Abstract
Objective
The study aimed to investigate the diurnal variation in cervical disc hydration and its relationship with cervical degeneration.
Materials and Methods
C3–C7 discs of 86 prospectively enrolled participants (37 males, 49 females; mean age ± standard deviation, 23.5 ± 2.5 years) were assessed using T2 mapping in the morning and evening. All discs were stratified by Miyazaki grade or C2–C7 Cobb angle and T2 values (T2). The degree of diurnal T2 variation (T2-DDV), defined as (morning T2 – evening T2)/morning T2 × 100%, was measured for the entire disc, annulus fibrosus (AF), nucleus pulposus (NP), and endplate zones.
Results
T2 of the entire disc decreased significantly after the daytime load (p < 0.001), with a T2-DDV of 13.3% for all discs and 16.0%, 12.2%, and 13.0% for healthy (grade I), mild degenerative (grade II), and advanced degenerative (grade III/IV) discs, respectively. T2 of regional NPs and AFs decreased significantly from morning to evening (p ≤ 0.049) except in the healthy anterior inner AF (p = 0.092). Compared with healthy discs, mild degenerative discs displayed lower T2 and T2-DDV in regional NPs (p < 0.001). Advanced degenerative discs showed higher T2-DDV in the anterior inner AF compared with healthy discs (p = 0.050). Significant diurnal T2 changes in the endplate zones were observed only in healthy discs (p = 0.013). Cervical discs in the low Cobb angle group showed higher T2-DDV in the anterior AFs and anterior NP and lower T2-DDV in the posterior AF than those in the high Cobb angle group (p ≤ 0.041).
Conclusion
This study characterized the diurnal variation in hydration of the cervical discs as assessed using T2 mapping and revealed early chemo-mechanical coupling dysfunction in degenerating discs. Cervical sagittal alignment on MRI can affect the diurnal stress patterns of the cervical discs. T2 mapping is sensitive to disc biomechanical dysfunction and offers translational potential from biomechanical research to clinical application.
Keywords: Magnetic resonance imaging, Spine, Intervertebral disc degeneration, Neck pain, Diurnal variation
INTRODUCTION
Neck pain is a severe public health problem worldwide and is associated with cervical spine disorders [1,2]. Early cervical degeneration is defined as biomechanical dysfunction, with cervical intervertebral disc degeneration occurring between 15 and 45 years of age [3,4]. An intervertebral disc comprises a fiber-reinforced annulus fibrosus (AF), gelatinous nucleus pulposus (NP), and cartilaginous endplate [5]. Cervical disc degeneration can be observed even in young individuals [6]. Evaluation of the cervical biomechanical environment may help establish new methods for early detection of cervical disc degeneration. Previous studies on cervical biomechanical properties mainly focused on in vitro stress profilometry or finite element analysis with few characterizations in vivo [7,8]. Thus, there remains a need for non-invasive methods to assess cervical biomechanical dysfunction in clinical settings.
Magnetic resonance imaging (MRI) is utilized for investigating the intervertebral discs in vivo. T2 relaxation time (T2) could be used to accurately quantify disc water content [9]. A previous study revealed diurnal variation in the hydration of normal lumbar discs and characterized their biomechanical characteristics using T2 mapping [10]. However, in vitro experiments have shown structural and functional differences between the cervical and lumbar discs [7,11]. The cervical disc has a more fibrous nucleus and thinner posterior annulus [7,11]. Its biomechanical characteristics in vivo, particularly the fluctuation in hydration under daytime stress and by the effect of cervical degeneration, are still unclear. Aberrant diurnal stress patterns may interact with cervical degeneration and mediate degeneration in a vicious circle [3]. Detection of biomechanical abnormalities in young adults could help avoid future severe neck pain through early biomechanical correction.
The goals of this study were [1] to investigate the diurnal variation in hydration of the cervical discs of young adults for characterization of cervical biomechanical properties using T2 mapping and [2] to evaluate the relationship between diurnal variation in hydration and disc degeneration or cervical sagittal alignment on MRI to reveal aberrant diurnal stress patterns relevant to future clinical practice.
MATERIAL AND METHODS
Study Population
Following approval by the Institutional Review Board, 86 participants were prospectively enrolled from our medical college (IRB No. TJ-IRB20210602). These included 49 females and 37 males with a mean age ± standard deviation (SD) of 23.5 ± 2.5 years (range: 19–33 years), mean height of 167.3 ± 8.4 cm, and mean weight of 60.0 ± 9.6 kg. The participants mostly lead a sedentary lifestyle. Informed consent was obtained from all participants. The exclusion criteria were participants who had metal implants, cervical radiculopathy, moderate to severe neck or shoulder pain, history of spinal surgery or injury, cervical spinal deformity, neck or shoulder treatment within the past 2 months, and strenuous exercise within the past 7 days.
Magnetic Resonance Imaging Protocol
All participants underwent MRI examination in the morning (6:47 to 8:53 am, within 60 minutes after rising) and in the evening (7:00 to 11:42 pm). The mean time interval between the two MRI scans was 14.3 ± 1.1 hours. The participants were required to continue normal daily activities without strenuous exercise or long-term bed rest before the evening MRI examination. A 3T MRI scanner (Magnetom Skyra; Siemens Healthineers) was used. To minimize the impact of posture, the participants were instructed to position their heads such that they were uniformly aligned to the coil grooves. The MRI protocol included midsagittal T2-weighted imaging (T2WI) and T2 mapping (repetition time/echo time: 1800/96, 84, 72, 60, 48, 36, 24, 12 ms; voxel size: 0.375 × 0.375 × 2.5 mm3) [10]. The body position and sequence location remained constant for both MRI examinations.
