Abstract
With regard to the indolent clinical characteristics of prostate cancer (PCa), the more selective detection of clinically significant PCa (CSC) has been emphasized in its diagnosis and management. Magnetic resonance imaging (MRI) has advanced technically, and recent international cooperation has provided a standardized imaging and reporting system for prostate MRI. Accordingly, prostate MRI has recently been investigated and utilized as a triage tool before biopsy to guide tissue sampling to increase the detection rate of CSC beyond the staging tool for patients in whom PCa was already confirmed on conventional systematic biopsy. Radiologists must understand the current paradigm shift for better PCa diagnosis and management. This article reviewed the recent literature, demonstrating the diagnostic value of pre-biopsy prostate MRI with targeted biopsy and discussed unsolved issues regarding the paradigm shift in the diagnosis of PCa.
Keywords: Prostate cancer, MRI, MRI-guided biopsy, Prostate biopsy, Target biopsy
INTRODUCTION
Prostate cancer (PCa) is the second-most prevalent cancer and the fifth leading cause of cancer-specific mortality in male, worldwide [1]. In the Republic of Korea, the incidence of PCa has rapidly increased over the last couple of decades, and it is the fourth-most prevalent cancer and seventh leading cause of cancer-specific mortality in male [2]. Considering the global prevalence in developed countries and the aging tendency in Korean demographics, PCa is expected to become more prevalent in the near future. In PCa epidemiology, it is remarkable that the cancer-specific mortality rate is relatively low compared with the incidence rate. This is because PCa predominantly occurs in elderly male who are at high risk of developing aging-related comorbidities. Furthermore, the introduction of serum prostate-specific antigen (PSA)-based screening tests has contributed to an increase in low-risk PCa detection associated with excellent prognosis [3].
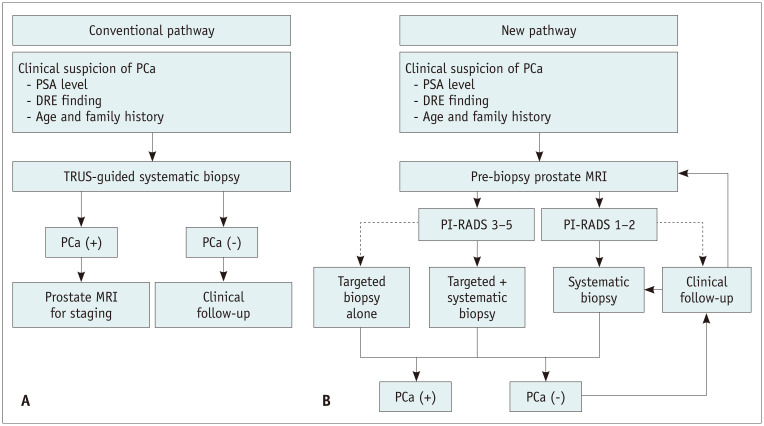
PCa is clinically suspected based on the results of digital rectal examination (DRE) and/or an elevated serum PSA level. Although abnormal DRE results are an indication for biopsy, PSA level is a more sensitive indicator of PCa than either DRE or transrectal ultrasound (TRUS) results [4]. The standard method for pathological diagnosis is TRUS-guided 10–12-core systematic biopsy, in male with clinical suspicion of PCa (Fig. 1A). However, conventional screening systems based on PSA levels and diagnostic strategies using systematic biopsy have several limitations. First, some PCa can be missed in relation to tumor size and location because TRUS is usually confined to the role of anatomical guidance, owing to its low capability of detecting PCa [5]. Second, the sampling power may decrease as prostate volume increases in association with the underlying benign prostatic hypertrophy. Finally, overdiagnosis of low-risk PCa is an important issue because serum PSA levels are not specific to clinically significant PCa (CSC). Low-risk PCa, characterized by an early stage and a low pathologic grade, has a substantially excellent prognosis [6,7]. Therefore, active management can be clinically doubtful for silent PCa, especially in elderly male with a short life expectancy; this is because radical prostatectomy, which is the standard therapeutic option for localized PCa, yields morbidity [8]. The conventional diagnostic pathway has been reported to be ineffective in the selective detection of CSC, despite its diagnostic ability to detect overall PCa. Several factors, such as volume, pathologic grade, and local extent of the index lesion, should be actively managed owing to its aggressiveness and poor prognosis [9]. CSC was defined based on surgical specimen findings as follows: tumor volume ≥ 0.5 cm3, Gleason score > 6, or presence of extraprostatic extension [10]. Among the variable criteria for CSC, tumors with group 2 International Society of Urological Pathology (ISUP) grade (i.e., Gleason score 3 + 4) or higher constitute the most common and important criteria for CSC in both biopsy and prostatectomy specimens [11].
