Abstract
The mass inoculation of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine to induce herd immunity is one of the most effective measures to fight COVID-19. The vaccination of pregnant women cannot only avoid or reduce the probability of infectious diseases, but also offers the most effective and direct protection for neonates by means of passive immunization. However, there is no randomized clinical data to ascertain whether the inactivated vaccination of pregnant women or women of childbearing age can affect conception and the fetus. We found that human angiotensin-converting enzyme 2 (hACE2) mice that were vaccinated with two doses of CoronaVac (an inactivated SARS-CoV-2 vaccine) before and during pregnancy exhibited normal weight changes and reproductive performance indices; the physical development of their offspring was also normal. Following intranasal inoculation with SARS-CoV-2, pregnant mice in the immunization group all survived; reproductive performance indices and the physical development of offspring were all normal. In contrast, mice in the non-immunization group all died before delivery. Analyses showed that inoculation of CoronaVac was safe and did not exert any significant effects on pregnancy, lactation, or the growth of offspring in hACE2 mice. Vaccination effectively protected the pregnant mice against SARS-CoV-2 infection and had no adverse effects on the growth and development of the offspring, thus suggesting that inoculation with an inactivated SARS-CoV-2 vaccine may be an effective strategy to prevent infection in pregnant women.
Keywords: Inactivated SARS-CoV-2 vaccine, Pregnant hACE2 mice, Safety, Protective capability
1. Introduction
The pandemic of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has brought tremendous pressure and disastrous consequences to public health and medical system on a global scale. The mass inoculation of a SARS-CoV-2 vaccine to induce herd immunity is considered as one of the most effective measures to fight Coronavirus Disease-2019 (COVID-19) [1]. The inactivated SARS-CoV-2 vaccination strategy is being conducted in an orderly manner in China, manifesting good safety and immunogenicity among healthy adults (18–59 years old) [2] and people aged above 60 years [3], and is being further expanded to cover the 3–17 years age group. To protect pregnant women and fetuses, however, pregnant women were not included in phase I, II & III clinical trials during the research and development of SARS-CoV-2 vaccines. There are few clinical data to ascertain whether the inactivated vaccination of pregnant women, or women of childbearing age, has side effects during pregnancy or for the fetus, and whether it can protect pregnant women and fetuses from SARS-CoV-2 infection. Pregnant women are prone to a higher risk of severe illness than non-pregnant women; this may not only lead to premature delivery, abortion, and other adverse pregnancy outcomes; it may also increase the probability of infection in infants after delivery [4], [5], [6], [7]. There is a serious concern that pregnant women would be one of the most at-risk groups once international exchanges resume.
The concept of immunizing pregnant women has been proposed for decades [8]. The vaccination of pregnant women cannot only avoid or reduce the probability of infectious diseases, but also offers the most effective and direct protection for neonates by means of passive immunization. A previous study reported that a pregnant woman successfully gave birth to a healthy infant after inoculation with CoronaVac (an inactivated vaccine developed by Sinovac Life Sciences) at 28 weeks and 32 weeks of gestation, and that neutralizing antibody (NAb) was detected in the blood sample of the infant [9]. Epidemiological studies reported no increased risk of threatened labor, miscarriage, pre-eclampsia, or any other pregnancy-related adverse events associated with COVID-19 mRNA vaccination in pregnancy [10], [11]. The safety data from more than 185,000 individuals inoculated COVID-19 mRNA vaccine during pregnancy since December 2020 also showed that there was no increased risk of any poor obstetric outcome, including stillbirth, preterm birth, small for gestational age, neonatal death, and congenital abnormalities [11]. Recent reports found that the humoral immunity and cellular immunity induced by mRNA vaccination in pregnant women and lactating women, were similar to that seen in non-pregnant controls; the antibody titer was higher after the actual infection of SARS-CoV-2, and a vaccine-elicited antibody was transported to umbilical cord blood and breast milk [12], [13], remaining detectable in the blood of infants at 6 months [14]. A follow-up in infants for an average of 134 days after birth, also reported no increased risk in the incidence of all-cause neonatal hospitalizations, postneonatal hospitalizations after birth, congenital anomalies, or infant mortality in infants born with vaccination during pregnancy [14]. In addition, fertility rates and pregnancy outcomes were unaffected by vaccination before pregnancy in global clinical trials of ChAdOx1 nCoV-19 [15]. Thus far, there is no evidence to suggest that a vaccine against SARS-CoV-2 is harmful to either lactating women or breastfed infants. Many international health organizations, including the Center for Disease Control and Prevention in the US, the Society for Maternal-Fetal Medicine, Gynecologists of Canada, and the American College of Obstetricians and Gynecologists, have recommended the administration of vaccines to pregnant and lactating individuals [16]. The WHO also suggested that pregnant women should discuss with their healthcare provider and obtain a vaccine when the benefits of vaccination outweigh the potential risks. However, vaccine hesitancy remains high among some pregnant and lactating individuals, particularly due to the perceived lack of data from randomized clinical trials. Furthermore, the influences of vaccination or post-immunization infection in pregnant women, or women of childbearing age, on conception or the fetus, have yet to be clarified.
