Abstract
Purpose
The requirement of zinc for the development and maturation of germ lines and reproductive systems is deeply conserved across evolution. The nematode Caenorhabditis elegans offers a tractable platform to study the complex system of distributing zinc to the germ line. We investigated several zinc importers to investigate how zinc transporters play a role in the reproductive system in nematodes, as well as establish a platform to study zinc transporter biology in germline and reproductive development.
Methods
Previous high throughput transcriptional datasets as well as phylogenetic analysis identified several putative zinc transporters that have a function in reproduction in worms. Phenotypic analysis of CRISPR-generated knockouts and tags included characterization of offspring output, gonad development, and protein localization. Light and immunofluorescence microscopy allowed for visualization of physiological and molecular effects of zinc transporter mutations.
Results
Disruption of two zinc transporters, ZIPT-2.4 and ZIPT-15, was shown to lead to defects in reproductive output. A mutation in zipt-2.4 has subtle effects on reproduction, while a mutation in zipt-15 has a clear impact on gonad and germline development that translates into a more pronounced defect in fecundity. Both transporters have germline expression, as well as additional expression in other cell types.
Conclusions
Two ZIP-family zinc transporter orthologs of human ZIP6/10 and ZIP1/2/3 proteins are important for full reproductive fecundity and participate in development of the gonad. Notably, these zinc transporters are present in gut and reproductive tissues in addition to the germ line, consistent with a complex zinc trafficking network important for reproductive success.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02495-z.
Keywords: Zinc transporter, Caenorhabditis elegans, Germline development, Fecundity, Germline gene expression
Introduction
Developing gametes and zygotes are sensitive to changes in nutritional status [1, 2], and disturbances in nutrient availability can have lasting impacts on the reproductive system and offspring [3, 4]. Natural and assisted reproductive success can be affected by excess or deficient dietary nutrients [5–9], as well as genetic defects that impact nutrient acquisition [10–12]. Therefore, it is crucial to understand how nutrients are mobilized to the reproductive system, and the impacts they have on molecular processes. Trace metals are of interest due to their fundamental requirement and toxicity in reproduction [13–18], particularly the transition metal zinc [19–23]. Zinc serves many different roles as a cofactor in metalloprotein sites where it can confer structural stability, as seen in zinc finger proteins [24–29], act as a substrate for membrane transporters [30–33], or serve as an essential catalytic cofactor [27–29, 34, 35]. More recently, a number of regulatory proteins have been identified as zinc receptors that play roles in signaling processes [29, 36–40]. While signaling roles for zinc are not well understood, there is clearly an abundance of possible receptor sites for zinc in mammalian cells. For instance, canonical binding sites for this inorganic cofactor have been predicted in 10% of the protein-coding genes in the human genome [41–43]. Given the abundance of genes that encode zinc-dependent biological functions, it is not surprising that systemic changes in zinc availability and cellular zinc content impact the functions of many physiological processes such as immunity [29, 36, 44], insulin production [29, 45, 46], and fertility [29, 47, 48]. Although it has been known for decades that zinc deficiency can impact reproductive function and development [20, 29, 49], mechanistic and signaling roles of zinc in reproduction are just being uncovered. Recent studies have shown that gametes must undergo significant shifts in zinc content, localization, and activity during meiotic maturation, meiotic progression, and oocyte development up to and including the point of fertilization. In mammalian eggs, the import of billions of zinc ions in the last few hours of maturation is essential for MII arrest and must occur before the egg can be fertilized. Approximately 15% of the zinc quota in an MII egg has been shown to be localized in a system of 8000 vesicles near the plasma membrane. Strikingly, fertilization induces a sequence of rapid zinc efflux events known as zinc sparks, which play several roles in the block to polyspermy [50–52]. Currently, it is not known how zinc is loaded into cortical vesicles or accumulated in early stages of oocyte maturation. However, zinc uptake by the maturing oocyte has been found to be mediated in part by genes from a class of proteins called zinc transporters [53].
Zinc transporters are part of a diverse group of transporters called solute carriers (SLCs), which are integral membrane proteins that transport ions and molecules across biological membranes [54, 55]. The twenty-four mammalian genes that encode zinc transporters fall into two distinct functional groups: fourteen are members of the ZRT, IRT-like proteins (ZIPs/SLC39) family that import zinc into the cytosol (either from outside the cell or from internal compartments), while ten are members of the cation diffusion facilitators (CDFs/SLC30, or alternatively, ZnTs) family that export zinc from the cytoplasm (either into compartmental sites or into the extracellular milieux). Each of these families are further divided into subfamilies; ZIPs have the ZIPI, ZIPII, LIV-1, and gufA subfamilies, while CDFs have the CDFI, CDFII, and CDFIII subfamilies. Zinc transporters are expressed in a multitude of cell types, can be found in membrane bound organelles, and have expression patterns ranging from ubiquitous to specific [56–59]. The mechanism by which CDFs transport zinc is well understood [60–62], while zinc import via ZIPs is still being debated [63–65], with a crystal structure only being solved recently [32, 66]. It is also not clear how the complementary zinc import and export processes are orchestrated. The net result is that total cellular zinc is maintained at near millimolar concentrations, while cytosolic concentrations of free zinc are typically maintained at sub-nanomolar levels. In many ways, this compartmentalization and set-point condition has parallels to calcium biology, where free calcium is maintained at low threshold conditions until fertilization induces a wave of free calcium availability in the cytosol [67–69]. While inherited mutations of specific zinc transporter genes have been associated with a number of diseases, including acrodermatitis enteropathica [70], breast cancer [71, 72], diabetes mellitus type 2 [73, 74], and many others [75–78], none has yet been linked with reproductive phenotypes. This is surprising given that zinc dietary status is important for multiple aspects of mammalian reproduction and early development.
Several mammalian zinc transporters are known to be associated with reproduction and development. Zinc transporters ZIP6 and ZIP10 are crucial for meiotic maturation of oocytes [53]. ZIP1, ZIP2, ZIP3, ZIP4, and ZIP8 have all been demonstrated to be important for fetal development [79–83], and mutations in ZnT2 in humans and ZnT4 in mice cause neonatal zinc deficiency due to low zinc content in breast milk [84, 85]. A number of zinc transporter genes are expressed in oocytes [53, 86], but it remains unclear where these transporters are expressed (i.e., oocyte or organelle membranes), when these transporters function (oogenesis, meiotic maturation, post fertilization), and the mechanistic impacts the zinc fluxes have on molecular processes in the oocyte.
Due to the number of interacting cell types and organ systems, it has proven difficult to isolate and interpret zinc trafficking proteins and pathways in mammals. Caenorhabditis elegans is emerging as a powerful model system to study reproductive phenomenon that can overcome drawbacks associated with using mammalian systems. Two independent studies established strong parallels between the roles of zinc in proper meiotic progression and offspring production in C. elegans and mammals [87, 88]. Both Hester et al. [87] and Mendoza et al. [88] found that zinc deficiency led to a pronounced reduction in the number of offspring produced, as well as several defects in oocyte maturation and development. A subsequent study found that zinc fluxes also occur in C. elegans oocytes following fertilization [89]. Zinc has also been shown to be critical for sperm activation in nematodes [90]. Furthermore, zipt-7.1 was recently identified as important for both hermaphrodite and male sperm activation and fertility [91, 92].
Notably, C. elegans has experimental advantages compared to mammals for studying the roles of zinc in reproduction; worms achieve reproductive maturity 3 days after hatching and can produce upwards of 300 offspring per animal [93]. C. elegans are predominantly hermaphroditic and therefore produce both male and female gametes (males are XO and are produced following nondisjunction of the X chromosome) [94]. Worms also have only 959 somatic cells, well characterized cell lineages [93, 95, 96], and because they are transparent, developmental defects can be tracked and imaged. The genome of C. elegans is also sequenced, and a significant number of orthologs exist between worms and humans [97, 98]. CRISPR-Cas9 genome editing has also been adapted for C. elegans, providing methods to quickly generate new strains [99–101]. These features allow for rapid and expansive studies into zinc biology in reproduction.
Although twenty-eight putative zinc transporters have been identified in C. elegans [102–109], many have yet to be characterized, only a few of them have been examined in the context of germline and oocyte development [91, 92, 110]. Therefore, we aimed to determine zinc transporters that are involved in C. elegans reproductive functions, and the impacts zinc trafficking has on gonad and germline development. Here we develop a general approach to evaluate reproductive phenotypes in predicted worm orthologs to the LIV-1 and ZIPII subfamilies, which include ZIPs such as human ZIP4/6/8/10 and ZIP1/2/3, respectively. These zinc importer subfamilies have been previously implicated in development of the oocyte and fetus [53, 79–83]. By generating deletion mutants, we identified several zinc transporters from these subfamilies that had impacts on C. elegans offspring production and gonad development. We found two zinc transporters expressed in the germ line and in somatic tissue that affect normal C. elegans reproductive biology.
Methods
Materials
Unless otherwise noted, all materials were obtained from MilliporeSigma. Wild-type C. elegans were a gift from the Morimoto Lab.
