Abstract
Background
In December 2021, the SARS-CoV-2 Omicron variant displaced the Delta variant and caused an unprecedented spike in the numbers of COVID-19 cases. This study reports the positivity rates of circulating non-SARS-CoV-2 respiratory viruses and evaluates coinfections of these viruses with SARS-CoV-2 during the Omicron surge.
Methods
Data from the multiplex respiratory panels used for diagnosis at the Johns Hopkins Microbiology Laboratory were used to assess positivity rates and respiratory virus coinfections in the time frame between November 2021 and February 2022. Clinical presentations and outcomes were assessed in the cohort of 46 patients who had SARS-CoV-2 coinfections with other respiratory viruses.
Results
Between November 2021 and February 2022, the high positivity of SARS-CoV-2 outcompeted enterovirus/rhinovirus and other circulating respiratory viruses and was associated with a notable decrease in influenza A infections. Coinfections represented 2.3% of the samples tested by the extended multiplex respiratory panel. SARS-COV-2 coinfections represented 25% of the coinfections in this time frame and were mostly SARS-COV-2/enterovirus/rhinovirus. Of the SARS-CoV-2 coinfection cohort, 3 patients were hospitalized and were coinfected with influenza-A (2) or RSV (1). Cough and shortness of breath were the most frequent symptoms (29%) followed by fever (28%).
Conclusions
The SARS-CoV-2 Omicron surge was associated with a change in the circulation of other respiratory viruses. Coinfections were most prevalent with viruses that showed the highest positivity in this time frame.
Keywords: Respiratory viruses, Influenza, Enterovirus/ rhinovirus, Coinfection
1. Introduction
In November 2021, the World Health Organization (WHO) designated the SARS-CoV-2 variant, Omicron (B.1.1.529), as a variant of concern (VOC) [1, 2]. Omicron rapidly displaced the circulating Delta variant and was associated with a remarkable increase in symptomatic and asymptomatic COVID-19 cases [3, 4]. The impact of Omicron on the circulation of other respiratory viruses, particularly influenza, has been an area of major interest.
We have previously reported the impact of SARS-CoV-2 on common respiratory viruses prior to the circulation of Omicron [5]. In our study we showed that shortly after the relaxation of the infection control measures in late spring of 2021, the circulation of respiratory viruses, including mainly respiratory syncytial virus (RSV) and enterovirus/ rhinovirus, showed a marked increase. In the same study, we reported that coinfections with SARS-CoV-2 were uncommon. In this study, we report the positivity rates of SARS-CoV-2 relative to other respiratory viruses in the time frame between November 2021 and February 2022. We also report viral coinfections with SARS-CoV-2 from November 2021 to January 2022.
2. Materials and methods
2.1. Respiratory viral testing
Clinical testing was performed on nasal/ nasopharyngeal samples collected in Universal Transport Media (UTM), at the Johns Hopkins Medical Microbiology laboratory. Testing for SARS-CoV-2 during the time frame of the study (November 2021 to February 2022) was performed using the following molecular assays: Xpert Xpress SARS-CoV-2/Flu/RSV (Cepheid, Sunnyvale, CA), NeuMoDx SARS-CoV-2 (Qiagen, Hilden, Germany), Cobas SARS-CoV-2 (Roche, Indianapolis, IN), ePlex RP2 (Roche, Indianapolis, IN), Aptima SARS-CoV-2 (Hologic) and Accula SARS-CoV-2 assays (ThermoFisher Scientific, Waltham, MA). Respiratory panels used for diagnosis include the Xpert Xpress SARS-CoV-2/Flu/RSV (Cepheid, Sunnyvale, CA) (used for Influenza A and B detection along with RSV and SARS-CoV-2) [6] in addition to the Respiratory Panel (RP2) of the ePlex platform which detects the following targets: Adenovirus, human coronavirus (HCoV)−229E, HCoV-HKU1, HCoV-NL63, HCoV-OC43, human Metapneumovirus (HMPV), enterovirus/rhinovirus, influenza A/A H1/A H1–2009/A H3, influenza B, parainfluenza (HPIV)1–4, RSV A, RSV B, Chlamydia pneumoniae, Mycoplasma pneumoniae, and SARS-CoV-2 [7]. Clinical testing follows ordering algorithms as previously described [5].