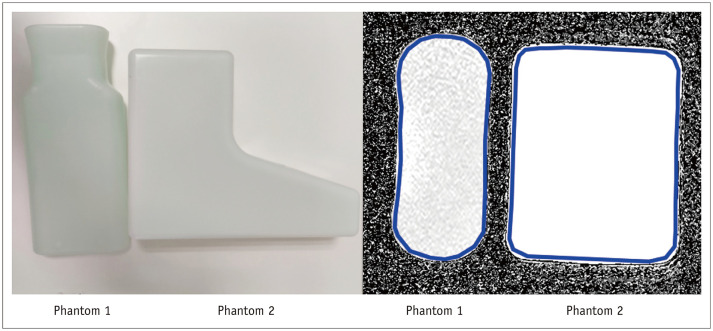
In the scan-rescan test assessing diurnal stability of T2 mapping, the same T2 mapping was performed on phantom 1 (Siemens, per 1000 g H2O: 3.75 g NiSO4 × 6H2O + 5 g NaCl) and phantom 2 (Siemens, per 1000 g H2O: 1.24 g NiSO4 × 6H2O + 2.62 g NaCl) (Fig. 1) in the morning and the evening for 10 days. The mean time interval ± SD between the morning and evening scans was 14.6 ± 1.9 hours.
Fig. 1. Phantoms for magnetic resonance imaging.
The figure shows the two phantoms (left) and the ROIs on the T2 maps (right; blue boundaries). The ROIs for each measurement were placed in the same position in the phantoms. The blue boundaries (right) represent the ROI outlines. The regions inside the boundaries were measured for the T2 relaxation time of the phantoms. ROI = region of interest
Image Analysis
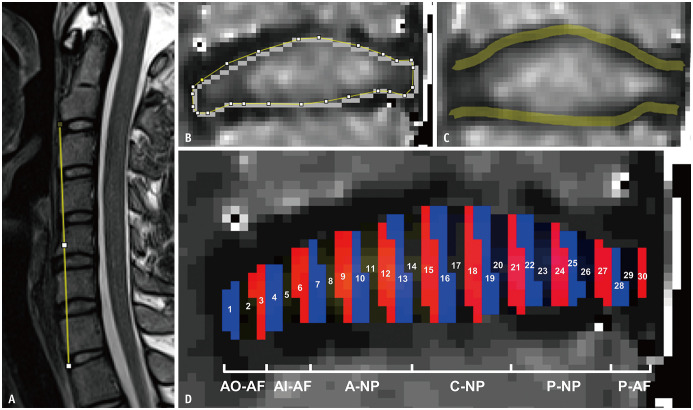
All MRI data from the morning and evening scans were assessed in a random order by two residents (experience of 5 years in musculoskeletal radiology), who were blinded to the clinical data, using ImageJ 1.53c (http://rsb.info.nih.gov/ij/). Ventral cervical height was defined as the distance between the anterior edges of the lower C2 vertebra and the upper T1 vertebra on T2WI (Fig. 2A). Cervical discs were assessed using T2WI, and grading was performed by consensus using the Miyazaki grading system [12]. Based on the interpretation of cervical disc degeneration according to the Miyazaki grading system, 344 C3–C7 discs were divided into the following three groups: grade I indicated healthy discs, grade II indicated discs with mild degeneration, and grade III/IV indicated discs with advanced degeneration [12]. Quantitative measurements were reserved for the C3–C7 segment [13]. T2 maps were processed in the MR workspace (Siemens Healthineers) using monoexponential decay models. The height of the entire disc on T2WI, T2 of the entire disc, and 30 regions of interest (ROIs) (Fig. 2) were measured using ROI Analyzer (https://github.com/tjrantal/RoiAnalyzer). The percentage degree of diurnal T2 variation (T2-DDV) was calculated using the following formula: (morning T2 – evening T2)/morning T2 × 100%. The cervical AF formed a thick crescent anteriorly and was thin posterolaterally. Therefore, we interpreted ROIs 1–3, ROIs 4–6, ROIs 7–13, ROIs 14–20, ROIs 21–27, and ROIs 28–30 as the anterior outer AF, anterior inner AF, anterior NP, central NP, posterior NP, and posterior AF, respectively [11]. Based on a previous study, T2 values of the superior and inferior endplate zones (EPZs) were measured with 0.75 mm wide lines and averaged for analysis (Fig. 2C) [14].
Fig. 2. Image analysis.
A-D. The figure shows the measurements on the mid-sagittal T2-weighted image (A) and T2 mapping (B-D). Cervical ventral height was defined as the distance between the anterior edges of the lower C2 vertebra and the upper T1 vertebra on a T2-weighted image (A). T2 values of the entire disc (B) and those of the superior and inferior endplate zones (C) were measured on T2 mapping. Segmentations of 30 ROIs were marked by 30 zones with different colors (D). Based on the anatomical structure of the cervical discs, ROIs 1–3, ROIs 4–6, ROIs 7–13, ROIs 14–20, ROIs 21–27, and ROIs 28–30 were interpreted as the AO-AF, AI-AF, A-NP, C-NP, P-NP, and P-AF, respectively. A = anterior, AF = annulus fibrosus, AI = anterior inner, AO = anterior outer, C = central, NP = nucleus pulposus, P = posterior, ROI = region of interest
The C2–C7 Cobb angles between the lower endplates of C2 and C7 were also assessed [4]. To facilitate the analysis, 344 discs were divided into the following two equal groups based on a median split: the high Cobb angle group (> 3.79°) and the low Cobb angle group (< 3.79°) [4].
ROI placement for the measurements of phantom images is shown in Figure 1.
Statistical Analysis
Intra-class correlation coefficients (ICCs; a two-way random model of absolute agreement) were evaluated to test inter-reader agreement. ICC values < 0.40, 0.40–0.59, 0.60–0.74, and 0.75–1.00 represented poor, fair, good, and excellent agreement, respectively [4]. Two measurements from the two residents were averaged for the main analysis. Data were tested for normal distribution using a Q-Q plot. Paired two-tailed t tests were performed to assess differences between the morning and evening measurements. One-way analysis of variance (ANOVA) with Bonferroni adjustments (no difference in variances) or Welch’s ANOVA with Games–Howell test (different variances) was performed to assess the differences in disc height, T2, and T2-DDV among the disc degeneration groups (healthy, mild, and advanced). Independent samples t tests were performed to determine the differences between males and females or between the high and low Cobb angle groups. Statistical analyses were performed using IBM SPSS statistics (version 26.0; IBM Corp.). The level of significance was set at p ≤ 0.05.
