Abstract
Patellofemoral instability (PFI) is common in pediatric knee injuries. PFI results from loss of balance in the dynamic relationship of the patella in the femoral trochlear groove. Patellar lateral dislocation, which is at the extreme of the PFI, results from medial stabilizer injury and leads to the patella hitting the lateral femoral condyle. Multiple contributing factors to PFI have been described, including anatomical variants and altered biomechanics. Femoral condyle dysplasia is a major risk factor for PFI. Medial stabilizer injury contributes to PFI by creating an imbalance in dynamic vectors of the patella. Increased Q angle, femoral anteversion, and lateral insertion of the patellar tendon are additional contributing factors that affect dynamic vectors on the patella. An imbalance in the dynamics results in patellofemoral malalignment, which can be recognized by the presence of patella alta, patellar lateral tilt, and lateral subluxation. Dynamic cross-sectional images are useful for in vivo tracking of the patella in patients with PFI. Therapeutic approaches aim to restore normal patellofemoral dynamics and prevent persistent PFI. In this article, the imaging findings of PFI, including risk factors and characteristic findings of acute lateral patellar dislocation, are reviewed. Non-surgical and surgical approaches to PFI in pediatric patients are discussed.
Keywords: Patellofemoral instability, Children, MRI
INTRODUCTION
Patellofemoral instability (PFI) encompasses a spectrum of diseases, ranging from mild maltracking to frank lateral patellar dislocation (LPD). LPD is relatively common, affecting 50 in 100000 children, with the first episode of dislocation generally occurring between the ages of 15 and 19 years [1,2]. LPD is a debilitating condition with a rate of re-dislocation up to 70% after conservative management or physical therapy [3]. Medial patellofemoral ligament reconstruction is the most widely used surgical management [1,4]; however, complications, including re-dislocation, are observed in up to 16% of patients [5]. Furthermore, subsequent surgery of the opposite non-surgically managed knee is required in one out of four patients [6]. The long-term risk of progressive cartilage damage and osteoarthritis (OA) after LPD is six times higher after the initial dislocation, placing many young patients at risk for OA in their 30s and 40s [7]. A lack of a comprehensive understanding of PFI is one of the major obstacles to restoring normal patellofemoral joint congruency. Multiple risk factors contributing to PFI have been identified, with improved diagnostic accuracy of MRI for PFI. The evaluation of these risk factors is a critical component of comprehensive assessment to determine the appropriate therapeutic approach. In this review, the risk factors for PFI and associated imaging findings are presented. Non-surgical and surgical approaches to the treatment of PFI and recent updates on pediatric PFI will also be reviewed.
Risk Factors
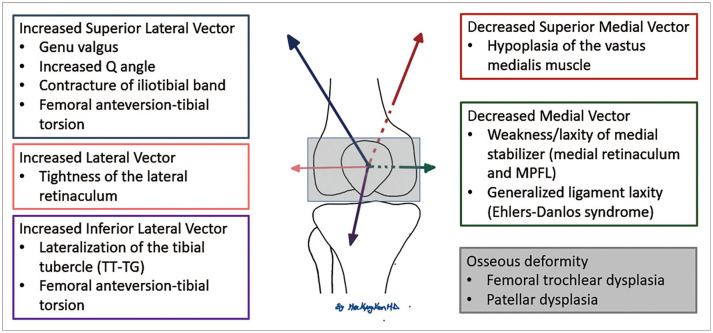
The risk factors for PFI are divided into two major categories: anatomical and alignment anomalies. Femoral trochlear dysplasia is the most important anatomical anomaly. Alignment anomalies include patellar alta, patellar lateral tilt, and subluxation. Patellofemoral malalignment occurs as a result of altered biomechanics due to medial stabilizer injury [8], increased Q angle, femoral anteversion, and lateralization of the patella tendon insertion. Risk factors for PFI are summarized in Figure 1 [9,10,11,12].
Fig. 1. Risk factors for patellofemoral instability.
MPFL = medial patellofemoral ligament, TT-TG = tibial tubercle-trochlear groove
Femoral Trochlear Dysplasia
Femoral trochlear dysplasia is a major risk factor for PFI [13]. Trochlear dysplasia refers to deformity of the femoral trochlea such that the articular surface is flattened instead of concave, which results in loss of lateral patellar tracking and predisposition to lateral dislocation of the patella. The more severe forms of dysplasia have a hypoplastic medial femoral condyle with a convex-shaped trochlear surface. Trochlear dysplasia is a developmental anomaly that usually involves the bilateral knee joints. A true lateral knee radiograph may demonstrate a crossing sign or double contour sign, reflecting flat and convex trochlear grooves, respectively (Fig. 2) [14]. Four different morphological types of trochlear dysplasia were described by Dejour et al. [13] and included Type A: shallow sulcus with preserved trochlear morphological structure; Type B: flat with horizontal orientation of the trochlear surface; Type C: flat with obliquely oriented trochlear surface; and Type D: type C associated with prominent protrusion of the trochlea on parasagittal images described as a “cliff” pattern (Fig. 3). Axial and sagittal MRI enable accurate identification of the type of femoral trochlear dysplasia [15]. Several quantitative methods have been proposed to diagnose trochlear dysplasia, but the best diagnostic accuracy and reproducibility have been achieved with MRI. MRI can provide information regarding the subchondral bone, articular cartilage, and non-ossified epiphyseal cartilage, which is a unique feature in skeletally immature children. Lateral trochlear inclination angle, trochlear facet asymmetry, and trochlear depth are all important factors in determining femoral trochlear dysplasia [16,17].
Fig. 2. Normal knee and femoral dysplasia with cross sign and double contour sign.
A normal 7 year old female.
