Abstract
Purpose
To determine whether embryo mosaicism prevalence in preimplantation genetic testing for aneuploidy (PGT-A) cycles is associated with the trophectoderm biopsy technique used (a. number of laser pulses or b. the use of flicking or pulling) or the time to tubing.
Methods
Prospective observational study performed in a single IVF-PGT-A setting from May 2019 to May 2021. Trophectoderm biopsies were analysed by next-generation sequencing. Mosaicism was analysed in relation to the biopsy methodology (number of laser pulses and pulling vs flicking), time elapsed from biopsy to tubing (min), and time of sample cryostorage from tubing to amplification (days). As a secondary objective, the number of laser pulses and biopsy methodology were studied in relation to clinical outcomes of transferred euploid blastocysts.
Results
None of the analysed variables were associated to mosaicism prevalence. Multivariable regression analysis demonstrated that mosaicism prevalence was comparable either when > 3 laser pulses were used as compared to ≤ 3 (13.9% vs 13.8%, aOR = 0.8726 [0.60–1.28]) and pulling compared to flicking (13.1% vs 14.0%, aOR = 0.86 [0.60–1.23]). Moreover, neither the number of laser pulses during biopsy (> 3 vs ≤ 3) nor the technique used (pulling vs flicking) were associated with clinical pregnancy after the transfer of frozen-thawed euploid blastocysts (54.9% vs 55.2%, aOR = 1.05 [0.53–2.09]; 61.1% vs 52.9%, aOR = 1.11 [0.55–2.25], respectively).
Conclusion
Our results suggest that, as long as the biopsy and tubing procedures are performed following standardized high quality procedures, no specific approach would increase the generation of artefactual mosaicism as a result of trophectoderm biopsy. Trophectoderm biopsies should be performed regardless of the methodology but always aiming on minimising blastocyst manipulation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02453-9.
Keywords: PGT-A, Blastocyst, Biopsy, Tubing, Mosaicism, Artefactual
Introduction
Embryo chromosomal mosaicism detection has been for a long time a bump in the road of preimplantation genetic testing programs. The transfer of mosaic embryos with a euploid cell line in coexistence with at least one aneuploid line has been proven to give rise to healthy pregnancies and live births [1, 2]. However, there are still many concerns regarding the associated potential risks of replacing mosaic embryos, making it difficult to provide counselling to patients that most of the times decide not to transfer them [3].
Mitotic errors during early preimplantation development are at the origin of this phenomenon and have been known to occur in in vitro preimplantation embryos for a very long time [4]. The molecular mechanisms that origin chromosomal mosaicism in human embryos have been widely studied and discussed [5, 6]. The fact that embryo mosaicism prevalence may vary among different patients and that it might be associated to specific patients’ characteristics has been assessed and discussed [7–9]. This kind of mosaicism has been referred to as intrinsic mosaicism. Furthermore, a wide variability in the prevalence of chromosomal mosaicism among different IVF laboratories has been reported. Interventions during the performance of an IVF cycle, such as ovarian stimulation and culture conditions, may increase the occurrence of chromosomal mosaicism as they may be affecting mitotic division. Some authors have actually evidenced that suboptimal culture conditions are associated with a higher prevalence of mosaicism [10, 11]. Therefore, a certain percentage of mosaicism observed in in vitro preimplantation embryos may be iatrogenic and could be reduced.
Some studies analysing the concordance of a mosaic trophectoderm biopsy with the results obtained from additional portions of the same embryo (including ICM) have evidenced that it is not unusual not to observe it in the latter samples [8, 12–14]. While this can be explained by low degree mosaicism being confined in a specific region of the embryo, some authors have speculated that mosaicism is actually not present in the embryo and an artefactual result caused by technique may be sometimes diagnosed [15–17]. This has been called artefactual or technical mosaicism.
In this sense, it has been discussed that the quality and integrity of the trophectoderm biopsy obtained may be related to artefactual mosaicism [18, 19]. Actually, the Preimplantation Genetic Diagnosis International Society (PGDIS) statement on mosaic embryo transfer warns that poor biopsy technique causing excessive cell damage or the biopsy of too few cells may affect chromosome profiles [20]. Therefore, the biopsy operator and its expertise may be key to minimize this issue. Moreover, the use of laser itself and the amount of laser pulses applied to obtain the biopsy has also been discussed as a potential source of technical mosaicism [21].