RESULTS
Reliability of Measurements
Reliability was excellent for the cervical ventral height or the disc height (ICC: 0.762–0.998) and good to excellent for T2 and T2-DDV measurements (ICC: 0.626–0.992) (Table 1). T2 mapping was stable with no statistically significant differences between the morning and evening T2 values of phantom 1 (92.81 ± 1.14 ms vs. 93.27 ± 1.24 ms, p = 0.061) and phantom 2 (386.62 ± 16.74 ms vs. 390.80 ± 22.58 ms, p = 0.393).
Table 1. Inter-Reader Agreement of Measurements of the Entire and Regional Cervical Discs.
| Measurement | ICC (95% Confidence Intervals) | ||
|---|---|---|---|
| Morning T2 Value | Evening T2 Value | T2-DDV | |
| Entire disc | 0.990 (0.987, 0.992) | 0.992 (0.990, 0.993) | 0.936 (0.921, 0.948) |
| Anterior outer AF | 0.626 (0.556, 0.687) | 0.815 (0.775, 0.848) | 0.733 (0.680, 0.778) |
| Anterior inner AF | 0.814 (0.774, 0.848) | 0.847 (0.814, 0.875) | 0.724 (0.669, 0.770) |
| Anterior NP | 0.985 (0.982, 0.988) | 0.985 (0.982, 0.988) | 0.889 (0.864, 0.909) |
| Central NP | 0.988 (0.984, 0.990) | 0.990 (0.988, 0.992) | 0.922 (0.904, 0.936) |
| Posterior NP | 0.964 (0.956, 0.971) | 0.963 (0.955, 0.970) | 0.868 (0.839, 0.891) |
| Posterior AF | 0.861 (0.822, 0.890) | 0.833 (0.782, 0.871) | 0.754 (0.705, 0.797) |
| Endplate zone | 0.967 (0.959, 0.979) | 0.955 (0.944, 0.963) | - |
AF = annulus fibrosus, ICC = intra-class correlation coefficients, NP = nucleus pulposus, T2-DDV = the degree of diurnal T2 values variation
Diurnal Variation in Magnetic Resonance Imaging Measurements
Cervical ventral height, disc height, and T2 of the entire disc for all participants decreased by 2.1%, 10.3%, and 13.3%; respectively (all p < 0.001) (Table 2) after daily activities. Males exhibited significantly higher disc height than females (p < 0.001). However, there was no significant sex difference in T2 and T2-DDV of the entire disc (p = 0.428–0.839) (Fig. 3). Table 2 shows the height and T2 data with sex differences in detail.
Table 2. The Morning and Evening Measurements in All Participants and Each Sex.
| Measurement | All (n = 86) | Sex Difference | |||
|---|---|---|---|---|---|
| Male (n = 37) | Female (n = 49) | P * | |||
| Cervical ventral height, mm | |||||
| Morning | 93.75 ± 7.27 | 99.54 ± 5.16 | 89.17 ± 5.10 | < 0.001 | |
| Evening | 91.74 ± 7.13 | 97.48 ± 5.30 | 87.20 ± 4.70 | < 0.001 | |
| p value† | < 0.001 | < 0.001 | < 0.001 | ||
| Disc height, mm | |||||
| Morning | 3.10 ± 0.48 | 3.30 ± 0.45 | 2.95 ± 0.45 | < 0.001 | |
| Evening | 2.78 ± 0.42 | 2.93 ± 0.41 | 2.67 ± 0.40 | < 0.001 | |
| p value† | < 0.001 | < 0.001 | < 0.001 | ||
| T2 of entire disc, ms | |||||
| Morning | 63.82 ± 13.41 | 64.02 ± 14.34 | 63.67 ± 12.68 | 0.810 | |
| Evening | 55.12 ± 11.06 | 55.25 ± 12.52 | 55.01 ± 9.79 | 0.839 | |
| p value† | < 0.001 | < 0.001 | < 0.001 | ||
| T2-DDV of entire disc, % | 13.3 ± 8.7 | 13.7 ± 9.1 | 12.9 ± 8.3 | 0.428 | |
Data are presented as mean ± standard deviation. *p value of the independent sample t tests to assess the gender difference, †p value for the morning and evening comparison of the paired t test. T2-DDV = the degree of diurnal T2 values variation
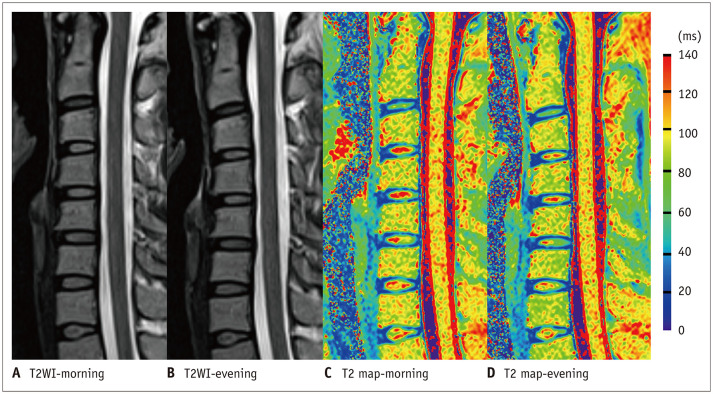
Fig. 3. An example of cervical diurnal variations.
The figure shows the cervical spine of a 24-year-old female on T2WI and T2 mapping with a MRI scan time interval of 15.17 hours. The cervical ventral height decreased from 95.44 mm in the morning to 92.44 mm in the evening on T2WI. The T2 maps show a visible decrease in the central high T2 values (red region) in the cervical discs on the evening MRI scan. MRI = magnetic resonance imaging, T2WI = T2-weighted imaging
Analysis according to Degeneration Grades
All discs were divided into the following three groups: 66 discs were classified as healthy, 136 discs were classified into the mild degeneration group, and 142 discs were classified into the advanced degeneration group (135 discs with grade III and 7 discs with grade IV). The diurnal variation in disc height and T2 of the cervical intervertebral discs according to different degeneration grades are presented in Table 3. No significant difference was observed in disc height among the groups (p = 0.224 and p = 0.258). After diurnal loading, disc height in the healthy, mild degeneration, and advanced degeneration groups decreased by 11.1%, 9.9%, and 9.5%; respectively (all p < 0.001). T2 of the entire disc and that of NPs significantly decreased in the presence of disc degeneration (all p < 0.001). No statistically significant differences were observed in T2 of AFs among the three groups (p = 0.064–0.776).