A. Lateral view of the knee radiograph demonstrates normal alignment of the lateral (blue) and medial (red) ridges of the condyle and the deepest portion of the trochlear groove (green). The contour of the trochlear groove (green) does not intersect the contour of the medial (red) or lateral femoral condyle (blue). B. Proton density axial MR image demonstrating a normal femoral trochlear groove. The deepest portion of the trochlear groove (green) is deep to the medial (red) and lateral (blue) ridges of the condyle. C, D. An 18-year-old female with acute patella dislocation with cross sign. C. Lateral view of the knee radiograph demonstrates intersection (arrow) of the contour of the lateral femoral condyle and that of the trochlear groove with “cross sign.” An incidental finding of the trochlea beak/spur is shown (arrowhead). D. Proton density axial MR image demonstrates a flat trochlear groove with medial (red) and lateral (blue) ridges and the deepest portion of the trochlear groove (green) at the same level. E, F. A 17-year-old male with recurrent patellar dislocation with a cross sign and double contour sign. E. Lateral view of the knee radiograph demonstrating a “double contour sign” formed by a significantly smaller medial femoral condyle (arrowheads). More inferiorly, the contour of the lateral femoral condyle is intersected by that of the trochlear groove with “cross sign” (arrow). F. Proton density axial MR image demonstrating medial femoral condyle hypoplasia (red dashes) with a convex-shaped trochlear groove. The deepest portion of the trochlear groove (green) is above the medial (red) and lateral (blue) ridges of the femoral condyle.
Fig. 3. Classification of trochlear dysplasia on magnetic resonance images.
A. Type A dysplasia. Axial T2 with fat suppression image demonstrating a shallow trochlear groove, but the trochlear structures (dashes) are maintained. B. Type B dysplasia. Axial T2 with fat suppression image demonstrating almost flat (dashes) and horizontally oriented trochlear surfaces. C. Type C dysplasia. The axial proton density image demonstrates a flat and obliquely (dashed) oriented trochlear surface. D. Type D dysplasia. Parasagittal proton density image demonstrating prominent bone protrusion (arrow). Lipohemarthrosis with lipid (black asterisk) fluid level (white asterisks) was identified.
Lateral Trochlear Inclination Angle
The lateral trochlear inclination angle is measured on the axial image. The superior section, showing the articular cartilage of the femoral trochlea, is selected. The angle between the line tangential to the articular surface of the lateral trochlear facet and the line tangential to the posterior aspect of the medial and lateral femoral condyles is measured (Fig. 4, red line) [6,18]. An inclination angle of < 11 degrees is an indication of trochlear dysplasia (93% sensitivity and 87% specificity) [17].
Fig. 4. Lateral trochlear inclination angle, trochlear facet asymmetry, and trochlear depth in a patient with patellofemoral instability.

The lateral inclination is measured by the angle between the two lines. The first line is placed on the posterior aspect of the femoral condyle (red line). The second line is placed on the lateral facet of the trochlea (red line). In this case, the lateral inclination angle was 10 degrees, which is abnormal (< 11 degrees). Trochlear facet asymmetry is measured in the same axial plane as that used for the lateral inclination angle. The length of the medial facet (yellow M) is divided by that of the lateral facet (yellow L). Trochlear facet asymmetry in this case was 33% (9.6/28.6 × 100) and abnormal (< 40%). The trochlear depth was measured using the same image. A line tangential to the posterior aspect of the femoral condyle was used as the reference line. The largest AP distance between the line and the medial (A) and lateral (C) facets, and the deepest portion of the sulcus (B) were measured. The trochlear depth is calculated using the formula ([A + C]/2) - B. Trochlear depth in this case is 2 mm ([56 mm + 58 mm]/2) - 55 mm and abnormal (< 3 mm).
Trochlear Facet Asymmetry
Trochlear facet asymmetry is measured on the same axial image used to measure the lateral trochlear inclination angle. It is the ratio of the length of the medial trochlear facet (M) to that of the lateral trochlear facet (L) (Fig. 4, yellow line) [6,18]. A trochlear facet ratio < 0.4 (40%) is an indication of trochlear dysplasia (100% sensitivity and 96% specificity) [17].
Trochlear Depth
Trochlear depth is also measured on the same axial image used to measure the lateral trochlear inclination angle. A line tangential to the posterior aspect of the medial and lateral femoral condyles is used as a reference line. Trochlear depth is calculated from the formula ([A + C]/2) - B where “A” is the length of a perpendicular line between the medial trochlear facet and the reference line, “C” is the length of a perpendicular line between the lateral trochlear facet and the reference line, and “B” is the length of a perpendicular line between the deepest portion of the sulcus and the reference line (Fig. 4, blue line) [6,18]. A trochlear depth of < 3 mm indicates trochlear dysplasia (100% sensitivity and 96% specificity) [17].
Alignment Anomalies
Patella Alta
Patella alta, or high-riding patella, is a normal variant that may be seen incidentally in the asymptomatic population [19] but is a contributing factor for PFI when associated with other risk factors. A higher-riding patella requires more flexion of the knee to be engaged in the trochlea than a normally positioned patella. At lower degrees of knee flexion, this results in a reduction of the contact area between the patella and the trochlea, and increased PFI. A higher incidence of patellar alta (up to 50%) is observed on radiographs in patients with PFI [20,21]. On plain radiographs, the patellar alta is measured based on the bony landmarks by measuring the patellar length and the distance between the patella and tibial tuberosity. MRI can provide more reliable measurements with true 3-dimensional distances between anatomical landmarks, including both osseous and ligamentous structures. The Insall-Salvati index is widely used to assess patella height; this is the ratio of patella tendon length (distance between the posterior inferior aspect of the patella apex and the patella tendon insertion site on the tibial tuberosity [T]) divided by the patella superior-inferior length (P) (Fig. 5, solid lines). A patella height ratio of > 1.3 indicates patella alta [19,22]. The Caton Deschamps index is another method used to document patellar alta and is calculated by measuring the distance from the inferior aspect of the patellar articular surface to the anterior aspect of the tibial plateau (T’) and dividing by the greatest length of the patellar articular surface (P’) (Fig. 5, dashes). A ratio of ≥ 1.2 is defined as patella alta [23].
Fig. 5. Patella alta.
Insall-Salvati index: the length of the patellar tendon (T) (from the patellar apex to the tibial insertion, solid white line) is divided by the length of the patella (P) (the largest distance of the patella, solid black line). Caton Deschamps index: the length of the patellar tendon (T’) (from the inferior margin of the articular surface of the patella, white dashes) is divided by the length of the articular surface of the patella (P’) (black dashes). The Insall-Salvati index is 1.71 (67 mm/39 mm) and abnormal (> 1.3). The Caton Deschamps index is 1.88 (66 mm/35 mm) and abnormal (> 1.3).