2.2. Clinical data
Patients’ information, age and clinical data including vaccination status, chief complaints and outcomes were collected by manual clinical chart reviews. For SARS-CoV-2 positive patients, full vaccination prior to infection was based on the CDC definition of positive test results more than 14 days post the second shot for pfizer/BioNTech BNT162b2 and Moderna mRNA-1273 or 14 days after the J&J/Janssen, but no booster doses.
3. Results
3.1. Circulating respiratory viruses and positivity rates
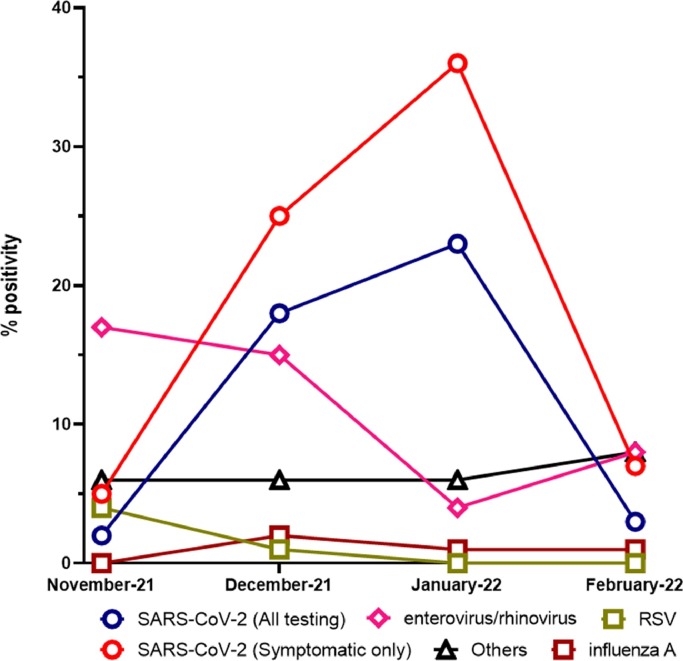
Respiratory viral testing and positivity trends were collected for samples tested at the Johns Hopkins system in the time frame from November 1st 2021 to February 28th 2022. A total of 39,122 respiratory samples were tested with both the Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV (35,809) and ePlex RP2 (3313) panels (Table 1 ). For SARS-CoV-2, a total of 120,508 specimens were tested in the same time frame with 13,799 positives in symptomatic patients (Table 1). Before the Omicron surge, and the peak of SARS-CoV-2 positivity in January 2022 (Fig. 1 ), enterovirus/rhinovirus positivity rate was the highest in November 2021 (17.73%), then started to decrease to 15.37% in December 2021 and 4.57% in January 2022, followed by a small increase in February (8.85%). Influenza A positivity increased in December 2021 (2.98%) and then remained at nearly the same level or slightly decreased to 1.79% in February 2022, correlating with the large increase in SARS-CoV-2 positivity. Other respiratory viruses showed minimal to no circulation, including RSV that showed a decrease from 4.8% to 0.45%, HPIV2 (1.59% – 0%), and HPIV3 (1.39% – 0.17%). On the other hand, adenovirus and HCoV positivity increased from 0.93% and 0.66% respectively in November 2021 – 3.3% and 2.43% in February 2022. Only very few positive Influenza B (0.01%) and no HPIV1 (0%) cases were reported (Table 1).
Table 1.
Respiratory panel testing, respiratory pathogens, and total positives per target at Johns Hopkins between November 2021 and February 2022 using the extended respiratory panel (ePlex GenMark, RP2) and the Xpert Xpress SARS-CoV-2/Flu/RSV (Cepheid). RSV (respiratory syncytial virus), HCoV (coronavirus; HKU1, NL63, 229E, OC43), HMPV (human metapneumovirus), HPIV (human parainfluenza virus).
