Abstract
Purpose
To determine whether granulocyte–macrophage colony-stimulating factor (GM-CSF)-containing medium could improve embryo-transfer outcomes in frozen-thawed blastocyst transfer.
Methods
Patients who underwent frozen-thawed blastocyst transfer (430 women, aged 30–39 years, 566 cycles) were analyzed. Frozen-thawed blastocysts were cultured in GM-CSF-containing medium or control medium for 3–5 h, followed by transfer to the uterus. The embryo-transfer outcomes in the two groups were measured and compared, and a propensity score matching (1:1) method was used to balance the differences in baseline characteristics. We analyzed 213 matched samples.
Results
In patients who underwent frozen-thawed blastocyst transfer with GM-CSF, the percentage of human chorionic gonadotropin-positive cases, biochemical pregnancies, clinical pregnancies, ongoing pregnancies, and live birth rates was 60.6%, 7.98%, 52.6%, 42.9%, and 40.9%, respectively, as compared with 45.1%, 3.29%, 41.8%, 31.1%, and 30.5%, respectively, for the control groups. The rates of human chorionic gonadotropin positivity (odds ratio [OR]: 1.87, 95% confidence interval: [CI]: 1.27–2.75), biochemical pregnancy (2.55, 1.04–6.29), clinical pregnancy (1.54, 1.05–2.27), ongoing pregnancy (1.64, 1.13–2.41), and live birth (1.67, 1.14–2.45) were significantly higher in the GM-CSF group than the control group. The incidence of pregnancy loss (22.3% vs. 27.0%) did not significantly differ between the groups.
Conclusion
The use of a GM-CSF-containing medium for blastocyst-recovery culture improved the live birth rate as a result of increased implantation rate in the frozen-thawed blastocyst-transfer cycle. The use of GM-CSF-containing medium following blastocyst thawing could be an effective choice for improving the blastocyst-transfer outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02493-1.
Keywords: Blastocyst transfer, Embryo cryopreservation, Granulocyte–macrophage colony-stimulating factor, Implantation, Neonatal outcome
Introduction
The live birth rate following blastocyst transfer in assisted reproduction technology (ART) ranges from 30 to 60% [1]. To increase the live birth rate, blastocysts with a high potential for live birth are selected using a morphological grading system, morphokinetic assessment with time-lapse technology, and preimplantation genetic testing for aneuploidy [2–4]. However, these are embryo-selection methods and not measures for improving the latent fertility of the embryo.
Granulocyte–macrophage colony-stimulating factor (GM-CSF) is a cytokine secreted by epithelial cells lining the female reproductive tract, including the fallopian tube and endometrium, in humans and other mammals [5–7]. Human and animal embryos express the GM-CSF receptor but cannot synthesize GM-CSF on their own, suggesting that preimplantation embryos receive GM-CSF secreted from maternal epithelial cells in a paracrine manner. Furthermore, human and mouse placental cells secrete GM-CSF after implantation. In human placental cells, GM-CSF promotes the proliferation and differentiation of the syncytiotrophoblast [8]. Serum GM-CSF levels are elevated during pregnancy; however, women experiencing recurrent spontaneous abortion exhibit low serum GM-CSF levels during pregnancy [9]. The implantation rate in GM-CSF knockout mice is comparable to that in wild-type mice; however, the number of pups decreases in GM-CSF knockout mice. This could be attributed to intrauterine fetal death owing to placental abnormalities [10]. These findings indicate that GM-CSF is an important cytokine in the reproductive system of both humans and animals.
The physiological environment contributes to the developmental potential of embryos; therefore, GM-CSF-containing media have long been investigated [11]. A randomized clinical trial [12] revealed that the use of a GM-CSF-containing medium for 3 days after fertilization increased the live birth rate by reducing pregnancy loss. GM-CSF improves blastulation, developmental speed, proliferation of the inner cell mass and trophectoderm (TE), and the implantation potential in human embryos in vitro [5], and is beneficial after blastocyst thawing in enhancing the re-expansion of murine blastocysts [13]. These findings suggest that GM-CSF improves blastocyst-transfer outcomes; however, the effects of GM-CSF on blastocysts in terms of pregnancy outcomes in ART remain unclear.
In this retrospective cohort study and in vitro experimental study, we aimed to clarify whether post-thawing culture in GM-CSF-containing medium just before blastocyst transfer improves blastocyst-transfer outcomes. The results indicated that short-term use of GM-CSF-containing medium for post-thawed blastocysts promoted the early implantation process, thereby increasing the live birth rate.