Increased Tibial Tubercle to Trochlear Groove (TT-TG) Distance
Normally, the tibial tubercle is located vertically inferior to the femoral sulcus; therefore, the patellar tendon applies an inferiorly directed vector on the patella. However, when the tibial tubercle is shifted laterally, patellar tendon insertion can cause lateral and inferior vectors on the patella, resulting in patellar lateral dislocation. Excessive lateral displacement of the tibial tubercle is a risk factor for PFI [24]. The tibial tubercle to trochlear groove (TT-TG) distance is measured on axial MRI by measuring the mediolateral distance between the tibial tuberosity and the deepest portion of the femoral trochlear groove (Fig. 6) [25]. In the adult population, a TT-TG > 20 mm is considered abnormal and associated with PFI. A TT-TG between 15 and 20 mm is borderline. In the pediatric population, TT-TG levels increase with age. In children with PFI, the median TT-TG distance (12.1 mm) is longer than that in controls (8.5 mm) but still within the borderline range, since reference values from the adult population are used. To identify abnormal TT-TG levels and develop an appropriate surgical approach, an age-based reference is necessary for pediatric patients during skeletal maturation [26].
Fig. 6. TT-TG distance.

A line tangential to the posterior aspect of the medial and lateral femoral condyles was used as a reference line (grey dashes). A perpendicular line crossing the deepest portion of the trochlear groove (black line) was drawn. Another line crossing the mid-portion of the tibial tubercle was also drawn (white line). The distance between the two lines was defined as the TT-TG distance (red line). TT-TG = tibial tubercle-trochlear groove
Bilateral patellar dislocation requiring bilateral MPFLR has been reported in up to 25% of children with patella dislocation [18]. Kim et al. [18] identified TT-TG and patellar height ratio as important risk factors for bilateral dislocation; TT-TG and patellar height ratios (patella alta) were significantly increased in a group with bilateral patellar dislocation compared with a group with unilateral patellar dislocation. The severity of femoral condyle dysplasia did not correlate with the severity of cartilage damage or bilateral involvement.
Increased Q Angle
An increased Q angle is one of the risk factors for PFI because it affects patellofemoral kinematics. The Q angle is calculated using two lines: a line drawn from the anterior superior iliac spine to the mid-portion of the patella and another line from the tibial tubercle to the mid-portion of the patella (Fig. 7A). An altered Q angle affects the patellofemoral joint by changing the load vectors of the quadriceps and patellar tendons, and an increase in the Q angle results in lateral patellar subluxation and tilt with knee flexion [27].
Fig. 7. Q angle and femoral anteversion.

A. The Q angle is measured between two lines. The first line is drawn from the anterior superior iliac spine to the mid portion of the patella (red line). The second line is from the tibial tubercle to the mid portion of the patella (blue line). B. Increased femoral anteversion (purple arrow in the first figure) results in inward rotation of the distal femur (purple arrow in the second figure). The patella is located laterally relative to the femoral condyle (second figure). To compensate for this, the distal tibia rotates externally (purple arrow in the third figure) resulting in an increase of the lateral vector of the patellar tendon on the patella.
Femoral Anteversion
Femoral anteversion is defined as inward rotation of the knee (distal femur) relative to the hip joint. As the distal femur and femoral trochlea rotate inward, the patella is pulled relatively outward and laterally (Fig. 7B). To compensate for the femoral anteversion, the distal tibia rotates outward, resulting in an increased lateral vector of the patellar tendon on the patella, which further adds to the complexity of the patellofemoral joint alignment. Increased femoral anteversion negatively affects therapeutic outcomes in PFI [28].
Imaging Findings
MRI findings of PFI vary depending on the severity and chronicity of the disease. Mild cases of PFI may demonstrate patellar maltracking as evidenced by edema of the superior lateral aspect of Hoffa’s fat pad, also known as patellofemoral fat impingement [29,30]. Patellofemoral fat impingement is strongly associated with other risk factors for PFI, including femoral condyle dysplasia, patellar alta, increased TT-TG distance, and patellar lateral tilting and subluxation. Longstanding patellar maltracking results in cartilage damage and early degeneration of the lateral patellofemoral joint [31,32,33].
Acute patellar lateral dislocation (APLD) is the most severe form of PFI. Plain radiographs demonstrate findings of acute injury, which may include joint effusion, occasional fat-fluid levels from lipohemarthrosis, osteochondral fracture of the medial aspect of the patella, patellar lateral tilt/subluxation (Fig. 8A), and a deep lateral sulcus sign from osteochondral impaction type injury of the lateral femoral condyle. Specific MRI findings of acute LPD include medial stabilizer injuries (seen in 96%), lateral patellar tilt or subluxation, osteochondral injury, and joint effusion (Fig. 8B, C) [34]. In most cases, the patella spontaneously reduces after the first dislocation. Up to 70% of patients experience recurrent dislocation and may develop chronic recurrent dislocation, in which case MRI may show findings of chronic tears of the medial stabilizers, a deformed medial aspect of the patella, ossification of the medial patella, patellofemoral fat impingement, cartilage damage, and degeneration of the lateral patellofemoral joint (Fig. 9) [35].
Fig. 8. A 15-year-old male with acute patella dislocation.
A. The patellar sunrise view demonstrates lateral tilt and lateral subluxation of the patella and medial patellar avulsion fracture (arrow). B. Axial T2 weighted image with fat suppression demonstrates bone marrow edema of the medial aspect of the patella (asterisk) and lateral femoral condyle (asterisk), complete tear of the medial stabilizer near the femoral insertion (white arrow), and partial tear near the patella insertion (open arrow). A small, avulsed bone fragment is seen at the medial aspect of the patella (arrowhead). Patellar lateral tilt and lateral subluxation are also seen. C. Coronal T1-weighted image demonstrates an osteochondral fracture of the lateral femoral condyle (arrow).
Fig. 9. A 19-year-old male with recurrent bilateral patellar dislocation for several years.