Table 3. Diurnal Disc Height and T2 Values of the Cervical Intervertebral Discs according to Different Degeneration Grades.
| Measurement | Degeneration Grade | P (ANOVA)* | P (Post Hoc Comparison)* | |||||
|---|---|---|---|---|---|---|---|---|
| Grade I (n = 66) | Grade II (n = 136) | Grade III/IV (n = 142) | I vs. II | II vs. III/IV | I vs. III/IV | |||
| Disc height, mm | ||||||||
| Morning | 3.17 ± 0.54 | 3.05 ± 0.49 | 3.13 ± 0.45 | 0.224 | ||||
| Evening | 2.80 ± 0.43 p < 0.001 | 2.74 ± 0.45 p < 0.001 | 2.82 ± 0.39 p < 0.001 | 0.258 | ||||
| Morning T2, ms | ||||||||
| Entire disc | 85.75 ± 14.08 | 63.93 ± 8.24 | 54.45 ± 5.35 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Anterior outer AF | 27.51 ± 6.03 | 27.65 ± 6.31 | 29.01 ± 6.25 | 0.122 | ||||
| Anterior inner AF | 39.65 ± 13.29 | 41.91 ± 12.29 | 41.06 ± 9.28 | 0.422 | ||||
| Anterior NP | 92.56 ± 28.75 | 72.09 ± 15.63 | 58.44 ± 8.75 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Central NP | 107.59 ± 18.68 | 75.15 ± 10.33 | 62.37 ± 6.52 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Posterior NP | 72.68 ± 15.26 | 56.12 ± 7.98 | 51.62 ± 7.22 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Posterior AF | 35.97 ± 8.88 | 35.11 ± 8.56 | 35.19 ± 7.85 | 0.776 | ||||
| Endplate zone | 26.88 ± 3.57 | 26.13 ± 3.89 | 25.62 ± 3.10 | 0.054 | ||||
| Evening T2, ms | ||||||||
| Entire disc | 71.34 ± 10.97 p < 0.001 | 55.90 ± 7.20 p < 0.001 | 47.18 ± 4.79 p < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Anterior outer AF | 24.45 ± 6.17 p < 0.001 | 26.61 ± 5.91 p = 0.049 | 26.30 ± 6.77 p < 0.001 | 0.065 | ||||
| Anterior inner AF | 37.59 ± 13.36 p = 0.092 | 38.05 ± 11.22 p < 0.001 | 35.29 ± 8.97 p < 0.001 | 0.064 | ||||
| Anterior NP | 79.04 ± 21.37 p < 0.001 | 63.27 ± 13.62 p < 0.001 | 51.11 ± 7.53 p < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Central NP | 87.60 ± 14.80 p < 0.001 | 64.82 ± 8.41 p < 0.001 | 53.55 ± 5.75 p < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Posterior NP | 56.91 ± 9.66 p < 0.001 | 48.77 ± 6.95 p < 0.001 | 44.41 ± 5.92 p < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Posterior AF | 31.73 ± 8.24 p = 0.001 | 31.51 ± 6.94 p < 0.001 | 30.72 ± 5.93 p < 0.001 | 0.493 | ||||
| Endplate zone | 25.80 ± 4.01 p = 0.013 | 25.64 ± 3.41 p = 0.093 | 25.43 ± 2.98 p = 0.459 | 0.743 | ||||
Data are presented as mean ± standard deviation. The superscript of the evening data: p value for the morning and evening comparison of the paired t test. *p value of one-way ANOVA with Bonferroni adjustments (no difference in variance) or Welch ANOVA with Games-Howell test (different variance). AF = annulus fibrosus, ANOVA = analysis of variance, NP = nucleus pulposus
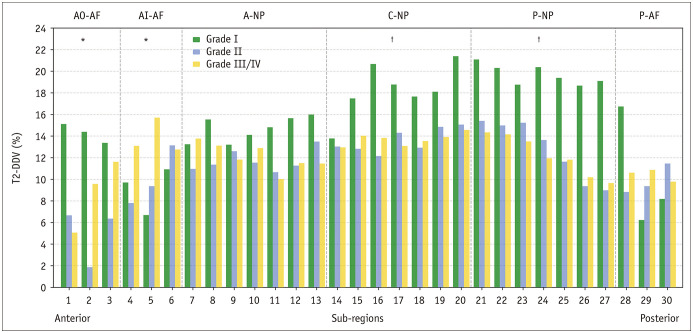
T2-DDV values for the entire disc in the healthy, mild degeneration, and advanced degeneration groups were 16.0%, 12.2%, and 13.0%; respectively (all p < 0.001). After the diurnal load, T2 in the regional NPs and Afs of the three groups decreased significantly (all p ≤ 0.049) except in the anterior inner AF of the healthy group (p = 0.092). Table 3 shows the differences in T2 among the groups and morning-evening comparisons. When compared with healthy discs, T2-DDV was lower in the central and posterior NPs of discs with mild and advanced degeneration (all p ≤ 0.001) and higher in the anterior inner AF of discs with advanced degeneration (p = 0.050). Details of the differences in T2-DDV among the groups are presented in Table 4 and Figure 4.
