Abstract
Purpose
The aim of this investigation was to design a perfusion-based decellularization protocol to provide whole human uterine bio-scaffolds with preserved structural and componential characteristics and to investigate the in vivo properties of the decellularized tissues.
Methods
Eight human uteri, donated by brain-dead patients, were decellularized by perfusion of sodium dodecyl sulfate (SDS) through the uterine arteries using a peristaltic pump. The bio-scaffolds were evaluated and compared with native human uterus regarding histological, immunohistochemical, structural, and bio-mechanical properties, in addition to CT angiographies to examine the preservation of the vascular networks. Subsequently, we obtained acellular patches and implanted them on uterine defects of female Wistar rats to investigate the bio-compatibility and regenerative potential of the bio-scaffolds. Finally, we performed immunostaining to investigate the potential role of circulating stem cells in recellularization of the implanted bio-scaffolds.
Results
The outcomes of this investigation confirmed the efficacy of the proposed protocol to provide whole human uterine scaffolds with characteristics and extra-cellular matrix components similar to the native human uterus. Subsequent in vivo studies demonstrated the bio-compatibility and the regenerative potential of the scaffolds and suggested a signaling pathway as an underlying mechanism for the regenerative process.
Conclusions
To the best of our knowledge, this investigation provides the first efficient perfusion-based decellularization protocol for the human uterus to obtain whole-organ scaffolds. The outcomes of this investigation could be employed in future human uterus tissue engineering studies which could ultimately result in the development of novel treatments for female infertile patients.
Keywords: Uterus, Extra-cellular matrix, Tissue engineering, Regeneration, Bio-scaffold
Introduction
The recent advancements in the field of tissue engineering provided new possibilities to rehabilitate or replace damaged organs. Previous investigations described the therapeutic potential of biological scaffolds as promising bio-materials for regeneration of different tissues and as substitutes for organ transplantation without the adverse effects of immunosuppressive therapies [1]. An optimized decellularization protocol sufficiently removes the nuclear components, providing scaffolds with low antigenicity and minimized immune reactions following transplants. Also, the bio-compatibility of the bio-scaffolds provides them with higher advantages for in vivo applications in comparison with synthetic materials [2]. The application of decellularized uterine tissues in animal models showed considerable regenerative potential and efficacy to repair full thickness uterine injuries [3]. Previous studies described different methods for decellularizing segments or the whole uterus of small and large animals. The investigations on perfusion-based decellularization of rat uterus resulted in the development of efficient protocols to obtain whole-organ bio-scaffolds. Subsequent in vitro and in vivo examinations confirmed the preservation of the extra-cellular matrix (ECM) with native-resembling characteristics as well as the regenerative potential and bio-compatibility of the provided bio-scaffolds [4, 5]. Furthermore, Hellström et al. reported successful pregnancies in rat uterine defect models receiving engineered scaffolds on the defected areas [6]. Recently, investigations have been focused on development of whole-organ bio-scaffolds from large animals’ uteri to provide the required basis for human studies [7–9]. In 2017, Campo et al. described a decellularization protocol for pig uterus. They also carried out preliminary in vivo studies by implanting engineered patches in rat models to determine the bio-compatibility of the provided bio-scaffolds [7]. Nonetheless, previous studies reported more similarities between ovine and human uterine tissues in comparison with other animal models [10]. In a previously published study, we compared three different perfusion-based decellularization techniques and determined an optimized protocol to provide whole-organ bio-scaffolds from the ovine uterus as a human-sized model [8]. Furthermore, Tiemann et al. evaluated three different decellularization protocols of the ovine uterus. The outcomes were in accordance with ours regarding the efficacy of sodium dodecyl sulfate (SDS) solution as the detergent agent to sufficiently remove the nuclear components. However, the application of 2% sodium deoxycholate (SDC) solution resulted in bio-scaffolds’ higher biomechanical strength [9]. These findings could pave the path for the development of novel treatments for female factor infertilities using bio-materials and regenerative medicine techniques, especially in patients who would benefit from uterus transplantations [11].
Based on the recent positive outcomes from previous studies on the development of whole uterus bio-scaffolds in small and large animals, in the present study we aimed to investigate the feasibility of utilizing these techniques on human donated organs to provide whole-organ scaffolds and design an efficient protocol for decellularization of the human uterus. Subsequently, we characterized the acellular organs and compared them with native human samples. Finally, we provided full-thickness patches from the bio-scaffolds and implanted them on the uterine defects of eight female Wistar rats, to evaluate the bio-compatibility and in vivo properties of the acellular tissues.
Materials and methods
The organs were donated by ten brain-dead female patients (30–55 years of age), who underwent operations for donation of other organs. The diagnosis of brain death was based upon the absence of breathing and cranial nerve reflexes. Four specialist physicians including a neurologist, an anesthesiologist, an internist, and a neurosurgeon evaluated and confirmed brain death of the patients according to national protocols. The research purpose and the protocols were explained to the donors’ families, and informed consent forms were collected in the presence of a legal medicine specialist. The participated animals had free access to food and water, and they were treated according to the guidelines of the “Guide for the Care and Use of Laboratory Animals”. The study protocol including the application of human organs for research purposes and experiments on animals were evaluated at Ethics Committee of National Institute of Health Researches and were approved with ethics approval number: IR NIMAD REC 1396–255.