Strains
SMW63: zipt-1(wig7) I
SMW64: zipt-2.4 (wig8) I
SMW65: zipt-2.4 (wig9 [ZIPT-2.4::3xFLAG]) I
SMW66: zipt-3 (wig10) II
SMW67: zipt-15 (wig11) IV
SMW68: zipt-15 (wig12 [ZIPT-15::3xFLAG]) IV
Maintenance
All strains were maintained in a 20 °C incubator on standard nematode growth media (NGM) dishes (1.7% w/v bacteriological agar, 0.25% w/v peptone, 51.33 mM sodium chloride, 1 mM magnesium sulfate, 1 mM calcium chloride, 5 mg/L cholesterol, and 25 mM potassium phosphate) and with E. coli strain OP50 as a food source, following established protocols [111]. For dishes used for worm maintenance, OP50 was grown overnight in LB and seeded at 350 μL volumes for 6 cm dishes, and 900 μL for 10 cm dishes. Under experimental conditions, an OP50 stock was grown overnight then inoculated at 1:100 volume into fresh LB and incubated until the culture was in growth phase (OD600 = 0.6). Additives such as N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN) were added after autoclaving the NGM and before pouring the media into dishes.
Strain generation
All crRNAs were designed using a prediction algorithm from either benchling.com or crispor.tefor.net [112, 113]. All crRNAs, tracrRNAs, rescue constructs, and Cas9 were ordered from Integrated DNA Technologies, and sequences can be found in Table S1. The injection mix was prepared by first mixing 0.48 μL 100 μM target gene crRNAs, 0.20 μL 100 μM dpy-10 crRNA, and 0.34 μL 200 μM tracrRNA and incubating at 95 °C for 5 min then 10 °C for 5 min. The tubes were centrifuged at 13,000 rpm for 30 s, then 1.92 μL 10 mg/mL Cas9 was added to each mix and incubated at room temperature for 5 min. Finally, 0.20 μL 100 μM target gene rescue template and 0.67 μL dpy-10 rescue template were added and diluted to 5 μL using nuclease-free water. This mixture was kept on ice. Wild-type animals were injected using a Leica DM IRB Inverted Microscope with a Femtojet attachment. Needles were generated using a Sutter P-87 Micropipette Puller and loaded with a CRISPR mix using a mouth pipette. Each animal was immersed in oil on a 3% agarose pad and injected in both gonad arms. Following injection, worms were placed onto a recovery dish and washed with a droplet of M9 (22.04 mM potassium phosphate monobasic, 42.27 mM sodium phosphate dibasic, 85.56 mM sodium chloride, and 1 mM magnesium sulfate) to remove any oil. After a 24-h recovery, surviving animals were isolated onto individual dishes. Animals were allowed a few days to produce offspring, then the progeny of the 3 dishes with highest number of roller/dumpy phenotypes were isolated. These animals were allowed to produce offspring for 1–2 days, then genotyped. Animals that contained the desired mutation were passaged for homozygosity, as well as absence of dpy-10 phenotypes [99, 114, 115].
Brood size assays
Brood size assays were performed as described previously with the following modifications [88]. Animals were synchronized by allowing adult worms to lay eggs on standard NGM dishes for 1–2 h. Due to the developmental delay in zipt-15 mutants, the synchronization started 24 h prior to other strains. When worms reached the L4 stage, individual animals were transferred to separate dishes. Every 24 h for 5 days, each worm was transferred to a new dish, and after the final day, the worm was removed. The first 5 days after the onset of reproduction represents the majority of offspring produced [116]. The progeny of untreated animals were allowed 3 days and TPEN-treated animals an additional 3 days to develop before capturing images for ease of counting. The offspring of TPEN-treated animals did not reach reproductive maturity at the time of imaging (data not shown). Worms were imaged in a dark box setup using IC Capture software with a DMK 23GP031 monochrome industrial camera (ImagingSource). Dishes were illuminated from below with LED lights. Images were analyzed using ImageJ [117].
Embryonic lethality assays
Animals were synchronized by transferring L4 stage worms onto individual dishes. Worms were transferred as described in the brood size assay protocol. Twenty-four hours after a worm was transferred off a dish, the eggs remaining on the plate were considered embryonic lethal offspring and counted. The living larvae were allowed to develop for an additional 48 h and imaged. Embryonic lethality was then calculated by taking the ratio of total eggs counted to the sum of the total living offspring and eggs counted. The embryonic lethality for zipt-15(wig11) mutants was only calculated using the first 2 days of reproductive adulthood due to most animals dying to bagging or bursted vulva phenotypes before the time course was completed.
Nuclear imaging
Animals were synchronized by egg laying as described for the brood size assays. When worms reached day 2 of adulthood, 10 animals were picked into a droplet of M9 on a glass slide and fixed with ethanol as described [118]. Briefly, excess M9 was whisked off the slide using Whatman paper, and a few drops of 200 proof ethanol were added onto the M9. Immediately after the ethanol evaporated, a few more drops of ethanol were added, and this was repeated for 3 ethanol washes. While the last wash of ethanol was evaporating, a 6 μL drop of a 1:1 mixture of mounting media (0.5% p-phenylenediamine in 90% glycerol, 20 mM Tris, pH 8.8) and 10 μg/mL Hoechst 33342 (hereby abbreviated as Hoechst) in M9 was added to a coverslip. Immediately after the last ethanol wash had evaporated, the slide was placed on the coverslip such that the mounting media covered the worms and then the coverslip was sealed with nail polish. The slides were imaged using a Leica DM6B Widefield fluorescence microscope and stitched with the Leica Application Suite X software. Images were analyzed and processed using ImageJ to draw regions of interest (ROIs) of the gonad arms using both the DIC and Hoechst channels as a guide [117]. The gonad ROI was determined as starting from the distal tip, indicated by a convergence of germline nuclei, and ending with the entrance to the spermatheca, indicated by the narrowing of the gonad preceding a cluster of sperm nuclei. The total area was determined by the perimeter of the worm in the DIC channel. The percent area of the gonad was calculated by dividing the area of the gonad to the area of the entire animal and represents a fractional area.
Immunofluorescence imaging
Animals were synchronized by allowing adults to lay eggs on a standard NGM dish for 1–2 h. When offspring reached day 1–2 of adulthood, greater than 30 worms were picked into a droplet of M9 on a poly-L lysine glass slide (Fisher 47-100) and immunofluorescence was performed as described [118]. Briefly, gonads were dissected into the droplet using a pair of needles. After the worms were dissected, a coverslip was added, and the slide was plunged in liquid nitrogen for 10 min. After removing the slide, the coverslip was quickly removed, and the worms were fixed using −20 °C methanol for 35 min. Slides were then washed twice for 5 min with PBS and blocked using AbDil (PBS plus 4% BSA, 0.1% Triton X-100, and 0.02% sodium azide) overnight at 4 °C. All antibody incubations were performed in a humid chamber. Abdil was aspirated off and a 2 μg/mL solution of mouse anti-FLAG antibody (Sigma #F1804, Lot #: SLCF9337) was added and incubated overnight at 4 °C. Between each antibody probe, the slides were washed 3 times with PBS-T. 4 μg/mL goat anti-mouse conjugated to Alexa FluorTM 647 (Fisher A21235) in Abdil was added overnight at 4 °C. 3 μg/mL FITC-conjugated mouse anti-ɑ-tubulin in Abdil was added for 90 min at room temperature. Finally, 10 μg/mL Hoechst in PBS-T was added for 10 min at room temperature. A coverslip with a 6 μL droplet of mounting media was added on top of the sample and sealed with nail polish. Slides were imaged on a DeltaVision Core microscope. Images were deconvolved using the SoftWorx software conservative deconvolution algorithm for 15 cycles and processed in ImageJ. Fluorescence intensity measurements were acquired by drawing ROIs using the rotated rectangle in ImageJ. Each region was first assessed if oocyte borders were discernable, and if so, 3 ROIs were drawn at the distal and proximal poles of the oocyte. If the outlines of two adjacent oocytes could not be resolved, 3 ROIs were drawn at the interface and labeled as a “junction” of two oocytes. Each ROI was drawn on a single slice. Once all ROIs were drawn on an image, the measure function of the ROI manager tool in ImageJ was used to provide the average intensity of each ROI. Data was normalized as a ratio over the background. Briefly, 3 proximal and 3 distal or 3 junction ROIs were averaged, then divided by the average of 3 background ROIs.
Statistics
Data analyses were performed in R using the RStudio graphical user interface [119, 120]. The stats package was used for repeated measures testing using one- or two-way analysis of variance (ANOVA), followed by a post hoc pairwise t-test with Bonferroni corrections as well as Tukey Honest Significant Difference (HSD) test. Additionally, normality was tested using the Shapiro-Wilk test. The car package was used to test homogeneity of variance between data using Levene’s test [121]. Data was presented using packages ggplot2 and ggpubr [122, 123]. Data was imported, arranged, and exported using the broom, dplyr, and readxl packages [124–126].
