Abstract
The sequence of events for secreting insulin in response to glucose in pancreatic β-cells is termed “stimulus-secretion coupling”. The core of stimulus-secretion coupling is a process which generates electrical activity in response to glucose uptake and causes Ca2+ oscillation for triggering exocytosis of insulin-containing secretory granules. Prior to exocytosis, the secretory granules are mobilized and docked to the plasma membrane and primed for fusion with the plasma membrane. Together with the final fusion with the plasma membrane, these steps are named the exocytosis process of insulin secretion. The steps involved in the exocytosis process are crucial for insulin release from β-cells and considered indispensable for glucose homeostasis. We recently confirmed a signature of defective exocytosis process in human islets and β-cells of obese donors with type 2 diabetes (T2D). Furthermore, cyclic AMP (cAMP) potentiates glucose-stimulated insulin secretion through mechanisms including accelerating the exocytosis process. In this mini-review, we aimed to organize essential knowledge of the secretory granule exocytosis and its amplification by cAMP. Then, we suggest the fatty acid translocase CD36 as a predisposition in β-cells for causing defective exocytosis, which is considered a pathogenesis of T2D in relation to obesity. Finally, we propose potential therapeutics of the defective exocytosis based on a CD36-neutralizing antibody and on Apolipoprotein A-I (ApoA-I), for improving β-cell function in T2D.
Keywords: Apolipoprotein A-I, β-Cell, CD36, Diabetes, Exocytosis, Insulin secretion, Type 2 diabetes
Introduction
Diabetes is a heterogenous metabolic disorder which is characterized by chronic hyperglycemia as a consequence of impaired insulin secretion and/or abnormal insulin action. Recently, adult-onset diabetes has been classified into five subtypes, i.e., one autoimmune and four non-autoimmune clusters, according to six clinical parameters (GAD autoantibodies, age at diabetes onset, HbA1c, BMI, and measures of insulin resistance and insulin secretion) [1]. Such a cluster analysis has been applied for individuals at a high risk of type 2 diabetes (T2D) and six clusters were identified according to variables of oral glucose tolerance tests, MRI-measured body fat distribution, liver fat content, and genetic risk [2]. The findings of two stratification studies point towards different underlying pathogenesis among T2D where over 90% of adults with diabetes are currently classified [3]. In general, the incidence of T2D is strongly associated with genetic factors but also several environmental factors, e.g., sedentary lifestyle and western diet, causing insulin resistance in peripheral tissues through obesity. Recent genome wide association studies (GWAS) have revealed that gene variants correlated with increased risk of T2D are associated with pancreatic islet cell function [4, 5], suggesting that predisposed individuals are at higher risk of developing T2D when exposed to the harmful environmental factors. Accordingly, β-cell dysfunction, especially impaired insulin secretion, is considered to be central to T2D etiology. Therefore, to know the regulatory mechanism of insulin secretion and to dissect molecular mechanisms disrupting the regulation are of great importance not only for better understanding T2D pathophysiology but also for the exploration of novel therapeutic strategies.
The sequence of events for secreting insulin in response to glucose in pancreatic β-cells is termed “stimulus-secretion coupling”. The final step of this process is calcium-dependent exocytosis, i.e., the fusion of the insulin-containing secretory granules with the plasma membrane and the release of insulin into the blood stream. Recent data point towards an involvement of defective exocytosis in the pathogenesis of T2D. Specifically, exocytosis, measured as an increase in membrane capacitance or by TIRF imaging, is reduced in human β-cells from T2D donors [5–7]. Moreover, expression levels of several genes involved in the exocytosis process are significantly reduced in islets from T2D donors compared to those from non-T2D (ND) donors [6, 8].
This mini-review aims to organize essential knowledge of the secretory granule exocytosis process, a crucial step in insulin release from the β-cell. Based on the knowledge, we put forward the hypothesis that defective exocytosis can be a pathogenesis of T2D in relation to obesity, due to that facilitated uptake of fatty acids (FAs) through CD36 disturbs the exocytosis process. Finally, after introducing our recent findings about effects of a CD36 antibody and of Apolipoprotein A-I (ApoA-I) on the exocytosis process, we briefly discuss the potential therapeutics for improving β-cell function, especially defective exocytosis, in T2D.
Islets of Langerhans and β-cells
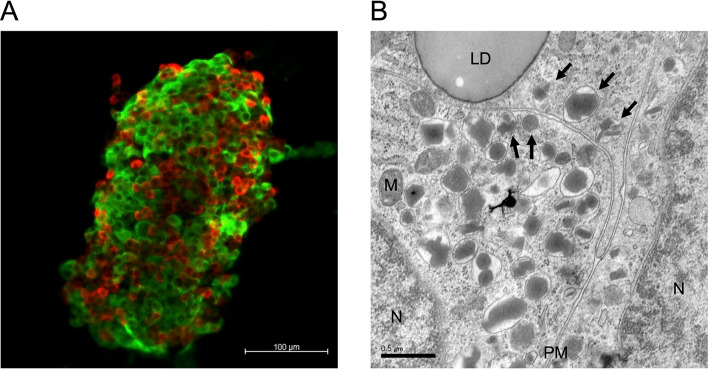
The islets constitute the endocrine part of pancreas and are clusters of ~ 2000 cells spread out in the exocrine pancreas. Within the islets, there are five major endocrine cell types, including the insulin-secreting β-cell, the glucagon-secreting α-cell, the somatostatin-secreting δ-cell, the pancreatic polypeptide (PP)-producing cell, and ghrelin-secreting ε-cell. Human islets contain 50–60% β-cells, 30–40% α-cells, 10% δ-cells and a small number of PP- and ε-cells [9]. The proportion of β-cells in human islets is smaller than that in rodent islets (60–80%) [10]. The human β-cells are scattered throughout the islet (Fig. 1A), which is different to rodents where the β-cells form a predominant central core of the islet surrounded by α- and δ-cells [11].