Organ preparation and decellularization
An expert gynecologist performed total hysterectomy surgeries on ten brain-dead patients and harvested the uteri with preserved vessels and the surrounding tissues. The obtained organs were placed in a container filled with steriled ice and were immediately transferred to the laboratory. Two organs were preserved to be evaluated as native uterus and eight uteri underwent the decellularization process. The macroscopic characteristics of the organs were evaluated. Subsequently, we removed the surrounding adipose and connective tissues from the uteri, cannulated the uterine arties on both sides using 18-G catheters, and connected them to a peristaltic pump (CESCO Bellofeeder 1300, CESCO Bioengineering, Taiwan) at flow rates of 50 ml/min for each artery. Based on previous findings from decellularizing the ovine uterus as a close model of the human organ [8], in this study we initially performed a preliminary investigation to design an optimized protocol to produce whole-organ human uterus scaffolds by perfusion of detergent agents through the uterine arties. We provided serial sections using needle biopsies throughout the decellularization process to determine the most efficient time length and detergent agents’ concentrations to sufficiently remove the nuclear components while preserving the tissue structure and ECM components. Initially, the organs were washed with distilled water for 5 days to remove any remaining blood clots. Subsequently, a 2% SDS solution was perfused through the arteries as the detergent agent to remove the nuclear components. We changed the solution every 24 h for 28 days. Finally, we carried out 5 days of perfusion with phosphate buffered saline (PBS) to remove the detergent agents’ remnants from the organ. We obtained full-thickness samples from the decellularized organs for further assessments.
Histological and immunohistochemical evaluations
The provided specimens were fixed by 10% neutral buffered formalin (Merck, Darmstadt, Germany), dehydrated by ethanol graded series, paraffin blocked, and sectioned into 5-µm slices for histological and immunohistochemical (IHC) evaluations. We performed histological studies before and after the decellularization process using hematoxylin and eosin (H&E) and Masson’s trichrome staining to investigate the efficacy of the protocol to remove the nuclear components from all layers of the tissues and to compare the samples regarding their ECM structure and content. In addition, IHC studies were carried using specific antibodies for collagen I (sc-59772; Santa Cruz Biotechnology, Dallas, USA), collagen III (sc-271249; Santa Cruz Biotechnology, Dallas, USA), elastin (ab9519; Abcam, Cambridge, UK), fibronectin (ab268020; Abcam, Cambridge, UK), and laminin (ab233389, Abcam, Cambridge, UK) to investigate the provided scaffolds’ ECM elements. Finally, we performed image analysis (pixel/μm2) using ImageJ software (Wayne Rasband Analytics, National Institutes of Health, USA) to quantify the data and to compare the specimens’ ECM contents before and after the decellularization process.
DAPI staining and DNA quantification
We carried out 4,6-diamidino-2-phenylindole (DAPI) staining and DNA quantification tests to confirm the efficacy of the decellularization process to remove the nuclear DNA. In order to perform DAPI staining, the samples were preserved in 1 μg/ml DAPI solution (Sigma, St Louis, MO, USA) for 15 min; they were subsequently washed with PBS for 15 min and were observed under a fluorescence microscope with a UV filter. Furthermore, we employed genomic DNA purification kit (Thermo Scientific, Lithuania) and Nanodrop spectrophotometry (Thermo scientific Nanodrop 1000) to extract and measure the DNA content of the specimens.
Scanning electron microscopy (SEM)
Initially, we provided samples from different segments of natural and acellular uterine tissues to investigate both endometrial and myometrial surfaces using SEM. Furthermore, we preserved the specimens in 2.5% glutaraldehyde at 4 °C for 45 min and dehydrated them using graded series of ethanol. The samples were gold-sputtered and visualized by SEM (Vega, TESCAN, Brno, Czech Republic) at 30 kV voltage to evaluate the ultra-structural features of the tissues before and after the decellularization process.
Biomechanical characterization
We obtained 20 × 10 mm2 full-thickness samples from acellular and native uterine tissues to investigate and compare their biomechanical properties by performing a tensile test using a dynamic servohydraulic testing machine (Zwick/Roell, Model: Hct 25–400, Germany) at 1 kN calibrated load cell and a rate of 10 mm/min. The ultimate tensile strength, elastic modulus, strain at failure, and stress–strain patterns were recorded and compared between the two groups.
Computer tomography (CT) angiography
Our research group employed CT angiography (BrightSpeed 16, Tampa, FL) to illustrate and compare the organs’ vascular networks before and after the decellularization and to determine the possible vascular damages of the organs’ conduits during the processes. We injected 50% iodixanol (GE Healthcare, Cork, Ireland) through the uterine arties as the radiocontrast agent to illustrate the conduits. The uteri were investigated in the arterial phase at 10 kV and 50 mA, and the images were reconstructed by a multi-segment algorithm.