Results
ZIPT-2.4 and ZIPT-15 are required for normal fecundity in C. elegans
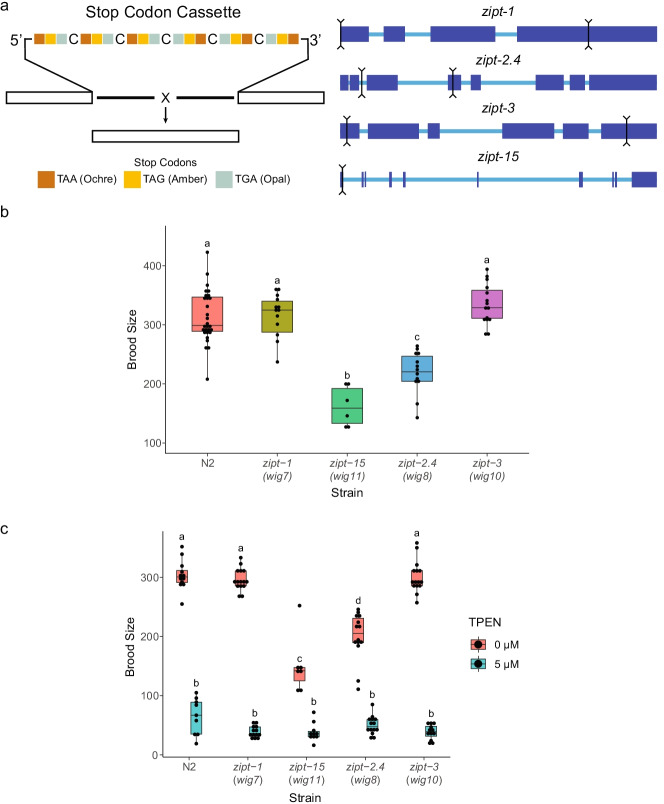
Evidence from mammalian literature suggested that identifying specific ZIP subfamilies in C. elegans would point to specific target genes to pursue as involved in reproduction. Both the LIV-1 and ZIPII subfamily of zinc importers have members that have defects in meiotic and zygotic development when disrupted [53, 80, 81, 127]. Of the 14 predicted and characterized ZIPs in C. elegans, 8 were found to belong to these two subfamilies by phylogenetic analysis (Fig. S1), in agreement with previous studies [57, 91, 105]. This set of candidate C. elegans genes was further narrowed by analyzing the expression levels in gonads and oocytes as reported in several microarray and RNA-seq datasets (Table S2) [128–130]. From these criteria, ZIPs ZIPT-1, ZIPT-2.4, ZIPT-3, and ZIPT-15 had both phylogenetic similarities to mammalian LIV-1 and ZIPII subfamilies and high expression in the germ line or oocytes. A CRISPR-Cas9 approach adapted from several studies was employed to make deletion mutants of these 4 genes by inserting a stop codon array in addition to deleting a significant portion of the gene (Fig. 1a) [99, 101, 114, 115]. The crRNA prediction algorithms for zipt-15 were unable to identify two cut sites that had both predicted high efficiency and low off-target rates, while also being within a few kilobases of each other. Therefore, in this instance, a single cut was made to insert the stop codon array.
Fig. 1.
Identification of brood size defects in zinc transporter mutants. a Cartoon depicting the stop codon array used to generate each zinc transporter mutant. Sets of stop codons were designed in every reading frame, and a perfect insertion of the cassette will also introduce a frameshift. The exons of each gene are depicted in the larger blue boxes. The black lines indicate the 5’ and 3’ cuts made to replace the intervening sequence with the stop codon array. For zipt-15, only one cut was made, and the cassette was inserted at this point. Box plots of the brood sizes of each zinc transporter mutant for the first 5 days of reproductive adulthood (b) and challenged with either 0 or 5 μM TPEN (c). Each plot represents 3 biological replicates with 5 technical replicates per experiment. Data for worms that died before the experiment completed were discarded. Box plots labeled with the same letter are statistically indistinguishable (p > 0.05) as determined by the post hoc Tukey HSD test
To determine which ZIPs have a role in reproduction, we first evaluated changes in brood size as a metric for identifying defects in the development and maturation of the germ line. The number of offspring produced by individual worms was determined over the first 5 days of reproductive maturity. Interestingly, during the period of maximum reproduction (days 1 and 2), zipt-15 mutants for day 1 and both zipt-2.4 and zipt-15 mutants for day 2 have a reduced brood (Fig. S2a), while the brood sizes of zipt-1 and zipt-3 mutants were similar to wild-type animals (Fig. S2b). This is also reflected in the 5-day output of these mutants (Fig. 1b), with zipt-2.4 mutants having a 32% reduced brood size and zipt-15 mutants having a 48% reduced brood size. When challenged by growth under zinc limiting conditions imposed by the addition of divalent metal chelator TPEN to the agar, the brood sizes for both wild-type and mutant animals were drastically reduced (Fig. 1c).
zipt-15 mutants have a defective gonad architecture
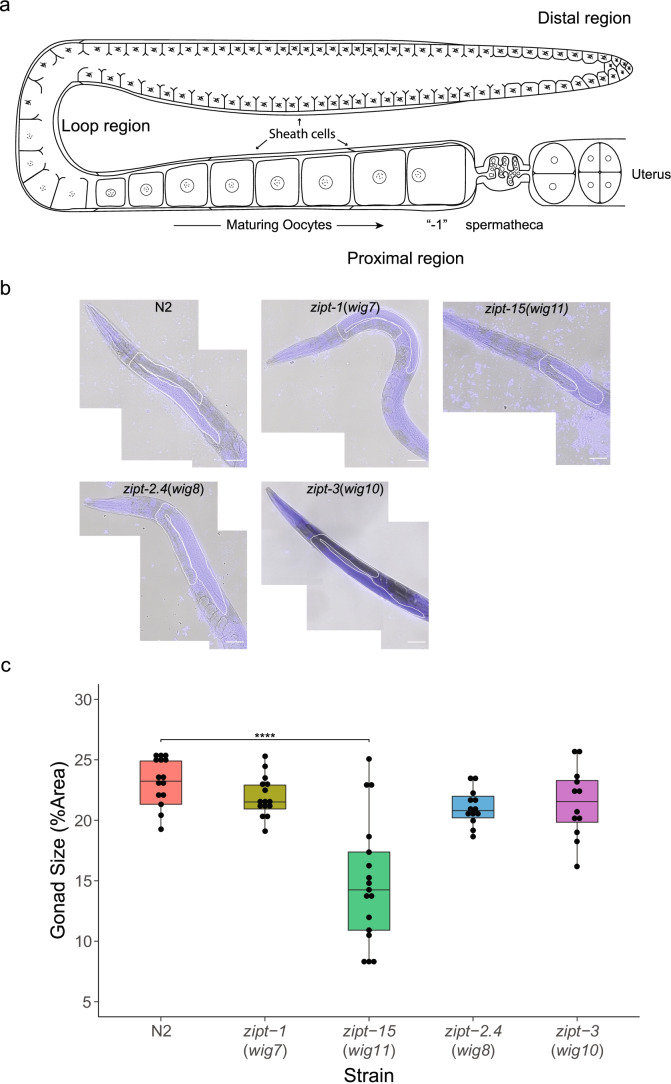
C. elegans gonads are readily observed in intact animals using DIC light microscopy. Each animal has two gonad arms; a schematic of one of the two arms is shown in Fig. 2a. Gonad boundaries are readily established as ROI after staining with Hoechst to delineate the location of germline nuclei (Fig. 2b). We therefore measured gonad size in our mutants, scored as the maximum area of a single gonad arm relative to the area of the whole worm (Fig. 2c). This measurement is a rough approximation of the relative volume of the gonad arm, under the assumption that the gonad and worm are both ellipsoids. Figure 2a illustrates the general architecture of a C. elegans gonad arm as well as represents the regions we captured with our measurements. The gonad sizes of zipt-1 and zipt-3 mutants are similar to wild type (Fig. 2b and c), consistent with the brood size results. Analysis of the zipt-2.4 mutant animals also revealed no significant difference in gonad size or appearance (Fig. 2b and c). In contrast, we found that zipt-15 mutant animals have significantly smaller gonads compared to their body size than wild-type animals. This could either be a result of zipt-15 mutants increasing in size or the gonads decreasing in size. To distinguish between these possibilities, we compared the areas of worms in each strain and found that these sizes did not significantly differ, demonstrating that the decrease in gonad arm size is not due to an increase in worm size (Fig. S3). Furthermore, zipt-15 mutants exhibit several other weakly penetrant phenotypes associated with defective egg-laying, including protruding vulva (1.9%), offspring hatching inside the parent (2.7%, also called “bagging”), and internal tissues bursting through the vulva (2.0%, Table S3, Fig. S4, and Movies S1–2).
Fig. 2.
