Abstract
For many years, the medical community has relied in clinical practice on historic data about the physiological changes that occur during pregnancy. However, some newer studies have disputed a number of assumptions in these data for not being evidence-based or derived from large prospective cohort-studies. Accurate knowledge of these physiological changes is important for three reasons: Firstly, it facilitates correct diagnosis of diseases during pregnancy; secondly, it enables us to answer questions about the effects of medication during pregnancy and the ways in which pregnancy alters pharmacokinetic and drug-effects; and thirdly, it allows for proper modeling of physiologically-based pharmacokinetic models, which are increasingly used to predict gestation-specific changes and drug–drug interactions, as well as develop new knowledge on the mode-of-action of drugs, the mechanisms underlying their interactions, and any adverse effects following drug exposure. This paper reviews new evidence regarding the physiologic changes during pregnancy in relation to existing knowledge.
Keywords: pharmacogenomics, pharmacokinetics, pharmacology, physiologic changes, pregnancy
Introduction
Pregnancy is associated with several structural and functional changes that influence the processes of drug absorption, distribution, metabolism, and excretion (ADME) [1]. For example, there is increased drug transit time in the gastro-intestinal tract, increased volume of distribution of drugs, increased renal filtration, and increased metabolism of most medications during pregnancy [1]. Besides these pregnancy-related changes attributable to alterations in maternal blood flow, increased fluid retention, and the effect of hormones (such as vasopressin, progesterone, relaxin, estrogen, and angiotensin II), transmembrane transporter function, expression, and regulation also significantly influencing the pharmacokinetics (PK) of many drugs during pregnancy [2, 3]. These physiologic and transporter changes that occur during pregnancy have critical effects on the PK profile of drugs during pregnancy.
Although several efforts have been made to understand the physiologic changes that occur during pregnancy and how these changes affect drug PK [4], [5], [6], [7], [8], [9], [10], [11], recent studies have disputed a number of assumptions in older studies for not being gestational-age specific, evidence-based, or derived from large prospective cohort studies [12, 13]. In 2018, for example, a New England Journal of Medicine publication demonstrated that platelet count consistently decreases throughout gestation in uncomplicated pregnancies [13], contrary to previously published reports that this decrease begins in the mid-second to third trimester and continues until time of delivery [14], [15], [16]. Other recent data from multicenter observational cohorts similarly dispute physiological reference values for blood pressure, heart rate, respiratory rate, and oxygen saturation during pregnancy [12]. In addition, there have been multiple publications on intestinal, hepatic, and renal transporter expression, regulation, and function during pregnancy [17], [18], [19], [20] which have not yet been systematically reviewed. Accurate knowledge of these physiological changes is important for three reasons: Firstly, it helps us correctly diagnose and treat diseases during pregnancy; secondly, it enables us to answer important questions about the effects of medication during pregnancy and the ways in which pregnancy alters PK and drug effects; and thirdly, it allows for proper modeling and validation of physiologically-based pharmacokinetic (PBPK) models, which are increasingly being used to predict gestation-specific changes and drug–drug interactions, as well as develop new knowledge on the mode of action of drugs, the mechanisms underlying their interactions, and any adverse effects following maternal drug exposure.
This paper reviews new evidence in relation to existing knowledge, focusing on the five major organ-systems with the greatest potential to alter the PK of drugs during pregnancy: cardiovascular, respiratory, hematologic, renal, and gastrointestinal systems. Data for this manuscript were obtained from a comprehensive review of new and old published literature on the physiologic changes that occur during normal pregnancy, in addition to relevant pregnancy pharmacology literature.
Methods
The methodology of this review conformed to the synthesis without meta-analyses (SWiM) guidelines [21]. The search included ClinicalTrials.gov, MEDLINE, EMBASE, Scopus, Web of Science, and the Cochrane Library for studies that met inclusion and exclusion criteria using a search strategy designed in collaboration with an experienced Librarian at the Johns Hopkins University School of Public Health. The search included controlled vocabulary terms (MeSH and free text keywords) for physiologic changes in pregnancy, transporters, drug therapy, patient monitoring, and pharmacokinetics. The search strategy included Boolean operators “OR” (for related term) and “AND” (combination of different concepts). Relevant synonyms and alternative spellings were identified via EMBASE’s controlled vocabulary, Emtree.
Using Covidence software (Veritas Health Innovation Ltd, Australia 2018), titles and abstracts were extracted for full-text review. Duplicate entries were removed by Covidence software based on matching titles, authors and journals, and then manually confirmed. Studies were categorized by titles and abstracts as “include” or “exclude” based on relevance to the study question. Titles and abstracts marked “include” were reviewed in full text. Each full-text article was read to assess for eligibility based on the predefined eligibility criteria. Full-text articles were categorized as “include” or “exclude.” Studies marked as “include” based on full-text review were included in the review.
Results
The search identified 332 bibliographic references, 274 through the PubMed database and 58 through Clinicaltrials.gov. After 12 duplicate papers were removed, there were a total of 320 records in title and abstract form available for further screening. 193 clearly irrelevant references were excluded through reading of the abstracts. Thus, 127 references were assessed for eligibility into the review. After careful scrutiny, 13 other references were excluded as they did not fulfil the inclusion criteria (did not discuss physiology of pregnancy, drug transporter, or related to drug pharmacokinetics). Subsequently, 114 references met the inclusion criteria for this review on topics related to physiologic changes in pregnancy, transporters, and pharmacokinetics.
Discussion
Cardiovascular changes and its influence on drug pharmacokinetics during pregnancy
Maternal heart rate and blood pressure changes
The cardiovascular system, consisting of the heart and blood vessels, undergo several changes during pregnancy that impact the PK of many drugs [22]. Recent data suggest new target ranges for maternal heart rate and blood pressure changes in pregnant women. For example, up until recently, it was thought that maternal heart rate increases by approximately 10–15 beats/min, and cardiac output increases by about 30–50% (due to increased blood volume and heart rate) during pregnancy [23, 24]. Maternal blood pressure falls by 10–15 mmHg due to a reduction in peripheral vascular resistance caused by progesterone-induced vasodilation, and a low resistance circuit during normal pregnancy [24]. While systolic ejection murmurs (grades 1 or 2) are the most common types of murmurs that occur during pregnancy [25], diastolic murmurs are rarely due to physiologic changes associated with pregnancy.
Recent data from over 36,000 healthy pregnant women have disputed some of these previously described changes as not gestational age specific nor evidence based, and were based on data from small retrospective studies [12, 26]. The results from newer studies demonstrate that changes in maternal heart rate and blood pressures are not as substantial as previously thought. Using data from the Pregnancy Physiology Pattern Prediction (4P) study, a multicenter observational cohort, University of Oxford investigators developed a database of prospective vital sign measurements using standardized measurement techniques throughout pregnancy in healthy women with singleton pregnancies [12]. The median maternal heart rate was noted to be lowest at about 12 weeks of gestation, at approximately 82 beats/min (3rd to 97th centile of 63–105 beats/min). The heart rate rose progressively until about 34.1 weeks of gestation to a maximum of 91 beats/min (3rd to 97th quartile of 68–115 beats/min), an increase of 9 beats/min when compared to the maternal heart rate at 12 weeks of gestation [12]. Maternal heart rate then minimally decreased to a median of 89 beats/min at 40 weeks [12] (Figure 1). This means that the range of heart rate during pregnancy is approximately between 63 and 115 beats/min, compared to a range of 60–100 beats/min as previously suggested. For blood pressure changes, diastolic blood pressure declined from 12 weeks of gestation (median of 70 mmHg), nadired to 69 mmHg at approximately 19.2 weeks, and subsequently increased to a maximum median of 78 mmHg at 40 weeks, a difference 9 mmHg from minimum to maximum diastolic blood pressure [12] (Figure 2). Systolic blood pressure also declined in parallel from 12 weeks of gestation (median of 114 mmHg), nadired to 113 mmHg at approximately 18.6 weeks, and subsequently increased to a maximum median of 121 mmHg at 40 weeks, a difference 7 mmHg from minimum to maximum systolic blood pressure during pregnancy [12] (Figure 2). These changes in blood pressure and heart rate differ slightly from prior knowledge, where it is thought that blood pressure decreases by 10–15 mmHg during mid-pregnancy.
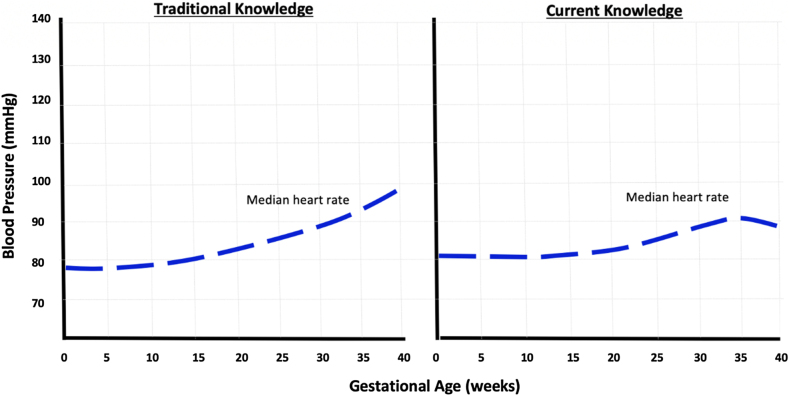
Figure 1:
Heart rate changes during normal pregnancy.
Figure illustrates heart rate changes during normal pregnancy (previously known knowledge on the left graph, and current knowledge on the right graph). On the right graph, the median heart rate was lowest at 12 weeks of gestation, at approximately 82 beats/min. The heart rate rose progressively until 34.1 weeks of gestation to a maximum of 91 beats/min, an increase of 9 beats/min when compared to the heart rate at 12 weeks of gestation. Maternal heart rate then minimally decreased to a median of 89 at 40 weeks. These changes in heart rate differ slightly from prior knowledge (left graph), as it is thought that heart rate increases by approximately 10–15 mmHg throughout gestation in normal pregnancy.
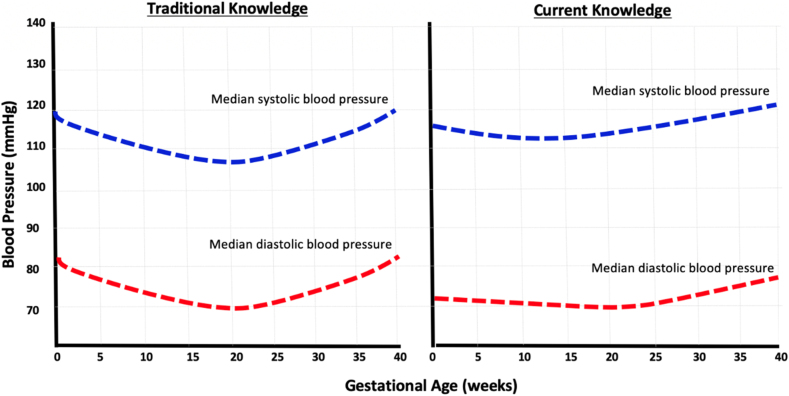
Figure 2:
Blood pressure changes during normal pregnancy.
