Abstract
Background & Aims:
The contribution of the abdominal muscles to normal defecation and disturbances thereof in defecatory disorders (DD) are unknown.
Methods:
In 30 healthy and 60 constipated women with normal rectal balloon expulsion time (BET) (n=26) or prolonged BET (ie, defecatory disorder, DD, n=34), seated anorectal pressures (manometry) and thickness (ultrasound) of the external and internal oblique and transversus abdominis muscles were simultaneously measured at rest, during hollowing, squeeze, evacuation, and a Valsalva maneuver.
Results:
Compared to healthy women with a normal BET, DD women had a lower rectal and greater anal pressure increase during evacuation (P ≤.05) and more activation of the internal oblique and the transversus abdominis muscles during squeeze (P<.05). The change in transversus abdominis thickness during a Valsalva maneuver vs hollowing (rho=0.5,P=.002) and separately vs evacuation (rho=0.7,P<.0001) were correlated in DD but not in healthy women with a normal BET. A principal component (PC) analysis of anorectal pressures and muscle thicknesses during evacuation uncovered a PC (PC3) that was associated with a prolonged BET. Higher PC3 scores were associated with low rectal and high anal pressures at rest and during evacuation, thinner external oblique, and thicker internal oblique muscle during evacuation. A greater PC3 score was associated with increased odds for DD versus health (OR[95% CI]=2[1.1–3.6]), and separately versus constipation with a normal BET (OR[95% CI]=3[1.6–5.7]).
Conclusion:
Taken together, these findings reveal three, possibly interrelated, disturbances suggestive of dyscoordination in DD: aberrant activation of abdominal muscles during squeeze in DD, dyscoordination of the abdominal muscles during various tasks in constipated women, and abdomino-anal dyscoordination.
Keywords: Constipation, dyssynergia, biofeedback therapy, manometry
BACKGROUND
Defecation is attributed to increased intra rectal pressure coordinated with relaxation of the anal sphincter and pelvic floor muscles1. Defecatory disorders (DD) result from inadequate intra rectal propulsive forces and/or impaired pelvic relaxation during defecation2, 3. However, the contribution of abdominal muscles to normal defecation or DD is unknown. This is a key question for 3 reasons. First, the pelvic floor muscles function synergistically with the abdominal muscles4, 5, chest wall5, and diaphragm6. The abdominal muscles responds rapidly to changes in intra-abdominal pressure and to the varied continence demands of daily living5. Hence, it is conceivable that the abdominal muscles participate in defecation. Second, excessive straining during defecation has been implicated in DD7. Third, pelvic floor biofeedback therapy, which is the cornerstone for managing DD, is, in part, designed to restore abdomino-pelvic coordination during defecation8, 9.
This study aimed to compare the activation of the abdominal muscles and anorectal pressures during defecation and other tasks and the relationship between these processes in healthy and constipated women. A better understanding of this topic is necessary to refine our understanding of the pathogenesis of DD and may lead to different biofeedback and other physical therapies to manage DD.
METHODS
Study Design and Participants
This study was approved by the Institutional Review Board at Mayo Clinic. From March 2015 to August 2020, 30 healthy women without and 60 women with Rome III symptom criteria for chronic constipation who had failed treatment with over-the-counter laxatives were enrolled. Major exclusion criteria for patients were systemic diseases that might interfere with the objectives of the study and/or pose safety concerns, opioid use, rectosigmoid surgery, inflammation, cancer, or pelvic radiation, inflammatory bowel disease, or a BMI greater than 32 kg/m2. A clinical interview and physical examination, bowel questionnaires10, the Hospital Anxiety and Depression Scale11, the Roland-Morris Disability Questionnaire12, transabdominal ultrasound, and anorectal manometry were performed.
Ultrasonography
Transabdominal ultrasonography identifies abdominal muscle activation by increased muscle thickness, which is correlated with electromyography, especially during low-level isometric muscle contractions such abdominal hollowing13. In most participants, images were acquired with a linear probe (Sonosite M Turbo, 7.5–15 MHz), which is adequate to visualize the wall up to 6–8 cm. In 6 participants, a curvilinear probe (Sonosite M Turbo, 3–6 MHz probe), which provides deeper penetration (24 cm) was necessary and used for optimal visualization.
With the patient supine, the transducer was positioned at the anterior axillary line midway between the costal margin and iliac crest in order to identify the location at which the abdominal muscle fascial planes were optimally. Thereafter, seated images were acquired at rest, during hollowing and squeeze (3 maneuvers each), evacuation (2 maneuvers), and a Valsalva maneuver14. Measurements were obtained when all three muscles were clearly visible; the average muscle thickness across each maneuver were analyzed. Before each study, participants were trained to as follows:
Rest. Participants were asked to take a deep breath, exhale, and hold their breath for 10s.
Hollowing. During hollowing, which entails an isometric activation of the transverse abdominis and internal oblique muscles, participants were asked to gently draw-in the abdomen without moving the spine 15. This maneuver is used to assess the ability to appropriately contact these muscles. While practicing this maneuver, the instructor placed a hand under the participant’s spine. If the spine (pelvis) moved into posterior tilt, indicating contraction of the oblique abdominal or rectus abdominis muscles, the maneuver was repeated until appropriate.
Valsalva maneuver. Participants generated a pressure of 20 mmHg while exhaling into a sphygmomanometer.
Defecation. Participants were asked to attempt expulsion of the anorectal catheter and balloon assembly during simulated defecation.