Table 4. T2-DDV of the Cervical Intervertebral Discs according to Different Degeneration Grades.
| T2-DDV (%) | Degeneration Grade | P (ANOVA)* | P (Post Hoc Comparison)* | ||||
|---|---|---|---|---|---|---|---|
| Grade I (n = 66) | Grade II (n = 136) | Grade III/IV (n = 142) | I vs. II | II vs. III/IV | I vs. III/IV | ||
| Entire disc | 16.0 ± 10.8 | 12.2 ± 8.3 | 13.0 ± 7.7 | 0.011 | 0.009 | 1.000 | 0.059 |
| Anterior outer AF | 9.0 ± 24.3 | 1.7 ± 22.7 | 7.9 ± 21.3 | 0.030 | 0.093 | 0.065 | 1.000 |
| Anterior inner AF | 2.0 ± 33.5 | 9.4 ± 19.5 | 12.5 ± 18.8 | 0.046 | 0.224 | 0.375 | 0.050 |
| Anterior NP | 13.8 ± 15.2 | 11.8 ± 11.5 | 11.9 ± 10.5 | 0.620 | |||
| Central NP | 18.6 ± 9.0 | 13.2 ± 9.1 | 13.7 ± 8.4 | < 0.001 | < 0.001 | 1.000 | 0.001 |
| Posterior NP | 19.5 ± 11.3 | 12.9 ± 11.6 | 13.2 ± 10.5 | < 0.001 | < 0.001 | 1.000 | < 0.001 |
| Posterior AF | 7.9 ± 26.3 | 7.1 ± 22.7 | 9.0 ± 23.1 | 0.796 | |||
Data are presented as mean ± standard deviation. *p value of one-way ANOVA with Bonferroni adjustments (no difference in variance) or Welch ANOVA with Games-Howell test (different variance). AF = annulus fibrosus, ANOVA = analysis of variance, NP = nucleus pulposus, T2-DDV = the degree of diurnal T2 values variation
Fig. 4. T2-DDV in the cervical intervertebral discs with different degeneration grades.
Degenerating cervical discs displayed a lower T2-DDV in the NP regions and a higher T2-DDV in the AI-AF regions when compared with healthy discs. T2-DDV data are presented as medians. One-way analysis of variance or Welch’s analysis of variance: *p ≤ 0.05, †p ≤ 0.001. A = anterior, AF = annulus fibrosus, AI = anterior inner, AO = anterior outer, C = central, NP = nucleus pulposus, P = posterior, T2-DDV = the degree of diurnal T2 values variation
No statistically significant differences were observed among the three groups in T2 of the EPZs (p = 0.054 and p = 0.743) (Table 3). After the daytime load, T2 of the EPZs from healthy discs decreased significantly (p = 0.013). No statistically significant diurnal T2 variations in the EPZs were observed in the mild and advanced degeneration groups (p = 0.093 and p = 0.459).
Analysis according to Cobb Angle Groups
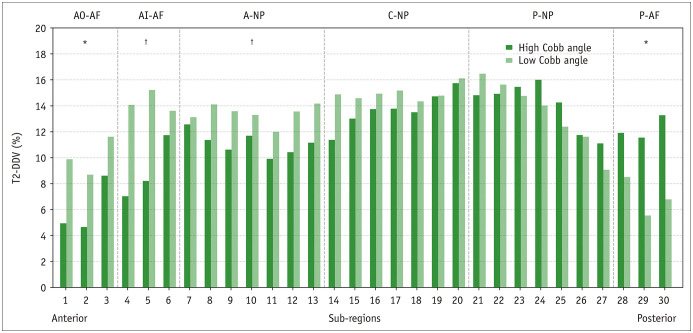
No statistically significant differences were observed between the high and low Cobb angle groups in the morning T2 of the NPs and AFs (p = 0.085–0.751) (Table 5). After the daytime load, T2 of the entire disc, regional NPs, and regional AFs of the two groups declined significantly (all p ≤ 0.021). When compared with the high Cobb angle group, the low Cobb angle group showed statistically lower evening T2 in the anterior AFs with higher evening T2 in the posterior NP and posterior AF (all p ≤ 0.049) (Table 5). The low Cobb angle group showed higher T2-DDV in the entire disc, anterior AFs, and anterior NP and lower T2-DDV in the posterior AF (all p ≤ 0.041) (Table 6, Fig. 5).
Table 5. Diurnal T2 Values of the Cervical Intervertebral Discs of the High Cobb Angle Group versus the Low Cobb Angle Group.
| T2 Value (ms) | High Cobb Angle Group (n = 172) | Low Cobb Angle Group (n = 172) | P * | |
|---|---|---|---|---|
| Morning | ||||
| Entire disc | 63.65 ± 13.23 | 64.31 ± 14.22 | 0.658 | |
| Anterior outer AF | 28.30 ± 6.53 | 28.08 ± 5.98 | 0.751 | |
| Anterior inner AF | 41.34 ± 11.95 | 40.92 ± 10.73 | 0.730 | |
| Anterior NP | 70.68 ± 19.59 | 68.21 ± 17.05 | 0.216 | |
| Central NP | 74.24 ± 16.41 | 76.99 ± 21.12 | 0.179 | |
| Posterior NP | 54.75 ± 7.96 | 56.47 ± 9.82 | 0.085 | |
| Posterior AF | 34.79 ± 7.80 | 35.83 ± 8.81 | 0.251 | |
| Evening | ||||
| Entire disc | 55.32 ± 10.81 p < 0.001 | 54.92 ± 11.34 p < 0.001 | 0.736 | |
| Anterior outer AF | 27.01 ± 6.75 p = 0.021 | 25.12 ± 5.81 p < 0.001 | 0.006 | |
| Anterior inner AF | 37.98 ± 10.71 p < 0.001 | 35.67 ± 10.92 p < 0.001 | 0.049 | |
| Anterior NP | 62.17 ± 16.13 p < 0.001 | 59.10 ± 14.86 p < 0.001 | 0.068 | |
| Central NP | 63.47 ± 12.58 p < 0.001 | 64.92 ± 16.56 p < 0.001 | 0.364 | |
| Posterior NP | 47.18 ± 7.52 p < 0.001 | 49.82 ± 9.11 p < 0.001 | 0.004 | |
| Posterior AF | 30.29 ± 6.81 p < 0.001 | 32.14 ± 6.70 p < 0.001 | 0.012 | |
Data are presented as mean ± standard deviation. The superscript of the evening data: p value for the morning and evening comparison of the paired t test. *Independent sample t test. AF = annulus fibrosus, NP = nucleus pulposus
Table 6. T2-DDV of the Cervical Intervertebral Discs of the High Cobb Angle Group versus Low Cobb Angle Group.
| T2-DDV (%) | High Cobb Angle Group (n = 172) | Low Cobb Angle Group (n = 172) | P * |
|---|---|---|---|
| Entire disc | 12.4 ± 9.6 | 14.3 ± 7.0 | 0.039 |
| Anterior outer AF | 2.2 ± 26.0 | 8.5 ± 19.6 | 0.012 |
| Anterior inner AF | 6.5 ± 22.9 | 13.3 ± 19.0 | 0.003 |
| Anterior NP | 10.1 ± 15.2 | 13.6 ± 9.2 | 0.009 |
| Central NP | 13.6 ± 9.9 | 15.2 ± 7.9 | 0.101 |
| Posterior NP | 14.8 ± 12.5 | 13.9 ± 10.1 | 0.463 |
| Posterior AF | 11.7 ± 20.3 | 6.8 ± 22.7 | 0.041 |
Data are presented as mean ± standard deviation. *Independent sample t test. AF = annulus fibrosus, NP = nucleus pulposus, T2-DDV = the degree of diurnal T2 values variation
Fig. 5. Comparison of T2-DDV in the cervical intervertebral discs between the low Cobb angle and high Cobb angle groups.