| Total Tested | November- 2021 | December- 2021 | January-2022 | February-2022 |
|---|---|---|---|---|
| ePlex (RP2) (3313) | 863 | 976 | 898 | 576 |
| Xpert Xpress SARS-CoV-2/Flu/RSV (35,809) | 7316 | 13,108 | 9582 | 5803 |
| SARS-CoV-2 (120,508) | 27,148 | 37,944 | 33,129 | 22,287 |
| Single targets | Total Positives (%) | |||
| influenza A | 53 (0.72) |
390 (2.98) |
130 (1.36) |
104 (1.79) |
| influenza B | 1 (0.01) |
1 (0.01) |
0 (0.00) |
1 (0.02) |
| RSV | 353 (4.83) |
238 (1.82) |
52 (0.54) |
26 (0.45) |
| adenovirus | 8 (0.93) |
19 (1.95) |
20 (2.23) |
19 (3.30) |
| HCoV | 5 (0.58) |
13 (1.33) |
15 (1.67) |
14 (2.43) |
| HMPV | 12 (1.39) |
19 (1.95) |
20 (2.23) |
11 (1.91) |
| enterovirus/rhinovirus | 153 (17.73) |
150 (15.37) |
41 (4.57) |
51 (8.85) |
| HPIV1 | 0 (0.00) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
| HPIV2 | 12 (1.39) |
2 (0.20) |
0 (0.00) |
0 (0.00) |
| HPIV3 | 12 (1.39) |
9 (0.92) |
1 (0.11) |
1 (0.17) |
| HPIV4 | 3 (0.35) |
6 (0.61) |
1 (0.11) |
1 (0.17) |
| SARS-CoV-2 | 726 (2.67) |
7078 (18.65) |
7838 (23.66) |
838 (3.76) |
| SARS-CoV-2 (Symptomatic only) | 592 (5.70) |
6236 (25.50) |
6438 (36.70) |
533 (7.59) |
| SARS-CoV-2 by RP2 (% to total tested by RP2) | 17 (1.97) | 99 (10.14) | 124 (13.81) | 19 (3.3) |
| SARS-CoV-2 by Xpert Xpress SARS-CoV-2/Flu/RSV (% to total tested by Xpert) | 115 (1.57) | 672 (5.13) | 861 (8.99) | 100 (1.72) |
Fig. 1.
Respiratory virus positivity rates from November 2021 through February 2022. Others include adenovirus, HPMV, influenza B, HCoV, and HPIV 1- 4. HCoV (coronavirus (229E, HKU1, NL63, OC43), RSV (respiratory syncytial virus), HMPV (human metapneumovirus), HPIV (human parainfluenza virus).
3.2. Coinfections with respiratory viruses
Only the data from the ePlex (RP2) were used to assess coinfections with two or more targets as detailed in Table 2 (to compare coinfections of SARS-CoV-2 with other respiratory viruses). Coinfections with two or more viruses reached a total of 72 out of 3313 total tested samples (2.3%) in the timeframe of November 2021 – February 2022 . Coinfections with enterovirus/rhinovirus were the most frequent (54, 75%, Table 2), followed by coinfections with adenovirus (26, 36%), then SARS-CoV-2 (19, 26%). Interestingly, no coinfections with SARS-CoV-2 and influenza A were detectable in this cohort.
Table 2.
Detailed coinfections using the extended respiratory panel (RP2). HCoV (coronavirus (229E, HKU1, NL63, OC43), RSV (respiratory syncytial virus), HMPV (human metapneumovirus), HPIV (human parainfluenza virus).