A. Bilateral patella sunrise view demonstrates a well-corticated bone fragment from a prior avulsion fracture (asterisks). B. Axial proton density image demonstrates medial stabilizer with fibrous healing (arrow). An ossified, avulsed bone fragment is seen at the medial patella (arrowhead). C. Sagittal intermediate image with fat suppression demonstrates edema of the patellofemoral fat, suggesting patellofemoral fat impingement (asterisk). No significant joint effusion is seen.
Medial Patellar Stabilizer Injuries
The medial retinaculum and MPFL are medial stabilizers of the patella that prevent lateral dislocation. The medial retinaculum and MPFL are seen as well-defined low T2 signal bands and are difficult to distinguish from each other on MRI; they have a broad attachment near the patellar insertion, joining the vastus medialis oblique muscle fibers, and a thin ligamentous insertion near the femoral attachment with a less well-depicted appearance [21]. MRI enables accurate diagnosis of medial stabilizer injuries. In a study of children with acute LPD, approximately 78% had medial patellar stabilizer injuries, which included injuries near the patellar insertion (31%), near the femoral insertion (14%), and at both the femoral and patellar insertion sites (33%) [36]. Medial stabilizer injuries are often associated with avulsion fractures of the patella or femoral epicondyle, which require surgical treatment to prevent recurrent patellar dislocation and PFI [37]. MRI findings of a full-thickness medial stabilizer tear include complete disruption of the ligament with adjacent soft tissue edema and wavy and retracted fibers surrounded by joint effusion, best seen on axial T2-weighted images with fat suppression (Fig. 8B) [34]. A partial tear is characterized by partial discontinuity and irregular appearance of medial stabilizers [34]. A chronic tear or tear with fibrous healing is difficult to diagnose by MRI because of the absence of soft tissue edema or joint effusion. A diffuse attenuated appearance of the medial stabilizer is an indication of a chronic tear. Thickened fibers with proliferation near the patellar or femoral insertion is a sign of remote injury with fibrous healing (Fig. 9) [29,30].
Patellar Lateral Subluxation and Lateral Tilt
After patellar lateral dislocation with medial stabilizer injury, the patella may not be completely reduced. Persistent patellar lateral subluxation or lateral tilt is observed in these patients (Fig. 8A, B) [34]. Lateral subluxation occurs when the patellar apex moves laterally and is not centered within the femoral trochlear groove. Lateral tilt is identified when the angle between the lateral facet of the patella and the anterior aspect of the lateral femoral condyle opens medially. Patellofemoral malalignment, as evidenced by patellar subluxation and tilt, is affected by the degree of knee flexion and amount of joint effusion [38].
Bone Marrow Edema and Deformities
LPD results in the medial aspect of the patella striking the lateral aspect of the femoral condyle, represented by low T1 and high T2 signal intensities on MRI (Fig. 8B, C). An MRI study in pediatric patients with a first patellar dislocation demonstrated that osteochondral or chondral injuries occurred at the patella in 76% of patients, the lateral femoral condyle in 24%, and both sites in 6.5% [39]. A concave-shaped deformity at the inferomedial aspect of the patella results from the impaction type of injury and is a specific sign of LPD. Avulsion injuries are represented by fragmented bones separated at the ligament insertion site (Figs. 8B, 9B). Recognition of osteochondral fractures with intra-articular loose body formation is important for determining a therapeutic plan.
Most patients with patellar dislocation have bone marrow edema at the anterior lateral femoral condyle due to an impaction type of injury. Bone marrow edema is best seen on T2-weighted images and resolves over time after acute dislocation. Cortical fracture of the lateral femoral condyle rarely occurs after lateral dislocation and is best seen on coronal T1-weighted image as a dark signal line surrounded by edema (Fig. 8C) [29,30].
Cartilage Damage
MRI is the most sensitive method for diagnosing a wide range of cartilage damage, including mild cartilage signal changes from softening and edema, partial and full thickness fissures, fractures, cartilage flaps, and delamination injuries. The medial facet of the patella is the most common location of cartilage damage, followed by the medial ridge, lateral facet of the patella, anterolateral aspect of the lateral femoral condyle, and lateral trochlea [39].
Several methods have been described for grading cartilage damage, the most widely accepted of which is the system developed by the International Cartilage Repair Society (ICRS), determined via arthroscopy. MRI classification uses a modified ICRS system as follows: grade 0, normal cartilage; grade 1, abnormal cartilage signal; grade 2 with, superficial cartilage lesion < 50% of the cartilage depth; grade 3 with, deep cartilage lesion > 50% of the cartilage depth; and grade 4 with full full-thickness osseous changes (Fig. 10) [40]. It is important to include information regarding cartilage damage in the MRI report, including the depth of cartilage damage and the two dimensions of cartilage size. Traditional spin-echo sequences or three-dimensional gradient-echo sequences are used for the evaluation of cartilage damage and are excellent for high-grade cartilage damage (grade 3 or 4). Quantitative MRI can provide excellent detection of low-grade cartilage damage and has enabled probing of microarchitecture changes that can precede low-grade cartilage damage (Fig. 10B, D) [41]. Several MR techniques have been applied in cartilage research to reflect each component and physiology of cartilage: T2 relaxation time mapping (Fig. 10B, D), T1 rho imaging, delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), and sodium imaging [42].
Fig. 10. International Cartilage Repair Society classification of cartilage damage.
A. Grade 0: normal. No significant abnormal signal is observed within the patellar cartilage on T2-weighted image with fat suppression. B. T2 relaxation time mapping (bottom) does not show any increase in T2 values on the color map. C. Grade 1: intra-substance high T2 signal in the patellar cartilage on T2-weighted image with fat suppression (arrowheads). D. Increased T2 relaxation time with hotter color (arrowheads) is shown on the T2 map. E. Grade 2: superficial cartilage fissure less than 50% of the depth. Axial T2 weighted image with fat suppression shows a superficial cartilage fissure (arrowhead). F. Grade 3: deep cartilage lesion more than 50% of the depth. Axial T2 weighted image with fat suppression shows cartilage damage involving almost the entire depth of the cartilage (arrowheads). G. Grade 4: full-thickness cartilage damage with underlying bone changes. Axial T2-weighted image with fat suppression showing full-thickness cartilage damage (arrowheads) with adjacent bone marrow edema (asterisk).
