Abstract
Background and Aims
Controversies exist regarding the benefits and most appropriate approach for preprocedural coronavirus disease 2019 (COVID-19) testing (eg, rapid antigen test, polymerase chain reaction, or real-time polymerase chain reaction) for outpatients undergoing diagnostic and therapeutic procedures, such as GI endoscopy, to prevent COVID-19 infections among staff. Guidelines for protecting healthcare workers (HCWs) from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection from outpatient procedures varies across medical professional organizations. This study provides an evidence-based decision support tool for key decision-makers (eg, clinicians) to respond to COVID-19 transmission risks and reduce the effect of personal biases.
Methods
A scoping review was used to identify relevant factors influencing COVID-19 transmission risk relevant for GI endoscopy. From 12 relevant publications, 8 factors were applicable: test sensitivity, prevalence of SARS-CoV-2 in the population, age-adjusted SARS-CoV-2 prevalence in the patient cohort, proportion of asymptomatic patients, risk of transmission from asymptomatic carriers, risk reduction by personal protective equipment (PPE), vaccination rates of HCWs, and risk reduction of SAE by vaccination. The probability of a serious adverse event (SAE), such as workplace-acquired infection resulting in HCW death, under various scenarios with preprocedural testing was determined to inform decision-makers of expected costs of reductions in SAEs.
Results
In a setting of high community transmission, without testing and PPE, 117.5 SAEs per million procedures were estimated to occur, and this was reduced to between .079 and 2.35 SAEs per million procedures with the use of PPE and preprocedural testing. When these variables are used and a range of scenarios are tested, the probability of an SAE was low even without testing but was reduced by preprocedural testing.
Conclusions
Under all scenarios tested, preprocedural testing reduced the SAE risk for HCWs regardless of the SARS-CoV-2 variant. Benefits of preprocedural testing are marginal when community transmission is low (eg, below 10 infections a day per 100,000 population). The proposed decision support tool can assist in developing rational preprocedural testing policies.
Abbreviations: COVID-19, coronavirus disease 2019; HCW, healthcare worker; PCR, polymerase chain reaction; PPE, personal protective equipment; QALY, quality-adjusted life-year; RAT, rapid antigen test; SAE, serious adverse event; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
At the beginning of the coronavirus disease 2019 (COVID-19) pandemic and subsequent surges, many high-volume clinical diagnostic and therapeutic services were restricted to urgent cases. This was because of the need to divert resources toward the care of COVID-19 patients and concerns regarding the spread of COVID-19 infections among clinical staff.1 , 2 In the context of endoscopy, it has been recognized that reduced capacity for endoscopic services has resulted in an increased rate of adverse outcomes related to delayed treatment of cancers and other conditions.3 In most jurisdictions, endoscopic services have resumed while the COVID-19 pandemic/endemic persists, and services are forced to transition to a new normal4 with added complexities to manage COVID-19–related risks for patients and staff. Evidence suggests that with appropriate precautions the risk to staff becoming infected with COVID-19 during an endoscopic procedure is limited.5
Occupational health and safety is a concern among healthcare workers (HCWs), which requires attention when providing clinical services in the setting of a pandemic. Data from the early phase of the pandemic suggest a low but appreciable risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission for HCWs and patients in the setting of GI endoscopy.5 , 6 In addition to appropriate vaccination of all HCWs, a variety of measures are suitable to mitigate the infection risk, which includes the use of appropriate personal protective equipment (PPE) such as KN95 masks, limiting staff in procedure rooms, screening patients for potential COVID-19 symptoms, and postponing/testing patients with symptoms, testing of all patients, or discontinuation of services.7 Considering the importance of avoiding delays in treatment of relevant conditions such as GI cancers,8 endoscopic services have restarted worldwide.
Guidelines from professional organizations, however, recommended different approaches. The most recent guideline from the American Gastroenterological Association favors screening all elective endoscopy patients for potential COVID-19–related symptoms and testing of symptomatic patients (eg, with polymerase chain reaction [PCR] or reverse transcriptase PCR), whereas no testing is recommended for asymptomatic patients.9 This is based on low transmission rates reported from endoscopy settings and assumes the use of appropriate PPE with N95 masks.5 , 10 In contrast, the European Society for Gastrointestinal Endoscopy recommends patients undergoing GI endoscopy should be fully vaccinated or have a negative PCR test,7 but the use of rapid antigen testing (RAT) was not recommended. Irrespective of these guidelines, many HCWs prefer preprocedural COVID-19 screening with RAT or PCR in all patients to minimize personal exposure risks.
Choice of an appropriate risk mitigation during the COVID-19 pandemic/endemic is based on a variety of factors; however, it is a decision that is chosen under uncertainty. The limitations facing decision-makers in the context of complex decision-making are well established.11 The decisions are not only relevant because resource allocation for specific risk-mitigation measures should result in relevant and cost-efficient reduction of risks, but some risk-mitigation measures may increase morbidity and mortality. This is further complicated by a variety of rapidly changing factors including the prevalence of community infection, vaccination rates and the efficiency of available vaccines in relation to the prevention of SARS-CoV-2 transmission, and adverse events. The COVID-19 pandemic provides a challenge for health systems and necessitates the modification or redesign of clinical practice. Changes in practice should be based on clinical judgement, available evidence, and the balance of probabilities that a measure achieves the intended outcome.12 In this rapidly changing environment, a new level of evidence-based medicine is urgently required that allows key decision-makers (eg, clinicians) to respond with agility but without personal biases.