The low Cobb angle group displayed a higher T2-DDV in the anterior discs and a lower T2-DDV in the posterior discs when compared with the high Cobb angle group. T2-DDV data are presented as medians. Independent samples t tests: *p ≤ 0.05, †p ≤ 0.01. A = anterior, AF = annulus fibrosus, AI = anterior inner, AO = anterior outer, C = central, NP = nucleus pulposus, P = posterior, T2-DDV = the degree of diurnal T2 values variation
DISCUSSION
Cervical ventral height and disc height exhibited 2.1% and 10.3% diurnal variations, respectively. After diurnal loading, T2 of the entire disc from the healthy, mild degeneration, and advanced degeneration groups decreased by 16.0%, 12.2%, and 13.0%; respectively. T2 in the regional NPs and AFs of the healthy and degeneration groups decreased significantly, except in the healthy anterior inner AF. Mild cervical disc degeneration was marked by lower T2 and T2-DDV in the regional NPs, with no significant diurnal T2 variation in the EPZs. Discs with advanced degeneration showed higher T2-DDV in the anterior inner AF when compared with healthy discs. Cervical discs in the low Cobb angle group displayed higher T2-DDV anteriorly and lower T2-DDV posteriorly when compared with those in the high Cobb angle group.
Water is an essential component of the intervertebral discs and is attracted by proteoglycans, which perpetuate the hyperosmolar and hydration status of the discs to reciprocally restrict water shifting and resist compression [5,15]. Diurnal loading increases disc pressure, resulting in a fluid shift and creation of osmotic swelling pressure to neutralize mechanical loading and maintain chemo-mechanical equilibrium [16]. NPs are responsible for absorbing daily loading and redistributing it radially to the AFs [15,17]. The nocturnal unloading recovery response of the discs relies solely on osmotic pressure [18]. The interplay between the chemical compositions and mechanical properties of the discs is defined as chemo-mechanical coupling [16]. Diurnal variation in the cervical ventral height was similar to that in the lumbar spine, and discs were implicated as a major factor contributing to this variation, which was mainly attributed to the viscoelastic displacement of the solid disc matrix and time-dependent fluid flow [10,19,20,21].
T2 can be biologically interpreted as a reflection of water content [9]. T2 values of the entire cervical disc complex and their sex differences in the present study are consistent with earlier findings in young adults [22,23]. The morning and evening T2 values reflect absolute and relative disc hydration before and after diurnal loading, respectively. Diurnal T2 variation is characterized by fluid shift (redistribution, inflow, or outflow) mediated by the chemo-mechanical balance [21,24]. In contrast to the previously observed T2 increase in the lumbar AFs with the highest T2-DDV in the posterior AFs after daily loading, a significant decrease in cervical T2 was observed in all of the assessed regional discs (except in the healthy anterior inner AF) [10]. This was mainly attributed to differences in the structure and function of the cervical and lumbar regions [7,11,25]. Cervical movement requires greater disc deformation than lumbar movement [25]. Healthy cervical NPs showed higher T2-DDV than AFs, suggesting that a healthy NP with abundant proteoglycans and water content could initiate high water shifts to regulate diurnal mechanical stress and osmotic pressure. Notably, the healthy anterior inner AF maintained a consistent diurnal T2, indicating that it could act as an elastically resistant vessel with load-induced water outflow and water inflow from the NP to tether the disc fluid [10]. Moreover, the healthy disc endplate was dehydrated during the day, indicating that its high hydraulic permeability functioned as a chemical transport channel with diurnal water shifts [26].
In the present study, degenerating cervical discs showed chemo-mechanical coupling failure due to water loss and aberrant stress-mediated water transfer. They exhibited significantly lower T2 in the NPs (rather than AFs) when compared with healthy discs, demonstrating earlier and greater water loss in the NPs when compared with AFs and the failure of nocturnal fluid inflow due to the disrupted osmotic pressure gradient [27]. Dehydration of the cervical NPs is also accompanied by chemo-mechanical coupling failure, as evidenced by a decreased T2-DDV in degenerating NPs. Interestingly, the anterior inner AF of discs with advanced degeneration displayed significantly higher T2-DDV than those of healthy discs. Reportedly, disc degeneration decreases the pressure in the nucleus with the load resisted by adjacent structures [7,17]. The anterior inner AF in degenerating discs might experience disproportionate stress concentrations with reduced water redistribution from the NP and serve a compensatory role for the NP in the mechanical functions of resisting pressure and absorbing shock [7]. Dysregulated diurnal water fluctuation in the degenerating disc endplate prior to considerable water loss showed early impairment of its capability for load-induced material exchange, potentially accelerating disc degeneration [26].
Cervical degeneration is commonly accompanied by alterations in cervical sagittal alignment [8]. Reportedly, the C2–C7 Cobb angle on supine MRI was reliable and highly correlated with upright radiographs, suggesting that the low Cobb angle in our MRI results corresponded to cervical straightening or kyphosis [4]. The higher T2-DDV of the entire disc in the low Cobb angle group than in the high Cobb angle group indicated a greater water shift induced by the increased diurnal stress, which was consistent with a previous finite element analysis examining increased disc stress in the straightened cervical spine [8]. Moreover, our study suggested that elongation of the cervical moment arm in the low Cobb angle group resulted in diurnal ventral stress concentration and dorsal stress shielding of the discs. This led to a greater water outflow in the anterior disc and lower water outflow in the posterior disc after diurnal loading.
