Abstract
We describe the multimodal management of a patient with proliferative diabetic retinopathy and diabetic macular edema associated with active acromegaly. A 61-year-old Japanese female who had had type 2 diabetic mellitus for > 10 years complained of deteriorated eyesight. She had distinct acromegalic features, and her visual acuity was 0.05 (right) and 0.4 (left) because of sub-capsular cataracts and proliferative diabetic retinopathy with macular edema. Anti-vascular endothelial growth factor treatments, cataract surgeries and retinal direct laser photocoagulation were performed together with gradual glycemic control with basal insulin to prevent worsening of the visual impairment. She was given an injection of a long-acting somatostatin analog (octreotide LAR) and began taking three bolus mealtime insulin shots with basal insulin beginning 1 month before undergoing a trans-sphenoidal adenomectomy. After this successful surgery, her blood glucose levels immediately decreased, and the rapid-acting insulin at mealtimes was discontinued with the observation of normal growth hormone and insulin-like growth factor (IGF)-1 levels, suggesting that her acromegaly was in remission. Her visual acuity improved without a worsening of diabetic retinopathy. Since the increased IGF-1 production in systemic circulation and local vitreous fluids may be one of the aggravating factors for diabetic retinopathy, our patient's acromegaly complicated with severe retinopathy presented an opportunity for multimodal management in close collaboration with an ophthalmologist, neurosurgeon, and endocrinologist. Our literature review revealed that the estimated prevalence of diabetic retinopathy in cases of acromegaly associated with diabetes mellitus is 12.5–42.9%.
Keywords: Acromegaly, Proliferative diabetic retinopathy, Growth hormone, Insulin-like growth factor 1, Vascular endothelial growth factor
Introduction
Acromegaly is a rare chronic disease characterized by the hypersecretion of growth hormone (GH) from a pituitary adenoma and an elevated level of insulin-like growth factor-1 (IGF-1) [1]. Diabetes mellitus (DM) is frequently associated with acromegaly, but diabetic complications, such as retinopathy and maculopathy (macular edema), are less frequently encountered in patients with acromegaly [2–6]. Severe forms of diabetic retinopathy (DR) have occasionally been reported, suggesting the causative roles of GH and IGF-1 in the progression of DR [7–9]. We encountered a patient with active acromegaly complicated with severe proliferative DR with diabetic macular edema (DME), and we report the details of the patient’s multimodal management, including anti-vascular endothelial growth factor (VEGF) therapy, surgeries for cataract, retinal direct laser photocoagulation, a pituitary adenomectomy with a preoperative long-acting somatostatin analog and careful glycemic control.
Case report
The patient was a 61-year-old Japanese woman who had DM for > 10 years. At the age of 53, her fasting blood glucose and glycosylated hemoglobin (HbA1c) levels were 202 mg/dL and 13.3%. Her fasting serum C-peptide (CPR) and immuno-reactive insulin (IRI) levels were 0.97 ng/mL (CPR-index 0.48) and 5.7 μU/mL (HOMA-IR 2.84), respectively. An ophthalmic examination revealed no diabetic retinopathy at that time. She had been treated with metformin (1500 mg/day), teneligliptin (20 mg/day), pioglitazone (15 mg/day), and insulin glargine (9 U/day). She had stopped taking her medication and seeing her physician at the age of 58, when her HbA1c level was 10.3%.
She visited our hospital because of bilateral visual disturbances. She had a clear family history of DM (her mother and two younger brothers) but no endocrinological disease. She had given birth to two very large infants (birth weights 4.040 g and 4.010 g) at the ages of 26 and 33, respectively, but we have no information as to whether she had gestational diabetes.
