Abstract
Purpose
To determine the utilization of planned oocyte cryopreservation (OC) in the year immediately prior to, and the year of, insurance coverage commencement for employees at our institution.
Methods
Patient demographics and cycle outcomes were retrospectively compared between the first OC cycles occurring in 2017 vs. 2018 according to insurance coverage and type, age, and the number of oocytes retrieved and cryopreserved. Continuous demographic variables including age, BMI, day 3 FSH and E2, AMH, gravidity, and parity were compared using student T-tests. Cycle outcomes, including the number of oocytes retrieved and cryopreserved were compared using linear regression models, adjusting for potential confounders including age, BMI, and ovarian reserve parameters.
Results
Between January 2017 and December 2018, 123 patients underwent planned OC at our institution. Patient age ranged from 23 to 44 years and did not significantly differ from 2017 to 2018 (mean 34.9 vs. 35.2). There was a 12% increase in planned OC utilization from 2017 (N = 58) to 2018 (N = 65). Significantly, more patients had any insurance coverage in 2018 vs. 2017 (71.9% vs. 40.4%, p = 0.001), a 78% increase. From 2017 to 2018, the number of patients with hospital-based insurance coverage undergoing planned OC increased by a factor of 8 (5 to 41.5%, p < 0.001), while the number of self-pay patients significantly decreased (p = 0.001). No differences were found regarding cycle outcomes.
Conclusion
A greater proportion of women at our institution had insurance coverage for planned OC in 2018 vs. 2017. Employer-based insurance coverage for planned OC was associated with a significant increase in utilization by hospital employees.
Keywords: Planned oocyte cryopreservation, Fertility preservation, Insurance coverage
Introduction
The average age of first-time mothers in the USA now stands between 28 and 30 years, a steep rise from the 21.4 years reported by the CDC in 1970 [1, 2]. The rise in the age of first-time motherhood is associated with career building, lack of a suitable partner, and/or personal and financial readiness [3, 4]. As the 20 s and 30 s are pivotal years for women to establish careers and expand their families, there remains an imbalance between a woman’s personal goals and her “biological clock.”
Women face biological inequity, a term coined to describe the limited biological reproductive lifespan of women compared to men. While men may continue to make viable sperm late into old age, women are believed to have a finite follicular pool, which diminishes in both quantity and quality with age. It is widely understood that older age at conception leads to a greater risk of fetal chromosomal abnormalities and pregnancy loss [5, 6]. While the use of donated oocytes can extend female reproductive potential, it precludes genetically related offspring [7].
Historically, medical oocyte cryopreservation was used to preserve fertility in the face of imminent medical conditions or gonadotoxic medications that threatened reproductive potential. The modern clinical approach has shifted toward supporting planned oocyte cryopreservation as a means of extending fertility and/or restoring fertility potential in the face of age-based fertility decline. Cryopreservation of oocytes offers women increased reproductive autonomy and choice, while simultaneously preserving egg quality. One study reported that following oocyte cryopreservation, 90% of women stated that they felt more secure about their reproductive future [8]. Medical advancements in the laboratory and stimulation techniques for oocyte cryopreservation, specifically the advent of vitrification, have led to similar success rates when using vitrified oocytes as compared to fresh oocytes [9–13]. In 2012, the American Society for Reproductive Medicine (ASRM) lifted the experimental label on vitrification of oocytes, and in 2018, they stated that planned oocyte cryopreservation was ethically permissible prior to the development of a medical indication, including reproductive aging, that may impact fertility [14].
The cost of oocyte preservation is a challenge for many women and remains a barrier to their access to care. Currently, most insurance companies are not mandated to provide coverage for planned oocyte cryopreservation, leading to unequal access to the procedure according to insurance benefits. In 2018, the largest hospital system in New York State began to include planned oocyte cryopreservation as a benefit for employees under their insurance plan. The purpose of this study is to compare the utilization of planned oocyte cryopreservation (planned OC) before and after the initiation of employer-based insurance coverage for this reproductive health benefit.
Materials and methods
Study population
All planned oocyte cryopreservation cycles from January 1, 2017, to December 31, 2018, were retrospectively reviewed after approval from an academic institutional review board. These data represent cycles from the year prior to initiation of employer-based insurance coverage for planned OC (1 January–31 December 2017) and cycles that occurred within the first year of planned OC coverage (1 January–31 December 2018). Patients’ insurance status information included either coverage for OC by our own hospital insurance (employees or their immediate family covered by the same insurance), coverage for OC by another insurance carrier, or self pay with no insurance coverage for planned OC. Patients with our hospital-based insurance were only covered for planned OC at our institution and would not have been covered for OC elsewhere. Only a patient’s first planned OC cycle was included. Cycles were excluded if the oocyte cryopreservation cycle was completed for an acute medical indication that placed a patient at risk for infertility, such as gonadotoxic therapy and oocyte donor cycles.