Cervical disc protrusion is related to disc degeneration and deteriorates with age [28]. Chemo-mechanical coupling dysfunction can disrupt disc homeostasis if aberrant diurnal stress persists [16,29]. It might accelerate disc degeneration and cause protrusion by damaging the microstructural integrity [16,29]. Our study showed that cervical degeneration with abnormal stress patterns is common among young adults with a sedentary lifestyle. To avoid future disabling symptoms, preventive strategies, such as exercise and posture correction, could be implemented if biomechanical abnormalities are recognized early enough. Evaluation of cervical biomechanical performance in vivo is expected to establish new diagnostic and therapeutic strategies at an early stage [1]. Subregional T2 and the degree of T2 variation could be early biomarkers of biomechanical dysfunction of the disc and can be utilized for clinical diagnosis and long-term monitoring. This study also lays the foundation for further research. Traction is used clinically for patients with neck pain, but its precise effect is unclear [30]. The degree of T2 variation before and after traction might be used to determine water content recovery following local stress to provide a theoretical basis for cervical traction and guide clinical treatment [31]. Further research on the relationship between cervical degeneration and neck pain using T2 mapping is warranted, and researchers must consider the diurnal variations in hydration of the cervical discs to avoid bias.
This study has several limitations. The histopathological correlation of MRI findings, scan-rescan test of the discs, and quantification of daily activity were impractical [10]. Nevertheless, the scan-rescan test results of the phantoms suggested that the diurnal T2 variations in the discs were not measurement variations but physiological phenomena. The participants continued normal daily activities without strenuous exercise before the evening scan. This makes our study representative of young adults, especially college students with a sedentary lifestyle. We did not perform radiographic examination, the gold standard to confirm cervical sagittal straightening or kyphosis, to avoid radiation exposure. However, the participants adopted a unified scanning position. A strong correlation between MRI and upright radiography has been reported [4]. We observed that a low Cobb angle on MRI is related to higher stress in the anterior disc and lower stress in the posterior disc.
In conclusion, this study characterized the diurnal variations in hydration of the cervical discs as assessed by T2 mapping of MRI and revealed early chemo-mechanical coupling dysfunction in degenerating discs. Cervical sagittal alignment on MRI can affect the diurnal stress patterns of the cervical discs. T2 mapping is sensitive to biomechanical dysfunction of the cervical discs. It offers translational potential from basic biomechanical research to clinical applications in order to help restore a normal cervical biomechanical environment.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Chanyuan Liu, Jingyi Wang.
- Data curation: Chanyuan Liu, Jingyi Wang, Bowen Hou, Yitong Li.
- Formal analysis: Chanyuan Liu, Jingyi Wang, Bowen Hou, Yitong Li.
- Funding acquisition: Jun Ran, Xiaoming Li.
- Investigation: Chanyuan Liu, Jingyi Wang, Bowen Hou, Yitong Li.
- Methodology: Chanyuan Liu, Jingyi Wang, Bowen Hou, Yitong Li.
- Project administration: Chanyuan Liu.
- Resources: Jun Ran, Xiaoming Li.
- Software: Jun Ran, Xiaoming Li, John N. Morelli.
- Supervision: Peisen Zhang, John N. Morelli, Jun Ran, Xiaoming Li.
- Validation: Peisen Zhang, Jun Ran. Xiaoming Li.
- Visualization: Chanyuan Liu.
- Writing—original draft: Chanyuan Liu.
- Writing—review & editing: John N. Morelli, Peisen Zhang, Jun Ran, Xiaoming Li.
Funding Statement: This study was supported by the National Natural Science Foundation of China (NSFC) (No. 31630025, No. 81930045 and No. 81901715).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Kolenkiewicz M, Włodarczyk A, Wojtkiewicz J. Diagnosis and incidence of spondylosis and cervical disc disorders in the university clinical hospital in olsztyn, in years 2011–2015. Biomed Res Int. 2018;2018:5643839. doi: 10.1155/2018/5643839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safiri S, Kolahi AA, Hoy D, Buchbinder R, Mansournia MA, Bettampadi D, et al. Global, regional, and national burden of neck pain in the general population, 1990-2017: systematic analysis of the Global Burden of Disease Study 2017. BMJ. 2020;368:m791. doi: 10.1136/bmj.m791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhoury J, Dowling T. Cervical degenerative disc disease. Treasure Island: Statpearls publishing; 2021. [PubMed] [Google Scholar]
- 4.Lee HD, Jeon CH, Chung NS, Kwon HJ. Comparative analysis of three imaging modalities for evaluation of cervical sagittal alignment parameters: a validity and reliability study. Spine (Phila Pa 1976) 2017;42:1901–1907. doi: 10.1097/BRS.0000000000002256. [DOI] [PubMed] [Google Scholar]
- 5.Yang B, O’Connell GD. Intervertebral disc swelling maintains strain homeostasis throughout the annulus fibrosus: a finite element analysis of healthy and degenerated discs. Acta Biomater. 2019;100:61–74. doi: 10.1016/j.actbio.2019.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Machino M, Ito K, Ando K, Kobayashi K, Nakashima H, Kato F, et al. Normative magnetic resonance imaging data of age-related degenerative changes in cervical disc morphology. World Neurosurg. 2021;152:e502–e511. doi: 10.1016/j.wneu.2021.05.123. [DOI] [PubMed] [Google Scholar]
- 7.Skrzypiec DM, Pollintine P, Przybyla A, Dolan P, Adams MA. The internal mechanical properties of cervical intervertebral discs as revealed by stress profilometry. Eur Spine J. 2007;16:1701–1709. doi: 10.1007/s00586-007-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei W, Liao S, Shi S, Fei J, Wang Y, Chen C. Straightened cervical lordosis causes stress concentration: a finite element model study. Australas Phys Eng Sci Med. 2013;36:27–33. doi: 10.1007/s13246-013-0182-4. [DOI] [PubMed] [Google Scholar]