Bio-scaffold implantation and evaluation of the recellularization process
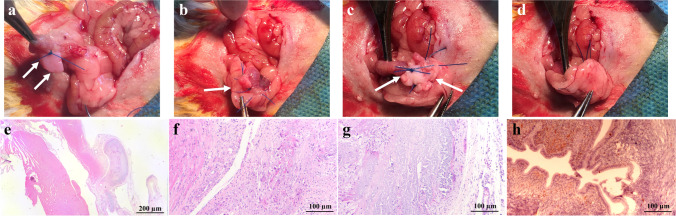
In order to evaluate the bio-compatibility and the in vivo features of the bio-scaffolds, we obtained 10 × 5 mm2 full-thickness segments from each of the acellular uteri and implanted them on the uterine defects of eight fertile female Wistar rats (220–250 g). The animals were anesthetized by intramuscular injection of ketamine (80 mg/kg) and xylazine (10 mg/kg), and the bladder was exposed by making a midline abdominal incision. We made 10-mm longitudinal incisions on the right horns of the uteri, placed the prepared scaffolds on the defects, and fixed the scaffolds using 5.0 non-absorbable stitches on the corners. Finally, the implanted tissues were carefully covered by the omentum to create sandwich-like structures, and the muscular layer and the skin were sutured (Fig. 6a−d).
Fig. 6.
Surgical implantation of bio-scaffold patches and histological evaluations of the harvested grafts. We made 10-mm longitudinal incisions on the right horns of the uteri, placed the prepared scaffolds on the defects, and fixed the scaffolds using 5.0 non-absorbable stitches on the corners (a–c, white arrows indicate the implant site). The implanted tissues were covered by the omentum to create sandwich-like structures surrounding the grafts (d). Histological studies by H&E staining of the implanted scaffolds (e–g) and control samples (h) showed signs of recellularization within the scaffolds, infiltration of acute and chronic inflammatory cells, and angiogenesis
We performed a second surgery after 10 days to harvest the implanted tissues and opposite uterine horns which we aimed to evaluate as control samples. The extracted specimens were fixed by 10% formalin (Merck, Germany) and were paraffin blocked for further examinations. We performed H&E staining on both groups samples. Furthermore, IHC studies were carried out for cytokeratin (MAD-211000Q, Master Diagnostica, Spain) and alpha-smooth muscle actin (α-SMA; M:0851, Dako, Denmark) markers to illustrate the endometrium and the smooth muscle layers. We also employed IHC staining for CD31 (M: 0823, Dako, Denmark) and Ki-67 (MAD-020310Q, Master Diagnostica, Spain) to determine the angiogenesis and the proliferative capacity of the implanted scaffolds. In addition, IHC staining was performed by specific antibodies for C‐X‐C motif chemokine ligand 12 (CXCL12; orb11353, Biorbyt, Cambridge, UK), C-X-C chemokine receptor type 4 (CXCR4; sc-53534, Santa Cruz Biotechnology, Dallas, USA), and C-X-C chemokine receptor type 7 (CXCR7; ab72100, Abcam, Cambridge, UK), the signaling axis influencing and promoting the chemoattraction of bone marrow-derived stem cells (BMDSCs) towards the endometrium, to investigate the possible role of circulating stem cells in recellularization of the implanted bio-scaffolds [12, 13].
Statistical analysis
Statistical Package for the Social Sciences (SPSS; version 20, SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the data. Numerical results were expressed as mean ± standard deviation (SD) and were tested by independent sample t-test and analysis of variance (ANOVA). The level of significance was considered as p value < 0.05.
Results
Evaluation of the decellularized uteri
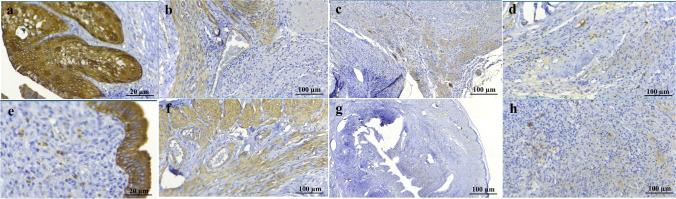
The organs were bleached after the decellularization process but the density and macroscopic structure of the uteri remained intact (Fig. 1a,b). Histological evaluation of the acellular tissues by H&E staining demonstrated complete removal of the nuclear components following the process. The ECM structure showed no deformities, and the fibers were preserved in all layers. The glandular elements of the endometrium were detectable in native and decellularized samples (Fig. 1c,d,g,h). Furthermore, bio-scaffolds’ collagen fibers deposition was maintained following decellularization as demonstrated by trichrome staining. The mean collagen deposition rate was 34.15 ± 2.02% for the native group and 38.61 ± 1.99% for the acellular group (Fig. 1e,f,i,j). IHC staining for collagen I and III fibers as predominant collagen types of the uterine tissue was suggestive for similarities between two groups of samples regarding the concentration and formation of these fibers. The mean collagen types I and III expressions were 32.45 ± 4.14% and 31.45 ± 4.71% in the native specimens and 30.21 ± 3.48% and 27.21 ± 6.57% in the acellular samples with no significant differences between the two groups. Furthermore, IHC studies confirmed the maintenance of ECM laminin, elastin, and fibronectin amount and formation in acellular specimens in comparison with native uterine tissues. Native and acellular groups IHC expressions were measured as 54.42 ± 2.34% and 46.13 ± 4.75% for elastin, 18.04 ± 2.35% and 16.82 ± 3.42% for fibronectin, and 22.41 ± 3.24% and 17.88 ± 3.11% for laminin as major ECM components with no significant differences between the two groups. Altogether, histological and immunohistochemical evaluations for major ECM elements confirmed the preservation of the ECM content and structure following the proposed decellularization protocol (Fig. 2).