Variable collection
The primary study outcome was the utilization of planned OC in 2017 vs. 2018. Secondary outcomes were the number of oocytes retrieved and the number of oocytes cryopreserved. Patient demographics were collected from the electronic medical record and included age, BMI, day 3 FSH and E2 levels, anti-müllerian hormone level, gravidity, parity, relationship status, insurance coverage and insurance type, and hospital-based employment status.
Statistical analysis
All statistical analyses were performed using “R” statistical software. Continuous numerical demographic variables including age, BMI, day 3 FSH and E2, AMH, gravidity, and parity were compared between 2017 and 2018 using the two-tailed student T-tests. Discrete variables, including relationship status, employment status, and insurance status, were presented as frequencies (percentage). Cycle outcomes, including the number of oocytes retrieved and the number of oocytes cryopreserved, were compared using a linear regression model adjusting for potential confounding factors including age, BMI, and ovarian reserve parameters. Statistical significance was set as p < 0.05.
Results
Between January 2017 and December 2018, 123 unique patients underwent planned OC, with 58 planned OC cycles occurring in 2017 and 65 in 2018. The mean age of patients who underwent a planned OC cycle in 2017 was 34.9 ± 4.2 years (range: 23–39 years) versus 35.2 ± 3.6 years (range: 26–44 years) in 2018 (p = 0.68, Table 1). The BMI of both groups was not significantly different (25.1 ± 5.8 vs. 24.4 ± 4.3 in 2017 and 2018, respectively). There was no significant difference in pretreatment hormone levels between 2017 and 2018 (day 3 FSH: 7.3 ± 2.9 vs. 7.1 ± 2.9, p = 0.68; day 3 E2: 41.8 ± 20.9 vs. 35.3 ± 16.6, p = 0.09; AMH: 2.9 ± 2.1 vs. 3.1 ± 2.1, p = 0.62; respectively, Table 1). Most patients reported relationship status as “single” in both years (73.2% and 81.3% in 2017 and 2018, respectively, p = 0.219). There were more nulligravid patients in 2018; however, no significant difference was found in gravidity and parity between the groups (Table 1). In summary, there were no statistically significant differences regarding patient demographics among groups.
Table 1.
Patient demographics
| 2017 | 2018 | p-value | |||
|---|---|---|---|---|---|
| (N = 58) | (N = 65) | ||||
| Avg/SD | Avg/SD | ||||
|
Age (y) Range Median |
35.0 (23–39) 36 |
4.2 |
35.2 (26–44) 34 |
3.6 | NS |
| BMI (kg/m2) | 25.1 | 5.8 | 24.4 | 4.3 | NS |
| Day 3 FSH (IU/mL) | 7.3 | 2.9 | 7.1 | 2.9 | NS |
| Day 3 E2 (pg/mL) | 41.8 | 20.9 | 35.3 | 16.6 | NS |
| AMH (ng/mL) | 2.9 | 2.1 | 3.1 | 2.1 | NS |
| %/N | %/N | ||||
| Gravidity | NS | ||||
| 0 | 67.2% | 39 | 81.5% | 53 | |
| 1 | 19.0% | 11 | 7.7% | 5 | |
| 2 + | 13.8% | 8 | 10.8% | 7 | |
| Parity | NS | ||||
| 0 | 93.1% | 54 | 98.5% | 64 | |
| 1 | 3.4% | 2 | 0.0% | 0 | |
| 2 + | 3.4% | 2 | 1.5% | 1 | |
| Relationship status | |||||
| Single | 73.2% | 41 | 81.3% | 52 | NS |
| Married | 17.9% | 10 | 15.6% | 10 | NS |
| Divorced | 8.9% | 5 | 3.1% | 2 | NS |
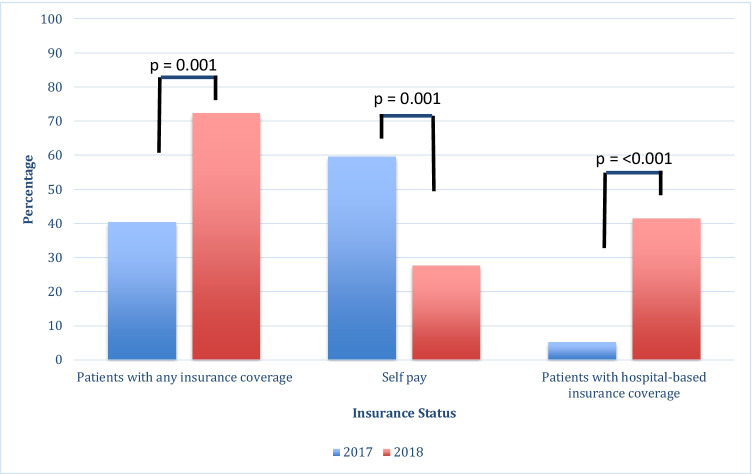
More patients had insurance coverage for planned OC in 2018 vs. 2017 (72.3% vs. 40.4%, p < 0.001), representing a 79% increase in insured patients. Accepted insurance providers between 2017 and 2018 remained the same, other than the addition of our hospital-based insurance plan in 2018. Of those with insurance coverage, 13% had hospital-based insurance coverage in 2017, while 57% had hospital-based coverage in 2018. From 2017 to 2018, the number of overall patients with hospital-based insurance coverage that underwent planned OC increased by a factor of 8 (5.3 to 41.5%, p < 0.001). In contrast, the proportion of self-pay patients significantly decreased from 2017 to 2018 (p = 0.001, Table 2/Fig. 1).