Examination of the gonad architecture of zinc transporter mutants. a Cartoon depicting the general structure of a gonad arm in C. elegans. The distal region contains the mitotic niche, as well as early meiotic cells. Except for the most distal region of the gonad, the germ line is encased by 5 sets of cells called the sheath cells. As the cells enter the loop region, their membrane completely encloses to form oocytes. As these oocytes enter the proximal region, they become prepared for fertilization, arresting at Prophase I of meiosis. The most mature oocyte is referred to as “-1.” Oocytes are processively pushed through the spermatheca, which houses the sperm, and subsequently ejected into a uterus that is shared between both gonad arms. b Representative slices of stitched images of N2, zipt-1, zipt-15, zipt-2.4, and zipt-3 mutants stained with Hoechst. The white lines represent a trace of the gonad arm. Scale bar represents 50 μm. c Box plots of the relative gonad arm size of each zinc transporter mutant. Each plot represents at least 10 technical replicates. Significance values shown as a Tukey HSD test relative to control. ****p < 1E-6
zipt-2.4 and zipt-15 have expression in the germ line as well as other tissues
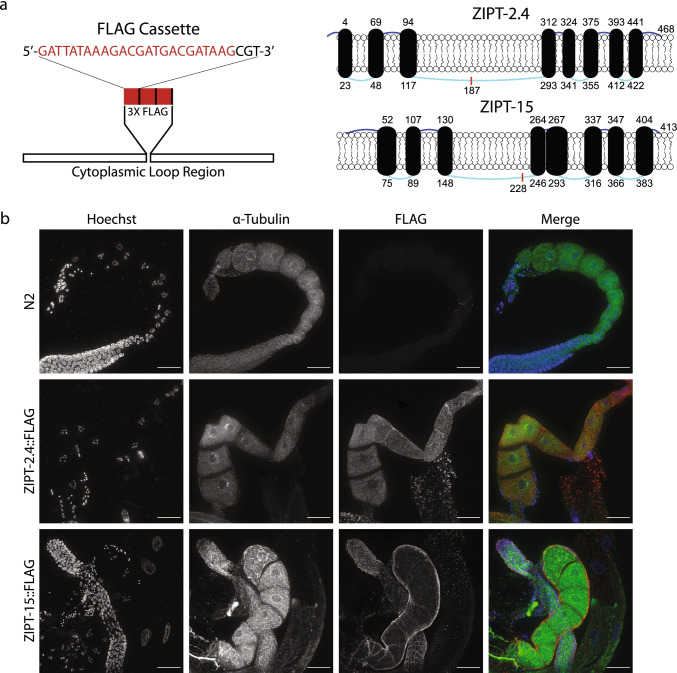
Although microarray and RNA-seq datasets indicate that both zipt-2.4 and zipt-15 have mRNA expression in reproductive cell types, it is unclear where each protein is localized and how that might impact zinc acquisition [128–130]. To address this, we inserted a FLAG epitope tag into the endogenous locus of these ZIP genes. These genes encode proteins with 8 transmembrane domains (TMDs), 7 loops between the TMDs, and N- and C-terminal domains that are present in extracellular space [32, 56]. Both ZIPT-2.4 and ZIPT-15 have a large cytoplasmic region between the third and fourth TMD, another feature common in ZIPs, and FLAG was inserted into this region (Fig. 3a) [131–133]. This region was chosen to minimize possible disruption of the TMD structure and any membrane localization sequence. Analysis of brood size and embryonic lethality of the ZIPT-2.4::FLAG and ZIPT-15::FLAG strains revealed no significant difference to wild type (Fig. S5), suggesting that the insertion does not substantially impact protein function, and thereby can be used to assess protein localization. Using an antibody to the FLAG epitope, the FLAG signal significantly increased in worms containing the FLAG array insertion compared to a wild-type strain (Fig. 3b). Additionally, when samples were not treated with FLAG antibody, there was an absence of signal in the FLAG channel, suggesting that the fluorescent signal is representative of antibody binding to the target epitope (Fig. S6). Multiple sections of the gonad were investigated and each of these regions had fluorescent signal (Fig. 4).
Fig. 3.
Generation of FLAG-tagged zinc transporter strains. a Cartoon representing the FLAG cassette used to tag ZIPT-2.4 and ZIPT-15. Each FLAG array (red) was separated by one arginine (black) and inserted in frame into the predicted cytoplasmic loop regions. The cylinders represent the transmembrane domains, which are connected by extracellular (dark blue) and intracellular (light blue) strands. Each number indicates the beginning and ending amino acid of the TM domains, and the lengths of the TM domains and loops are scaled to their size relative to the whole protein, with the exception of the N terminal strand of ZIPT-2.4 which was increased for clarity. The red line indicates the location of the FLAG insertion. b Representative maximum projections of wild-type (N2) and FLAG-tagged strains probed with FLAG (red) and tubulin (green) antibodies, as well as Hoechst to label DNA (blue). All images were taken under the same exposure conditions, and all images are displayed using the same minimum and maximum intensity values. Scale bar represents 25 μm
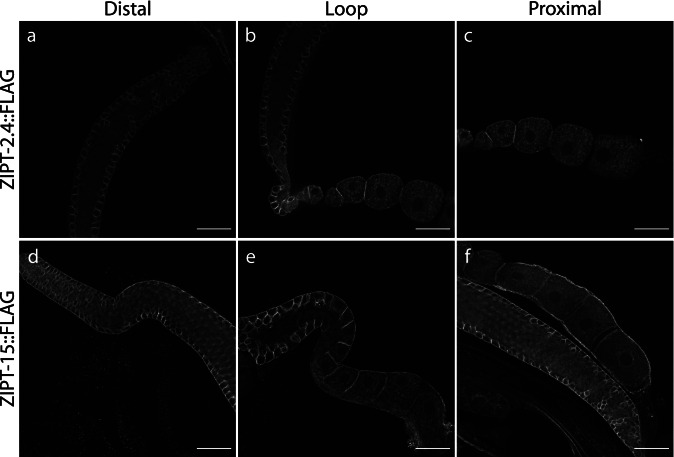
Fig. 4.
ZIPT-2.4 and ZIPT-15 are expressed in multiple regions of the gonad. Representative slices of the distal (a, d), loop (b, e), and proximal (c, f) regions in the gonads of the ZIPT-2.4::FLAG (top) and ZIPT-15::FLAG (bottom) strains, stained with an anti-FLAG antibody. All images were exposed and processed under equivalent conditions. Scale bar represents 25 μm
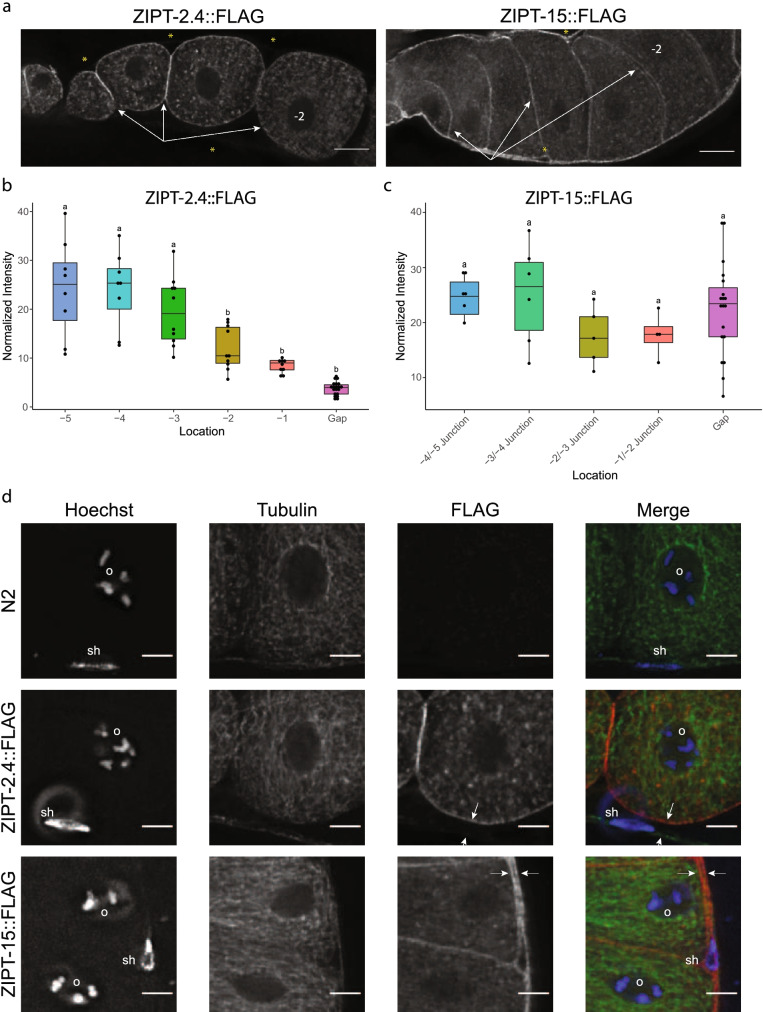
Upon closer inspection of the different regions of the gonad, similar and distinct expression patterns were observed between ZIPT-2.4 and ZIPT-15. Both ZIPs appear to localize to the membranes of germ cells from the distal through the proximal region of the gonad (Fig. 4 and Movies S3–8). We assessed the membrane fluorescence of the FLAG antibody in the proximal region of the gonad, which contains mature oocytes (Fig. S7). ZIPT-2.4::FLAG showed a significant decrease in signal from the -3 to -1 oocyte (Figs. 5a, b, S8a, and Movies S9–11). ZIPT-15::FLAG had no significant decrease in the same region (Figs. 5a, b, and S8a). Furthermore, the ZIPT-15::FLAG worms exhibited significant signal in the surrounding gonad membranes that are not in contact with oocyte membranes (Figs. 5c, d, S8b, and Movies S12–14). The cells closest to this region are sheath cells, which encase the germ line, suggesting that ZIPT-15 may be localized to sheath cell membranes as well. In addition, ZIPT-2.4::FLAG showed signal in vesicular-like structures in the intestine, and ZIPT-15::FLAG had punctate staining in this region that may also be vesicular, although the patterning is not as clear (Fig. S9 and Movies S15–16). Together, these results indicate that ZIPT-2.4 and ZIPT-15 are expressed in reproductive and somatic tissue, where they may promote normal C. elegans reproduction.