At her admission, the following data were obtained: height, 154.0 cm; body weight, 55.7 kg; body mass index, 23.4 kg/m2; blood pressure, 113/69 mmHg. Her body weight had not changed since the age of 58. She exhibited enlarged hands and feet, and distinct acromegalic features, including her facial appearance. Her fasting plasma glucose was 296 mg/dL, and her HbA1c level was 12.5%. Her fasting serum CPR level was 1.77 ng/mL (CPR-index 0.6), her IRI level was 7.6 μIU/mL (HOMA-IR 5.55), and her anti-glutamic acid decarboxylase antibody was negative. An X-ray examination revealed thickening of the cranium and tufting of the terminal phalanges. The basal serum GH value was 32.3 ng/mL, and the IGF-1 level was 513 ng/mL (+ 6.2 SD for a 61-year-old female; reference range 69–198 ng/mL).
Brain magnetic resonance imaging (MRI) showed a 10-mm-diameter pituitary adenoma extending inferiorly into the sphenoid sinus and into the right cavernous sinus: Knosp grade 2. The patient's visual fields were full, but her visual acuity was 0.05 in the right eye and 0.4 in the left. There were posterior sub-capsular cataracts in both eyes and vitreous hemorrhage in the right eye. The fundus examination showed proliferative DR in both eyes and macular edema in the right eye (Fig. 1). Optical coherence tomography (OCT) showed serous retinal detachment with increased central foveal thickness in the right eye (Fig. 1). The patient's deep tendon reflex responses had diminished, and the motor conduction velocities of the right and left tibial nerves were slightly diminished to 39.4 m/s and 41.2 m/s, respectively (reference ranges 49.4 ± 5.2 m/s). The urinary albumin excretion was 155.1 mg/g creatinine. The diagnosis was type 2 DM complicated with diabetic proliferative retinopathy, nephropathy (microalbuminuria), and multiple peripheral neuropathies, and coexisting active acromegaly due to the pituitary adenoma.
Fig. 1.
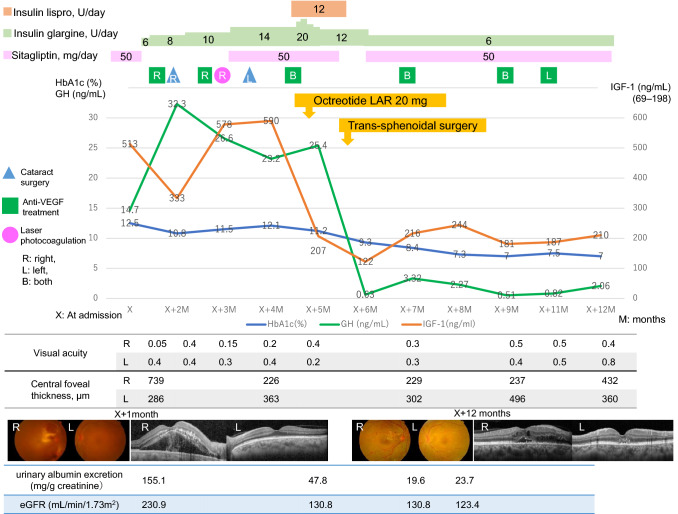
Clinical course of the patient with acromegaly complicated with proliferative diabetic retinopathy. The time courses of the levels of IGF-1 (orange line, scale on the right axis; reference range 69–198 ng/mL), HbA1c (blue line, scale on the left axis; reference range 4.6–6.2%) and GH (green line, scale on the left axis; reference range 0.13–9.88 ng/mL) are depicted. Fundus photographs at 1 month after admission show retinal hemorrhages in both eyes and macular edema with hard exudates in the right eye. The fundus photographs are slightly hazy due to the cataracts and vitreous hemorrhage. The fundus photographs at 12 months post-admission show the absorption of macular edema with mild hard exudates. Optical coherence tomography (OCT) images at 1 month post-admission show serous retinal detachment with increased central foveal thickness in the right eye. OCT images at 12 months post-admission show the resolution of the macular edema and decreased central foveal thickness. The timing of the surgeries for cataracts (triangles), anti-VEGF treatments (squares) for diabetic macular edema, and retinal direct laser photocoagulation (circles) is shown
The patient's clinical course with multimodal management is depicted in Fig. 1. For glycemic control, a low dose of basal insulin glargine was started; the dose was gradually increased to avoid inducing hypoglycemia. At 1 month before the patient's scheduled pituitary surgery, three bolus mealtime insulin lispro injections were added to the basal insulin to sustain good glycemic control. Intraocular administration of aflibercept, an anti-VEGF antibody agent, for the patient's DME, cataract surgeries and retinal direct laser photocoagulation were performed to prevent the worsening of her DR during glycemic control and to improve the visual impairment. A long-acting somatostatin analog, octreotide LAR (long-acting release: 20 mg) was administered intramuscularly once to reduce the GH hypersecretion and improve perioperative metabolic derangements. Multiple daily insulin injection was started at the same time as octreotide LAR, and the fasting and postprandial blood glucose levels decreased to 83 mg/dL and 156 mg/dL, respectively. The patient’s basal GH and IGF-1 levels were 25.4 ng/mL and 207 ng/mL (+ 2.2 SD), respectively, 5 days after octreotide LAR administration. Due to concern about a potential change in consistency of the pituitary mass, the neurosurgeon chose to administer octreotide LAR only once.
