---|---|---|---|---|---|---|---|
| Control (N = 431) | Probucol (N = 400) | Control (N = 102) | Probucol (N = 92) | Control (N = 533) | Probucol (N = 492) | P value† | ||
| No. of patients (%) | No. of patients (%) | No. of patients (%) | ||||||
| Country | Japan | 431 (100.0) | 400 (100.0) | 0 (0.0) | 0 (0.0) | 431 (80.9) | 400 (81.3) | 0.986 |
| Korea | 0 (0.0) | 0 (0.0) | 48 (47.1) | 44 (47.8) | 48 (9.0) | 44 (8.9) | ||
| China | 0 (0.0) | 0 (0.0) | 54 (52.9) | 48 (52.2) | 54 (10.1) | 48 (9.8) | ||
| Sex | Male | 319 (74.0) | 291 (72.8) | 84 (82.4) | 78 (84.8) | 403 (75.6) | 369 (75.0) | 0.828 |
| Female | 112 (26.0) | 109 (27.3) | 18 (17.6) | 14 (15.2) | 130 (24.4) | 123 (25.0) | ||
| LDL-C | <140 mg/dL | 418 (97.0) | 388 (97.0) | 96 (94.1) | 86 (93.5) | 514 (96.4) | 474 (96.3) | 0.859 |
| ≥ 140 mg/dL | 13 (3.0) | 12 (3.0) | 3 (2.9) | 4 (4.3) | 16 (3.0) | 16 (3.3) | ||
| Diabetes mellitus | 181 (42.0) | 168 (42.0) | 42 (41.2) | 36 (39.1) | 223 (41.8) | 204 (41.5) | 0.949 | |
| Hypertension | 342 (79.4) | 325 (81.3) | 68 (66.7) | 63 (68.5) | 410 (76.9) | 388 (78.9) | 0.498 | |
| Peripheral artery disease | 14 (3.2) | 15 (3.8) | 1 (1.0) | 0 (0.0) | 15 (2.8) | 15 (3.0) | 0.855 | |
| Smoking status | Current smoker | 34 (7.9) | 15 (3.8) | 31 (30.4) | 27 (29.3) | 65 (12.2) | 42 (8.5) | 0.065 |
|
Nonsmoker (Ex-smoker/Never) |
396 (91.9) | 385 (96.3) | 71 (69.6) | 65 (70.7) | 467 (87.6) | 450 (91.5) | ||
| Coronary heart disease‡ | Myocardial infarction | 160 (37.1) | 138 (34.5) | 30 (29.4) | 24 (26.1) | 190 (35.6) | 162 (32.9) | 0.392 |
| Angina pectoris | 266 (61.7) | 258 (64.5) | 47 (46.1) | 43 (46.7) | 313 (58.7) | 301 (61.2) | 0.444 | |
| Cerebrovascular disease | 27 (6.3) | 25 (6.3) | 3 (2.9) | 8 (8.7) | 30 (5.6) | 33 (6.7) | 0.516 | |
†: Fisher’s exact test was used to evaluate the significance of differences between groups.
‡: Multiple choices were allowed.
LDL-C, low-density lipoprotein cholesterol
Table 2. Baseline demographics and other characteristics (quantitative variables).
| Characteristic | PROSPECTIVE | IMPACT | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Probucol | Control | Probucol | |||||
| N | mean±SD or median (IQR) | N | mean±SD or median (IQR) | N | mean±SD or median (IQR) | N | mean±SD or median (IQR) | |
| Age (years) | 431 | 70.0±9.4 | 400 | 70.0±9.0 | 102 | 63.5±6.8 | 92 | 64.1±7.1 |
| Height (cm) | 431 | 160.2±8.5 | 400 | 160.5±8.8 | 102 | 166.5±7.4 | 92 | 166.8±7.3 |
| Weight (kg) | 431 | 63.2±11.4 | 400 | 63.7±12.0 | 102 | 71.0±8.8 | 92 | 70.4±11.2 |
| TC (mg/dL) | 431 | 169.5±28.7 | 400 | 169.0±28.6 | 102 | 151.3±30.7 | 92 | 151.4±34.7 |
| HDL-C (mg/dL) | 431 | 54.4±14.4 | 400 | 53.6±13.6 | 102 | 46.6±12.4 | 92 | 46.4±11.8 |
| LDL-C (mg/dL) | 431 | 90.4±23.5 | 400 | 89.6±22.7 | 99 | 75.2±25.7 | 90 | 77.2±30.0 |
| TG (mg/dL) | 431 | 107 (80-154) | 400 | 114 (83-158) | 102 | 129 (92-185) | 92 | 116 (83-165) |
| hs-CRP (ng/mL) | 69 | 570 (216-1640) | 56 | 698 (409-1585) | 102 | 685 (300-1160) | 92 | 665 (270-2145) |
| No. of cigarettes smoked on average daily (only current smokers) | 34 | 20 (10-20) | 15 | 10 (7-20) | 31 | 10 (10-20) | 27 | 10 (10-20) |
| Characteristic | PROSPECTIVE+IMPACT | |||||||
| Control | Probucol | P value† | ||||||
| N | mean±SD or median (IQR) | N | mean±SD or median (IQR) | |||||
| Age (years) | 533 | 68.7±9.3 | 492 | 68.9±9.0 | 0.965 | |||
| Height (cm) | 533 | 161.4±8.7 | 492 | 161.7±8.9 | 0.649 | |||
| Weight (kg) | 533 | 64.7±11.4 | 492 | 64.9±12.1 | 0.942 | |||
| TC (mg/dL) | 533 | 166.0±29.9 | 492 | 165.7±30.6 | 0.694 | |||
| HDL-C (mg/dL) | 533 | 52.9±14.4 | 492 | 52.3±13.6 | 0.588 | |||
| LDL-C (mg/dL) | 530 | 87.6±24.7 | 490 | 87.3±24.7 | 0.724 | |||
| TG (mg/dL) | 533 | 113 (83-157) | 492 | 114 (83-158) | 0.753 | |||
| hs-CRP (ng/mL) | 171 | 660 (254-1360) | 148 | 669 (327-1720) | 0.220 | |||
| No. of cigarettes smoked on average daily (only current smokers) | 65 | 15 (10-20) | 42 | 10 (10-20) | 0.196 | |||
†: Two-sample Wilcoxon test was used to evaluate the significance of differences between groups.
*: The level of significance was set at p<0.05 (2-sided).
hs-CRP, high-sensitivity C-reactive protein; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride
There were no significant differences in the background characteristics between the control and probucol groups, except there tended to be more current smokers in the control group than in the probucol group (p=0.065) ( Table 1 ) ; note that the daily number of cigarettes smoked on average for current smokers was not significantly different between the two groups ( Table 2 ) .