Fig. 5.
ZIPT-2.4::FLAG and ZIPT-15::FLAG have distinct expression patterns in the gonad. a Representative slices of ZIPT-2.4::FLAG and ZIPT-15::FLAG signal. In the images shown, oocyte maturity (age) is increasing from left to right, with the -2 oocyte labeled in each image. White arrows indicate oocyte membrane signal, which decreases from left to right. Yellow asterisks indicate either locations where sheath cell FLAG signal is weak or absent (ZIPT-2.4) or strong (ZIPT-15). b, c Box plots of normalized fluorescence intensity values of ZIPT-2.4::FLAG (b) and ZIPT-15::FLAG (c) associated with oocyte membranes and non-oocyte membranes adjacent to the oocyte. Box plots labeled with the same letter are statistically indistinguishable (p > 0.05) as determined by the post hoc Tukey HSD test. d Representative Z slices of zoomed insets of N2, ZIPT-2.4::FLAG, and ZIPT-15::FLAG immunofluorescence images. Shown are FLAG (red), tubulin (green), and DNA (blue). Each image contains an oocyte nucleus (labeled “o”), as well as genetic material not associated with oocytes; nuclei putatively associated with sheath cells are labeled “sh.” The white arrows indicate two membranes, signifying another cell type contacting the oocyte, likely the sheath cell, as well as the outer membrane of the putative sheath cell. All images were exposed and processed under equivalent conditions. Scale bar represents 5 μm
Discussion
Regulatory fluxes in zinc concentration and localization play a number of stage-specific roles in mammalian reproduction including meiotic maturation, the egg-to-embryo transition, and fertilization. Several of these roles for zinc are conserved in amphibians and invertebrates and thus span a large part of the evolutionary timeline [87, 88, 134–137]. Zinc fluxes in gametes involve coordination of zinc uptake, compartmentalization, and efflux processes, some of which have been shown to be mediated by integral membrane cation transport proteins. These zinc transporters are known to have an impact on several aspects of reproduction, ranging in scope from molecular and cellular to physiological [53, 58, 79, 81, 82, 91, 138]. Here we identify two zinc transporters in C. elegans that have impacts on reproduction: these factors belong to subsets of the zinc importing ZIP family of proteins that have known roles in mammalian reproduction. The protein encoded by the C. elegans zipt-15 gene appears to be from the ZIP subfamily LIV-1, based on our phylogenetic analysis (Fig. S1). The LIV-1 subfamily has members in mammals (ZIP6 and ZIP10) that drive a 50% increase of zinc quota of mouse eggs required for the progression from the GV to MII stage. The zipt-2.4 gene appears to belong to the ZIPII subfamily of zinc transporters, which have documented effects on fetal growth and development [79–81].
Many of the phenotypes observed with the zipt-15 mutant were associated with defects in development of the worm reproductive system (Figs. 2 and S2), which is consistent with the expression pattern we observed for ZIPT-15::FLAG (Figs. 3 and 4) if the site of action of ZIPT-15 is in these tissues. We note that although the penetrance of these phenotypes is low, wild-type animals very rarely display these phenotypes under normal growth conditions [139, 140]. Moreover, these penetrance values are representative of mutants in early adulthood, and a high number of mutant animals succumb to bursted vulvas or bagging as they age (77% of zipt-15 mutant adults perished before the end of day 5 in our experiments). This suggests a serious structural or mechanical defect in the vulva or with components of the reproductive system. Sheath cells are known to impact the development of the germ line, and therefore, a zinc insufficiency in the developing sheath precursors could result in defects in gonad size and germline quantity [141–144]. Although our immunofluorescence images revealed ZIPT-15 expression in the adult gonad, the vulval phenotypes suggest a role for this transporter in early gonad development, and future studies can uncover the temporal and spatial expression of both ZIPT-2.4 and ZIPT-15. The C. elegans reproductive system, which includes both the germ line and somatic gonad, arises from 4 cells, with a pair of these cells forming the anterior germ line and gonad and the other pair forming the posterior arm [96, 145]. Within each pair, one cell will give rise to the germ line, and the other will be responsible for forming several somatic gonad structures, including the sheath cells, spermatheca, and the anchor cell. Critically, the anchor cell functions to induce and pattern the development of the vulva [146, 147]. Therefore, if ZIPT-15 is important for early proliferation of these cell types, we would anticipate gonadal and vulval deformities. Additionally, zinc transporters have been previously implicated in vulval development. CDF-1 and SUR-7, both zinc exporters, impact the Ras signaling pathway, which is part of how the anchor cell dictates vulval development [106, 107].
Conversely, ZIPT-2.4, which has expression in both the intestine and germ line, does not appear to affect reproductive development; we did not detect substantial embryonic lethality or changes in gonad size in zipt-2.4 mutants. Therefore, it will be interesting in the future to further investigate the function of ZIPT-2.4 to understand why the mutants have a reduced brood size. Given that oocytes from mouse to worm must take up significant amounts of zinc just before fertilization [89] and that ZIPT-2.4 is expressed in the oocyte plasma membrane, one possibility is that defective zinc import during this critical period of development may impact the oocyte’s ability to acquire the necessary zinc requirement to progress through meiosis. However, if ZIPT-2.4 were the primary transporter mediating zinc import in the oocyte, we expect that there would be higher embryonic lethality in the zipt-2.4 mutant. Thus, we speculate that either there is a different cause of the reduced brood size or, if ZIPT-2.4 does mediate zinc uptake, that there are redundant transporters that can compensate for its absence [105]. Redundancy may also explain why the zinc transporter mutants were not hypersensitive to TPEN treatments. Several ZIPs have increased expression when worms are exposed to TPEN [105], which could overcome deficiencies in a single transporter. The intestinal expression may also be impacting the success of the germ line. In C. elegans, the intestine is the site for nutrient entry and storage, and in this context, zinc transport has been well characterized [102, 103, 108]. The vesicular expression of ZIPT-2.4 may also suggest that there is a defect in a compartment that is delivered to the oocytes. Interestingly, ZIPT-15 also has expression in the intestine; however, the structures observed were less clearly vesicular. Gut granules have been previously noted to adopt a “bi-lobed” appearance under high zinc conditions [102], which serve as an additional storage site to accommodate high dietary zinc levels. Based on this phenomenon, ZIPT-15 could facilitate zinc fluxes out of this compartment, which would not be inflated under normal conditions, consistent with the observations in the ZIPT-15::FLAG strain.
Although it has been documented that zinc deficiency can impact reproductive growth and maturation, fertility, and fetal development, there are still many functional roles to be established at the molecular and physiological level [50, 51, 80, 81, 135, 137]. Recent studies have implicated a handful of mechanisms by which zinc could be acting to facilitate late oocyte maturation or post fertilization events [134, 148]. Because zinc levels change drastically over the course of these processes, zinc transporters are becoming a critical piece of understanding how zinc fits into reproductive biology. Emerging evidence in C. elegans shows that zinc transporters influence several facets of the reproductive axis. A recent report describes the critical role of zipt-7.1 in sperm activation [91, 108], and here we provide evidence for zipt-2.4 and zipt-15 being important for gonad development and fecundity. Because both ZIPT-2.4 and ZIPT-15 are expressed in multiple cell types both within and outside the reproductive system, this underscores the scope of zinc in impacting reproductive health. Interestingly, although zipt-1 and zipt-3 were promising targets, these genes appear to either have redundant counterparts or are dispensable for normal reproduction: multiple knockout studies may be useful in the future to address their function. A recent study by Lee et al. in Arabidopsis observed a similar phenomenon, where three separate knockouts were required to expose physiological defects [149]. Furthermore, the authors found that the triple and quadruple mutants had partially defective seed production, which they postulate could be a result from poor zinc mobilization to the plant from the roots or directly to the endosperm and embryo. Some of the mutants tested also contained phylogenetically distinct ZIPs, emphasizing a strongly redundant system of transporters. Similar requirements between C. elegans and other systems for zinc and zinc transport for reproduction indicate a deep conservation in function, and therefore, discoveries in C. elegans have implications in reproductive processes that are shared across evolution.