Different methodologies for trophectoderm biopsy have been described [19]. On one hand, the biopsy can be obtained by a combination of aspiration and pulling the cells to be biopsied while applying a few intercellular laser pulses to separate them from the rest of the embryo. Alternatively, the biopsy can be retrieved from the embryo by aspirating the cells to be biopsied inside the biopsy pipette and flicking this pipette with the holding pipette to detach the cells. This method can be complemented by the use of laser pulses before flicking to ease the biopsy. These different approaches may lead to trophectoderm biopsies of different characteristics and DNA integrity that may, in turn, lead to different kinds of results in terms of artefactual mosaicism. Notably, the lapse of time between biopsy and tubing and between tubing and whole-genome amplification (WGA) have been paid very little attention, though they could also influence DNA quality and therefore artefactual mosaicism.
The aim of the present study was to evaluate in depth the potential role of different interventions during trophectoderm biopsy and tubing prior to WGA in the generation of technical mosaicism. As a secondary objective, we also assessed whether the biopsy technique influences clinical outcomes after euploid blastocyst transfer.
Material and methods
This is a prospective observational study performed in a single IVF-preimplantation genetic testing (PGT) setting from May 2019 to May 2021 analysing the chromosomal constitution of 1341 biopsied blastocysts. The prevalence of mosaicism detected in trophectoderm biopsies was analysed in relation to the number of laser pulses used for biopsy, the biopsy technique (flicking vs pulling), the time lapsed from biopsy to tubing, and time of sample storage (from tubing to WGA). Additionally, the number of laser pulses and biopsy technique were analysed with regard to clinical outcomes.
All cycles included underwent comprehensive chromosome screening for PGT-A for one of the following reasons (advanced maternal age, recurrent miscarriages, severe male factor, repeated implantation failure, no indication).
Study design was approved and reviewed by our centre’s institutional review board.
IVF cycle
Standardized protocols were used for ovarian stimulation [22]. Pituitary suppression was achieved using gonadotrophin-releasing hormone analogues (agonists or antagonists) and multiple follicular recruitment, and growth was performed under gonadotrophins at clinician’s discretion according to patient’s characteristics.
Oocyte retrieval was performed 36 h after ovulation triggering. Denudation was conducted before insemination of metaphase-II oocytes by ICSI 40 h after triggering. Injected oocytes were left in culture in single-step media (G-TL™, Vitrolife®, Göteborg, Sweden) in a time-lapse incubator (Geri®) (Merck, Darmstadt, Germany) with low oxygen tension (5%). All media used for gamete and embryo handling and culture were G-series media (Vitrolife®, Göteborg, Sweden).
Biopsy procedure
Zona pellucida opening was performed on day 3 developing embryos using laser thermolysis [23]. Embryos reaching the blastocyst stage were biopsied between day 5 and day 7. Only hatching or fully hatched blastocysts with a defined inner cell mass and a cohesive multiple-cell trophectoderm epithelium were considered suitable for biopsy.
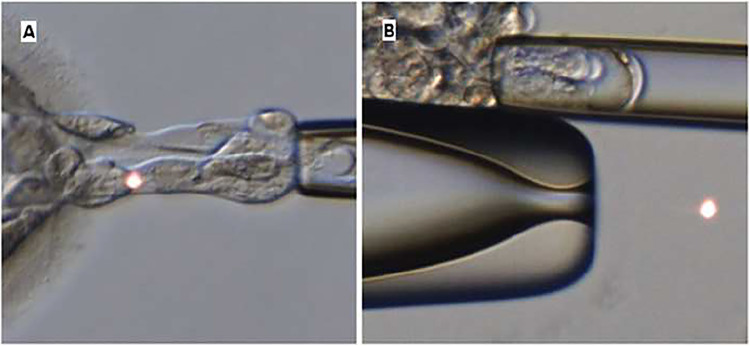
Trophectoderm biopsy was performed by a combination of intercellular laser pulses and pulling and aspiration [24], or by laser and flicking. Laser pulses were performed with OCTAX Navilase™ (Olympus, Tokyo, Japan) using trophectoderm biopsy mode. Flicking or pulling methodology (Fig. 1) was performed for each embryo biopsy at operator’s discretion and aiming to minimize manipulation according to the following criteria. Pulling was the preferred strategy for biopsy and was generally performed to blastocysts with few hatched cells and when biopsy could be obtained without needing an excessive number of laser pulses and excessive manipulation. In cases of half hatching blastocysts or more, flicking was preferred to avoid induced full hatching of the blastocyst. Fully hatched blastocysts were always biopsied by flicking. Holding pipette used for procedure was a 35° angled Small Holding (CooperSurgical®, Connecticut, USA), and biopsy pipette was 35° angled, flat, and polished with an inner diameter of 13–15 µm (CooperSurgical®, Connecticut, USA). Characteristics of the biopsy procedure (operator, number of laser pulses, time of biopsy) were annotated by the operator immediately after biopsy.