Fig. 1. Diagnostic pathway for prostate cancer.
A, B. Conventional (A) and new MRI-based diagnostic pathway (B) for PCa detection. Dashed arrow means controversial pathway with weak level of evidence. DRE = digital rectal examination, PCa = prostate cancer, PI-RADS = Prostate Imaging Reporting and Data System, PSA = prostate-specific antigen, TRUS = transrectal ultrasonography
Over the last twenty years, prostate magnetic resonance imaging (MRI) has advanced technologically and has been widely investigated in PCa detection, localization, and characterization. With increasing knowledge on prostate MRI, to compensate for the limitations of conventional diagnostic strategies, several researchers have suggested the potential of prostate MRI before biopsy in PCa diagnosis. However, heterogeneity in imaging protocols and interpretive methods has been recognized as an obstacle in utilizing prostate MRI beyond cancer staging. Recently, global collaboration has attempted to standardize the protocol and interpretation of multiparametric prostate MRI (mpMRI), and efforts have brought about promising results, such as the formation of international guidelines termed the Prostate Imaging Reporting and Data System (PI-RADS) [12]. Positive results may induce a change in PCa diagnosis using prostate MRI before biopsy to identify specific target lesions or even to determine whether to perform a biopsy (Fig. 1B). The current paradigm shift in PCa diagnosis aims at the following points: improvement in CSC detection, reduction in the number of unnecessary biopsies or biopsy cores, and prevention of the over-detection of clinically insignificant PCa.
The purpose of this review is to introduce the achievements of current investigations in association with pre-biopsy MRI and MRI-targeted biopsy, for PCa diagnosis and providing insight into the unsolved issues of this paradigm shift.
Pre-Biopsy MRI and MRI-Targeted Biopsy in Patients with Prior Negative Biopsy
Persistent or even increased PSA levels are clinically dilemmatic in patients with prior negative biopsy results. Conventionally, a repetitive systematic TRUS-guided biopsy is the only diagnostic approach. However, repetitive systematic biopsies yield a decreasing PCa detection rate at each subsequent sampling. In a previous study of 2526 patients, the cancer detection rates of serial systematic biopsies after initial biopsy were 17%, 14%, 11%, and 7%, respectively [13]. Similarly, in another study, the detection rates of PCa on the first and second biopsies in 1051 male with prior negative biopsy were 10% and 5%, respectively [14]. Therefore, previous guidelines recommend at least a single session of TRUS-guided biopsy for patients with an initial negative biopsy result. To improve the detection rate of PCa in repetitive biopsies, a study performed saturation biopsies with a markedly increased number of cores [15]. In this study, the PCa detection rate was 34% on the first repeat biopsy in male with a prior negative biopsy. However, limitation of saturation biopsy is the necessity for general anesthesia or conscious sedation, which is not mandatory in conventional biopsy. Furthermore, the increased detection rate of PCa is primarily attributed to the increased detection of clinically insignificant PCa.
