Abstract
We used an assay based on the uptake of SYTOX Green, an organic compound that fluoresces upon interaction with nucleic acids and penetrates cells with compromised plasma membranes, to investigate membrane permeabilization in fungi. Membrane permeabilization induced by plant defensins in Neurospora crassa was biphasic, depending on the plant defensin dose. At high defensin levels (10 to 40 μM), strong permeabilization was detected that could be strongly suppressed by cations in the medium. This permeabilization appears to rely on direct peptide-phospholipid interactions. At lower defensin levels (0.1 to 1 μM), a weaker, but more cation-resistant, permeabilization occurred at concentrations that correlated with the inhibition of fungal growth. Rs-AFP2(Y38G), an inactive variant of the plant defensin Rs-AFP2 from Raphanus sativus, failed to induce cation-resistant permeabilization in N. crassa. Dm-AMP1, a plant defensin from Dahlia merckii, induced cation-resistant membrane permeabilization in yeast (Saccharomyces cerevisiae) which correlated with its antifungal activity. However, Dm-AMP1 could not induce cation-resistant permeabilization in the Dm-AMP1-resistant S. cerevisiae mutant DM1, which has a drastically reduced capacity for binding Dm-AMP1. We think that cation-resistant permeabilization is binding site mediated and linked to the primary cause of fungal growth inhibition induced by plant defensins.
Plant defensins are a family of small (45 to 54 amino acids), usually basic, peptides occurring in various plant species (2, 3). Many plant defensins can inhibit the growth of a broad range of fungi at micromolar concentrations but are nontoxic to both mammalian and plant cells (18, 19). In some plant tissues, the expression of defensin genes is induced in response to fungal infection (21), whereas in other tissues they are expressed constitutively (31). All plant defensins share a common three-dimensional folding pattern, stabilized by eight disulphide-linked cysteines (2, 3). Plant defensins are structurally related to antibacterial insect defensins (8) and drosomycin, an antifungal peptide found in insects (15). The plant defensin family may be divided into two main groups (A and B), sharing only 25% similarity (11). Group A can be further divided into four subfamilies (A1, A2, A3, and A4) with at least 50% similarity within each subfamily. Members of subfamily A2, formerly termed nonmorphogenic plant defensins (3), including Dm-AMP1 from Dahlia merckii, reduce hyphal elongation without affecting fungal morphology. In contrast, members of subfamilies A3 and A4, including Rs-AFP2 from Raphanus sativus and Hs-AFP1 from Heuchera sanguinea, respectively, induce tip ballooning and branch formation on susceptible fungi (19). These plant defensins have therefore been termed morphogenic plant defensins (3).
Plant defensins induce ion fluxes across the plasma membranes of living fungal hyphae (28). Unlike insect (8) and mammalian (12) defensins, plant defensins neither form ion-permeable pores in artificial membranes nor change the electrical properties of artificial lipid bilayers (28). This finding indicates that a direct interaction with lipid components of the plasma membrane, a mechanism proposed to explain the antimicrobial effects of insect defensins or mammalian defensins (8, 12), is unlikely for plant defensins. In addition, specific, high-affinity binding sites for plant defensins on fungal cells and microsomal membranes have been identified based on studies with radiolabeled plant defensins (29, 30). Binding of plant defensins to fungal cells and plasma membrane fractions is partially reversible. A mutant of the yeast Saccharomyces cerevisiae has been identified which, in contrast to the wild-type strain, is not sensitive to the plant defensin Dm-AMP1. The capacity of this mutant, called DM1, to bind Dm-AMP1 to its plasma membrane is more than 10-fold less than that of the wild type, suggesting that binding of Dm-AMP1 to a specific binding site is a prerequisite to the antifungal activity of this plant defensin (30). Combined, these observations suggest that the ion fluxes may result from (i) the interaction of the plant defensins with a binding site that transduces a signal to endogenous ion channels in the membrane or (ii) binding-site-mediated insertion of the plant defensins into the membrane, with subsequent ion channel formation.
To distinguish these models, we studied the influx of SYTOX Green, a high-affinity nucleic acid stain that fluoresces upon nucleic acid binding and for which no endogenous channels exist. SYTOX Green penetrates cells with compromised plasma membranes but does not cross the membranes of noncompromised living cells (17, 23, 34). SYTOX Green uptake was measured fluorimetrically in Neurospora crassa and S. cerevisiae treated with different plant defensins. Our results are consistent with a model for plant defensin action involving binding-site-mediated insertion of the defensins into the plasma membrane.
MATERIALS AND METHODS
Materials.
The antifungal peptides Dm-AMP1, Hs-AFP1, and Ah-AMP1 were isolated as described previously (19). Rs-AFP2, Rs-AFP2(Y38G), and the membrane-active antifungal α-purothionin (α-PT) found in wheat seeds were isolated as described previously by Terras et al. (26), De Samblanx et al. (9), and Redman and Fisher (22), respectively. SYTOX Green was obtained from Molecular Probes (Eugene, Oreg.). Carbonyl cyanide m-chlorophenylhydrazone (CCCP) was purchased from Sigma (St. Louis, Mo.). All other reagents were of reagent grade and were obtained from commercial sources.