In this study, human angiotensin-converting enzyme 2 (hACE2) mice, known to be susceptible to SARS-CoV-2, were used to investigate the effects of CoronaVac on pregnancy, lactation, and the growth of offspring in hACE2 mice. CoronaVac is based on the SARS-CoV-2 virus, which was harvested, inactivated with β-propiolactone, concentrated, purified, and finally adsorbed onto aluminum hydroxide. Mice were immunized with CoronaVac on day 0 and day 28 with a dose of 100 μL each time via intramuscular injection [17]. By observing immunized pregnant mice (day 13 post-pregnancy; 13 dpp) that were subsequently inoculated intranasally with SARS-CoV-2 at 100 50% tissue culture infectious dose (TCID50), we were able to investigate the protective effects of CoronaVac on pregnant mice and the growth and development of their offspring. This research aimed to provide reference for the immunization of pregnant women, and women of childbearing age, with an inactivated SARS-CoV-2 vaccine.
2. Methods
2.1. Ethics statement
Mice infection experiments involving SARS-CoV-2 were performed in an animal biosafety level 3 (ABSL-3) facility with high-efficiency particulate air (HEPA)-filtered isolators. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Laboratory Animal Science (ILAS), Peking Union Medical College (PUMC) (Reference: GH21001).
2.2. Viruses and vaccines
The SARS-CoV-2 virus designated as SARS-CoV-2/WH-09/human/2020/CHN (GenBank: MT093631.2) was provided by ILAS, PUMC, China. The inactivated SARS-CoV-2 Vaccine (batch No.20200411, labeling amount 1200 SU/0.5 ml, specification 0.5 ml/dose), was produced by Sinovac Life Sciences.
2.3. Live virus neutralization assays
Virus titers were measured by a TCID50 assay. Serum samples were heat inactivated at 56 °C for 30 min and diluted with cell culture medium in two steps. The heat-inactivated sera were mixed with a viral suspension of 100 TCID50 in 96-well plates at a ratio of 1:1, followed by 2 h incubation at 36.5 °C in a 5% CO2 incubator. Then 1 × 104 Vero cells were added to the serum-virus mixture, and the plates were cultured for 3 days in an incubator. The cytopathic effect (CPE) was recorded in each by microscopy, and the neutralizing titer was calculated by the dilution number of 50% protective condition.
2.4. Vaccine safety experiments
16–18-week-old female transgenic (K18-hACE2) mice were provided by ILAS, PUMC, China. Five transgenic mice in immunization group (M/V group) were injected two times (on day 0 and 28) with CoronaVac via intramuscular injection; 50 μL of CoronaVac (with a single dose of 100 μL) was separately injected into the right and left hind legs. Five transgenic mice in the control group (the M/M group) were given an equal volume of PBS. Two groups of mice were mated and became pregnant on 23 days after the first vaccination (days post-vaccination; dpv). These mice were monitored, and body weight and reproductive performance indices during immunization, pregnancy, and lactation were recorded. Blood samples were collected pre-delivery (13 days post-pregnancy; 13 dpp) and post-delivery (0 days post-delivery [0 dpd] and on 21 dpd) to detect the presence of S1-specific IgG antibodies against the SARS-CoV-2 antigen by enzyme-linked immunosorbent assay (ELISA) and NAbs by CPE. Physical development indices and the gross morphology of offspring during lactation were recorded after delivery. The histopathological characteristics of offspring at 0 dpd were analyzed. The titers of S1-specific IgG and NAb in serum at 21dpd were also detected.