It is important to note that although this study provides evidence for zipt-2.4 and zipt-15 having a role in reproduction, we currently cannot distinguish the impacts these genes have on reproductive function at the cell autonomous and cell non-autonomous levels, particularly considering that these genes are expressed in multiple tissues. Furthermore, we chose to assess our mutants during a specific stage, reproductive adulthood, meaning that development of reproductive cells and tissues was not captured. Finally, the changes to zinc levels or fluxes as a result of mutating these genes are not yet known. Thorough investigations of zipt-2.4 and zipt-15 in future studies will clarify exactly where and how these genes function. Rescuing the transporter using tissue-specific drivers [150, 151] will reveal how the zinc transporters function in specific cell types. Combined with zinc probes [52, 152], this will give insight into changes in zinc distribution upon restoring zinc transporter function, and whether this has cell non-autonomous effects. Following changes in zinc distribution and tissue morphology throughout development in these mutants or in mutants with tissue-specific rescues will also demonstrate how reproductive cells and tissues depend on the function of zinc transport. Here, we provide evidence that two zinc transporters impact germline and gonad development and provide a platform to deeply investigate the role of zinc transport in reproductive function.
Supplementary information
(PDF 1491 kb)
(ZIP 482796 kb)
Acknowledgements
The authors would like to thank Dr. Mendoza for many helpful discussions regarding C. elegans zinc transporter biology, R. Brielmann and the Morimoto lab for instruction and use of their microinjection setup, and Dr. Zhang, Dr. Crombie, and the Andersen lab for assistance and use of their worm plate imaging setup. Microscopy was performed at the Biological Imaging Facility at Northwestern University (RRID:SCR_017767), graciously supported by the Chemistry for Life Processes Institute, the NU Office for Research, and the Department of Molecular Biosciences. Dr. Hornick and Dr. Antonova were helpful in resolving microscopy issues and recommending imaging parameters.
Author contribution
All authors designed the research and experiments. ACS performed the experiments and all authors contributed to data analysis and interpretation. ACS drafted the initial manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Research in this study was supported by National Institute of Health grants R01GM115848 (TKW and TVO), U54CA193419 (TVO), R01GM038784 (TVO), and R01GM124354 (SMW).
Data availability
The data presented in this article will be shared on request to the corresponding author.
Code availability
The code scripts used in this article will be shared on request to the corresponding author.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aaron C. Sue, Email: aaronsue2017@u.northwestern.edu
Sarah M. Wignall, Email: s-wignall@northwestern.edu
Teresa K. Woodruff, Email: tkw@msu.edu
Thomas V. O’Halloran, Email: ohallor8@msu.edu
References
- 1.Quesada-Candela C, Loose J, Ghazi A, Yanowitz JL. Molecular basis of reproductive senescence: insights from model organisms. J Assist Reprod Genet. 2021;38:17–32. doi: 10.1007/s10815-020-01959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu L, et al. Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci. 2015;72:251–271. doi: 10.1007/s00018-014-1739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin YJ, et al. Detrimental effect of maternal and post-weaning high-fat diet on the reproductive function in the adult female offspring rat: roles of insulin-like growth factor 2 and the ovarian circadian clock. J Assist Reprod Genet. 2017;34:817–826. doi: 10.1007/s10815-017-0915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587:905–915. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaskins AJ, et al. Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet Gynecol. 2014;124:801–809. doi: 10.1097/AOG.0000000000000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavarro JE, Schlaff WD. Introduction: impact of nutrition on reproduction: an overview. Fertil Steril. 2018;110:557–559. doi: 10.1016/j.fertnstert.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Gaskins AJ, et al. Substantial weight gain in adulthood is associated with lower probability of live birth following assisted reproduction. J Nutr. 2021;151:649–656. doi: 10.1093/jn/nxaa371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagliardini L, et al. High prevalence of vitamin D deficiency in infertile women referring for assisted reproduction. Nutrients. 2015;7:9972–9984. doi: 10.3390/nu7125516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu YH, et al. Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum Reprod. 2018;33:156–165. doi: 10.1093/humrep/dex335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman HJ. Reproductive changes associated with celiac disease. World J Gastroenterol. 2010;16:5810–5814. doi: 10.3748/wjg.v16.i46.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham TW. Trace element deficiencies in cattle. Vet Clin North Am Food Anim Pract. 1991;7:153–215. doi: 10.1016/s0749-0720(15)30816-1. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Botella A, et al. Impact of heavy metals on human male fertility—an overview. Antioxidants (Basel) 2021;10:1473. doi: 10.3390/antiox10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloom MS, et al. Associations between toxic metals in follicular fluid and in vitro fertilization (IVF) outcomes. J Assist Reprod Genet. 2012;29:1369–1379. doi: 10.1007/s10815-012-9882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aitken RJ, et al. Potential importance of transition metals in the induction of DNA damage by sperm preparation media. Hum Reprod. 2014;29:2136–2147. doi: 10.1093/humrep/deu204. [DOI] [PubMed] [Google Scholar]
- 16.Roussou P, Tsagarakis NJ, Kountouras D, Livadas S, Diamanti-Kandarakis E. Beta-thalassemia major and female fertility: the role of iron and iron-induced oxidative stress. Anemia. 2013;2013:617204. doi: 10.1155/2013/617204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza TL, et al. Multigenerational analysis of the functional status of male reproductive system in mice after exposure to realistic doses of manganese. Food Chem Toxicol. 2019;133:110763. doi: 10.1016/j.fct.2019.110763. [DOI] [PubMed] [Google Scholar]
- 18.Christian P. Micronutrients and reproductive health issues: an international perspective. J Nutr. 2003;133:1969S–1973S. doi: 10.1093/jn/133.6.1969S. [DOI] [PubMed] [Google Scholar]
- 19.Sommer AL, Lipman CB. Evidence on the indispensable nature of zinc and boron for higher green plants. Plant Physiol. 1926;1:231–249. doi: 10.1104/pp.1.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad AS, Miale A, Jr, Farid Z, Sandstead HH, Schulert AR. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J Lab Clin Med. 1963;61:537–549. [PubMed] [Google Scholar]
- 21.Nasiadek M, Stragierowicz J, Klimczak M, Kilanowicz A. The role of zinc in selected female reproductive system disorders. Nutrients. 2020;12:2464. doi: 10.3390/nu12082464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apgar J. Zinc and reproduction. Annu Rev Nutr. 1985;5:43–68. doi: 10.1146/annurev.nu.05.070185.000355. [DOI] [PubMed] [Google Scholar]
- 23.Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: an integrative review. J Res Med Sci. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- 24.Cox EH, McLendon GL. Zinc-dependent protein folding. Curr Opin Chem Biol. 2000;4:162–165. doi: 10.1016/s1367-5931(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 25.Kochanczyk T, et al. Metal-coupled folding as the driving force for the extreme stability of Rad50 zinc hook dimer assembly. Sci Rep. 2016;6:36346. doi: 10.1038/srep36346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Zhang J, Wang J, Wang W. Metal-coupled folding of Cys2His2 zinc-finger. J Am Chem Soc. 2008;130:892–900. doi: 10.1021/ja075302g. [DOI] [PubMed] [Google Scholar]
- 27.Lee YM, Lim C. Physical basis of structural and catalytic Zn-binding sites in proteins. J Mol Biol. 2008;379:545–553. doi: 10.1016/j.jmb.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Pace NJ, Weerapana E. Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules. 2014;4:419–434. doi: 10.3390/biom4020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King JC, et al. Biomarkers of nutrition for development (BOND)-zinc review. J Nutr. 2016. 10.3945/jn.115.220079. [DOI] [PMC free article] [PubMed]
- 30.Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- 31.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T, et al. Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway. Sci Adv. 2017;3:e1700344. doi: 10.1126/sciadv.1700344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calmettes C, et al. The molecular mechanism of zinc acquisition by the neisserial outer-membrane transporter ZnuD. Nat Commun. 2015;6:7996. doi: 10.1038/ncomms8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JK, et al. Elucidating the role of metal ions in carbonic anhydrase catalysis. Nat Commun. 2020;11:4557. doi: 10.1038/s41467-020-18425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theorell H, Mc KJ. Mechanism of action of liver alcohol dehydrogenase. Nature. 1961;192:47–50. doi: 10.1038/192047a0. [DOI] [PubMed] [Google Scholar]