Fig. 1.
A Image of a blastocyst being biopsied by pulling technique. B Image of an embryo being biopsied by flicking technique
Tubing of the sample was performed with UV-sterilized material with sterile gown and sterile gloves. Biopsied sample was thoroughly washed in 1 × phosphate-buffered saline (PBS) with 0.1% polyvinyl alcohol (PVA) droplets before isolation in 0.2 ml PCR tubes with 1.5 µl 1 × PBS. A 80-µm capillary was used for sample handling. Placement of the biopsy into the tube was confirmed through stereomicroscope. Isolated samples were centrifuged at 1200 rpm during 3 min and immediately stored at − 80 °C freezer.
Biopsied blastocysts were vitrified as previously reported [25].
PGT protocol and results
WGA was achieved using Sureplex Amplification System (Illumina®, California, USA). The cytogenetic analysis of the biopsies was performed by NGS using the VeriSeq™PGS-MiSeq® platform (Illumina, California, USA) following the manufacturer’s protocols and guidelines.
Embryos were diagnosed as euploid, aneuploid, or mosaic. A mosaicism event was called when a deviation from the normal copy number of 30 to 70% was observed. In the context of the present study and analysis, mosaic embryo group includes embryos with at least one mosaic anomaly regardless of them being euploid or aneuploid for the remaining chromosomes.
Euploid embryo transfer
Euploid embryos were transferred in a deferred cycle. Hormonal replacement for endometrial preparation and frozen embryo transfer was performed following the established protocols in our centre [26]. Embryo transfer of warmed surviving blastocysts was conducted under ultrasound guidance [27]. Luteal phase support treatment plan has been previously reported [28].
Statistical analysis
Continuous variables were described with mean and SD, and categorical variables were described with number and percentage. Chi-squared test was used to compare categorical variables, and t-test or Wilcoxon Mann–Whitney test was used to compare continuous variables according to the necessary assumptions.
A logistic mixed multivariable model with random intercepts was applied to estimate the association between mosaicism and different interventions during trophectoderm biopsy and tubing adjusted by female and male age, as well as embryo quality. Patient, IVF cycle, and biopsy operator were treated as random effects.
To evaluate the association between clinical pregnancy and the interventions during biopsy, a logistic regression model was adjusted.
R software [29] was used for statistical analyses. For all comparisons, a statistical significance was set at P < 0.05. Package MASS [30] was used to adjust the mixed model.
Results
Patients’ demographics and cycles’ characteristics
A total of 474 cycles undergoing PGT with comprehensive chromosome screening from May 2019 to May 2021 were included. Patients’ demographics and cycles’ characteristics are shown in Table 1. Indications for PGT were advanced maternal age (70.0%; 332/474), recurrent miscarriages (9.9%; 47/474), repeated implantation failure (9.5%; 45/474), PGT-A with no indication (9.3%; 44/474), and severe male factor (1.3%; 6/474).
Table 1.
Patients’ demographics and cycles’ characteristics
| Mean ± SD | |
|---|---|
| Female age (y) | 38.4 ± 4.4 |
| Male age (y) | 41.0 ± 5.3 |
| Antral follicle count (AFC) | 12.8 ± 6.9 |
| Female body-mass index (BMI) | 23.5 ± 4.7 |
| Ovarian stimulations per PGT-A cycle | 1.1 ± 0.3 |
| Inseminated oocytes per PGT-A cycle | 9.3 ± 5.4 |
| Biopsied embryos per PGT-A cycle | 3.0 ± 2.1 |
A trophectoderm biopsy was performed in 1341 developing blastocysts, achieving a conclusive diagnosis for 1318 of them (98.3%). The distribution of diagnostic rate was similar among the studied variables (Supplementary Table 1). A euploidy rate of 40.2% (530/1318) was observed, while 45.9% (606/1318) of embryos were aneuploid, 6.5% (86/1318) aneuploid with at least one additional mosaic anomaly (aneuploid-aneuploid mosaic), and 7.3% (96/1318) were euploid-aneuploid mosaic.
From 182 mosaic blastocysts (86 aneuploid-aneuploid mosaic + 96 euploid-aneuploid mosaic), 89 (48.9%) exhibited whole-chromosome mosaicism, 87 (47.8%) segmental mosaicism, and 6 (3.3%) presented a combination of both.
