Abstract
Both infection with and vaccination against SARS-CoV-2 trigger a complex B-cell and T-cell response. Methods for the analysis of the B-cell response are now well established. However, reliable methods for measuring the T-cell response are less well established and their usefulness in clinical settings still needs to be proven. Here, we have developed and validated a T-cell proliferation assay based on 3H thymidine incorporation. The assay is using SARS-CoV-2 derived peptide pools that cover the spike (S), the nucleocapsid (N) and the membrane (M) protein for stimulation. We have compared this novel SARS-CoV-2 lymphocyte transformation test (SARS-CoV-2 LTT) to an established ELISA assay detecting Immunoglobulin G (IgG) antibodies to the S1 subunit of the SARS-CoV-2 spike protein. The study was carried out using blood samples from both vaccinated and infected health care workers as well as from a non-infected control group. Our novel SARS-CoV-2 LTT shows excellent discrimination of infected and/or vaccinated individuals versus unexposed controls, with the ROC analysis showing an area under the curve (AUC) of > 0.95. No false positives were recorded as all unexposed controls had a negative LTT result. When using peptide pools not only representing the S protein (found in all currently approved vaccines) but also the N and M proteins (not contained in the vast majority of vaccines), the novel SARS-CoV-2 LTT can also discriminate T-cell responses resulting from vaccination against those induced by infection.
Keywords: SARS-CoV-2, Lymphocyte transformation test, T-cell response, Humoral and cellular immune responses
1. Introduction
It is well established that infection with and vaccination against SARS-CoV-2, the virus causing COVID-19, trigger complex B-cell and T-cell responses. The production of IgG antibodies from B-cells requires cooperation from T-cells that also recognize SARS-CoV-2 antigens (‘T-cell help’). Concomitantly, T-cells may lyse virus infected cells and are instrumental in clearing SARS-CoV-2 from the organism [1], [2]. In our current understanding, a robust SARS-CoV-2-specific T-cell response is important for preventing severe disease [3], [4], [5]. Conversely, it is highly likely that T-cells contribute to the severe tissue damage seen in the lungs of patients requiring respiratory support. This may result from the fact that in previously unexposed cases without HCoV-induced cross-reactive T-immunity, or those with a weakened immune system, the T-cell response does not get into full swing until the time when the virus has reached the lungs. Having a robust SARS-CoV-2-specific (or cross-reactive) T-cell response prior to infection, by contrast, might prevent the virus from even getting this far. Authors of a recent study in asymptomatic cases believe that strong cross-reactive T-cell responses may explain that some individuals can be exposed and infected without ever showing symptoms or even a positive PCR test [6].Also, T-cell responses to HCoVs causing the common cold were recently shown to contribute to protection as a result of cross-reactivity [7].
The above-mentioned mechanistic considerations are just some reasons why reliable methods to monitor T-cell response to SARS-CoV-2 infections are needed. In addition, measuring T-cell responses may be a promising alternative (or at least suitable addition) to measuring antibody levels for validation of effective vaccination and for predicting the optimal time for a booster vaccination. Several methods for the analysis of the T-cell response to SARS-CoV-2 infection and vaccination have been reported [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. However, the usefulness of the respective T-cell tests in routine clinical settings has not been proven yet.
Here, we report the development and validation of an assay for the detection of SARS-CoV-2-specific T-cells which is adapted from a traditional T-cell proliferation assay referred to as lymphocyte transformation test (LTT) based on 3H thymidine incorporation. The LTT format has been in use for measuring T-cell immunity to a range of different T-cell target antigens over several decades [20]. We adapted this assay in early 2020 to detect SARS-CoV-2 spike protein-specific T-cells based on the stimulation with two peptide pools together covering the entire spike protein (SARS-CoV-2 LTT). We show that the assay can efficiently distinguish infected and vaccinated from non-infected, non-vaccinated study participants. In addition, we show that by using peptide pools against the S, N and M proteins, the novel SARS-CoV-2 LTT can be expanded to discriminate between T-cell responses resulting from vaccination and those induced by infection.
2. Methods
2.1. Study populations
The study was approved by the ethics committee of the association of physicians in the province of Brandenburg, Germany (Ärztekammer Brandenburg). All study participants provided written, informed consent.
After the COVID-19 outbreak in the Ernst von Bergmann Hospital (EvB), Potsdam, Germany, in March and April 2020, all health care workers underwent twice weekly PCR testing (nasal-oral swab) for possible SARS-CoV-2 infection. Participants were recorded from two cohorts between July and October 2020 (4–6 months following the outbreak):
Cohort 1: 69 hospital staff of the EvB who had not been infected during the observation period and were fully vaccinated with their second shot of a SARS-CoV-2 vaccine administered between May and July 2021 (for details, see Table 1 ). EvB staff were vaccinated twice with either the BioNTech, BNT162b2, or Astrazeneca, ChAdOx1, vaccine.
Table 1.