Microorganisms.
N. crassa FGSC 2489 was grown on six-cereal agar, and conidia were harvested as described previously (4). Conidium stocks in 20% (vol/vol) glycerol were at a final concentration of 2 × 107 conidia/ml. Yeast (S. cerevisiae) was grown and stored following standard protocols (1). S. cerevisiae strains used were W303-1A (genotype, MATa leu2-3/112 ura3-1 trp1-1 his3-11/15 ade2-1 can1-100 GAL SUC2) and the W303-1A-derived mutant DM1 (30).
Antifungal activity assay.
Activity against N. crassa was assayed by microspectrophotometry (4, 26). N. crassa was grown at an inoculum density of 3 × 105 conidia/ml in 12 g of potato dextrose broth per liter (Difco, Detroit, Mich.) with continuous shaking (200 rpm) at 22°C. After 20 h of incubation, hyphae were washed with either SMF1 or SMF1 supplemented with either 50 mM KCl or 5 mM MgCl2 · 6H2O, (SMF1 is synthetic medium for fungi, containing 50 μM MgSO4 · 7H2O, 50 μM CaCl2 · 2H2O, 5 μM FeSO4 · 7H2O, 0.1 μM CoCl2, 0.1 μM CuSO4 · 5H2O, 2 μM Na2MoO4 · 2H2O, 0.5 μM H3BO3, 0.1 μM KI, 0.5 μM ZnSO4 · 7H2O, 0.1 μM MnSO4 · 1H2O, 10 g of glucose per liter, 1 g of asparagine per liter, 20 mg of methionine per liter, 2 mg of myo-inositol per liter, 0.2 mg of biotin per liter, 1 mg of thiamine-HCl per liter, 0.2 mg of pyridoxine-HCl per liter, 0.5 mM K2HPO4 · 3H2O). Ninety-microliter aliquots of the suspension of N. crassa hyphae in the appropriate medium were mixed with 10 μl of the same medium containing antifungal peptides and incubated in transparent 96-well microtiter plates. After incubation for 24 h at 22°C without shaking, the absorbance at 595 nm was determined with an enzyme-linked immunosorbent assay reader (26). The absorbance values served as a measure of microbial growth (26). The concentration of the antifungal protein that is required to inhibit 50% of the fungal growth was calculated from the dose-response curves with twofold dilution steps (26).
The activity of protein samples against S. cerevisiae was determined in an analogous manner. Briefly, 10 μl of the protein sample was mixed in a well of a 96-well microplate with 90 μl of yeast minimal medium (YMM) (0.8 g of complete supplement mixture [CSM] per liter [BIO 101, La Jolla, Calif.], 6.5 g of yeast nitrogen base without amino acids per liter [Difco], 20 g of glucose per liter), containing about 2 × 106 yeast cells per ml. The microplates were incubated at 30°C without shaking, and the absorbance at 595 nm was recorded after 20 h of incubation.
SYTOX Green uptake assay.
N. crassa was grown in potato dextrose broth as described above. After 20 h of incubation, hyphae were washed with either water buffered with 100 μM HEPES-KOH (pH 6.5), SMF1, or SMF1 supplemented with either 50 mM KCl or 5 mM MgCl2. Ninety-microliter aliquots of the suspension of N. crassa hyphae, supplemented with 0.2 μM SYTOX Green, were mixed with 10 μl of the same medium containing antifungal peptides and incubated in white 96-well microplates (PE white; Perkin-Elmer, Norwalk, Conn.). After incubation for 10 min to 6 h at 22°C with periodic agitation, fluorescence emitted by the cells in the microplates was measured with a Perkin-Elmer LS 50 B fluorescence spectrometer at an excitation wavelength of 488 nm (slit, 10 nm) and an emission wavelength of 540 nm (slit, 5 nm). Fluorescence values of the samples were corrected by subtracting the fluorescence value of a culture in the same medium without peptides but with SYTOX Green. Absolute values of fluorescence did not differ more than 50% in independent tests performed under identical conditions. SYTOX Green uptake in S. cerevisiae was measured similarly except that the medium used was either YMM or YMM supplemented with 5 mM MgCl2, the cell density was approximately 2 × 108 cells per ml, and incubation was at 30°C.
Fluorescence microscopy.
N. crassa hyphae, grown as described for the antifungal activity assay, were suspended in SMF1 containing 5 mM MgCl2 and 0.2 μM SYTOX Green, in either the presence or absence of antifungal peptides. After 360 min of incubation, fluorescence was viewed with a Nikon Optiphot (Tokyo, Japan) fluorescence microscope equipped with a B-2A filter set (Nikon) for fluorescein detection (excitation wavelength, 450 to 490 nm; emission wavelength, 520 nm).
Statistical analysis.
To determine a possible correlation between SYTOX Green uptake and antifungal activity, P values of the corresponding data were calculated with Microsoft Excel software, by calculating the analysis of variance. If the P value of the data was lower than 0.05, it was concluded that the data were significantly correlated.
RESULTS
Permeabilization of N. crassa and S. cerevisiae suspended in water.