2.5. Vaccine protection experiments
16–18-week-old female transgenic (K18-hACE2) mice (M/CV group, n = 8) were immunized two times (on day 0 and 28) with CoronaVac via intramuscular injection to the hind legs. The control group (M/CM group, n = 5) were given an equal volume of PBS. Two groups of mice were mated and became pregnant on day 23 after the first immunization. To evaluate the protective capability of CoronaVac on pregnant mice, two groups of pregnant mice were then intranasally infected with 100 TCID50 SARS-CoV-2 on day 13 dpp. Infected animals were monitored and body weight, percent survival, and reproductive performance indices, were recorded. Lung samples were collected to quantify the viral RNA load at 5 days post infection (dpi) (all mice in the M/CM group died; three mice in the M/CV group were sacrificed). Blood samples were collected pre-SARS-CoV-2 (0 dpi) and post-SARS-CoV-2 (0 dpd, 21 dpd) to detect S1-specific IgG by ELISA and NAbs by CPE. Next, we analyzed the viral RNA load, antibody titer, physical development index, and histopathological characteristics of the offspring. The viral RNA loads of the offspring were quantified by RT-PCR at 5 dpi. The titers of S1-specific IgG and NAb in serum at 21 dpd were also detected. Physical development indices and the gross morphology of offspring during lactation were recorded. The histopathological characteristics of offspring at 5 dpi were analyzed.
2.6. Statistical analysis
GraphPad Prism 8.0 software was used for data analysis. The two-tailed unpaired Student's t test was used to make comparisons between two groups while one-way analysis of variance (ANOVA) was used to compare data between the three groups. *p < 0.05, ** p < 0.01 indicated statistically significant differences.
3. Results
3.1. Inoculation with an inactivated SARS-CoV-2 vaccine had no effect on the pregnancies period of hACE2 mice
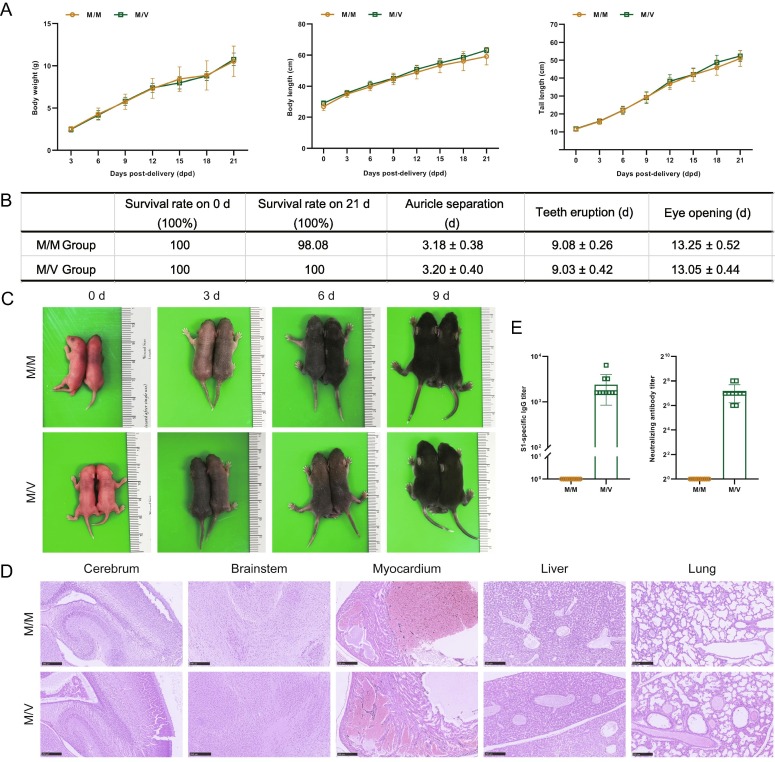
To investigate the effect of vaccination on pregnancy and lactation, 10 hACE2 mice were randomly divided into two groups. Five mice in the immunization group (M/V group) were injected two times (on day 0 and 28) with CoronaVac via intramuscular injection to the hind legs with a single dose of 100 μL. The control group consisted of five mice (M/M group, n = 5) that were given PBS (Fig. 1 A). Compared with the M/M group, the M/V group showed no significant differences in terms of body weight during immunization, pregnancy, and lactation (Fig. 1B) or reproductive performance indices (including pregnancy rate, birth rate, live birth rate, weaning rate, pregnancy cycle, litter weight, and the number of fetuses) (Fig. 1C). To evaluate the immune effect, blood samples were collected pre-delivery (13 dpp) and post-delivery (0 dpd, 21 dpd) to determine the titers of S1-specific IgG antibodies by ELISA and NAbs by CPE. The S1-specific IgG titers to SARS-CoV-2 from the M/V group were 6400–25600 arbitrary units (AU) pre-delivery and 3200–12800 AU post-delivery; the NAb titers were 256–512 AU pre-delivery and 64–256 AU post-delivery (Fig. 1D). Further analysis indicated that the S1-specific IgG titers and NAb titers against SARS-CoV-2 in the M/V group showed no statistically significant difference in pre-delivery and post-delivery, and that these two antibodies were not detected in the M/M group (Fig. 1D).