Comparison of characteristics between SARS-CoV-2 LTT positive and negative cases in vaccinated health care workers.
|
Parameters |
LTT_N positive (n = 66) |
LTT_N negative (n = 3) |
P value |
|---|---|---|---|
| Age (years) | 44.6 ± 13.7 | 64.3 ± 11.7 | 0.037 |
| Gender (M/F) | 17/49 | 1/2 | 0.772 |
| BMI | 25.5 ± 4.8 | 25.4 ± 3.1 | 0.823 |
| Diabetes (yes/no) | 4/61 | 0/3 | 0.660 |
| Hypertension (yes/no) | 13/43 | 2/1 | 0.095 |
| COPD (yes/no) | 0/65 | 0/3 | 1 |
| Asthma (yes/no) | 6/59 | 2/1 | 0.003 |
| Smoking (yes/no) | 12/53 | 0/3 | 0.416 |
| SARS-CoV-2 IgG-Ab (S1) (BAU/ml) | 556.4 ± 289.3 | 157.0 ± 44.9 | 0.018 |
| SARS surrogate neutralization test (%) | 81.2 ± 25.3 | 73.0 ± 10.4 | 0.143 |
| T-cell responses to SARS-CoV-2 Spike-N-Term (SI) | 8.83 ± 8.61 | 1.63 ± 0.21 | 0.004 |
| T-cell responses to SARS-CoV-2 Spike-C-Term (SI) | 6.75 ± 6.44 | 1.53 ± 0.29 | 0.004 |
Continuous variables are given as mean (SD) or numbers. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. COPD, chronic obstructive pulmonary disease.
Cohort 2: 54 hospital staff of the EvB who had been infected, but without being vaccinated at the time of blood taking (for details, see Table 2 ). Infection with SARS-CoV-2 was proven by PCR.
Table 2.
Comparison of characteristics between LTT_N positive and negative cases in infected health care workers.
| Parameters | LTT_N positive (n = 49) | LTT_N negative (n = 5) | P value |
|---|---|---|---|
| Age (years) | 50.0 (37.0, 60.0) | 31.0 (26.5, 49.5) | 0.092 |
| Gender (M/F) | 19/30 | 2/3 | 0.958 |
| BMI | 24.8 (23.1, 29.2) | 21.5 (20.7, 35.6) | 0.622 |
| Diabetes (yes/no) | 7/42 | 0/5 | 0.369 |
| Hypertension (yes/no | 20/29 | 1/4 | 0.368 |
| COPD (yes/no) | 2/47 | 0/5 | 0.648 |
| Asthma (yes/no) | 7/41 | 0/5 | 0.364 |
| Smoking (yes/no) | 2/47 | 0/5 | 0.648 |
| SARS-CoV-2 IgG-Ab (S1) (BAU/ml) | 129.0 (71.9, 553.5) | 26.2 (16.2, 35.6) | 0.002 |
| SARS surrogate neutralization test (%) | 63.0 (45.0, 90.0) | 26.0 (15.5, 29.5) | 0.001 |
| T-cell responses to SARS-CoV-2 Spike-N-Term (SI) | 6.5 (4.1, 13.3) | 1.4 (1.2, 1.7) | <0.001 |
| T-cell responses to SARS-CoV-2 Spike-C-Term (SI) | 5.1 (3.3, 9.8) | 1.5 (1.4, 2.0) | 0.001 |
| Time from infection to blood collection in days | 201 (131, 432) | 200 (176, 398) | 0.637 |
Continuous variables are given as median (interquartile range) or numbers. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. COPD, chronic obstructive pulmonary disease.
As control group, a total of 88 individuals of mixed age and sex who had no detectable SARS-CoV-2 antibodies (IgG (S1)-negative) were recruited prior to vaccination. Blood samples were drawn into heparinized blood collection tubes by cubital venous phlebotomy.
All study participants were examined/interviewed by a study physician. The following parameters were recorded in all individuals: age, sex, BMI, and resting blood pressure. The medical interview explored the presence/a history of type 1 or 2 diabetes, COPD, and asthma. Hypertension was established according to the European Society of Hypertension guidelines [21] or based on the use of hypertensive medication. With respect to cohort 1 we recorded the time from complete vaccination (second dose) to blood collection and whether vaccine side effects were observed. With respect to cohort 2 we recorded the time from PCR detection of SARS-CoV-2 infection to blood collection and whether infection was symptomatic or not.
Cohort 3: An additional 35 members of staff of the Institute of Medical Diagnostics Berlin (IMD Berlin, Germany), aged 28–56 years, were recruited for examination of cellular immune responses to different immunodominant SARS-CoV-2 antigens. None of them exhibited any preexisting condition that would be considered as risk factor for an adverse COVID-19 outcome. This group included 3a) those recovered from PCR proven SARS-CoV-2 infection (n = 12); 3b) those uninfected but vaccinated against SARS-CoV-2 (mRNA vaccine) with the second dose obtained between May and July 2021 (n = 11); 3c) those without infection and without vaccination (n = 12). With samples from these participants the LTT was performed using three separate peptide pools (covering the S, N and M protein of SARS-CoV-2, respectively).