At 1 month after the octreotide LAR injection, trans-sphenoidal surgery was performed. At surgery, right cavernous sinus invasion of the adenoma was confirmed. The pathological findings showed a densely granulated somatotroph adenoma. The patient's blood glucose levels decreased further after the surgery, and the mealtime insulin was stopped. At 3 months post surgery, the patient's basal GH and IGF-1 levels were normalized to 0.51 ng/mL and 181 ng/mL (+ 1.8 SD), respectively. The nadir GH level during 75 g oral glucose tolerance test was 0.54 ng/mL. Her acromegaly was considered to be in remission. Her visual acuities improved (0.4 for the right eye and 0.8 for the left eye) 7 months after surgical treatment. A fundus examination showed that mild hard exudate remained, but the macular edema was alleviated (Fig. 1). OCT showed that the serous retinal detachment was absorbed and the central foveal thickness had decreased (Fig. 1). Her HbA1c levels had been stabilized below 7% by low doses of insulin glargine and sitagliptin 50 mg/day after surgery. Her fasting plasma glucose was 106 mg/dL, and her fasting serum CPR and IRI levels were 1.42 ng/mL (CPR-index 1.34) and 4.9 μIU/mL (HOMA-IR 1.28), respectively, showing the improvement of her remaining beta cell function and insulin resistance. Her DME still requires intraocular injections of anti-VEGF, but her DR has not been worsened. Her urinary albumin excretion was markedly decreased to 19.6 mg/g creatinine.
Discussion
We have described a case of active acromegaly with DM complicated with proliferative DR. The facts that the patient had a clear family history of DM and had twice given birth to a large infant suggest that she had type 2 DM. Her retinopathy had progressed to the advanced stage over the recent 3 years, while her nephropathy was mild in degree and improved by glycemic control, and her peripheral neuropathy was stable. We conjecture that GH hypersecretion may have induced insulin resistance and worsened the glucose metabolism in our patient. The multivariate analyses performed to identify risk factors for impaired glucose tolerance associated with acromegaly showed that family history of diabetes and IGF-1 levels were associated with hyperglycemia, and body mass index and IGF-1 predicted insulin resistance [10]. The single injection of octreotide LAR together with intensive insulin therapy decreased her GH and IGF-1 levels and stabilized her glycemic control before the trans-sphenoidal adenomectomy. The rapid improvement in glycemic control after pituitary surgery may have been due to the decrease in GH/IGF-1. More time may be necessary to alleviate her proliferative DR. It would be difficult to estimate to what extent the excess GH/IGF-1 was involved in the long-term persistence of hyperglycemia in our patient.