Biopsy technique, sample handling, and mosaicism
Trophectoderm biopsies were performed by 5 different senior operators and were distributed as follows: 34.1% Operator 1 (449/1318), 28.4% Operator 2 (375/1318), 20.5% Operator 3 (270/1318), 9.8% Operator 4 (129/1318), and 7.2% Operator 5 (95/1318). The number of laser pulses applied was ≤ 3 in 79.2% of biopsies with results (1044/1318), while > 3 pulses were applied in the remaining cases. The mean number of pulses was 3.24 ± 0.55 with a range from 2 to 8. Additionally, flicking methodology was more frequently applied than pulling (74.6% vs 25.4%). With regard to the tubing procedure, the mean time lapsed from biopsy was 53.8 ± 40.4 min. Samples were cryostored 13.4 ± 19.1 days before amplification.
The univariate analysis of the biopsy and tubing procedure characteristics in relation to mosaicism prevalence observed in the biopsies did not evidence any statistically significant association (Table 2).
Table 2.
Univariate analysis of mosaicism association with biopsy and tubbing procedures
| Mosaicism NO (n = 1136) | Mosaicism YES (n = 182) | OR (95%CI) | p-value | |
|---|---|---|---|---|
| Biopsy operator: | 0.798 | |||
| Operator 1 | 392 (87.5%) | 56 (12.5%) | Ref | |
| Operator 2 | 320 (85.1%) | 56 (14.9%) | 1.22 (0.82;1.83) | |
| Operator 3 | 229 (84.8%) | 41 (15.2%) | 1.25 (0.81;1.93) | |
| Operator 4 | 113 (87.6%) | 16 (12.4%) | 1.00 (0.53;1.77) | |
| Operator 5 | 82 (86.3%) | 13 (13.7%) | 1.12 (0.56;2.09) | |
| Number of laser pulses: | 1.000 | |||
| ≤ 3 | 900 (86.2%) | 144 (13.8%) | Ref | |
| > 3 | 236 (86.1%) | 38 (13.9%) | 1.01 (0.68;1.47) | |
| Biopsy technique: | 0.747 | |||
| Flicking | 845 (86.0%) | 138 (14.0%) | Ref | |
| Pulling | 291 (86.9%) | 44 (13.1%) | 0.93 (0.64;1.33) | |
| Time to tubing (min): | 0.215 | |||
| (1,22) | 284 (85.3%) | 49 (14.7%) | Ref | |
| (22,45) | 292 (85.4%) | 50 (14.6%) | 0.99 (0.65;1.52) | |
| (45,71) | 267 (84.5%) | 49 (15.5%) | 1.06 (0.69;1.64) | |
| (> 71) | 293 (89.6%) | 34 (10.4%) | 0.67 (0.42;1.07) | |
| Time to amplification (d): | 13.5 (19.1) | 13.3 (20.3) | 1.00 (0.99;1.01) | 0.90 |
When a subanalysis was performed differentiating between embryos with whole-chromosome mosaicism (n = 89) and those with segmental mosaicism (n = 87), no differences in the parameters analysed were observed either (Supplementary Table 2).
The multivariable analysis in a mixed-model including the biopsy related variables with patient, cycle, and biopsy operator as random effects and adjusted for female and male age, and embryo quality, confirmed the results observed in the univariate analysis (Table 3).
Table 3.
Multivariable analysis of mosaicism association with biopsy and tubbing procedures. *Odds ratios adjusted for female and male age and embryo quality with patient, cycle, and biopsy operator as random effects
| aOR* | 95% CI | |
|---|---|---|
| Number of laser pulses | ||
| ≤ 3 | Ref | Ref |
| > 3 | 0.87 | 0.60–1.28 |
| Biopsy technique | ||
| Flicking | Ref | Ref |
| Pulling | 0.86 | 0.60–1.23 |
| Time to tubing | 1.00 | 0.99–1.00 |
| Time to amplification | 1.00 | 0.99–1.01 |
Biopsy technique and clinical outcomes
From all embryos included in this study, to date, 213 euploid blastocysts have been warmed for transfer. Survival rate was 95.3% (203/213). No differences were found in the survival rate with regard to the number of laser pulses used for biopsy, nor to the biopsy technique (Table 4).
Table 4.
Warming results according to the biopsy methodology used
| Survival NO (n = 10) | Survival YES (n = 203) | OR (95%CI) | p-value | |
|---|---|---|---|---|
| Number of laser pulses | 0.270 | |||
| ≤ 3 | 6 (3.75%) | 154 (96.2%) | Ref | |
| > 3 | 4 (7.55%) | 49 (92.5%) | 0.47 (0.13;2.00) | |
| Biopsy technique | 0.725 | |||
| Flicking | 7 (4.46%) | 150 (95.5%) | Ref | |
| Pulling | 3 (5.36%) | 53 (94.6%) | 0.80 (0.21;4.05) | |
Currently, we have data on the clinical pregnancies from 194 single euploid blastocyst transfers with a clinical pregnancy rate of 55.1% (107/194). Our data did not evidence differences regarding how the biopsy was performed (Table 5). A multivariable analysis adjusting the results for embryo quality, biopsy day, and hatching status of the embryo (hatching vs hatched) showed results in the same line (Table 6).