2.2. Measurement of T-cell responses to SARS-CoV-2 with the SARS-CoV-2 LTT
Heparinized venous blood was processed by density gradient centrifugation to obtain peripheral blood mononuclear cells (PBMCs). After washing the cells twice with PBS (Sigma-Aldrich), the cell pellet was resuspended to obtain a cell count of 1 × 106/ml in cell culture medium (RPMI 1640; Biowest) supplemented with 2 mM L-glutamine, 100 μg/ml gentamicin (all from Biowest) and 5% autologous serum. Specific T-cell reactions were assessed by a lymphocyte proliferation assay (LTT). For that, 2 × 105 PBMCs were either incubated with peptide pool 1 or peptide pool 2 of the SARS-CoV-2 spike glycoprotein (PM-WCPV-S from JPT), or with the peptide pool of SARS-CoV-2 nucleocapsid (PM-WCPV-NCAP-2) or membrane protein (PM-WCPV-VME-2) using a concentration of 1µg/ml per peptide, together with 1µg/ml anti-CD28 Abs (clone CD28.2 from BD Biosciences). All pools contained 15 amino acid long peptides that overlapped by 11 amino acids, respectively, and in total spanned the entire SARS-CoV-2 S, N, or M protein. The N-terminal part of S, containing the RBD-region, was covered by pool 1 (N-Term) and the C-terminal part of the spike protein was covered by pool 2 (C-Term). Detailed information about the peptide pools is given below and were also published recently [22].
Two positive control experiments were performed: a) by stimulating cells with a mixture of recall-antigens, containing tetanus, influenza and candida albicans (antigen control), and b) by stimulating cells with pokeweed mitogen (mitogen control). For measuring base-level proliferation control cells were left unstimulated. All stimulations were performed in triplicates in 96-well plates for 5 days at 37 °C and 5% CO2 atmosphere. Cells were labeled with 3H-thymidine (1 μCi/ml, Hartmann Analytic) 12 h prior to cell harvesting. A cell harvester (PerkinElmer) was used to harvest cells on glass fiber filters. The incorporated 3H-thymidine activity was measured as “counts per minute” (cpm) using a solid phase beta counter (PerkinElmer). Mean values of the triplicates were calculated for data analysis. The results for all stimulations were given as a ‘stimulation index’ (SI; ratio of cpm of stimulated over unstimulated samples). The threshold SI for positivity was set at 1.9 (an SI > 1.9 was considered positive).
2.3. PepMixTM peptide pools
Five different peptide pools were prepared as follows: Individual peptides were synthesized by standard solid phase peptide synthesis (SPPS) [23]. Each peptide was purified by HPLC using different gradients of solvent A (0.1% TFA in water) and solvent B (0.1% TFA in MeCN) on a preparative HPLC system. After drying of the individual peptides by lyophilization, the peptides were redissolved, combined and lyophilized again to furnish peptide pools containing equal amounts of each peptide. This yielded the peptide pools for the following antigens: Spike Glycoprotein N-term and Spike Glycoprotein C-term (158 and 157 peptides, purity > 90%); NCAP (102 peptides, purity > 70%); VME1 (53 peptides, purity > 70%).
2.4. Measurement of humoral immune response to SARS-CoV-2
For quantitative detection of IgG against SARS-CoV-2 spike glycoprotein 1 (S1 subunit) an enzyme-linked immunosorbent assay (ELISA; EUROIMMUN) was performed on an automated ANALYZER system (QuantiVac, EUROIMMUN) according to manufactureŕs instructions. The assay relies on 6 calibrators in order to quantify the IgG (S1)-concentration given as BAU/ml (Binding Antibody Units) and highly correlates with the “First WHO International Standard“ (NIBSC code: 20/136). Values between 25.6 and 35.2 BAU/ml were considered borderline, while values above 35.2 BAU/ml were interpreted as positive. The assay is based on a previously established semi-quantitative assay, which has been already described [24].
2.5. Statistical analysis
Results across groups are shown as median (interquartile range), mean+/-SD, or simple percentages. Non-parametric tests (Mann-Whitney U or χ2 test) were applied as appropriate to determine statistical significance. Receiver Operating Characteristic (ROC) analysis was used to derive ROC curves and measure test performance. All statistical analyses were performed using SPSS 25.0 software (IBM SPSS Statistics, Version 25.0. Armonk, NY, USA). If individual values were above the upper validated limit of quantification according to the supplier of the assay, these values were replaced with substitute values (twice the highest measurable value in a dataset). The level of significance was set at p < 0.05.