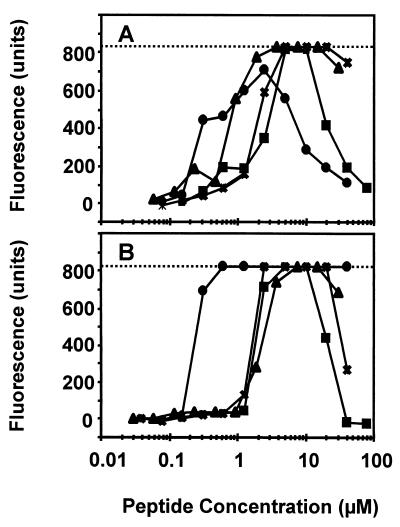
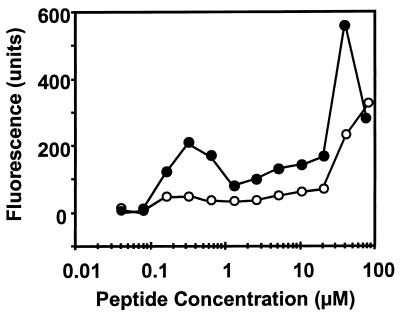
We tested the ability of the plant defensins to permeabilize N. crassa hyphae in water (buffered with 100 μM HEPES [pH 6.5]). SYTOX Green permeabilization was assessed 30 min after the addition of SYTOX Green and the peptides. α-PT, Rs-AFP2, Hs-AFP1, and Dm-AMP1 caused an increase in SYTOX Green influx (Fig. 1A). At high peptide concentrations (>5 μM), the relative increase in SYTOX Green uptake decreased, with the greatest decreases for α-PT and Rs-AFP2.
FIG. 1.
Membrane permeabilization induced by plant defensins and α-PT in N. crassa and S. cerevisiae in water. Dose-response curves of membrane permeabilization measured by SYTOX Green fluorescence of N. crassa hyphae (A) and S. cerevisiae wild-type cells (B) are shown. Fungal cells were suspended in distilled water buffered with 100 μM HEPES (pH 6.5) and treated with α-PT (●), Rs-AFP2 (■), Hs-AFP1 (X), or Dm-AMP1 (▴) for 30 min, whereupon fluorescence was measured. The dotted line indicates the upper limit of fluorescence detection. Values correspond to one representative experiment of three.
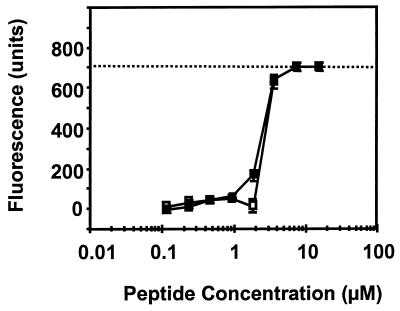
Similar observations were made for S. cerevisiae cells suspended in water buffered with 100 μM HEPES (pH 6.5). α-PT, Rs-AFP2, Hs-AFP1, and Dm-AMP1 all increased the influx of SYTOX Green (Fig. 1B), with a drop at high concentrations of the plant defensins. Dm-AMP1 permeabilized the S. cerevisiae mutant DM1, known to be resistant to Dm-AMP1 and to lack Dm-AMP1 binding sites (30), to essentially the same extent as wild-type cells, indicating that this type of permeabilization is not linked to the presence of specific binding sites for Dm-AMP1 (Fig. 2).
FIG. 2.
Membrane permeabilization induced by Dm-AMP1 in S. cerevisiae wild-type cells and S. cerevisiae DM1 cells in water. Dose-response curves of membrane permeabilization measured by SYTOX Green fluorescence of S. cerevisiae wild-type cells (□) and S. cerevisiae DM1 cells (■) are shown. Fungal cells were suspended in distilled water buffered with 100 μM HEPES (pH 6.5) and treated with Dm-AMP1 for 30 min, whereupon fluorescence was measured. The dotted line indicates the upper limit of fluorescence detection. Values are averages with standard errors of triplicate measurements and correspond to one representative experiment of three.
Permeabilization of N. crassa in growth medium.