We outline a decision tool that can be used to inform choices of whether to test or not, enabling decision-makers to consider the estimated financial cost per expected serious adverse event (SAE), which we define as a workplace-acquired infection with subsequent death of the HCW. We argue this financial cost is like the value used to justify decisions for funding of new therapies or procedures in relation to adding a quality-adjusted life-year (QALY), which is a measure that combines the length of life with the quality of life.13 For discussion, we illustrate the argument of an SAE as equivalent to the loss of 1 QALY. As with all choices, decision-makers may differ with respect to these and other judgements based on the parameters they observe; however, our decision support tool expands the existing literature by allowing decision-makers to choose their own parameters to inform their decisions on preprocedural asymptomatic testing. This approach may not only be suitable to determine the benefit of preprocedural testing versus not testing in various scenarios but could also allow the appropriate timepoint to discontinue preprocedural testing when community transmission decreases, in line with observations by Ebigbo et al.14
Methods
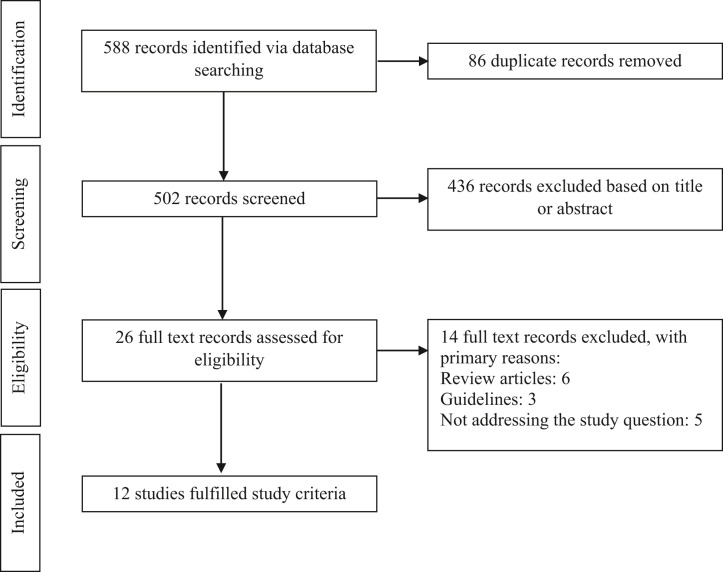
To determine the factors that influence the risk of an SAE, we conducted a scoping review, which is a map of synthesized research on a particular topic.15 We performed an electronic database search of MEDLINE and EMBASE, using the search terms in Appendix 1 (available online at www.giejournal.org), searching for peer-reviewed publications up to January 2022 for original studies providing information related to risk of COVID-19 and endoscopic services. The search was restricted to English and German articles involving humans. Articles were excluded if they did not involve a GI procedure, HCW, and COVID-19 transmission. We identified 588 publications (Appendix 2, available online at www.giejournal.org), which returned several themes surrounding risk factors for SARS-CoV-2 transmission. After discussion with clinicians, we identified an additional theme: risk modifier in the patient population. Institutional review board approval and patient consent were not required for this study.
SARS-CoV-2 transmission risk factors
Sensitivity of the screening test
The ability of screening tests to correctly identify asymptomatic carriers of disease is highly variable, with PCR as the criterion standard.16, 17, 18 Lower sensitivity rates for RAT kits have been observed in a clinical setting, with 1 indicating a 65% clinical sensitivity.19 This sensitivity varies further when comparing asymptomatic with symptomatic patients (63% vs 89%).20
Population prevalence of SARS-CoV-2 infections in the respective area
The prevalence of COVID-19 infections in the population greatly influences the probability that a patient referred for a procedure is COVID-19 positive.1
Risk modifier for the a priori risk of a SARS-CoV-2 infection in the referral population
The risk of infection is influenced by sociodemographic factors.21 In a setting where a large proportion of the population is vaccinated (eg, 80%-90%), the infection risk is usually highest in people younger than age 40 years,22 if most endoscopy patients are above 50 and below 85 years of age and the risk of infection is lower.
Proportion of asymptomatic SARS-CoV-2 carriers
A proportion of infected subjects is initially or for the entire course of the infection asymptomatic.23, 24, 25 This proportion increases in vaccinated subjects.26
Transmission rates from asymptomatic carriers
The risk to transmit a SARS-CoV-2 infection is related to symptom status because asymptomatic carriers have a lower viral load.23 With the variation in test sensitivity, the possibility of a false-negative result exists for asymptomatic patients.24
Risk of transmission in the setting of GI endoscopy with appropriate PPE
The use of PPE is highly recommended to reduce the transmission of COVID-19 in endoscopy procedures.1 , 17 , 27, 28, 29 The risk of transmission from an asymptomatic carrier to appropriately protected staff with N95 masks is low.6 Although good data support the efficacy of surgical and cloth masks in preventing COVID-19 infections in the community setting, data are limited on the efficacy of surgical masks to prevent infections of exposed HCWs in the clinical setting.30
Risk reduction of infection by vaccination
Vaccinations have been proven to reduce the risk of contracting SARS-CoV-2.27 Vaccinated HCWs have a >90% reduction in the risk of a SARS-CoV-2 infection.31 The risk reduction will differ based on the SARS-CoV-2 variant, which have different levels of transmissability.32
Risk reduction of SAE by vaccination
There is a reduced risk of an SAE in vaccinated HCWs.27 A vaccinated individual has a 70% to 78% reduction in the risk of an SAE, in terms of mortality.31 , 33
Decision-making tool comparing test sensitivities
To understand the risk and impact of SARS-CoV-2 infection for staff providing endoscopic services with or without preprocedural testing, we calculated the risk of an SAE in HCWs based on probabilities related to SARS-CoV-2 transmission and community infection in Australia. Given the small probabilities, we present estimates of the risk per 1 million endoscopy procedures. We considered factors identified in the scoping review.