The prevalence of DR in acromegaly has been reported to be anywhere from 2.2 to 20% [2–6]. However, acromegalic patients have shown various degrees of metabolic derangement (DM), impaired glucose tolerance (IGT), and normal glucose tolerance (NGT). We conducted a literature search and re-analyzed the reported data. As shown in Table 1, the calculated prevalence of DR in acromegaly associated with DM was 12.5–42.9%, and the prevalence in acromegaly with DM/IGT was 3.8–30%. Macular abnormalities had been reported in 4 of 46 cases of acromegaly with DM [6]. The numbers of reported cases of proliferative and non-proliferative DR were 9 and 12 cases, respectively. Persistent hyperglycemia in patients with acromegaly may explain the high prevalence of DR. The severe forms of DR [7–9] may be linked to the higher prevalence of proliferative DR in acromegaly associated with DM, or this may simply be the result of publication bias. However, these case reports and cross-sectional prevalence studies do not provide details on the duration of acromegaly or diabetes, the degree of glycemic control, or other diabetic complications. Therefore, a prospective epidemiological study will be needed to determine whether DR in simple type 2 DM differs from DR in DM associated with acromegaly in terms of the pathophysiology and precise prevalence.
Table 1.
Prevalence of diabetic retinopathy (DR) in acromegaly associated with diabetes mellitus (DM) or diabetes mellitus + impaired glucose tolerance (DM + IGT)
| References | Acromegaly (n) Total (DM/IGT/NGT) |
DR (n) Total (PDR/NPDR) |
DR (%) in DM/DM + IGT |
Macular abn n (%) |
|---|---|---|---|---|
| Amemiya et al. [2] | 15 (10/0/5) | 3 (0/3) | 30.0%/30.0% | n.d |
| Ballintine et al. [3] | 44 (6/20/18) | 1 (0/1) | 16.7%/3.8% | n.d |
| Azzoug et al. [4] | 40 (40/–/–) | 5 (2/3) | 12.5%/– | n.d |
| Wu et al. [5] | 43 (16/11/16) | 4 (4/0) | 18.8%/14.8% | n.d |
| Fuchtbauer et al. [6] | G 26 (7/6/13) | 3 (0/3) | 42.9%/23.1% | 2 (7.7%) |
| R 39 (39/–/–) | 5 (3/2) | 12.8%/– | 2 (5.1%) | |
| G + R 65 (46/6/13) | 8 (3/5) | 17.4%/15.4% | 4 (6.2%) |
abn abnormalities, DM diabetes mellitus, G Gothenberg cohort, IGT impaired glucose tolerance, n.d. not determined, NGT normal glucose tolerance, NPDR non-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy, R Rotterdam cohort
The pathophysiological roles of GH, IGF-1, and somatostatin in DR have been extensively reviewed [11, 12]. DR-like macular edema was reported as a rare complication of GH therapy in non-diabetic patients [13, 14]. Hypoxia-stimulated retinal neovascularization is inhibited in mice expressing a GH antagonist gene or in normal mice given an inhibitor of GH secretion [15]. GH and IGF-1 modulate the function of retinal endothelial precursor cells and drive retinal angiogenesis in response to hypoxia. VEGF is an essential cytokine in hypoxia-induced proliferative retinopathy, and IGF-1 may accompany VEGF to stimulate new vessel growth [16]. Treatment of ischemia-induced mice with an IGF-1 receptor antagonist has been shown to prevent retinal neovascularization [17]. IGF-1 can also disrupt the blood-retina barrier and increase retinal vascular permeability. Somatostatin is known to be anti-angiogenic and neuroprotective for the retina [18], and two pilot studies using subcutaneous octreotide slowed the progression of DR, decreased the need for laser treatment, and reduced the occurrence of vitreous hemorrhages [19, 20]. However, a randomized double-blinded placebo-controlled trial using octreotide LAR was unable to confirm these findings in patients with moderate-to-severe non-proliferative DR to low-risk proliferative DR [21, 22]. Therefore, preoperative octreotide LAR therapy may not have modulated DR in our case.