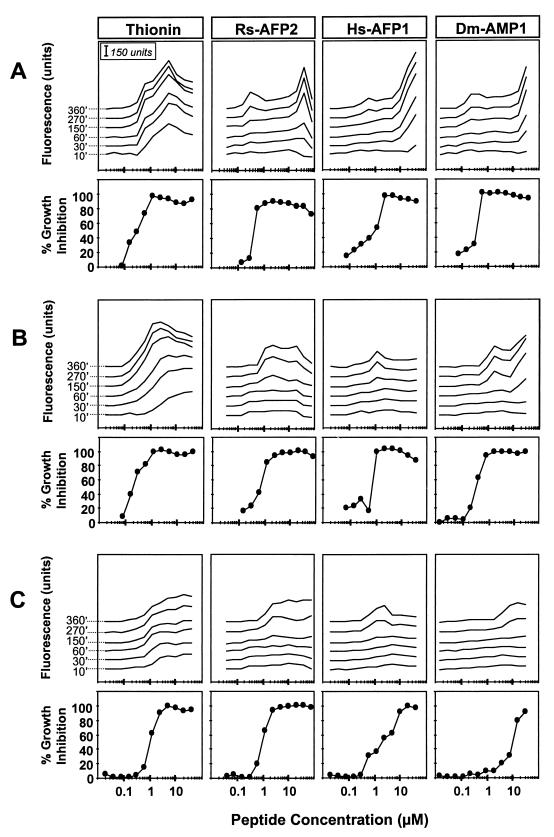
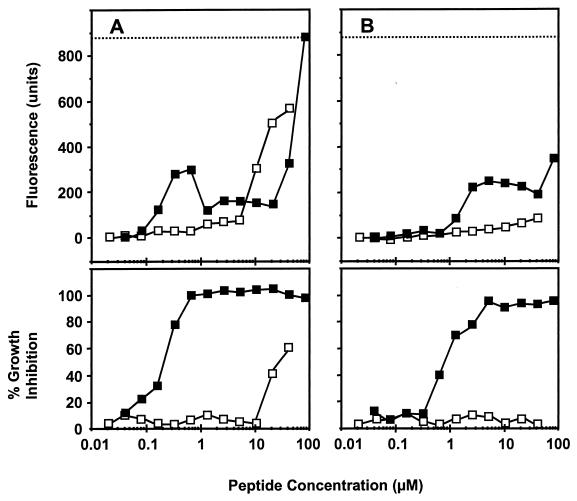
When N. crassa hyphae were suspended in the growth medium SMF1, membrane permeabilization to SYTOX Green induced by α-PT (Fig. 3A) could be detected after 10 min of incubation and increased with longer incubation times. A significant correlation (α = 0.05) between the induced hyphal permeabilization and the antifungal activity of α-PT could be observed. Weak permeabilization by the plant defensins (Rs-AFP2, Hs-AFP1, and Dm-AMP1) in the 0.1 to 1 μM range could be detected only after 2 to 4 h of incubation, whereas stronger permeabilization, induced at levels greater than 10 μM, could be detected after only 30 min of incubation. The concentrations of plant defensins inducing the weak permeabilization effect were significantly correlated (α = 0.05) with the concentrations required for growth inhibition.
FIG. 3.
Membrane permeabilization and growth inhibition induced by plant defensins and α-PT in N. crassa in low- and high-ionic-strength growth media. Dose-response curves of membrane permeabilization measured by SYTOX Green fluorescence and growth inhibition of N. crassa suspended in SMF1 (A), SMF1 plus 50 mM KCl (B), and SMF1 plus 5 mM MgCl2 (C) are shown. Fungal hyphae were treated with α-PT, Rs-AFP2, Hs-AFP1, or Dm-AMP1, and fluorescence was measured at different time points. Time points (in minutes) are indicated at the left of the α-PT permeabilization curves. Values correspond to one representative experiment of three.
Similar experiments were performed in growth medium with increased ionic strength, SMF1 supplemented with 50 mM KCl or 5 mM MgCl2 (Fig. 3B and C). Previous work has established that the presence of monovalent cations and especially of divalent cations in the growth medium reduces the antifungal activity of plant defensins, and that little or no antifungal activity is observed at concentrations of monovalent or divalent cations above 100 or 10 mM, respectively (19, 26, 27). α-PT permeabilized N. crassa irrespective of the ionic strength of the medium, although the effect was less pronounced in the 5 mM MgCl2 treatment than in the 50 mM KCl treatment. In all tested media, the dose-response curves of permeabilization by α-PT correlated significantly (α = 0.05) with those of growth inhibition, supporting the earlier hypothesis that thionins affect fungal growth by causing membrane permeabilization (5, 6, 28).
For the three plant defensins tested, permeabilization depended upon the cation composition of the medium. The strong permeabilization, observed after 30 min under low-ionic-strength conditions at plant defensin concentrations above 10 μM, was drastically reduced in the presence of either 50 mM KCl or 5 mM MgCl2. The weak permeabilization, detected at lower doses of plant defensins (0.1 to 1 μM), was not affected or only weakly affected by the presence of cations in the medium. In addition, this weaker and more cation-resistant permeabilization correlated significantly (α = 0.05) with the antifungal activity of the plant defensins.
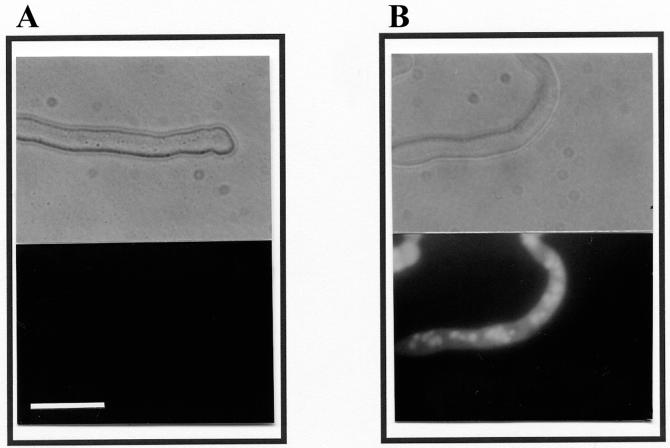
When viewed with a fluorescence microscope, N. crassa cells suspended in SMF1 supplemented with 5 mM MgCl2 in the presence of 20 μM plant defensins showed strong SYTOX Green fluorescence in the cytosol and especially in the nuclei (Fig. 4). This observation confirmed that the plant defensins cause intracellular uptake of the dye under these conditions.