Anorectal manometry
After rectal cleansing with 1–2 sodium phosphate (Fleets®) enemas, anorectal pressures were measured with high resolution manometry (HRM) (Manoscan™, Medtronic Inc) in the seated position at rest, during squeeze, evacuation, and a Valsalva maneuver but during hollowing. Thereafter participants had up to 3 minutes to expel a 4-cm-long balloon filled with 50 ml water from the rectum in privacy while seated on a commode. The balloon expulsion time (BET) was noted. For this balloon, a BET greater than 60 seconds is prolonged 16, 17.
Statistical Analysis
Demographic features, anorectal pressures, and thicknesses of the external oblique, internal oblique, and transversus abdominis muscles at rest and the change from rest in these variables during maneuvers (squeeze, evacuation, hollowing, and Valsalva maneuver) were compared among healthy women, constipated women with a normal BET, and constipated women with an abnormal BET. The absolute changes in anorectal pressures 18, 19 and the relative changes in muscle thickness during maneuvers were used for analysis 20. Quantitative and qualitative variables were compared using Kruskal-Wallis and Fisher’s exact tests; pairwise comparisons were performed with the Wilcoxon rank sum test. Correlations were assessed with Spearman’s coefficient.
These variables are inter-correlated across maneuvers. Hence, a principal component (PC) analysis of 10 variables (rectal and anal pressures at rest and during evacuation, abdominal muscle thickness at rest and during evacuation) was used to identify patterns of abdomino-anal activation and coordination. This analysis identifies specific weighted linear combinations of the variables that are mutually uncorrelated and progressively explain different dimensions of variation among participants. Specifically, the first PC score (or PC1) is a weighted linear combination of the 10 variables that accounts for the maximum between-subject variation. The weight (loading) for a specific variable is the coefficient multiplier used for that variable in the given PC score. The second linear combination (PC2) explains the maximum possible remaining variation and is uncorrelated with PC1. Prior to principal component analyses, all variables were Winsorized and center scaled using RStudio software (2020). During Winsorization, a predefined quantum, that is, 5% of the smallest and the largest values were respectively replaced by the next smallest and largest extreme values to control for outliers. Variables (x) were center-scaled by the formula x = (x – mean [x])/(2*standard deviation [x]). Because correlations between anorectal parameters vs age and vs body mass index (BMI) can affect the discriminant analysis, age and BMI were partialled out, i.e., only that portion of anorectal variables that was not explained by age and BMI were incorporated in the PCs used in the discriminant model. The correlations between PC scores and the original HRM variables were computed.
A logistic regression model used PC scores to predict group status, yielding estimates of the odds for constipation with normal BET versus health, constipation with abnormal BET versus health, and constipation with abnormal BET versus constipation with normal BET. These analyses were performed using JMP software (JMP Pro, version 14.1.0 SAS Institute Inc, Cary, NC). The Supplementary material contains the sample size assessment.
RESULTS
Demographic and clinical features
The constipated women had a lower BMI (P=.004) and a longer BET (P=.01) than healthy women (Table 1); 24 (41%) had constipation-predominant IBS and 35 (58%) had functional constipation; one patient did not complete the questionnaire. The mean Roland-Morris Disability Questionnaire score was greater in constipated than healthy women (P=.0003). Thirty-eight patients (63%) had anxiety and/or depression. Obstetric variables were not significantly different between healthy and constipated women (Table 1).
Table 1.
Demographic Variables, Clinical Features, Obstetric History, and Balloon Expulsion Time of Study Participants
| Parameter | Healthy women (N= 30) | Constipated women (N= 60) | p-value |
|---|---|---|---|
| Age, y | 29 (25–48) | 40 (28–52) | .06 |
| BMI, kg/m2 | 26 (22–28) | 22 (20–24) | .004 |
| Hip-waist circumference ratio | 1.1 (1.08–1.17) | 1.06 (1.04–1.16) | .8 |
| BET, s | 16 (10–122) | 119 (14–180) | .01 |
| Abnormal BET (>60s), n (%) | 8 (27%) | 34 (57%) | .008 |
| Symptoms | |||
| < 3 bowel movements/week, n (%) | 7 (23%) | 40 (67%) | .0001 |
| Incomplete evacuation, n (%) | 1 (3%) | 49 (82%) | <.0001 |
| Straining, n (%) | 1 (3%) | 48 (80%) | <.0001 |
| Hard stool, n (%) | 4 (13%) | 38 (63%) | <.0001 |
| Sensation of blockage, n (%) | 0 (0%) | 41 (68%) | <.0001 |
| Manual evacuation, n (%) | 0 (0%) | 14 (23%) | <.0001 |
| Functional constipation, n (%) | 0 (0%) | 35 (58%) a | <.0001 |
| IBS-C, n (%) | 0 (0%) | 24 (41%) a | <.0001 |
| RMDQ total score, mean (SD) b | 0.3 (1.1) | 3.1 (4.7) | .0003 |
| Depression, n (%) | 0 (0%) | 14 (23%) | .002 |
| Anxiety, n (%) | 4 (13%) | 37 (62%) | <.0001 |
| Live births, mean (SD) | 1.1 (1.4) | 1.2 (1.3) | .7 |
| Vaginal deliveries, mean (SD) | 0.7 (1.3) | 0.9 (1.3) | .4 |
| Cesarean section, mean (SD) | 0.4 (0.9) | 0.2 (0.7) | .2 |
| Vaginal deliveries requiring pelvic sutures, mean (SD) | 0.4 (0.9) | 0.6 (0.9) | .3 |
Values are Median (IQ range) unless specified otherwise.