Figure illustrates blood pressure changes during normal pregnancy (previously known knowledge on the left graph, and current knowledge on the right graph). On the right graph, diastolic blood pressure declined from 12 weeks of gestation (median of 70 mmHg), nadired at to 69 mmHg at 19.2 weeks, and subsequently increased to a maximum median of 78 mmHg at 40 weeks, a difference 9 mmHg from minimum to maximum diastolic blood pressure. Systolic blood pressure declined from 12 weeks of gestation (median of 114 mmHg), nadired at to 113 mmHg at 18.6 weeks, and subsequently increased to a maximum median of 121 mmHg at 40 weeks, a difference 7 mmHg from minimum to maximum systolic blood pressure during pregnancy. These changes in blood pressure differ slightly from prior knowledge (left graph), as it is thought that blood pressure decreases by 10–15 mmHg during mid-pregnancy.
There is additional recent research on newer blood pressure targets for pregnant women [27]. In 2018, based on results from the Systolic blood Pressure Intervention Trial (SPRINT) [28], the American Heart Association (AHA) and the American College of Cardiology (ACC) updated the threshold for diagnosis of hypertension in adults to 130/80 mmHg. However, a blood pressure threshold of 140/90 mmHg is still being used by many Obstetrical societies across the world to diagnose hypertension during pregnancy, despite the known reduction in blood pressure during pregnancy. Although the Obstetrics and Gynecologic community have not yet adopted the AHA/ACC new criteria for diagnosing high blood pressure, the study by Green et al. [12] suggesting that over 90% of women at 12 weeks of gestation have a blood pressure of ≤130 mmHg systolic and ≤80 mmHg diastolic, suggest that the AHA-ACC criteria for stage I hypertension are plausible in pregnant women. The pharmacologic importance of these cardiovascular changes is critical for diagnosis and pharmacologic management of some diseases during pregnancy, like pre-eclampsia and sepsis. For example, the third percentile for systolic blood pressure in the University of Oxford study was between 94 and 96 mmHg after 30 weeks of gestation in all groups. These thresholds are above the <90 mmHg systolic blood pressure threshold used to recognize and pharmacologically treat hypotension and sepsis during pregnancy [29]. In addition, data from the Control of Hypertension in Pregnancy Study (CHIPS) randomized trial that compared the effectiveness of tight (target diastolic blood pressures of 85 mmHg) vs. less-tight blood pressure control (target diastolic blood pressures of 100 mmHg) in improving pregnancy outcomes among women with mild chronic or gestational hypertension between 14 and 33 weeks of gestation, demonstrate that tight blood pressure control (similar to the updated physiological blood pressure changes in pregnancy as described above), might be the best clinical option because it minimizes maternal risk without increasing perinatal risk [30]. The 85 mmHg diastolic blood pressure goal from the CHIP trial is similar to the median diastolic blood pressure (78 mmHg) reported in the 2020 4P University of Oxford study [12].
Plasma volume during pregnancy and its impact on drug PK
Pregnancy is associated with about 40–50% rise in blood volume, reaching a peak at approximately 32 weeks of gestation [22]. Increased maternal plasma volume is thought to be related to the combined effect of mineralocorticoid and vasopressin activity, causing sodium and water retention during pregnancy [31]. This increase in plasma volume allows adequate perfusion of vital organs, and may provide some needed survival benefit in anticipation of blood loss at the time of vaginal delivery or cesarean section. In addition, there is maternal weight gain and increased body fat (a mean increase of 12–20 kg) from baseline during the course of pregnancy [32]. The PK consequence of increased maternal blood volume and increased fat and body mass during pregnancy is that hydrophilic drugs tend to be relatively minimally distributed (low volume of distribution), while lipophilic drugs tend to have a large volume of distribution, particularly into fatty tissues during pregnancy compared to the nonpregnant state. These physiologic changes may result in increased loading and maintenance medication dosage requirements, as well as the potential for subtherapeutic drug dosing during pregnancy. For example, buprenorphine is highly lipophilic and is highly (96%) protein bound [33], therefore, has a large volume of distribution (188–335 L). In a dose optimization PK study, the PK of buprenorphine in pregnant and nonpregnant individuals differed significantly [34]. Due to buprenorphine’s high volume of distribution, persistently low buprenorphine plasma concentrations were demonstrated during the second and third trimesters of pregnancy, suggesting that pregnant women may need buprenorphine dose adjustments during pregnancy, due to its very high apparent volume of distribution and protein binding [34]. Similarly, body weight and increased volume of distribution during pregnancy impacted the PK of enoxaparin, requiring dose adjustments, corrected for body weight in pregnant women, compared to non-pregnant women [35]. Several other drugs are affected by the increased volume of distribution that occurs during pregnancy. For example, analgesics (e.g., oxycodone) [36] and antihypertensives (e.g., metoprolol [37]) have extensive volumes of distribution of approximately 2.6 L/kg (range 1.8–3.7) [36] and 3.2–5.6 L/kg respectively that can affect drug disposition during pregnancy. Digoxin, while very low-protein bound (25%), distributes into muscle and fat, with an apparent volume of distribution of approximately 475–500 L in non-pregnant adults, but reaches a volume of distribution of approximately 680–730 L during pregnancy [38]. Similarly, metformin has a larger apparent volume of distribution at steady-state during pregnancy compared to postpartum [39]. Due to these pregnancy-induced changes in the PK of drugs, clinicians who prescribe these medications often make significant dosage changes when inadequate responses are encountered in clinical practice.
Consequences of pregnancy on the pharmacokinetics of high and low plasma-protein bound drugs
The synthesis of plasma proteins during pregnancy (primarily albumin and alpha 1-acid glycoprotein) are increased, but the increase in plasma volume results in a dilutional decrease in plasma protein concentrations, decreased protein binding ability, and increased free-drug (non-protein bound pharmacologically active drug fractions during pregnancy that correlates with therapeutic and adverse effects), as well as trans-placental drug transfer. Therefore, measuring total plasma concentrations of highly protein bound drugs is not a good proxy for free plasma drug concentrations [40]. A critical PK consequence of changes in plasma proteins is that drugs that are highly protein-bound (>80%) may show variations in protein binding during pregnancy. For drugs with significant protein binding, clearance is often dependent on the drug’s total concentration (a sum of the free and protein-bound concentrations), and not just the free drug concentrations. During pregnancy, there is decreased clearance of extensively protein-binding drugs (because there is less free pharmacologically available drug for metabolism). Conversely, there is increased clearance of low plasma protein-binding drugs due to increase the free-fraction of a drug from the decreased plasma protein concentrations and protein-binding ability during pregnancy. Decreased serum concentrations of drugs potentially reduce total drug concentrations needed for a desired therapeutic effect. For example, carbamazepine is an anticonvulsant medication that is approximately 80–85% protein-bound. Several studies have demonstrated decreased carbamazepine plasma concentrations during pregnancy [41, 42], with the largest prospective open label nonrandomized study of pregnant and postpartum women reporting a decline in total carbamazepine concentrations of 9–12% during the second and third trimesters of pregnancy [43]. Similarly, the serum concentration of phenytoin, which is approximately 90% bound to serum proteins, also decreases steadily from the first trimester of pregnancy, upto the third trimester, when the total serum concentrations of phenytoin are decreased by approximately 55–61% [44]. These physiological changes can lead to subtherapeutic plasma concentrations of these antiepileptic medications during pregnancy, resulting in worsening of epilepsy symptoms during pregnancy. Increased clearance of a drug due to decreased plasma protein is only true for drugs with low-extraction ratios (see hepatic changes and hepatic transporter expression during pregnancy for description of drug-extraction ratios). Although directly measuring free (unbound) drug concentrations either by dialysis or ultrafiltration remain the optimal strategy to quantify plasma drug concentrations for highly protein bound drugs [45, 46], PK studies in pregnant women continue to rely on plasma concentrations of total drug concentrations (bound plus unbound) drug for decision making during clinical care, as development of bioanalytical methods for measuring free fractions are extremely complicated, exceedingly time-consuming, difficult to validate, and exorbitantly expensive [46, 47].
It is crucial to note that while it is important to understand the impact individual organ-system changes can have on drug disposition during pregnancy, the final plasma drug concentration during pregnancy depends on a complex relationship (net effect) between so many PK variables, including the fraction of drug absorbed, the physicochemical properties governing diffusion across membranes, drug bioavailability, protein binding and unbound fractions of drug, volume of distribution, intrinsic organ clearance, organ extraction ratio (hepatic or renal), and several other PK variables. For example, darunavir (a protease inhibitor) is approximately 85% bound to plasma-proteins, but plasma proteins decrease during pregnancy, so the bound fraction of darunavir is expected to decrease. Darunavir has a low hepatic extraction ratio (<0.3), is metabolized extensively by cytochrome P450-3A (which increases in pregnancy), and eliminated (>90%) by the liver through hepatic clearance [40]. During pregnancy, darunavir protein binding decreases to approximately 30% (compared to pre-pregnancy), while the free-fraction (active drug) would be expected to increase. The increases in darunavir hepatic drug metabolism, volume of distribution, and clearance during pregnancy account for the low plasma darunavir concentrations observed during pregnancy [48], [49], [50], [51], [52], [53].
Respiratory system physiologic changes during pregnancy
Several functional changes that occur in the respiratory system due to increased estrogen and progesterone during pregnancy, affect drug PK. The increase in estrogen concentrations during pregnancy result in increased vascularity and edema of the upper respiratory tract [54]. Additionally, progesterone, a known stimulant of respiration and respiratory drive, directly induces an increase in alveolar ventilation during pregnancy [55]. As a result, the minimum alveolar concentration (alveolar concentration of an inhaled anesthetic that is required to prevent a response to a standardized painful stimulus in 50% of patients) of drugs, is reduced during pregnancy [56]. This means that a lower amount of inhalational agent is required to produce a response with inhalational agents during pregnancy compared to the non-pregnant state. Progesterone’s stimulant effect is also responsible for a 40–50% increase in minute ventilation, beginning in the first trimester, and reaching 20–50% above pre-pregnancy values at term (Table 1). The increased minute ventilation occurring during pregnancy is primarily due to an increase in tidal volume (by 30–35%) and secondarily due to a negligible increase in respiratory rate (by 10–15%) above nonpregnant levels – minute ventilation is respiratory rate times the tidal volume [57]. The peak expiratory flow rate (PEFR) and forced expiratory volume in 1 s (FEV1) are largely unaffected during pregnancy, while vital capacity and total lung capacity undergo minimal decrease (Table 1). There is a 15–25% decrease in functional residual capacity due to a decrease in residual volume and expiratory reserve volume during the third trimester of pregnancy [57, 58]. There is also an increase in oxygen consumption (by 20–30%) due to resting maternal metabolic rate and fetal metabolic demands [58]. The effect of pregnancy on other physiologic parameters in the respiratory system is as shown in Table 1.