Table 2.
Employment and insurance status
| 2017 | 2018 | p-value | |||
|---|---|---|---|---|---|
| (N = 58) | (N = 65) | ||||
| %/N | %/N | ||||
| Insurance status | |||||
| Patients with any insurance coverage | 40.4% | 23 | 72.3% | 47 | < 0.001 |
| Self-pay patients | 59.6% | 34 | 27.7% | 18 | < 0.001 |
| Patients with hospital-based insurance coverage | 5.3% | 3 | 41.5% | 27 | < 0.001 |
Fig. 1.
Utilization of planned oocyte cryopreservation by insurance status before and after implementation of hospital-based insurance coverage of planned OC
From 2017 to 2018, the utilization of planned OC increased by 12%, with 58 cycles completed in 2017 and 75 cycles completed in 2018 (Table 3). There was no significant difference in planned OC outcomes by year, with the average number of oocytes retrieved (14.6 ± 8.0 vs. 16.2 ± 8.2 for 2017 and 2018, respectively; p = 0.17) and the average number of oocytes cryopreserved per cycle (11.6 ± 7.1 vs. 12.5 ± 7.1 for 2017 and 2018, respectively; p = 0.33) similar among groups (Table 3).
Table 3.
Planned OC cycle outcomes
| 2017 | 2018 | 95% CI | p-value | |||
|---|---|---|---|---|---|---|
| Avg/SD | Avg/SD | |||||
| Total number of women with planned OC cycles | 58 | 65 | ||||
| Oocytes retrieved/cycle | 14.6 | 8.0 | 16.2 | 8.2 | (1.19)–(6.54) | 0.17 |
| Oocytes cryopreserved/cycle | 11.6 | 7.1 | 12.5 | 7.1 | (1.81)–(5.24) | 0.33 |
Discussion
Our data suggests that institutional coverage of planned OC may be a factor in the increasing utilization of planned OC by reproductive-aged women. There was a statistically significant increase in the number and percentage of patients with insurance coverage for planned OC in 2018 compared to 2017, as well as an increase in the number of patients utilizing their insurance benefits. Specifically, employer-based insurance coverage for planned OC was associated with a significant increase in utilization by hospital-system employees.
Prior work related to this study has focused on large corporations and their motivations behind offering coverage of planned OC, rather than the impact insurance coverage of planned OC may have on usage of this technology by reproductive-aged women [15–17]. Our study is one of the first to evaluate the utilization of insurance coverage for planned OC among reproductive-aged women. The majority of patients utilizing planned OC in both years were nulligravid and single. We observed an increase in the percentage of nulligravid women utilizing planned oocyte cryopreservation once insurance coverage was offered (66% of patients in 2017 and 81.5% in 2018), although this finding did not reach statistical significance (p = 0.052). Additionally, the majority of patients in 2017 and in 2018 who used planned OC identified as single and likely assumed the financial burden of planned OC, whether through self pay or their own insurance coverage. One study by Mertes et al. presented an in-depth discussion of various financial options for planned OC and highlighted that full coverage, at least up to age 36, may be the preferred strategy as it allows women to freeze eggs at a younger age, wherein quality is higher, but disposable income may be lower [18].