Fig. 1.
Body weight, reproductive performance indices, and antibody detection data for hACE2 mice inoculated with CoronaVac. (A) Experimental design and sample collection. Ten mice were used in this study. Five mice in the immunization group (M/V group) were injected two times (on day 0 and 28) with CoronaVac via intramuscular injection to the hind legs; 50 μL of CoronaVac (with a single dose of 100 μL) was separately injected into the right and left hind legs. The control group (M/M group, n = 5) were given an equal volume of PBS. All mice were pregnant on day 23 after the first immunization. (B) Body weight at the indicated time points. (C) Reproductive performance indices. (D) The titers of S1-specific IgG antibodies against SARS-CoV-2 antigen and NAb in hACE2 pregnant mice.
3.2. Inoculation with an inactivated SARS-CoV-2 vaccine had no effect on the growth of offspring from hACE2 pregnant mice
Next, we determined the effect of vaccinating hACE2 mice with regards to the growth of their offspring. Compared with the M/M group, offspring from the M/V group showed no statistically significant changes in body weight, body length, and tail length, during lactation (Fig. 2 A). There were no significant changes in a range of physical development indices, including survival rate, the time of auricle separation, tooth eruption, and eye opening (Fig. 2B); gross morphology (Fig. 2C) did not show any obvious signs of dysplasia. Histopathological analyses at 0 dpd did not reveal any significant abnormalities in the cerebrum, brainstem, myocardium, liver, and lungs of the M/V offspring (Fig. 2D). Blood samples taken from the offspring at 21 dpd were collected to detect whether serum samples contained S1-specific IgG to SARS-CoV-2 and NAbs. The S1-specific IgG titers from the M/V offspring were 1600–6400 AU and the NAb titers were 64–256 AU; neither of these were detected in the M/M group (Fig. 2E).
Fig. 2.
Physical development indices, gross morphology, histopathological characteristics, and antibody detection in the offspring. (A) Growth of body weight, body length, and tail length, during lactation period. (B) Physical development indices. (C) Gross morphology at the indicated time points during lactation period. (D) Histopathological characteristics of offspring at 0 dpd. (E) Detection the titers of S1-specific IgG to SARS-CoV-2 and NAb at 21 dpd.
3.3. Inoculation with an inactivated vaccine protected pregnant hACE2 mice from infection with SARS-CoV-2
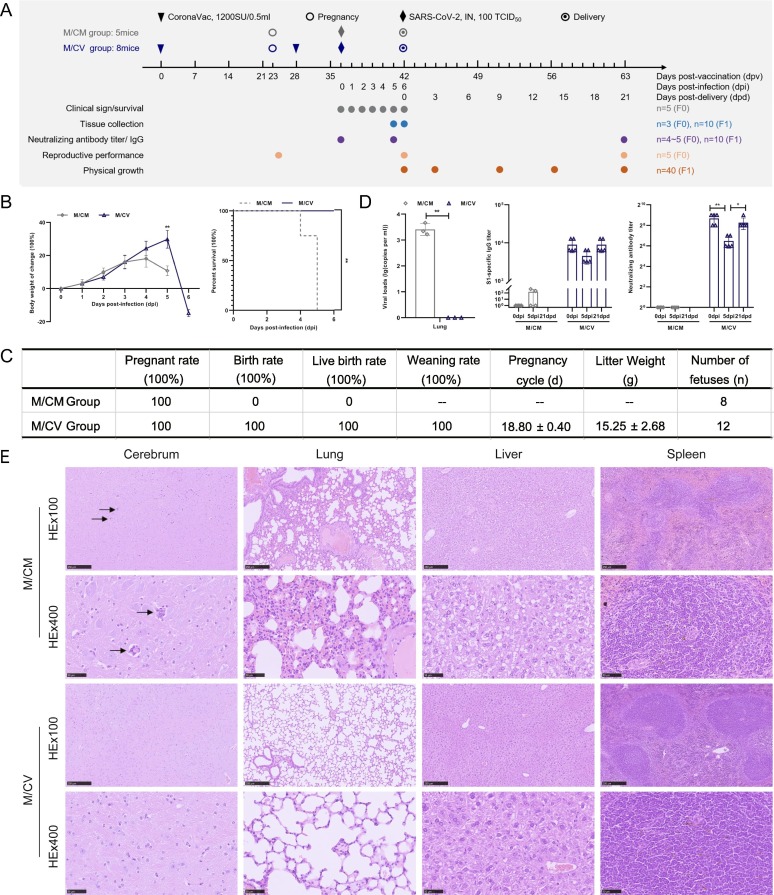