Table 5.
Univariate analysis showing the association of biopsy methodology with clinical outcomes
| Clinical pregnancy NO (n = 87) |
Clinical pregnancy YES (n = 107) |
OR (95%CI) | p-value | |
|---|---|---|---|---|
| Number of laser pulses | 1.000 | |||
| ≤ 3 | 64 (44.8%) | 79 (55.2%) | Ref | |
| > 3 | 23 (45.1%) | 28 (54.9%) | 0.99 (0.52;1.89) | |
| Biopsy technique | 0.136 | |||
| Flicking | 66 (47.1%) | 74 (52.9%) | Ref | |
| Pulling | 21 (38.9%) | 33 (61.1%) | 1.40 (0.74;2.68) | |
Table 6.
Multivariable logistic model for biopsy methodology adjusted for embryo quality, biopsy day, and hatching status of the embryo (hatching vs hatched)
| aOR | 95% CI | |
|---|---|---|
| Number of laser pulses | ||
| ≤ 3 | Ref | Ref |
| > 3 | 1.05 | 0.53–2.09 |
| Biopsy technique | ||
| Flicking | Ref | Ref |
| Pulling | 1.11 | 0.55–2.25 |
To date, data on pregnancy follow-up is available for 104 pregnancies. Three cases are lost to follow-up. No significant differences were observed in miscarriage rate neither between the use of ≤ 3 pulses vs > 3 pulses (13.2%, 10/66 vs 25.0%, 7/21; p = 0.229) nor between the use of flicking vs pulling (14.1%, 10/61 vs 21.2%, 7/26; p = 0.52).
Discussion
The potential diagnosis of artefactual mosaicism has become an important concern among PGT scientific community. Some authors have suggested that the true incidence of mosaicism in trophectoderm biopsies might be much lower than what is actually diagnosed [14]. The fact that we might be overestimating the prevalence of mosaicism in trophectoderm biopsies is an important issue to be addressed as diagnosis of mosaicism in preimplantation embryos leads, in most cases, to patients finally discarding such embryos for transfer [3]. The trophectoderm biopsy procedure has been suggested as a clear candidate for technical mosaicism generation [7, 21, 31]. However, to date, data actually assessing this issue are scarce. Therefore, in this study, we have performed a comprehensive analysis around the role that biopsy and tubing procedure may have on the generation of artefactual mosaicism.
The experience of the biopsy operator has always been a matter of concern regarding PGT results. The biopsy operator skills could affect the viability of embryos due to a detrimental biopsy, but could also be inducing artefactual mosaicism by altering the sample. The results obtained in this study confirm our previous reports [9] and agree with other authors that senior biopsy operators equally trained do not differ in their results [32]. However, the situation might change in the case of inexpert operators [33].
The implementation of laser-assisted trophectoderm biopsy has so far been proven safe and harmless for the embryo [34, 35]. However, it is not clear whether DNA damage on the biopsied cells may be induced by the use of laser and result in technical mosaicism at diagnosis. Our results do not evidence an increased prevalence of mosaicism in biopsies that had been obtained by using > 3 laser pulses compared to ≤ 3 (mean of 3.24 laser pulses). It should be taken into account that most of the biopsies were obtained by applying 3 or 4 pulses and that the range of pulses applied is quite narrow as it was always intended to minimize manipulation during biopsy. We cannot rule out that the use of different number of pulses and intensities might compromise sample quality, although this has not been observed in previous reports [36, 37]. While all these studies have compared different laser methodologies, recent data have pointed to differences in mosaicism rates when comparing biopsies obtained with and without laser, with less mosaicism prevalence in the latter [38]. More data would be needed to confirm these results. Performing a biopsy without laser use should be considered with caution as it should be confirmed that it does not induce more damage to both the embryo and the sample.
Additionally to the use of laser, two biopsy methods have been proposed: flicking and pulling. Both methods have been used depending on centres’ or practitioners’ criteria. However, little is known about the results obtained with such methodologies and, to date, no published studies have addressed this issue. Our results regarding mosaicism prevalence using each of these strategies do not evidence any difference. It would seem that flicking is more rough and could lead to more cell damage than pulling strategy where sampling is more gentle. In fact, some authors have reported poorer quality of biopsy samples obtained by flicking [39]. However, while flicking may be inducing more cell damage, according to our data, this does not seem to be related to artefactual mosaicism.