- 36.Kim B, Lee WW. Regulatory role of zinc in immune cell signaling. Mol Cell. 2021;44:335–341. doi: 10.14348/molcells.2021.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99:1515–1522. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- 39.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 40.Gilston BA, et al. Structural and mechanistic basis of zinc regulation across the E. coli Zur regulon. PLoS Biol. 2014;12:e1001987. doi: 10.1371/journal.pbio.1001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 42.Passerini A, Andreini C, Menchetti S, Rosato A, Frasconi P. Predicting zinc binding at the proteome level. BMC Bioinform. 2007;8:39. doi: 10.1186/1471-2105-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maret W. Zinc and the zinc proteome. Met Ions Life Sci. 2013;12:479–501. doi: 10.1007/978-94-007-5561-1_14. [DOI] [PubMed] [Google Scholar]
- 44.Maywald M, Wessels I, Rink L. Zinc signals and immunity. Int J Mol Sci. 2017;18:2222. doi: 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emdin SO, Dodson GG, Cutfield JM, Cutfield SM. Role of zinc in insulin biosynthesis. Some possible zinc-insulin interactions in the pancreatic B-cell. Diabetologia. 1980;19:174–182. doi: 10.1007/BF00275265. [DOI] [PubMed] [Google Scholar]
- 46.Cruz KJC, et al. Zinc and insulin resistance: biochemical and molecular aspects. Biol Trace Elem Res. 2018;186:407–412. doi: 10.1007/s12011-018-1308-z. [DOI] [PubMed] [Google Scholar]
- 47.Fallah A, Mohammad-Hasani A, Colagar AH. Zinc is an essential element for male fertility: a review of Zn roles in men’s health, germination, sperm quality, and fertilization. J Reprod Infertil. 2018;19:69–81. [PMC free article] [PubMed] [Google Scholar]
- 48.Grieger JA, et al. Maternal selenium, copper and zinc concentrations in early pregnancy, and the association with fertility. Nutrients. 2019;11:1609. doi: 10.3390/nu11071609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams RB, Mills CF. The experimental production of zinc deficiency in the rat. Br J Nutr. 1970;24:989–1003. doi: 10.1079/bjn19700102. [DOI] [PubMed] [Google Scholar]
- 50.Kim AM, Vogt S, O’Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim AM, et al. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Que EL, et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem. 2015;7:130–139. doi: 10.1038/nchem.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong BY, et al. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol Hum Reprod. 2014;20:1077–1089. doi: 10.1093/molehr/gau066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai X, Moraes TF, Reithmeier RAF. Structural biology of solute carrier (SLC) membrane transport proteins. Mol Membr Biol. 2017;34:1–32. doi: 10.1080/09687688.2018.1448123. [DOI] [PubMed] [Google Scholar]
- 55.Colas C, Ung PM, Schlessinger A. SLC transporters: structure, function, and drug discovery. Medchemcomm. 2016;7:1069–1081. doi: 10.1039/C6MD00005C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeong J, Eide DJ. The SLC39 family of zinc transporters. Mol Asp Med. 2013;34:612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 58.Hara T, et al. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J Physiol Sci. 2017;67:283–301. doi: 10.1007/s12576-017-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kambe T, Taylor KM, Fu D. Zinc transporters and their functional integration in mammalian cells. J Biol Chem. 2021;296:100320. doi: 10.1016/j.jbc.2021.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shusterman E, et al. ZnT-1 extrudes zinc from mammalian cells functioning as a Zn(2+)/H(+) exchanger. Metallomics. 2014;6:1656–1663. doi: 10.1039/c4mt00108g. [DOI] [PubMed] [Google Scholar]
- 61.Ohana E, et al. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J Biol Chem. 2009;284:17677–17686. doi: 10.1074/jbc.M109.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao Y, Fu D. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. J Biol Chem. 2004;279:17173–17180. doi: 10.1074/jbc.M400208200. [DOI] [PubMed] [Google Scholar]
- 63.Lin W, Chai J, Love J, Fu D. Selective electrodiffusion of zinc ions in a Zrt-, Irt-like protein, ZIPB. J Biol Chem. 2010;285:39013–39020. doi: 10.1074/jbc.M110.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Girijashanker K, et al. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008;73:1413–1423. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaither LA, Eide DJ. Functional expression of the human hZIP2 zinc transporter. J Biol Chem. 2000;275:5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 66.Zhang T, Sui D, Hu J. Structural insights of ZIP4 extracellular domain critical for optimal zinc transport. Nat Commun. 2016;7:11979. doi: 10.1038/ncomms11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilkey JC, Jaffe LF, Ridgway EB, Reynolds GT. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol. 1978;76:448–466. doi: 10.1083/jcb.76.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tokmakov AA, Stefanov VE, Iwasaki T, Sato K, Fukami Y. Calcium signaling and meiotic exit at fertilization in Xenopus egg. Int J Mol Sci. 2014;15:18659–18676. doi: 10.3390/ijms151018659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takayama J, Fujita M, Onami S. In vivo live imaging of calcium waves and other cellular processes during fertilization in Caenorhabditis elegans. Bio Protoc. 2017;7:e2205. doi: 10.21769/BioProtoc.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kury S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 71.Ziliotto S, et al. Activated zinc transporter ZIP7 as an indicator of anti-hormone resistance in breast cancer. Metallomics. 2019;11:1579–1592. doi: 10.1039/c9mt00136k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor KM, et al. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology. 2008;149:4912–4920. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 73.H. Diabetes Genetics Initiative of Broad Institute of et al Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 74.Boesgaard TW, et al. The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients—the EUGENE2 study. Diabetologia. 2008;51:816–820. doi: 10.1007/s00125-008-0955-6. [DOI] [PubMed] [Google Scholar]
- 75.Lazarczyk M, et al. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J Exp Med. 2008;205:35–42. doi: 10.1084/jem.20071311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zou J, Milon BC, Desouki MM, Costello LC, Franklin RB. hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of ras responsive element binding protein-1 (RREB-1) Prostate. 2011;71:1518–1524. doi: 10.1002/pros.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JH, et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156:730–743. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 78.Giunta C, et al. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome—an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am J Hum Genet. 2008;82:1290–1305. doi: 10.1016/j.ajhg.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andrews GK, Wang H, Dey SK, Palmiter RD. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis. 2004;40:74–81. doi: 10.1002/gene.20067. [DOI] [PubMed] [Google Scholar]
- 80.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis. 2006;44:239–251. doi: 10.1002/dvg.20211. [DOI] [PubMed] [Google Scholar]
- 81.Kambe T, Geiser J, Lahner B, Salt DE, Andrews GK. Slc39a1 to 3 (subfamily II) zip genes in mice have unique cell-specific functions during adaptation to zinc deficiency. Am J Phys Regul Integr Comp Phys. 2008;294:R1474–R1481. doi: 10.1152/ajpregu.00130.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galvez-Peralta M, et al. ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero. PLoS One. 2012;7:e36055. doi: 10.1371/journal.pone.0036055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dufner-Beattie J, et al. The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum Mol Genet. 2007;16:1391–1399. doi: 10.1093/hmg/ddm088. [DOI] [PubMed] [Google Scholar]
- 84.Itsumura N, et al. Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant. PLoS One. 2013;8:e64045. doi: 10.1371/journal.pone.0064045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 86.Menezo Y, et al. Zinc concentrations in serum and follicular fluid during ovarian stimulation and expression of Zn2+ transporters in human oocytes and cumulus cells. Reprod BioMed Online. 2011;22:647–652. doi: 10.1016/j.rbmo.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 87.Hester J, Hanna-Rose W, Diaz F. Zinc deficiency reduces fertility in C. elegans hermaphrodites and disrupts oogenesis and meiotic progression. Comp Biochem Physiol C Toxicol Pharmacol. 2017;191:203–209. doi: 10.1016/j.cbpc.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mendoza AD, Woodruff TK, Wignall SM, O’Halloran TV. Zinc availability during germline development impacts embryo viability in Caenorhabditis elegans. Comp Biochem Physiol C Toxicol Pharmacol. 2017;191:194–202. doi: 10.1016/j.cbpc.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mendoza AD, Sue A, Antipova O, Vogt S, Woodruff TK, Wignall SM, O'Halloran TV. Dynamic zinc fluxes regulate meiotic progression in C. elegans oocytes. Biol Reprod. 2022. 10.1093/biolre/ioac064. [DOI] [PMC free article] [PubMed]
- 90.Liu Z, Chen L, Shang Y, Huang P, Miao L. The micronutrient element zinc modulates sperm activation through the SPE-8 pathway in Caenorhabditis elegans. Development. 2013;140:2103–2107. doi: 10.1242/dev.091025. [DOI] [PubMed] [Google Scholar]
- 91.Zhao Y, et al. The zinc transporter ZIPT-7.1 regulates sperm activation in nematodes. PLoS Biol. 2018;16:e2005069. doi: 10.1371/journal.pbio.2005069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan CH, Kornfeld K. Zinc is an intracellular signal during sperm activation in Caenorhabditis elegans. Development. 2021;148:dev199836. doi: 10.1242/dev.199836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corsi AK, Wightman B, Chalfie M. A transparent window into biology: a primer on Caenorhabditis elegans. Genetics. 2015;200:387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lints R, Halls DH. Reproductive system, overview. WormAtlas. 2009. 10.3908/wormatlas.1.21.