3. Results
3.1. Vaccinated EvB health care workers
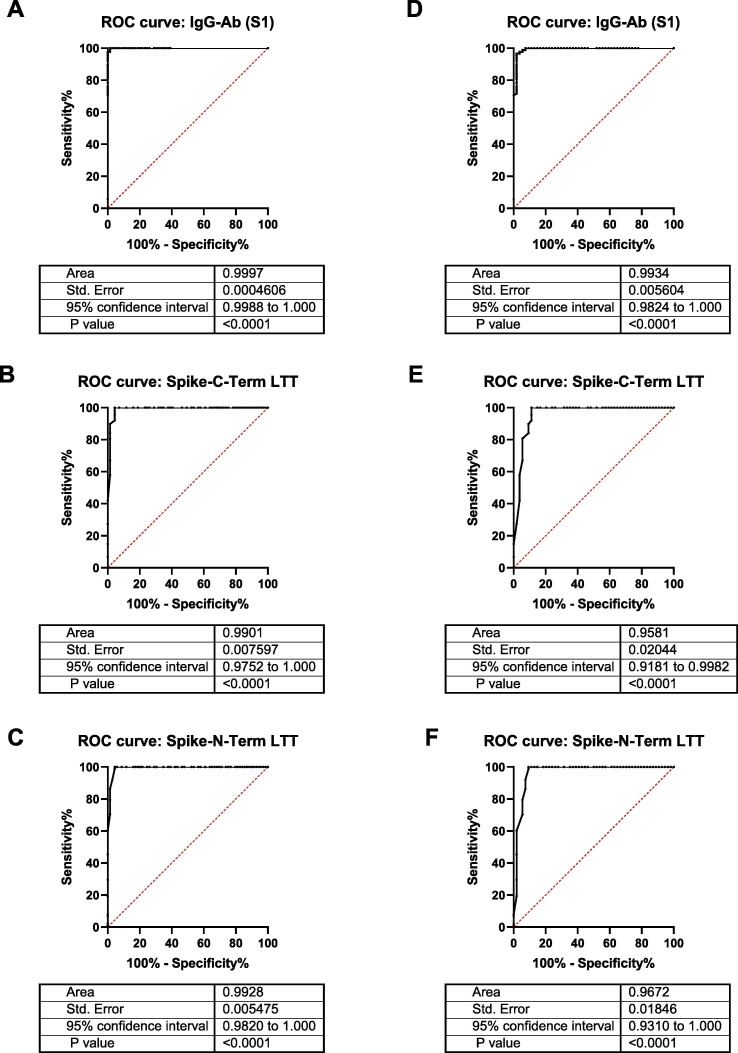
Humoral and cellular immune responses in vaccinated health care workers (cohort 1) were assessed 4–8 weeks after the 2nd vaccination. ROC analysis showed an excellent performance of the SARS-CoV-2 LTT (Fig. 1 A-C) with an AUC of > 0.95 for both studied peptide pools (Spike-N-Term and Spike-C-Term).
Fig. 1.
ROC curves showing discrimination between vaccinated and unvaccinated health care workers (A)-(C), or infected and uninfected health-care workers (D)-(F), based on detection of IgG against spike S1 (A, D) or LTT with the C-terminal (B, E) or N-terminal (C, F) spike peptide pools.
As it was important for us to avoid false positives (and thus to reach 100% specificity), the threshold SI for positivity was set at 1.9 (an SI > 1.9 was considered positive), because 1.9 was the highest value that was measured in the control cohort of 88 non-vaccinated patients without evidence of SARS-CoV-2 infection (Supplementary Table 1). This led to 66/69 positive test in the vaccinated group (cohort 1), thus inferring to 95.7% sensitivity at 100% specificity.
The clinical characteristics of the LTT positive and the LTT negative groups are shown in Table 1. Asthma appeared more common among those who did not develop a cellular response after vaccination. All other clinical characteristics were not different (Table 1). 97.1% of the vaccinated group had SARS-CoV-2 S1 IgG antibodies after vaccination and 95.7% of the group had T-cellular response after vaccination. It should be noted that vaccinated health care workers not developing antibodies were not identical with health care workers not developing cellular immunity to SARS-CoV-2. Nevertheless, those without a humoral response generally had a weak T-cell response and vice versa.
3.2. Infected EvB health care workers
Humoral and cellular immunity in infected health care workers (cohort 2) was examined 200 days (median) after PCR proven infection. ROC analysis showed an excellent performance of the SARS-CoV-2 LTT (Fig. 1D-1F) with an AUC of > 0.95 for both studied peptide pools (Spike-N-Term and Spike-C-Term).
The threshold SI for positivity was set at 1.9 for the same reasons as described above. This led to 49/54 positive test in the infected group (cohort 2), thus inferring to 90.7% sensitivity at 100% specificity.
The clinical characteristics of the LTT positive and the LTT negative groups and the performance of the SARS-CoV-2 LTT test are shown in Table 2. 48 out of 54 (88.9%) of the infected study group had SARS-CoV-2 S1 IgG antibodies and 49 out of 54 (90.7%) had developed T-cell response. Three of the five infected health care workers with no S1 antibodies also had no cellular response. In those with no humoral response the T-cell response was generally weak and vice versa.