FIG. 4.
Fluorescence microscopy of fungal cells in the presence of SYTOX Green and Dm-AMP1. N. crassa cells were suspended in SMF1 supplemented with 5 mM MgCl2 and 0.2 μM SYTOX Green and treated for 360 min in the absence (A) or presence (B) of 20 μM Dm-AMP1. Upper panels are light microscopic images; lower panels are fluorescence microscopic images. Bar, 25 μm.
Carbendazim, an inhibitor of microtubule formation, did not increase SYTOX Green uptake in the concentration range tested (0.1 to 1 mM), although it completely inhibited growth of N. crassa (results not shown). Hence, growth inhibition by itself is not sufficient to increase permeabilization of fungal cells to SYTOX Green.
Permeabilization of various antimicrobial agents has previously been shown to be abolished by treating cells with a membrane-depolarizing agent (10). Treating N. crassa cells with plant defensins in the presence of 2 μM CCCP significantly reduced permeabilization to SYTOX Green, especially with lower doses of plant defensins (0.1 to 1 μM) (Fig. 5).
FIG. 5.
Effect of CCCP on membrane permeabilization induced by Rs-AFP2 in N. crassa. Dose-response curves of membrane permeabilization measured by SYTOX Green fluorescence of N. crassa suspended in SMF1 in the absence (●) or presence (○) of 2 μM CCCP are shown. Fluorescence was measured 4 h after the addition of SYTOX Green, CCCP, and Rs-AFP2. Values correspond to one representative experiment of two.
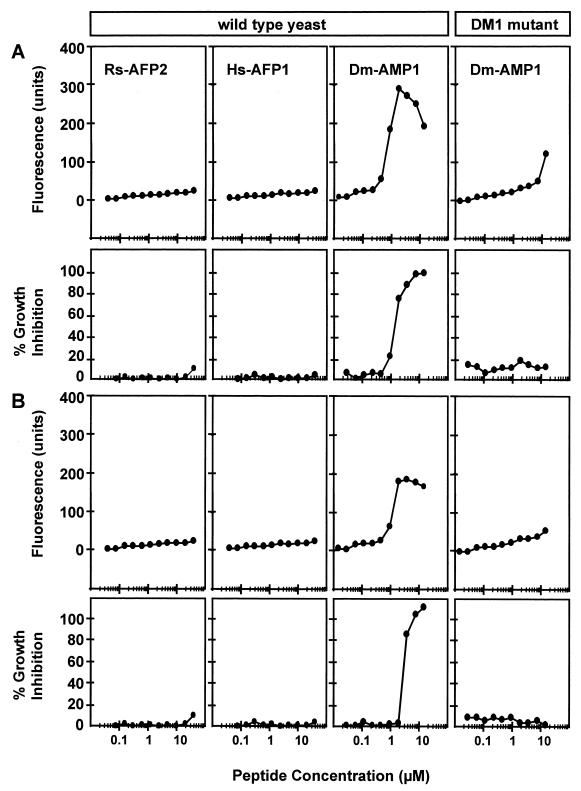
Rs-AFP2(Y38G) is a variant of Rs-AFP2 with substantially decreased antifungal activity (9). Membrane permeabilization induced by Rs-AFP2(Y38G) under low-ionic-strength conditions could be detected only at concentrations above 10 μM (Fig. 6A). Under high-ionic-strength conditions, however, no permeabilization could be detected (Fig. 6B), indicating that Rs-AFP2(Y38G) induces only cation-sensitive membrane permeabilization. This cation-sensitive permeabilization correlates significantly (α = 0.05) with the antifungal activity of Rs-AFP2(Y38G) in low-ionic-strength medium.
FIG. 6.
Membrane permeabilization and growth inhibition induced by Rs-AFP2 and Rs-AFP2(Y38G) in N. crassa in low- and high-ionic-strength growth media. Dose-response curves of membrane permeabilization measured by SYTOX Green fluorescence and growth inhibition of N. crassa suspended in SMF1 (A) and SMF1 plus 5 mM MgCl2 (B) are shown. Fungal hyphae were incubated with Rs-AFP2 (■) and Rs-AFP2(Y38G) (□) for 4 h, whereupon fluorescence was measured. The dotted line indicates the upper limit of fluorescence detection. Values correspond to one representative experiment of two.
Permeabilization of S. cerevisiae suspended in growth medium.
Rs-AFP2 and Hs-AFP1 did not inhibit growth of S. cerevisiae in YMM and also failed to cause permeabilization to SYTOX Green (Fig. 7). Dm-AMP1 induced membrane permeabilization in S. cerevisiae after 12 h of incubation, irrespective of the presence of cations in the medium. This cation-resistant permeabilization correlated significantly (α = 0.05) with the antifungal activity. No such permeabilization was induced by Dm-AMP1 in the DM1 mutant.
FIG. 7.