Currently, some guidelines recommend the use of RAT and/or PCR testing to asymptomatic patients, and therefore we examined the probability of SAEs of HCWs under several scenarios: no testing, no PPE, and high community transmission; low test sensitivity and high community transmission (worst-case RAT); high test sensitivity and low community transmission (best-case RAT); high community transmission (worst-case PCR); low community transmission (best-case PCR); and an alternate scenario faced by governments at the beginning of the pandemic: PCR test, low community transmission, and no PPE. Only scenarios 2 through 4 use PPE, and this is classified as the use of N95 masks.
The scenarios explore the effects of the variation in RAT sensitivity rates, baseline risk of an SAE, and the availability of PPE when testing asymptomatic patients. For PCR, we used 95% sensitivity. Although the sensitivity of RAT is based on the Australian Therapeutic Goods Administration recommendation of 90% sensitivity, we did not use the Australian Therapeutic Goods Administration’s 95% recommendation because RAT kits do not have the same sensitivity as PCR. In the worst-case RAT scenario a sensitivity of 60% was used because of RAT kits having a lower clinical sensitivity for asymptomatic patients.20 , 34 Similarly, we considered the situation of community prevalence of COVID-19 over a range of 1.36% to 20.36%. In terms of risk reduction because of vaccination, we varied the risk of an SAE at 20%, 30%, and 40% because by doing so we can vary the risk of an SAE for SARS-CoV-2 variants.
We calculated the probability of an SAE based on the aforementioned factors under different scenarios and used this to determine the cost to avoid an SAE in an HCW for a million procedures with 10 staff exposed per endoscopy procedure (for the calculation, see Appendix 3, available online at www.giejournal.org). For our calculations, the cost of RAT was U.S.$6.70 and of PCR was U.S.$67.
The initial model was stress tested with a variety of scenarios35 and reviewed to develop a final model. Stress testing the model with a variety of extreme assumptions (Appendix 4, Supplementary Figures 1 and 2, available online at www.giejournal.org) revealed that the risk of SAEs for staff involved in the delivery of endoscopic services was incredibly low and likely smaller than the risk associated with SARS-CoV-2 exposure in daily life (eg, dining at a restaurant36). Nevertheless, preprocedural testing had a protective effect. Thus, the cost–benefit analysis in relation to avoiding SAEs can be assessed. A QALY can be used as a benchmark to determine the efficient and effective testing recommendation. Because there is no official threshold in Australia, we used a benchmark of U.S.$18,765 to U.S.$33,510 per SAE avoided to be recommended as cost-effective.37, 38, 39
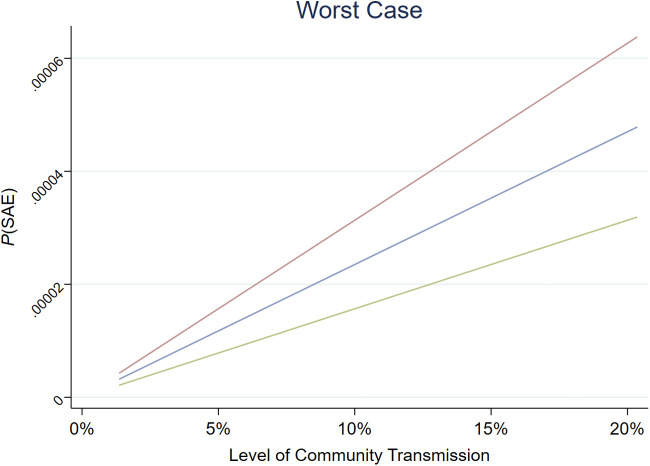
Supplementary Figure 1.
Probability of an occurrence of an SAE by community transmission rates for worst case: no personal protective equipment, no testing. Adjusted to the baseline risk of SAE for vaccinated individuals. Blue line, risk of SAE = 30%; green line, risk of SAE = 20%; red line, risk of SAE = 40%. SAE, Serious adverse event.
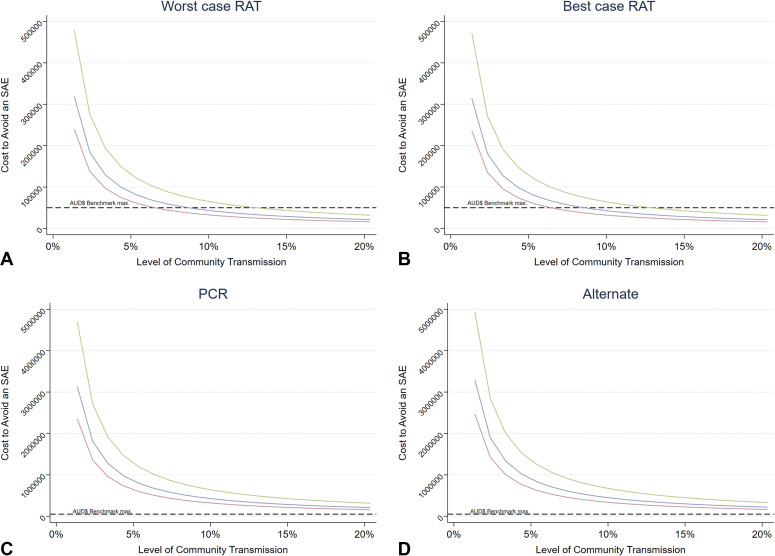
Supplementary Figure 2.