Missing values in one patient
Four constipated women did not complete the Roland-Morris Disability Questionnaire (RMDQ)
In healthy women, the BET was normal in 22(73%) and abnormal in 8(27%) participants. Among constipated women, the BET was normal (“C-normal”) in 26 (43%) and abnormal in the remaining patients, suggestive of a DD. Compared to C-normal patients, more DD patients had infrequent bowel movements (88% versus 48%, P=.001); other constipation symptoms were not different between these groups (data not shown) or between healthy women with a normal vs abnormal BET (Supplementary Table 1). Eight of 24 (33%) FC patients and 25 of 35 IBS patients (71%) had a DD (P=0.007)
Anorectal pressures
There was less anal relaxation during evacuation in DD than in all healthy women (n=30) (P=.008) (Table 2). During a Valsalva maneuver, the anal pressure increment was lower in DD than in C-normal patients (P=.048). The anal pressure increased during a Valsalva maneuver but declined during evacuation (P<.0001).
Table 2.
Comparison of Anorectal Pressures and Muscle Thickness in Healthy and Constipated Women
| Parameter | Healthy women (N= 30) | Constipated women, normal BET (N=26) | Constipated women, prolonged BET (N=34) | P value, Healthy vs constipated abnormal BET | P value, Constipated normal vs abnormal BET |
|---|---|---|---|---|---|
| Anorectal pressures, mmHg | |||||
| Anal pressure, rest | 78 (64–103) | 82 (64–99) | 89 (61–101) | .71 | .78 |
| Rectal pressure, rest | 26 (15–34) | 30 (19–40) | 20 (11–29) | .36 | .009 |
| Anal pressure (squeeze–rest) | 38 (24–71) | 46 (24–88) | 43 (20–88) | .49 | .89 |
| Rectal pressure (squeeze–rest) | 5.5 (1.5–12) | 4 (2–15) | 2 (0.2–5) | .06 | .04 |
| Anal pressure (evacuation–rest) | −10 (−19 – −1) | −3 (−21–10) | 0.05 (−9–14) | .008 | .15 |
| Rectal pressure (evacuation–rest) | 16 (6–30) | 26 (14–42) | 9 (2–22) | .26 | .004 |
| Anal pressure (Valsalva maneuver–rest) | 13 (2–21) | 17 (13–26) | 11 (2–20) | .87 | .048 |
| Rectal pressure (Valsalva maneuver–rest) | 15 (11–26) | 26 (13–37) | 10 (2–18) | .03 | .0001 |
| Abdominal muscle thickness, mm | |||||
| External oblique, rest | 5.3 (4.9–6.6) | 5.3 (3.7–6.2) | 3.8 (3.3–5.2) | .0004 | .06 |
| Internal oblique, rest | 8.1 (6.6–12.3) | 7.6 (5.7–8.4) | 6.9 (5.04–9.4) | .049 | .60 |
| Transversus abdominis, rest | 3.8 (2.6–5.1) | 3.7 (2.7–5.2) | 3.9 (2.8–4.9) | .86 | .99 |
| External oblique (squeeze–rest)/rest | 0.007 (−0.2–0.2) | −0.006 (−0.2–0.2) | −0.03 (−0.1–0.4) | .99 | .83 |
| Internal oblique, (squeeze–rest)/rest | −0.05 (−0.2–0.2) | −0.04 (−0.2–0.2) | 0.1 (0.03–0.3) | .01 | .046 |
| Transversus abdominis, (squeeze–rest)/rest | −0.07 (−0.3–0.2) | 0.2 (−0.1–0.5) | 0.1 (−0.1–0.4) | .09 | .44 |
| External oblique (evacuation–rest)/rest | 0.06 (−0.2–0.2) | 0.005 (−0.3–0.4) | −0.01 (−0.2–0.1) | .82 | .64 |
| Internal oblique, (evacuation–rest)/rest | 0.02 (−0.1–0.3) | 0.1 (−0.05–0.5) | 0.1 (−0.06–0.6) | .12 | .60 |
| Transversus abdominis, (evacuation–rest)/rest | 0.09 (−0.2–0.3) | 0.3 (−0.2–0.7) | 0.06 (−0.2–0.4) | .91 | .22 |
| External oblique, (Valsalva maneuver–rest)/rest | −0.05 (−0.4 – −0.001) | 0.02 (−0.3–0.4) | −0.1 (−0.3–0.06) | .93 | .21 |
| Internal oblique, (Valsalva maneuver–rest)/rest | 0.4 (0.08–0.6) | 0.2 (0.08–0.6) | 0.4 (0.05–0.96) | .70 | .21 |
| Transversus abdominis, (Valsalva maneuver–rest)/rest | 0.5 (0.0007–0.9) | 0.5 (−0.1–0.9) | 0.3 (0.03–0.7) | .39 | .52 |
| External oblique, (hollowing–rest)/rest | −0.04 (−0.3–0.1) | −0.04 (−0.2–0.1) | −0.06 (−0.2–0.05) | .87 | .93 |
| Internal oblique, (hollowing–rest)/rest | 0.08 (−0.1–0.4) | 0.01 (−0.06–0.4) | 0.05 (−0.03–0.3) | .78 | .74 |
| Transversus abdominis, (hollowing–rest)/rest | 0.5 (0.09–0.8) | 0.4 (0.06–0.8) | 0.2 (0.07–0.4) | .08 | .13 |
Values are Medians (IQR)
Compared to C-normal patients, the rectal pressure at rest (P=.009), the increase during evacuation (P=.004), and during a Valsalva maneuver (P=.0001) were lower in DD (P≤ .04, Table 2). However, differences between healthy women with normal vs abnormal BET were not significant (Supplementary Table 2).