Materials and methods
Study design and population
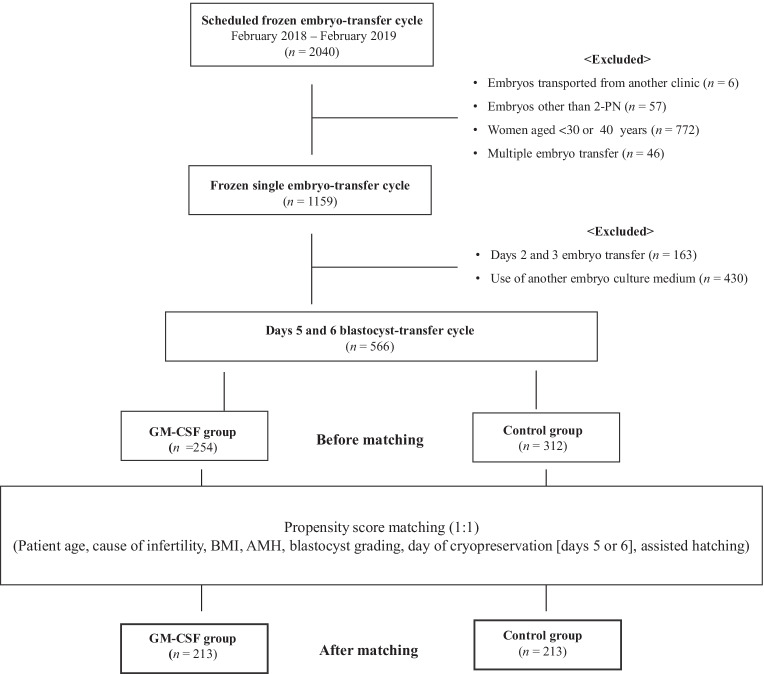
We routinely used a GM-CSF-free medium between February 2018 and August 2018 and a GM-CSF-containing medium between September 2018 and February 2019 for post-thawing culture before embryo transfer (ET). In this retrospective cohort study, we retrospectively analyzed ET outcomes obtained from patient medical records at Takahashi Women’s Clinic from February 2018 to February 2019. The inclusion criteria were as follows: (i) frozen-thawed cycles, (ii) ET using day-5 and day-6 blastocysts, (iii) women who underwent ART using autologous oocytes, (iv) women aged 30 to 39 years, and (v) use of the same fertilization medium and single-step culture medium without GM-CSF. The exclusion criteria were as follows: (i) women aged < 30 and > 40 years; (ii) cycles with more than one blastocyst transferred; (iii) ET using days 2, 3, and 7 embryos; (iv) use of a different culture medium; (v) use of embryos transported from another clinic; and (vi) use of embryos other than two-pronuclear (2-PN) embryos. We analyzed a total of 566 cycles in 430 couples who underwent ART at our clinic. None of the patients underwent preimplantation genetic testing for aneuploidy.
Ovarian stimulation, oocyte retrieval, fertilization method, and pronuclei appearance assessment
Ovarian stimulation was performed using gonadotropin-releasing hormone (GnRH) long protocol, GnRH short protocol, or GnRH antagonist protocol. For the GnRH long protocol, 400 µg of nafarelin acetate (NAFARELIL; Fuji Pharma, Toyama, Japan) was nasally administered daily from the mid-luteal phase of the pre-oocyte-retrieval cycle until final oocyte maturation. For the GnRH short protocol, 400 µg of buserelin acetate (BUSERECUR; Fuji Pharma) was nasally administered daily from day 3 of the follicular phase until final oocyte-maturation induction. Ovarian stimulation was performed by daily injection of 150 or 300 IU/mL human menopausal gonadotropin or recombinant follicle-stimulating hormone from day 3 of the follicular phase for the GnRH short protocol and GnRH antagonist protocol or from day 6 of the follicular phase for the GnRH long protocol. For the GnRH antagonist protocol, 0.25 mg of ganirelix acetate (Ganirest; MSD, Tokyo, Japan) was administered from the time when the reading follicle was > 15 mm until final oocyte maturation. Final oocyte maturation was induced with human chorionic gonadotropin (hCG) (3,000 or 5,000 IU; Fuji Pharma) and a nasal GnRH agonist spray (BUSERECUR; Fuji Pharma) when the size of each follicle was > 20 mm, and serum estradiol level was approximately ≥ 300 pg/mL/follicle. After 34 h from the induction of oocyte maturation, oocytes were collected under transvaginal ultrasound guidance, washed with a HEPES-buffered medium (P + HEPES medium; Naka Medical, Tokyo, Japan), transferred into insemination medium (P + insemination medium; Naka Medical), and subsequently precultured in an environment of 6% CO2, 5% O2, and 89% N2 for 2 to 6 h at 37 °C in a mixed gas incubator (Cook Medical, Bloomington, IN, USA) until the insemination procedure. Conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) was performed based on the sperm parameters and history of fertilization failure in conventional IVF. Oocytes were inseminated with 10 to 40 million sperms in a culture dish and co-incubated until fertilization assessment. For ICSI, the oocytes from cumulus cells were denuded using hyaluronidase (CooperSurgical, Trumbull, CT, USA), and the sperms were injected into metaphase II oocytes.
After 19 to 20 h of the insemination procedure, fertilization assessment was conducted by checking 2-PNs. The 2-PN embryos were individually cultured in 30–50 µL of GM-CSF-free medium (Naka ONESTEP Medium; Naka Medical) covered with mineral oil (OVOIL; Vitrolife, Gothenburg, Sweden) in a multi-gas incubator (CooperSurgical) at 37 °C with 6% CO2, 5% O2, and 89% N2 under a dry condition for 5–6 days. Embryo morphology was evaluated according to Gardner’s criteria, and blastocysts at days 5 and 6 (grade 3CC or higher according to Gardner’s criteria) were cryopreserved using the vitrification method with the Cryotop safety kit (Kitazato Corp., Fuji, Japan) according to the manufacturer’s instructions.