Membrane permeabilization induced by plant defensins and α-PT in S. cerevisiae wild-type cells and S. cerevisiae DM1 cells in low- and high-ionic-strength growth media. Dose-response curves of membrane permeabilization measured by SYTOX Green fluorescence and growth inhibition of S. cerevisiae wild-type cells and S. cerevisiae DM1 cells suspended in YMM (A) and YMM plus 5 mM MgCl2 (B) are shown. Fungal cells were incubated with Rs-AFP2, Hs-AFP1, or Dm-AMP1 for 12 h, whereupon fluorescence was measured. Values correspond to one representative experiment of two.
DISCUSSION
Plant defensins have a biphasic effect on the membrane permeabilization of N. crassa depending on the plant defensin dose. At high doses, strong permeabilization can be detected within 30 to 60 min of the addition of the plant defensins. This effect depends on the ionic constitution of the medium: it is most pronounced in water but is almost undetectable in the presence of either 50 mM K+ or 5 mM Mg2+. In SMF1, cation-sensitive permeabilization occurs at plant defensin doses approximately 10-fold higher than those causing fungal growth inhibition. These findings are consistent with previous observations that plant defensins cause detectable permeabilization of N. crassa in a low-ionic-strength growth medium to the nonmetabolizable compound isoaminobutyric acid only at doses that are an order of magnitude above those required for antifungal activity (28). Hence, cation-sensitive permeabilization does not appear to be the primary cause of growth inhibition in hyphae treated with plant defensins.
At lower plant defensin doses, permeabilization is relatively weak (as it requires at least 2 to 4 h of incubation to be detected) and much less affected by either 50 mM K+ or 5 mM Mg2+. The doses at which weak cation-resistant permeabilization occurs correlate significantly with those for growth inhibition, suggesting that this effect is linked to the primary cause of defensin-induced growth inhibition.
In the case of S. cerevisiae, two types of membrane permeabilization were observed: (i) a cation-sensitive membrane permeabilization which is induced by all plant defensins in water-suspended cells and (ii) a cation-resistant membrane permeabilization that was observed only with Dm-AMP1. Since the growth of S. cerevisiae is inhibited only by Dm-AMP1 and not by Hs-AFP1 or Rs-AFP2 (30), the cation-sensitive permeabilization cannot play a role in the growth inhibition of S. cerevisiae cells suspended in growth medium. The doses required for the cation-resistant membrane permeabilization of S. cerevisiae by Dm-AMP1 in growth medium correlated significantly with those required for antifungal activity, again suggesting that this kind of membrane permeabilization is linked to the primary cause of fungal growth inhibition. Although our data clearly reveal similarities in the way plant defensins act on the yeast S. cerevisiae and the filamentous fungus N. crassa, we do not yet know if the underlying mechanistic details are the same in both organisms.
Evidence that the first step to fungal growth inhibition by plant defensins is the binding to a binding site located in the plasma membranes of fungal hyphae has been previously presented (29, 30). We now propose that the interaction of plant defensins with such a binding site subsequently enables them to insert into the plasma membrane, causing a structural disruption and alteration of the membrane’s permeability to ions such as Ca2+ and K+ and organic molecules like SYTOX Green. This process of binding-site-mediated membrane insertion and disruption does not appear to be highly influenced by the presence of cations in the medium. Binding-site-mediated insertion in plasma membranes has previously been proposed to explain the antimicrobial activity of a number of proteins (7, 14, 24, 32). However, this idea has so far not gained wide support, mainly due to poor experimental evidence.
Our most compelling evidence for binding-site-mediated cation-resistant membrane permeabilization is the observation that cation-resistant permeabilization was caused by Dm-AMP1 in wild-type S. cerevisiae but not in the isogenic Dm-AMP1-resistant DM1 mutant (30). Moreover, Rs-AFP2(Y38G), a variant of Rs-AFP2 with substantially decreased antifungal activity, did not induce cation-resistant membrane permeabilization in N. crassa. Rs-AFP2(Y38G) does not compete with binding sites for Hs-AFP1 on either N. crassa cells or N. crassa microsomes (29). A single amino acid substitution might change the three-dimensional structure of Rs-AFP2 such that interaction with its receptor no longer occurs. Although the presence of binding sites appears to be a prerequisite for the antifungal effect of plant defensins under high-ionic-strength conditions, it does not appear to be sufficient; S. cerevisiae possesses specific binding sites for Hs-AFP1, but its growth is not affected by this plant defensin (20).
Binding of Hs-AFP1 and Dm-AMP1 to fungal microsomes is only partially reversible (29) and not reversible (30), respectively. These findings are fully consistent with our model, as insertion of plant defensins into the plasma membrane following binding would make them inaccessible for competition. The binding of plant defensins to their binding sites is affected by membrane-depolarizing agents (29). Permeabilization to Ca2+ (28) and SYTOX Green (Fig. 5), however, appears to require a polarized membrane, as these processes are abolished by treating cells with the membrane-depolarizing agent CCCP. The apparent dependency of permeabilization on membrane polarization might explain why the dose-response curves for SYTOX Green uptake declined at doses above those providing maximum effect. This phenomenon was also observed when fungal cells were treated with detergents such as sodium dodecyl sulphate or Triton X-100 (results not shown). The permeabilization of fungal membranes treated with plant defensins will result in depolarization (28), which may counteract membrane permeabilization. The net result may be strongly influenced by the plant defensin dose.