| Year | Month (Total coinfections, ≥2 targets = 72) | Target 1 | Target 2 | Target 3 | Total |
|---|---|---|---|---|---|
| 2021 | November (23) | enterovirus/rhinovirus | HPIV2 (6), HPIV3 (3), RSVA (1), RSVB (1), | 11 | |
| Adenovirus | enterovirus/rhinovirus (5), HCoV (1) | 6 | |||
| HMPV | enterovirus/rhinovirus (3) | 3 | |||
| SARS-CoV-2 | RSVB (1), HPIV3(1) | 2 | |||
| HPIV2 | RSVB (1) | 1 | |||
| December (25) | Adenovirus | enterovirus/rhinovirus (6), HMPV (1), HCoV (1) | 8 | ||
| enterovirus/rhinovirus | RSVB (2), HPIV2 (1), HPIV3 (2), HPIV4 (1) | 6 | |||
| SARS-CoV-2 | enterovirus/rhinovirus (4) | HPIV3 (1) | 5 | ||
| HCoV | SARS-CoV-2(2), enterovirus/rhinovirus (1) | 3 | |||
| HPIV3 | RSVB (1) | 1 | |||
| Influenza B | Chlamydia pneumoniae (1) | 1 | |||
| HMPV | enterovirus/rhinovirus (1) | 1 | |||
| 2022 | January (15) | Adenovirus | enterovirus/rhinovirus (3), SARS-CoV-2 (2),influenza A(1) | 6 | |
| SARS-CoV-2 | HMPV (1), enterovirus/rhinovirus (4) | 5 | |||
| enterovirus/rhinovirus | HMPV (2) | 2 | |||
| HCoV | HMPV (1), SARS-CoV-2 (1) | 2 | |||
| February (9) | Adenovirus | enterovirus/rhinovirus (5), HCoV (1) | 6 | ||
| SARS-CoV-2 | enterovirus/rhinovirus (2) | 2 | |||
| enterovirus/rhinovirus | HPIV 3 (1) | 1 |
3.3. Clinical and metadata analysis of patients with SARS-CoV-2 coinfections
SARS-CoV-2 coinfections with other respiratory viruses in the time frame November 2021 to January 2022 were collected from both the cepheid Xpert Xpress SARS-CoV-2/Flu/RSV and the ePlex (RP2) to assess the clinical presentations and outcomes of SARS-CoV-2 coinfections. A total of 46 patients had SARS-CoV-2 coinfections with other respiratory viruses in this time frame from both assays. Coinfections with Influenza A were the most prevalent (30 out of 46, 65%) (Table 3 ). Within this cohort, 22% were less than 2 years old (10/46), 35% ranged from 2 to 18 (16/46), and the rest were above 18 (20/46). Fifty-five percent of all SARS-CoV-2 coinfections were 18 years or younger with the vast majority of SARS-CoV-2 coinfections being with influenza A in this age group (31%). Forty-two patients (89%) with SARS-CoV-2 coinfections were symptomatic. The chief complaints were shortness of breath and cough (29%), fever (28%), upper respiratory symptoms (runny nose, ear pain, sore throat) (11%), and exacerbation of asthma (wheezes and retractions) (2%). Three patients (6.5%) were admitted for COVID-19 (Table S1), two had SARS-CoV-2/influenza A coinfection and one had SARS-CoV-2 RSV coinfection. Assessment of the COVID-19 vaccination status revealed that 76% of our cohort (35/46) were unvaccinated, 63% (22/35) of which had SARS-CoV-2/ Influenza A coinfections while 20% (7/35) had SARS-CoV-2/enterovirus/rhinovirus coinfections (Table S1). Eleven patients were fully vaccinated; of these, 72% (8/11) had SARS-CoV-2/Influenza A coinfections (Table S1).
Table 3.
Forty-six SARS-CoV-2 coinfections with respiratory viral pathogens (Cepheid (Xpert Xpress SARS-CoV-2/Flu/RSV) and the extended respiratory panel (RP2)) in the time frame between November 2021 and January 2022. HCoV (coronavirus (229E, HKU1, NL63, OC43), RSV (respiratory syncytial virus), HMPV (human metapneumovirus), HPIV3 (human parainfluenza virus 3).
| Coinfection | Count | % |
|---|---|---|
| SARS-CoV-2/influenza A | 30 | 65 |
| SARS-CoV-2/ enterovirus-rhinovirus | 9 | 20 |
| SARS-CoV-2/Adenovirus | 1 | 2 |
| SARS-CoV-2/HCoV | 3 | 7 |
| SARS-CoV-2/RSV | 1 | 2 |
| SARS-CoV-2/HMPV | 1 | 2 |
| SARS-CoV-2/HPIV3 | 1 | 2 |
| Total | 46 | 100 |
4. Discussion
Omicron was associated with the largest increase in SARS-CoV-2 positivity rates since the beginning of the pandemic [8]. The positivity rate for SARS-CoV-2 reached 36.6% in symptomatic patients in January 2022 and was associated with a steady low-level influenza positivity and a reduction in the positivity of other respiratory viruses (adenovirus, RSV, HCoV, HMPV, HPIV1–4 and enterovirus/rhinoviruses).