Lipid and hs-CRP Data
The total cholesterol, LDL-C, HDL-C, and TG levels at baseline were not significantly different between the probucol and control groups ( Table 2 ) . The total cholesterol, LDL-C, HDL-C, and TG levels at 3 months and 3 years were significantly decreased in the probucol group compared with the control group ( Table 3 ) .
Table 3. Lipids and hs-CRP.
| Characteristic | PROSPECTIVE | IMPACT | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Probucol | Control | Probucol | |||||
| N | mean±SD or median (IQR) | N | mean±SD or median (IQR) | N | mean±SD or median (IQR) | N | mean±SD or median (IQR) | |
| TC (mg/dL) | ||||||||
| Baseline | 431 | 169.5±28.7 | 400 | 169.0±28.6 | 102 | 151.3±30.7 | 92 | 151.4±34.7 |
| 3 months | 407 | 168.8±28.9 | 382 | 145.8±32.5 | 102 | 157.2±37.1 | 91 | 127.2±39.6 |
| 3 years | 343 | 167.9±29.6 | 314 | 139.8±28.9 | 97 | 154.5±39.3 | 85 | 133.8±41.2 |
| LDL-C (mg/dL) | ||||||||
| Baseline | 431 | 90.4±23.5 | 400 | 89.6±22.7 | 99 | 75.2±25.7 | 90 | 77.2±30.0 |
| 3 months | 406 | 89.3±23.2 | 375 | 81.8±23.9 | 100 | 82.4±32.1 | 88 | 69.7±33.3 |
| 3 years | 340 | 88.1±23.9 | 310 | 79.6±24.5 | 96 | 80.0±36.5 | 83 | 74.6±32.4 |
| HDL-C (mg/dL) | ||||||||
| Baseline | 431 | 54.4±14.4 | 400 | 53.6±13.6 | 102 | 46.6±12.4 | 92 | 46.4±11.8 |
| 3 months | 416 | 54.9±14.4 | 386 | 39.4±13.5 | 100 | 46.5±11.1 | 90 | 32.8±10.6 |
| 3 years | 353 | 54.8±14.1 | 317 | 37.3±12.7 | 97 | 47.6±13.3 | 85 | 35.0±14.3 |
| TG | ||||||||
| Baseline | 431 | 107 (80-154) | 400 | 114 (83-158) | 102 | 129 (92-185) | 92 | 116 (83-165) |
| 3 months | 415 | 112 (78-157) | 387 | 103 (74-150) | 102 | 128 (97-163) | 91 | 106 (73-156) |
| 3 years | 353 | 111 (76-154) | 317 | 98 (73-142) | 97 | 113 (96-166) | 84 | 107 (74-150) |
| hs-CRP | ||||||||
| Baseline | 69 | 570 (216-1640) | 56 | 698 (409-1585) | 102 | 685 (300-1160) | 92 | 665 (270-2145) |
| 3 months | 62 | 447 (229-1220) | 44 | 771 (370-1960) | 100 | 685 (360-1305) | 92 | 880 (470-1880) |
| 3 years | 39 | 478 (250-1400) | 31 | 683 (474-1680) | 97 | 890 (360-2200) | 85 | 1060 (560-2160) |
| Characteristic | PROSPECTIVE+IMPACT | |||||||
| Control | Probucol | P value† | ||||||
| N | mean±SD or median (IQR) | N | mean±SD or median (IQR) | |||||
| TC (mg/dL) | ||||||||
| Baseline | 533 | 166.0±29.9 | 492 | 165.7±30.6 | 0.694 | |||
| 3 months | 509 | 166.4±31.0 | 473 | 142.2±34.7 | <0.001* | |||
| 3 years | 440 | 164.9±32.4 | 399 | 138.6±31.9 | <0.001* | |||
| LDL-C (mg/dL) | ||||||||
| Baseline | 530 | 87.6±24.7 | 490 | 87.3±24.7 | 0.724 | |||
| 3 months | 506 | 88.0±25.3 | 463 | 79.5±26.3 | <0.001* | |||
| 3 years | 436 | 86.3±27.4 | 393 | 78.5±26.4 | <0.001* | |||
| HDL-C (mg/dL) | ||||||||
| Baseline | 533 | 52.9±14.4 | 492 | 52.3±13.6 | 0.588 | |||
| 3 months | 516 | 53.3±14.2 | 476 | 38.1±13.2 | <0.001* | |||
| 3 years | 450 | 53.2±14.2 | 402 | 36.9±13.1 | <0.001* | |||
| TG | ||||||||
| Baseline | 533 | 113 (83-157) | 492 | 114 (83-158) | 0.753 | |||
| 3 months | 517 | 114 (81-158) | 478 | 104 (74-151) | 0.010* | |||
| 3 years | 450 | 112 (80-160) | 401 | 101 (73-143) | 0.005* | |||
| hs-CRP | ||||||||
| Baseline | 171 | 660 (254-1360) | 148 | 669 (327-1720) | 0.220 | |||
| 3 months | 162 | 555 (280-1280) | 136 | 870 (440-1880) | 0.003* | |||
| 3 years | 136 | 666 (320-1585) | 116 | 930 (480-2110) | 0.046* | |||
†: Two-sample Wilcoxon test was used to evaluate the significance of differences between groups.
*: The level of significance was set at p<0.05 (2-sided).
TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; hs-CRP, high- sensitivity C-reactive protein
The hs-CRP levels at 3 months and 3 years were also significantly increased in the probucol group compared with the control group, although the sample numbers were limited ( Table 3 ) .
Mean Carotid IMT
There were no significant differences between the control and probucol groups in the mean IMT of the carotid arteries and their changes ( Table 4 ) ; note that the number of patients for whom ultrasonography of the carotid arteries was performed was small, and the measurements of the mean and max IMT at the same point in each patient may have had limited accuracy.