Embryo thawing, post-warming embryo culture, assisted hatching, and ET
Embryos were thawed using the Cryotop safety kit (Kitazato Corp.) according to the manufacturer’s instructions. Assisted hatching was performed on some blastocysts based on the condition of the embryos and previous ET results. The warmed blastocysts were shrunk in sucrose-containing TCM199 (0.2 M; Kitazato Corp.), and an actual hole was made using a laser in the zona pellucida. After assisted hatching, the embryos were washed three to four times and placed in 0.5 mL of fresh medium. SAGE 1-Step GM-CSF (CooperSurgical) was used for the GM-CSF group and Naka ONESTEP Medium for the control group. In both groups, the embryos were cultured for 3–5 h as a recovery culture in a multi-gas incubator (Astec, Fukuoka, Japan) at 37 °C with 6% CO2, 5% O2, and 89% N2 until ET. Luteal support was provided in all cases by oral administration of 3 mg/day estradiol (Julina, BAYER, Leverkusen, Germany) and 30 mg/day dydrogesterone (Duphaston, Mylan Inc., Canonsburg, PA, USA) when the endometrium thickness was confirmed as ≥ 8 mm until the eighth week of pregnancy. All embryos were transferred with a minimal amount of the same type of culture medium used in the previous step (< 1 µL; Fig. S1) using an ET catheter (Kitazato Corp.) under transabdominal ultrasound guidance.
ET outcomes
A positive hCG result was defined as serum hCG level ≥ 20 IU/L at 2 weeks after ET. A clinical pregnancy was defined as an observation of the gestational sac in transvaginal ultrasonography. An ongoing pregnancy was defined as the continuation of pregnancy for > 22 weeks. A biochemical pregnancy was defined as the absence of the gestational sac despite a positive hCG result. Pregnancy loss was defined as failure to achieve an ongoing pregnancy. Live birth was recorded based on a patient-reported outcome questionnaire that was returned via mail. If there was no written report of birth, the patients were called to check the birth status.
Neonatal outcomes of blastocyst transfer
Neonatal outcomes were recorded based on a patient-reported outcome questionnaire that was returned via mail. If there was no written report of neonatal outcomes, the patients were called, or the birth status was checked from their obstetrician.
Blastocyst outgrowth assay
A blastocyst outgrowth assay was performed according to the method described by Uneo et al. [14]. Poor-grade expanded blastocysts (grade 4 according to Gardner’s criteria) derived from patients aged 30–39 years that were cryopreserved on day 6 were used. The warmed blastocysts were shrunk in 0.2 M sucrose-containing TCM199, and the zona pellucida was completely removed from the blastocysts using laser-assisted hatching, followed by gentle pipetting. The blastocysts were divided into three groups and cultured in Naka ONESTEP Medium, SAGE 1-Step (CooperSurgical), or SAGE 1-Step GM-CSF for 3 h. Subsequently, the embryos were washed twice with fresh GM-CSF-free medium and transferred into a 20-µL drop of GM-CSF-free medium on a dish (Sarstedt K.K., Nümbrecht, Germany) coated with fibronectin (Sigma-Aldrich, St. Louis, MO, USA) and cultured at 37 °C with 6% CO2, 5% O2, and 89% N2. The presence or absence of visible adhesion to the dish 24 h later was examined, and adhesion rates (adhesive blastocysts/cultured blastocysts) in the control and treatment groups were compared.
Statistical analysis
All statistical analyses were performed using JMP Pro 15 statistical software (SAS Institute Inc., Cary, NC, USA). Continuous variables were analyzed using Student’s t test or Wilcoxon test. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. Multi-logistic regression analysis using confounding variables (age at embryo cryopreservation, causes of infertility, body mass index [BMI], serum anti-Müllerian hormone [AMH] level, number of previous failed ETs, assisted hatching, blastocyst grade, and day at embryo vitrification) was used for calculating the propensity scores of the embryo-transfer medium. We performed propensity scoring to match the cycles that used GM-CSF-containing medium with others at a 1:1 ratio. We used nearest-neighbor matching using a caliper of 0.95 (~ 0.2 standard deviations of the logit of the propensity score). Additionally, we performed blastocyst grading-stratified analysis according to the following three groups: excellent-quality blastocysts (3/4/5/6 AA), good-quality blastocysts (3/4/5/6 AB, BA, BB), and poor-quality blastocysts (3/4/5/6 AC, CA, BC, CB, CC).The propensity scores were calculated, and nearest-neighbor matching was performed with a caliper of 0.11, 0.19, or 0.53 for excellent-quality, good-quality, or poor-quality blastocysts, respectively. We calculated E-values using an E-value calculator (https://www.evalue-calculator.com/) to identify the influence of unmeasured confounders [15]. For the analysis of neonatal outcomes, male ratio, cesarean section, and congenital anomaly were evaluated using the risk ratio. Results with P < 0.05 were considered statistically significant.
Results
Patient characteristics in the unmatched and matched cohorts
In the unmatched cohort, 566 frozen-thawed blastocyst-transfer cycles from 430 patients were performed (Fig. 1). The mean age, BMI, AMH level, previous number of ETs, causes of infertility, inner cell mass (ICM) grade, TE grade, and day of vitrification were similar between the GM-CSF and control groups (Table 1). The cycles with assisted hatching were higher in the GM-CSF group than those in the control group (P < 0.0001). Propensity score matching resulted in well-balanced control (n = 213) and GM-CSF (n = 213) groups. After matching, none of the cycle characteristics differed significantly between the groups (Table 1), and the bias of assisted hatching was eliminated by propensity score matching in the matched cohort.
Fig. 1.
Flowchart for patient selection. PN, pronuclear; GM-CSF, granulocyte–macrophage colony-stimulating factor
Table 1.