Comparison of muscle thickness during the same maneuver
At rest, the external oblique and internal oblique muscles were thicker in all healthy women (n=30, P=.004 and P=.049 respectively, Table 2) than in DD. However, the resting thickness of the transversus abdominis was not different among groups.
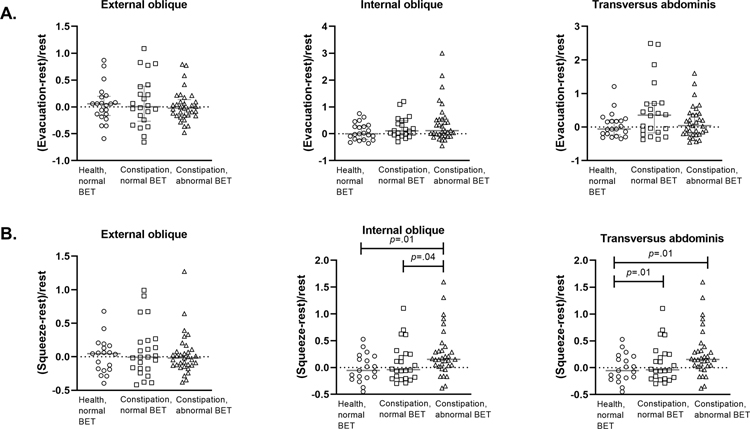
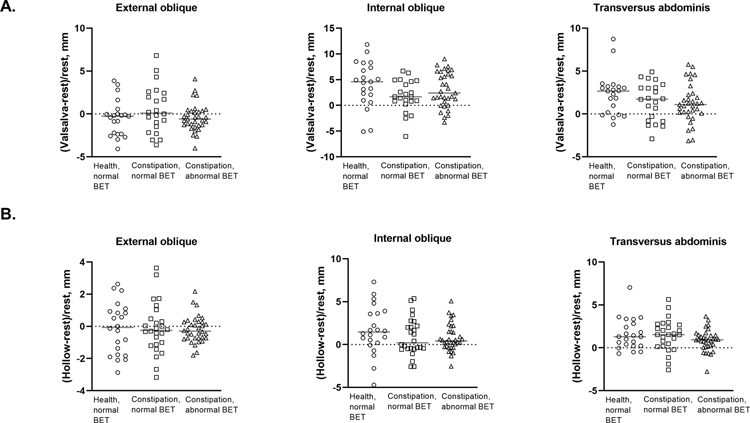
The change in muscle thickness during maneuvers was compared in constipated women vs all healthy women (Table 2) and vs healthy women with a normal BET (Figures 2 and 3). During squeeze, the internal oblique muscle thickness increased to a greater extent in DD vs healthy women (P=.01) and vs C-normal patients (P=.046) (Table 2). These differences were also significant for DD vs healthy women with a normal BET (Figure 1a). Compared to healthy women with a normal BET, the change in transversus abdominis muscle thickness during squeeze was greater in C-normal and DD patients (P=.01, Figure 1a). For all muscles, the change in thickness during evacuation, Valsalva maneuver, and hollowing (relative to rest) were comparable among the groups (Table 2, Figure 1b).
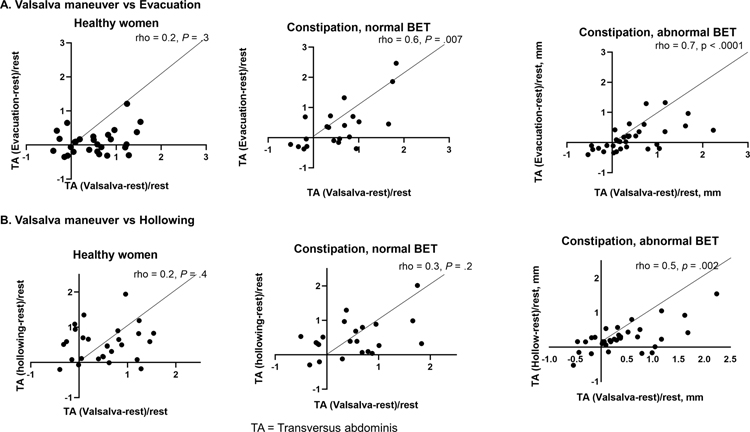
Figure 2.