3.3. Discrimination between infected and vaccinated people based on responses to N and M proteins
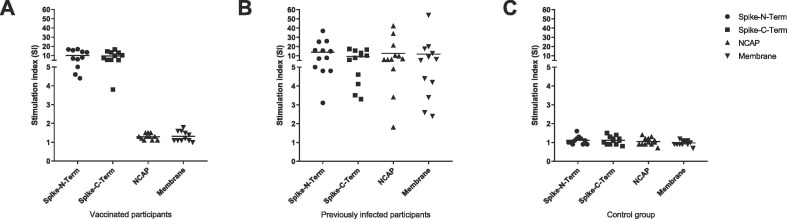
SARS-CoV-2t LTT responses in infected (cohort 3a, n = 12) versus vaccinated (cohort 3b, n = 11) volunteers were examined based on stimulation with peptide pools covering the S, N, and M proteins. As expected, since currently all in the EU approved vaccines are exclusively based on the spike-glycoprotein, all vaccinated participants (n = 11) showed positive responses (SI > 1.9) to both S peptide sub-pools, but no responses to the M or N peptide pools (SI < 2, Fig. 2 ). In contrast, all previously infected participants (n = 12) showed positive responses to the S peptide pools and in addition to the M and/or the N peptide pools (Fig. 2). Taken together, the LTT method efficiently differentiates between infected and vaccinated individuals and offers the possibility of detecting a prior SARS-CoV-2 infection even after subsequent vaccination.
Fig. 2.
SARS-CoV-2 antigen-specific T-cell responses in vaccinated or infected volunteers (cohort 3) were examined using the SARS-CoV-2 LTT based on peptide pools covering the S, N, and M proteins. (A) All vaccinated participants (cohort 3b, n = 11) showed positive responses (SI ≥ 2) to the S peptide pools but no responses to the N or M pools (SI < 2). (B) All previously infected participants (cohort 3a, n = 12) showed positive responses to the S and, in addition, to both the N and M pools. (C) Participants without infection and without vaccination (cohort 3c, n = 12) showed no LTT response (SI < 2) to any of the peptide pools used for stimulation.
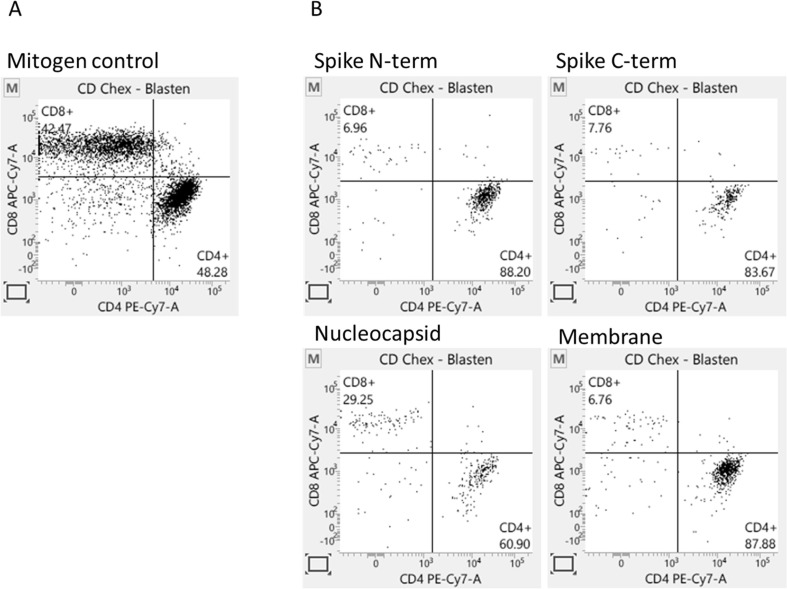
Depending on the particular antigen chosen as stimulant for the T cell-assay, antigen-specific CD4 or CD8 T cells are stimulated in varying proportions.. To address the question, which T-cells do proliferate after 5d stimulation with described SARS-CoV-2 peptide pool in our LTT-assay, we additionally analyzed these 5d-cell cultures using flow cytometry. We examined the proportion of CD4 or CD8 T-cells, respectively after gating on CD45 + CD3 + T-cells and as well as gating on T-cell blasts, since dividing cells are blastic. In contrast to the relatively balanced CD4/CD8 T-cell proportions after mitogen stimulation, SARS-CoV-2-specific stimulations predominantly induced CD4 + T-cell responses (Fig. 3 ). Raw data of our experiments are in supplementary Table 1.
Fig. 3.
SARS-CoV-2-specific T cell-response is more prominent for CD4 + T cells. After five-days stimulation PBMCs were stained for CD45, CD3, CD4 and CD8. CD45 + CD3 + blasts were calculated for CD4+- and CD8 + proportion. A: PBMCs were stimulated with Pokeweed mitogen. B: PBMCs were stimulated with SARS-CoV-2 specific peptide pools spanning different total virus specific proteins.
4. Discussion
Our novel SARS-CoV-2 LTT was designed for the detection of vaccine and/or infection-induced cellular immune responses to SARS-CoV-2. The test shows excellent discrimination between infected/vaccinated individuals and unexposed controls. Since the threshold for positive SARS-CoV-2 LTT responses had been set in such a way that all 88 unexposed controls (Supplementary Table 1) had a negative LTT, specificity was 100% by design. False negatives, however, did occur (sensitivity 95.7% and 90.7% for cohort 1 and cohort 2, respectively), but were most likely not test-related but due to the lacking or weak immunity in those infected or vaccinated.