Cycle characteristics
| Parameter | Unmatched | Matched | ||||
|---|---|---|---|---|---|---|
| Control | GM-CSF | P | Control | GM-CSF | P | |
| No. of patients | 233 | 196 | — | — | ||
| No. of ET cycles | 312 | 254 | — | 213 | 213 | — |
| Mean agea (years) | 35.4 ± 2.7 | 35.2 ± 2.7 | 0.2651 | 35.3 ± 2.7 | 35.2 ± 2.7 | 0.5787 |
| BMIa | 21.2 ± 3.2 | 21.0 ± 2.5 | 0.3897 | 20.9 ± 2.5 | 21.1 ± 2.6 | 0.3315 |
| AMHa (ng/mL) | 3.93 ± 3.09 | 4.26 ± 3.34 | 0.2368 | 3.95 ± 3.18 | 4.20 ± 2.97 | 0.4041 |
| No. of previous failed ETsa | 2.4 ± 1.8 | 2.2 ± 1.6 | 0.2485 | 2.4 ± 1.8 | 2.2 ± 1.7 | 0.4667 |
| Causes | ||||||
| Male factors (%) | 38 (12.2) | 29 (11.4) | 0.7801 | 28 (13.2) | 25 (11.7) | 0.6597 |
| Tubal factors (%) | 19 (6.1) | 15 (5.9) | 0.9269 | 15 (7.0) | 13 (6.1) | 0.6958 |
| Ovarian disorders (%) | 26 (8.3) | 22 (8.7) | 0.8892 | 15 (7.0) | 18 (8.45) | 0.5866 |
| Endometriosis (%) | 11 (3.5) | 6 (2.4) | 0.4677 | 5 (2.4) | 4 (1.9) | 0.7362 |
| Uterine factors (%) | 84 (17.3) | 66 (20.9) | 0.2822 | 39 (18.3) | 42 (19.7) | 0.7111 |
| Multiple factors (%) | 64 (26.9) | 48 (26.0) | 0.8013 | 56 (26.3) | 59 (27.7) | 0.7434 |
| Unexplained (%) | 80 (25.6) | 63 (24.8) | 0.8195 | 55 (25.8) | 52 (24.4) | 0.7375 |
| ICM grade | ||||||
| A (%) | 185 (59.3) | 160 (63.0) | 0.3698 | 128 (60.1) | 133 (62.4) | 0.6190 |
| B (%) | 82 (26.3) | 61 (24.0) | 0.5371 | 56 (26.3) | 49 (23) | 0.4313 |
| C (%) | 45 (14.4) | 33 (13.0) | 0.6233 | 29 (13.6) | 31 (14.6) | 0.7806 |
| TE grade | ||||||
| A (%) | 188 (60.3) | 164 (64.6) | 0.2929 | 129 (60.6) | 138 (64.8) | 0.3673 |
| B (%) | 87 (27.9) | 63 (24.8) | 0.4087 | 58 (27.2) | 52 (24.4) | 0.5065 |
| C (%) | 37 (11.9) | 27 (10.6) | 0.6461 | 26 (12.2) | 23 (10.8) | 0.6487 |
| Day 5 vitrified blastocysts (%) | 229 (73.4) | 193 (76.0) | 0.4822 | 155 (72.8) | 159 (74.7) | 0.6598 |
| Assisted hatched blastocyst (%) | 82 (26.3) | 118 (46.5) | < 0.0001 | 81 (38.0) | 77 (36.2) | 0.6883 |
P values in bold typeface indicate statistical significance
aMean ± SD
GM-CSF, granulocyte–macrophage colony-stimulating factor; BMI, body mass index; FSH, follicle-stimulating hormone; AMH, anti-Müllerian hormone; ET, embryo transfer; ICM, inner cell mass; TE, trophectoderm
Blastocyst-transfer outcomes in the unmatched and matched cohorts
In the unmatched cohort, the rates of a positive hCG result, clinical pregnancy, ongoing pregnancy, and live birth were significantly higher in the GM-CSF group than in the control group (Table 2). Additionally, the rate of biochemical pregnancy tended to be higher in the GM-CSF group than in the control group (P = 0.0972). After matching, the rates of a positive hCG result, biochemical pregnancy, clinical pregnancy, ongoing pregnancy, and live birth were significantly higher in the GM-CSF group than in the control group. Moreover, the E-values for a positive hCG result, biochemical pregnancy, clinical pregnancy, ongoing pregnancy, and live birth in the matched analysis were 2.08, 4.54, 1.79, 1.88, and 1.91, respectively. This result demonstrates that an unmeasured confounder has to be associated with embryo-transfer outcomes by an odds ratio (OR) of ≥ 1.8 beyond the measured confounders to fully explain the results.
Table 2.