Comparison of change in transversus abdominis thickness during evacuation, hollowing, and Valsalva maneuver among healthy women, constipated women with a normal, and abnormal BET, respectively none, 1, and both correlations were significant. TA= transversus abdominis muscle
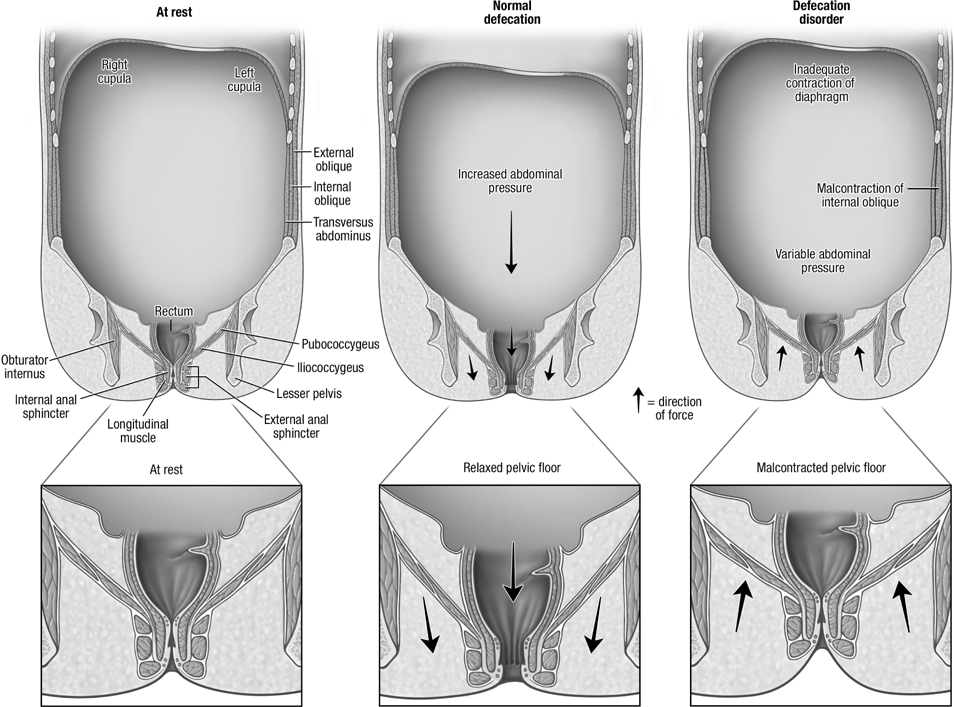
Figure 3.
Schematic representation of abdominopelvic floor configuration at rest (left panel), during defecation in healthy persons (center panel) and in DD (right panel). Among DD patients, the change in abdominal pressure during defecation is either normal or reduced, infrequently increased.
Figure 1.
a. Change in abdominal muscle thickness during evacuation (Panel A) and during squeeze (Panel B). BET = balloon expulsion time.
b. Change in abdominal muscle thickness during a Valsalva maneuver (Panel A) and during hollowing (Panel B)
Comparison of muscle activation across maneuvers
During selected maneuvers, there was differential activation of the abdominal muscles in healthy but not in constipated women. Hence, during evacuation in healthy women with a normal BET, the relative change in thickness of the (1) transversus abdominis muscle was lower than during a Valsalva maneuver or hollowing (P < .01, Supplementary Table 3) and (2) internal oblique muscle was lower than during a Valsalva maneuver (P=.002, Supplementary Table 3).
The relative change in muscle thickness during maneuvers also differed among healthy women with a normal BET, C-normal and DD patients. The change in transversus abdominis muscle thickness during a Valsalva maneuver was significantly correlated with the change in thickness during evacuation and during hollowing in DD but not in healthy women (Figure 2). The change in transversus abdominis muscle thickness during a Valsalva maneuver and evacuation were also correlated in C-normal patients (Figure 2).
The internal oblique muscle thickness during a Valsalva maneuver was significantly correlated with the change in thickness during evacuation in all groups (Supplementary Figure 1). By contrast, the internal oblique muscle thickness during a Valsalva maneuver was correlated with the change during hollowing only in healthy women and not in C-normal or DD patients.
Phenotypes
The PC analysis, which was performed in 22 healthy women with a normal BET and all constipated women, generated 10 scores (or PCs) that explained 29%, 21%, 14%, 11%, 7%, 5%, 5%, 4%, 2%, and 2% (a sum of 100%), of the total between participant variation in the 10 response variables. PCs 1– 4, which explained 75% of the variance, are shown in Table 3; correlation coefficients that were 0.4 or greater are considered biologically relevant. Of note, PCs 1 and 2 were respectively correlated with the thickness of all abdominal muscles and all anorectal pressures at rest and during evacuation (Table 3). PC3 was (1) correlated with anal pressures and the thickness of only the internal oblique muscle during evacuation, (2) inversely correlated with rectal pressure and the external oblique muscle thickness during evacuation and (3) correlated with the BET. By contrast to PCs 1 and 2, which respectively represent dimensions predominantly characterized by the average muscle thickness and anorectal pressures, PC3 is characterized by contrasts between rectal and anal pressures and separately between thickness of the internal and external oblique muscles. Greater values of PC3 were correlated with lower rectal pressure at rest and during evacuation (rho=−0.4, P<.0001), higher anal pressure at rest and during evacuation (rho=0.4, P<.0001), thinner external oblique during evacuation (rho=−0.4, P<.0001), thicker internal oblique during evacuation (rho=0.5, P<.0001), and a longer BET (rho=0.4, P< .0001). PC4 was most strongly correlated with anal pressure and external oblique thickness during evacuation and inversely correlated with internal oblique thickness during evacuation.
Table 3.