Table 4. Mean carotid IMT.
| Characteristic | PROSPECTIVE | IMPACT | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Probucol | Control | Probucol | |||||
| N | mean±SD | N | mean±SD | N | mean±SD | N | mean±SD | |
| Mean carotid IMT, mm | ||||||||
| Baseline | 84 | 0.86±0.25 | 88 | 0.91±0.27 | 102 | 1.27±0.42 | 92 | 1.26±0.34 |
| 1 year | 77 | 0.85±0.22 | 68 | 0.87±0.29 | 102 | 1.21±0.39 | 92 | 1.16±0.36 |
| 2 years | 73 | 0.83±0.22 | 67 | 0.88±0.27 | 100 | 1.15±0.37 | 86 | 1.17±0.37 |
| 3 years | 62 | 0.80±0.18 | 55 | 0.87±0.27 | 97 | 1.16±0.40 | 84 | 1.15±0.38 |
| Changes in mean IMT, mm | ||||||||
| Baseline to 1 year | 72 | -0.01±0.16 | 66 | 0.00±0.18 | 102 | -0.06±0.25 | 92 | -0.10±0.29 |
| Baseline to 2 years | 67 | -0.02±0.16 | 64 | 0.00±0.15 | 100 | -0.13±0.33 | 86 | -0.10±0.27 |
| Baseline to 3 years | 54 | -0.05±0.19 | 52 | -0.01±0.17 | 97 | -0.12±0.36 | 84 | -0.10±0.32 |
| Characteristic | PROSPECTIVE+IMPACT | |||||||
| Control | Probucol | P value† | ||||||
| N | mean±SD | N | mean±SD | |||||
| Mean carotid IMT, mm | ||||||||
| Baseline | 186 | 1.09±0.41 | 180 | 1.09±0.36 | 0.652 | |||
| 1 year | 179 | 1.06±0.37 | 160 | 1.04±0.36 | 0.525 | |||
| 2 years | 173 | 1.01±0.35 | 153 | 1.04±0.36 | 0.450 | |||
| 3 years | 159 | 1.02±0.38 | 139 | 1.04±0.37 | 0.421 | |||
| Changes in mean IMT, mm | ||||||||
| Baseline to 1 year | 174 | -0.04±0.22 | 158 | -0.06±0.25 | 0.597 | |||
| Baseline to 2 years | 167 | -0.09±0.28 | 150 | -0.06±0.23 | 0.204 | |||
| Baseline to 3 years | 151 | -0.10±0.31 | 136 | -0.06±0.28 | 0.373 | |||
†: Two-sample Wilcoxon test was used to evaluate the significance of differences between groups.
*: The level of significance was set at p<0.05 (2-sided).
IMT, intima-media thickness
Efficacy Endpoints
The adjusted HRs of the probucol group compared with the control group were 0.75 and 0.37 in the PROSPECTIVE and IMPACT studies, respectively. The HR directions were consistent between the studies. In the integrated analysis, which was stratified by the study, the adjusted HR and 95% CI were 0.67 and 0.44–1.03, respectively. We observed a tendency for the difference in the numbers of cerebrocardiovascular events to occur, although there was no statistical significance (p=0.052) between 35 patients in the probucol group (1.98/100 person-years) and 55 patients in the control group (2.92/100 person-years) ( Table 5 , Fig.2 ) .
Table 5. The incidence of cerebrocardiovascular events.
| Study | Group | Patient | Cerebrocardiovascular events | Incidence rate (/100 persons-year) |
Adjusted HR† (95% confidence interval) |
P value† |
|---|---|---|---|---|---|---|
| PROSPECTIVE | Control | 431 | 44 | 2.75 | 0.75 (0.47 - 1.18) | 0.184 |
| Probucol | 400 | 31 | 2.06 | |||
| IMPACT | Control | 102 | 11 | 3.88 | 0.37 (0.12 - 1.16) | 0.073 |
| Probucol | 92 | 4 | 1.52 | |||
| Integrated analysis | Control | 533 | 55 | 2.92 | 0.67 (0.44 - 1.03) | 0.052 |
| Probucol | 492 | 35 | 1.98 |
†: The adjusted hazard ratio was calculated by a proportional hazards model with baseline LDL-C, the presence or absence of diabetes, and the presence or absence of hypertension as covariates. The p-value was calculated by the stratified log-rank test (with baseline LDL-C, the presence or absence of diabetes, and the presence or absence of hypertension). In the integrated analysis, the study was added as a stratified factor to the model.
Fig.2. Kaplan–Meier estimates of the 5-year incidences of endpoint events after randomization into the probucol and control groups.
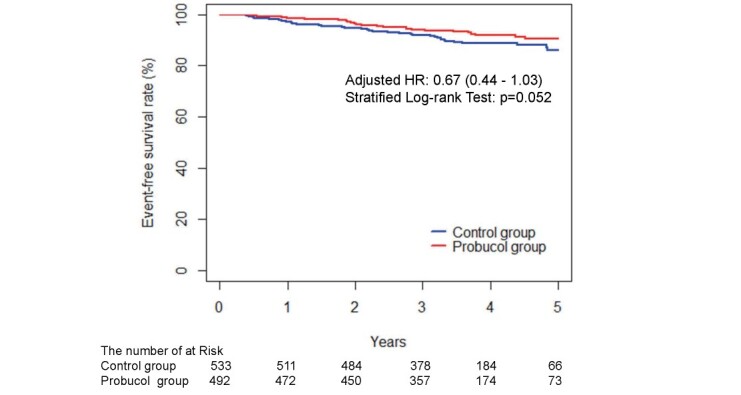
The adjusted HR was calculated using a stratified proportional hazards model (with baseline LDL-C, the presence or absence of diabetes, and the presence or absence of hypertension as covariates and the study as a stratified factor). The p-value was calculated using the stratified log-rank test (stratified by the study, baseline LDL-C, the presence or absence of diabetes, and the presence or absence of hypertension).
HDL-C Reduction and Efficacy Endpoints in the Probucol Group
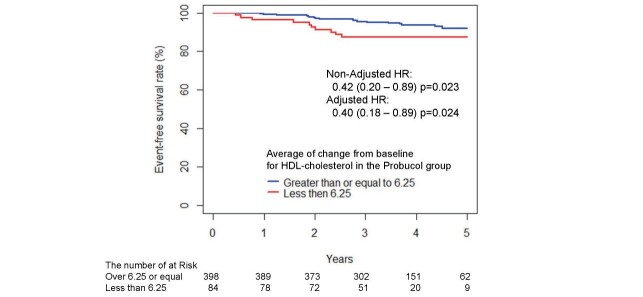
Additionally, the relationships of the reduction levels of HDL-C with cerebrocardiovascular events were analyzed in the probucol group ( Fig.3 ) . The analysis using the two groups separated by 6.25 mg/dL, an efficient cutoff value obtained from the ROC curve analysis, showed a significant decrease in efficacy endpoints in patients with reduced HDL-C levels (≥ 6.25 mg/dL) compared with those with levels <6.25 mg/dL (23 cases in 398 patients vs. 10 cases in 84 patients; adjusted HR, 0.40; p=0.024).
Fig.3. Kaplan–Meier estimates of the 5-year incidences of endpoint events after randomization into the probucol group with an average decrease in HDL cholesterol from baseline of 6.25 mg/dL or more and <6.25 mg/dL.