Fig. 1.
A representative confocal micrograph of human pancreatic islets of Langerhans immunolabelled for glucagon (red) and insulin (green) (A) and a representative electron micrograph of human β-cells (B). The secretory granules, pointed with black arrows, are considered docked or almost docked with the plasma membrane. LD, lipid droplet; M, mitochondrion; N, nucleus; PM, plasma membrane
Insulin biosynthesis
Insulin is synthesized exclusively in β-cells and stored in secretory granules. The insulin gene encodes an insulin-precursor preproinsulin, which is a single chain molecule containing a N-terminal signal peptide with 110 amino acids. After the translation of the mRNA, preproinsulin translocates across the rough endoplasmic reticulum (ER) membrane to the ER lumen where it is cleaved by a signal peptidase to form proinsulin [12]. Proinsulin maturation in the ER involves folding and formation of three disulfide bonds to get the native structure, followed by transportation to the Golgi complex and a coordinated assembly of a secretory granule. Once departed from the Golgi complex, the granules need to be translocated along microtubules to the plasma membrane where they can undergo the process of exocytosis. Therefore, β-cells are equipped with a dense actin-myosin web beneath the plasma membrane [13, 14].
The mature proinsulin consists of A and B chains, and the C-peptide. The proinsulin is cleaved in the granular lumen by the prohormone convertase enzymes, PC1/3 and PC2, to yield insulin (with A and B chains covalently linked by disulfide bonds) and C-peptide [15]. This step requires a low pH and the entry of H+ to the granular lumen is thus vital for optimal conversion [16]. In this process, Cl− needs to counteract the electrostatic gradient across the granule membrane formed by the entry of H+ [17]. Insulin and C-peptide are stored together in the mature secretory granules along with small amounts of intact proinsulin and intermediate products, as described in the section below. The mature insulin can be stored in the granules for several days before being released or degraded by a process called crinophagy, i.e., the intracellular destruction of the secretory granules in the lysosomes [18].
Insulin-containing secretory granules
Every β-cell contains 9,000–13,000 secretory granules with diameters of ~ 350 nm (Fig. 1B) [19, 20]. Mature secretory granules contain insulin molecules as a crystalline form bound to zinc (Zn2-insulin6 crystal), which makes the so called “electron-dense core” of the granular lumen detectable by Transmission electron microscopy (TEM) [20]. A single granule contains ~ 1.6 amol of insulin (8 fg or 106 molecules of insulin) [21]. Along with insulin, C-peptide, and a small amount of intact proinsulin, the granules also contain ~ 50 different polypeptides such as islet amyloid polypeptide (IAPP) and chromogranin A. A number of low-molecular weight compounds such as ATP, GABA, serotonin and glutamate, and high concentrations of metal ions including Zn2+ and Ca2+ are also stored in the granules [12, 22, 23]. The ultrastructure of the granules in rodent β-cells is round and homogeneous with the electron-dense core of insulin surrounded by a halo of less dense mantle in a phospholipid bilayer envelope, while granules in human β-cells are more irregular with a variety of core shapes (Fig. 1B) [24].