As of January 1, 2020, New York State fertility insurance coverage laws mandate that in vitro fertilization be covered by health insurance provided in the large group market, and medically necessary fertility preservation be covered in a large group, small group, and individual markets [19]. Medically necessary fertility preservation includes “medical treatments for people facing iatrogenic infertility, that is, infertility caused by a medical intervention, such as radiation, medication, or surgery.” Planned OC is not included in this updated mandate. Stoop et al. argued that planned OC, or “oocyte banking for anticipated gamete exhaustion” should be considered medically necessary, as it is used to mitigate the impact of reproductive aging that leads to infertility later in life, and therefore should be considered preventative medicine [20]. Several studies have already been conducted highlighting the positive impact of removing barriers to access to medical egg freezing through cost reduction and universal insurance coverage [21–24].
A major strength of this study is that it uniquely underscores the impact that insurance coverage may have on planned OC utilization rates in just 1 year. It is the first to address the association between employer-based insurance coverage and utilization of planned OC. Additionally, our institution has offered planned OC as a fertility treatment since 2012, with the only major change from 2017 to 2018 being the addition of our hospital-based insurance coverage for employees. Importantly, no marketing was done to promote this new insurance benefit over the study’s time frame. Limitations to this study include the small sample size as well as the retrospective study design at a single fertility clinic, which may limit generalizability. The retrospective design is prone to selection bias and misclassification bias, specifically from missing or inaccurate data that may be unaccounted for. Additionally, only an association, not causation, can be determined. Only patients who actually underwent a cryopreservation cycle were included in our review. The number of patients who presented for a new patient visit but did not actually undergo planned OC could not be accurately obtained. Therefore, the focus of this present study was on the actual utilization of the procedure. While the sample size was limited, this study may serve as a pilot study for future research on this important topic. As more insurance companies and large employers are likely to add planned OC as a potential benefit for employees, it is valuable to consider how that may change the utilization of the technology.
In the year following the introduction of insurance coverage for planned OC at our institution, there was a 12% increase in the utilization of the procedure. The results of this study suggest that insurance coverage of planned OC may be an important factor in increasing access to, and utilization of this, technology. Legislation concerning insurance coverage of medically necessary fertility preservation services represents an important step forward in ensuring equal access to reproductive health care. As awareness of coverage increases and other employers begin to expand benefits, we anticipate that planned OC utilization rates will continue to rise among reproductive-aged women desiring future fertility.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arielle S. Yeshua, Email: ayeshua@northwell.edu
Baruch Abittan, Email: Babittan2@northwell.edu.
Liron Bar-El, Email: Lbarel1@northwell.edu.
Christine Mullin, Email: Cmullin1@northwell.edu.
Randi H. Goldman, Email: Rgoldman4@northwell.edu
References
- 1.OECD Family Database, SF2.3: mean age of mothers at first childbirth. 2019. Last updated: Available at: https://www.oecd.org/els/soc/SF_2_3_Age_mothers_childbirth.pdf
- 2.NCHS Pressroom - 2002 News Release - Mean age of mother.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 20 Aug. 2014, Available at: www.cdc.gov/nchs/pressroom/02news/ameriwomen.htm#:~:text=In%201970%20the%20average%20age,over%20the%20past%20three%20decades.).