Correlation Coefficients for Principal Component (PC) Scores vs Anorectal Pressures and Abdominal Muscle thickness
| Variables a | Correlation coefficients | |||
|---|---|---|---|---|
| PC1 (29%) | PC2 (21%) | PC3 (14%) | PC4 (11%) | |
| Pressure, mmHg | ||||
| Anal pressure, rest | 0.07 | 0.73 *** | 0.44 *** | 0.29** |
| Rectal resting pressure | 0.22* | 0.73 *** | −0.41 *** | −0.25* |
| Anal pressure, evacuation | −0.05 | 0.64 *** | 0.49 *** | 0.39 *** |
| Rectal pressure, evacuation | 0.18 | 0.70 *** | −0.43 *** | −0.18 |
| Muscle thickness, mm | ||||
| External oblique, rest | 0.65 *** | −0.21* | −0.13 | 0.32** |
| Internal oblique, rest | 0.67 *** | −0.21* | 0.32** | −0.005 |
| Transversus abdominis, rest | 0.73 *** | −0.002 | 0.06 | −0.24* |
| External oblique, evacuation | 0.50 *** | −0.10 | −0.37 *** | 0.50 *** |
| Internal oblique, evacuation | 0.56 *** | −0.002 | 0.47 *** | −0.48 *** |
| Transversus abdominis, evacuation | 0.71 *** | 0.08 | −0.05 | −0.03 |
Correlation coefficients with an absolute value of 0.4 or greater, which indicates that the variable is correlated substantially with the factor, are shown in bold.
p<.05
p<.01
p<.001
Higher PC3 scores were associated with increased odds of DD vs healthy women with normal BET (OR[95% CI]=1.84[1.05–3.23]) and vs C-normal (OR[95% CI]=3.64[1.73–7.69], Supplementary Table 4). The PC3 yielded an area under the ROC curve of 0.72 (P=.007) and 0.79 (P<.0001) to discriminate respectively between DD vs health with normal BET and DD vs C-normal BET.
DISCUSSION
Confirming previous studies, women with DD had lower rectal and higher anal pressures during evacuation than in healthy women19, 21, 22. While excessive straining has been implicated to predispose to DD, the pathogenesis of DD, indeed the contribution of abdominal motion to normal defecation is poorly understood because abdominal muscle function during evacuation has not been studied in health or disease. These findings reveal three, possibly interrelated, disturbances suggestive of dyscoordination in DD: aberrant activation of abdominal muscles during squeeze, altered patterns of firing of individual abdominal muscles during various tasks, and abdomino-anal dyscoordination during defecation.
The activation of the individual abdominal muscles during evacuation, hollowing, and Valsalva maneuver were not significantly different between DD and healthy women. Perhaps this is true. Alternatively, overall differences may not be significant because such disturbances may only affect a subset of patients. The PC analysis was used to concurrently explore abdominal and anal motion and abdomino-anal coordination. PC1 and PC2 together explained 50% of the total variation among participants. By design, the PC analysis identifies the linear combination of weighted variables that explains the maximum variance among participants. Hence, it is anticipated that PCs 1 and 2 would be correlated most strongly with the anorectal pressures and muscle thickness. Neither PC1 nor PC2 were correlated with the BET or discriminated among groups. Of the residual variance, PC3 uncovered a dimension, which by contrast to PCs 1 and 2 was differentially correlated with rectal and anal pressures during evacuation. Higher scores of PC3 were associated with lower rectal and higher anal pressures at rest and during evacuation, a thicker internal oblique muscle at rest, a thinner external oblique and thicker internal oblique during evacuation (Figure 3). Lo and behold, PC3 was also correlated with a longer BET, which was not included in the PC analysis. PC3 was associated with an increased odds of DD vs health and vs C-normal BET. These observations suggest that greater activation of the internal oblique muscle at rest and during evacuation is accompanied by higher anal and lower rectal pressures at rest and during evacuation in DD. Perhaps exaggerated activation of the internal oblique muscle is associated with co-contraction of the external anal sphincter, which increases anal pressure, in a subset of DD women19.
In healthy women with a normal BET, there was no significant change in abdominal thickness during evacuation. We recently observed that rectal evacuation is associated with abdominal expansion and increased rectal pressure which were respectively measured with MRI and manometry23. Moreover, abdominal expansion, increased rectal pressure, and perineal descent were correlated with each other in healthy and constipated women with normal evacuation. These findings suggest that diaphragmatic descent, which is accompanied by increased abdominal pressure24, 25, at least partly explains increased rectal pressure during defecation. This abdominal pressure increment varies inversely with abdominal wall compliance25. When the wall is absent, it is infinitely compliant; diaphragm descent will not increase abdominal pressure. Conversely, during a Valsalva maneuver, diaphragm descent is associated with a contracted and less compliant abdominal wall; hence greater abdominal and likely rectal pressure26. However, a Valsalva maneuver is associated with co-contraction of pelvic floor muscles26 that may hinder evacuation. Indeed, compared to evacuation, a Valsalva maneuver was accompanied by a comparable rectal but substantially greater anal pressure increment in this study27.
During anal contraction (squeeze), anal pressure but not abdominal muscle thickness increased in asymptomatic women. Compared to healthy women, the internal oblique and transversus abdominis muscles became even thicker during squeeze in DD women. This suggests by contrast to healthy women, DD women co-contract other muscles when they try to contract the anal sphincter. Such substitution also occurs in women with urinary incontinence28 and back pain29.
The individual abdominal muscles are differentially activated among maneuvers. Among healthy women, activation of the internal oblique and transversus abdominis muscles was most pronounced during a Valsalva maneuver followed by hollowing. In healthy women, there was comparable activation of the internal oblique during a Valsalva maneuver vs hollowing and vs evacuation. By contrast, in the transversus abdominis muscle, these correlations were only significant in DD. This suggests that similar to women with urinary incontinence, DD women find it difficult to be task specific or to differentiate between tasks28. Conceivably, this disturbance is explained by the following disturbances: differences in cortical control30, awareness of contraction of pelvic floor muscles31, mal-coordination of the deep and superficial parts of the pelvic floor31 and mal-coordination of the pelvic floor and abdominal muscles31.