Secretory granule exocytosis
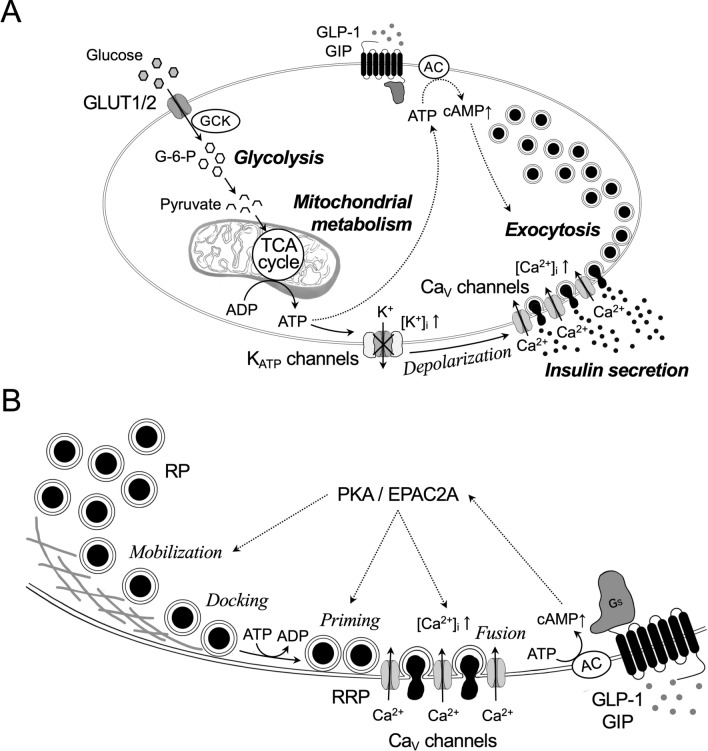
The secretory granules are released from β-cells in response to the main stimuli, glucose. During the process, uptake of glucose by β-cells and glucose metabolism lead to the generation of ATP, closure of ATP-sensitive K+ (KATP) channels, membrane depolarization, opening of voltage-dependent Ca2+ (CaV) channels, and finally exocytosis for insulin release from the secretary granules (Fig. 2A). These components are shared by rodent and human β-cells. In human β-cells, opening of voltage-dependent Na+ channels following after the closure of KATP channels is important for further depolarization to open CaV channels [25]. The final step, exocytosis, is principally dependent on Ca2+ influx through the L-type and P/Q-type CaV channels in rodent and human β-cells, respectively [26]. The process of exocytosis includes following four steps: (1) translocation of insulin granules to the plasma membrane (mobilization), (2) docking of insulin granules at the plasma membrane (docking), (3) priming of insulin granules for making them release-compatible (priming), and (4) actual fusion with the plasma membrane and extracellular release of the granular content (fusion) (Fig. 2B) [22]. Notably, the priming step is tightly regulated by Ca2+, ATP, and temperature, and is the rate-limiting step in exocytosis [27–29].
Fig. 2.
Schematic presentation of stimulus-secretion coupling (A), and secretory granule exocytosis and its amplification by cyclic AMP (cAMP) in β-cells (B). A: Glucose transporters (primarily, GLUT1 and GLUT2) facilitate diffusion of glucose into β-cells and start the stimulus-secretion coupling process. Glucose is phosphorylated by glucokinase (GCK) to generate glucose-6-phosphate (G-6-P), followed by glycolysis to yield pyruvate. Pyruvate is then actively transported and metabolized in the mitochondria through the tricarboxylic acid (TCA) cycle for yielding ATP by the stimulation of the respiratory chain. Generation of ATP at the expense of ADP leads to the closure of the ATP-sensitive K+ (KATP) channels, membrane depolarization, opening of voltage-dependent Ca2+ (CaV) channels, and finally exocytosis of secretory granules and release of insulin. Cyclic AMP (cAMP) potentiates insulin secretion through mechanisms including accelerating the exocytosis process. B: Majority of secretory granules belongs to the reserve pool (RP). A small part of secretory granules exists in the readily releasable pool (RRP). The secretory granules must undergo physical translocation (mobilization) from the RP for docking with the plasma membrane and attain release competence by priming. Fusion is principally dependent on Ca2+ influx through voltage dependent Ca2+ (CaV) channels. Enhancers like incretins (GLP-1 and GIP) bind to G-protein (Gs)-coupled receptors and generate cAMP from ATP by activating adenylyl cyclase (AC). cAMP potentiates glucose-stimulated insulin secretion by accelerating the exocytosis process through PKA-dependent and PKA-independent mechanism (EPAC2A)
Biphasic pattern of exocytosis
Exocytosis in β-cells measured by capacitance recordings upon rapid increase in intracellular Ca2+ is characterized as a biphasic pattern which consists of a rapid ATP-independent component followed by sustained more prolonged component [20]. The separable phases of exocytosis could be explained by distinct functional pools of secretory granule, i.e., readily releasable pool (RRP, ~ 5% of the total pool of granules) and reserve pool (RP, 95–99% of the total pool of granules) (Fig. 2B) [30]. In fact, ultrastructural analyses have revealed that ~ 600 granules per cell are docked with the plasma membrane and further ~ 1500 granules are situated within less than one granule diameter form the plasma membrane (almost docked) [20, 31]. The RRP is assumed to represent a small subset (~ 200 granules) of the docked granule pool that are primed for exocytosis and situated in close vicinity of the CaV channels [21, 32]. Recruitment of CaV channel clusters to the primed granules is dependent on the synaptic protein Munc13-1; however, this organization is disturbed in human β-cells of T2D donors [33]. Moreover, long-term exposure of β-cells to palmitate has been shown to elongate the distance between CaV channels and granules within the RRP [34]. After the rapid fusion of granules from the RRP, the subsequent supply of new granules from the RP account for a sustained release through ATP-dependent processes.
Insulin secretion in response to constant glucose stimulation occurs in a biphasic manner with an early rapid peak (the first phase) and a following gradual increase (the second phase) [35]. This resembles (and can possibly be explained by) the biphasic pattern of β-cell exocytosis as described above. Indeed, the first phase insulin secretion can occur by mere membrane depolarization using K+ or arginine in the absence of metabolic stimulus forming ATP, which suggests that the primed granules are released in the first phase. In contrast, the second-phase insulin secretion requires glucose or another generator of ATP for granule docking and priming.