The adjusted HR was calculated using a stratified proportional hazards model (with baseline LDL-C, the presence or absence of diabetes, and the presence or absence of hypertension as covariates and the study as a stratified factor).
Discussion
The current study was an integrated analysis of the effect of probucol on cardiovascular events in patients from the PROSPECTIVE study 30) and IMPACT study 31) . In the PROSPECTIVE study, patients were randomly assigned to the control and probucol (500 mg/day) groups. The primary endpoint was a composite of cerebrovascular and cardiovascular events (cardiovascular disease death including sudden death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, hospitalization for heart failure, or coronary revascularization). The secondary endpoint in a subset of patients was the carotid intima-media thickness (IMT). Probucol showed a tendency to reduce cardiovascular events in patients with CAD treated with statins, but the difference was not statistically significant. In contrast, in the IMPACT study, subjects were randomized to three groups and received study drugs for 3 years: control with statin alone (control group); statin and probucol (probucol group); and statin, probucol, and cilostazol (combination group). The primary endpoint was the change in the mean carotid IMT at 3 years. The secondary endpoints were biomarkers, major adverse cerebrocardiovascular events, and safety. Therefore, the primary and secondary endpoints were opposite in these two trials.
When these two trials were combined and an integrated analysis was performed, probucol was demonstrated to reduce cerebrocardiovascular events in patients with CAD, with marginal significance. The results were basically similar between patients from Japan and Korea/China; therefore, the preventive effects of probucol on secondary cerebrocardiovascular events in patients with CAD may be consistent regardless of the population. In the integrated analysis, probucol reduced LDL-C by only 10.1% at 3 years compared with control therapy. It is possible that the reduction in LDL-C by probucol may be one of the reasons why the number of cardiovascular events tended to decrease. However, the reduction in serum LDL-C was very small compared with that by statins, and our analysis demonstrated that the preventive effect of probucol on secondary cerebrocardiovascular events in patients with CAD was not dependent on the reduction in serum LDL-C levels. In addition, in both studies, physicians were asked not to change the statin dose as much as possible. Therefore, this small reduction of LDL-C is not associated with the change of other lipid-lowering drugs. In fact, 93.1% and 94.5% of the patients in the control and probucol arms were found to continue the original dose of statins, respectively.
In the IMPACT study, probucol or probucol and cilostazol with a statin did not reduce the carotid IMT compared with a statin alone; however, major adverse cerebrocardiovascular events were less frequent in the probucol or combination group than in the control group, without a significant difference. Regarding the results of the IMT in the integrative analysis, the IMT was not significantly reduced by probucol treatment; however, the number of participants who were subjected to IMT analysis may not have been enough to draw a conclusion.
Probucol has potent antioxidant activity and strongly protects LDL from oxidation. LDL particles from probucol-treated patients are strongly protected from oxidation 21 , 32) . We speculate that the antioxidative properties of probucol may be one of the reasons for its effects on the prevention of cerebrocardiovascular events. Furthermore, the probucol-mediated reduction in serum HDL-C might reflect enhanced reverse cholesterol transport (RCT). Probucol was shown to regress tendon xanthomas and xanthelasmas in patients with FH despite a reduction in serum HDL-C 21 , 33) . Probucol was shown to decrease serum HDL-C levels by enhancing plasma CETP activity 34) and the hepatic expression of scavenger receptor class B type I (SR-BI) 35) , which accelerates the excretion of cholesterol into the bile and feces 36) . Therefore, the reduction in HDL-C by probucol could be explained by both enhanced CETP-mediated transfer of cholesteryl esters from HDL to apolipoprotein B-containing lipoproteins and increased hepatic SR-BI-mediated selective uptake of cholesteryl esters from HDL. Furthermore, probucol enhances the formation of lipid-poor prebeta-1 HDL, which is involved in cellular cholesterol efflux. Intriguingly, our subanalysis ( Fig.3 ) showed a significant decrease in efficacy endpoints in patients with reduced HDL-C levels (≥ 6.25 mg/dL) compared with those without. However, because of the small sample size, concluding that less decrease of HDL-C by probucol treatment is associated with the lack of beneficial effect of probucol would be difficult.
In contrast to probucol, which reduces serum HDL-C levels, many CETP inhibitors have been developed to increase serum HDL-C levels and decrease serum LDL-C levels. However, as mentioned above, most CETP inhibitors disappeared from drug development because of negative results that show cardiovascular risk reduction in patients treated with statins. The failure of CETP inhibitors could be attributed to their strategy to increase serum HDL-C levels by inhibiting the transfer of cholesteryl ester from HDL particles, resulting in the formation of very large cholesteryl ester/apoE/apoC-III-rich HDL particles with increased oxidized phospholipids and impaired antiatherogenic functions. In our integrative analysis of the PROSPECTIVE 30) and IMPACT 31) studies, we demonstrated that the reduction in cerebrocardiovascular events by probucol was likely independent of the degree of LDL-C reduction but rather dependent on the degree of HDL-C reduction. The degree of serum HDL-C reduction by probucol was correlated with cardiovascular risk reduction. Previous epidemiological studies have clearly demonstrated that decreased serum HDL-C levels are strongly linked to the cardiovascular event rate. Therefore, the probucol-induced reduction in serum HDL-C levels is so marked and thus may be predicted to remarkably enhance the cardiovascular event rate. Nevertheless, our current study clearly demonstrated that lowering HDL-C levels by probucol use may predict satisfactory cardiovascular outcomes. Thus, the reduction in serum HDL-C by probucol may not be harmful and might rather suggest the enhancement of RCT via increased CETP and hepatic SR-BI.
In addition to the level of serum HDL-C, probucol has been shown to modify the lipid and protein compositions of HDL particles. HDL particles from probucol-treated patients are small and poor in cholesteryl ester, possessing potent antiatherogenic activity against foam cell formation 37) and strong antioxidant activity partly due to an increase in antioxidant paraoxonase 1 activity 38) , although we have seen a significant increase of hs-CRP only in the probucol group in this pooled analysis, which was an unexpected finding. A recent study showed that serum oxidized HDL levels were reduced in patients with FH treated with probucol 39) . Probucol was also demonstrated to have anti-inflammatory effects via the inhibition of VCAM1 and MCP 40 , 41) and attenuated the progression of atherosclerosis in animal models 42 , 43) .