Overall and embryo-transfer outcomes during the study period
| Outcome | Unmatched | Matched | |||||
|---|---|---|---|---|---|---|---|
| Control | GM-CSF | P (OR [95% CI]) | Control | GM-CSF | P (OR [95% CI]) | E-value | |
| No. of ET cycles | 312 | 254 | 213 | 213 | |||
| Positive hCG result (%) | 148 (47.4) | 159 (62.6) | 0.0003 (1.85 [1.32–2.60]) | 96 (45.1) | 129 (60.6) | 0.0014 (1.87 [1.27–2.75]) | 2.08 |
| Biochemical pregnancy (%) | 15 (4.81) | 21 (8.27) | 0.0972 (1.78 [0.90–3.54]) | 7 (3.29) | 17 (7.98) | 0.0356 (2.55 [1.04–6.29]) | 4.54 |
| Clinical pregnancy (%) | 133 (42.6) | 138 (54.3) | 0.0057 (1.60 [1.15–2.24]) | 89 (41.8) | 112 (52.6) | 0.0256 (1.54 [1.05–2.27]) | 1.79 |
| Ongoing pregnancy, week 22 (%) | 97(31.1) | 109 (42.9) | 0.0057 (1.64 [1.16–2.32]) | 97 (31.1) | 109 (42.9) | 0.0256 (1.64 [1.13–2.41]) | 1.88 |
| Pregnancy lossa (%) | 36 (27.1) | 29 (21.0) | 0.2443 (0.72 [0.41–1.30]) | 24 (27.0) | 25 (22.3) | 0.4462 (0.78 [0.41–1.48]) | — |
| Ectopic pregnancy | 3 | 0 | — | 2 | — | — | |
| Induced abortion | 0 | 1 | — | 0 | 0 | — | — |
| Live birth (%) | 97 (31.1) | 109 (42.9) | 0.0038 (1.67 [1.18–2.35]) | 65 (30.5) | 87 (40.9) | 0.0261 (1.67 [1.14–2.45]) | 1.91 |
| Live baby | 98 | 110 | — | 65 | 88 | — | — |
| Twin | 1 | 1 | — | 0 | 1 | — | — |
P values in bold typeface indicate statistical significance
aPercentage of the number of patients with clinical pregnancy
GM-CSF, granulocyte–macrophage colony-stimulating factor; hCG, human chorionic gonadotropin; OR, odds ratio; CI, confidence interval
Blastocyst morphological grading-stratified analysis
After matching, there was no significant difference in cycle characteristics in each analysis (Table S1).
For all stratified blastocyst groups, the rates of positive hCG results, clinical pregnancy, ongoing pregnancy, and live birth were higher in the GM-CSF group than in the control group (Table 3). However, we found that all embryo-transfer outcomes significantly increased but only in poor-quality blastocysts. These results indicate that detecting the implantation-promoting effect of GM-CSF-containing medium was easy with poor-quality blastocysts. For poor-quality blastocysts, the E-values were 4.2, 5.15, 4.03, and 4.03 for positive hCG results, clinical pregnancy, ongoing pregnancy, and live birth, respectively.
Table 3.
Blastocyst morphology-stratified analysis of blastocyst-transfer outcomes
| Outcome | Excellent-quality blastocysts | Good-quality blastocysts | Poor-quality blastocysts | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | GM-CSF |
P (OR [95% CI]) |
Control | GM-CSF |
P (OR [95% CI]) |
Control | GM-CSF |
P (OR [95% CI]) |
|
| No. of blastocyst transfers | 102 | 102 | — | 61 | 61 | — | 40 | 40 | — |
| Positive hCG result (%) | 63 (61.8) | 74 (72.6) |
0.101 (1.63 [0.91–2.95]) |
25 (41.0) | 36 (59.0) |
0.0464 (2.07 [1.01–4.27]) |
7 (17.5) | 22 (55.0) |
0.0005 (5.76 [2.06–16.1]) |
| Clinical pregnancy (%) | 57 (55.9) | 65 (63.7) |
0.1598 (1.39 [0.79–2.43]) |
22 (36.1) | 31 (50.8) |
0.1002 (1.83 [0.89–3.78]) |
4 (10.0) | 19 (47.5) |
0.0002 (8.14 [2.44–27.2]) |
| Ongoing pregnancy, week 22 (%) | 44 (43.14) | 57 (55.9) |
0.0687 (1.67 [0.96–3.24]) |
15 (24.6) | 22 (36.1) |
0.1680 (1.73 [0.79–3.78]) |
3 (7.50) | 12 (30.0) |
0.0099 (5.29 [1.36–20.5]) |
| Live birth (%) | 44 (43.14) | 57 (55.9) |
0.0687 (1.67 [0.96–3.24]) |
15 (24.6) | 22 (36.1) |
0.1680 (1.73 [0.79–3.78]) |
3 (7.50) | 12 (30.0) |
0.0099 (5.29 [1.36–20.5]) |
P values in bold typeface indicate statistical significance
aPercentage of the number of patients with clinical pregnancy
GM-CSF, granulocyte–macrophage colony-stimulating factor; OR, odds ratio; CI, confidence interval; hCG, human chorionic gonadotropin
Neonatal outcomes of blastocyst transfer
The neonatal outcomes for the unmatched cohort are summarized in Table 4. No differences were observed in the rate of male babies, pregnancy duration, and body weight between the control and GM-CSF groups. Congenital anomaly was observed in two of 98 babies in the control group and one of 110 babies in the GM-CSF group. There was one stillbirth in the control group.
Table 4.