Molecular machinery of exocytosis
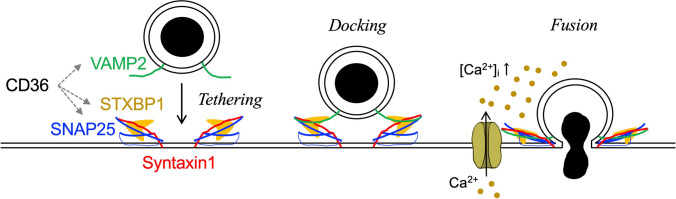
The molecular machinery of exocytosis whereby secretory granules dock, prime, and fuse with the plasma membrane requires the presence of several proteins. In β-cells, like in neurons, the process involves the formation of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, which brings the vesicle membrane in close contact with the plasma membrane for bridging the membranes for fusion (Fig. 3). The SNARE complex is composed of two plasma membrane SNARE proteins (t-SNAREs), syntaxin 1 and synaptosomal-associated protein-25 (SNAP25), and one vesicular SNARE protein (v-SNAREs), vesicle-associated membrane protein-2 (VAMP2) [36]. Apart from the SNARE proteins, the Sec1/Munc18 (SM) proteins and the different isoforms of Synaptotagmins (SYTs) are also involved in the exocytosis process. Munc18-1, also known as syntaxin-binding protein 1 (STXBP1), has been shown to play roles in both docking and priming [37–39]. SYTs possess two C2 domains (C2A and C2B), which make them either Ca2+-sensitive or Ca2+-insensitive according to their capacity to bind Ca2+. In β-cells the Ca2+-sensitive SYTs, SYT 5, 7 and 9, have been shown to play an important role in insulin secretion [40, 41]. On the other hand, specific roles of Ca2+-insensitive SYTs in β-cells are largely unknown, however expression levels of the Ca2+-insensitive SYT11 and SYT13 are both reduced in islets of T2D donors [8], indicating a direct or an indirect role in insulin secretion. Certain Rab proteins are also specifically important for the exocytosis process in the β-cell. This includes Rab3A and Rab27a essential for mobilization, docking and fusion [42, 43]. Hence, although the SNARE complex is the central part of β-cell exocytosis, a large number of other proteins play important roles for the individual steps in the exocytosis process.
Fig. 3.
Schematic presentation on how the SNARE complex forms and works on the exocytosis process, with a focus on docking and fusion with the plasma membrane, as well as the point where CD36 affect the SNARE complex. The secretory granule approaches the plasma membrane and the vesicular protein VAMP2 pairs with their plasma membrane binding partners syntaxin 1 and SNAP25, giving rise to a tight complex that tethers the granule to the plasma membrane of the β-cell. Fusion of the two membranes then occurs following a localized increase in [Ca2+]i during the opening of Cav channels. STXBP1 regulates granule docking and fusion by interacting with the SNARE complex. CD36 overexpression reduced expression levels of SNAP25, VAMP2 and STXBP1 in INS-1 cells
Amplification of insulin secretion by cAMP
An increase in intracellular cyclic AMP (cAMP) is essential to augment insulin release. cAMP is the intracellular mediator of the incretin effect in which glucagon-like peptide 1 (GLP-1) and gastric insulinotropic polypeptide (GIP) enhance glucose-stimulated insulin secretin (GSIS) [44]. GLP-1 and GIP bind to stimulatory G proteins (Gs)-coupled receptors (GLP1R and GIPR) and activate adenylyl cyclase to generate cAMP from ATP (Fig. 2A) [45, 46]. Glucose is also suggested to contribute to the increase in cAMP via the generation of ATP [44]. cAMP potentiates GSIS through mechanisms that include increased Ca2+-influx via the CaV channels, activating Ca2+ release from intracellular stores and enhancing several steps in the exocytosis process (Fig. 2B) [47, 48]. These effects are mediated by cAMP-binding protein kinase A (PKA) and EPAC2A (or cAMP-GEFII), a guanine nucleotide exchange factor for the Rap family of small GTPase. In the exocytosis process, PKA promotes the refilling of RRP by stimulating mobilization of granules and increases the number of granules that are highly sensitive to Ca2+ [29, 49]. EPAC2A on the other hand has a key role in the priming of granules [50, 51]. EPAC2A has been shown to interact directly with the plasma membrane receptor SUR1 [50, 52] and the granular machinery of ion channels needed for granular acidification in the β-cell [50, 53]. Moreover, EPAC2A interacts with several proteins involved in exocytosis, including SNAP25 [54] and a RIM2-piccolo complex [55]. Overall, it demonstrates a complex network of proteins involved in granule priming and all being regulated by cAMP. The action of cAMP is balanced via production by adenylyl cyclase and via degradation by phosphodiesterase (PDE) [44].