A recent randomized controlled trial (PICASSO) examined the efficacy and safety of cilostazol versus aspirin, with and without probucol, in patients with ischemic stroke with a high risk of cerebral hemorrhage 44) . Patients with ischemic stroke and a history of or imaging findings of intracerebral hemorrhage or two or more microbleeds were randomly assigned to receive the following: 1) 200 mg/day cilostazol, 2) 100 mg/day aspirin, 3) cilostazol plus 500 mg/day probucol, or 4) aspirin plus probucol. The incidence of vascular events was significantly lower in the probucol group than in the non-probucol group (HR, 0.69; 95% CI, 0.50–0.97; p=0.0316). In a recent post hoc analysis of the PICASSO study 45) , the mean HDL-C level was significantly lower in the probucol group than in the non-probucol group, and no significant interaction was shown between HDL-C changes and the assigned treatment regarding the risk of the efficacy endpoint in either study arm. These findings are partly similar to the results of our current integrated analysis and strongly suggest that probucol may be beneficial in reducing the incidence of cardiovascular events in patients with CAD and ischemic stroke.
In the current study, we also showed that probucol treatment in high-risk patients treated with statins was well tolerated and safe despite a reduction in serum HDL-C. No significant difference was observed in the frequencies of serious adverse events between the probucol-treated and control groups. Furthermore, ventricular arrhythmias were not increased in probucol-treated patients. Similarly, previous studies, including the POSITIVE 28) and PICASSO 44) study and a study by Kasai et al. 29) , demonstrated no significant increases in severe ventricular arrhythmia. Therefore, QT prolongation by probucol may not affect the occurrence of lethal ventricular arrhythmias, although this should be further addressed in a larger study because of a few events in this study.
In conclusion, in this integrated analysis of the PROSPECTIVE and IMPACT studies, we demonstrated a marginal effect of probucol on cerebrocardiovascular events in Asian patients with CAD, with reasonable safety profiles. Further study may be needed to show the effect of probucol in a larger sample size of high-risk patients.
Acknowledgements
This study was funded by Foundation for Biomedical Research and Innovation at Kobe, a third-party organization which is independent of investigators’ institution and was responsible for on-site monitoring in addition to data collection, data management and data analysis. The Foundation for Biomedical Research and Innovation at Kobe has received research grant from several pharmaceutical companies, which was not specific for this study.
Declaration of Interests
Dr. Arai reports personal fees from Sanofi, MSD K.K., Pfizer, Kowa Company, Ltd., Daiichi-Sankyo Company, Ltd., Takeda Pharmaceutical Company, Ltd., UCB Japan, and Otsuka Pharm. Ltd. Dr. Bujo has nothing to disclose. Dr. Masuda reports grants and personal fees from Nippon Boehringer Ingelheim Co., Ltd., MSD K.K., Takeda Pharmaceutical Company, Ltd., Daiichi-Sankyo Company, Ltd., Mochida Pharmaceutical Company, Ltd., Kowa Company Ltd., Kissei Pharmaceutical Co., Ltd.; grants from Otsuka Pharmaceutical Co Ltd.; personal fees from Kowa Company, Ltd., Bayer Yakuhin, Ltd, Kyowa Medex Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Ono Pharmaceutical Company, Ltd., Astellas Pharma Inc., AstraZeneca K.K., non-financial support from Skylight Biotec, Inc., Pfizer Japan Inc., Amgen Astellas Biopharma K.K., Sanofi K.K., grants from Shionogi & Co., Ltd., grants from Bayer Yakuhin, Ltd., grants from Sanwa Kagaku Kenkyusho Co., Ltd., grants from Astellas Pharma Inc., grants from Hayashibara Co., Ltd., Teijin Pharma Limited, Kaken Pharmaceutical Co., Ltd.. Dr. Ishibashi, Mr. Nakagawa, Mr. Kagimura, Mr. Tanabe, Dr. Kang, Dr. Kim, Dr. Sung, Dr. Kim, Dr. Kim, Dr. Park, Dr. Ge, Dr. Oh, Dr. Kita, Dr. Saito, Dr.Matsuzawa, and Dr. Fukushima have nothing to disclose. Ltd., Dr. Yamashita reports personal fees from MSD K.K., and Kowa Company, Ltd.
Appendix
PROSPECTIVE Study
The PROSPECTIVE study is a randomized (1:1), prospective, open-label, multicenter clinical trial conducted on patients with hyper-LDL-cholesterolemia with a prior history of coronary events as CAD (30). Patients on conventional lipid-lowering therapy (LLT) within 6 weeks after registration from participating faculties were screened using the inclusion/exclusion criteria. A web-based central registration system was used to automatically and randomly assign patients to either the control group (conventional LLT continued) or the test group (LLT with probucol 500 mg/day, 250 mg twice daily) at a 1:1 allocation rate based on the registered patient’s data. In randomization, LDL-C levels (140 mg/dL and more vs. less than 140 mg/dL), diabetes (with vs. without), and hypertension (with vs. without) were dynamically balanced between the two groups as adjusted allocation factors.
The primary efficacy endpoint was the presence and time from registration until the first occurrence of a major cerebrovascular and cardiovascular event (MACCE; CV-related death, nonfatal MI, nonfatal cerebral stroke, hospital admission due to unstable angina/heart failure and PCI/CABG), and the other six parameters were used as the secondary efficacy and safety endpoints. Patient background; all cerebrovascular and cardiovascular events; all adverse events; the levels of total cholesterol, TG, and HDL-C; and the confirmation of survival were determined before protocol treatment and at 3 months and 1, 2, and 3 years after registration. The maximum (max)/minimum (min) IMT of the carotid arteries was measured by each participating faculty member using the same evaluation protocol, and the obtained images were evaluated by the Imaging Evaluation Committee.
The patient population in the statistical analysis was a full analysis set (FAS) based on the intention-to-treat principle. A total of 876 patients with CAD underwent randomization at 82 sites in Japan, and 831 of 876 patients were assigned to either the control group (conventional LLT continued, 431 patients) or the test group (LLT plus probucol 500 mg/day, 400 patients). The primary endpoint occurred in 44 patients (10.2%) in the control group and in 31 patients (7.8%) in the test group (adjusted hazard ratio, 0.746; 95% CI, 0.471 to 1.182; stratified log-rank test, p=0.1839). There was no significant difference between the two groups in the incidence of the primary endpoint or in the key composites of cerebrovascular and cardiovascular events, and there was no significant difference in the secondary efficacy and safety endpoint or the changes in serum hs-CRP and adiponectin concentrations. Translational Research Center for Medical Innovation, Foundation for Biomedical Research and Innovation at Kobe is responsible for randomization and statistical analysis.