A-D, Probability of an occurrence of an SAE by community transmission rates across different testing scenarios. Adjusted to the baseline risk of SAE for vaccinated individuals. A, The expected cost to avoid an SAE under the worst case RAT scenario. B, The expected cost to avoid an SAE under the best case RAT scenario. C, The expected cost to avoid an SAE under the PCR test scenario. D, The expected cost to avoid an SAE under the alternate scenario. Blue line, risk of SAE = 30%; green line, risk of SAE = 20%; red line, risk of SAE = 40%. SAE, Serious adverse event; RAT, rapid antigen testing; PCR, polymerase chain reaction.
Results
Costs and benefits of testing
When comparing the risk of an adverse outcome for an HCW between no asymptomatic testing versus RAT (with variable RAT sensitivities) or PCR testing for a million endoscopic procedures, the occurrence of an SAE is <1 in a million procedures when community transmission is low for RAT screening or PPE and PCR testing are used (Table 1 ). The worst-case RAT scenario had an expected occurrence of 2.35 SAEs in a million procedures involving 10 staff members during every procedure (eg, staff in the procedure room, recovery staff). Although the lowest number of expected SAEs (n = .079) was in the best-case PCR scenario, the value of using PPE was demonstrated when comparing the occurrence of an SAE in the alternate strategy and best-case PCR scenario, with the SAE per million procedures reducing from 1.59 to .079, respectively.
Table 1.
Factors influencing the risk of COVID-19–related adverse outcomes for healthcare workers in the endoscopy setting in Australia
| Pandemic/endemic scenario |
||||||
|---|---|---|---|---|---|---|
| No test, high community transmission, no PPE | Worst-case RAT: low test sensitivity and high community transmission | Best-case RAT: high test sensitivity and low community transmission | Worst-case PCR: high community transmission | Best-case PCR: low community transmission | Alternate: PCR, high community transmission, no PPE | |
| Factors | ||||||
| Test sensitivity,18,33 % | 0 | 60 | 90 | 95 | 95 | 95 |
| Population infected with SARS-CoV-2, % | 5 | 5 | 1.36 | 5 | 1.36 | 1.36 |
| Age-adjusted risk of SARS-CoV-2 infection in endoscopy patient population (age >50 y), % | 15.89 | 15.89 | 15.89 | 15.89 | 15.89 | 15.89 |
| Proportion of SARS-CoV-2 cases without symptoms,22 % | 17 | 17 | 17 | 17 | 17 | 17 |
| Transmission rates | ||||||
| Risk of infection from asymptomatic carrier compared with symptomatic carrier,22 % | 58 | 58 | 58 | 58 | 58 | 58 |
| Full PPE use (prevents >95% of transmissions), % | 100 | 5 | 5 | 5 | 5 | 100 |
| Risk reduction of infection for vaccinated,30 % | 95 | 95 | 95 | 95 | 95 | 95 |
| Risk reduction for SAE for vaccinated,30 % | 70 | 70 | 70 | 70 | 70 | 70 |
| No. of endoscopy staff exposed per procedure | 10 | 10 | 10 | 10 | 10 | 10 |
| No. of SAEs per 1,000,000 procedures | 117.50 | 2.35 | .159 | .293 | .079 | 1.598 |
| Cost per test, U.S.$ | — | 6.70 | 6.70 | 67 | 67 | 67 |
| Cost to avoid 1 COVID-19 staff fatality, & | — | 86,838.41 | 85,217.53 | 853,149.26 | 851,595.48 | 862,749.79 |
COVID-19, Coronavirus disease 2019; PCR, polymerase chain reaction; PPE, personal protective equipment; RAT, rapid antigen test; SAE, serious adverse event; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; —, calculation not required.
The direct cost of preprocedural COVID-19 testing in endoscopic patients will vary based on the current level of transmission among the community and patient population (Fig. 1 ). When considering higher community infection rates with subsequent high transmission rates (eg, 18.3% community transmission) in nonhealthcare environments, the cost to avoid 1 SAE using RAT with 60% sensitivity is U.S.$58199.10 at 5% community transmission when compared with a no-testing strategy. This cost remains above the Australian benchmark of U.S.$18,765 for mortality-related QALY gained to $33,510 per QALY for pharmaceuticals; however, as the level of community transmission increases, the cost to avoid an SAE will be below the benchmark (Fig. 1). For preprocedural PCR, the cost of avoiding an SAE remains greater than the benchmark no matter the rate of community transmission when PCR tests are U.S.$67.
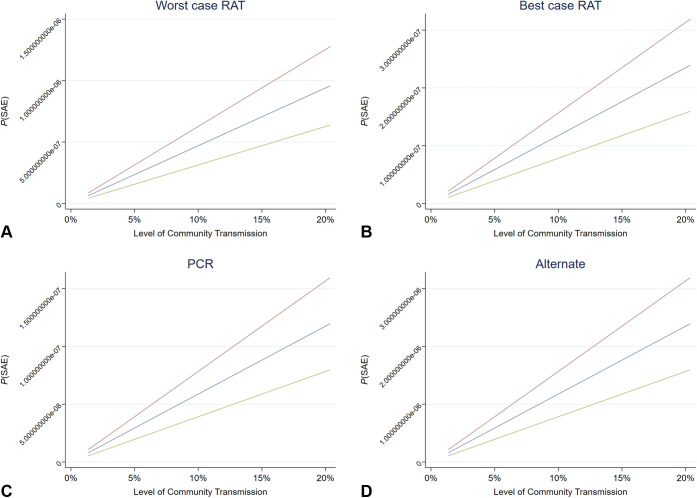
Figure 1.