Perinatal outcomes of live birth babies
| Parameter | Control (n = 98) | GM-CSF (n = 110) | P | RR [95% CI] |
|---|---|---|---|---|
| Male (%) | 50 (51.0) | 58 (52.7) | 0.8057 | 1.03 [0.79–1.34] |
| Cesarean section (%) | 40 (40.8) | 42 (38.2) | 0.6979 | 0.94 [0.67–1.31] |
| Pregnancy durationa (weeks) | 38.5 ± 1.8 | 38.8 ± 1.4 | 0.2558 | — |
| Weighta (g) | 3037 ± 437 | 3133 ± 466 | 0.1281 | — |
| Congenital anomaly (%) | 2 (2.04) | 1 (0.91) | 0.6026 | 0.45 [0.04–4.84] |
aMean ± SD
GM-CSF, granulocyte–macrophage colony-stimulating factor; RR, risk ratio; CI, confidence interval
Effects of short-term stimulation by GM-CSF-containing medium on blastocyst adhesion in vitro
The outgrowth assay results are presented in Table 5. The donor characteristics did not significantly differ among the control, SAGE 1-Step, and SAGE 1-Step GM-CSF groups. After 24 h of culture, the adhesion rate for blastocysts did not significantly differ between the NAKA ONESTEP group and the SAGE 1-STEP group; however, the adhesion rate of the SAGE 1-Step GM-CSF group was significantly higher than that of the NAKA ONESTEP group.
Table 5.
Results of the outgrowth assay
| Parameter | NAKA ONESTEP | SAGE 1-Step | SAGE 1-Step GM-CSF |
P (NAKA ONESTEP vs. SAGE 1-Step) |
P (NAKA ONESTEP vs. SAGE 1-Step GM-CSF) |
|---|---|---|---|---|---|
| Age (years)a | 35.5 ± 3.2 | 33.7 ± 1.8 | 33.9 ± 3.0 | 0.0977 | 0.2083 |
| No. of blastocysts cultured | 13 | 18 | 16 | — | — |
| No. of adhered blastocysts (%) | 2 (15.6) | 7 (38.9) | 9 (56.3) | 0.1548 | 0.0241 |
aMean ± SD
Discussion
The use of GM-CSF-containing medium for frozen-thawed blastocysts increased the live birth rate by ~ 10.4% and the hCG-positive cycles by 15.5%, indicating that GM-CSF stimulation to blastocysts promoted the early implantation process. GM-CSF-containing medium had a more potent effect in good- or poor-quality blastocysts and had no adverse effects on the babies.
To the best of our knowledge, this is the first clinical study showing that GM-CSF-containing medium improved the live birth potential in the frozen-thawed blastocyst-transfer cycle. Additionally, the blastocyst morphological grading-stratified analysis revealed that GM-CSF-containing medium was more effective for good- or poor-quality blastocysts than for excellent-quality blastocysts. Generally, chromosomal normality is the strongest predictor of live birth; however, > 30% of euploid blastocysts do not implant [16, 17]. Abnormal embryo morphology and developmental delay are potential causes of implantation failure in addition to other maternal factors, such as un-synchronizing endometrium, maternal immunity, and chronic endometrial inflammation [18–22]. The neonatal outcomes of poor-quality embryos did not differ from those of good-quality embryos; however, poor-quality blastocysts can be actively transferred or discarded in some facilities because of a low pregnancy potential [23]. The present study offers insights into the effective use of morphologically poor-quality blastocysts that may otherwise be discarded.
There is still no consensus on the usefulness of GM-CSF supplementation of IVF medium. In this study, the use of GM-CSF-containing medium increased the live birth rate as a result of the improved implantation potential of the blastocysts. Several studies have shown that the use of GM-CSF-containing medium from the zygote stage to day 3 of embryonic development increases the live birth rate by decreasing the rates of early pregnancy loss [12] and biochemical pregnancy [24]. Agerhorm et al. [25] reported that GM-CSF has no effect on the chromosomal constitution in the oocyte-donation cycles, whereas Economou et al. [26] showed that GM-CSF improves embryo-developmental potential and chromosomal competencies in calcium ionophore-induced artificial oocyte activation after ICSI in humans. Recently, Rose et al. [27] reported that a GM-CSF-containing sequential medium for human blastocyst culture significantly reduced the blastulation rate and embryo quality. Colony-stimulating factor 2 receptor subunit alpha (CSF2RA) encodes the GM-CSF receptor alpha chain, and its mRNA level fluctuates depending on the developmental stage of the preimplantation embryos [28, 29]. CSF2RA mRNA is most highly expressed in zygotes; however, its levels subsequently decrease, and it is re-expressed in blastocysts. This fluctuation in mRNA levels may indicate that GM-CSF exhibits embryo-stage-specific function in humans.
The addition of GM-CSF to culture medium should be assessed for its potential adverse effects on newborn babies [24]. There are a few reports of babies born using the GM-CSF-containing medium, and they have concluded that GM-CSF has no adverse effects on babies [12, 24]. In the present study, we analyzed 110 babies born from embryos treated with GM-CSF-containing medium. Our study is a second-scale study following the study conducted by Ziebe et al. [12]. There were no significant differences in the male-to-female ratio, congenital anomaly rate, cesarean section rate, and pregnancy duration between the GM-CSF and control groups, suggesting that GM-CSF supplementation after blastocyst thawing is safe. However, further case accumulation and research are required to ensure the safety of GM-CSF supplementation of IVF medium.