Defective exocytosis: pathogenesis of type 2 diabetes in obesity
Obesity is one of the strong risk factors of T2D [56]. In obesity, lipid accumulation is commonly observed not only in adipose tissue but also in ectopic tissues such as the liver and skeletal muscles, where it may cause insulin resistance. However, pancreatic β-cells can adjust, to a certain extent, for an increasing demand of insulin even under the condition of insulin resistance. Accordingly, those who cannot adapt to the extra demand for increased insulin secretion in the insulin resistance state are considered to be prone for developing T2D. To address the mechanism leading to a reduced capability to adapt the level of insulin secretion, we recently characterized human islets and β-cells of obese donors with either established T2D or being ND [7]. Among the obese donors, islets from T2D individuals showed reduced GSIS in the perifusion study, which is an ex vivo model for monitoring the dynamic insulin release from pancreatic islets in response to glucose stimulation, while the basal insulin secretion level was similar to that of islets from obese ND. A static incubation with K+ also showed reduced insulin secretion in the islets from obese T2D individuals, suggesting that defects exist downstream of the KATP channels in the triggering pathway. Indeed, capacitance recordings on single β-cells using the patch-clamp technique revealed that exocytosis was reduced by ~ 50% in the obese T2D group compared to obese ND.
CD36 as a cause of defective exocytosis
We previously reported that increased accumulation of lipid droplets in human β-cells is observed with increased body mass index (BMI) [5]. The role of lipids in β-cell function is complex. During short term exposure, FAs increase insulin secretion through increased Ca2+-influx and augmentation of granule priming [57]. In contrast, long-term exposure of islets to FAs diminishes GSIS due to the dissociation of CaV channel clusters at the release site of secretory granules [34]. Therefore, chronic presence of FAs (and/or triacylglycerols) inside β-cells will lead to defective exocytosis as observed in β-cells of obese T2D donors. In the islets, we recently identified an increased protein expression level of the FA translocase CD36, which can facilitate FA uptake into β-cells [7]. The higher gene expression level of CD36 has also been identified in islets of obesity-induced diabetes-prone Oikawa-Nagao (ON) mice, which in addition shows lower expression levels of exocytotic proteins [58, 59]. Accordingly, an increased level of CD36 in the obese state is a likely contributor to β-cell dysfunction in T2D in relation to obesity. We therefore investigated the effect of CD36 on β-cell function using INS-1 cells. CD36 overexpression led to decreased insulin secretion and defective exocytosis due to a reduced number of docked granules (by ~ 50%). These effects were linked to reduced expression levels of SNAP25, VAMP2, and STXBP1 (Fig. 3). The findings are highly relevant since, as discussed above, patients with T2D often have a loss of the first phase insulin secretion due to the reduced RRP, possibly associated with lower expression levels of exocytotic proteins [8, 60]. In contrast to the reduced number of docked granules, the granule pool away from the plasma membrane was enlarged by CD36 overexpression. Such secretory granules stuck in the RP, that do not undergo exocytosis, can be degraded intracellularly through two mechanisms, crinophagy and autophagy [61]. Overall, obese individuals who have higher CD36 expression in β-cells may have a limited insulin secretion capacity through defective exocytosis, which consequently leads to increased risk for developing T2D.
Potential therapeutics for defective exocytosis
Treatment of T2D has clearly improved over the last decade. However, significant unmet medical needs are yet to be addressed, many of which are in relation to obesity. Exploration of biological mechanism that links obesity to T2D etiology has a potential of providing novel drug targets. To further build our knowledge on the role of CD36 in β-cell dysfunction, we investigated if blockade of CD36 function can ameliorate exocytosis and GSIS on human EndoC-βH1 cells [7]. The long-term treatment (72 h) of the EndoC-βH1 cells with a CD36-neutralizing IgG antibody (FA6-152) increased expression levels of the exocytotic proteins ~ 1.5-fold, enlarged the RRP, and improved the early phase insulin secretion. In a separate study, we have also reported positive effects of ApoA-I, the primary structural protein of high-density lipoprotein (HDL), on exocytosis and GSIS in β-cells [62]. Pre-incubation of INS-1 cells or mouse islets with ApoA-I increased secretory granules at the RRP, and consequently improved GSIS. These effects were partly explained by the promotion of insulin biosynthesis due to increased PC1/3 expression. Considering the fact that ApoA-I was internalized into β-cells via endocytosis [62] and its character of high affinity binding to lipids [63], ApoA-I may work for improving exocytosis and GSIS in β-cells even under the condition of facilitating FA uptake through CD36. Further explorations of CD36 modulation and ApoA-I biology, including peptides based on the ApoA-I protein [64, 65], are needed to clarify their potential to treat T2D.
Concluding remarks
By organizing essential knowledge of secretory granule exocytosis and its amplification by cAMP, we put forward the hypothesis that abnormal lipid handlings in β-cells, including the facilitated FA uptake, can contribute to defective exocytosis and impaired insulin secretion in the pathogenesis of T2D. We suggest here CD36 as a molecular candidate to disrupt the exocytosis process in β-cells. However, it should be emphasized that several factors may contribute to the etiology of T2D. Even for the cause of the reduced expression of exocytotic proteins in islets of T2D, transcription factors, micro-RNAs, and epigenetic factors have been also suggested [66]. Therefore, further studies will be needed to investigate which mechanisms are the main causes for the β-cell dysfunctions observed in human islets of T2D. In relation to this, the recently established comprehensive databases for genotypic phenotypic information of human islets, such as “Islet Gene View” [67] and “TIGER” [68], will provide a new insight into the etiology of T2D. Taken together, further understanding for the regulatory mechanism of insulin secretion and the molecular mechanisms disrupting the regulation will ultimately lead to the invention of better prevention and treatment strategies for T2D.