Concerning laser pulses and biopsy technique, we considered important to assess whether they were related to clinical outcomes. An increased number of laser pulses or the use of a less precise technique such as flicking may be harming the embryo and thus affecting its implantation ability. Our data do not show any association between the methodology used with the survival rate after warming, the clinical pregnancy rate, or the miscarriage rate. As this was a secondary aim of the study, results should be considered with caution due to the limited sample size.
Tubing procedure is a key step in PGT. While the importance of a proper and standardized tubing procedure is evident [19], this step has been neglected with regard to artefactual mosaicism generation. The tubing of damaged cells may lead to inconclusive results, but also to artefactual mosaicism. This can be minimized throughout extensive washings. We hypothesised that extended time from biopsy to tubing may be helpful to detach such cellular debris from the biopsy sample and reduce artefacts that could be interpreted as mosaicism. However, this hypothesis was not confirmed by our results, as there is no association between mosaicism prevalence and time from biopsy to tubing. Additionally, storage at − 80 °C is known to be the optimal strategy for maintenance of DNA quality [40]. In tune with that, the length of time the sample was stored at − 80 °C until amplification was not associated to mosaicism either.
As previously reported, the aetiology of mosaicism seems to be different when considering separately segmental and whole-chromosome mosaicism [9]. In this sense, we wanted to confirm whether a determinate kind of mosaicism was more influenced by the biopsy procedure than the other. Due to its nature, segmental mosaicism may be more prone to be artefactually generated through the biopsy procedure. Our results did not evidence differences regarding the type of mosaicism either.
Even though our results show that no biopsy methodology is related to mosaicism when biopsies are performed by experienced operators, this does not exclude that artefactual mosaicism can be generated during biopsy or tubing. Consequently, it is key to keep the biopsy and tubing operations at the highest quality standards. Moreover, it has to be considered that aneuploidy calling in most PGT-A protocols is based on DNA obtained after whole-genome amplification (WGA) and processing, and results are obtained through algorithms. All this steps could also, at some point, generate artefacts that may be interpreted as mosaicism [17, 18]. While most artefacts originated at this point are technique-related and difficult to avoid, it is still important to work under optimal conditions [19]. However, even taking all precautions, artefactual mosaicism might always be present in some cases and artefacts from biological origin due to cells being in S-phase will be impossible to prevent [18].
Data from this study are of great interest as they shed light on one of the most discussed yet less assessed topics around mosaicism: the biopsy technique. The fact that our results are obtained in a single centre ensures standardized conditions. However, sample size is limited and data should be considered with caution. Moreover, being a prospective observational study, the choice of flicking or pulling was not randomized. Results regarding clinical outcomes should be considered as preliminary and must be confirmed after more data are available.
Conclusions
To the best of our knowledge, this is the first study to assess in detail the role of biopsy methodology and sample handling on the diagnosis of mosaicism. Our results evidence that under standardized high-quality procedures, neither the use of flicking nor pulling nor the number of laser pulses are related to mosaicism incidence.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was performed under the auspices of the Càtedra d’Investigació en Obstetrícia i Ginecologia of the Department of Obstetrics and Gynecology, Hospital Universitari Dexeus, Universitat Autònoma de Barcelona. The authors want to specially thank all the “trophectoderm biopsy team” (Gemma Arroyo, Beatriz Carrasco, Yolanda Gil, Mònica Parriego, and Miquel Solé) for their patience and dedication in the annotation of the characteristics of each biopsy.
Author contribution
LC and MP conceived and designed the study. All the authors analyzed and interpreted the data. LC, MP, and BC contributed to data collection. IR performed the statistical analysis. All authors revised the article for important intellectual content. LC and MP wrote the article. All the authors approved the final version of the manuscript.