Inclusion Criteria
This study included male and female patients older than 20 years with all of the following eight clinical statuses:
1) diagnosis of dyslipidemia with a high LDL-C level (≥ 140 mg/dL) without any medication;
2) treatment using any lipid-lowering drug, including statins, for more than 8 weeks before providing informed consent;
3) a serum LDL-C level less than 200 mg/dL within 8 weeks before providing informed consent, as calculated by Friedewald’s formula (LDL-C=total cholesterol – HDL-cholesterol − triglycerides (TG)/5);
4) a history of acute myocardial infarction or angina pectoris more than 3 months before providing informed consent, old myocardial infarction, CABG more than 3 months earlier, PCI more than 9 months earlier, or PCI with no restenosis that was diagnosed by follow-up coronary angiography at 6–9 months after PCI;
5) normal cardiac function and mild or moderate heart failure (NYHA classification I or II);
6) older than 20 years at the time informed consent was provided;
7) no severe hepatic or renal dysfunction (AST<100 IU/L, ALT<100 IU/L, and serum creatinine <1.5 mg/dL) within 4 weeks before providing informed consent; and
8) signed written informed consent for participation in this study.
Exclusion Criteria
The exclusion criteria were the presence of the following clinical statuses at the time informed consent was provided:
1) ongoing treatment with probucol within 6 months before the time informed consent was provided;
2) ongoing treatment with cyclosporine;
3) a history of hypersensitivity reactions to probucol;
4) a diagnosis of FH based on the NICE clinical guidelines [1];
5) a very high TG level (>400 mg/dL) within 8 weeks before providing informed consent;
6) a markedly high HbA1c level (≥ 8%) on the most recent blood test;
7) frequent multifocal ventricular arrhythmia;
8) Af including paroxysmal Af;
9) a long QTc interval on resting electrocardiogram (>450 ms in males or >470 ms in females);
10) congestive heart failure (NYHA III or IV) or unstable angina;
11) participation in other clinical trials;
12) women who were pregnant, lactating, might become pregnant, or wish to become pregnant within the study period; and
13) inappropriate candidates for participation as assessed by doctors in the current study.
Serious Adverse Events (SAEs)
SAEs were designated as death, events that could lead to death, events that required hospital admission or extended hospitalization, disorders, events that could lead to disorders, other events that were serious as well as former adverse events, or a congenital disease or abnormality in postgenerations.
Endpoints
The primary efficacy endpoint was the presence and time from registration until the first occurrence of a cerebrovascular and cardiovascular event, as follows:
1) cardiovascular death including cardiac sudden death;
2) nonfatal myocardial infarction;
3) nonfatal cerebral stroke excluding transient ischemic attack;
4) hospital admission due to unstable angina;
5) hospital admission due to heart failure; and
6) all coronary revascularizations with either PCI or CABG.
The secondary efficacy and safety endpoints were as follows:
1) all-cause death;
2) all cerebrovascular and cardiovascular diseases;
3) event-free survival time;
4) mean IMT of the carotid arteries and their changes;
5) max IMT in the common or internal carotid arteries and their changes; and
6) severe adverse events and their frequency.
Carotid IMT Measurements
The mean carotid IMT was measured by averaging the values from 100 points within 1 cm from each side of the site giving the maximal thickness of the carotid IMT. For measurement of the carotid IMT, a linear probe with 7 MHz or greater was used, with scanner conditions adjusted to a depth of 30 mm, a dynamic range of 65 dB, and 40 frames per second. For the same lesion, the same means of measurement and conditions, such as ultrasound equipment, patient position, and ultrasound probe angle, were applied, and the same examiner obtained the carotid ultrasound images. Intima Scope software (Soft Medical, Tokyo, Japan), was used to measure the carotid artery, and IMT measurements were performed by an independent, blinded central laboratory (Matsuo Clinic, Osaka, Japan).
IMPACT Study
The IMPACT study is a prospective, randomized, multinational, open-blinded endpoint study, and the long-term effects of probucol or probucol and cilostazol with statins on the carotid mean intima media thickness (IMT), as well as cardiovascular and cerebrovascular events, were evaluated; this study was conducted in South Korea and China31). Hypercholesterolemic patients who had the following clinical features and did not meet the exclusion criteria were enrolled: age ≥ 20 years, chronic stable coronary disease, taking a statin, max IMT ≥ 1.2 mm, and LDL-C< 200 mg/dL. In total, 558 patients from five South Korean and 10 Chinese centers were enrolled between February 2011 and January 2013. Ultimately, 355 participants were randomized to one of three groups: 1) a control group (received a statin), 2) a probucol group (received probucol 250 mg twice daily and a statin), or 3) a combination group (received probucol 250 mg twice daily and cilostazol 100 mg twice daily with a statin). Randomization was stratified by country and the maximal carotid IMT (≥ 2.0 mm or <2 mm). The planned treatment and observation duration of the study was 3 years.
The primary efficacy endpoint of the IMPACT study was a change in the mean carotid IMT at 3 years. Biomarkers, major adverse cerebrocardiovascular events (MACCEs; a composite of cardiovascular-related death, myocardial infarction, cerebral infarction, unstable angina or heart failure requiring hospitalization, or revascularization) and safety were secondary endpoints. The carotid IMT was measured at baseline and then at 1, 2, and 3 years by each participating faculty member of the Imaging Evaluation Committee using the same evaluation protocol and the same protocol as in the PROSPECTIVE study. The secondary efficacy endpoints were the time to a major adverse cerebrocardiovascular event, occurrence of MACCEs, changes in biomarkers, and safety. Biomarkers were measured at baseline and at 3 months and 3 years. The following biomarkers were included: total cholesterol, LDL-C, HDL-C, triglyceride, high-sensitivity C-reactive protein (hs-CRP), monocyte chemoattractant protein-1 (MCP-1), and oxidized LDL-C. Safety was evaluated by reported adverse events, physical examinations, laboratory tests, and electrocardiograms.
The modified intention to treat (ITT) group was planned to be used for primary efficacy analysis and was defined as subjects who received at least one dose of the study drug and had baseline and at least one follow-up carotid IMT measurement. Two hundred eighty-one patients were randomized into three groups. Among the modified ITT group, 102 patients were assigned to the control group, 92 patients to the probucol group and 87 patients to the combination group. The decrease in the mean carotid IMT was significantly greater in the combination group than in the control group at 1 year; however, there were no significant differences in changes in the mean carotid IMT between the groups at 3 years (control; -0.12±0.36 mm vs. probucol; -0.11±0.32 mm vs. combination; -0.16±0.38 mm). There was no significant difference in the frequency of MACCEs between the three groups (control; 10.8% vs. probucol; 4.4% vs. combination; 6.9%, p=0.35). Probucol and cilostazol were well tolerated as long-term treatments, without serious drug-related adverse reactions. This study is funded by Otsuka pharma. LTD. ADM Korea is a randomized center of this study.