Acknowledgements
We thank our present and former colleagues at Lund University Diabetes Centre (LUDC) and Nippon Medical School for contributions to the research introduced in this review and Yoshimi Nagao for supporting figure illustration. We acknowledge the human tissue laboratory at LUDC/Exodiab and the Nordic Centre for Islet Transplantation for the delivery of islets from human donors. We are grateful for support to our research in this area from the JSPS KAKENHI Grant Numbers 17KK0184, 19K23872 and 21K05453, the European Foundation for the Study of Diabetes and the Japan Diabetes Society, the Diabetes Wellness Sverige, the Uehara Memorial Foundation, the Scandinavia-Japan Sasakawa Foundation, the Sumitomo Life Welfare Foundation, the Ono Medical Research Foundation, the Swedish Research Council (LE project grant, JOL project grant, and an SRA grant SFO-EXODIAB (2009-1039)), ALF-Region Scania, Sweden, The Swedish Diabetes Foundation and the Swedish Foundation for Strategic Research (IRC-LUDC; IRC15-0067).
Declarations
Conflict of interest
JOL is an employee of Novo Nordisk A/S, DK. The authors have no conflict of interest.
Ethical statement
The work using human islets was approved by the ethics committee in Malmö/Lund, Sweden: project No. 2011/263, date of approval 2011–05-03. All the procedures on the human islets were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and/or with the Helsinki Declaration of 1964 and later versions. Donors or their relatives had given their written consent to donate organs for biomedical research upon admission to the intensive care unit.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mototsugu Nagao, Email: s8067@nms.ac.jp.
Lena Eliasson, Email: lena.eliasson@med.lu.se.
References
- 1.Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 2.Wagner R, Heni M, Tabak AG, Machann J, Schick F, Randrianarisoa E, et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med. 2021;27(1):49–57. doi: 10.1038/s41591-020-1116-9. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas 2021. 10 ed. 2021. [PubMed]
- 4.Lawlor N, Khetan S, Ucar D, Stitzel ML. Genomics of Islet (Dys)function and Type 2 Diabetes. Trends Genet. 2017;33(4):244–255. doi: 10.1016/j.tig.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosengren AH, Braun M, Mahdi T, Andersson SA, Travers ME, Shigeto M, et al. Reduced insulin exocytosis in human pancreatic beta-cells with gene variants linked to type 2 diabetes. Diabetes. 2012;61(7):1726–1733. doi: 10.2337/db11-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandasi NR, Yin P, Omar-Hmeadi M, Ottosson Laakso E, Vikman P, Barg S. Glucose-dependent granule docking limits insulin secretion and is decreased in human type 2 diabetes. Cell Metab. 2018;27(2):470–478. doi: 10.1016/j.cmet.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Nagao M, Esguerra JLS, Asai A, Ofori JK, Edlund A, Wendt A, et al. Potential protection against type 2 diabetes in obesity through lower CD36 expression and improved exocytosis in beta-cells. Diabetes. 2020;69(6):1193–1205. doi: 10.2337/db19-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson SA, Olsson AH, Esguerra JL, Heimann E, Ladenvall C, Edlund A, et al. Reduced insulin secretion correlates with decreased expression of exocytotic genes in pancreatic islets from patients with type 2 diabetes. Mol Cell Endocrinol. 2012;364(1–2):36–45. doi: 10.1016/j.mce.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Seino S, Bell GI. Pancreatic Beta Cell in Health and Disease. Kaneohe: Springer; 2010. [Google Scholar]
- 10.Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53(9):1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 11.Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–145. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53. doi: 10.2174/157339913804143225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orci L, Gabbay KH, Malaisse WJ. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972;175(4026):1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- 14.Ivarsson R, Obermuller S, Rutter GA, Galvanovskis J, Renstrom E. Temperature-sensitive random insulin granule diffusion is a prerequisite for recruiting granules for release. Traffic. 2004;5(10):750–762. doi: 10.1111/j.1600-0854.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc Natl Acad Sci U S A. 2002;99(16):10299–10304. doi: 10.1073/pnas.162352799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson HW, Rhodes CJ, Hutton JC. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988;333(6168):93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- 17.Barg S, Huang P, Eliasson L, Nelson DJ, Obermuller S, Rorsman P, et al. Priming of insulin granules for exocytosis by granular Cl(-) uptake and acidification. J Cell Sci. 2001;114(Pt 11):2145–2154. doi: 10.1242/jcs.114.11.2145. [DOI] [PubMed] [Google Scholar]
- 18.Schnell AH, Swenne I, Borg LA. Lysosomes and pancreatic islet function A quantitative estimation of crinophagy in the mouse pancreatic B-cell. Cell Tissue Res. 1988;252(1):9–15. doi: 10.1007/BF00213820. [DOI] [PubMed] [Google Scholar]
- 19.Dean PM. Ultrastructural morphometry of the pancreatic -cell. Diabetologia. 1973;9(2):115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- 20.Olofsson CS, Gopel SO, Barg S, Galvanovskis J, Ma X, Salehi A, et al. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflugers Arch. 2002;444(1–2):43–51. doi: 10.1007/s00424-002-0781-5. [DOI] [PubMed] [Google Scholar]