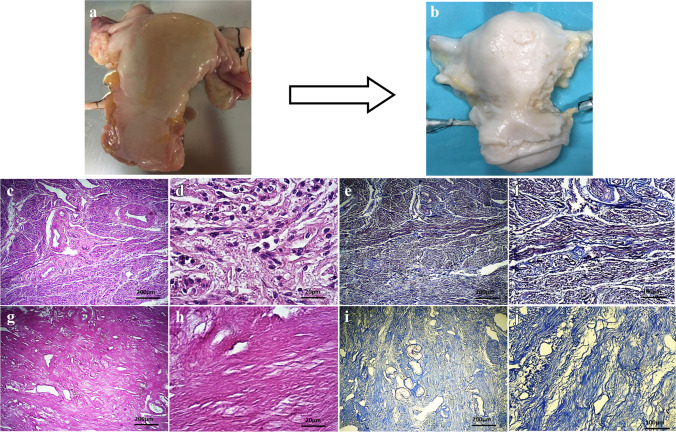
Fig. 1.
Macroscopic and histological evaluation of native and decellularized human uterus. Macroscopic examinations and comparison between native uterus (a) and acellular uterus (b) showed that the organs were whitened in decellularized group but gross formation and density of the tissues remained intact. H&E staining of native (c, d) and acellular (g, h) uteri was suggestive for complete removal of the nuclear components and preserved extra-cellular matrix (ECM) structure showed and the fibers in all layers. Trichrome staining in native (e, f) and decellularized (i, j) groups demonstrated that bio-scaffolds’ collagen fibers deposition were maintained following decellularization
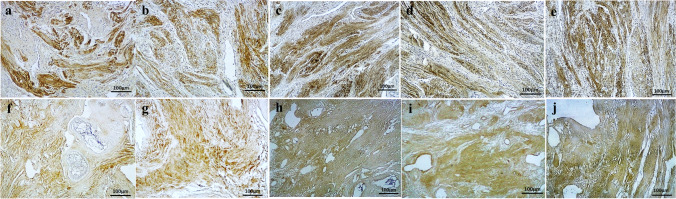
Fig. 2.
Immunohistochemical assessment of native and decellularized human uterus. Immunohistochemical evaluations for collagen I in native (a) and acellular (f) samples, in addition to collagen III in native (b) and decellularized (g) specimens showed similar contents comparing the two groups of samples. Immunostaining for elastin (c: native, h: acellular), fibronectin (d: native, i: acellular), and laminin (e: native, j: acellular) as major components of extra-cellular matrix showed with no significant differences between the two groups and confirmed the preservation of the contents and structures following the decellularization protocol
DAPI staining of the acellular samples depicted complete removal of the nuclei after the decellularization as compared with control group (Fig. 3). In addition, DNA quantification of decellularized group samples (40.11 ± 20.59 ng/mg) demonstrated a significant decrease in comparison with the control group (2463.73 ± 320.87 ng/mg), confirming efficient nuclear components removal (p value < 0.05) following decellularization. These findings approved the efficacy of the studied protocol to provide decellularized whole human uterine bio-scaffolds with preserved ECM.
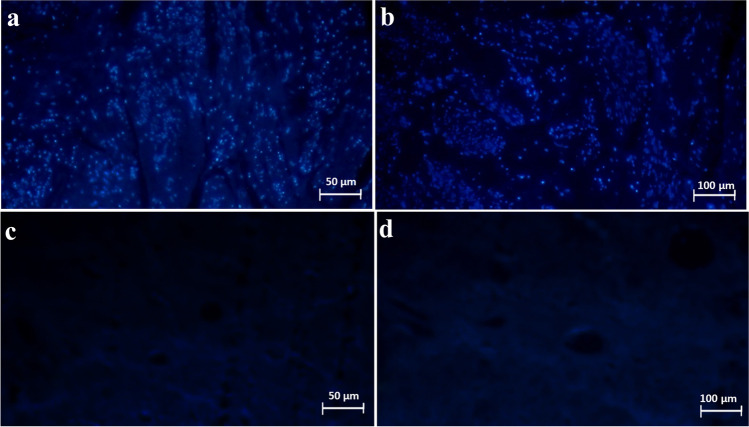
Fig. 3.