As observed previously14, 16, some asymptomatic healthy women had a prolonged BET. The rectal pressure increment during evacuation was greater in C-normal than healthy women. Perhaps this finding partly reflects excessive straining in an attempt to overcome increased anal pressure32 and/or explains a normal BET despite pelvic floor dysfunction. Perhaps defecography might uncover a DD in some C-normal women33. Indeed, a comparison of change in muscle thickness between maneuvers (Supplementary Table 3) suggests that the differential activation of the abdominal muscles during various tasks is also impaired in C-normal women.
These findings have clinical implications. Currently, biofeedback therapy for DD focuses on anorectal techniques, is not widely available, and only effective in approximately 60% of patients 9, 34. Patients are variably encouraged to practice diaphragmatic breathing and to push adequately, but not excessively; abdomino-pelvic coordination is encouraged. However, this guidance is informed by biofeedback from rectal pressure, not from the abdominal muscles. Prompted by these findings, future studies should investigate the efficacy of biofeedback therapy guided by transabdominal ultrasound to reduce abdominal malcontraction during evacuation and diaphragmatic breathing during evacuation.35 While seemingly counterintuitive, biofeedback therapy to improve selective contraction of the anal sphincter may also improve abdomino-anal coordination in DD. Perhaps patients will find it more convenient to practice biofeedback therapy guided by abdominal rather than anorectal activity.
This study has several strengths and limitations. Abdominal muscle thickness and anorectal pressures were measured in the upright position. Unlike needle electromyography, transabdominal ultrasound is non-invasive; inter-observer variability is greater than intra-observer variability but within acceptable limits36. The correlation between muscle activation measured with electromyography and ultrasound is excellent in the transversus abdominis and internal oblique muscles but lower in the external oblique muscle13, 37, which is arguably less relevant to the pathogenesis of DD. The non-significant age difference between healthy and constipated women may affect the findings. However, the PC analysis adjusted for age.
In conclusion, these findings uncover further evidence for dysccordination, including abdomino-anal dyscoordination during defecation in women with DD.
Supplementary Material
Background and context
Defecatory disorders (DD) result from inadequate intra rectal propulsive forces and/or impaired pelvic relaxation during defecation. Although the pelvic floor muscles function synergistically with the chest wall, diaphragm, and abdominal muscles in several tasks, the contribution of abdominal muscles to normal defecation or DD is unknown.
Findings
There are three, possibly interrelated, disturbances suggestive of dyscoordination in DD: aberrant activation of abdominal muscles during squeeze in DD, dyscoordination of the abdominal muscles during various tasks in constipated women, and abdomino-anal dyscoordination.
Implications for Patient Care
Currently, biofeedback therapy for DD is exclusively based on anorectal techniques. These findings provide the basis for augmenting biofeedback therapy with techniques designed to improve abdomino-anal coordination in DD.
Financial Support:
The project was supported by USPHS NIH Grant R01 DK78924
Footnotes
Conflict of interest: Dr. Bharucha holds a patent for an anorectal catheter fixation clip that was used in this study but has not received any royalties. No other author has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Heitmann PT, Vollebregt PF, Knowles CH, et al. Understanding the physiology of human defaecation and disorders of continence and evacuation. Nature Reviews Gastroenterology & Hepatology 2021;09:09. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE, Lacy BE. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020;158:1232–1249.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deb B, Prichard DO, Bharucha AE. Constipation and Fecal Incontinence in the Elderly. Current Gastroenterology Reports 2020;22:54. [DOI] [PubMed] [Google Scholar]
- 4.Bo K, Stien R. Needle EMG registration of striated urethral wall and pelvic floor muscle activity patterns during cough, Valsalva, abdominal, hip adductor, and gluteal muscle contractions in nulliparous healthy females. Neurourology & Urodynamics 1994;13:35–41. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JA, O’Sullivan PB, Briffa K, et al. Assessment of pelvic floor movement using transabdominal and transperineal ultrasound. International Urogynecology Journal 2005;16:285–92. [DOI] [PubMed] [Google Scholar]
- 6.Hemborg B, Moritz U, Hamberg J, et al. Intra-abdominal pressure and trunk muscle activity during lifting. III. Effect of abdominal muscle training in chronic low-back patients. Scandinavian Journal of Rehabilitation Medicine 1985;17:15–24. [PubMed] [Google Scholar]
- 7.Palit S, Lunniss PJ, Scott SM. The physiology of human defecation. Digestive Diseases & Sciences 2012;57:1445–64. [DOI] [PubMed] [Google Scholar]
- 8.Rao SS, Sadeghi P, Beaty J, et al. Ambulatory 24-h colonic manometry in healthy humans. American Journal of Physiology - Gastrointestinal & Liver Physiology 2001;280:G629–39. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan SP, Bharucha AE. A Practical Guide to Biofeedback Therapy for Pelvic Floor Disorders. Current Gastroenterology Reports 2019;21:21. [DOI] [PubMed] [Google Scholar]
- 10.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- 11.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 12.Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000;25:3115–24. [DOI] [PubMed] [Google Scholar]