- 21.Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46(8):1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 22.Eliasson L, Abdulkader F, Braun M, Galvanovskis J, Hoppa MB, Rorsman P. Novel aspects of the molecular mechanisms controlling insulin secretion. J Physiol. 2008;586(14):3313–3324. doi: 10.1113/jphysiol.2008.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutton JC, Penn EJ, Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983;210(2):297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rorsman P, Ashcroft FM. Pancreatic beta-cell electrical activity and insulin secretion: of mice and men. Physiol Rev. 2018;98(1):117–214. doi: 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57(6):1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 26.Braun M, Ramracheya R, Johnson PR, Rorsman P. Exocytotic properties of human pancreatic beta-cells. Ann N Y Acad Sci. 2009;1152:187–193. doi: 10.1111/j.1749-6632.2008.03992.x. [DOI] [PubMed] [Google Scholar]
- 27.Eliasson L, Renstrom E, Ding WG, Proks P, Rorsman P. Rapid ATP-dependent priming of secretory granules precedes Ca(2+)-induced exocytosis in mouse pancreatic B-cells. J Physiol. 1997;503(Pt 2):399–412. doi: 10.1111/j.1469-7793.1997.399bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renstrom E, Eliasson L, Bokvist K, Rorsman P. Cooling inhibits exocytosis in single mouse pancreatic B-cells by suppression of granule mobilization. J Physiol. 1996;494(Pt 1):41–52. doi: 10.1113/jphysiol.1996.sp021474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502(Pt 1):105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straub SG, Sharp GW. Hypothesis: one rate-limiting step controls the magnitude of both phases of glucose-stimulated insulin secretion. Am J Physiol Cell Physiol. 2004;287(3):C565–C571. doi: 10.1152/ajpcell.00079.2004. [DOI] [PubMed] [Google Scholar]
- 31.Straub SG, Shanmugam G, Sharp GW. Stimulation of insulin release by glucose is associated with an increase in the number of docked granules in the beta-cells of rat pancreatic islets. Diabetes. 2004;53(12):3179–3183. doi: 10.2337/diabetes.53.12.3179. [DOI] [PubMed] [Google Scholar]
- 32.Barg S, Ma X, Eliasson L, Galvanovskis J, Gopel SO, Obermuller S, et al. Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic B cells. Biophys J. 2001;81(6):3308–3323. doi: 10.1016/S0006-3495(01)75964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandasi NR, Yin P, Riz M, Chibalina MV, Cortese G, Lund PE, et al. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. J Clin Invest. 2017;127(6):2353–2364. doi: 10.1172/JCI88491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoppa MB, Collins S, Ramracheya R, Hodson L, Amisten S, Zhang Q, et al. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca(2+) channels from secretory granules. Cell Metab. 2009;10(6):455–465. doi: 10.1016/j.cmet.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Prato S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia. 2003;46(Suppl 1):M2–8. doi: 10.1007/s00125-002-0930-6. [DOI] [PubMed] [Google Scholar]
- 36.Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem. 1999;259(1–2):3–17. doi: 10.1046/j.1432-1327.1999.00043.x. [DOI] [PubMed] [Google Scholar]
- 37.Toonen RF, Verhage M. Munc18-1 in secretion: lonely Munc joins SNARE team and takes control. Trends Neurosci. 2007;30(11):564–572. doi: 10.1016/j.tins.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Tomas A, Meda P, Regazzi R, Pessin JE, Halban PA. Munc 18–1 and granuphilin collaborate during insulin granule exocytosis. Traffic. 2008;9(5):813–832. doi: 10.1111/j.1600-0854.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 39.Yin P, Gandasi NR, Arora S, Omar-Hmeadi M, Saras J, Barg S. Syntaxin clusters at secretory granules in a munc18-bound conformation. Mol Biol Cell. 2018;29(22):2700–2708. doi: 10.1091/mbc.E17-09-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iezzi M, Kouri G, Fukuda M, Wollheim CB. Synaptotagmin V and IX isoforms control Ca2+ -dependent insulin exocytosis. J Cell Sci. 2004;117(Pt 15):3119–3127. doi: 10.1242/jcs.01179. [DOI] [PubMed] [Google Scholar]
- 41.Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, et al. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A. 2008;105(10):3992–3997. doi: 10.1073/pnas.0711700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, et al. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest. 2005;115(2):388–396. doi: 10.1172/JCI22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, et al. Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J Biol Chem. 2003;278(11):9715–9721. doi: 10.1074/jbc.M211352200. [DOI] [PubMed] [Google Scholar]
- 44.Tengholm A, Gylfe E. cAMP signalling in insulin and glucagon secretion. Diabetes Obes Metab. 2017;19(Suppl 1):42–53. doi: 10.1111/dom.12993. [DOI] [PubMed] [Google Scholar]
- 45.Ahren B. Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia. 2000;43(4):393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 46.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–179. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 47.Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide 1 (7–36) amide stimulates exocytosis in human pancreatic beta-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes. 1998;47(1):57–65. doi: 10.2337/diab.47.1.57. [DOI] [PubMed] [Google Scholar]