Table 1:
Respiratory changes during normal pregnancy show the respiratory changes during normal pregnancy.
| Variable | Normal values | |
|---|---|---|
| Pregnant (updated) | Non-pregnant women | |
| Lung volumes and ventilation | ||
| Respiratory rate, mL/min | No change/minimal increase | 14–20 |
| Tidal volume, mL | Increased (30–35% at term) | 500–600 |
| Minute ventilation, mL | Increased (40–50% at term) | 5,000–8,000 |
| Inspiratory capacity, mL | Increased (by 10–15% at term) | 1,900–2,400 |
| Vital capacity, mL | Unchanged/minimal decrease | 2,800–3,200 |
| Inspiratory reserve volume, mL | Increased (by 5–8% at term) | 1,800–2,200 |
| Expiratory reserve volume, mL | Decreased (10–30% at term) | 700–900 |
| Residual volume, mL | Decreased (15–25% at term) | 1,000–1,200 |
| Functional residual capacity, mL | Decreased (15–25% at term) | 1,600–2,000 |
| Total lung capacity, mL | No change/minimal (4–6%) decrease | 4,000–4,400 |
| Spirometry | ||
| Forced expiratory volume in 1 s (FEV1) | No change | 2,500–3,250 |
| Forced vital capacity (FVC) | No change | 3,250–3,750 |
| (FEV1)/FVC | No change | >70% |
| Blood gases | ||
| pH | Slight respiratory alkalosis (7.4–7.47) | 7.35–7.45 |
| Bicarbonate, mmol/L | Decreased by 10–15% (18–22) | 22–26 |
| Arterial pO2, mmHg | Increased by 10–12% (97–98) [12] | 90–100 |
| Arterial pCO2, mmHg | Decreased by 15–30% (28–32) | 35–40 |
| Base excess | No change | +2 to −2 |
Recent literature has demonstrated that although the respiratory rate does not change significantly during pregnancy compared to the nonpregnant state (an average of 15 beats/min), the pO2 decreases from a median of 98% at 12 weeks of gestation, to reach a minimum of 97% at 40 weeks of gestation. This small decrease in pO2 differ from previously published reports that suggest moderate reduction in pO2 in the second and third trimesters of pregnancy
The stimulatory effect of progesterone also results in maternal hyperventilation and physiological changes in acid-base balance, including increased arterial partial pressure of oxygen (pO2 of 95–105 mmHg), decrease arterial carbon dioxide partial pressure (pCO2 of 28–32 mmHg), a compensatory decrease in serum bicarbonate (18–22 mmol/L) due to increased renal excretion, and a mild compensatory respiratory alkalosis that occur during pregnancy [59, 60], maintaining the maternal arterial pH in the range of 7.40–7.47 (Table 1). Up until recently, it was thought that the partial pressure of oxygen can approach 106 mmHg in the first trimester (12 weeks), and then decreases to approximately 100 mmHg at term [59]. However, recent literature have demonstrated that although the respiratory rate does not change significantly during pregnancy compared to the non-pregnant state (an average of 15 beats/min), the pO2 decreases from a median of 98% at 12 weeks of gestation, to reach a minimum of 97% at 40 weeks of gestation [12]. This small decrease in pO2 differs from previously published reports that suggest moderate reduction in pO2 in the second and third trimesters of pregnancy [59].
The pharmacologic consequence of these pulmonary changes applies mainly to the PK of drugs used to treat respiratory diseases affecting pregnancy, and the use of inhalational pharmacologic agents in pregnant women. For example, respiratory alkalosis is associated with decreased PaCO2 (hypocapnia) and an increased bicarbonate to PaCO2 ratio, with increase in pH. This increase in pH affect the absorption of inhalational agents, as lung uptake of basic drugs like halothane, enflurane and isoflurane increases with increase in pH due to more of the drug rapidly absorbed in the non-ionized lipophilic form [61]. The more rapidly these basic drugs are being absorbed and equilibrate in blood in the setting of respiratory alkalosis, the more quickly the drugs passes into the brain to produce anesthetic effects, and the lower the dose needed to produce anesthetic effects in pregnant women compared to non-pregnant women. In a study by Chan et al. the minimum alveolar concentration (MAC) of isoflurane in pregnant women (at 8–12 weeks gestation) was 28% less than the MAC measured in non-pregnant controls [62]. In another study in pregnant women during the first trimester (at 8–13 weeks gestation), the median percentage decreases in MAC for halothane and enflurane were 27 and 30%, respectively, compared with those in nonpregnant women [56]. The disposition of asthmatic drugs is affected by physiologic changes of pregnancy as well. For example, in a study of the effect of pregnancy on quantitative medication use relating to asthma exacerbations during pregnancy, a third of asthmatics had optimal control of their asthma despite discontinuing anti-asthmatics during pregnancy when compared to non-pregnant asthmatics [63]. Asthma medications were rapidly reduced at the beginning of pregnancy, and then slowly increased to pre-pregnant levels in the postpartum period [63]. In another study, there was a 23% decrease in the use of inhalational drugs used in treatment of acute asthmatic attacks, and a 13% decrease in the use of short-acting beta-2 agonists for asthma treatment during pregnancy [64]. The decreased medication uses and dosing in asthmatics, and decreased requirements for general inhalational anesthetics are because of the respiratory physiologic changes in pregnancy as discussed above. In addition, the increase in placental-mediated steroid hormone production during pregnancy (free and conjugated cortisol) are associated with antiinflammatory effects, bronchial smooth muscle relaxation, and enhanced secretion of prostaglandin E2 (PGE2), leading to improvement in the symptoms of asthma (dyspnea, wheezing), and reduction in asthmatic flares during pregnancy. The impact of respiratory changes during pregnancy has yet to be included in many pregnancy PBPK models in current use.
Hematopoietic system physiologic changes during pregnancy
Pregnant women undergo many changes in the hematologic system that impact the PK of drugs. Recent literature demonstrate that platelet count consistently decrease throughout gestation in uncomplicated pregnancies [13], contrary to previously published reports that this decrease begins in the mid-second to third trimester and continues until time of delivery [14], [15], [16] (Table 2). This recent evidence also suggests that the largest physiological decrease in platelet count occur at term, with return to pre-pregnancy levels occurring at mean of seven weeks postpartum [13]. The normal range of platelet count during pregnancy is 150,000 to 450,000/µL. Therefore, a decrease in platelet count below 150,000/µL is termed thrombocytopenia, with counts below 150,000/µL but not less than 100,000/µL classified as mild thrombocytopenia, and levels <100,000/µL and <50,000/µL classified as moderate and severe thrombocytopenia respectively [65]. Gestational thrombocytopenia, a diagnosis of exclusion with platelet counts below 150,000/µL, occurs as a result of hemodilution from increased plasma volume, which peaks in the third trimester of pregnancy (between 28 and 32 weeks of gestation) [65].
Table 2:
Hematologic changes during normal pregnancy.
| Coagulation factor | Change during pregnancy |
|---|---|
| Factor 1 (fibrinogen) | Increased (>50–200% above pre-pregnancy levels) [109] |
| Factor II (prothrombin) | Increased [109] |
| Factor III (tissue thromboplastin) | Increased [110, 111] |
| Factor IV (calcium ions) | No change [112, 113] |
| Factor V (labile factor) | No change |
| Factor VII (stable factor) | Increased (upto 1,000% above pre-pregnancy levels) [109] |
| Factor VIII (antihemophilic factor) | Increased (>100% above pre-pregnancy levels) |
| Factor IX (christmas factor) | Increased (>100% above pre-pregnancy levels) [109] |
| Factor X (Stuart–Prower factor) | Increased (>100% above pre-pregnancy levels) |
| Factor XI (plasma thromboplastin antecedent) | Variable |
| Factor XII (hageman factor) | Increased (>100% above pre-pregnancy levels) [109] |
| Factor XIII (fibrin stabilizing factor) | Decreased (upto 50% of non-pregnant levels at term) [114] |
| Von Willebrands factor, vWF | Increased (>100% above pre-pregnancy levels) |
| Anti-thrombin III | No change/slightly decreased (10–20%) [115] |
| Activated partial thromboplastin time, APTT | No change/slightly decreased |
| D-dimer | Increased (upto 400%) [116] |
| Plasminogen activator inhibitor 1 | Increased |
| Plasminogen activator inhibitor 2 | Increased |
| Protein C | No change [117] |
| Protein S | Decreased (upto 50%) [109, 117] |
| Prothrombin time, PT | No change |
| Platelets | Decreased by 10–20% [13] |
During pregnancy, the plasma volume increases by approximately 40–50% above baseline, while the red blood cell volume increases concomitantly by approximately 20–30% [66]. Hemodilution that occurs from an increase in plasma volume relative to increase in red blood cell volume that occur during the second trimester and third trimesters of pregnancy (with a peak at about 32 weeks of gestation) account for the so called ‘physiologic anemia of pregnancy’ [67]. Pregnancy is a ‘hypercoagulable state’ because most of the blood clotting factors in the intrinsic and extrinsic pathways of the clotting cascade increase during pregnancy, with the potential for increased risk of thrombosis in pregnant patients predisposed to coagulation, either due to a genetic predisposition (for example, prothrombin gene G20210A homozygous mutation) or as a result of other factors (Table 2). The changes in the coagulant system are usually balanced by the anticoagulant system (protein C, anti-thombin III, and protein S) and by the fibrinolytic system changes during pregnancy.
The pharmacologic consequence of hematologic changes during pregnancy applies mainly to antiplatelet and anticoagulation therapy. Administration of low molecular weight heparin (LMWH) or unfractionated heparin (UFH) at the time of labor or cesarean delivery is usually dependent on platelet count, with moderate to severe thrombocytopenia being a relative contraindication for use of heparin or other anticoagulants. Another pharmacologic consideration is drugs that induce thrombocytopenia during pregnancy. A common example is heparin-induced thrombocytopenia (HIT), a severe adverse effect that can occur with the use of heparin. In the setting of HIT, direct thrombin inhibitors (bivalirudin and danaparoid), or factor Xa inhibitors (fondaparinux) are used for thombo-prophylaxis or treatment, as continued use of heparin can worsen thrombocytopenia and predispose to thrombosis.