The strength of this study is that blastocysts, which were grown in the absence of GM-CSF until the blastocyst stage, were the target of our ET protocol. Frozen-thawed blastocyst transfer has become a common procedure, similar to ET in the early cleavage stage [30]. Adaption of this protocol by other facilities could potentially improve the implantation rate of frozen blastocysts yet to be transferred. However, this study had several limitations. First, this was a single-center, retrospective study with a small sample size. Second, chromosomal abnormalities in embryos were not considered; however, the live birth rate among babies was analyzed. Biochemical pregnancies significantly increase with the use of GM-CSF-containing medium; therefore, the implantation-improving effect of GM-CSF-containing medium could possibly extend to aneuploid blastocysts. Moreover, reducing biochemical pregnancies could be possible by assessing chromosomal abnormalities using preimplantation genetic testing for aneuploidies. Finally, the basic chemical composition of the culture medium (salt concentration and glucose concentration) in the control group differed from that of the GM-CSF-containing culture medium. During the process of using multiple types of culture medium to improve routine clinical laboratory work, we found that GM-CSF-containing medium promoted blastocyst implantation. The outgrowth assay results support the benefit of GM-CSF supplementation of IVF medium; however, it is necessary to confirm whether similar results can be obtained in clinical practice. Therefore, a prospective multicenter study is required to clarify the benefits of GM-CSF-containing medium for poor-quality blastocysts.
Conclusion
In summary, we found that the use of a GM-CSF-containing medium for blastocyst-recovery culture and ET improved the live birth rate as a result of an improved implantation rate during the frozen-thawed blastocyst-transfer cycle. Moreover, GM-CSF-containing medium improved the initial attachment process of implantation in poor-quality blastocysts in vivo and in vitro. These results were specific for blastocysts, and the effects of GM-CSF on preimplantation embryos may differ depending on the embryonic development stage. However, the application of this protocol may potentially improve the outcomes of frozen blastocyst transfer, which is being widely used worldwide.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the staff of Takahashi Women’s Clinic. We also thank Editage (http://www.editage.com) for editing and reviewing this manuscript for language.
Author contribution
All authors contributed to the study conception and design. Data collection and analysis were performed by MO-K, TK, TS, MF, and KT. In vitro experiments were performed by MO-K and MS. The first draft of the manuscript was written by TK and MO-K. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Data availability
The datasets analyzed in this study are not published in order to protect personal information but are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
This study was approved by the institutional review board of Takahashi Women’s Clinic (protocol number: TWC20-001). The in vitro experimental study was approved by the institutional review board of Takahashi Women’s Clinic (protocol number: TWC20-002) and registered at the Japan Society of Obstetrics and Gynecology.
Consent to participate
Consent for this study was obtained in the form of opt-out through our clinic website and a bulletin board. For the in vitro experiments, written consent was obtained from all patients, and the discarded, cryopreserved blastocysts were used for the outgrowth assay.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Miki Okabe-Kinoshita, Email: miki_okabe_0319@yahoo.co.jp.
Tatsuya Kobayashi, Email: tatsu.kobayashi@chiba-u.jp.
Masashi Shioya, Email: khg032638@gmail.com.
Tomoharu Sugiura, Email: rtd@aioros.ocn.ne.jp.
Maki Fujita, Email: monyomplease@yahoo.co.jp.
Keiichi Takahashi, Email: kei-taka@y4.dion.ne.jp.
References
- 1.Glujovsky D, Farquhar C. Cleavage-stage or blastocyst transfer: what are the benefits and harms? Fertil Steril. 2016;106:244–250. doi: 10.1016/j.fertnstert.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 3.ESHRE Working Group on Time-Lapse Technology. Apter S, Ebner T, Freour T, Guns Y, Kovacic B, Le Clef N, Marques M, Meseguer M, Montjean D, Sfontouris I. Good practice recommendations for the use of time-lapse technology. Hum Reprod Open. 2020;2020:hoaa008. doi: 10.1093/hropen/hoaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simopoulou M, Sfakianoudis K, Maziotis E, Tsioulou P, Grigoriadis S, Rapani A, Giannelou P, Asimakopoulou M, Kokkali G, Pantou A, Nikolettos K. PGT-A: who and when? Α systematic review and network meta-analysis of RCTs. J Assist Reprod Genet. 2021;38:1939–1957. doi: 10.1007/s10815-021-02227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjöblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum Reprod. 1999;14:3069–3076. doi: 10.1093/humrep/14.12.3069. [DOI] [PubMed] [Google Scholar]
- 6.Dobbs KB, Khan FA, Sakatani M, Moss JI, Ozawa M, Ealy AD, Hansen PJ. Regulation of pluripotency of inner cell mass and growth and differentiation of trophectoderm of the bovine embryo by colony stimulating factor 2. Biol Reprod. 2013;89:141. doi: 10.1095/biolreprod.113.113183. [DOI] [PubMed] [Google Scholar]
- 7.Jeong W, Kim J, Bazer FW, Song G. Proliferation-stimulating effect of colony stimulating factor 2 on porcine trophectoderm cells is mediated by activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase. PLoS One. 2014;9:e88731. doi: 10.1371/journal.pone.0088731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Lloret MI, Morrish DW, Wegmann TG, Honore L, Turner AR, Guilbert LJ. Demonstration of functional cytokine-placental interactions: CSF-1 and GM-CSF stimulate human cytotrophoblast differentiation and peptide hormone secretion. Exp Cell Res. 1994;214:46–54. doi: 10.1006/excr.1994.1232. [DOI] [PubMed] [Google Scholar]