DAPI staining of native and acellular uteri. DAPI staining of the control samples (a, b) and acellular samples (c, d) depicted complete and homogenous removal of the nuclei and confirmed the efficacy of the proposed protocol to provide decellularized whole uterine bio-scaffolds. These findings were also confirmed by DNA quantification of the samples
CT angiography
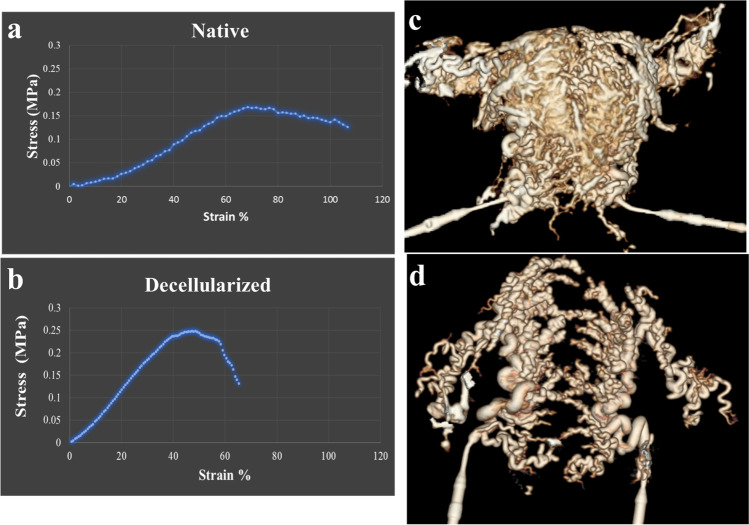
We investigated the vascular network of the uteri before and after the decellularization by CT angiography to determine the impact of the detergent agent perfusion on organs’ conduits. CT angiography with contrast injection and 3-D reconstruction of the provided images confirmed the integrity of the uterine arteries and their branches on both sides of the scaffolds, in addition to maintained vascular anastomoses. Moreover, the conduits were filled with the contrast agent in both groups and no leakage was detected. Comparing the reconstructed images of native organs and whole uterus scaffolds was suggestive for the preservation of the uterine vascular network after the decellularization process (Fig. 4c,d).
Fig. 4.
Biomechanical tests, CT angiography of native and acellular samples. Biomechanical properties evaluations illustrated ultimate tensile strength as 168 ± 0.51 MPa for the native group (a) and 0.247 ± 0.18 MPa for acellular samples (b). Young’s modulus was calculated as 1.2 ± 0.21 kPa for native and 4.57 ± 1.1 kPa for acellular specimens. CT angiography with contrast injection and 3-D reconstruction of native (c) and decellularized (d) human uterus confirmed the integrity of the uterine arteries and their branches, in addition to maintained vascular anastomoses following decellularization
Biomechanical properties
The ultimate tensile strength, elastic modulus, and strain at failure for the decellularized and native tissues were calculated. The ultimate tensile strength for native tissues was reported as 168 ± 0.51 MPa for the native group and 0.247 ± 0.18 MPa for acellular samples. Young’s modulus was calculated as 1.2 ± 0.21 kPa for native and 4.57 ± 1.1 kPa for acellular specimens (Fig. 4a,b).
Scanning electron microscopy
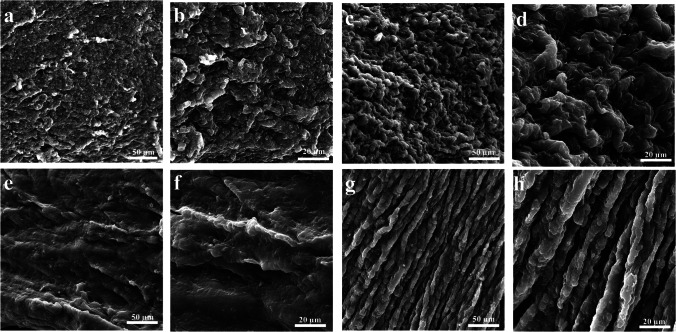
We obtained samples from different anatomical locations of the bio-scaffolds and the native uteri to determine the impact of the detergent agents and the proposed decellularization protocol on human uterine tissues at ultra-structural levels. The decellularized uterine samples lacked the cobblestone-resembling structures, commonly interpreted as cellular components, in all samples confirming complete decellularization of the specimens. The remaining ECM of the scaffolds were homogenously smoothened in endometrial and myometrial scanned areas. The acellular group specimens did not demonstrate any structural distortion or malformation. These findings were in accordance with histological and immunohistochemical data regarding the efficacy of the decellularization protocol to preserve ECM structural properties (Fig. 5).
Fig. 5.