Inclusion Criteria
Hypercholesterolemic patients who met the following inclusion criteria were enrolled in the study:
1) age ≥ 20 years;
2) chronic stable coronary disease (≥ 3 months);
3) receiving a statin;
4) maximal carotid IMT ≥ 1.2 mm; and
5) low-density lipoprotein (LDL) cholesterol 200 mg/dL.
Exclusion Criteria
The exclusion criteria were as follows:
1) patients with homozygous familial hypercholesterolemia;
2) uncontrolled diabetes mellitus;
3) symptomatic heart failure; and
4) a prolonged QTc interval.
Safety
SAE safety was evaluated by reported adverse events, physical examinations, laboratory tests, and electrocardiograms.
Endpoints
The primary efficacy endpoint was the change in the carotid mean IMT at the end of the study (3-year follow-up or the last test during the study) from baseline. The carotid IMT was measured at baseline and then at 1, 2, and 3 years.
Secondary efficacy endpoints were as follows:
1) time to a major adverse cerebrocardiovascular event (MACCE; a composite of cardiovascular-related death, myocardial infarction, cerebral infarction, unstable angina or heart failure requiring hospitalization, or revascularization);
2) occurrence of a MACCE;
3) changes in the following biomarkers: total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, high-sensitivity C-reactive protein (hs-CRP), monocyte chemoattractant protein-1 (MCP-1), and oxidized LDL cholesterol (measured at baseline, 3 months, and 3 years); and
4) safety: reported adverse events, physical examinations, laboratory tests, and electrocardiograms.
Carotid IMT Measurements
Carotid IMT measurements were performed in the same manner as in the PROSPECTIVE study.
References
- 1).Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet, 2015; 385: 1397-1405 [DOI] [PubMed] [Google Scholar]
- 2).Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, H. Darius H, B.S. Lewis, T.O. Ophuis, J.W. Jukema, G.M. De Ferrari, W. Ruzyllo, P. De Lucca, K. Im, E.A. Bohula, C. Reist, S.D. Wiviott, A.M. Tershakovec, T. Musliner, E. Braunwald, R.M. Califf,. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med, 2015; 372: 2387-2397 [Google Scholar]
- 3).Ouchi Y, Sasaki J, Arai H, Yokote K, Harada K, Katayama Y, Urabe T, Uchida Y, Hayashi M, Yokota N, Nishida H, Otonari T, Arai T, Sakuma I, Sakabe K, Yamamoto M, Kobayashi T, Oikawa S, Yamashita S, Rakugi H, Imai T, Tanaka S, Ohashi Y, Kuwabara M, Ito H. Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older (EWTOPIA 75): A Randomized, Controlled Trial. Circulation, 2019; 140: 992-1003 [DOI] [PubMed] [Google Scholar]
- 4).Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 5).Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med, 2018; 379: 2097-2107 [DOI] [PubMed] [Google Scholar]
- 6).Lorenzatti AJ, Toth PP. New Perspectives on Atherogenic Dyslipidaemia and Cardiovascular Disease. Eur Cardiol, 2020; 15: 1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C, Laakso M. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet, 2005; 366: 1849-1861 [DOI] [PubMed] [Google Scholar]
- 8).ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med, 2010; 362: 1563-1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J, Perkovic V. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet, 2010; 375: 1875-1884 [DOI] [PubMed] [Google Scholar]
- 10).Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 11).Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med, 2019; 380: 11-22 [DOI] [PubMed] [Google Scholar]
- 12).ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med, 2018; 379: 1540-1550 [DOI] [PubMed] [Google Scholar]
- 13).Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med, 2007; 357: 2109-2122 [DOI] [PubMed] [Google Scholar]
- 14).Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med, 2012; 367: 2089-2099 [DOI] [PubMed] [Google Scholar]
- 15).Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N Engl J Med, 2017; 376: 1933-1942 [DOI] [PubMed] [Google Scholar]
- 16).Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, Revkin JH, Grobbee DE, Riley WA, Shear CL, Duggan WT, Bots ML. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med, 2007; 356: 1620-1630 [DOI] [PubMed] [Google Scholar]
- 17).Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, Shear CL, Duggan WT, Vicari RM, Grobbee DE, Kastelein JJ. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet, 2007; 370: 153-160 [DOI] [PubMed] [Google Scholar]
- 18).Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med, 2007; 356: 1304-1316 [DOI] [PubMed] [Google Scholar]
- 19).HPS3/TIMI55–REVEAL Collaborative Group, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N Engl J Med, 2017; 377: 1217-1227 [DOI] [PubMed] [Google Scholar]
- 20).Krishna R, Gheyas F, Liu Y, Hagen DR, Walker B, Chawla A, Cote J, Blaustein RO, Gutstein DE. Chronic Administration of Anacetrapib Is Associated With Accumulation in Adipose and Slow Elimination. Clin Pharmacol Ther, 2017; 102: 832-840 [DOI] [PubMed] [Google Scholar]
- 21).Yamamoto A, Matsuzawa Y, Yokoyama S, Funahashi T, Yamamura T, Kishino B. Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol, 1986; 57: 29H-35H [DOI] [PubMed] [Google Scholar]
- 22).Walldius G, Erikson U, Olsson AG, Bergstrand L, Hadell K, Johansson J, Kaijser L, Lassvik C, Mölgaard J, Nilsson S, Schafer-Elinder L, Stenport G, Holme I. The effect of probucol on femoral atherosclerosis: the Probucol Quantitative Regression Swedish Trial (PQRST). Am J Cardiol, 1994; 74: 875-883 [DOI] [PubMed] [Google Scholar]
- 23).Yamashita S, Matsuzawa Y. Where are we with probucol: a new life for an old drug? Atherosclerosis, 2009; 207: 16-23 [DOI] [PubMed] [Google Scholar]
- 24).Yamashita S, Masuda D, Matsuzawa Y. Did we abandon probucol too soon? Curr Opin Lipidol, 2015; 26: 304-316 [DOI] [PubMed] [Google Scholar]