The excellent performance of the SARS-CoV-2 LTT in discriminating between those infected or vaccinated and controls is reflected by ROC analysis (Fig. 1) showing areas under the curve (AUC) of > 0.95. The obtained results favorably compare to other T-cell tests for SARS-CoV-2 infection or vaccination, e.g. Interferon-gamma release assay (IGRA) T-cell tests that reported a cut-off dependent sensitivity of 75.4–89.6% with a specificity of 96.3–100% [19]; a sensitivity of 73–88% with a specificity of 85–97% [18]; or a sensitivity of 90–100% with a specificity of 96–90% [17]; and an assay based on cumbersome T-cell receptor (TCR) sequencing that reported 97.1% sensitivity and 100% specificity in a preprint manuscript [11].
For optimal activation, T cells need different kinds of signals from the antigen presenting cell (APC). Together with the initial MHC-TCR signal, further co-stimulatory signals promote proliferation and differentiation of T cells. An important co-stimulatory molecule on T cells is CD28 [25]. As peptides in contrast to proteins are not undergoing active processing by APCs in order to be presented on MHC molecules, APCs provide less co-stimulatory signals when using peptides for in vitro stimulation. Optimizing co-stimulatory signaling by adding anti-CD28 can improve assay sensitivity. We mentioned this in the discussion of the revised version of the manuscript.
Few study participants showed neither antibodies nor a positive LTT despite vaccination or infection. Their frequency roughly corresponded to the rate of non-responders after infection or vaccination reported in the literature [26], [27], [28]. With respect to the vaccinated or infected healthcare workers, in participants with a positive antibody test and a negative LTT or vice versa, usually one of the tests was just above the threshold while the other test was just below. There were 3 individuals in the infected group with no detectable IgG (S1) antibodies but a clear T-cell response. This may be due to the fact that antibody titers usually drop within a few months after infection or vaccination whereas T-cell response appears to be more persistent [29], [30]. As a result, detection of SARS-CoV-2-specific T-cells may represent a better way of determining the presence of SARS-CoV-2-specific immunity than SARS-CoV-2 S1 protein subunit-specific antibodies in subjects who have been infected for some time. Antibodies to the N protein were not measured in this study, and while the presence of such antibodies would point to infection, their kinetics are similar to those of S1-specific antibodies, dropping off after a few months.
The currently most frequently used read-out for T-cell activation assays are been cytokine production measured intracellularly (ICS), in the supernatant (ELISA or equivalent), or detected by Elispot [31]. T-cell surface marker upregulation is being used by some authors and has also been applied to SARS-CoV-2 [22]. In particular the Elispot method depends at least partially on subjective judgments on test results and has thus a huge interobserver variability. T-cell proliferation assays have a more definitive end-point and are thus independent of the experience of an observer. Moreover, cytokine production upon stimulation within the first 23–48 h - as it is analyzed in the cytokine dependent methods – detects also T-cell clones that might be activated in the early phase of the immune response but not in later stages [32]. However, the T-cell subsets arising from proliferating T-cell clones - as measured by the LTT - are the ones that will ultimately deal with the infection. In addition, it has to be noted that the experimental conditions such as the availability of IL-2, for example, have a critical role in ex-vivo testing. The conditions used in our experiments appear to have favored positive responses in those infected or vaccinated with very few false negatives. This is noteworthy since the threshold for positivity was set in such a way that the negative control group included no positives at all. Setting the threshold slightly lower, i.e., allowing for a few false positives, would have further reduced the false negative rate.
To date, T-cell proliferation assays for SARS-CoV-2 specific T-cells appear to represent a niche in the diagnostic market and their clinical usefulness still has to be explored in more detail. The LTT assay presented here is an important contribution to this field and will help to answer a number of questions that are of particular relevance: What is the long-term course of SARS-CoV-2-specific T-cell immunity after vaccination and/or infection in light of antibody responses declining within months? How do SARS-CoV-2 specific T-cell responses fare compared to antibody responses in patients receiving biologicals in rheumatoid arthritis, for example? Are SARS-CoV-2-specific T-cell responses weaker in HIV patients, transplant recipients, or oncological patients undergoing chemotherapy?