Scanning electron microscopy of native and acellular uteri. Scanning electron microscopic studies in native (a–d) and acellular (e–h) showed that the scaffolds were smoothened in endometrial and myometrial scanned areas without any structural distortion and malformations. Also, the removal of cobble-stoned formations indicated cell removal from the specimens after the process
Evaluation of bio-scaffolds’ in vivo properties
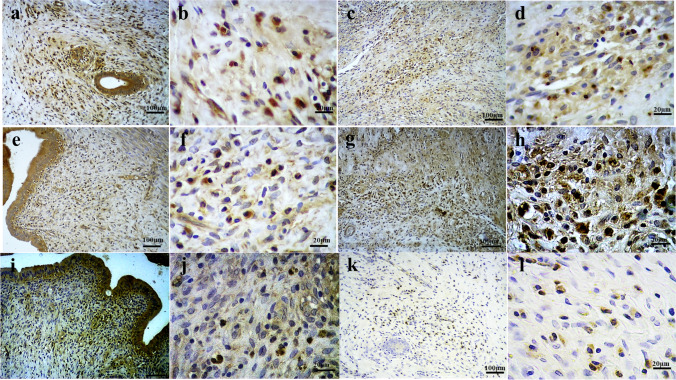
After 10 days of follow-up, the implanted scaffolds were harvested and compared with intact rat uterine tissues as control samples. No mortalities or complications were observed in the operated animals during the follow-up time. Histological studies of the extracted samples depicted recellularization of the implanted scaffolds, in addition to infiltration of acute and chronic inflammatory cells. Angiogenesis were detectable within the scaffolds as well as the host tissues adjacent to the grafts (Fig. 6e−h). Furthermore, IHC examinations revealed scattered presence of cytokeratin and α-SMA positive-stained cells within the harvested scaffolds, suggestive for the initiation of a regenerative process in endometrial and myometrial layers of the implanted tissues. Cytokeratin staining, used to determine the endometrium in implant and control groups, showed reduced positive reaction in the middle portion of the endometrial surface of the grafts. The endothelium of the vessels was determined by IHC staining for CD31, indicative of increased angiogenesis in implant sites as well as the grafts, especially the peripheral areas, in comparison with control rat uteri. Also, Ki-67 staining was carried out to investigate the proliferative processes in both groups of samples which showed prominent increased proliferative activity in lower layers of the grafted tissues. Altogether, the IHC findings of the implanted bio-scaffolds and comparison with normal rat uterine tissues were in accordance with the primary histological outcomes regarding the recellularization capacity of the scaffolds, initiation of a regenerative process, and increased vascularization in the grafted samples (Fig. 7).
Fig. 7.
Immunohistochemical (IHC) evaluations of the harvested grafts and comparison with control tissue. IHC examinations for cytokeratin marker in grafts (a) and control samples (e) revealed the scattered presence of cytokeratin positive-stained cells within the harvested scaffolds. Similar findings were detectable in IHC staining for α-SMA in implanted tissues (b) and control group (f). IHC staining for CD31 in grafts (c) and control (g) specimens was indicative of increased angiogenesis in implant sites and the grafts, especially the peripheral areas. Ki-67 marker staining in implant (d) and control (h) groups was suggestive for prominent increased proliferative activity in lower layers of the grafted tissues
IHC evaluations for CXCR4, CXCR7, and CXCL12 markers, as a signaling pathway to attract BMDSCs and stimulate cell proliferation and angiogenesis, revealed increased positive reactions for CXCL12 and reduced CXCR4 and CXCR7 positive reaction in the implanted grafts in comparison with normal uterine tissues. Furthermore, CXCL12 and CXCR7 IHC studies demonstrated higher concentration of positive-stained cells in the endometrial layers of all samples. IHC staining for CXCR4 in implanted scaffolds was associated with scarce positive reaction (Fig. 8).
Fig. 8.
Immunohistochemical (IHC) evaluations for CXCR4, CXCR7, and CXCL12 markers. IHC studies for CXCL12 in control samples (a, b) and grafted scaffolds (c, d) revealed increased positive reactions in the implant group (67.49% vs 34.43% in control group). Reduced CXCR7 positive reaction in the implanted grafts (34.50%; g, h) in comparison with normal uterine tissues (54.20%; e, f) was detectable. IHC staining for CXCR4 in implanted scaffolds (k, l) was associated with scarce positive reaction (8.25%), as compared with control samples (25.70%) (i, j)
Discussion
In this study, we initially developed a decellularization protocol for the human uterus by perfusion of chemical detergent agents through the uterine arteries that efficiently removes the nuclear components of all tissue layers and provides whole-organ bio-scaffolds with similar ECM structure and content to the native human uterus. Histological and immunohistochemical studies confirmed the preservation of major ECM components, and SEM assessment of native tissues and provided bio-scaffolds depicted the ECM integrity at ultra-structural level following decellularization. A maintained vascular network is a necessity for whole-organ bio-scaffolds that provides the capability of in vitro recellularization and various in vivo applications [14]. The obtained results from CT angiography assessments with 3-D reconstruction in both groups were indicative of preservation of major uterine arteries and conduits network in whole decellularized uteri, as compared with normal organs’ vasculature. Nevertheless, biomechanical tests were suggestive for reduced elasticity and increased ultimate tensile strength and resistance against tensile force in acellular samples as compared with the control samples. In the second phase of this investigation, we implanted acellular patches on uterine defects of Wistar rats according to a previously described method [8] to confirm the bio-compatibility and the regenerative capacity of the scaffolds. We exercised a previously described surgical method to employ rich vascular network of the peritoneum as a natural bio-reactor for in vivo recellularization by creating a sandwich like structure surrounding the grafts [15, 16]. Evaluation of the harvested grafts was suggestive for increased proliferation and angiogenesis within the bio-scaffolds and the implant sites, as well as the initiation of a regenerative process in endometrial and myometrial layers. Furthermore, IHC studies were carried out for CXCR4, CXCR7, and CXCL12 to investigate the underlying mechanisms for recellularization of the implanted scaffolds, indicating of CXCL12-positive cells and reduction of CXCR4 and CXCR7 expressions within the scaffolds. Previous studies illustrated the role of CXCL12 expressed by the endometrial stromal cells as a chemo-attractive and migration of stem cells. Also, increased CXCL12 and decreased expression of CXCR4 have been detected in leiomyomas and the myometrium of patients with leiomyoma in comparison with normal uterus [13, 17]. In addition, CXCL12 treatment has been proposed in severe uterine damages and as a therapeutic option for Asherman’s syndrome due to its ability to prevent fibrosis and improve the organ’s function through recruitment of stem cell populations [18]. These findings are in accordance with the outcomes of the present study which confirmed our hypothesis regarding the mediating role of CXCL12/CXCR4 and CXCR7 signaling pathways stem cells in chemo-attraction, proliferation, and initiation of a recellularizing process. Previous investigations on uterus tissue engineering therapeutics illustrated the advantages of application of biological scaffolds in regeneration of injured uterine tissues and rehabilitation of the organ’s function [19–21]. Considering the positive outcomes of recent studies on development and utilization of uterine bio-scaffolds from large animal models [7–9], we hereby introduced an efficient perfusion-based method to provide whole human uterine scaffolds with similar features and structure to the native organ with the capacity of in vivo recellularization.