- 95.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 96.Kimble J, Hirsh D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- 97.C. e. S. Consortium Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 98.Kim W, Underwood RS, Greenwald I, Shaye DD. OrthoList 2: a new comparative genomic analysis of human and Caenorhabditis elegans genes. Genetics. 2018;210:445–461. doi: 10.1534/genetics.118.301307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paix A, Folkmann A, Rasoloson D, Seydoux G. High efficiency, homology-directed genome editing in caenorhabditis elegans using CRISPR-Cas9 ribonucleoprotein complexes. Genetics. 2015;201:47–54. doi: 10.1534/genetics.115.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friedland AE, et al. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods. 2013;10:741–743. doi: 10.1038/nmeth.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim H, et al. A co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics. 2014;197:1069–1080. doi: 10.1534/genetics.114.166389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roh HC, Collier S, Guthrie J, Robertson JD, Kornfeld K. Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans. Cell Metab. 2012;15:88–99. doi: 10.1016/j.cmet.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roh HC, et al. ttm-1 encodes CDF transporters that excrete zinc from intestinal cells of C. elegans and act in a parallel negative feedback circuit that promotes homeostasis. PLoS Genet. 2013;9:e1003522. doi: 10.1371/journal.pgen.1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roh HC, et al. A modular system of DNA enhancer elements mediates tissue-specific activation of transcription by high dietary zinc in C. elegans. Nucleic Acids Res. 2015;43:803–816. doi: 10.1093/nar/gku1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dietrich N, Schneider DL, Kornfeld K. A pathway for low zinc homeostasis that is conserved in animals and acts in parallel to the pathway for high zinc homeostasis. Nucleic Acids Res. 2017;45:11658–11672. doi: 10.1093/nar/gkx762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bruinsma JJ, Jirakulaporn T, Muslin AJ, Kornfeld K. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev Cell. 2002;2:567–578. doi: 10.1016/s1534-5807(02)00151-x. [DOI] [PubMed] [Google Scholar]
- 107.Yoder JH, Chong H, Guan KL, Han M. Modulation of KSR activity in Caenorhabditis elegans by Zn ions, PAR-1 kinase and PP2A phosphatase. EMBO J. 2004;23:111–119. doi: 10.1038/sj.emboj.7600025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Earley BJ, Mendoza AD, Tan CH, Kornfeld K. Zinc homeostasis and signaling in the roundworm C. elegans. Biochim Biophys Acta Mol Cell Res. 2021;1868:118882. doi: 10.1016/j.bbamcr.2020.118882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davis DE, et al. The cation diffusion facilitator gene cdf-2 mediates zinc metabolism in Caenorhabditis elegans. Genetics. 2009;182:1015–1033. doi: 10.1534/genetics.109.103614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chapman EM, et al. A conserved CCM complex promotes apoptosis non-autonomously by regulating zinc homeostasis. Nat Commun. 2019;10:1791. doi: 10.1038/s41467-019-09829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stiernagle T. Maintenance of C. elegans. WormBook. 2006;1-11. 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed]
- 112.Hsu PD, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Concordet JP, Haeussler M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018;46:W242–W245. doi: 10.1093/nar/gky354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arribere JA, et al. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics. 2014;198:837–846. doi: 10.1534/genetics.114.169730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Richardson CD, Ray GJ, DeWitt MA, Curie GL, Corn JE. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat Biotechnol. 2016;34:339–344. doi: 10.1038/nbt.3481. [DOI] [PubMed] [Google Scholar]
- 116.Muschiol D, Schroeder F, Traunspurger W. Life cycle and population growth rate of Caenorhabditis elegans studied by a new method. BMC Ecol. 2009;9:14. doi: 10.1186/1472-6785-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wolff ID, Divekar NS, Wignall SM. Methods for investigating cell division mechanisms in C. elegans. Methods Mol Biol. 2022;2415:19–35. doi: 10.1007/978-1-0716-1904-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Version 4.4.1. 2021. https://www.R-project.org/.
- 120.RStudio Team. RStudio: Integrated development for R. Boston, MA: RStudio, PBC. Version 1.4.1717. 2021. https://www.rstudio.com.
- 121.Fox J, Weisberg S. An {R} companion to applied regression. 3rd Ed. Thousand Oaks, CA: Sage 2019.
- 122.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 123.Kassambara A. ggpubr: ‘ggplot2’ based publication ready plots. R package version 0.4.0. 2020. https://CRAN.R-project.org/package=ggpubr.
- 124.Robinson D, Hayes A, Couch S. broom: convert statistical objects into tidy tibbles. R package version 0.7.9. 2021. https://CRAN.R-project.org/package=broom.
- 125.Wickham H, François R, Henry L, Müller K. dplyr: a grammar of data manipulation. Version 1.0.7. 2021. https://CRAN.R-project.org/package=dplyr.
- 126.Wickham H, Bryan J. readxl: read excel files. R package version 1.3.1. 2019. https://CRAN.R-project.org/package=readxl.
- 127.Taylor KM, et al. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem J. 2016;473:2531–2544. doi: 10.1042/BCJ20160388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- 129.Ortiz MA, Noble D, Sorokin EP, Kimble J. A new dataset of spermatogenic vs. oogenic transcriptomes in the nematode Caenorhabditis elegans. G3 (Bethesda) 2014;4:1765–1772. doi: 10.1534/g3.114.012351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stoeckius M, et al. Global characterization of the oocyte-to-embryo transition in Caenorhabditis elegans uncovers a novel mRNA clearance mechanism. EMBO J. 2014;33:1751–1766. doi: 10.15252/embj.201488769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim Biophys Acta. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 132.Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005;579:427–432. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 133.Bin BH, et al. Biochemical characterization of human ZIP13 protein: a homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J Biol Chem. 2011;286:40255–40265. doi: 10.1074/jbc.M111.256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seeler JF, et al. Metal ion fluxes controlling amphibian fertilization. Nat Chem. 2021;13:683–691. doi: 10.1038/s41557-021-00705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tian X, Diaz FJ. Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Dev Biol. 2013;376:51–61. doi: 10.1016/j.ydbio.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu Q, et al. Zinc dynamics during drosophila oocyte maturation and egg activation. iScience. 2020;23:101275. doi: 10.1016/j.isci.2020.101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Beaver LM, et al. Adverse effects of parental zinc deficiency on metal homeostasis and embryonic development in a zebrafish model. J Nutr Biochem. 2017;43:78–87. doi: 10.1016/j.jnutbio.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Croxford TP, McCormick NH, Kelleher SL. Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J Nutr. 2011;141:359–365. doi: 10.3945/jn.110.131318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eisenmann DM, Kim SK. Protruding vulva mutants identify novel loci and Wnt signaling factors that function during Caenorhabditis elegans vulva development. Genetics. 2000;156:1097–1116. doi: 10.1093/genetics/156.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hurd DD, Kemphues KJ. PAR-1 is required for morphogenesis of the Caenorhabditis elegans vulva. Dev Biol. 2003;253:54–65. doi: 10.1006/dbio.2002.0866. [DOI] [PubMed] [Google Scholar]
- 141.Seydoux G, Schedl T, Greenwald I. Cell-cell interactions prevent a potential inductive interaction between soma and germline in C. elegans. Cell. 1990;61:939–951. doi: 10.1016/0092-8674(90)90060-r. [DOI] [PubMed] [Google Scholar]
- 142.McCarter J, Bartlett B, Dang T, Schedl T. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev Biol. 1997;181:121–143. doi: 10.1006/dbio.1996.8429. [DOI] [PubMed] [Google Scholar]
- 143.Killian DJ, Hubbard EJ. Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev Biol. 2005;279:322–335. doi: 10.1016/j.ydbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 144.Korta DZ, Hubbard EJ. Soma-germline interactions that influence germline proliferation in Caenorhabditis elegans. Dev Dyn. 2010;239:1449–1459. doi: 10.1002/dvdy.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 146.Schindler AJ, Sherwood DR. Morphogenesis of the caenorhabditis elegans vulva. Wiley Interdiscip Rev Dev Biol. 2013;2:75–95. doi: 10.1002/wdev.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hwang BJ, Sternberg PW. A cell-specific enhancer that specifies lin-3 expression in the C. elegans anchor cell for vulval development. Development. 2004;131:143–151. doi: 10.1242/dev.00924. [DOI] [PubMed] [Google Scholar]
- 148.Que EL, et al. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr Biol (Camb) 2017;9:135–144. doi: 10.1039/c6ib00212a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee S, et al. Redundant roles of four ZIP family members in zinc homeostasis and seed development in Arabidopsis thaliana. Plant J. 2021. 10.1111/tpj.15506. [DOI] [PMC free article] [PubMed]
- 150.Merritt C, Rasoloson D, Ko D, Seydoux G. 3’ UTRs are the primary regulators of gene expression in the C. elegans germline. Curr Biol. 2008;18:1476–1482. doi: 10.1016/j.cub.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Haenni S, et al. Analysis of C. elegans intestinal gene expression and polyadenylation by fluorescence-activated nuclei sorting and 3’-end-seq. Nucleic Acids Res. 2012;40:6304–6318. doi: 10.1093/nar/gks282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garwin SA, et al. Interrogating intracellular zinc chemistry with a long stokes shift zinc probe ZincBY-4. J Am Chem Soc. 2019;141:16696–16705. doi: 10.1021/jacs.9b06442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1491 kb)
(ZIP 482796 kb)
Data Availability Statement
The data presented in this article will be shared on request to the corresponding author.
The code scripts used in this article will be shared on request to the corresponding author.