In conclusion, the present study shows that the novel SARS-CoV-2 LTT may improve the identification of those with infection or vaccine-induced immunity to the coronavirus and our data suggests that this new approach can be rolled out to a range of clinical situations. The SARS-CoV-2 LTT can not only differentiate between acquired immunity due to natural infection or vaccination, but it may also help us examine and understand the complexity of the cellular immune response at an individual level.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2022.05.025.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Mahajan S., Kode V., Bhojak K., Karunakaran C., Lee K., Manoharan M., Ramesh A., Hv S., Srivastava A., Sathian R., Khan T., Kumar P., Gupta R., Chakraborty P., Chaudhuri A. Immunodominant T-cell epitopes from the SARS-CoV-2 spike antigen reveal robust pre-existing T-cell immunity in unexposed individuals. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-92521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winkler E.S., Gilchuk P., Yu J., Bailey A.L., Chen R.E., Chong Z., Zost S.J., Jang H., Huang Y., Allen J.D., Case J.B., Sutton R.E., Carnahan R.H., Darling T.L., Boon A.C.M., Mack M., Head R.D., Ross T.M., Crowe J.E., Diamond M.S. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell. 2021;184(7):1804–1820.e16. doi: 10.1016/j.cell.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuo J., Dowell A.C., Pearce H., Verma K., Long H.M., Begum J., Aiano F., Amin-Chowdhury Z., Hoschler K., Brooks T., Taylor S., Hewson J., Hallis B., Stapley L., Borrow R., Linley E., Ahmad S., Parker B., Horsley A., Amirthalingam G., Brown K., Ramsay M.E., Ladhani S., Moss P. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021;22(5):620–626. doi: 10.1038/s41590-021-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassaniti I., Percivalle E., Bergami F., Piralla A., Comolli G., Bruno R., et al. SARS-CoV-2 specific T-cell immunity in COVID-19 convalescent patients and unexposed controls measured by ex vivo ELISpot assay. Clin. Microbiol. Infect. 2021;27(7):1029–1034. doi: 10.1016/j.cmi.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung M.K., Shin E.-C. Phenotypes and Functions of SARS-CoV-2-Reactive T Cells. Mol. Cells. 2021;44(6):401–407. doi: 10.14348/molcells.2021.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swadling L., Diniz M.O., Schmidt N.M., Amin O.E., Chandran A., Shaw E., et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. 2021 doi: 10.1038/s41586-021-04186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loyal L., Braun J., Henze L., Kruse B., Dingeldey M., Reimer U., Kern F., Schwarz T., Mangold M., Unger C., Dörfler F., Kadler S., Rosowski J., Gürcan K., Uyar-Aydin Z., Frentsch M., Kurth F., Schnatbaum K., Eckey M., Hippenstiel S., Hocke A., Müller M.A., Sawitzki B., Miltenyi S., Paul F., Mall M.A., Wenschuh H., Voigt S., Drosten C., Lauster R., Lachman N., Sander L.-E., Corman V.M., Röhmel J., Meyer-Arndt L., Thiel A., Giesecke-Thiel C. Cross-reactive CD4(+) T cells enhance SARS-CoV-2 immune responses upon infection and vaccination. Science. 2021;374(6564) doi: 10.1126/science:abh1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruse M., Dark C., Aspden M., Cochrane D., Competiello R., Peltz M., Torres L., Wrighton-Smith P., Dudek M. Performance of the T-SPOT.COVID test for detecting SARS-CoV-2-responsive T cells. Int. J .Infect. Dis. 2021;113:155–161. doi: 10.1016/j.ijid.2021.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaganathan S., Stieber F., Rao S.N., Nikolayevskyy V., Manissero D., Allen N., Boyle J., Howard J. Preliminary Evaluation of QuantiFERON SARS-CoV-2 and QIAreach Anti-SARS-CoV-2 Total Test in Recently Vaccinated Individuals. Infect. Dis. Ther. 2021;10(4):2765–2776. doi: 10.1007/s40121-021-00521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheridan C. COVID-19 testing turns to T cells. Nat. Biotechnol. 2021;39(5):533–534. doi: 10.1038/s41587-021-00920-9. [DOI] [PubMed] [Google Scholar]
- 11.S.C. Dalai, J.N. Dines, T.M. Snyder, R.M. Gittelman, T. Eerkes, P. Vaney, et al., Clinical Validation of a Novel T-cell Receptor Sequencing Assay for Identification of Recent or Prior SARS-CoV-2 Infection, (2021) 2021.01.06.21249345. [DOI] [PMC free article] [PubMed]
- 12.Zelba H., Worbs D., Harter J., Pieper N., Kyzirakos-Feger C., Kayser S., Seibold M., Bartsch O., Ködding J., Biskup S. A Highly Specific Assay for the Detection of SARS-CoV-2-Reactive CD4(+) and CD8(+) T Cells in COVID-19 Patients. J. Immunol. 2021;206(3):580–587. doi: 10.4049/jimmunol.2000811. [DOI] [PubMed] [Google Scholar]