A-D, Expected costs of testing asymptomatic patients under different levels of risk of an SAE. A, The expected cost to avoid an SAE under the worst case RAT scenario. B, The expected cost to avoid an SAE under the best case RAT scenario. C, The expected cost to avoid an SAE under the PCR test scenario. D, The expected cost to avoid an SAE under the alternate scenario. Blue line, risk of SAE = 30%; green line, risk of SAE = 20%; red line, risk of SAE = 40%. SAE, Serious adverse event; RAT, rapid antigen testing; PCR, polymerase chain reaction.
We observed that at any rate of transmission in the community and patient population for the probability of an SAE, a no-testing strategy using PPE would be more cost-effective than testing when no PPE is used regardless of test sensitivity level, community transmission, and the risk of an SAE or infection of a vaccinated HCW in comparison with no testing. In the case of a 5% level of transmission, when no testing occurs but PPE is used, there is a P(SAE) = 1.59809E-07. Despite the variation in probabilities of an SAE, when we make decisions on who should have testing, we must consider the opportunity costs to the allocation of testing resources and the opportunity cost of the financial resource allocation. The decision tool, which is a template of the calculations described above, is available online at www.giejournal.org.
Opportunity costs of testing
Screening for COVID-19 is an essential health provision when infections are rising. However, this screening is constrained by the health system’s resources to facilitate the demand for testing. The supply of PCR tests is restricted by laboratory capacity and RAT by supply, and in periods of high demand, the infrastructure for testing is unable to meet demand. As such, specific decisions on who and when individuals are required to be tested are made, often with the intention of reducing transmission for target groups. The criteria for those who are eligible for testing do come with trade-offs.
If there are no constraints on the availability of RAT or PCR, then the test with the greatest sensitivity is the optimal choice. At current levels of RAT sensitivity, it is likely that if preprocedural testing for asymptomatic patients is conducted, PCR testing outperforms RAT. However, this assumption of greater test sensitivity is limited because we assume a negative PCR 3 days to 24 hours before the procedure implies there is no risk of infecting others during the procedure. In our tool, the reliability of PCR testing can be adjusted to accommodate the risk of infection within the usual delay of PCR tests available and the risk of becoming infected between test and procedure, which could change the choice of RAT versus PCR.
Finally, when community transmission is high, the direct opportunity cost of not conducting a procedure because of a positive preprocedural test result should be considered. Although it has been established that 2020 closures of screening procedures are associated with adverse health impacts because of delayed diagnosis of, for example, colon cancers,40 we should consider that a positive test reduces the capacity to provide endoscopies and increases health costs because of delayed screening procedures.
Discussion
The COVD-19 pandemic necessitates decisions in a rapidly changing environment. For example, the use of preprocedural testing mitigates the risk of SARS-CoV-2 infections in HCWs. This choice to screen or not creates a challenging set of decisions for standard medical testing and screening procedures, such as colon cancer screening procedures conducted in endoscopy departments. Because many of these decisions involve uncertainty, caused by small probabilities, decision-makers are highly likely to be affected by cognitive biases. The calculations and simulations we have provided in this tool aim to enable clinicians to consider relevant factors and facilitates the ability to compare choices for COVID-19 testing and screening policies and adjust for changes to relevant parameters as they change over time. Furthermore, this tool can be adapted for use in other settings, because the principle of the tool is to guide decision-making. This tool is developed from a cost-effectiveness analysis; however, instead of comparing between different testing strategies, the tool allows decision-makers to make an informed choice on the testing strategy based on the different sensitivities of testing strategies compared with no preprocedural testing. The tool enables one to test a variety of scenarios and different types of SAEs (eg, hospitalization) using relevant information for their hospital, region, and risk profile and inform their judgement on test reliability and costs. Thus, the decision-maker can make a more evidence-based decision rather than one affected by behavioral biases regarding underlying risks. Such a tool can be used to navigate through unknown situations, thereby supporting the decision-maker to achieve better outcomes, without affecting their ability to make the decision while taking other considerations (ie, political, emotional, or organizational) into account.
Most practices have followed the guidelines set out by their respective professional association or societies and stratify based on symptoms, which do not focus on asymptomatic patients. However, many clinicians still prefer preprocedural COVID-19 screening for asymptomatic patients. The recommended use of preprocedural RAT and PCR testing in endoscopic patients may be unnecessarily delaying procedures and diverting resources to activities with no or marginal benefits. This is an administrative decision that should be based on the characteristics of the underlying situation with respect to the risks, benefits, and costs involved of testing for COVID-19. Although some factors are inherently uncertain, we now have reasonably reliable data about the probabilities of COVID-19 infection and the risk of SAEs such as COVID-19–related death. Careful considerations of all these parameters should help to overcome potential risks of overweighting small probabilities and consequences to choose appropriate screening strategies.
Although direct costs of administering tests do seem to be high compared with other health expenditures, considering avoidance of SAEs, once the potential opportunity costs are considered, the recommended decision of testing asymptomatic patients may change. For instance, during a period of high rates of community infection, testing capacity may be limited. A test for asymptomatic patients who are due for an endoscopy may reduce the opportunity to test people who do not require an endoscopy with symptoms or other indications. Our arguments and the tool can provide the relevant information to make these decisions while accounting for medical, administrative, and political/social considerations. Hence, this tool focuses on environmental factors and not the underlying cost of different strategies (eg, RAT vs PCR).