- 3.Matthews TJ, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief. 2009;21:1–8. [PubMed] [Google Scholar]
- 4.Heck KE, Schoendorf KC, Ventura SJ, Kiely JL. Delayed childbearing by education level in the United States, 1969–1994. Maternal and child health journal. 1997;1(2):81–88. Availabel at: 10.1023/a:1026218322723 [DOI] [PubMed]
- 5.Hook EB. Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol. 1981;58(3):282–285. [PubMed] [Google Scholar]
- 6.Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ (Clinical research ed) 2000;320(7251):1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauer MV, Paulson RJ, Lobo RA. Pregnancy after age 50: application of oocyte donation to women after natural menopause. Lancet (London, England) 1993;341(8841):321–323. doi: 10.1016/0140-6736(93)90132-z. [DOI] [PubMed] [Google Scholar]
- 8.Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100(5):1343–1349. doi: 10.1016/j.fertnstert.2013.07.201. [DOI] [PubMed] [Google Scholar]
- 9.Cobo A, Pérez S, De los Santos MJ, Zulategui J, Domingo J, Remohí J. Effect of different cryopreservation protocols on the metaphase II spindle in human oocytes. Reprod Biomed Online. 2008;17(3):350–359. doi: 10.1016/s1472-6483(10)60218-0. [DOI] [PubMed] [Google Scholar]
- 10.Cobo A, Kuwayama M, Pérez S, Ruiz A, Pellicer A, Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89(6):1657–1664. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 11.Cobo A, Meseguer M, Remohí J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod (Oxford, England) 2010;25(9):2239–2246. doi: 10.1093/humrep/deq146. [DOI] [PubMed] [Google Scholar]
- 12.Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, Colamaria S, Sapienza F, Ubaldi F. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod (Oxford, England) 2010;25(1):66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parmegiani L, Cognigni GE, Bernardi S, Cuomo S, Ciampaglia W, Infante FE, Tabarelli de Fatis C, Arnone A, Maccarini AM, Filicori M. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reprod Biomed Online. 2011;23(4):505–512. doi: 10.1016/j.rbmo.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Ethics Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org, & Ethics Committee of the American Society for Reproductive Medicine Planned oocyte cryopreservation for women seeking to preserve future reproductive potential: an Ethics Committee opinion. Fertil Steril. 2018;110(6):1022–1028. doi: 10.1016/j.fertnstert.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Zoll M, Mertes H, Gupta J. Corporate giants provide fertility benefits: have they got it wrong? Eur J Obstet Gynecol Reprod Biol. 2015;195:A1–A2. doi: 10.1016/j.ejogrb.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Bennett J. Egg freezing: Facebook, Apple, Citibank and delaying childbearing. Time. 2014. https://time.com/3509930/company-paid-egg-freezing-will-be-the-great-equalizer/#:~:text=Company%2DPaid%20Egg%20Freezing%20Will%20Be%20the%20Great%20Equalizer,-Egg%20storage&text=Updated%20on%20October%2016%20at%2011%3A25%20am.&text=It%20was%20a%20free%20seminar,already%20be%20reduced%20by%20half
- 17.Mertes H. Does company-sponsored egg freezing promote or confine women’s reproductive autonomy? J Assist Reprod Genet. 2015;32(8):1205–1209. doi: 10.1007/s10815-015-0500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mertes H, Pennings G. Elective oocyte cryopreservation: who should pay? Human Reprod (Oxford, England) 2012;27(1):9–13. doi: 10.1093/humrep/der364. [DOI] [PubMed] [Google Scholar]
- 19.NY State Senate Bill S719. NY State Senate. (2020, January 9). https://www.nysenate.gov/legislation/bills/2019/s719.
- 20.Stoop D, van der Veen F, Deneyer M, Nekkebroeck J, Tournaye H. Oocyte banking for anticipated gamete exhaustion (AGE) is a preventive intervention, neither social nor nonmedical. Reprod Biomed Online. 2014;28(5):548–551. doi: 10.1016/j.rbmo.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Inhorn MC, Birenbaum-Carmeli D, Westphal LM, Doyle J, Gleicher N, Meirow D, Raanani H, Dirnfeld M, Patrizio P. Medical egg freezing: how cost and lack of insurance cover impact women and their families. Reprod Biomed Soc. 2018;5:82–92. doi: 10.1016/j.rbms.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn GP, Vadaparampil ST, Bell-Ellison BA, Gwede CK, Albrecht TL (2008). Patient-physician communication barriers regarding fertility preservation among newly diagnosed cancer patients. Soc Sci Med 1982, 66(3), 784–789. 10.1016/j.socscimed.2007.09.013 [DOI] [PubMed]
- 23.Ajala T, Rafi J, Larsen-Disney P, Howell R. Fertility preservation for cancer patients: a review. Obstet Gynecol Int. 2010;2010:160386. doi: 10.1155/2010/160386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rashedi AS, de Roo SF, Ataman LM, Edmonds ME, Silva AA, Scarella A, Horbaczewska A, Anazodo A, Arvas A, Ramalho de Carvalho B, Sartorio C, Beerendonk C, Diaz-Garcia C, Suh CS, Melo C, Yding Andersen C, Motta E, Greenblatt EM, Van Moer E, Zand E, Woodruff TK. Survey of fertility preservation options available to patients with cancer around the globe. JCO Glob Oncol. 2020, 6, JGO.2016.008144. 10.1200/JGO.2016.008144 [DOI] [PMC free article] [PubMed]