To further investigate the protective capability of CoronaVac during pregnancy, pregnant hACE2 mice were divided into two groups. The experimental design and longitudinal sampling plan are shown in Fig. 3 A. Eight mice (M/CV group) were immunized with CoronaVac; PBS-immunized mice (M/CM group, n = 5) served as a control group. All mice were inoculated intranasally with SARS-CoV-2 at 100 TCID50 and observed successively for changes in body weight and percent survival for 6 days after infection. Compared with the M/V group, the mice in the M/CV group all exhibited normal weight changes and gave birth to offspring at 6 dpi at which point there was obvious weight loss (Fig. 3B); these mice showed normal reproductive performance (Fig. 3C). All pregnant hACE2 mice in the M/CV group survived after infection with SARS-CoV-2 (Fig. 3B). Compared with the M/CV group, the weight of the M/CM group had decreased significantly at 5 dpi; there was a significant difference in percent survival in which the mice all died at 4 dpi or 5 dpi without childbirth (p < 0.01) (Fig. 3B). Compared with pre-infection (6400–12800 AU), the S1-specific IgG titers to SARS-CoV-2 in the M/CV group showed no significant differences at 5 dpi (3200–6400 AU) and 21 dpd (6400–12800 AU) (Fig. 3D). However, the NAb titers in the M/CV group (64–128 AU) had significantly reduced by 4.5-fold at 5 dpi to neutralize viruses compared with pre-infection titers (256–512 AU) (p < 0.01), and then increased to the pre-infection levels at 21 dpd (256–512 AU) to maintain neutralization efficacy (Fig. 3D). The mean viral RNA loads of pregnant mice from the M/CM group were 103.41 copies/ml at 5 dpi (Fig. 3D), but it was not possible to detect SARS-CoV-2 viral RNA loads in the M/CV group. The histopathological analysis of pregnant mice at 5 dpi showed that mild interstitial pneumonia was observed in the M/CM group, including focal thickened alveolar septum and infiltration of mainly lymphocytes and mononuclear cells around the bronchioles and blood vessels (Fig. 3E). There was also a small amount of inflammatory cell infiltration around the cerebral vessels, vacuolar degeneration of hepatocytes and slight atrophy of splenic white pulp in the M/CM group (Fig. 3E); however, no significant abnormality was found in M/CV group (Fig. 3E), thus indicating that vaccination had protected the M/CV pregnant mice from SARS-CoV-2 infection.
Fig. 3.
Clinical characteristics, reproductive performance, virus replication, antibody detection and histopathological characteristics in hACE2 pregnant mice infected with SARS-CoV-2. (A) Experimental design and sample collection. Thirteen mice were used in this study. Eight pregnant mice (M/CV group, n = 8) were inoculated with CoronaVac and challenged intranasally with SARS-CoV-2 at 100 TCID50. The control group (M/CM group, n = 5) were infected intranasally with the same dose of SARS-CoV-2. (B) Infected pregnant mice were observed for changes in body weight and percent survival at the indicated time points. (C) Reproductive performance indices. (D) The viral RNA loads of pregnant mice were quantified by RT-PCR at 5dpi; the titers of S1-specific IgG and NAb in hACE2 pregnant mice were also tested. (E) Histopathological characteristics of pregnant mice at 5 dpi were analyzed.