- 9.Perricone R, De Carolis C, Giacomelli R, Guarino MD, De Sanctis G, Fontana L. GM-CSF and pregnancy: evidence of significantly reduced blood concentrations in unexplained recurrent abortion efficiently reverted by intravenous immunoglobulin treatment. Am J Reprod Immunol. 2003;50:232–237. doi: 10.1034/j.1600-0897.2003.00083.x. [DOI] [PubMed] [Google Scholar]
- 10.Robertson SA, Roberts CT, Farr KL, Dunn AR, Seamark RF. Fertility impairment in granulocyte-macrophage colony-stimulating factor-deficient mice. Biol Reprod. 1999;60:251–261. doi: 10.1095/biolreprod60.2.251. [DOI] [PubMed] [Google Scholar]
- 11.Chronopoulou E, Harper JC. IVF culture media: past, present and future. Hum Reprod Update. 2015;21:39–55. doi: 10.1093/humupd/dmu040. [DOI] [PubMed] [Google Scholar]
- 12.Ziebe S, Loft A, Povlsen BB, Erb K, Agerholm I, Aasted M, Gabrielsen A, Hnida C, Zobel DP, Munding B, Bendz SH. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil Steril. 2013;99:1600–1609. doi: 10.1016/j.fertnstert.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 13.Papayannis M, Eyheremendy V, Sanjurjo C, Blaquier J, Raffo FG. Effect of granulocyte-macrophage colony stimulating factor on growth, resistance to freezing and thawing and re-expansion of murine blastocysts. Reprod Biomed Online. 2007;14:96–101. doi: 10.1016/s1472-6483(10)60770-5. [DOI] [PubMed] [Google Scholar]
- 14.Ueno S, Ezoe K, Yabuuchi A, Uchiyama K, Okimura T, Okuno T, Kobayashi T, Kato K. Complete zona pellucida removal from vitrified-warmed human blastocysts facilitates earlier in-vitro attachment and outgrowth. Reprod Biomed Online. 2016;33:140–148. doi: 10.1016/j.rbmo.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 15.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 16.Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Electronic address: ASRM@asrm.org. Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429–36. doi: 10.1016/j.fertnstert.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Popovic M, Dhaenens L, Boel A, Menten B, Heindryckx B. Chromosomal mosaicism in human blastocysts: the ultimate diagnostic dilemma. Hum Reprod Update. 2020;26:313–334. doi: 10.1093/humupd/dmz050. [DOI] [PubMed] [Google Scholar]
- 18.Kliman HJ, Frankfurter D. Clinical approach to recurrent implantation failure: evidence-based evaluation of the endometrium. Fertil Steril. 2019;111:618–628. doi: 10.1016/j.fertnstert.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Kimura F, Takebayashi A, Ishida M, Nakamura A, Kitazawa J, Morimune A, Hirata K, Takahashi A, Tsuji S, Takashima A, Amano T. Review: chronic endometritis and its effect on reproduction. J Obstet Gynaecol Res. 2019;45:951–960. doi: 10.1111/jog.13937. [DOI] [PubMed] [Google Scholar]
- 20.Kwak-Kim JY, Chung-Bang HS, Ng SC, Ntrivalas EI, Mangubat CP, Beaman KD, Beer AE, Gilman-Sachs A. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod. 2003;18:767–773. doi: 10.1093/humrep/deg156. [DOI] [PubMed] [Google Scholar]
- 21.Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, Xu K, Rosenwaks Z. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107:664–670. doi: 10.1016/j.fertnstert.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Irani M, O’Neill C, Palermo GD, Xu K, Zhang C, Qin X, Zhan Q, Clarke RN, Ye Z, Zaninovic N, Rosenwaks Z. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110:95–102.e1. doi: 10.1016/j.fertnstert.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Kirillova A, Lysenkov S, Farmakovskaya M, Kiseleva Y, Martazanova B, Mishieva N, Abubakirov A, Sukhikh G. Should we transfer poor quality embryos? Fertil Res Pract. 2020;6:2. doi: 10.1186/s40738-020-00072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou W, Chu D, Sha W, Fu L, Li Y. Effects of granulocyte-macrophage colony-stimulating factor supplementation in culture medium on embryo quality and pregnancy outcome of women aged over 35 years. J Assist Reprod Genet. 2016;33:39–47. doi: 10.1007/s10815-015-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agerholm I, Loft A, Hald F, Lemmen JG, Munding B, Sørensen PD, Ziebe S. Culture of human oocytes with granulocyte-macrophage colony-stimulating factor has no effect on embryonic chromosomal constitution. Reprod Biomed Online. 2010;20:477–484. doi: 10.1016/j.rbmo.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Economou KA, Christopikou D, Tsorva E, Davies S, Mastrominas M, Cazlaris H, Koutsilieris M, Angelogianni P, Loutradis D. The combination of calcium ionophore A23187 and GM-CSF can safely salvage aged human unfertilized oocytes after ICSI. J Assist Reprod Genet. 2017;34:33–41. doi: 10.1007/s10815-016-0823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose RD, Barry MF, Dunstan EV, Yuen SM, Cameron LP, Knight EJ, Norman RJ, Hull ML. The BlastGen study: a randomized controlled trial of blastocyst media supplemented with granulocyte-macrophage colony-stimulating factor. Reprod Biomed Online. 2020;40:645–652. doi: 10.1016/j.rbmo.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Sjöblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod. 2002;67:1817–1823. doi: 10.1095/biolreprod.101.001503. [DOI] [PubMed] [Google Scholar]
- 29.Blakeley P, Fogarty NM, Del Valle I, Wamaitha SE, Hu TX, Elder K, Snell P, Christie L, Robson P, Niakan KK. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development. 2015;142:3613. doi: 10.1242/dev.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh B, Reschke L, Segars J, Baker VL. Frozen–thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril. 2020;113:252–257. doi: 10.1016/j.fertnstert.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in this study are not published in order to protect personal information but are available from the corresponding author upon reasonable request.