When considering the opportunity cost, the logic of testing changes, because under low community infection rates the cost per avoided SAE among staff is higher but still within range compared with other health policy decisions, whereas when there are few positive test results, the effect of lost capacity is much lower as well. Once community infection rates are higher, testing significantly affects a hospital’s ability to operate at capacity, and the decision may change. In addition, a potential shortage of test capacity within a health system may further weaken the case for a testing policy but would require the determination of where a healthcare resource in limited supply provides the greatest benefit. Furthermore, we did not differentiate for N95 or flat surgical masks because the guidelines of the European Society for Gastrointestinal Endoscopy did not assume a difference based on a systematic review and meta-analysis.41 Finally, the decision support tool can provide evidence to comply with or diverge from recommended guidelines. For example, the European Society for Gastrointestinal Endoscopy7 recommends that no testing is provided to fully vaccinated individuals, but by using the decision support tool, a facility can determine the appropriate level of risk they are willing to take if a vaccinated individual is asymptomatic, especially if the opportunity cost of having staff on leave is greater in some areas than others (eg, consider low- and middle-income countries). Similarly, the guidelines from the American Gastroenterological Association9 recommend routine preprocedure testing for SARS-CoV2 in patients scheduled to undergo endoscopy. Although these experts placed a high value on minimizing additional delays in patient care and potential downstream effects in relation to delays of patient care, staff safety and the costs associated with preprocedural testing were not considered.
However, our approach with an adaptable decision support tool is also not without limitations. We have specified the setting of endoscopic procedures, but the risk of transmission was not broken down into aerosol-generating versus nonaerosol-generating procedures. A breakdown of this risk will lead to a greater understanding of the specific risk of individual procedures, but at the current level the risk would be a slight overestimation. Moreover, most endoscopists provide a mix of services, and the available data do not allow an allocation to different risks to lower or upper endoscopy. Additionally, our approach focused on the worst possible outcome (fatality) of a workplace-related SARS-CoV-2 transmission to HCWs. Thus, we did not include other adverse effects of SARS-CoV-2 transmission, such as sick leave of infected staff, impact on service capacity, hospitalization, and so on. We also did not consider the death of a patient as the outcome of interest, because most focus of health department governance has been protecting HCWs. Furthermore, we did not incorporate transactional costs to collect swabs or fluid samples for further testing or the burden of canceled or missed procedures because of a patient’s positive COVID-19 test. We also did not consider the costs on the staffing capacity of the endoscopy unit if infection or SAE does occur. If asymptomatic testing does not occur and a procedurally transmitted COVID-19 infection does occur, then HCW shortages will impact the capacity of the unit to provided services. Using such SAEs is a reasonable simplification considering these costs (and benefits) are linear yet highly variable across systems and could be relatively easily incorporated in local modeling.
We did not incorporate the relevance of testing for the service delivery (eg, additional resources required to perform the tests), including delay of services until the test results are available. Additionally, the possibility of false positives and negatives might alter decision-making and complicate the processes. On the other hand, the proposed approach provides a tool that allows the incorporation and adjustment for a multitude of factors to provide decision support. This tool is rapidly adoptable to inform choices by incorporating clinical judgement, available evidence, and the balance of probabilities of the intended outcome. Thus, the proposed decision tool provides support in complex and potentially rapidly changing environments. Instead of proposing a specific measure (eg, use of a specific test before endoscopic procedures), future guidelines could articulate the acceptable risk level. This would provide a decision framework that avoids biases and allows rational allocation of resources to maximize benefits in relation to staff safety and patient outcomes.
Footnotes
DISCLOSURE: The following authors disclosed financial relationships: N. J. Talley: Nonfinancial supportfromHVN National Science Challenge NZ; Personal fees for consulting fromAviro Health,Anatara Life Sciences,Brisbane,Allakos,Bayer,Planet Innovation,Viscera Labs,Progenity Inc,Glutagen,IsoThrive,BluMaiden,Rose Pharma,Intrinsic Medicine, andComvita Mānuka Honey; Patent-holder for Nepean Dyspepsia Index, Biomarkers of IBS, Licensing Questionnaires Talley Bowel Disease Questionnaire licensed to Mayo/Talley, Nestec European, Singapore Provisional Patent and “Diagnostic marker for functional gastrointestinal disorders” Australian Provisional Patent Application 2021901692; Committee member for OzSage, Rome V Working Team Member (Gastroduodenal Committee) (2021-), Australian Medical Council, Australian Telehealth Integration Programme, International Plausibility Project Co-Chair (Rome Foundation funded) (2021-), COVID-19 vaccine forum member (by invitation only) (2021-), and NHMRC Principaland Committee Asia Pacific Association of Medical Journal Editors; Community groups, Advisory Board, IFFGD (International Foundation for Functional GI Disorders) and AusEE; Editor for Medical Journal of Australia (Editor in Chief), Up to Date (Section Editor), Precision and Future Medicine, Sungkyunkwan University School of Medicine, South Korea, and Journal of Cell Press; Research funding from the National Health and Medical Research Council to the Centre for Research Excellence in Digestive Health, NHMRHC Investigator. All other authors disclosed no financial relationships.