- 13.Riou C., Schäfer G., du Bruyn E., Goliath R.T., Stek C., Mou H., Hung D., Wilkinson K.A., Wilkinson R.J. Rapid, simplified whole blood-based multiparameter assay to quantify and phenotype SARS-CoV-2-specific T-cells. Eur. Respir. J. 2022;59(1):2100285. doi: 10.1183/13993003.00285-202110.1183/13993003.00285-2021.Supp110.1183/13993003.00285-2021.Shareable1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan A.T., Lim J.M.E., Le Bert N., Kunasegaran K., Chia A., Qui M.D.C., Tan N., Chia W.N., de Alwis R., Ying D., Sim J.X.Y., Ooi E.E., Wang L.-F., Chen M.-C., Young B.E., Li Yang Hsu, Low J.G.H., Lye D.C., Bertoletti A. Rapid measurement of SARS-CoV-2 spike T cells in whole blood from vaccinated and naturally infected individuals. J. Clin. Invest. 2021;131(17) doi: 10.1172/JCI15237910.1172/JCI152379DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Gallo M., Esperalba J., Pujol-Borrell R., Sandá V., Arrese-Muñoz I., Fernández-Naval C., Antón A., Cardona V., Labrador-Horrillo M., Pumarola T., Hernandéz-González M. Commercialized kits to assess T-cell responses against SARS-CoV-2 S peptides. Med. Clín. 2021 doi: 10.1016/j.medcli.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrone L., Petruccioli E., Vanini V., Cuzzi G., Najafi Fard S., Alonzi T., Castilletti C., Palmieri F., Gualano G., Vittozzi P., Nicastri E., Lepore L., Antinori A., Vergori A., Caccamo N., Cantini F., Girardi E., Ippolito G., Grifoni A., Goletti D. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin. Microbiol. Infect. 2021;27(2):286.e7–286.e13. doi: 10.1016/j.cmi.2020.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murugesan K., Jagannathan P., Pham T.D., Pandey S., Bonilla H.F., Jacobson K., et al. Interferon-gamma Release Assay for Accurate Detection of Severe Acute Respiratory Syndrome Coronavirus 2 T-Cell Response. Clin. Infect. Dis. 2021;73(9):e3130–e3132. doi: 10.1093/cid/ciaa1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brand I., Gilberg L., Bruger J., Gari M., Wieser A., Eser T.M., et al. Broad T Cell Targeting of Structural Proteins After SARS-CoV-2 Infection: High Throughput Assessment of T Cell Reactivity Using an Automated Interferon Gamma Release Assay. Front. Immunol. 2021;12:688436. doi: 10.3389/fimmu.2021.688436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D. Huzly, M. Panning, F. Smely, M. Enders, J. Komp, D. Steinmann, Validation and performance evaluation of a novel interferon-γ release assay for the detection of SARS-CoV-2 specific T-cell response, (2021) 2021.07.17.21260316. [DOI] [PMC free article] [PubMed]
- 20.Sachs B., Fatangare A., Sickmann A., Glässner A. Lymphocyte transformation test: History and current approaches. J. Immunol. Methods. 2021;493:113036. doi: 10.1016/j.jim.2021.113036. [DOI] [PubMed] [Google Scholar]
- 21.Stergiou G.S., Palatini P., Parati G., O’Brien E., Januszewicz A., Lurbe E., Persu A., Mancia G., Kreutz R. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J. Hypertens. 2021;39(7):1293–1302. doi: 10.1097/HJH.0000000000002843. [DOI] [PubMed] [Google Scholar]
- 22.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F., Baysal E., Mangold M., Henze L., Lauster R., Mall M.A., Beyer K., Röhmel J., Voigt S., Schmitz J., Miltenyi S., Demuth I., Müller M.A., Hocke A., Witzenrath M., Suttorp N., Kern F., Reimer U., Wenschuh H., Drosten C., Corman V.M., Giesecke-Thiel C., Sander L.E., Thiel A. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 23.Made V., Els-Heindl S., Beck-Sickinger A.G. Automated solid-phase peptide synthesis to obtain therapeutic peptides. Beilstein. J. Org. Chem. 2014;10:1197–1212. doi: 10.3762/bjoc.10.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., Lamers M.M., Sikkema R.S., de Bruin E., Chandler F.D., Yazdanpanah Y., Le Hingrat Q., Descamps D., Houhou-Fidouh N., Reusken C.B.E.M., Bosch B.-J., Drosten C., Koopmans M.P.G., Haagmans B.L. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020;26(7):1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa S., Abe R. Signal Transduction Via Co-stimulatory and Co-inhibitory Receptors. Adv. Exp. Med. Biol. 2019;1189:85–133. doi: 10.1007/978-981-32-9717-3_4. [DOI] [PubMed] [Google Scholar]
- 26.Dekervel M., Henry N., Torreggiani M., Pouteau L.-M., Imiela J.-P., Mellaza C., Garnier A.-S., Dujardin A., Asfar M., Ducancelle A., Paquin A., Blanchi S., Besson V., Piccoli G.B., Augusto J.-F. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 2021;14(11):2349–2355. doi: 10.1093/ckj/sfab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.L. Timmermann, B. Globke, G. Lurje, M. Schmelzle, W. Schöning, R. Öllinger, et al., Humoral Immune Response following SARS-CoV-2 Vaccination in Liver Transplant Recipients, 9(12) (2021) 1422. [DOI] [PMC free article] [PubMed]
- 28.Vályi-Nagy I., Matula Z., Gönczi M., Tasnády S., Bekő G., Réti M., Ajzner É., Uher F. Comparison of antibody and T cell responses elicited by BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) vaccines against SARS-CoV-2 in healthy adult humans. Geroscience. 2021;43(5):2321–2331. doi: 10.1007/s11357-021-00471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., Milo R., Alroy-Preis S., Ash N., Huppert A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Channappanavar R., Fett C., Zhao J., Meyerholz D.K., Perlman S., Sandri-Goldin R.M. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014;88(19):11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kern F., LiPira G., Gratama J.W., Manca F., Roederer M. Measuring Ag-specific immune responses: understanding immunopathogenesis and improving diagnostics in infectious disease, autoimmunity and cancer. Trends Immunol. 2005;26(9):477–484. doi: 10.1016/j.it.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhan Y., Carrington E.M., Zhang Y., Heinzel S., Lew A.M. Life and Death of Activated T Cells: How Are They Different from Naive T Cells? Front. Immunol. 2017;8:1809. doi: 10.3389/fimmu.2017.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.