Renal system changes and renal transporter expression during pregnancy
During pregnancy, both kidney bipolar diameters increase in size by an average of 1.5 cm, and the right ureter dilates in approximately 80% of women during the duration of pregnancy [68], with concomitant increases in the size of the renal calyces and ureters (Table 3). These renal changes begin to occur as early as the first trimester of pregnancy, between 6 and 10 weeks of gestation. The renal blood flow rises to about 70–80% from its baseline value at 20–22 weeks of gestation, peaks around 32–24 weeks of gestation, and then falls to about 60–70% above pre-pregnancy levels towards the end of pregnancy, while the glomerular filtration rate (GFR) rises in parallel to about 40–50% of its baseline values at 20–22 weeks, then continues to increase through most of the third trimester, up to 36–38 weeks of gestation, when it declines steadily until the time of delivery [69, 70] (Figure 3). A 2019 systematic review of serum creatinine concentrations during pregnancy from 49 studies that measured over 4,000 serum creatinine concentrations in healthy pregnant women demonstrated that the mean values for serum creatinine in the first, second, and third trimesters were 16, 23, and 20% lower when compared to baseline values in non-pregnant adults [71] (Figure 3). The increased renal blood flow and increased glomerular hyper-filtration are responsible for the increased creatinine clearance, decreased blood urea nitrogen levels, and increased protein excretion (up to 300 mg/day) during pregnancy [9] (Table 3). These changes return to pre-pregnancy levels about 2–10 weeks postpartum. The renal changes associated with normal pregnancy (Table 3) are largely responsible for the decreased serum concentrations of several drugs during the second and third trimesters of pregnancy [72], [73], [74], [75], [76], [77], [78], with many drugs requiring dose adjustments during pregnancy to prevent subtherapeutic levels [50, 72, 73].
Table 3:
Renal changes during normal pregnancy.
| Variable | Direction and magnitude of change in pregnancy |
|---|---|
| Renal, calyceal, and ureter size | Increases in bipolar diameter (1.0–2.5 cm); kidney volume increases by 25–30% |
| pH | Increases by about 0.02–0.06 |
| Renal blood flow | Increases by 60–80% |
| Glomerular filtration rate | Increases by 40–50% |
| Renal osmolality | Decreases by approximately 10 mOsm/kg of water |
| Urine protein excretion | Increases (up to 300 mg/day) |
| Serum creatinine | Decreases by 16, 23 and 20% in first, second & third trimester (normal pregnancy range: 0.4–0.6 mg/dL) |
| Blood urea nitrogen levels | Decreases to 8–10 mg/dL |
| Serum bicarbonate | Decreases (by 4–5 mEq/L) due to compensatory respiratory alkalosis |
| Renal albumin excretion | Increases |
| Renal AAG-1 excretion | Increases |
|
Unchanged to mild decreases Decreases to approximately 130–135 mmol/L Decreases by (18–22 mmol/L is normal range in pregnancy) Unchanged Unchanged Unchanged |
| Uric acid (serum) | Decreases by approximately 130–135 mEq/L |
| Aldosterone | Increases |
| Renin–angiotensin | Increases |
|
Slight increase in BRCP [20, 118] Unchanged [81] Decreased to unchanged expression of MRP2 in pregnant rats [81, 119] Increased MRP3 (31–225%) [81, 118] Decreased MRP4 [81, 89] Decreased MRP5 [81] Unchanged [81] Decreased expression (by 20–40%) of MATE1 [118] Increased expression of MATE2-K Unchanged to slight decrease MDR1 expression [20, 118] Decreased expression of OAT1 [118] Increased OAT2 expression [118] Decreased expression of OAT3 [118] Decreased OAT5 [118] Decreased expression [118] Increased expression [118] Decreased OAT3A1 [118] Increased during pregnancy Increased OCT1 expression [118] Increased OCT2 expression [118] Decreased OCT3 expression [118] Decreased OCTN1 [118] Decreased OCTN2 [118] No change in renal P-gp expression during pregnancy [120] Increased PEPT1 expression [118] Decreased PEPT2 expression [118] Decreased expression of URAT1 [121] |
BRCP, breast cancer resistance protein; MATE, multidrug and toxin extrusion transporter; MDR, multidrug resistance protein; MRP, multidrug resistance-associated protein; OAT, organic anionic transporter; OATP, organic anionic transporting protein; OCT, organic cationic transporter; OCTN, organic cation/carnitine transporter; PEPT, peptide transporter; Pgp, P glycoprotein; URAT, urate transporter.
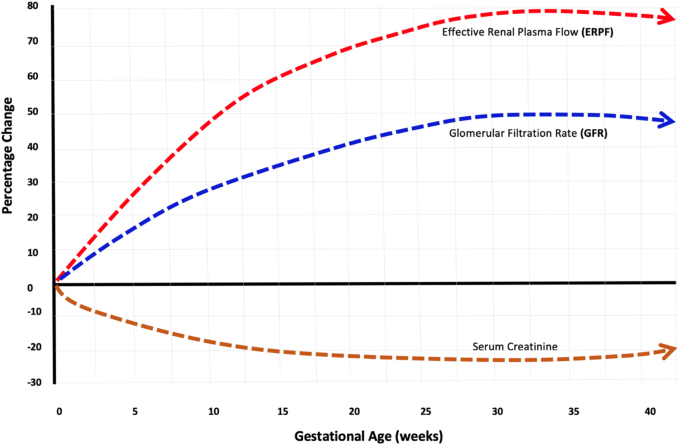
Figure 3:
Effective renal plasma flow, glomerular filtration rate, and creatinine changes during pregnancy.
Figure illustrates the changes in effective renal plasma flow, glomerular filtration rate, and serum creatinine concentrations during normal pregnancy the renal blood flow rises to about 70–80% from its baseline value at 20–22 weeks of gestation, and then falls to about 60–70% above pre-pregnancy levels towards the end of pregnancy, while the glomerular filtration rate (GFR) rises in parallel to about 40–50% of its baseline values at 20–22 weeks, then continues to increase through most of the third trimester, up to 36–38 weeks of gestation, when it declines steadily until the time of delivery.
A 2019 systematic review of serum creatinine concentrations during pregnancy from 49 studies that measured over 4,000 serum creatinine concentrations in healthy pregnant women demonstrated that the mean values for serum creatinine in the first, second, and third trimesters were 16, 23, and 20% lower when compared to baseline values in nonpregnant adults (as shown in figure above).
In addition to increased renal blood flow and increased renal clearance, changes in transmembrane receptor function, expression, and regulation also alter the PK of drugs during pregnancy. Transmembrane transporter function in the kidneys is the most studied transporter changes during pregnancy. Renal elimination of drugs occurs through a combination of glomerular filtration, tubular secretion, and efflux into urine [79]. Although glomerular filtration and tubular secretion increases during pregnancy, tubular reabsorption appears unchanged [69]. Literature is increasing on how pregnancy alters the function of renal transporters in secretion and/or reabsorption of drugs [80]. Generally, the changes in these renal transporter functions are modest. For example, a 2012 study showed that pregnancy had minimal effect on streptozocin-mediated changes in renal function [81]. To date, more than 20 renal transporters have been studied and described in pregnant humans, mice, and rats (Table 3). Some of these renal transporters are predominant on the renal basolateral membranes (separating the renal tubular cells and maternal blood), while others are located on the apical luminal membranes of the kidneys. Pregnancy has been shown to decrease the renal expression of multi-drug resistance mutation 1 (MDR1), multi-drug resistance protein 4 and 5 (MRP4 and MRP5), and increase the mRNA and protein expression of MRP3 by 50–60% [81] (Table 3). These renal transmembrane transporter changes are critical to explain some of the PK changes that occur during pregnancy. For example, breast cancer receptor protein (BCRP) is the primary uptake transporter for glyburide during pregnancy, and BCRP activity increases during pregnancy (Table 3). Therefore, it is plausible that due to the increase in BCRP expression in the renal tubules during pregnancy, systemic intrinsic renal clearance of glyburide increases [82]. The increased expression of MATE-1, OCT-2, and MATE-2-K might contribute to increased creatinine clearance during pregnancy, as creatinine is a substrate of these transporters [83]. Incorporating the effect of these drug efflux and influx transporters in maternal and fetal PBPK models would improve the predictive abilities of these models in estimating the PK of drugs, and the effect of drugs on medical diseases during pregnancy.
Gastrointestinal changes and intestinal transporter expression during pregnancy
Pregnancy is characterized by several changes in the gastrointestinal system that affects the PK of drugs. For example, pregnancy results in prolongation of gastric emptying, increase in gastric residence times, an increase in gastric pH, and increased small bowel transit times [84]. These pregnancy-related changes are mainly driven by progesterone-mediated inhibition of smooth muscle motility [85]. Progesterone levels increase exponentially during pregnancy. The corpus luteum of the ovaries initially produces progesterone, but at about 8–12 weeks of gestation, the placenta completely takes over the progesterone production to maintain pregnancy. These progesterone-induced pregnancy associated smooth muscle relaxant effects may lead to reflux, nausea and vomiting, constipation, and exaggerated heartburn due to increased gastric acidity and excessive production of gastrin during pregnancy [85]. These gastrointestinal changes also affect the PK of many orally administered drugs by delaying their absorption and onset of action. For drugs that are rapidly absorbed, the gastrointestinal changes would decrease bioavailability, while the opposite effect is seen in slowly absorbed drugs during pregnancy. Due to the exaggerated gastric reflux during pregnancy, the use of proton pump inhibitors and H2-receptor blockers are used widely in pregnancy to prevent and treat acid reflux, as well as moderate to severe gastroesophageal reflux in about 50% of pregnant women [86]. In addition, increased pH of the stomach during pregnancy will alter absorption of pH dependent drugs, with the potential for lower efficacy during pregnancy.