- 13.Hodges PW, Pengel LHM, Herbert RD, et al. Measurement of muscle contraction with ultrasound imaging. Muscle & Nerve 2003;27:682–92. [DOI] [PubMed] [Google Scholar]
- 14.Oblizajek NR, Gandhi S, Sharma M, et al. Anorectal pressures measured with high-resolution manometry in healthy people—Normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterology and Motility : the official journal of the European Gastrointestinal Motility Society 2019:e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hides J, Hughes B, Stanton W. Magnetic resonance imaging assessment of regional abdominal muscle function in elite AFL players with and without low back pain. Man Ther 2011;16:279–84. [DOI] [PubMed] [Google Scholar]
- 16.Ratuapli S, Bharucha AE, Harvey D, et al. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterology and Motility 2013;25:e813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazor Y, Prott G, Jones M, et al. Anorectal physiology in health: A randomized trial to determine the optimum catheter for the balloon expulsion test. Neurogastroenterology & Motility 2019;31:e13552. [DOI] [PubMed] [Google Scholar]
- 18.Sharma M, Muthyala A, Feuerhak K, et al. Improving the Utility of High Resolution Manometry for the Diagnosis of Defecatory Disorders in Women with Chronic Constipation. Neurogastroenterol and Motility 2020. 32:e13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan SG, Sharma M, Feuerhak K, et al. A comparison of rectoanal pressures during Valsalva maneuver and evacuation uncovers rectoanal discoordination in defecatory disorders. Neurogastroenterology & Motility 2021:e14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teyhen DS, Childs JD, Stokes MJ, et al. Abdominal and lumbar multifidus muscle size and symmetry at rest and during contracted States. Normative reference ranges. J Ultrasound Med 2012;31:1099–110. [DOI] [PubMed] [Google Scholar]
- 21.Rao SS, Mudipalli RS, Stessman M, et al. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterology & Motility 2004;16:589–96. [DOI] [PubMed] [Google Scholar]
- 22.Ratuapli S, Bharucha AE, Noelting J, et al. Phenotypic Identification and Classification of Functional Defecatory Disorders Using High Resolution Anorectal Manometry Gastroenterology 2013;144:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deb B, Sharma M, Srinivasan SG, et al. Phenotypic Characterization of Functional Defecatory Disorders using Simultaneous MR Defecography and Manometry. Gastroenterology 2021;160:S101–S102. [Google Scholar]
- 24.Accarino A, Perez F, Azpiroz F, et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009;136:1544–51. [DOI] [PubMed] [Google Scholar]
- 25.Gartman EJ, Koo P, McCool FD. Dependence of Diaphragm Function on Abdominal Compliance. Annals of the American Thoracic Society 2019;16:381–386. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JA, O’Sullivan PB, Briffa NK, et al. Differences in muscle activation patterns during pelvic floor muscle contraction and Valsalva maneuver. Neurourology & Urodynamics 2006;25:148–55. [DOI] [PubMed] [Google Scholar]
- 27.Bharucha AE, Croak AJ, Gebhart JB, et al. Comparison of rectoanal axial forces in health and functional defecatory disorders. American Journal of Physiology - Gastrointestinal & Liver Physiology 2006;290:G1164–9. [DOI] [PubMed] [Google Scholar]
- 28.Thompson JA, O’Sullivan PB, Briffa NK, et al. Altered muscle activation patterns in symptomatic women during pelvic floor muscle contraction and Valsalva manouevre. Neurourology & Urodynamics 2006;25:268–76. [DOI] [PubMed] [Google Scholar]
- 29.O’Sullivan P, Twomey L, Allison G, et al. Altered patterns of abdominal muscle activation in patients with chronic low back pain. Aust J Physiother 1997;43:91–98. [DOI] [PubMed] [Google Scholar]
- 30.Gunnarsson M, Ahlmann S, Lindström S, et al. Cortical magnetic stimulation in patients with genuine stress incontinence: correlation with results of pelvic floor exercises. Neurourol Urodyn 1999;18:437–44; discussion 444–5. [DOI] [PubMed] [Google Scholar]
- 31.Devreese A, Staes F, De Weerdt W, et al. Clinical evaluation of pelvic floor muscle function in continent and incontinent women. Neurourol Urodyn 2004;23:190–7. [DOI] [PubMed] [Google Scholar]
- 32.Mazor Y, Hansen R, Prott G, et al. The importance of a high rectal pressure on strain in constipated patients: implications for biofeedback therapy. Neurogastroenterology and Motility 2017;29. [DOI] [PubMed] [Google Scholar]
- 33.Prichard DO, Lee T, Parthasarathy G, et al. High-resolution Anorectal Manometry for Identifying Defecatory Disorders and Rectal Structural Abnormalities in Women. Clinical Gastroenterology & Hepatology 2017;15:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patcharatrakul T, Valestin J, Schmeltz A, et al. Factors Associated With Response to Biofeedback Therapy for Dyssynergic Defecation. Clinical Gastroenterology & Hepatology 2017;27:27. [DOI] [PubMed] [Google Scholar]
- 35.Halland M, Bharucha AE, Crowell MD, et al. Effects of Diaphragmatic Breathing on the Pathophysiology and Treatment of Upright Gastroesophageal Reflux: A Randomized Controlled Trial. Am J Gastroenterol 2021;116:86–94. [DOI] [PubMed] [Google Scholar]
- 36.Park SD. Reliability of Ultrasound Imaging of the Transversus Deep Abdominial, Internal Oblique and External Oblique Muscles of Patients with Low Back Pain Performing the Drawing-in Maneuver. Journal of physical therapy science 2013;25:845–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.John EK, Beith ID. Can activity within the external abdominal oblique be measured using real-time ultrasound imaging? Clinical Biomechanics 2007;22:972–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.