Vitreous IGF-1 may be derived from diffusion of serum IGF-1 or from local production, and IGF-1 levels in the vitreous cavity were reported to be elevated in patients with proliferative DR compared to those in non-diabetics [23, 24]. An even higher level of vitreous IGF-1 was reported in a patient with acromegaly with severe proliferative DR [7]. In addition, a high IGF-1 level was observed in the sub-retinal fluid of a diabetic patient with acromegaly who underwent surgery for retinal detachment [25]. Normoglycemic/normoinsulinemic transgenic mice overexpressing IGF-1 in the retina developed most alterations seen in human diabetic eye disease [26]. A paracrine effect of IGF-1 in the retina initiated vascular alterations that progressed from non-proliferative to proliferative retinopathy and finally retinal detachment. We may speculate that an excessive production of local IGF-1 and/or the breakdown of the blood–retina barrier (which results in the diffusion of serum IGF-1 into the vitreous cavity) may occur in acromegalic patients who experience an accelerated progression of DR. Our patient needed to continue anti-VEGF therapy even though GH/IGF-1 had been decreased to normal range after remission by the successful surgery. Since DR is associated with multiple factors, such as long-term glucotoxicities, oxidative stress, inflammation, cytokines and growth factors [11, 16], GH/IGF-1 is considered to one of the factors affecting non-proliferative and proliferative DR.
Our patient had advanced diabetic complications due to the intervals required for the diagnosis of acromegaly and her long-term hyperglycemic condition, including the treatment interruption period. Our patient's pituitary surgery resulted in a complete remission of her acromegaly and maintained good glycemic control without worsening her DR. The present case of acromegaly represents a model of multimodal management in close collaboration with an ophthalmologist, neurosurgeon, and endocrinologist.
Acknowledgements
The authors gratefully thank Dr Akimichi Saito from Department of Cardiovascular Medicine, Health Sciences University of Hokkaido Hospital, for providing clinical information.
Declarations
Conflict of interest
None of the authors have any potential conflicts of interest associated with this research.
Ethical standard
This article does not contain any studies with human or animal subjects performed by any of the authors. The identity of the patient has been protected. The patient provided informed consent for this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM, et al. Acromegaly. Nat Rev Dis Primers. 2019;5(1):20. doi: 10.1038/s41572-019-0071-6. [DOI] [PubMed] [Google Scholar]
- 2.Amemiya T, Toibana M, Hashimoto M, Oseko F, Imura H. Diabetic retinopathy in acromegaly. Ophthalmologica. 1978;176(2):74–80. doi: 10.1159/000308696. [DOI] [PubMed] [Google Scholar]
- 3.Ballintine EJ, Foxman S, Gorden P, Roth J. Rarity of diabetic retinopathy in patients with acromegaly. Arch Intern Med. 1981;141(12):1625–1627. doi: 10.1001/archinte.1981.00340130069016. [DOI] [PubMed] [Google Scholar]
- 4.Azzoug S, Chentli F. Diabetic retinopathy in acromegaly. Indian J Endocrinol Metab. 2014;18(3):407–409. doi: 10.4103/2230-8210.131207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu TE, Chen HS. Increased prevalence of proliferative retinopathy in patients with acromegaly. J Chin Med Assoc. 2018;81(3):230–235. doi: 10.1016/j.jcma.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Füchtbauer L, Olsson DS, Coopmans EC, Bengtsson BÅ, Norrman LL, Neggers SJCMM, et al. Increased number of retinal vessels in acromegaly. Eur J Endocrinol. 2020;182(3):293–302. doi: 10.1530/EJE-19-0778. [DOI] [PubMed] [Google Scholar]