Appendix
Appendix 1. Scoping Review Search Terms
Search terms
PubMed:
COVID-19 MESH
AND
Transmission MESH
AND
ENDOSCOPY MESH
AND
Vaccine MESH
AND Efficacy
Add together 502 together
(((COVID 19) AND (Transmission)) AND (Endoscopy)) AND (Vaccines) = 13
(((COVID 19) AND (Transmission)) AND (Endoscopy)) = 502
(Prevention) AND ((((COVID 19) AND (Transmission)) AND (Endoscopy))) =386
Other MESH terms
MeSH terms
-
•
COVID-19 / prevention & control∗
-
•
COVID-19 / transmission
-
•
COVID-19 Vaccines∗
-
•
Endoscopy, Digestive System∗
-
•
Humans
-
•
Infection Control
-
•
Infectious Disease Transmission, Patient-to-Professional / prevention & control∗
-
•
Pandemics
-
•
Personal Protective Equipment∗
Appendix 2
Scoping review Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram
Appendix 3
Calculations for the probability of serious adverse events
Here we calculate the probability of a serious adverse event (SAE) and the costs to avoid a SAE under the different levels of sensitivity. First, we consider the probability of infection and an adverse outcome using the probability of infection based on aforementioned factors, multiplied by the number of staff members involved in the procedure, which is then multiplied to represent a million procedures. The probability (P) of a severe adverse outcome is calculated as follows:
The absolute risk per staff involved in a procedure is then calculated as
To put the risk of an SAE for healthcare workers into perspective, we then multiply the risk by a million procedures. This is then the number of SAEs per million procedures.
To measure the direct cost of avoiding an SAE at different levels of testing sensitivity, we use the cost of the individual tests and probability of an SAE to determine the expected number of SAEs. This is then used to determine the incremental cost of avoiding an SAE between no testing and the different sensitivities of testing kits.
where, C ij is the cost to avoid 1 SAE between a no-testing strategy i and an alternate strategy j. C i is the cost for no testing by a million procedures, and C j is the cost of a screening strategy with varying sensitivity for a million procedures. The number of SAEs is determined by using the P(SAE) for strategy i or j per million procedures.
Appendix 4
Demonstrated probability of serious adverse events
The probability of a serious adverse event for variations in population infection levels of severe acute respiratory syndrome coronavirus 2 and baseline risk of a serious adverse event.
References
- 1.Chiu P.W.Y., Ng S.C., Inoue H., et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements) Gut. 2020;69:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gralnek I.M., Hassan C., Dinis-Ribeiro M. COVID-19 and endoscopy: implications for healthcare and digestive cancer screening. Nat Rev Gastroenterol Hepatol. 2020;17:444–446. doi: 10.1038/s41575-020-0312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samani S., Mir N., Naumann D.N., et al. COVID-19 and endoscopic services: the impact of delays in therapeutic colonoscopies on patients. Gut. 2021;70:2019–2020. doi: 10.1136/gutjnl-2021-324112. [DOI] [PubMed] [Google Scholar]
- 4.Holtmann G., Quigley E.M., Shah A., et al. "It ain't over … till it's over!" Risk-mitigation strategies for patients with gastrointestinal diseases in the aftermath of the COVID-19 pandemic. J Gastroenterol Hepatol. 2020;35:1117–1123. doi: 10.1111/jgh.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Repici A., Aragona G., Cengia G., et al. Low risk of COVID-19 transmission in GI endoscopy. Gut. 2020;69:1925–1927. doi: 10.1136/gutjnl-2020-321341. [DOI] [PubMed] [Google Scholar]
- 6.Hayee B.H., The SCOTS II Project group, Bhandari P., et al. COVID-19 transmission following outpatient endoscopy during pandemic acceleration phase involving SARS-CoV-2 VOC 202012/01 variant in UK. Gut. 2021;70:2227–2229. doi: 10.1136/gutjnl-2021-324354. [DOI] [PubMed] [Google Scholar]
- 7.Gralnek I.M., Hassan C., Ebigbo A., et al. ESGE and ESGENA position statement on gastrointestinal endoscopy and COVID-19: updated guidance for the era of vaccines and viral variants. Endoscopy. 2022;54:211–216. doi: 10.1055/a-1700-4897. [DOI] [PubMed] [Google Scholar]
- 8.Quigley E.M.M. Colon cancer detection and prevention in the age of COVID-19 [editorial] Curr Opin Gastroenterol. 2021;37:37–38. doi: 10.1097/MOG.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 9.Sultan S., Siddique S.M., Singh S., et al. AGA rapid review and guideline for SARS-CoV2 testing and endoscopy post-vaccination: 2021 update. Gastroenterology. 2021;161:1011–1029. doi: 10.1053/j.gastro.2021.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somerville C.C., Shoaib M., Kuschner C.E., et al. Prospective analysis of SARS-CoV-2 dissemination to environmental surfaces during endoscopic procedures. Endosc Int Open. 2021;9:E701–E705. doi: 10.1055/a-1395-6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushniruk A.W. Analysis of complex decision-making processes in health care: cognitive approaches to health informatics. J Biomed Inform. 2001;34:365–376. doi: 10.1006/jbin.2001.1021. [DOI] [PubMed] [Google Scholar]
- 12.Carley S., Horner D., Body R., et al. Evidence-based medicine and COVID-19: what to believe and when to change. Emerg Med J. 2020;37:572–575. doi: 10.1136/emermed-2020-210098. [DOI] [PubMed] [Google Scholar]