Previous studies on the application of SDS solution as a detergent agent for decellularization to provide whole-organ bio-scaffolds showed promising results in various organs and tissues [22–25]. The recellularization of the scaffolds provided by these techniques was associated with positive outcomes regarding cell adherence, proliferation, and differentiation. They also provide an optimal platform for ex vivo cell culture and toxicologic studies [23, 24]. Moreover, Shimoda et al. demonstrated that implantation of decellularized liver scaffolds in partial hepatectomy porcine models positively affects structural reorganization and liver regeneration [26]. Our study described an efficient protocol for human uterus decellularization and demonstrated the bio-compatibility of these scaffolds in small animal models. Further studies are needed to confirm the compatibility of these biomaterials in larger animal models. Also, the impact and therapeutic potential of in vitro recellularization of uterine scaffolds prior to implantation merits more investigations. Although our primary evaluations indicated that the required time for whole decellularization of the human uterus could be considerably longer than the previously studied animal models due to its structural characteristics and stiffness, the time length of the introduced protocol could be considered as a limitation to this study. The hormonal cycles and alterations of the graft receiving animals were not determined which could considerably impact the in vivo processes and the final outcomes. Nevertheless, this investigation was a preliminary effort to generate and utilize whole human bio-scaffolds, and various aspects of this novel bio-materials merit further assessment. We suggest the future studies to investigate the scaffolds’ capacity for perfusion-based in vitro recellularization as an important step for engineering of the human uterus. Also, further investigations could focus on reducing the time period required to achieve complete decellularization using more potent detergent agents.
Conclusion
Conclusively, in this investigation we introduced an efficient protocol to provide whole human uterine bio-scaffolds with ECM characteristics and components similar to the native uterus. To best of our knowledge, this is the first study to decellularize whole human uterus using a perfusion-based method. Our investigations confirmed the bio-compatibility and the regenerative potential of the obtained scaffolds by in vivo assessment in Wistar rats. The outcomes of this study also demonstrated the role of a signaling pathway as one of the possible underlying mechanisms of the regenerative process.
Acknowledgements
We would like to express our gratitude to National Institute for Medical Research Development (NIMAD) for supporting this investigation.
Author contribution
SS D: study design, execution, analysis, manuscript drafting and critical discussion.
K F: study design, execution, analysis, manuscript drafting and critical discussion.
F G: execution, analysis, manuscript drafting and critical discussion.
M D: execution, manuscript drafting and critical discussion.
Y Z: execution, manuscript drafting and critical discussion.
A O: execution, manuscript drafting and critical discussion.
S Z: execution, manuscript drafting and critical discussion.
M MZ: execution, manuscript drafting and critical discussion.
SM T: execution, manuscript drafting and critical discussion.
AM K: study design, execution, analysis, manuscript drafting and critical discussion.