Before the Omicron surge, enterovirus/rhinovirus was the most frequently circulating. We previously showed that enterovirus/rhinovirus circulation was not affected by SARS-CoV-2 [5] which might indicate that the reduction associated with the Omicron surge was likely related to the seasonality of enterovirus/rhinoviruses, which are known to circulate more in the summer months [9]. We though speculate that the reduction in influenza circulation was secondary to the marked circulation of SARS-CoV-2, and viral interference could be hypothesized as a possible contributor [10, 11]. Interestingly, we show 30 coinfections of SARS-CoV-2 with influenza A in a time frame of extensive SARS-CoV-2 circulation (November 2021 – January 2022, 1.82% of SARS-CoV-2 positive tests (1648) diagnosed by the Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV), in the same time frame. When only the data from extended respiratory panel (ePlex RP2) was used for assessing coinfections with other respiratory viruses, no coinfections with influenza A were seen and most of the coinfections were with enterovirus/ rhinovirus.
Our study was limited by the overall small numbers of coinfections which did not allow for a proper assessment of the contribution of influenza A coinfection to the severity of COVID-19. In our SARS-CoV-2 coinfection cohort, most of the patients were outpatients and only 3 patients were admitted for COVID-19. We conclude though that SARS-CoV-2 coinfections remain infrequent in a time frame that witnessed a large increase in SARS-CoV-2 infections. Surveillance of respiratory pathogens in different phases of the COVID-19 pandemic is essential for our understanding of the contribution of SARS-CoV-2 and other respiratory viruses to symptomatic disease. Future epidemiological studies will shed more light on the trends of respiratory viral circulation after the Omicron wave.
Funding
The Johns Hopkins University.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chen J., Wang R., Gilby N.B., Wei G.W. Omicron (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. ArXiv. 2021 doi: 10.1021/acs.jcim.1c01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannan S., Shaik Syed Ali P., Sheeza A. Omicron (B.1.1.529) - variant of concern - molecular profile and epidemiology: a mini review. Eur. Rev. Med. Pharmacol. Sci. 2021;25(24):8019–8022. doi: 10.26355/eurrev_202112_27653. [DOI] [PubMed] [Google Scholar]
- 3.Petersen E., Ntoumi F., Hui D.S., et al. Emergence of new SARS-CoV-2 variant of concern Omicron (B.1.1.529) - highlights Africa's research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int. J. Infect. Dis. 2022;114:268–272. doi: 10.1016/j.ijid.2021.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fall A., Eldesouki R.E., Sachithanandham J., et al. The displacement of the SARS-CoV-2 variant Delta with Omicron: an investigation of hospital admissions and upper respiratory viral loads. EBioMedicine. 2022;79 doi: 10.1016/j.ebiom.2022.104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhteg K., Amadi A., Forman M., Mostafa H.H. Circulation of non- SARS-CoV-2 respiratory pathogens and coinfection with SARS-CoV-2 amid the COVID-19 pandemic. Open Forum Infect. Dis. 2021 doi: 10.1093/ofid/ofab618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mostafa H.H., Carroll K.C., Hicken R., et al. Multi-center evaluation of the Cepheid Xpert(R) Xpress SARS-CoV-2/Flu/RSV Test. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00926-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarrett J., Uhteg K., Forman M.S., et al. Clinical performance of the GenMark Dx ePlex respiratory pathogen panels for upper and lower respiratory tract infections. J. Clin. Virol. 2021;135 doi: 10.1016/j.jcv.2021.104737. [DOI] [PubMed] [Google Scholar]
- 8.Fall A., Eldesouki R.E., Sachithanandham J., et al. A quick displacement of the SARS-CoV-2 variant Delta with Omicron: unprecedented spike in COVID-19 cases associated with fewer admissions and comparable upper respiratory viral loads. medRxiv. 2022 doi: 10.1016/j.ebiom.2022.104008. 2022.01.26.22269927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lugo D., Krogstad P. Enteroviruses in the early 21st century: new manifestations and challenges. Curr. Opin. Pediatr. 2016;28(1):107–113. doi: 10.1097/MOP.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han S., Zhang T., Lyu Y., et al. Influenza's plummeting during the COVID-19 pandemic: the roles of mask-wearing, mobility change, and SARS-CoV-2 interference. Engineering (Beijing) 2022 doi: 10.1016/j.eng.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halfmann P.J., Nakajima N., Sato Y., et al. SARS-CoV-2 interference of influenza virus replication in Syrian hamsters. J. Infect. Dis. 2021;225(2):282–286. doi: 10.1093/infdis/jiab587. [DOI] [PMC free article] [PubMed] [Google Scholar]