At high concentrations of plant defensins (10 to 40 μM), a different type of permeabilization is observed which is highly suppressible by cations in the medium. We propose that permeabilization at high plant defensin doses and low concentrations of cations is due to interaction with the membrane in a binding-site-independent way. In this case, the interaction involves only plant defensins and the phospholipid components of the fungal plasma membranes. Cations, especially divalent cations, stabilize membrane phospholipid structures (33) and their presence will counteract the insertion of plant defensins in membranes. The binding-site-independent nature of this permeabilization is supported by the observation that permeabilization of water-suspended S. cerevisiae cells by Dm-AMP1 is equally strong in the wild type and in the Dm-AMP1-resistant isogenic mutant. Direct interaction with membrane lipids and subsequent membrane permeabilization may explain the antimicrobial effects of the structurally related insect (8) and mammalian (12) defensins and several linear antimicrobial peptides, such as magainins, found in Xenopus skin (16); indolicidin found in the cytoplasmic granules of bovine neutrophils (13); and sticholysin I, a cytolysin found in the sea anemone Stichodactyla helianthus (25).
The specificity of plant defensins is dependent on the ionic strength of the growth medium (19, 26, 27). In low-ionic-strength medium, plant defensins inhibit most fungi. This observation is consistent with the proposed binding-site-independent membrane insertion of plant defensins. However, in high-ionic-strength medium, the spectrum of plant defensin activity is narrowed considerably and shows more variation among the different types of plant defensins. Under these conditions, binding-site-mediated insertion prevails and the specificity of the interaction of plant defensins with their binding site dictates the specificity of the antifungal spectrum of activity.
ACKNOWLEDGMENTS
This research was supported in part by the Commission of the European Union (AIR2-CT94-1356) and by a grant from the Fonds voor Wetenschappelijk Onderzoek—Vlaanderen. K.T. is Postdoctoral Researcher of the Onderzoeksfonds of the Katholieke Universiteit Leuven, Belgium.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Struhl K. Saccharomyces cerevisiae. In: Ausubel F M, editor. Current protocols in molecular biology. Somerset, N.Y: John Wiley & Sons, Inc.; 1993. pp. 13.1.1–13.11.4. [Google Scholar]
- 2.Broekaert W F, Cammue B P A, De Bolle M F C, Thevissen K, De Samblanx G W, Osborn R W. Antimicrobial peptides from plants. Crit Rev Plant Sci. 1997;16:297–323. [Google Scholar]
- 3.Broekaert W F, Terras F R G, Cammue B P A, Osborn R W. Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol. 1995;108:1353–1358. doi: 10.1104/pp.108.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broekaert W F, Terras F R G, Cammue B P A, Vanderleyden J. An automated quantitative assay for fungal growth. FEMS Microbiol Lett. 1990;69:55–60. [Google Scholar]
- 5.Caaveiro J M, Molina A, Gonzalez-Manas J M, Rodriguez-Palenzuela P, Garcia-Olmedo F, Goni F M. Differential effects of five types of antipathogenic plant peptides on model membranes. FEBS Lett. 1997;410:338–342. doi: 10.1016/s0014-5793(97)00613-3. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco L, Vazquez D, Hernandez-Lucas C, Carbonero P, Garcia-Olmedo F. Thionins: plant peptides that modify membrane permeability in cultured mammalian cells. Eur J Biochem. 1981;116:185–189. doi: 10.1111/j.1432-1033.1981.tb05317.x. [DOI] [PubMed] [Google Scholar]
- 7.Chikindas M L, Garcia-Garcera M J, Driessen A J, Ledeboer A M, Nissen-Meyer J, Nes I F, Abee T, Konings W N, Venema G. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol. 1993;59:3577–3584. doi: 10.1128/aem.59.11.3577-3584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cociancich S, Ghazi A, Hetru C, Hoffmann J A, Letellier L. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J Biol Chem. 1993;268:19239–19245. [PubMed] [Google Scholar]
- 9.De Samblanx G W, Goderis I J, Thevissen K, Raemaekers R, Fant F, Borremans F, Acland D P, Osborn R W, Patel S, Broekaert W F. Mutational analysis of a plant defensin from radish (Raphanus sativus L.) reveals two adjacent sites important for antifungal activity. J Biol Chem. 1997;272:1171–1179. doi: 10.1074/jbc.272.2.1171. [DOI] [PubMed] [Google Scholar]
- 10.Falla T J, Karunaratne D N, Hancock R E W. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 11.Harrison S J, Marcus J P, Goulter K C, Green J L, Maclean D J, Manners J M. An antimicrobial peptide from the Australian native Hardenbergia violacea provides the first functionally characterised member of a subfamily of plant defensins. Aust J Plant Physiol. 1997;24:571–578. [Google Scholar]