- 7.Inokuchi N, Ikeda T, Yasuda F, Shirai S, Uchinori Y. Severe proliferative diabetic retinopathy associated with acromegaly. Br J Ophthalmol. 1999;83(5):629–630. doi: 10.1136/bjo.83.5.628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran HA, Petrovsky N, Field AJ. Severe diabetic retinopathy: a rare complication of acromegaly. Intern Med J. 2002;32(1–2):52–54. doi: 10.1046/j.1445-5994.2002.00149.x. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra C. Proliferative diabetic retinopathy in acromegaly. Oman J Ophthalmol. 2010;3(2):96–97. doi: 10.4103/0974-620X.64237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexopoulou O, Bex M, Kamenicky P, Mvoula AB, Chanson P, Maiter D. Prevalence and risk factors of impaired glucose tolerance and diabetes mellitus at diagnosis of acromegaly: a study in 148 patients. Pituitary. 2014;17(1):81–89. doi: 10.1007/s11102-013-0471-7. [DOI] [PubMed] [Google Scholar]
- 11.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson-Berka JL, Wraight C, Werther G. The role of growth hormone, insulin-like growth factor and somatostatin in diabetic retinopathy. Curr Med Chem. 2006;13(27):3307–3317. doi: 10.2174/092986706778773086. [DOI] [PubMed] [Google Scholar]
- 13.Koller EA, Green L, Gertner JM, Bost M, Malozowski SN. Retinal changes mimicking diabetic retinopathy in two nondiabetic, growth hormone-treated patients. J Clin Endocrinol Metab. 1998;83(7):2380–2383. doi: 10.1210/jcem.83.7.4953. [DOI] [PubMed] [Google Scholar]
- 14.Paterson WF, Kelly B, Newman W, Savage MO, Camacho-Hubner C, Dutton GN, et al. Deterioration of visual acuity associated with growth hormone therapy in a child with extreme short stature and high hypermetropia. Horm Res. 2007;67(2):67–72. doi: 10.1159/000096088. [DOI] [PubMed] [Google Scholar]
- 15.Smith LE, Kopchick JJ, Chen W, Knapp J, Kinose F, Daley D, et al. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276(5319):1706–1709. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- 16.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5(12):1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 18.Vasilaki A, Thermos K. Somatostatin analogues as therapeutics in retinal disease. Pharmacol Ther. 2009;122(3):324–333. doi: 10.1016/j.pharmthera.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Grant MB, Mames RN, Fitzgerald C, Hazariwala KM, Cooper-DeHoff R, Caballero S, et al. The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy: a randomized controlled study. Diabetes Care. 2000;23(4):504–509. doi: 10.2337/diacare.23.4.504. [DOI] [PubMed] [Google Scholar]
- 20.Boehm BO, Lang GK, Jehle PM, Feldman B, Lang GE. Octreotide reduces vitreous hemorrhage and loss of visual acuity risk in patients with high-risk proliferative diabetic retinopathy. Horm Metab Res. 2001;33(5):300–306. doi: 10.1055/s-2001-15282. [DOI] [PubMed] [Google Scholar]
- 21.Palii SS, Caballero S, Jr, Shapiro G, Grant MB. Medical treatment of diabetic retinopathy with somatostatin analogues. Expert Opin Investig Drugs. 2007;16(1):73–82. doi: 10.1517/13543784.16.1.73. [DOI] [PubMed] [Google Scholar]
- 22.Simó-Servat O, Hernández C, Simó R. Somatostatin and diabetic retinopathy: an evolving story. Endocrine. 2018;60(1):1–3. doi: 10.1007/s12020-018-1561-0. [DOI] [PubMed] [Google Scholar]
- 23.Grant M, Russell B, Fitzgerald C, Merimee TJ. Insulin-like growth factors in vitreous. Studies in control and diabetic subjects with neovascularization. Diabetes. 1986;35(4):416–420. doi: 10.2337/diab.35.4.416. [DOI] [PubMed] [Google Scholar]
- 24.Burgos R, Mateo C, Cantón A, Hernández C, Mesa J, Simó R. Vitreous levels of IGF-I, IGF binding protein 1, and IGF binding protein 3 in proliferative diabetic retinopathy: a case-control study. Diabetes Care. 2000;23(1):80–83. doi: 10.2337/diacare.23.1.80. [DOI] [PubMed] [Google Scholar]
- 25.van Setten G, Brismar K, Algvere P. Elevated intraocular levels of insulin-like growth factor I in a diabetic patient with acromegaly. Orbit. 2002;21(2):161–167. doi: 10.1076/orbi.21.2.161.7186. [DOI] [PubMed] [Google Scholar]
- 26.Ruberte J, Ayuso E, Navarro M, Carretero A, Nacher V, Haurigot V, et al. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J Clin Investig. 2004;113(8):1149–1157. doi: 10.1172/JCI19478. [DOI] [PMC free article] [PubMed] [Google Scholar]