Declarations
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. New Engl J Med. 2015;373:2089–2090. doi: 10.1056/NEJMc1500421. [DOI] [PubMed] [Google Scholar]
- 2.Viotti M, Victor AR, Barnes FL, Zouves CG, Besser AG, Grifo JA, et al. Using outcome data from one thousand mosaic embryo transfers to formulate an embryo ranking system for clinical use. Fertil Steril. 2021;115:1212–1224. doi: 10.1016/j.fertnstert.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 3.Besser AG, McCulloh DH, Grifo JA. What are patients doing with their mosaic embryos? Decision making after genetic counseling. Fertil Steril. 2019;111:132–137.e1. doi: 10.1016/j.fertnstert.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Delhanty JDA. Mechanisms of aneuploidy induction in human oogenesis and early embryogenesis. Cytogenet Genome Res. 2005;111:237–244. doi: 10.1159/000086894. [DOI] [PubMed] [Google Scholar]
- 5.Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20:571–581. doi: 10.1093/humupd/dmu016. [DOI] [PubMed] [Google Scholar]
- 6.McCoy RC. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33:448–463. doi: 10.1016/j.tig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munné S, Wells D. Detection of mosaicism at blastocyst stage with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;107:1085–1091. doi: 10.1016/j.fertnstert.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Popovic M, Dheedene A, Christodoulou C, Taelman J, Dhaenens L, Van Nieuwerburgh F, et al. Chromosomal mosaicism in human blastocysts: the ultimate challenge of preimplantation genetic testing? Hum Reprod. 2018;33:1342–1354. doi: 10.1093/humrep/dey106. [DOI] [PubMed] [Google Scholar]
- 9.Coll L, Parriego M, Mateo S, García-Monclús S, Rodríguez I, Boada M, et al. Prevalence, types and possible factors influencing mosaicism in IVF blastocysts: results from a single setting. Reprod Biomed Online. 2021;42:55–65. doi: 10.1016/j.rbmo.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Katz-Jaffe M, Parks J, McReynolds S, Henry L, Schoolcraft WB. Chromosomal mosaicism is impacted by compromised embryo culture conditions. Fertil Steril. 2018;110:e431. doi: 10.1016/j.fertnstert.2018.08.037. [DOI] [Google Scholar]
- 11.Swain JE. Controversies in ART: can the IVF laboratory influence preimplantation embryo aneuploidy? Reprod Biomed Online. 2019;39:599–607. doi: 10.1016/j.rbmo.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Victor AR, Griffin DK, Brake AJ, Tyndall JC, Murphy AE, Lepkowsky LT, et al. Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Hum Reprod. 2019;34:181–192. doi: 10.1093/humrep/dey327. [DOI] [PubMed] [Google Scholar]
- 13.Treff NR, Marin D. The, “mosaic” embryo: misconceptions and misinterpretations in preimplantation genetic testing for aneuploidy. Fertil Steril. 2021;116:1205–1211. doi: 10.1016/j.fertnstert.2021.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Wu L, Jin L, Chen W, Liu JM, Hu J, Yu Q, et al. The true incidence of chromosomal mosaicism after preimplantation genetic testing is much lower than that indicated by trophectoderm biopsy. Hum Reprod. 2021;36:1691–1701. doi: 10.1093/humrep/deab064. [DOI] [PubMed] [Google Scholar]
- 15.Capalbo A, Ubaldi FM, Rienzi L, Scott R, Treff N. Detecting mosaicism in trophectoderm biopsies: current challenges and future possibilities. Hum Reprod. 2017;32:492–498. doi: 10.1093/humrep/dew250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodrich D, Xing T, Tao X, Lonczak A, Zhan Y, Landis J, et al. Evaluation of comprehensive chromosome screening platforms for the detection of mosaic segmental aneuploidy. J Assist Reprod Genet. 2017;34:975–981. doi: 10.1007/s10815-017-0924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popovic M, Dhaenens L, Boel A, Menten B, Heindryckx B. Chromosomal mosaicism in human blastocysts: the ultimate diagnostic dilemma. Hum Reprod Update. 2020;26:313–334. doi: 10.1093/humupd/dmz050. [DOI] [PubMed] [Google Scholar]
- 18.Coonen E, Rubio C, Christopikou D, Dimitriadou E, Gontar J, Goossens V, et al. ESHRE PGT Consortium good practice recommendations for the detection of structural and numerical chromosomal aberrations†. Hum Reprod Open. 2020;2020:hoaa017. doi: 10.1093/hropen/hoaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokkali G, Coticchio G, Bronet F, Celebi C, Cimadomo D, Goossens V, et al. ESHRE PGT Consortium and SIG Embryology good practice recommendations for polar body and embryo biopsy for PGT†. Hum Reprod Open. 2020;2020:hoaa020. doi: 10.1093/hropen/hoaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PGDIS. PGDIS position statement on the transfer of mosaic embryos 2021. 2021. https://pgdis.org/pgd_position.html. Accessed 29 Dec 2021