- 13.Prieto L., Sacristán J.A. Problems and solutions in calculating quality-adjusted life years (QALYs) Health Qual Life Outcomes. 2003;1:80. doi: 10.1186/1477-7525-1-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebigbo A., Römmele C., Bartenschlager C., et al. Cost-effectiveness analysis of SARS-CoV-2 infection prevention strategies including pre-endoscopic virus testing and use of high risk personal protective equipment. Endoscopy. 2021;53:156–161. doi: 10.1055/a-1294-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colquhoun H., Levac D., O'Brien K., et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67:1291–1294. doi: 10.1016/j.jclinepi.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Treggiari D., Piubelli C., Caldrer S., et al. SARS-CoV-2 rapid antigen test in comparison to RT-PCR targeting different genes: a real-life evaluation among unselected patients in a regional hospital of Italy. J Med Virol. 2022;94:1190–1195. doi: 10.1002/jmv.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalal A., Sonika U., Kumar M., et al. COVID-19 rapid antigen test: role in screening prior to gastrointestinal endoscopy. Clin Endosc. 2021;54:522–525. doi: 10.5946/ce.2020.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krüger L.J., Tanuri A., Lindner A.K., et al. Accuracy and ease-of-use of seven point-of-care SARS-CoV-2 antigen-detecting tests: a multi-centre clinical evaluation. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jegerlehner S., Suter-Riniker F., Jent P., et al. Diagnostic accuracy of a SARS-CoV-2 rapid antigen test in real-life clinical settings. Int J Infect Dis. 2021;109:118–122. doi: 10.1016/j.ijid.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turcato G., Zaboli A., Pfeifer N., et al. Rapid antigen test to identify COVID-19 infected patients with and without symptoms admitted to the emergency department. Am J Emerg Med. 2022;51:92–97. doi: 10.1016/j.ajem.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H., Wang S., Zhong F., et al. Age-dependent risks of incidence and mortality of COVID-19 in Hubei Province and other parts of China. Front Med. 2020;7:190–195. doi: 10.3389/fmed.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Australian Institute of Health and Welfare. Welfare AIoHa . Australia’s youth: COVID-19 and the impact on young people. Australian Government; 2021. Available at: https://www.aihw.gov.au/reports/children-youth/covid-19-and-young-people. Accessed January 30, 2022. [Google Scholar]
- 23.Byambasuren O., Cardona M., Bell K., et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. JAMMI. 2020;5:223–234. doi: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peery A.F., Arora S., Shaheen N.J. Reviving routine gastrointestinal endoscopy in the COVID-19 era. Am J Gastroenterol. 2020;115:1376–1379. doi: 10.14309/ajg.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S., Shahidi N., Gilroy N., et al. Proposal for the return to routine endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020;92:735–742. doi: 10.1016/j.gie.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonelli M., Penfold R.S., Merino J., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elli L., Rimondi A., Tontini E., et al. Endoscopy during the COVID-19 pandemic: Is it time to down-grade personal protective equipment for vaccinated personnel? Dig Liver Dis. 2021;53:801–802. doi: 10.1016/j.dld.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddique S.M., Sultan S., Lim J.K., et al. Spotlight: COVID-19 PPE and endoscopy. Gastroenterology. 2020;159:759. doi: 10.1053/j.gastro.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zellmer S., Ebigbo A., Kahn M., et al. Evaluation of the ESGE recommendations for COVID-19 pre-endoscopy risk-stratification in a high-volume center in Germany. Endosc Int Open. 2021;9:E1556–E1560. doi: 10.1055/a-1526-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard J., Huang A., Li Z., et al. An evidence review of face masks against COVID-19. Proc Natl Acad Sci USA. 2021;118:1–12. doi: 10.1073/pnas.2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas E.J., Angulo F.J., McLaughlin J.M., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore J.P., Offit P.A. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325:821–822. doi: 10.1001/jama.2021.1114. [DOI] [PubMed] [Google Scholar]
- 33.Jabłońska K., Aballéa S., Toumi M. The real-life impact of vaccination on COVID-19 mortality in Europe and Israel. Public Health. 2021;198:230–237. doi: 10.1016/j.puhe.2021.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Department of Health and Aged Care, Australian Government . Therapeutic Goods Administration; 2022. COVID-19 rapid antigen self-tests that are approved in Australia. Available at: https://www.tga.gov.au/products/covid-19/covid-19-tests/covid-19-rapid-antigen-self-tests-home-use/covid-19-rapid-antigen-self-tests-are-approved-australia. Accessed July 1, 2022. [Google Scholar]
- 35.Barbieri M.M., Berger J.O. Optimal predictive model selection. Ann Stat. 2004;32:870–897. [Google Scholar]
- 36.Galmiche S., Charmet T., Schaeffer L., et al. Exposures associated with SARS-CoV-2 infection in France: a nationwide online case-control study. Lancet Region Health Europe. 2021;7:100148. doi: 10.1016/j.lanepe.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edney L.C., Haji Ali Afzali H., et al. Estimating the reference incremental cost-effectiveness ratio for the australian health system. PharmacoEconomics. 2017;36:239–252. doi: 10.1007/s40273-017-0585-2. [DOI] [PubMed] [Google Scholar]
- 38.George B., Harris A., Mitchell A. Cost-effectiveness analysis and the consistency of decision making: evidence from pharmaceutical reimbursement in Australia (1991 to 1996) PharmacoEconomics. 2001;19:1103–1109. doi: 10.2165/00019053-200119110-00004. [DOI] [PubMed] [Google Scholar]
- 39.Raftery J.P. Paying for costly pharmaceuticals: regulation of new drugs in Australia, England and New Zealand. Med J Australia. 2008;188:26–28. doi: 10.5694/j.1326-5377.2008.tb01500.x. [DOI] [PubMed] [Google Scholar]
- 40.Rutter M.D., Brookes M., Lee T.J., et al. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database analysis. Gut. 2021;70:537–543. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 41.Bartoszko J.J., Faroogi M.A.M., Alhazzani W., et al. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020;14:365–373. doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]