According to a previous study by Kim et al. [18], important risk factors that correlate with the severity of cartilage damage include patellar alta, medial stabilizer injury, and presence of bone marrow edema. The severity of femoral trochlear dysplasia is not a predictor of cartilage damage.
Joint Effusion
Knee joint effusion is the most common finding immediately after acute patellar dislocation, although it is non-specific. The volume of joint effusion decreases over time after trauma. In the presence of hemorrhage, fluid-fluid levels resulting from sedimentation of blood may be seen on T2-weighted or gradient echo sequences, or lipid-fluid levels from lipohemarthrosis may be noted (Fig. 3D). In patients with chronic recurrent dislocation, joint effusion is often absent because re-dislocation rarely causes new traumatic injuries to the patellofemoral joint (Fig. 9).
Dynamic Imaging Studies
Patellofemoral tracking is a dynamic process that occurs as the knee flexes and is most pronounced between knee extension and the first 30 degrees of flexion. Kinematic knee CT and MRI have been employed to evaluate the biomechanics of the patellofemoral joint. Cine-sequences or combined static images are obtained while increasing the knee flexion angle, and have enabled probing of the maltracking of the patella in the femoral trochlear groove, measured with bisect offset and patellar tilt (Fig. 11) [43]. By applying weight bearing to the knee during flexion and extension, dynamic MRI can more closely replicate the PFI. Teng et al. [44] identified that the lateral trochlear inclination angle was a better predictor of PFI than TT-TG. Burke et al. [45] utilized real-time radial gradient echo images during knee flexion from 0–30 degrees and suggested that a lateral subluxation of > 3 mm may be the threshold for PFI. A recent study performed during volitional isometric contraction of the quadriceps muscle demonstrated that proximal migration of the patella in midsagittal images is a good indicator of PFI. The feasibility of ultrafast dynamic MRI has been demonstrated in the pediatric population with an acceptable range of imaging quality and acquisition time for the evaluation of children with PFI [46,47].
Fig. 11. Dynamic knee MR imaging in patient with patellofemoral instability.
A. Axial proton density image of both knees with knee extension. Patellar tilt is the angle between a line crossing the largest portion of the patella and a line placed on the posterior aspect of the femoral condyles (yellow lines). The bisect offset (the proportion of the patella lying lateral to the midline) was also measured. A line tangential to the posterior femoral condyles was used as the reference line (yellow dashes) and a perpendicular line crossing the apex of the patella was drawn (green). The distance from the lateral patellar edge to the perpendicular line was measured (sky blue) and divided by the length of the entire patella (blue) at this level. The patellar tilt angle was 8 degrees on the right and 9 degrees on the left. The bisect offset was 49% on the right and 50% on the left. B. Axial proton density images of both knees with 30 degrees of knee flexion. The patellar tilt angle was increased to 26 degrees on the right and 16 degrees on the left, and the bisect offset was 64% on the right and 60% on the left. Degenerative changes in the patella with subchondral cysts and sclerosis are seen on the left.
Treatment
Non-Surgical Treatment
Most acute patellar dislocations are transient and spontaneously reduced. At times, the patella is manually reduced onsite by the patient, family, friend, coach, or trainer. If the patient presents to the emergency department with a dislocated patella, conscious sedation is administered. Closed reduction of the patella is achieved by gradual extension of the leg. Once reduced, the knee joint is examined clinically for other injuries.
The standard treatment for first-time patellar dislocation is non-surgical [48]. A brief period (2–4 weeks) of immobilization in a splint or knee immobilizer allows pain control and initial tissue healing after an acute episode. During this time, weight bearing is allowed with crutches. Following this, a patellar stabilizing brace is used for activities. Physical therapy is administered to regain motion, strength, and limb control. The patient is typically allowed to return to sports approximately 3 months after the initial episode. Beyond this point, wearing a brace is optional.
Surgical Treatment
In more than 30% of patients, the first episode of patellar dislocation is associated with significant knee effusion [39]. MRI is warranted to identify the presence of osteochondral fractures in such cases. The most common location for these fractures is the medial patella or lateral femoral condyle. Surgical treatment is usually recommended in the presence of intra-articular fractures. During surgery, the osteochondral fracture piece is either removed or fixed based on the size of the fractured fragment and quality of the cartilage. When the size of the osteochondral fracture is ≥ 15 mm, fracture fixation is considered rather than excision. Such fixation is performed using an open approach with metal screws, bioabsorbable pins, or sutures. There is a trend for concomitant surgical stabilization of the patella by performing either medial-sided repair or MPFL reconstruction at the time of fracture treatment [49]. If metal screws are used for fracture fixation, they may have to be removed later by another surgical procedure.
In patients with recurrent patellar instability or those who have failed an adequate trial of conservative treatment after a first-time patellar dislocation, surgical stabilization of the patella is recommended. There are two schools of thought regarding the best approach for patellar stabilization. The first approach is to perform an isolated MPFL reconstruction. The MPFL is the primary restraint for lateral patellar subluxation; hence, its reconstruction would provide the required stability for the patella. MPFL reconstruction is commonly performed using quadriceps tendon autografts, hamstring tendon autografts, or allografts. The success rate of isolated MPFL reconstruction in restoring patellar stability is more than 95% and is irrespective of the graft choice [50]. The most common complications of MPFL reconstruction are knee stiffness, patellar fracture, and recurrent patellar instability [5].
The second approach addresses the risk factors for patellar instability, along with MPFL reconstruction. During this approach, anatomical risk factors for patellar instability, including trochlear dysplasia, patellar alta, and increased TT-TG distance, are identified on radiographs and CT/MRI. Once identified, some or all of the risk factors are surgically corrected. Trochlear dysplasia is addressed using trochleoplasty, wherein the trochlear groove is deepened (Fig. 12A). Trochleoplasty is a less popular option in the United States because it involves violation of the articular cartilage, and there is a theoretical risk for avascular necrosis or arthritis in the future. Patellar alta or increased patellar height is addressed by tibial tubercle distalization. To increase the TT-TG distance, tibial tubercle medialization or anteromedialization is performed (Fig. 12B). Complications of tibial tubercle osteotomy include nonunion, painful hardware, and loss of reduction and fracture of the tubercle. Lateral retinaculum release is performed for a tight lateral retinaculum, as manifested by increased patellar tilt. Lateral release complications include persistent swelling and iatrogenic medial instability of the patella.