Funding
This study was funded by National Institute for Medical Research Development (NIMAD), Grant number: 963486.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Ethics approval
Ethics Committee of National Institute for Medical Research Development (NIMAD) approved and overseen this study (IR.NIMAD.REC.1396.255). We certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gargus ES, Rogers HB, McKinnon KE, Edmonds ME, Woodruff TK. Engineered reproductive tissues. Nat Biomed Eng. 2020;4(4):381–393. doi: 10.1038/s41551-020-0525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Sun J, Li X, Mao L, Zhou Y, Cui L, Bai W. Antifibrotic effects of decellularized and lyophilized human amniotic membrane transplant on the formation of intrauterine adhesion. Exp Clin Transplant. 2018;17(2):236–242. doi: 10.6002/ect.2017.0284. [DOI] [PubMed] [Google Scholar]
- 3.Hellström M, Bandstein S, Brännström M. Uterine tissue engineering and the future of uterus transplantation. Ann Biomed Eng. 2017;45:1718–1730. doi: 10.1007/s10439-016-1776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellström M, El-Akouri R, Sihlbom C, Olsson B, Lengqvist J, Bäckdahl H, et al. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater. 2014;10:5034–5042. doi: 10.1016/j.actbio.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki K, Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials. 2014;35:8791–8800. doi: 10.1016/j.biomaterials.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 6.Hellström M, Moreno-Moya JM, Bandstein S, Bom E, Akouri RR, Miyazaki K, Maruyama T, Brännström M. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil Steril. 2016;106(2):487–496. doi: 10.1016/j.fertnstert.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Campo H, Baptista PM, López-Pérez N, Faus A, Cervelló I, Simón C. De- and recellularization of the pig uterus: a bioengineering pilot study. Biol Reprod. 2017;96:34–45. doi: 10.1095/biolre/bio143396. [DOI] [PubMed] [Google Scholar]
- 8.Daryabari SS, Kajbafzadeh A-M, Fendereski K, Ghorbani F, Dehnavi M, Rostami M, et al. Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J Assist Reprod Genet. 2019;36:1211–1223. doi: 10.1007/s10815-019-01463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiemann T, Padma A, Sehic E, Bäckdahl H, Oltean M, Song M, et al. Towards uterus tissue engineering: a comparative study of sheep uterus decellularisation. Mol Hum Reprod. 2020;26:167–178. doi: 10.1093/molehr/gaaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andraus W, Ejzenberg D, Santos RMNd, Mendes LRBC, Arantes RM, Baracat EC, et al. Sheep model for uterine transplantation: the best option before starting a human program. Clinics. 2017;72:178–82. doi: 10.6061/clinics/2017(03)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brännström M. Uterus transplantation and beyond. J Mater Sci Mater Med. 2017;28:70. doi: 10.1007/s10856-017-5872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu C, Yong X, Li C, Lü M, Liu D, Chen L, et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J Surg Res. 2013;183:427–434. doi: 10.1016/j.jss.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Mamillapalli R, Mutlu L, Du H, Taylor HS. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15(1):14–22. doi: 10.1016/j.scr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porzionato A, Stocco E, Barbon S, Grandi F, Macchi V, De Caro R. Tissue-engineered grafts from human decellularized extracellular matrices: a systematic review and future perspectives. Int J Mol Sci. 2018;19(12):4117. doi: 10.3390/ijms19124117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashemi J, Pasalar P, Soleimani M, Khorramirouz R, Fendereski K, Enderami SE, et al. Application of a novel bioreactor for in vivo engineering of pancreas tissue. J Cell Physiol. 2018;233:3805–3816. doi: 10.1002/jcp.26004. [DOI] [PubMed] [Google Scholar]
- 16.Han Q, Du Y. Advances in the application of biomimetic endometrium interfaces for uterine bioengineering in female infertility. Front Bioeng Biotechnol. 2020;28(8):153. doi: 10.3389/fbioe.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moridi I, Mamillapalli R, Kodaman PH, Habata S, Dang T, Taylor HS. CXCL12 attracts bone marrow-derived cells to uterine leiomyomas. Reprod Sci. 2020;27(9):1724–1730. doi: 10.1007/s43032-020-00166-x. [DOI] [PubMed] [Google Scholar]
- 18.Ersoy GS, Zolbin MM, Cosar E, Moridi I, Mamillapalli R, Taylor HS. CXCL12 promotes stem cell recruitment and uterine repair after injury in Asherman’s syndrome. Mol Ther Methods Clin Dev. 2017;17(4):169–177. doi: 10.1016/j.omtm.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo C-Y, Baker H, Fries MH, Yoo JJ, Kim PC, Fisher JP. Bioengineering strategies to treat female infertility. Tissue Eng B Rev. 2017;23:294–306. doi: 10.1089/ten.teb.2016.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Hu S, Wang S, Cheng K. Cell and biomaterial-based approaches to uterus regeneration. Regen Biomater. 2019;6:141–148. doi: 10.1093/rb/rbz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahanbani Y, Davaran S, Ghahremani-Nasab M, Aghebati-Maleki L, Yousefi M. Scaffold-based tissue engineering approaches in treating infertility. Life Sci. 2020;240:117066. doi: 10.1016/j.lfs.2019.117066. [DOI] [PubMed] [Google Scholar]
- 22.Ferng AS, Connell AM, Marsh KM, Qu N, Medina AO, Bajaj N, et al. Acellular porcine heart matrices: whole organ decellularization with 3D-bioscaffold & vascular preservation. J Clin Transl Res. 2017;3(2):260–270. [PMC free article] [PubMed] [Google Scholar]
- 23.Pennarossa G, Ghiringhelli M, Gandolfi F, Brevini TAL. Whole-ovary decellularization generates an effective 3D bioscaffold for ovarian bioengineering. J Assist Reprod Genet. 2020;37(6):1329–1339. doi: 10.1007/s10815-020-01784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennarossa G, De Iorio T, Gandolfi F, Brevini TA. Ovarian decellularized bioscaffolds provide an optimal microenvironment for cell growth and differentiation in vitro. Cells. 2021;10(8):2126. doi: 10.3390/cells10082126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33(31):7756–7764. doi: 10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Shimoda H, Yagi H, Higashi H, Tajima K, Kuroda K, Abe Y, et al. Decellularized liver scaffolds promote liver regeneration after partial hepatectomy. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-48948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.