3.4. Inoculation with an inactivated vaccine and subsequent infection with SARS-CoV-2 had no effect on the growth of offspring from hACE2 pregnant mice
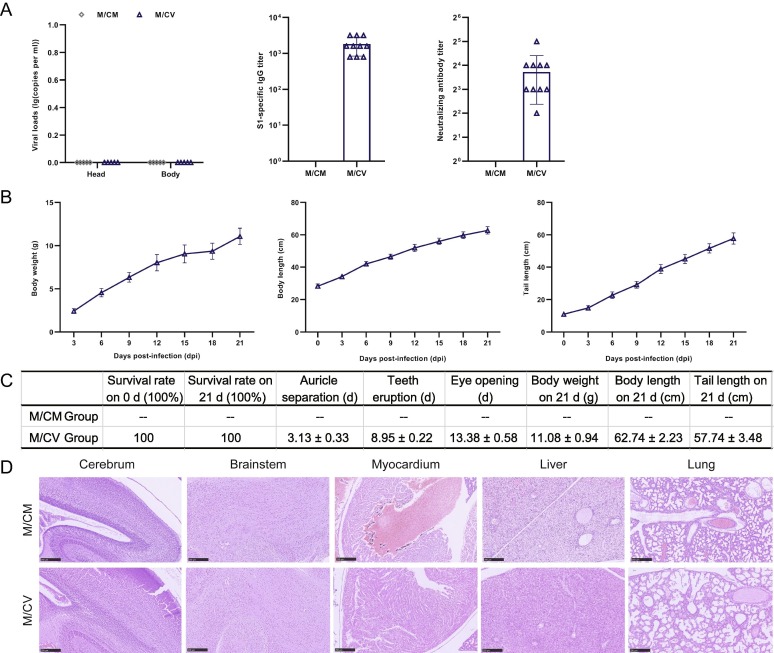
Finally, we studied the effect of SARS-CoV-2 infection on the growth of offspring derived from pregnant hACE2 that had been mice vaccinated with CoronaVac. There were no SARS-CoV-2 viral RNA loads in either of the two groups after infection with SARS-CoV-2 (Fig. 4 A). Blood samples taken from the offspring at 21 dpd were collected to detect S1-specific IgG to SARS-CoV-2 and NAbs. In the M/CV group, the IgG titers were 800–3200 AU, and the NAb titers were 4–32 AU (Fig. 4A). Compared with the M/V group, the IgG titers in the M/CV group showed no significant reduction, while the NAb titers were reduced significantly by approximately 10-fold (p < 0.01). Compared with the M/V group, there was no significant difference in the body weight, body length, or tail length (Fig. 4B), or physical development indices of the M/CV group during lactation (Fig. 4C). Histopathological analysis of offspring at 5 dpi showed that periventricular cells in the M/CM offspring were immature and densely arranged, thus indicating delayed cerebral cortex development. In addition, there were no obvious abnormalities in the morphology of the brain stem, and myocardium, except for hepatocyte edema and alveolar space narrowing. The development of the M/CV offspring was not significantly abnormal (Fig. 4D).
Fig. 4.
Virus replication, antibody detection, physical development indices, and histopathological characteristics of offspring. (A) The viral RNA loads in the heads and bodies of offspring were detected by RT-PCR at 5dpi (n = 3); the titers of S1-specific IgG and NAb in the offspring were tested at 21dpd (M/CM; no data). (B) Growth of body weight, body length, and tail length, during lactation (M/CM; no data). (C) Physical development indices (M/CM; no data). (D) Histopathological characteristics of offspring at 5 dpi were also analysed.
4. Discussion
COVID-19 has triggered a range of serious public health events thus far. One of the most recognized and most efficacious measures to fight against COVID-19 is to induce herd immunity via mass SARS-CoV-2 vaccination [1]. However, for the sake of protection, pregnant women and fetuses, were not included in phase I, II & III clinical trials during the research and development of SARS-CoV-2 vaccines, so there is limited validity and safety data during pregnancy. Although the absolute risks of a severe outcome for women were low, pregnant women were at increased risk for severe COVID-19–associated illness [5]. Evidence is accumulating that SARS-CoV-2 infection during pregnancy is associated with several adverse pregnancy outcomes including preeclampsia, preterm birth, and stillbirth, especially among pregnant women with severe cases of COVID-19. In addition to the direct impact of COVID-19 on pregnancy outcomes, there is evidence that the pandemic, and its effects on healthcare systems, can exert many adverse effects on the outcomes of pregnancy, such as increased stillbirths and maternal deaths [18].
The vaccination of pregnant women is intended to provide direct individual protection against infectious disease, and its side effects, during course of pregnancy as well as passive immunity for the newborn [19]. Multiple countries now recommend that females should be inoculated with inactivated influenza, tetanus and pertussis vaccines during pregnancy, as these vaccines exhibit preferable safety, tolerance, and validity benefits for both pregnant women and their infants [20]. In addition to conventional suggestions, pregnant women can be inoculated with other vaccines based on existing risk factors, including hepatitis A and B vaccines, yellow fever vaccine, and meningococcal vaccine [21]. Most vaccines can be given during pregnancy if the benefits of vaccination exceed the potential risks. There are some clinical data supporting that the mRNA vaccine produced strong humoral immunity and cellular immunity in pregnant and lactating women, with levels of immunogenicity and reactogenicity that were similar to those observed in non-pregnant women [12], [19], [22], [23], thus indicating that vaccination can provide maternal and perinatal immunization. Currently, there are rarely reports available with regards to inactivated SARS-CoV-2 vaccines in pregnant women. As this is a brand-new vaccine, there are limited data relating to its safety and validity during pregnancy, and pregnant women clearly feel uneasy about being vaccinated.