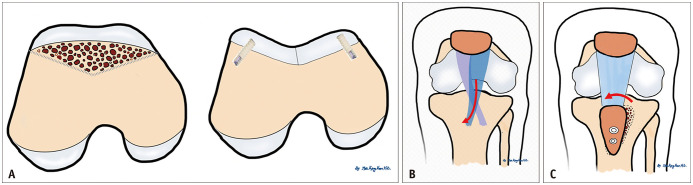
Fig. 12. Illustration of surgical approaches including trochleoplasty, tibial tubercle medialization, and the Roux-Goldthwait procedure.
A. Trochleoplasty. The preoperative image shows a flat trochlear groove. The trochlear joint area was removed and drilled. Subchondral bone was shaped to create a new groove. A surgical screw was placed below the cartilage. B. Tibial tubercle medialization. Tibial tuberosity osteotomy was performed, and the proximal portion of the osteotomy was medialized. Distal osteotomy was fixed using screws. C. Roux-Goldthwait procedure. The lateral half of the patellar tendon was transferred medially and anchored.
In skeletally immature patients, several procedures are either contraindicated or modified because of the presence of a physis. The femoral attachment point for the MFPL is just below the distal femoral physis [13]. Hence, MPFL reconstruction in skeletally immature patients should be performed under strict fluoroscopic guidance to ensure safe drilling of the femoral tunnel. Injury to the distal femoral physis can lead to deformity that may or may not require surgical correction [51]. Similarly, injury to the proximal tibial apophysis can lead to deformity, particularly in the genu recurvatum. Therefore, tibial tubercle osteotomy is contraindicated in patients with an open proximal tibial apophysis. Instead, the patellar tendon can be transposed medially, either fully or partially. When the lateral half of the patellar tendon is transferred medially, the procedure is known as the Roux-Goldthwait procedure (Fig. 12C).
Coronal and rotational limb alignments should be evaluated in all patients undergoing surgery for patellar instability. Increased genu valgum, excessive femoral anteversion, and increased external tibial torsion are risk factors for patellar instability. In skeletally immature patients, guided growth should be considered when addressing genu valgum use. A transphyseal screw or tension-band plate can be placed across the medial aspect of the distal femoral physis to allow gradual correction [52]. Coronal or rotational malalignment requires osteotomy for correction in skeletally mature patients. The indication for correction of genu valgum is > 10 degrees of deformity, and the indication for correction of rotational malalignment is more than 20 degrees of deformity [53].
Complex patellar instability patterns are encountered in young children (< 10 years of age). These include fixed or habitual patellar dislocations. Several syndromes, such as Downs syndrome, nail patella syndrome, Kabuki syndrome, and Rubinstein Taybi syndrome, have patellar instability as a component. It is important to recognize that isolated MPFL reconstruction would not be sufficient to address these complex patterns since the primary pathology is a laterally positioned, and sometimes shortened, quadriceps mechanism. Wide lateral releases and quadricepsplasty are required to address these issues [52]. During quadricepsplasty, the quadriceps mechanism is reoriented and/or lengthened. In cases of neglect or late presentation, these complex instability patterns may be encountered later in life.
Summary
• PFI is a wide spectrum of diseases ranging from mild maltracking to lateral dislocation, and results from risk factors, including femoral trochlear dysplasia and malignancy.
• Femoral trochlear dysplasia is measured using lateral inclination angle (< 11 degrees), trochlear facet asymmetry (facet ratio < 0.4), and trochlear depth (< 3 mm).
• Patellofemoral malalignment is measured using patella alta (patella height ratio ≥ 1.2), increased TT-TG (> 1 cm), increased Q angle, and increased femoral anteversion.
• MRI findings of acute LPD include medial stabilizer injury, osteochondral injury of the patella and lateral femoral condyle with bone marrow edema, and joint effusion.
• Osteochondral fracture in PFI requires surgical treatment with MPFL reconstruction.
Acknowledgments
Min Seo Kim, an undergraduate student at Johns Hopkins University, reviewed and edited of this article. The authors wish to thank Min-Seo Kim for collecting references, proofreading this paper and correcting the authors’ English.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: all authors.
- Writing—original draft: all authors.
- Writing—review & editing: all authors.