A fundamental aspect of drug PK during pregnancy is the role of intestinal membrane transporters in drug absorption. Majority of transporters that impact the bioavailability of drugs are localized in the small intestine, and the evidence from pregnant humans, mice and rats suggest that the activity of some of these transporters change during pregnancy (Table 4). The most extensively studied intestinal transporters belong to the ATP binding cassette (ABC) family. These include ABCB1, also known as P-glycoprotein (P-gp), ABCG2, also known as breast cancer resistance protein, and ABCC2, called multidrug resistance-associated protein 2 (MRP2) [87]. Some of the other gastrointestinal transmembrane transporters, and changes that occur during pregnancy, are shown in Table 4. Gastrointestinal changes in these intestinal transmembrane transporters may have significant effect on drug disposition within the gastrointestinal tract, as inhibition of these transporters can lead to decreased or increased efflux of drugs from the gastrointestinal tract, and alter drug absorption [88]. MRP4 is a primary uptake transporter for bile acids during pregnancy, and MRP4 activity decreases during pregnancy. Therefore, it is plausible that due to the decrease in MRP4 expression in the liver/bile ducts during pregnancy, systemic clearance of bile acids during pregnancy are likely decreased, especially in pregnancies complicated by intrahepatic cholestasis of pregnancy [89]. Intestinal membrane transporters are also particularly valuable in PBPK modeling, as most current PBPK models lack information on transmembrane transporter expression, regulation, and function [90, 91]. As research in this area is still growing in humans, most data from membrane transporter expression, regulation and function are derived from mice and rats. The international Transporter Consortium (ITC), formed in 2007, meet on a yearly basis to discuss the role of transporters in drug safety and efficacy [92].
Table 4:
Gastrointestinal system, hepatic enzymes and transporter changes during normal pregnancy.
| Gastrointestinal changes | Direction and magnitude of change in pregnancy |
|---|---|
|
Decreased [122] Increased BRCP expression in pregnant mice (30–55%) [123] GLUT2 is increased during pregnancy Increased expression of P-gp in pregnant mice [120] Decreased MRP1 expression in pregnant mice (30–40%) [123] Decreased MRP2 expression in pregnant mice (30–40%) [123] Decreased expression of MRP3 in pregnant mice No change in OSTα expression [123] No change/minimal increase in OSTβ expression [123] No change in expression of intestinal CYP3A4 [120, 124] No change in expression of intestinal CYP3A5 [120, 124] |
| Hepatic changes | Direction and magnitude of change in pregnancy |
|
| |
|
Decrease in CYP1A2 during pregnancy [94] Increases Increases Decrease in CYP2C19 during pregnancy Increases in CYP2D6 during pregnancy [94] Increases Increases [94] Increases [94] Increases [94] Increases [94] Increases [125] Expression of cathepsin A increase during pregnancy [17] Expression of CES1 is unchanged during pregnancy [18] >50% decrease in expression of ABCG5 in hepatocytes of pregnant mice [88] >50% decrease in expression of ABCG8 in hepatocytes of pregnant mice [88] ATP8B1 decrease in pregnant mice [88] and women with cholestasis [126] Decreased expression in hepatocytes of pregnant mice [88] Decreases expression of BSEP in hepatocytes of pregnant mice [88] >50% decreased expression of ENT1 in pregnant mice [88] Decreased expression of ENT2 in pregnancy Decreased expression in pregnant mice [88] Bile acid transporter (MDR3) Unchanged in hepatocytes of pregnant rats [127] Decreased expression of MRP2 in biliary system of pregnant rats [119]. 50% decreased expression of MRP3 in hepatocytes of pregnant rats [127] 63% increased expression of MRP4 in hepatocytes of pregnant rats [88] 60% decreased expression of MRP6 in hepatocytes of pregnant rats [127] 70% increased expression of OAT2 in hepatocytes of pregnant mice [88] OAT7 is only found in the liver (hepatic specific). Relatively unchanged of OATP1B1 in hepatocytes of pregnant rats and humans [19, 127] 50% decreased expression of OATP1B2 in hepatocytes of pregnant rats [127] Decreased expression of OAT1B3 in pregnant women with cholestasis [19] Decreased expression of OATP2B1 in hepatocytes of pregnant mice [88] >50% decreased expression of OCT1 in hepatocytes of mice [88] Decreased expression of OST (alpha and beta) during pregnancy Unchanged MRD1 expression in the liver [120] |
ABC, ATP binding cassette; ABCG, ATP-binding cassette super-family G; BCRP, Breast cancer receptor protein; CYP, cytochrome P450; ENT, equilibrative nucleoside transporters; GLUT, glucose transporter; MATE, Multiantimicrobial extrusion protein; MDR, multi-drug resistance mutation, MRP, multidrug resistance protein; OAT, organic anionic transporter; OCT, organic cationic transporter; OST, Organic solute and steroid transporter; UGT, uridine 5′-diphospho-glucuronosyltransferase.
Hepatic changes and hepatic transporter expression during pregnancy
The activity of most phase I cytochrome P450 (except CYP1A2 and CYP2C19) and phase 2 drug metabolic enzymes are increased during pregnancy [1] (Table 4). The changes in activity of these metabolic enzymes account for altered drug metabolism and clearance during pregnancy. For example, the activity of hepatic cytochrome P450 3A (CYP3A), the most abundant CYP in humans [93], increase by about 2 to 3 fold during pregnancy [94]. The resultant effect of increased activity of these enzymes is an increase in metabolic clearance, requiring increased doses of medications during pregnancy. The hepatic elimination of many anti-seizure medications, opioids, antibiotics, antimicrobials, antiviral agents, cancer chemotherapeutics, antiprotozoal, and immune-therapeutic drugs, are increased during pregnancy [95, 96]. Contrarily, decreased activity of CYP1A2 and CYP2C19 during pregnancy results in decreased metabolism and elimination of drugs that are substrates for CYP1A2 and CYP2C19, requiring dose reductions to avoid toxicity during pregnancy. For example, the elimination half-life of caffeine, a substrate of CYP1A2, was shown to increase during pregnancy [97]. The doses of drugs metabolized by CYP1A2 and 2C19 (for example, theophylline, caffeine, clozapine, olanzapine, ondansetron, and cyclobenzaprine), should ideally be reduced during pregnancy to prevent potential toxicity from decreased activity of by CYP1A2 and 2C19. Other hepatic enzyme changes that occur during pregnancy include minimal changes in serum levels of aspartate aminotransferase, alanine aminotransferase, bilirubin, and γ-glutamyl transferase, and a 4-fold increase in the concentration of alkaline phosphatase (mainly due to increased placental production), reaching a peak during the third trimester [98].
Hepatic changes in transporter function (uptake and efflux) may have significant effect on drug PK during pregnancy [88]. The impact of these during pregnancy can be significant, as inhibition of efflux transporters can lead to accumulation of metabolites within the liver. The three transporters most commonly expressed within hepatocytes are organic anion transporters (OAT) – OATP1B1, OATP1B2, and OATP1B3 [99]. During the third trimester, pregnant mice have been shown to exhibit decreased expression of several uptake OATP transporters, as well as downregulation of several efflux transporters (MRP2, MRP3, and MRP6) [88]. These transporters mediate bidirectional movement of substrates (efflux and reabsorption) through the liver, and can affect the PK of drugs during pregnancy. For example, OATP1B1 and OATP1B3 are responsible for hepatic uptake of TAF [100]. Decreased expression of OATP1B3 during pregnancy [19] potentially causes reduced TAF uptake into the liver in during pregnancy. Some of the other hepatic transmembrane transporters, and changes that occur during pregnancy, are shown in Table 4.
Pregnancy affects the organ extraction ratio of drugs, defined as the fractional unit of a drug cleared from plasma as it traverses a particular organ of drug elimination (e.g., liver or kidney) [101]. The hepatic extraction ratio is the amount of drug uptake from the liver cells (hepatocytes) per unit time, usually expressed as one pass (perfusate) through the liver. Based on how much drug is cleared from the plasma, the hepatic extraction ratio of drugs can be classified as high (>0.7), medium (0.3–0.7), or low (<0.30) [101]. The effect of pregnancy on a drug’s hepatic extraction ratio is directly dependent on three critical factors: hepatic blood flow (perfusion rate), protein binding (fraction of unbound drug in plasma), and the action of hepatic metabolic enzymes (hepatic intrinsic clearance of unbound drug). Drugs with high extraction ratio (e.g., morphine, lidocaine, verapamil, metoprolol, and propranolol) have decreased oral bioavailability and increased hepatic clearance during pregnancy, because drugs with high extraction ratios are dependent on hepatic blood flow (perfusion-rate dependent), which increases during pregnancy [102]. Contrarily, drugs with low extraction ratio (e.g., digoxin, cyclosporine, methadone, phenytoin, and warfarin) are dependent on protein binding and intrinsic action of hepatic metabolic enzymes, which are altered during pregnancy. A rise in fraction of unbound for drugs with low extraction ratio increases hepatic metabolic clearance, whereas for drugs with high extraction ratio, this does not affect metabolic clearance. The extraction ratio of drugs therefore depends on the properties of the drug and the effect of physiological changes during pregnancy.
Effect of the physiologic changes during pregnancy on phase I and phase II metabolic enzymes, and pharmacogenomic polymorphisms
Understanding biotransformation (complex processes occurring in hepatocytes responsible for facilitating drug metabolism) and the effect of pregnancy on biotransformation (through phase I and phase II drug-metabolizing enzymes) are critical to personalized medicine and pharmacotherapeutic decision-making in pregnant women. Pregnancy affects several phase I (oxidation via cytochrome P450, reduction, hydrolysis) and phase II (glucuronidation, acetylation, and sulfation) reactions (Table 4). CYP2D6, the most widely and extensively studied highly polymorphic enzyme (>100 types), accounting for metabolism of approximately 20–25% of currently available drugs [103], is a cytochrome P450 enzyme whose activity is increased during pregnancy. The pharmacokinetic consequence of increased CYP2D6 activity is an increase in metabolism of drugs that are substrates of CYP2D6, including but not limited to codeine, dihydrocodeine, tramadol, dextromethorphan, clomipramine, nortriptyline, and selective serotonin receptor inhibitors (fluoxetine, fluvoxamine, and paroxetine) [103]. Due to the high polymorphism exhibited by the CYP2D6 gene, the activity of this enzyme varies greatly among pregnant women. Phenotypically, women are classified on the basis of their enzyme activity as poor metabolizers (PMs), expressing very little of the enzyme due to the presence of two nonfunctional alleles or entire deletion of genes; extensive metabolizers (EM), expressing a normally functioning enzyme due to one or two normal functioning alleles; intermediate metabolizers (IM) due to the expression of one nonfunctional allele and another allele with reduced function; and ultra-rapid metabolizers (UMs), expressing one or multiple extra-functional genes [103, 104]. These genetic differences in CYP2D6 activity in pregnant women have the potential to increase or decrease serum concentration of drugs during pregnancy, thereby determining the effectiveness of therapy and potential for serious adverse effects. For example, the PK changes of codeine in a pregnant woman would depend on if the woman is a PM, IM, EM, or UM of codeine. Codeine is metabolized by O-demethylation to the active drug, morphine by CYP2D6 [105]. A PM will experience reduced analgesia due to low serum morphine concentrations from decreased metabolism of codeine (and less adverse events), while an UM will experience quick and effective pain relief due to high morphine concentrations in plasma from rapid metabolism of codeine to morphine, but potentially more adverse effects compared to a PM. CYP2D6 activity of EM and UM increases further during pregnancy [106], and can have major consequences for pregnant women taking drugs that are CYP2D6 substrates.