- 25).Yamashita S, Ruscica M, Macchi C, Corsini A, Matsuzawa Y, Sirtori CR. Cholesteryl ester transfer protein: An enigmatic pharmacology - Antagonists and agonists. Atherosclerosis, 2018; 278: 286-298 [DOI] [PubMed] [Google Scholar]
- 26).Yamashita S, Matsuzawa Y. Re-evaluation of cholesteryl ester transfer protein function in atherosclerosis based upon genetics and pharmacological manipulation. Curr Opin Lipidol, 2016; 27: 459-472 [DOI] [PubMed] [Google Scholar]
- 27).Yamashita S, Masuda D, Matsuzawa Y. New Horizons for Probucol, an Old, Mysterious Drug. J Atheroscler Thromb, 2021; 28: 100-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Yamashita S, Bujo H, Arai H, Harada-Shiba M, Matsui S, Fukushima M, Saito Y, Kita T, Matsuzawa Y. Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb, 2008; 15: 292-303 [DOI] [PubMed] [Google Scholar]
- 29).Kasai T, Miyauchi K, Kubota N, Kajimoto K, Amano A, Daida H. Probucol therapy improves long-term (>10-year) survival after complete revascularization: a propensity analysis. Atherosclerosis, 2012; 220: 463-469 [DOI] [PubMed] [Google Scholar]
- 30).Yamashita S, Arai H, Bujo H, Masuda D, Ohama T, Ishibashi T, Yanagi K, Doi Y, Nakagawa S, Yamashiro K, Tanabe K, Kita T, Matsuzaki M, Saito Y, Fukushima M, Matsuzawa Y. Probucol Trial for Secondary Prevention of Atherosclerotic Events in Patients with Coronary Heart Disease (PROSPECTIVE). J Atheroscler Thromb, 2021; 28: 103-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Kang HJ, Kim MH, Sung J, Kim SH, Kim CH, Park JE, Ge J, Oh BH. Effect of Probucol and/or Cilostazol on Carotid Intima Media Thickness in Patients with Coronary Heart Disease: A Randomized, Multicenter, Multinational Study. J Atheroscler Thromb, 2021; 28: 124-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Parthasarathy S, Young SG, Witztum JL, Pittman RC, Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest, 1986; 77: 641-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Yamamoto A, Takaichi S, Hara H, Nishikawa O, Yokoyama S, Yamamura T, Yamaguchi T. Probucol prevents lipid storage in macrophages. Atherosclerosis, 1986; 62: 209-217 [DOI] [PubMed] [Google Scholar]
- 34).Franceschini G, Sirtori M, Vaccarino V, Gianfranceschi G, Rezzonico L, Chiesa G, Sirtori CR. Mechanisms of HDL reduction after probucol. Changes in HDL subfractions and increased reverse cholesteryl ester transfer. Arteriosclerosis, 1989; 9: 462-469 [DOI] [PubMed] [Google Scholar]
- 35).Hirano K, Ikegami C, Tsujii K, Zhang Z, Matsuura F, Nakagawa-Toyama Y, Koseki M, Masuda D, Maruyama T, Shimomura I, Ueda Y, Yamashita S. Probucol enhances the expression of human hepatic scavenger receptor class B type I, possibly through a species-specific mechanism. Arterioscler Thromb Vasc Biol, 2005; 25: 2422-2427 [DOI] [PubMed] [Google Scholar]
- 36).Tawara K, Tomikawa M, Abiko Y. Mode of action of probucol in reducing serum cholesterol in mice. Jpn J Pharmacol, 1986; 40: 123-133 [DOI] [PubMed] [Google Scholar]
- 37).Ishigami M, Yamashita S, Sakai N, Hirano K, Arai T, Maruyama T, Takami S, Koyama M, Kameda-Takemura K, Matsuzawa Y. High-density lipoproteins from probucol-treated patients have increased capacity to promote cholesterol efflux from mouse peritoneal macrophages loaded with acetylated low-density lipoproteins. Eur J Clin Invest, 1997; 27: 285-292 [DOI] [PubMed] [Google Scholar]
- 38).Inagaki M, Nakagawa-Toyama Y, Nishida M, Nakatani K, Nakaoka H, Kawase M, Kawase R, Tsubakio-Yamamoto K, Masuda D, Ohama T, Matsuyama A, Ishigami M, Komuro I, Yamashita S. Effect of probucol on antioxidant properties of HDL in patients with heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2012; 19: 643-656 [DOI] [PubMed] [Google Scholar]
- 39).Okada T, Sumida M, Ohama T, Katayama Y, Saga A, Inui H, Kanno K, Masuda D, Koseki M, Nishida M, Sakata Y, Yamashita S. Development and Clinical Application of an Enzyme-Linked Immunosorbent Assay for Oxidized High-Density Lipoprotein. J Atheroscler Thromb, 2020; in press. doi: https: //doi.org/10.5551/jat.56887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Fruebis J, Gonzalez V, Silvestre M, Palinski W. Effect of probucol treatment on gene expression of VCAM-1, MCP-1, and M-CSF in the aortic wall of LDL receptor-deficient rabbits during early atherogenesis. Arterioscler Thromb Vasc Biol, 1997; 17: 1289-1302 [DOI] [PubMed] [Google Scholar]
- 41).Zapolska-Downar D, Zapolski-Downar A, Markiewski M, Ciechanowicz A, Kaczmarczyk M, Naruszewicz M. Selective inhibition by probucol of vascular cell adhesion molecule-1 (VCAM-1) expression in human vascular endothelial cells. Atherosclerosis, 2001; 155: 123-130 [DOI] [PubMed] [Google Scholar]
- 42).Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A, 1987; 84: 5928-5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Yoshikawa T, Mitani K, Kotosai K, Nozako M, Miyakoda G, Yabuuchi Y. Antiatherogenic effects of cilostazol and probucol alone, and in combination in low density lipoprotein receptor-deficient mice fed with a high fat diet. Horm Metab Res, 2008; 40: 473-478 [DOI] [PubMed] [Google Scholar]
- 44).Kim BJ, Lee EJ, Kwon SU, Park JH, Kim YJ, Hong KS, Wong LKS, Yu S, Hwang YH, Lee JS, Lee J, Rha JH, Heo SH, Ahn SH, Seo WK, Park JM, Lee JH, Kwon JH, Sohn SI, Jung JM, Navarro JC, Kang DW. Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): a multicentre, randomised controlled trial. Lancet Neurol, 2018; 17: 509-518 [DOI] [PubMed] [Google Scholar]
- 45).Lee EJ, Kwon SU, Park JH, Kim YJ, Hong KS, Yu S, Hwang YH, Lee JS, Lee J, Rha JH, Heo SH, Ahn SH, Seo WK, Park JM, Lee JH, Kwon JH, Sohn SI, Jung JM, Kim HY, Kim EG, Kim SH, Cha JK, Park MS, Nam HS, Kang DW. Changes in High-Density Lipoprotein Cholesterol and Risks of Cardiovascular Events: A Post Hoc Analysis from the PICASSO Trial. J Stroke, 2020; 22: 108-118 [DOI] [PMC free article] [PubMed] [Google Scholar]