It is vital that we conduct non-clinical safety research on inactivated SARS-CoV-2 vaccines in pregnant animals to provide reference for the application of vaccination on pregnant women. It is well known that hACE2 mice are susceptible to SARS-CoV-2 [24], [25]; they also exhibit short pregnancy and growth cycles and are highly suitable for the study of reproductive toxicity in response to inactivated SARS-CoV-2 vaccination. The use of this model would also allow us to investigate whether the vaccine is safe and protective during pregnancy and lactation period in hACE2 mice following vaccination and allow us to study the growth of their offspring. According to the results of our present research, the inoculation of hACE2 mice with inactivated SARS-CoV-2 vaccine was safe with regards to pregnancy, lactation period, and the growth of their offspring. A 34 years old pregnant woman who inoculated two doses of CoronaVac at the gestational age of 28 weeks and 32 weeks has not reported any vaccine-related adverse effects after either the first or second dose of the vaccine; these are consistent with the findings of the present research [9]. Data on adverse events related to vaccination during pregnancy were little reported in historical research or in pregnant women who received immunizations as part of mass immunization campaigns [20]. Furthermore, the analysis showed that vaccination induced anti-spike IgG to SARS-CoV-2 and NAb. Some macromolecular transfer such as IgG takes place in the postnatal intestinal transfer via milk during the entire suckling period in mice [26], [27]. The detection of antibodies on day 21 post-natally is entirely consistent with transfer via milk, as expected. Moreover, no adverse reactions were observed during this period, and the infants exhibited normal clinical manifestations; these are consistent with the findings of the present research [9]. All of the pregnant hACE2 mice that had been inoculated with the vaccine survived after being infection with SARS-CoV-2. They also exhibited normal growth and reproductive performance; their offspring also developed normally. These data indicated that inoculation of the SARS-CoV-2 vaccine could protect pregnant mice from SARS-CoV-2 infection. Previous studies have shown that mothers infected with SARS-CoV-2 at least 14 days before delivery are more prone to seroconversion and a higher titer of IgG at the time of delivery [28], [29]. Although the level of SARS-CoV-2 S protein-specific IgG in the serum is identical to that in the umbilical cord blood of the mother, the level of NAb declines in the umbilical cord blood. The NAb titers of the offspring from hACE2 mice infected with SARS-CoV-2 post-immunization were lower than that in the immunization group; these data are consistent with clinical results.
In summary, the vaccination of hACE2 mice had no effect on pregnancy, lactation period, or offspring growth. Furthermore, vaccination effectively protected the pregnant mice against SARS-CoV-2 infection, thus reminding that pregnant women can be vaccinated with inactivated SARS-CoV-2 vaccine to prevent potential infections when the risk of infection is high.
Funding statement
This work was supported by the National Key Research and Development Project of China (grant number 2020YFA0707603, 2021YFC0863300).
CRediT authorship contribution statement
Kaili Lin: Investigation, Writing – original draft. Meixuan Liu: Investigation. Linlin Bao: Investigation, Methodology, Writing – review & editing. Qi Lv: Investigation. Hua Zhu: Investigation. Dan Li: Investigation. Yanfeng Xu: Investigation. Zhiguang Xiang: Investigation. Jiangning Liu: Investigation. Xujian Liang: Investigation. Yunlin Han: Investigation. Zhe Cong: Investigation. Ruixue Liu: Investigation. Ran Deng: Investigation. Siyuan Wang: Investigation. Zhi Guo: Investigation. Lu Sun: Investigation. Qiang Wei: Investigation. Hongwei Qiao: Investigation. Shunyi Wang: Investigation. Sidan Pan: Investigation. Hong Gao: Conceptualization, Resources, Supervision, Methodology, Investigation, Writing – review & editing, Funding acquisition. Chuan Qin: Conceptualization, Resources, Supervision, Methodology, Investigation, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Frederiksen L.S.F., Zhang Y., Foged C., Thakur A. The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front Immunol. 2020;11:1817. doi: 10.3389/fimmu.2020.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., Hu Y., Xu M., Chen Z., Yang W., Jiang Z., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodworth K.R., Olsen E.O., Neelam V., Lewis E.L., Galang R.R., Oduyebo T., et al. Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy - SET-NET, 16 Jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020; 370: m3320. Published 2020 Sep 1. doi:10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed]