Other enzymes that are encoded by highly polymorphic genes that can potentially be affected by pregnancy physiology include CYP2C19 [107], (the second most extensively studied gene in pharmacogenomics) responsible for the hepatic metabolism of clopidogrel, tricyclic antidepressants, omeprazole, propranolol, and diazepam; vitamin K epoxide reductase complex subunit 1 (VCORC1) responsible for the pharmacogenomic polymorphic changes and risk of hemorrhage in women using warfarin (especially in PMs); and thiopurine methyltransferase (TPMT), involved in the inactivation of chemotherapeutic drugs like azathioprine and 6-mercaptopurine. Reduced TPMT CYP2D6 activity in PMs increases further during pregnancy, leading to excessive levels of 6-mercaptopurine serum concentrations, with the potential to result in altered efficacy, severe bone marrow suppression and myelo-toxicity [108].
Conclusions
In conclusion, accurate knowledge of physiological changes that occur during pregnancy is critical to understanding the manner in which pregnancy alters the PK of drugs, because it aids in making correct diagnosis of diseases, and help in understanding the mechanisms in which pregnancy alters the PK of drugs. Additionally, a detailed knowledge of physiological changes during pregnancy allow for proper modeling and validation of PBPK models, which are increasingly being used during pregnancy to guide drug dosing. While prior knowledge of some of the physiologic changes during pregnancy were based on small retrospective studies, this paper presented an update of the currently available evidence based on the use of gestational-age specific, evidence based large datasets to derive these physiologic changes, with emphasis on the five major organ-systems with the greatest potential to alter the PK of drugs during pregnancy: cardiovascular, respiratory, hematologic, renal, and gastrointestinal systems. Although most of the transporter information discussed in this manuscript was data from animal studies, a PBPK model in humans can be developed from these data, starting with all available data and what is understood at the organ or tissue level, to build a physiological model that fits the data, pending future understanding of the various transporters in humans. Future research should focus on encouraging studies in membrane transporter expression, regulation, and function in women.
Footnotes
Research funding: The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH) under the Award Number 1K23HD104517 provided overall support for this work. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Author contributions: AE wrote the entire paper and reviewed it. The findings and conclusions of this manuscript are those of the author and do not necessarily represent the official position of any organization. The author does not have any conflicts of interest. The author has accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: No conflict of interest.
Informed consent: Not applicable, as this is a review.
Ethical approval: Not applicable, as this is a review.
References
- 1.Sheffield JS, Siegel D, Mirochnick M, Heine RP, Nguyen C, Bergman KL, et al. Designing drug trials: considerations for pregnant women. Clin Infect Dis. 2014;59:S437–44. doi: 10.1093/cid/ciu709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG. Placental drug transporters and their role in fetal protection. Placenta. 2012;33:137–42. doi: 10.1016/j.placenta.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Kumar P, Magon N. Hormones in pregnancy. Niger Med J. 2012;53:179–83. doi: 10.4103/0300-1652.107549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald-Wallis C, Silverwood RJ, Fraser A, Nelson SM, Tilling K, Lawlor DA, et al. Gestational-age-specific reference ranges for blood pressure in pregnancy: findings from a prospective cohort. J Hypertens. 2015;33:96–105. doi: 10.1097/hjh.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikuro M, Obara T, Metoki H, Ohkubo T, Yamamoto M, Akutsu K, et al. Blood pressure measured in the clinic and at home during pregnancy among nulliparous and multiparous women: the BOSHI study. Am J Hypertens. 2013;26:141–8. doi: 10.1093/ajh/hps002. [DOI] [PubMed] [Google Scholar]
- 6.van Oppen AC, van der Tweel I, Alsbach GP, Heethaar RM, Bruinse HW. A longitudinal study of maternal hemodynamics during normal pregnancy. Obstet Gynecol. 1996;88:40–6. doi: 10.1016/0029-7844(96)00069-5. [DOI] [PubMed] [Google Scholar]
- 7.Capeless EL, Clapp JF. Cardiovascular changes in early phase of pregnancy. Am J Obstet Gynecol. 1989;161:1449–53. doi: 10.1016/0002-9378(89)90902-2. [DOI] [PubMed] [Google Scholar]
- 8.Sturgiss SN, Dunlop W, Davison JM. Renal haemodynamics and tubular function in human pregnancy. Bailliere Clin Obstet Gynaecol. 1994;8:209–34. doi: 10.1016/s0950-3552(05)80319-0. [DOI] [PubMed] [Google Scholar]
- 9.Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18:152–61. doi: 10.1038/ki.1980.124. [DOI] [PubMed] [Google Scholar]
- 10.Dunlop W. Serial changes in renal haemodynamics during normal human pregnancy. Br J Obstet Gynaecol. 1981;88:1–9. doi: 10.1111/j.1471-0528.1981.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 11.LoMauro A, Aliverti A. Respiratory physiology of pregnancy: physiology masterclass. Breathe. 2015;11:297–301. doi: 10.1183/20734735.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green LJ, Mackillop LH, Salvi D, Pullon R, Loerop L, Tarassenko L, et al. Gestation-specific vital sign reference ranges in pregnancy. Obstet Gynecol. 2020;135:653–64. doi: 10.1097/aog.0000000000003721. [DOI] [PubMed] [Google Scholar]
- 13.Reese JA, Peck JD, Deschamps DR, McIntosh J, Knudtson EJ, Terrell DR, et al. Platelet counts during pregnancy. N Engl J Med. 2018;379:32–43. doi: 10.1056/nejmoa1802897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reese JA, Peck JD, McIntosh JJ, Vesely SK, George JN. Platelet counts in women with normal pregnancies: a systematic review. Am J Hematol. 2017;92:1224–32. doi: 10.1002/ajh.24829. [DOI] [PubMed] [Google Scholar]
- 15.Cairns JW, Mahon A, Waters DA, Chanarin I. Platelet levels in pregnancy. J Clin Pathol. 1977;30:392. doi: 10.1136/jcp.30.4.392-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obisesan KA, Adeyemo AA, Okunade MA. Haematological values in pregnancy in Ibadan, Nigeria. Afr J Med Med Sci. 1998;27:9–11. [PubMed] [Google Scholar]
- 17.Kurzatkowski W, Ostrowska H, Doroszko M. [Serum cathepsin A activity in pregnant, parturient and puerperal patients] Zentralbl Gynakol. 1990;112:227–9. [PubMed] [Google Scholar]
- 18.Beigi RH, Han K, Venkataramanan R, Hankins GD, Clark S, Hebert MF, et al. Pharmacokinetics of oseltamivir among pregnant and nonpregnant women. Am J Obstet Gynecol. 2011;204:S84–88. doi: 10.1016/j.ajog.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Yan Z, Dong M, Zhu X, Wang H, Wang Z. Alteration in placental expression of bile acids transporters OATP1A2, OATP1B1, OATP1B3 in intrahepatic cholestasis of pregnancy. Arch Gynecol Obstet. 2012;285:1535–40. doi: 10.1007/s00404-011-2183-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, et al. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab. 2006;291:E1295–304. doi: 10.1152/ajpendo.00193.2006. [DOI] [PubMed] [Google Scholar]
- 21.Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. doi: 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahendru AA, Everett TR, Wilkinson IB, Lees CC, McEniery CM. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. J Hypertens. 2014;32:849–56. doi: 10.1097/hjh.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 24.Scantlebury DC, Schwartz GL, Acquah LA, White WM, Moser M, Garovic VD. The treatment of hypertension during pregnancy: when should blood pressure medications be started? Curr Cardiol Rep. 2013;15:412. doi: 10.1007/s11886-013-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Northcote RJ, Knight PV, Ballantyne D. Systolic murmurs in pregnancy: value of echocardiographic assessment. Clin Cardiol. 1985;8:327–8. doi: 10.1002/clc.4960080604. [DOI] [PubMed] [Google Scholar]
- 26.Loerup L, Pullon RM, Birks J, Fleming S, Mackillop LH, Gerry S, et al. Trends of blood pressure and heart rate in normal pregnancies: a systematic review and meta-analysis. BMC Med. 2019;17:167. doi: 10.1186/s12916-019-1399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinkey RG, Oparil S. Lower blood pressure thresholds raise the bar in pregnancy. Circ Res. 2019;125:195–7. doi: 10.1161/circresaha.119.315384. [DOI] [PubMed] [Google Scholar]
- 28.Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of Intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins PC, Stokes SM, Fakoyeho S, Bell TM, Zarzaur BL. Clinical indicators of hemorrhagic shock in pregnancy. Trauma Surg Acute Care Open. 2017;2:e000112. doi: 10.1136/tsaco-2017-000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magee LA, von Dadelszen P, Singer J, Lee T, Rey E, Ross S, et al. Hypertension. Vol. 68. Dallas, Tex ; 2016. The CHIPS randomized controlled trial (control of hypertension in pregnancy study): is severe hypertension just an elevated blood pressure? pp. 1153–9. 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scaife PJ, Mohaupt MG. Salt, aldosterone and extrarenal Na(+) - sensitive responses in pregnancy. Placenta. 2017;56:53–8. doi: 10.1016/j.placenta.2017.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute of M, National Research Council Committee to Reexamine IOMPWG . The National Academies Collection: reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 33.Elkader A, Sproule B. Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet. 2005;44:661–80. doi: 10.2165/00003088-200544070-00001. [DOI] [PubMed] [Google Scholar]
- 34.Bastian JR, Chen H, Zhang H, Rothenberger S, Tarter R, English D, et al. Dose-adjusted plasma concentrations of sublingual buprenorphine are lower during than after pregnancy. Am J Obstet Gynecol. 2017;216:64.e61. doi: 10.1016/j.ajog.2016.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lebaudy C, Hulot JS, Amoura Z, Costedoat-Chalumeau N, Serreau R, Ankri A, et al. Changes in enoxaparin pharmacokinetics during pregnancy and implications for antithrombotic therapeutic strategy. Clin Pharmacol Ther. 2008;84:370–7. doi: 10.1038/clpt.2008.73. [DOI] [PubMed] [Google Scholar]