Funding Statement: None
Availability of Data and Material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
- 1.Fithian DC, Paxton EW, Stone ML, Silva P, Davis DK, Elias DA, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32:1114–1121. doi: 10.1177/0363546503260788. [DOI] [PubMed] [Google Scholar]
- 2.Waterman BR, Belmont PJ, Jr, Owens BD. Patellar dislocation in the United States: role of sex, age, race, and athletic participation. J Knee Surg. 2012;25:51–57. doi: 10.1055/s-0031-1286199. [DOI] [PubMed] [Google Scholar]
- 3.Moiz M, Smith N, Smith TO, Chawla A, Thompson P, Metcalfe A. Clinical outcomes after the nonoperative management of lateral patellar dislocations: a systematic review. Orthop J Sports Med. 2018;6:2325967118766275. doi: 10.1177/2325967118766275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40:606–611. doi: 10.1249/MSS.0b013e318160740f. [DOI] [PubMed] [Google Scholar]
- 5.Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41:1030–1038. doi: 10.1177/0363546513482085. [DOI] [PubMed] [Google Scholar]
- 6.Kim HK, Greenstein R, Plemmons A, Rajdev N, Parikh S, Kim DH. Patellofemoral instability in children: correlation between patellofemoral incongruence, mechanism of injury, and cartilage damage. AJR Am J Roentgenol. 2019;213:182–190. doi: 10.2214/AJR.18.20778. [DOI] [PubMed] [Google Scholar]
- 7.Sanders TL, Pareek A, Johnson NR, Stuart MJ, Dahm DL, Krych AJ. Patellofemoral arthritis after lateral patellar dislocation: a matched population-based analysis. Am J Sports Med. 2017;45:1012–1017. doi: 10.1177/0363546516680604. [DOI] [PubMed] [Google Scholar]
- 8.Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82:1639–1650. [PubMed] [Google Scholar]
- 9.Beasley LS, Vidal AF. Traumatic patellar dislocation in children and adolescents: treatment update and literature review. Curr Opin Pediatr. 2004;16:29–36. doi: 10.1097/00008480-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bandy WD, Irion JM, Briggler M. The effect of static stretch and dynamic range of motion training on the flexibility of the hamstring muscles. J Orthop Sports Phys Ther. 1998;27:295–300. doi: 10.2519/jospt.1998.27.4.295. [DOI] [PubMed] [Google Scholar]
- 11.Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM, et al. Patellar dislocations in children, adolescents and adults: a comparative MRI study of medial patellofemoral ligament injury patterns and trochlear groove anatomy. Eur J Radiol. 2011;79:415–420. doi: 10.1016/j.ejrad.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 12.Balcarek P, Jung K, Frosch KH, Stürmer KM. Value of the tibial tuberosity-trochlear groove distance in patellar instability in the young athlete. Am J Sports Med. 2011;39:1756–1761. doi: 10.1177/0363546511404883. [DOI] [PubMed] [Google Scholar]
- 13.Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2:19–26. doi: 10.1007/BF01552649. [DOI] [PubMed] [Google Scholar]
- 14.Strauss M, Kyle Martin R, Lind M. 32—Trochleoplasty. sciencedirect.com Web site. [Accessed May 7, 2021]. https://www.sciencedirect.com/science/article/pii/B9780323713108000323 .
- 15.Salzmann GM, Weber TS, Spang JT, Imhoff AB, Schöttle PB. Comparison of native axial radiographs with axial MR imaging for determination of the trochlear morphology in patients with trochlear dysplasia. Arch Orthop Trauma Surg. 2010;130:335–340. doi: 10.1007/s00402-009-0912-y. [DOI] [PubMed] [Google Scholar]
- 16.Carrillon Y, Abidi H, Dejour D, Fantino O, Moyen B, Tran-Minh VA. Patellar instability: assessment on MR images by measuring the lateral trochlear inclination-initial experience. Radiology. 2000;216:582–585. doi: 10.1148/radiology.216.2.r00au07582. [DOI] [PubMed] [Google Scholar]
- 17.Pfirrmann CW, Zanetti M, Romero J, Hodler J. Femoral trochlear dysplasia: MR findings. Radiology. 2000;216:858–864. doi: 10.1148/radiology.216.3.r00se38858. [DOI] [PubMed] [Google Scholar]
- 18.Kim HK, Shiraj S, Kang CH, Anton C, Kim DH, Horn PS. Patellofemoral instability in children: correlation between risk factors, injury patterns, and severity of cartilage damage. AJR Am J Roentgenol. 2016;206:1321–1328. doi: 10.2214/AJR.15.15217. [DOI] [PubMed] [Google Scholar]
- 19.Shabshin N, Schweitzer ME, Morrison WB, Parker L. MRI criteria for patella alta and baja. Skeletal Radiol. 2004;33:445–450. doi: 10.1007/s00256-004-0794-6. [DOI] [PubMed] [Google Scholar]
- 20.Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167:339–341. doi: 10.2214/ajr.167.2.8686598. [DOI] [PubMed] [Google Scholar]
- 21.Elias DA, White LM, Fithian DC. Acute lateral patellar dislocation at MR imaging: injury patterns of medial patellar soft-tissue restraints and osteochondral injuries of the inferomedial patella. Radiology. 2002;225:736–743. doi: 10.1148/radiol.2253011578. [DOI] [PubMed] [Google Scholar]
- 22.Escala JS, Mellado JM, Olona M, Giné J, Saurí A, Neyret P. Objective patellar instability: MR-based quantitative assessment of potentially associated anatomical features. Knee Surg Sports Traumatol Arthrosc. 2006;14:264–272. doi: 10.1007/s00167-005-0668-z. [DOI] [PubMed] [Google Scholar]
- 23.Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34:305–309. doi: 10.1007/s00264-009-0929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsujimoto K, Kurosaka M, Yoshiya S, Mizuno K. Radiographic and computed tomographic analysis of the position of the tibial tubercle in recurrent dislocation and subluxation of the patella. Am J Knee Surg. 2000;13:83–88. [PubMed] [Google Scholar]
- 25.Schoettle PB, Zanetti M, Seifert B, Pfirrmann CW, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13:26–31. doi: 10.1016/j.knee.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Dickens AJ, Morrell NT, Doering A, Tandberg D, Treme G. Tibial tubercle-trochlear groove distance: defining normal in a pediatric population. J Bone Joint Surg Am. 2014;96:318–324. doi: 10.2106/JBJS.M.00688. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno Y, Kumagai M, Mattessich SM, Elias JJ, Ramrattan N, Cosgarea AJ, et al. Q-angle influences tibiofemoral and patellofemoral kinematics. J Orthop Res. 2001;19:834–840. doi: 10.1016/S0736-0266(01)00008-0. [DOI] [PubMed] [Google Scholar]