- 9.Yang B, Wendland MF, O’Connell GD. Direct quantification of intervertebral disc water content using MRI. J Magn Reson Imaging. 2020;52:1152–1162. doi: 10.1002/jmri.27171. [DOI] [PubMed] [Google Scholar]
- 10.Zhu T, Ai T, Zhang W, Li T, Li X. Segmental quantitative MR imaging analysis of diurnal variation of water content in the lumbar intervertebral discs. Korean J Radiol. 2015;16:139–145. doi: 10.3348/kjr.2015.16.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercer S, Bogduk N. The ligaments and annulus fibrosus of human adult cervical intervertebral discs. Spine (Phila Pa 1976) 1999;24:619–626. doi: 10.1097/00007632-199904010-00002. discussion 627-628. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki M, Hong SW, Yoon SH, Zou J, Tow B, Alanay A, et al. Kinematic analysis of the relationship between the grade of disc degeneration and motion unit of the cervical spine. Spine (Phila Pa 1976) 2008;33:187–193. doi: 10.1097/BRS.0b013e3181604501. [DOI] [PubMed] [Google Scholar]
- 13.Bogduk N, Mercer S. Biomechanics of the cervical spine. I: normal kinematics. Clin Biomech (Bristol Avon) 2000;15:633–648. doi: 10.1016/s0268-0033(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 14.Hebelka H, Miron A, Kasperska I, Brisby H, Lagerstrand K. Axial loading during MRI induces significant T2 value changes in vertebral endplates-a feasibility study on patients with low back pain. J Orthop Surg Res. 2018;13:18. doi: 10.1186/s13018-018-0727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silagi ES, Shapiro IM, Risbud MV. Glycosaminoglycan synthesis in the nucleus pulposus: dysregulation and the pathogenesis of disc degeneration. Matrix Biol. 2018;71-72:368–379. doi: 10.1016/j.matbio.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derrouiche A, Zaouali A, Zaïri F, Ismail J, Chaabane M, Qu Z, et al. Osmo-inelastic response of the intervertebral disc. Proc Inst Mech Eng H. 2019;233:332–341. doi: 10.1177/0954411919827983. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Fu P, Wu H, Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017;370:53–70. doi: 10.1007/s00441-017-2613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connell GD, Jacobs NT, Sen S, Vresilovic EJ, Elliott DM. Axial creep loading and unloaded recovery of the human intervertebral disc and the effect of degeneration. J Mech Behav Biomed Mater. 2011;4:933–942. doi: 10.1016/j.jmbbm.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt H, Reitmaier S, Graichen F, Shirazi-Adl A. Review of the fluid flow within intervertebral discs - How could in vitro measurements replicate in vivo? J Biomech. 2016;49:3133–3146. doi: 10.1016/j.jbiomech.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Bezci SE, Lim S, O’Connell GD. Nonlinear stress-dependent recovery behavior of the intervertebral disc. J Mech Behav Biomed Mater. 2020;110:103881. doi: 10.1016/j.jmbbm.2020.103881. [DOI] [PubMed] [Google Scholar]
- 21.Vergroesen PA, van der Veen AJ, Emanuel KS, van Dieën JH, Smit TH. The poro-elastic behaviour of the intervertebral disc: a new perspective on diurnal fluid flow. J Biomech. 2016;49:857–863. doi: 10.1016/j.jbiomech.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Huang M, Han Z, Shao L, Xie Y, Wu J, et al. Quantitative T2 magnetic resonance imaging compared to morphological grading of the early cervical intervertebral disc degeneration: an evaluation approach in asymptomatic young adults. PLoS One. 2014;9:e87856. doi: 10.1371/journal.pone.0087856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menezes-Reis R, Salmon CE, Bonugli GP, Mazoroski D, Tamashiro MH, Savarese LG, et al. Lumbar intervertebral discs T2 relaxometry and T1ρ relaxometry correlation with age in asymptomatic young adults. Quant Imaging Med Surg. 2016;6:402–412. doi: 10.21037/qims.2016.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zander T, Krishnakanth P, Bergmann G, Rohlmann A. Diurnal variations in intervertebral disc height affect spine flexibility, intradiscal pressure and contact compressive forces in the facet joints. Comput Methods Biomech Biomed Engin. 2010;13:551–557. doi: 10.1080/10255840903337855. [DOI] [PubMed] [Google Scholar]
- 25.Scott JE, Bosworth TR, Cribb AM, Taylor JR. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J Anat. 1994;184(Pt 1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 26.Ashinsky BG, Bonnevie ED, Mandalapu SA, Pickup S, Wang C, Han L, et al. Intervertebral disc degeneration is associated with aberrant endplate remodeling and reduced small molecule transport. J Bone Miner Res. 2020;35:1572–1581. doi: 10.1002/jbmr.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massey CJ, van Donkelaar CC, Vresilovic E, Zavaliangos A, Marcolongo M. Effects of aging and degeneration on the human intervertebral disc during the diurnal cycle: a finite element study. J Orthop Res. 2012;30:122–128. doi: 10.1002/jor.21475. [DOI] [PubMed] [Google Scholar]
- 28.Nakashima H, Yukawa Y, Suda K, Yamagata M, Ueta T, Kato F. Cervical disc protrusion correlates with the severity of cervical disc degeneration: a cross-sectional study of 1211 relatively healthy volunteers. Spine (Phila Pa 1976) 2015;40:E774–E779. doi: 10.1097/BRS.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 29.Tavakoli J, Elliott DM, Costi JJ. Structure and mechanical function of the inter-lamellar matrix of the annulus fibrosus in the disc. J Orthop Res. 2016;34:1307–1315. doi: 10.1002/jor.23306. [DOI] [PubMed] [Google Scholar]
- 30.Lee CH, Heo SJ, Park SH, Jeong HS, Kim SY. The functional and morphological changes of the cervical intervertebral disc after applying lordotic curve controlled traction: a double-blind randomized controlled study. Int J Environ Res Public Health. 2019;16:2162. doi: 10.3390/ijerph16122162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu ZZ, Wen HQ, Zhu YQ, Zhao BL, Kong QC, Chen JY, et al. Short-term effect of lumbar traction on intervertebral discs in patients with low back pain: correlation between the T2 value and ODI/VAS score. Cartilage. 2021;13(1_suppl):414S–423S. doi: 10.1177/1947603521996793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.