- 21.Aoyama N, Kato K. Trophectoderm biopsy for preimplantation genetic test and technical tips: a review. Reprod Med Biol. 2020;19:222–231. doi: 10.1002/rmb2.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez F, Rodriguez I, Devesa M, Buxaderas R, Gómez MJ, Coroleu B. Should progesterone on the human chorionic gonadotropin day still be measured? Fertil Steril. 2016;105:86–92. doi: 10.1016/j.fertnstert.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Boada M, Carrera M, De La Iglesia C, Sandalinas M, Barri PN, Veiga A. Successful use of a laser for human embryo biopsy in preimplantation genetic diagnosis: report of two cases. J Assist Reprod Genet. 1998;15:302–307. doi: 10.1023/A:1022548612107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veiga A, Sandalinas M, Benkhalifa M, Boada M, Carrera M, Santaló J, et al. Laser blastocyst biopsy for preimplantation diagnosis in the human. Zygote. 1997;5:351–354. doi: 10.1017/S0967199400003920. [DOI] [PubMed] [Google Scholar]
- 25.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–308. doi: 10.1016/S1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 26.Gaggiotti-Marre S, Martinez F, Coll L, Garcia S, Álvarez M, Parriego M, et al. Low serum progesterone the day prior to frozen embryo transfer of euploid embryos is associated with significant reduction in live birth rates. Gynecol Endocrinol. 2019;35:439–442. doi: 10.1080/09513590.2018.1534952. [DOI] [PubMed] [Google Scholar]
- 27.Coroleu B, Barri PN, Carreras O, Martínez F, Veiga A, Balasch J. The usefulness of ultrasound guidance in frozen-thawed embryo transfer: a prospective randomized clinical trial. Hum Reprod. 2002;17:2885–2890. doi: 10.1093/humrep/17.11.2885. [DOI] [PubMed] [Google Scholar]
- 28.Álvarez M, Gaggiotti-Marre S, Martínez F, Coll L, García S, González-Foruria I, et al. Individualised luteal phase support in artificially prepared frozen embryo transfer cycles based on serum progesterone levels: a prospective cohort study. Hum Reprod. 2021;36:1552–1560. doi: 10.1093/humrep/deab031. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. https://www.R-project.org/.
- 30.Venables WN, Ripley BD. Modern applied statistics with S. 4. New York: Springer; 2002. [Google Scholar]
- 31.Fragouli E, Munne S, Wells D. The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies. Hum Reprod Update. 2019;25:15–33. doi: 10.1093/humupd/dmy036. [DOI] [PubMed] [Google Scholar]
- 32.Capalbo A, Ubaldi FM, Cimadomo D, Maggiulli R, Patassini C, Dusi L, et al. Consistent and reproducible outcomes of blastocyst biopsy and aneuploidy screening across different biopsy practitioners: a multicentre study involving 2586 embryo biopsies. Hum Reprod. 2016;31:199–208. doi: 10.1093/humrep/dev294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yap WY, Lim MW, Lee CSS. Is mosaicism affected by an embryologist’s experience in biopsy? Hum Reprod Abstract Book. 2021;36(Suppl 1):O-204 i114. [Google Scholar]
- 34.Scott RT, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100:697–703. doi: 10.1016/j.fertnstert.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 35.Tiegs AW, Tao X, Zhan Y, Whitehead C, Kim J, Hanson B, et al. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril. 2021;115:627–637. doi: 10.1016/j.fertnstert.2020.07.052. [DOI] [PubMed] [Google Scholar]
- 36.Kelk DA, Sawarkar SS, Liu Y, Dufton M, Ribustello L, Munne S. Does laser assisted biopsy introduce mosaic or chaotic changes to biopsied cells? Fertil Steril. 2017;108:e88. doi: 10.1016/j.fertnstert.2017.07.272. [DOI] [Google Scholar]
- 37.Johnson D, Haimowitz Z, Arifova M, Akopians A, Welch C, Barritt J. Repeated high intensity laser biopsy pulses do not alter genetic testing results nor increases mosaicism following human embryo blastocyst biopsy of trophectoderm cells. Repro Biomed Online. 2019;39:e4–5. doi: 10.1016/j.rbmo.2019.07.013. [DOI] [Google Scholar]
- 38.Yelke HK, KumtepeColakoglu Y, Yukssel B, Cetinkaya M, Kahraman S. Usage of laser during trophectoderm biopsy and its affect on PGT-A results and mosaicism. Hum Reprod Abstract Book. 2021;36:O-202 i113. [Google Scholar]
- 39.Benavent M, Escriba M, Miret C, Vanrell I, Costa-Borges N, Calderón G, et al. Evaluation of the impact of the pulling and flicking trophectoderm biopsy procedures on the integrity of the biopsied cells and their correlation to PGT-A results. Fertil Steril. 2019;112:e242. doi: 10.1016/j.fertnstert.2019.07.1377. [DOI] [Google Scholar]
- 40.Smith S, Morin PA. Optimal storage conditions for highly dilute DNA samples: a role for trehalose as a preserving agent. J Forensic Sci. 2005;50:1101–1108. doi: 10.1520/JFS2004411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.