- 36.Kinnunen M, Piirainen P, Kokki H, Lammi P, Kokki M. Updated clinical pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacokinet. 2019;58:705–25. doi: 10.1007/s40262-018-00731-3. [DOI] [PubMed] [Google Scholar]
- 37.Ryu RJ, Eyal S, Easterling TR, Caritas SN, Venkataraman R, Hankins G, et al. Pharmacokinetics of metoprolol during pregnancy and lactation. J Clin Pharmacol. 2016;56:581–9. doi: 10.1002/jcph.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin-Suarez A, Sanchez-Hernandez JG, Medina-Barajas F, Pérez-Blanco SJ, Lanao JM, Garcia-Cuenllas Alvarez L, et al. Pharmacokinetics and dosing requirements of digoxin in pregnant women treated for fetal supraventricular tachycardia. Expet Rev Clin Pharmacol. 2017;10:911–7. doi: 10.1080/17512433.2017.1344096. [DOI] [PubMed] [Google Scholar]
- 39.Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GDV, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38:833–40. doi: 10.1124/dmd.109.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metsu D, Toutain PL, Chatelut E, Delobel P, Gandia P. Antiretroviral unbound concentration during pregnancy: piece of interest in the puzzle? J Antimicrob Chemother. 2017;72:2407–9. doi: 10.1093/jac/dkx176. [DOI] [PubMed] [Google Scholar]
- 41.Battino D, Binelli S, Bossi L, Canger R, Croci D, Cusi C, et al. Plasma concentrations of carbamazepine and carbamazepine 10,11-epoxide during pregnancy and after delivery. Clin Pharmacokinet. 1985;10:279–84. doi: 10.2165/00003088-198510030-00007. [DOI] [PubMed] [Google Scholar]
- 42.Yerby MS, Friel PN, Miller DQ. Carbamazepine protein binding and disposition in pregnancy. Ther Drug Monit. 1985;7:269–73. doi: 10.1097/00007691-198507030-00005. [DOI] [PubMed] [Google Scholar]
- 43.Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Epilepsy and pregnancy: a prospective study of seizure control in relation to free and total plasma concentrations of carbamazepine and phenytoin. Epilepsia. 1994;35:122–30. doi: 10.1111/j.1528-1157.1994.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 44.Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Disposition of carbamazepine and phenytoin in pregnancy. Epilepsia. 1994;35:131–5. doi: 10.1111/j.1528-1157.1994.tb02922.x. [DOI] [PubMed] [Google Scholar]
- 45.Wright JD, Boudinot FD, Ujhelyi MR. Measurement and analysis of unbound drug concentrations. Clin Pharmacokinet. 1996;30:445–62. doi: 10.2165/00003088-199630060-00003. [DOI] [PubMed] [Google Scholar]
- 46.Schalkwijk S, Greupink R, Burger D. Free dug concentrations in pregnancy: bound to measure unbound? Br J Clin Pharmacol. 2017;83:2595–8. doi: 10.1111/bcp.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musteata FM. Monitoring free drug concentrations: challenges. Bioanalysis. 2011;3:1753–68. doi: 10.4155/bio.11.187. [DOI] [PubMed] [Google Scholar]
- 48.Pope R, Kashuba A. Darunavir for use in pregnant women with HIV. Expet Rev Clin Pharmacol. 2017;10:1317–27. doi: 10.1080/17512433.2017.1390428. [DOI] [PubMed] [Google Scholar]
- 49.Lambert J, Jackson V, Else L, Lawless M, McDonald G, Le Blanc D, et al. Darunavir pharmacokinetics throughout pregnancy and postpartum. J Int AIDS Soc. 2014;17:19485. doi: 10.7448/ias.17.4.19485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eke AC, Stek AM, Wang J, Kreitchmann R, Shapiro DE, Smith E, et al. Darunavir pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2020;83:373–80. doi: 10.1097/QAI.0000000000002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stek A, Best B, Capparelli E. Pharmacokinetics of increased dose darunavir during late pregnancy and postpartum. Boston, Massachussetts, London: i-base Publishers; 2016. 23rd Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- 52.Stek A, Best BM, Wang J, Capparelli EV, Burchett SK, Kreitchmann R, et al. Pharmacokinetics of once versus twice daily darunavir in pregnant HIV-infected women. J Acquir Immune Defic Syndr. 2015;70:33–41. doi: 10.1097/QAI.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crauwels HM, Kakuda TN, Ryan B, Zorrilla C, Osiyemi OO, Yasin S, et al. Pharmacokinetics of once-daily darunavir/ritonavir in HIV-1-infected pregnant women. HIV Med. 2016;17:643–52. doi: 10.1111/hiv.12366. [DOI] [PubMed] [Google Scholar]
- 54.Taylor M. An experimental study of the influence of the endocrine system on the nasal respiratory mucosa. J Laryngol Otol. 1961;75:972–7. doi: 10.1017/s0022215100058746. [DOI] [PubMed] [Google Scholar]
- 55.Milne JA. The respiratory response to pregnancy. Postgrad Med. 1979;55:318–24. doi: 10.1136/pgmj.55.643.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan MT, Mainland P, Gin T. Minimum alveolar concentration of halothane and enflurane are decreased in early pregnancy. Anesthesiology. 1996;85:782–6. doi: 10.1097/00000542-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Cugell DW, Frank NR, Gaensler EA, Badger TL. Pulmonary function in pregnancy. I. Serial observations in normal women. Am Rev Tubercul. 1953;67:568–97. doi: 10.1164/art.1953.67.5.568. [DOI] [PubMed] [Google Scholar]
- 58.Gee JB, Packer BS, Millen JE, Robin ED. Pulmonary mechanics during pregnancy. J Clin Invest. 1967;46:945–52. doi: 10.1172/jci105600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Templeton A, Kelman GR. Maternal blood-gases, PAo2--Pao2), hysiological shunt and VD/VT in normal pregnancy. Br J Anaesth. 1976;48:1001–4. doi: 10.1093/bja/48.10.1001. [DOI] [PubMed] [Google Scholar]
- 60.Andersen GJ, James GB, Mathers NP, Smith EL, Walker J. The maternal oxygen tension and acid-base status during pregnancy. J Obstet Gynaecol Br Commonw. 1969;76:16–9. doi: 10.1111/j.1471-0528.1969.tb09444.x. [DOI] [PubMed] [Google Scholar]
- 61.Pavek P, Ceckova M, Staud F. Variation of drug kinetics in pregnancy. Curr Drug Metabol. 2009;10:520–9. doi: 10.2174/138920009788897993. [DOI] [PubMed] [Google Scholar]
- 62.Gin T, Chan MT. Decreased minimum alveolar concentration of isoflurane in pregnant humans. Anesthesiology. 1994;81:829–32. doi: 10.1097/00000542-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Koo SM, Kim Y, Park C, Park GW, Lee M, Won S, et al. Effect of pregnancy on quantitative medication use and relation to exacerbations in asthma. BioMed Res Int. 2017;2017:8276190. doi: 10.1155/2017/8276190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enriquez R, Wu P, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, et al. Cessation of asthma medication in early pregnancy. Am J Obstet Gynecol. 2006;195:149–53. doi: 10.1016/j.ajog.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 65.Ciobanu AM, Colibaba S, Cimpoca B, Peltecu G, Panaitescu AM. Thrombocytopenia in pregnancy. Maedica. 2016;11:55–60. [PMC free article] [PubMed] [Google Scholar]
- 66.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14:601–12. doi: 10.1016/s0308-2261(21)00496-3. [DOI] [PubMed] [Google Scholar]
- 67.Vricella LK. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am J Clin Nutr. 2017;106:1620s–5s. doi: 10.3945/ajcn.117.155903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bailey RR, Rolleston GL. Kidney length and ureteric dilatation in the puerperium. J Obstet Gynaecol Br Commonw. 1971;78:55–61. doi: 10.1111/j.1471-0528.1971.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 69.Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chron Kidney Dis. 2013;20:209–14. doi: 10.1053/j.ackd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindheimer MD, Davison JM, Katz AI. The kidney and hypertension in pregnancy: twenty exciting years. Semin Nephrol. 2001;21:173–89. doi: 10.1053/snep.2001.20937. [DOI] [PubMed] [Google Scholar]
- 71.Wiles K, Bramham K, Seed PT, Nelson-Piercy C, Lightstone L, Chappell LC. Serum creatinine in pregnancy: a systematic review. Kidney Int Rep. 2019;4:408–19. doi: 10.1016/j.ekir.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eke AC, Mirochnick MH. Cobicistat as a pharmacoenhancer in pregnancy and postpartum: progress to date and next steps. J Clin Pharmacol. 2019;59:779–83. doi: 10.1002/jcph.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eke AC, Wang J, Amin K, Shapiro DE, Stek A, Smith E, et al. Fosamprenavir with ritonavir pharmacokinetics during pregnancy. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.02260-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eke AC, McCormack SA, Best BM, Stek AM, Wang J, Kreitchmann R, et al. Pharmacokinetics of increased Nelfinavir plasma concentrations in women during pregnancy and postpartum. J Clin Pharmacol. 2019;59:386–93. doi: 10.1002/jcph.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eke AC, Dooley KE, Sheffield JS. Pharmacologic research in pregnant women - time to get it right. N Engl J Med. 2019;380:1293–5. doi: 10.1056/nejmp1815325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eke AC, Chakhtoura N, Kashuba A, Best BM, Sykes C, Wang J, et al. Rilpivirine plasma and Cervicovaginal concentrations in women during pregnancy and postpartum. J Acquir Immune Defic Syndr. 2018;78:308–13. doi: 10.1097/QAI.0000000000001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eke AC, Mirochnick M. Ritonavir and cobicistat as pharmacokinetic enhancers in pregnant women. Expet Opin Drug Metabol Toxicol. 2019;15:523–5. doi: 10.1080/17425255.2019.1628947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eke AC, Brooks KM, Gebreyohannes RD, Sheffield JS, Dooley KE, Mirochnick M. Tenofovir alafenamide use in pregnant and lactating women living with HIV. Expet Opin Drug Metabol Toxicol. 2020;16:333–42. doi: 10.1080/17425255.2020.1738384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eke AC, Brooks KM, Gebreyohannes RD, Sheffield JS, Dooley KE, Mirochnick M. Tenofovir alafenamide use in pregnant and lactating women living with HIV. Expet Opin Drug Metabol Toxicol. 2020 doi: 10.1080/17425255.2020.1738384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brouwer KL, Aleksunes LM, Brandys B, Giacoia GP, Knipp G, Lukacova V, et al. Human ontogeny of drug transporters: review and recommendations of the pediatric transporter working group. Clin Pharmacol Ther. 2015;98:266–87. doi: 10.1002/cpt.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yacovino LL, Aleksunes LM. Renal efflux transporter expression in pregnant mice with Type I diabetes. Toxicol Lett. 2012;211:304–11. doi: 10.1016/j.toxlet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou L, Zhang Y, Hebert MF, Unadkat JD, Mao Q. Increased glyburide clearance in the pregnant mouse model. Drug Metab Dispos. 2010;38:1403–6. doi: 10.1124/dmd.110.033837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Warren MS, Zhang X, Diamond S, Williams B, Punwani N, et al. Impact on creatinine renal clearance by the interplay of multiple renal transporters: a case study with INCB039110. Drug Metab Dispos. 2015;43:485–9. doi: 10.1124/dmd.114.060673. [DOI] [PubMed] [Google Scholar]