- 7.Galang RR, Newton SM, Woodworth KR, et al. Risk Factors for Illness Severity Among Pregnant Women With Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 Infection-Surveillance for Emerging Threats to Mothers and Babies Network, 22 State, Local, and Territorial Health Departments, 29 March 2020-5 March 2021. Clin Infect Dis. 2021; 73(Suppl 1): S17-S23. doi: 10.1093/cid/ciab432. [DOI] [PMC free article] [PubMed]
- 8.Rasmussen S.A., Watson A.K., Kennedy E.D., Broder K.R., Jamieson D.J. Vaccines and pregnancy: past, present, and future. Semin Fetal Neonatal Med. 2014;19(3):161–169. doi: 10.1016/j.siny.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Soysal A., Bilazer C., Gönüllü E., Barın E., Çivilibal M. Cord blood antibody following maternal SARS-CoV-2 inactive vaccine (CoronaVac) administration during the pregnancy. Hum Vaccin Immunother. 2021;17(10):3484–3486. doi: 10.1080/21645515.2021.1947099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadali R.A.K., Janagama R., Peruru S.R., Racherla S., Tirumala R., Madathala R.R., et al. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms. Am J Obstet Gynecol. 2021;225(4):458–460. doi: 10.1016/j.ajog.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Male V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat Rev Immunol. 2022;22(5):277–282. doi: 10.1038/s41577-022-00703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collier A.-R., McMahan K., Yu J., Tostanoski L.H., Aguayo R., Ansel J., et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA. 2021;325(23):2370. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldshtein I., Steinberg D.M., Kuint J., Chodick G., Segal Y., Shapiro Ben David S., et al. Association of BNT162b2 COVID-19 Vaccination During Pregnancy With Neonatal and Early Infant Outcomes [published online ahead of print, 2022 Feb 10] JAMA Pediatr. 2022;176(5):470. doi: 10.1001/jamapediatrics.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillson K., Clemens S.C., Madhi S.A., Voysey M., Pollard A.J., Minassian A.M. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. Lancet. 2021;398(10312):1683–1684. doi: 10.1016/S0140-6736(21)02282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorgensen S.C.J., Burry L., Tabbara N. Role of maternal COVID-19 vaccination in providing immunological protection to the newborn. Pharmacotherapy. 2022;42(1):58–70. doi: 10.1002/phar.2649. [DOI] [PubMed] [Google Scholar]
- 17.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson D.J., Rasmussen S.A. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226(2):177–186. doi: 10.1016/j.ajog.2021.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.COVID-19 vaccines. In: Drugs and Lactation Database (LactMed). Bethesda (MD): National Library of Medicine (US); March 21, 2022.
- 20.Chu H.Y., Englund J.A. Maternal immunization. Birth Defects Res. 2017;109(5):379–386. doi: 10.1002/bdra.23547. [DOI] [PubMed] [Google Scholar]
- 21.Vojtek I., Dieussaert I., Doherty T.M., Franck V., Hanssens L., Miller J., et al. Maternal immunization: where are we now and how to move forward? Ann Med. 2018;50(3):193–208. doi: 10.1080/07853890.2017.1421320. [DOI] [PubMed] [Google Scholar]
- 22.Young B.E., Seppo A.E., Diaz N., Rosen-Carole C., Nowak-Wegrzyn A., Cruz Vasquez J.M., et al. Association of Human Milk Antibody Induction, Persistence, and Neutralizing Capacity With SARS-CoV-2 Infection vs mRNA Vaccination. JAMA Pediatr. 2022;176(2):159. doi: 10.1001/jamapediatrics.2021.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg-Friedman M., Kigel A., Bahar Y., Werbner M., Alter J., Yogev Y., et al. BNT162b2 mRNA vaccine elicited antibody response in blood and milk of breastfeeding women. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-26507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583(7818):830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 25.Bao L., Deng W., Qi F., Lv Q.i., Song Z., Liu J., et al. Sequential infection with H1N1 and SARS-CoV-2 aggravated COVID-19 pathogenesis in a mammalian model, and co-vaccination as an effective method of prevention of COVID-19 and influenza. Signal Transduct Target Ther. 2021;6(1) doi: 10.1038/s41392-021-00618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weström B., Arévalo Sureda E., Pierzynowska K., Pierzynowski S.G., Pérez-Cano F.J. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front Immunol. 2020;11:1153. doi: 10.3389/fimmu.2020.01153. Published 2020 Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pentšuk N., van der Laan J.W. An interspecies comparison of placental antibody transfer: new insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res B Dev Reprod Toxicol. 2009;86(4):328–344. doi: 10.1002/bdrb.20201. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza M., Garcia‐Ruiz I., Maiz N., Rodo C., Garcia‐Manau P., Serrano B., et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127(11):1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherer ML, Lei J, Creisher P, et al. Dysregulated immunity in SARS-CoV-2 infected pregnant women. Preprint. medRxiv. 2020;2020.11.13.20231373. Published 2020 Nov 16. doi:10.1101/2020.11.13.20231373.