- 12.Kagan B L, Selsted M E, Ganz T, Lehrer R I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladokhin A S, Selsted M E, White S H. Bilayer interactions of indolicidin, a small antimicrobial peptide rich in tryptophan, proline, and basic amino acids. Biophys J. 1997;72:794–805. doi: 10.1016/s0006-3495(97)78713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakey J H, van der Goot F G, Pattus F. All in the family: the toxic activity of pore-forming colicins. Toxicology. 1994;87:85–108. doi: 10.1016/0300-483x(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 15.Landon C, Sodano P, Hetru C, Hoffmann J, Ptak M. Solution structure of drosomycin, the first inducible antifungal protein from insects. Protein Sci. 1997;6:1878–1884. doi: 10.1002/pro.5560060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuzaki K, Sugishita K, Harada M, Fujii N, Miyajima K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim Biophys Acta. 1997;1327:119–130. doi: 10.1016/s0005-2736(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki T, Suzuki T, Fujikura K, Takata K. Nuclear staining for laser confocal microscopy. Acta Histochem Cytochem. 1997;30:309–314. doi: 10.1177/002215549704500107. [DOI] [PubMed] [Google Scholar]
- 18.Moreno M, Segura A, Garcia-Olmedo F. Pseudothionin-St1, a potato peptide active against potato pathogens. Eur J Biochem. 1994;223:135–139. doi: 10.1111/j.1432-1033.1994.tb18974.x. [DOI] [PubMed] [Google Scholar]
- 19.Osborn R W, De Samblanx G W, Thevissen K, Goderis I, Torrekens S, Van Leuven F, Attenborough S, Rees S B, Broekaert W F. Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett. 1995;368:257–262. doi: 10.1016/0014-5793(95)00666-w. [DOI] [PubMed] [Google Scholar]
- 20.Osborn, R. W., and K. Thevissen. 1997. Unpublished data.
- 21.Penninckx I A, Eggermont K, Terras F R, Thomma B P, De Samblanx G W, Buchala A, Metraux J P, Manners J M, Broekaert W F. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redman D G, Fisher D. Purothionin analogues from barley flour. J Sci Food Agric. 1969;20:427–432. [Google Scholar]
- 23.Roth B, Poot M, Yue S, Millard P. Bacterial viability and antibiotic susceptibility testing with SYTOX Green nucleic acid stain. Appl Environ Microbiol. 1997;63:2421–2431. doi: 10.1128/aem.63.6.2421-2431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt M J, Compain P. Killer-toxin-resistant kre12 mutants of Saccharomyces cerevisiae: genetic and biochemical evidence for a secondary K1 membrane receptor. Arch Microbiol. 1995;164:435–443. doi: 10.1007/BF02529742. [DOI] [PubMed] [Google Scholar]
- 25.Tejuca M, Serra M D, Ferreras M, Lanio M E, Menestrina G. Mechanism of membrane permeabilization by sticholysin I, a cytolysin isolated from the venom of the sea anemone Stichodactyla helianthus. Biochemistry. 1996;35:14947–14957. doi: 10.1021/bi960787z. [DOI] [PubMed] [Google Scholar]
- 26.Terras F R, Schoofs H M, De Bolle M F, Van Leuven F, Rees S B, Vanderleyden J, Cammue B P, Broekaert W F. Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J Biol Chem. 1992;267:15301–15309. [PubMed] [Google Scholar]
- 27.Terras F R G, Schoofs H M E, Thevissen K, Osborn R W, Vanderleyden J, Cammue B P A, Broekaert W F. A new family of basic cysteine-rich plant antifungal proteins from Brassicaceae species. FEBS Lett. 1993;316:233–240. doi: 10.1016/0014-5793(93)81299-f. [DOI] [PubMed] [Google Scholar]
- 28.Thevissen K, Ghazi A, De Samblanx G W, Brownlee C, Osborn R W, Broekaert W F. Fungal membrane responses induced by plant defensins and thionins. J Biol Chem. 1996;271:15018–15025. doi: 10.1074/jbc.271.25.15018. [DOI] [PubMed] [Google Scholar]
- 29.Thevissen K, Osborn R W, Acland D P, Broekaert W F. Specific, high affinity binding sites for an antifungal plant defensin on Neurospora crassa hyphae and microsomal membranes. J Biol Chem. 1997;272:32176–32181. doi: 10.1074/jbc.272.51.32176. [DOI] [PubMed] [Google Scholar]
- 30.Thevissen, K., R. W. Osborn, D. P. Acland, and W. F. Broekaert. Specific binding sites for an antifungal plant defensin from dahlia (Dahlia merckii) on fungal cells are required for antifungal activity. Submitted for publication. [DOI] [PubMed]
- 31.Thomma B P H J, Broekaert W F. Tissue-specific expression of plant defensin genes PDF2.1 and PDF2.2 in Arabidopsis thaliana. Plant Physiol Biochem. 1998;36:533–537. [Google Scholar]
- 32.van Belkum M J, Kok J, Venema G, Holo H, Nes I F, Konings W N, Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol. 1991;173:7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Oss C J. Hydrophobicity and hydrophilicity of biosurfaces. Curr Opin Coll Interf Sci. 1997;2:503–512. [Google Scholar]
- 34.Veldhuis M J W, Cucci T L, Sieracki M E. Cellular DNA content of marine phytoplankton using two new fluorochromes: taxonomic and ecological implications. J Phycol. 1997;33:527–541. [Google Scholar]