- 48.Ammala C, Ashcroft FM, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993;363(6427):356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- 49.Wan QF, Dong Y, Yang H, Lou X, Ding J, Xu T. Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+ J Gen Physiol. 2004;124(6):653–662. doi: 10.1085/jgp.200409082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eliasson L, Ma X, Renstrom E, Barg S, Berggren PO, Galvanovskis J, et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol. 2003;121(3):181–197. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibasaki T, Sunaga Y, Seino S. Integration of ATP, cAMP, and Ca2+ signals in insulin granule exocytosis. Diabetes. 2004;53(Suppl 3):S59–62. doi: 10.2337/diabetes.53.suppl_3.s59. [DOI] [PubMed] [Google Scholar]
- 52.Seino S, Takahashi H, Takahashi T, Shibasaki T. Treating diabetes today: a matter of selectivity of sulphonylureas. Diabetes Obes Metab. 2012;14(Suppl 1):9–13. doi: 10.1111/j.1463-1326.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 53.Edlund A, Esguerra JL, Wendt A, Flodstrom-Tullberg M, Eliasson L. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014;12:87. doi: 10.1186/1741-7015-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vikman J, Svensson H, Huang YC, Kang Y, Andersson SA, Gaisano HY, et al. Truncation of SNAP-25 reduces the stimulatory action of cAMP on rapid exocytosis in insulin-secreting cells. Am J Physiol Endocrinol Metab. 2009;297(2):E452–E461. doi: 10.1152/ajpendo.90585.2008. [DOI] [PubMed] [Google Scholar]
- 55.Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, et al. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFIIRim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277(52):50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 56.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women The Nurses' Health Study. Am J Epidemiol. 1997;145(7):614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 57.Olofsson CS, Salehi A, Holm C, Rorsman P. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J Physiol. 2004;557(Pt 3):935–948. doi: 10.1113/jphysiol.2004.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagao M, Asai A, Inaba W, Kawahara M, Shuto Y, Kobayashi S, et al. Characterization of pancreatic islets in two selectively bred mouse lines with different susceptibilities to high-fat diet-induced glucose intolerance. PLoS ONE. 2014;9(1):e84725. doi: 10.1371/journal.pone.0084725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagao M, Esguerra JLS, Wendt A, Asai A, Sugihara H, Oikawa S, et al. Selectively Bred Diabetes Models: GK Rats, NSY Mice, and ON Mice. Methods Mol Biol. 2020;2128:25–54. doi: 10.1007/978-1-0716-0385-7_3. [DOI] [PubMed] [Google Scholar]
- 60.Daniel S, Noda M, Straub SG, Sharp GW. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes. 1999;48(9):1686–1690. doi: 10.2337/diabetes.48.9.1686. [DOI] [PubMed] [Google Scholar]
- 61.Marsh BJ, Soden C, Alarcon C, Wicksteed BL, Yaekura K, Costin AJ, et al. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol Endocrinol. 2007;21(9):2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 62.Nilsson O, Del Giudice R, Nagao M, Gronberg C, Eliasson L, Lagerstedt JO. Apolipoprotein A-I primes beta cells to increase glucose stimulated insulin secretion. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3):165613. doi: 10.1016/j.bbadis.2019.165613. [DOI] [PubMed] [Google Scholar]
- 63.Saito H, Dhanasekaran P, Nguyen D, Deridder E, Holvoet P, Lund-Katz S, et al. Alpha-helix formation is required for high affinity binding of human apolipoprotein A-I to lipids. J Biol Chem. 2004;279(20):20974–20981. doi: 10.1074/jbc.M402043200. [DOI] [PubMed] [Google Scholar]
- 64.Edmunds SJ, Liebana-Garcia R, Nilsson O, Domingo-Espin J, Gronberg C, Stenkula KG, et al. ApoAI-derived peptide increases glucose tolerance and prevents formation of atherosclerosis in mice. Diabetologia. 2019;62(7):1257–1267. doi: 10.1007/s00125-019-4877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edmunds SJ, Liebana-Garcia R, Stenkula KG, Lagerstedt JO. A short peptide of the C-terminal class Y helices of apolipoprotein A-I has preserved functions in cholesterol efflux and in vivo metabolic control. Sci Rep. 2020;10(1):18070. doi: 10.1038/s41598-020-75232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eliasson L, Esguerra JLS, Wendt A. Lessons from basic pancreatic beta cell research in type-2 diabetes and vascular complications. Diabetol Int. 2017;8(2):139–152. doi: 10.1007/s13340-017-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asplund O, Storm P, Chandra V, Ottosson-Laakso E, Hatem G, Mansour-Aly D, et al. Islet Gene View - a tool to facilitate islet research. BioRxiv. 2020 doi: 10.1101/435743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alonso L, Piron A, Moran I, Guindo-Martinez M, Bonas-Guarch S, Atla G, et al. TIGER: The gene expression regulatory variation landscape of human pancreatic islets. Cell Rep. 2021;37(2):109807. doi: 10.1016/j.celrep.2021.109807. [DOI] [PMC free article] [PubMed] [Google Scholar]