- 84.Gomes CF, Sousa M, Lourenço I, Martins D, Torres J. Gastrointestinal diseases during pregnancy: what does the gastroenterologist need to know? Ann Gastroenterol. 2018;31:385–94. doi: 10.20524/aog.2018.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Everson GT. Gastrointestinal motility in pregnancy. Gastroenterol Clin N Am. 1992;21:751–76. doi: 10.1016/s0889-8553(21)00599-9. [DOI] [PubMed] [Google Scholar]
- 86.Gerson LB. Treatment of gastroesophageal reflux disease during pregnancy. Gastroenterol Hepatol. 2012;8:763–4. [PMC free article] [PubMed] [Google Scholar]
- 87.Glavinas H, Krajcsi P, Cserepes J, Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv. 2004;1:27–42. doi: 10.2174/1567201043480036. [DOI] [PubMed] [Google Scholar]
- 88.Aleksunes LM, Yeager RL, Wen X, Cui JY, Klaassen CD. Repression of hepatobiliary transporters and differential regulation of classic and alternative bile acid pathways in mice during pregnancy. Toxicol Sci. 2012;130:257–68. doi: 10.1093/toxsci/kfs248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D. Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am J Physiol Gastrointest Liver Physiol. 2006;290:G640–9. doi: 10.1152/ajpgi.00354.2005. [DOI] [PubMed] [Google Scholar]
- 90.Eke AC, Gebreyohannes RD. Physiologically based pharmacokinetic modeling (PBPK’s) prediction potential in clinical pharmacology decision making during pregnancy. Int J Gynaecol Obstet. 2020;150:414–16. doi: 10.1002/ijgo.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ladumor MK, Thakur A, Sharma S, Rachapally A, Mishra S, Bobe P, et al. A repository of protein abundance data of drug metabolizing enzymes and transporters for applications in physiologically based pharmacokinetic (PBPK) modelling and simulation. Sci Rep. 2019;9:9709. doi: 10.1038/s41598-019-45778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang SM, Zhang L, Giacomini KM. The International Transporter Consortium: a collaborative group of scientists from academia, industry, and the FDA. Clin Pharmacol Ther. 2010;87:32–6. doi: 10.1038/clpt.2009.236. [DOI] [PubMed] [Google Scholar]
- 93.Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 94.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192:633–9. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 95.Jeong H. Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes. Expet Opin Drug Metabol Toxicol. 2010;6:689–99. doi: 10.1517/17425251003677755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hodge LS, Tracy TS. Alterations in drug disposition during pregnancy: implications for drug therapy. Expet Opin Drug Metabol Toxicol. 2007;3:557–71. doi: 10.1517/17425255.3.4.557. [DOI] [PubMed] [Google Scholar]
- 97.Knutti R, Rothweiler H, Schlatter C. Effect of pregnancy on the pharmacokinetics of caffeine. Eur J Clin Pharmacol. 1981;21:121–6. doi: 10.1007/bf00637512. [DOI] [PubMed] [Google Scholar]
- 98.Mutua DN, Njagi ENM, Orinda G. Liver function tests in normal pregnant women. J Liver. 2018;7:1–4. doi: 10.4172/2167-0889.1000228. [DOI] [Google Scholar]
- 99.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–87. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murakami E, Wang T, Park Y, Hao J, Lepist E, Babusis D, et al. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob Agents Chemother. 2015;59:3563–9. doi: 10.1128/aac.00128-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benet LZ, Zia-Amirhosseini P. Basic principles of pharmacokinetics. Toxicol Pathol. 1995;23:115–23. doi: 10.1177/019262339502300203. [DOI] [PubMed] [Google Scholar]
- 102.Nakai A, Sekiya I, Oya A, Koshino T, Araki T. Assessment of the hepatic arterial and portal venous blood flows during pregnancy with Doppler ultrasonography. Arch Gynecol Obstet. 2002;266:25–9. doi: 10.1007/pl00007495. [DOI] [PubMed] [Google Scholar]
- 103.Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89–295. doi: 10.1080/03602530902843483. [DOI] [PubMed] [Google Scholar]
- 104.Chowbay B, Zhou S, Lee EJ. An interethnic comparison of polymorphisms of the genes encoding drug-metabolizing enzymes and drug transporters: experience in Singapore. Drug Metab Rev. 2005;37:327–78. doi: 10.1081/dmr-200028805. [DOI] [PubMed] [Google Scholar]
- 105.Carranza-Leon D, Dickson AL, Gaedigk A, Stein CM, Chung CP. CYP2D6 genotype and reduced codeine analgesic effect in real-world clinical practice. Pharmacogenomics J. 2021 doi: 10.1038/s41397-021-00226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wadelius M, Darj E, Frenne G, Rane A. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther. 1997;62:400–7. doi: 10.1016/s0009-9236(97)90118-1. [DOI] [PubMed] [Google Scholar]
- 107.Betcher HK, George AL. Pharmacogenomics in pregnancy. Semin Perinatol. 2020;44:151222. doi: 10.1016/j.semperi.2020.151222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Katara P, Kuntal H. TPMT polymorphism: when shield becomes weakness. Interdiscipl Sci Comput Life Sci. 2016;8:150–5. doi: 10.1007/s12539-015-0111-1. [DOI] [PubMed] [Google Scholar]
- 109.Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16:153–68. doi: 10.1016/s1521-6926(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 110.Prochazkova J, Slavik L, Ulehlova J, Prochazka M. The role of tissue factor in normal pregnancy and in the development of preeclampsia: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:192–6. doi: 10.5507/bp.2014.061. [DOI] [PubMed] [Google Scholar]
- 111.Lockwood CJ, Krikun G, Runic R, Schwartz LB, Mesia AF, Schatz F. Progestin-epidermal growth factor regulation of tissue factor expression during decidualization of human endometrial stromal cells. J Clin Endocrinol Metab. 2000;85:297–301. doi: 10.1210/jcem.85.1.6292. [DOI] [PubMed] [Google Scholar]
- 112.Pitkin RM. Calcium metabolism in pregnancy: a review. Am J Obstet Gynecol. 1975;121:724–37. doi: 10.1016/0002-9378(75)90481-0. [DOI] [PubMed] [Google Scholar]
- 113.Pitkin RM, Gebhardt MP. Serum calcium concentrations in human pregnancy. Am J Obstet Gynecol. 1977;127:775–8. doi: 10.1016/0002-9378(77)90256-3. [DOI] [PubMed] [Google Scholar]
- 114.Sharief LT, Lawrie AS, Mackie IJ, Smith C, Peyvandi F, Kadir RA. Changes in factor XIII level during pregnancy. Haemophilia. 2014;20:e144–8. doi: 10.1111/hae.12345. [DOI] [PubMed] [Google Scholar]
- 115.James AH, Rhee E, Thames B, Philipp CS. Characterization of antithrombin levels in pregnancy. Thromb Res. 2014;134:648–51. doi: 10.1016/j.thromres.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 116.Gutiérrez García I, Pérez Cañadas P, Martínez Uriarte J, García Izquierdo O, Angeles Jódar Pérez M, García de Guadiana Romualdo L. D-dimer during pregnancy: establishing trimester-specific reference intervals. Scand J Clin Lab Invest. 2018;78:439–42. doi: 10.1080/00365513.2018.1488177. [DOI] [PubMed] [Google Scholar]
- 117.Warwick R, Hutton RA, Goff L, Letsky E, Heard M. Changes in protein C and free protein S during pregnancy and following hysterectomy. J R Soc Med. 1989;82:591–4. doi: 10.1177/014107688908201008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yacovino LL, Gibson CJ, Aleksunes LM. Down-regulation of brush border efflux transporter expression in the kidneys of pregnant mice. Drug Metab Dispos. 2013;41:320–5. doi: 10.1124/dmd.112.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aleksandrova MI, Sirotina NS, Smirnova OV. Expression of multidrug resistance protein 2 (mrp2) in the liver and kidney cells of female rats with modeled cholestasis of pregnancy. Bull Exp Biol Med. 2015;158:748–52. doi: 10.1007/s10517-015-2853-5. [DOI] [PubMed] [Google Scholar]
- 120.Zhang H, Wu X, Wang H, Mikheev AM, Mao Q, Unadkat JD. Effect of pregnancy on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: mechanisms, tissue specificity, and time course. Mol Pharmacol. 2008;74:714–23. doi: 10.1124/mol.107.043851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karimian Pour N, McColl ER, Piquette-Miller M. Impact of viral inflammation on the expression of renal drug transporters in pregnant rats. Pharmaceutics. 2019;11 doi: 10.3390/pharmaceutics11120624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shuster DL, Bammler TK, Beyer RP, Macdonald JW, Tsai JM, Farin FM, et al. Gestational age-dependent changes in gene expression of metabolic enzymes and transporters in pregnant mice. Drug Metab Dispos. 2013;41:332–42. doi: 10.1124/dmd.112.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moscovitz JE, Yarmush G, Herrera-Garcia G, Guo GL, Aleksunes LM. Differential regulation of intestinal efflux transporters by pregnancy in mice. Xenobiotica. 2017;47:989–97. doi: 10.1080/00498254.2016.1250292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mathias AA, Maggio-Price L, Lai Y, Gupta A, Unadkat JD. Changes in pharmacokinetics of anti-HIV protease inhibitors during pregnancy: the role of CYP3A and P-glycoprotein. J Pharmacol Exp Therapeut. 2006;316:1202–9. doi: 10.1124/jpet.105.095406. [DOI] [PubMed] [Google Scholar]
- 125.Chen S, Yueh MF, Evans RM, Tukey RH. Pregnane-x-receptor controls hepatic glucuronidation during pregnancy and neonatal development in humanized UGT1 mice. Hepatology. 2012;56:658–67. doi: 10.1002/hep.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mullenbach R, Bennett A, Tetlow N, Patel N, Hamilton G, Cheng F, et al. ATP8B1 mutations in British cases with intrahepatic cholestasis of pregnancy. Gut. 2005;54:829–34. doi: 10.1136/gut.2004.058115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cao J, Stieger B, Meier PJ, Vore M. Expression of rat hepatic multidrug resistance-associated proteins and organic anion transporters in pregnancy. Am J Physiol Gastrointest Liver Physiol. 2002;283:G757–66. doi: 10.1152/ajpgi.00126.2002. [DOI] [PubMed] [Google Scholar]