- 28.Franciozi CE, Ambra LF, Albertoni LJ, Debieux P, Rezende FC, Oliveira MA, et al. Increased femoral anteversion influence over surgically treated recurrent patellar instability patients. Arthroscopy. 2017;33:633–640. doi: 10.1016/j.arthro.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30:961–981. doi: 10.1148/rg.304095755. [DOI] [PubMed] [Google Scholar]
- 30.Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46:618–636. doi: 10.1007/s00247-015-3520-8. [DOI] [PubMed] [Google Scholar]
- 31.Macri EM, Neogi T, Tolstykh I, Widjajahakim R, Lewis CE, Torner JC, et al. Relation of patellofemoral joint alignment, morphology, and radiographic osteoarthritis to frequent anterior knee pain: data from the multicenter osteoarthritis study. Arthritis Care Res (Hoboken) 2020;72:1066–1073. doi: 10.1002/acr.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campagna R, Pessis E, Biau DJ, Guerini H, Feydy A, Thevenin FS, et al. Is superolateral Hoffa fat pad edema a consequence of impingement between lateral femoral condyle and patellar ligament? Radiology. 2012;263:469–474. doi: 10.1148/radiol.12111066. [DOI] [PubMed] [Google Scholar]
- 33.Jibri Z, Martin D, Mansour R, Kamath S. The association of infrapatellar fat pad oedema with patellar maltracking: a case-control study. Skeletal Radiol. 2012;41:925–931. doi: 10.1007/s00256-011-1299-8. [DOI] [PubMed] [Google Scholar]
- 34.Kirsch MD, Fitzgerald SW, Friedman H, Rogers LF. Transient lateral patellar dislocation: diagnosis with MR imaging. AJR Am J Roentgenol. 1993;161:109–113. doi: 10.2214/ajr.161.1.8517287. [DOI] [PubMed] [Google Scholar]
- 35.Jerabek SA, Asnis PD, Bredella MA, Ouellette HA, Poon SK, Gill TJ., 4th Medial patellar ossification after patellar instability: a radiographic finding indicative of prior patella subluxation/dislocation. Skeletal Radiol. 2009;38:785–790. doi: 10.1007/s00256-008-0644-z. [DOI] [PubMed] [Google Scholar]
- 36.Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32:145–155. doi: 10.1097/BPO.0b013e3182471ac2. [DOI] [PubMed] [Google Scholar]
- 37.Sillanpää PJ, Peltola E, Mattila VM, Kiuru M, Visuri T, Pihlajamäki H. Femoral avulsion of the medial patellofemoral ligament after primary traumatic patellar dislocation predicts subsequent instability in men: a mean 7-year nonoperative follow-up study. Am J Sports Med. 2009;37:1513–1521. doi: 10.1177/0363546509333010. [DOI] [PubMed] [Google Scholar]
- 38.Laurin CA, Lévesque HP, Dussault R, Labelle H, Peides JP. The abnormal lateral patellofemoral angle: a diagnostic roentgenographic sign of recurrent patellar subluxation. J Bone Joint Surg Am. 1978;60:55–60. [PubMed] [Google Scholar]
- 39.Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33:511–518. doi: 10.1097/BPO.0b013e318288b7a0. [DOI] [PubMed] [Google Scholar]
- 40.Forney M, Subhas N, Donley B, Winalski CS. MR imaging of the articular cartilage of the knee and ankle. Magn Reson Imaging Clin N Am. 2011;19:379–405. doi: 10.1016/j.mric.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Kang CH, Kim HK, Shiraj S, Anton C, Kim DH, Horn PS. Patellofemoral instability in children: T2 relaxation times of the patellar cartilage in patients with and without patellofemoral instability and correlation with morphological grading of cartilage damage. Pediatr Radiol. 2016;46:1134–1141. doi: 10.1007/s00247-016-3574-2. [DOI] [PubMed] [Google Scholar]
- 42.Choi JA, Gold GE. MR imaging of articular cartilage physiology. Magn Reson Imaging Clin N Am. 2011;19:249–282. doi: 10.1016/j.mric.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jibri Z, Jamieson P, Rakhra KS, Sampaio ML, Dervin G. Patellar maltracking: an update on the diagnosis and treatment strategies. Insights Imaging. 2019;10:65. doi: 10.1186/s13244-019-0755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teng HL, Chen YJ, Powers CM. Predictors of patellar alignment during weight bearing: an examination of patellar height and trochlear geometry. Knee. 2014;21:142–146. doi: 10.1016/j.knee.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Burke CJ, Kaplan D, Block T, Chang G, Jazrawi L, Campbell K, et al. Clinical utility of continuous radial magnetic resonance imaging acquisition at 3 T in real-time patellofemoral kinematic assessment: a feasibility study. Arthroscopy. 2018;34:726–733. doi: 10.1016/j.arthro.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regalado G, Lintula H, Eskelinen M, Kokki H, Kröger H, Svedström E, et al. Dynamic KINE-MRI in patellofemoral instability in adolescents. Knee Surg Sports Traumatol Arthrosc. 2014;22:2795–2802. doi: 10.1007/s00167-013-2679-5. [DOI] [PubMed] [Google Scholar]
- 47.Biyani R, Elias JJ, Saranathan A, Feng H, Guseila LM, Morscher MA, et al. Anatomical factors influencing patellar tracking in the unstable patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:2334–2341. doi: 10.1007/s00167-014-3195-y. [DOI] [PubMed] [Google Scholar]
- 48.Liu JN, Steinhaus ME, Kalbian IL, Post WR, Green DW, Strickland SM, et al. Patellar instability management: a survey of the International Patellofemoral Study Group. Am J Sports Med. 2018;46:3299–3306. doi: 10.1177/0363546517732045. [DOI] [PubMed] [Google Scholar]
- 49.Gurusamy P, Pedowitz JM, Carroll AN, Johnson K, Chambers HG, Edmonds EW, et al. Medial patellofemoral ligament reconstruction for adolescents with acute first-time patellar dislocation with an associated loose body. Am J Sports Med. 2021;49:2159–2164. doi: 10.1177/03635465211013543. [DOI] [PubMed] [Google Scholar]
- 50.Schneider DK, Grawe B, Magnussen RA, Ceasar A, Parikh SN, Wall EJ, et al. Outcomes after isolated medial patellofemoral ligament reconstruction for the treatment of recurrent lateral patellar dislocations: a systematic review and meta-analysis. Am J Sports Med. 2016;44:2993–3005. doi: 10.1177/0363546515624673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24:680–685. doi: 10.1016/j.knee.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Parikh SN, Redman C, Gopinathan NR. Simultaneous treatment for patellar instability and genu valgum in skeletally immature patients: a preliminary study. J Pediatr Orthop B. 2019;28:132–138. doi: 10.1097/BPB.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 53.White GR, Mencio GA. Genu valgum in children: diagnostic and therapeutic alternatives. J Am Acad Orthop Surg. 1995;3:275–283. doi: 10.5435/00124635-199509000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.