Although prostate MRI has been used for staging pathologically confirmed PCa on TRUS-guided systematic biopsy, technical development and accumulated data on prostate MRI have enabled the utilization of MRI before re-biopsy, to potentially solve the limitations of both repetitive systematic biopsy and saturation biopsy. Hambrock et al. [16] reported a superior PCa detection rate of MRI with targeted biopsy compared with systematic TRUS-guided biopsy in male with a prior negative biopsy. In the study by Portalez et al. [17], more targeted cores were found to be PCa than random systematic cores (36.3% vs. 4.9%) in patients with prior negative biopsy. In another study, the positive biopsy yield was higher in MRI-prompted biopsies than in systematic samplings (92% vs. 23%), and 77% of tumors were exclusively detected in MRI-prompted zones [18]. Furthermore, the authors demonstrated that the anterior and apical regions contained most of the tumors that were missed by prior systematic TRUS-guided biopsy. In a study by Sonn et al. [19], more CSC was detected on targeted biopsy than on systematic biopsy, and the degree of suspicion on MRI was the most powerful predictor of CSC in male with prior negative biopsy. In a systematic review and meta-analysis of the abovementioned studies, MRI-targeted biopsy improved both overall PCa and CSC detection rates (relative sensitivity, 1.62 and 1.22, respectively) compared with systematic TRUS-guided biopsy in male with prior negative biopsy [20]. The added value of MRI-targeted biopsy is related to the tumor location, where contact between biopsy needles and PCa can be easily avoided during systematic biopsy. According to accumulating data, recent international guidelines recommend pre-biopsy MRI and targeted biopsy in patients with prior negative biopsy [21,22,23,24]. Therefore, pre-biopsy MRI should be considered in repeat biopsy cases if both quality-controlled prostate MRI and experienced operators are available for targeted biopsies.
Pre-Biopsy MRI and MRI-Targeted Biopsy in Biopsy-Naïve Patients
In biopsy-naïve patients with clinically suspicious PCa, there has been growing interest in adopting pre-biopsy MRI and MRI-targeted biopsy (Table 1). Panebianco et al. [25] reported the results of pre-biopsy MRI and targeted biopsy in a randomized prospective analysis of 1140 male who were initially evaluated for PCa. In their study, the proportion of male with an overall PCa diagnosis was higher in those randomized to the MRI-first strategy than in those randomized to the standard TRUS-guided biopsy. However, another prospective randomized study by Tonttila et al. [26] did not find a significant difference between the pre-biopsy MRI group and the standard TRUS-guided biopsy group among 113 biopsy-naïve patients, although the pre-biopsy MRI group showed a slightly higher detection rate for both overall PCa and CSC than the standard TRUS-guided biopsy group. Similarly, there was no significant difference in the detection rates of the two biopsy strategies, for overall PCa and CSC, in a study by Baco et al. [27]. However, the authors emphasized the utility of MRI-targeted biopsy because the majority of CSCs (87%) were detected by targeted biopsy. In a more recently published study by Porpiglia et al. [28], the diagnostic pathway using pre-biopsy MRI was stated to be superior to the standard pathway, in detecting both overall PCa and CSC. This topic was further investigated in multicenter-based studies, such as in a prospective study including 626 biopsy-naïve men by van der Leest et al. [29], where the MRI pathway (i.e., pre-biopsy MRI with MRI-targeted biopsy) showed an identical detection rate for CSC as the standard pathway (25% vs. 23%). The analysis showed that the MRI pathway enabled biopsy avoidance in 49% of the enrolled patients, at the cost of missing CSC in 4% [30]. In another prospective study including 576 male without a previous biopsy by Ahmed et al. [30] (PROMIS trial), MRI-targeted biopsy was more sensitive and less specific in detecting CSC (sensitivity, 93%; specificity, 41%) than TRUS-guided biopsy (sensitivity, 48%; specificity, 96%). Triage using MRI allowed 27% of the patients to avoid biopsy. Furthermore, a recent study including 500 biopsy-naïve male (PRECISION trial) reported similar results [31], as MRI-targeted biopsy was superior to standard TRUS-guided biopsy (38% vs. 26%), and fewer patients were diagnosed with clinically insignificant PCa in the MRI pathway than in the standard pathway (adjusted difference, -13%). Another prospective study (MRI-FIRST trial) in 251 biopsy-naïve patients demonstrated that targeted biopsy was similar to systematic biopsy and added value to systematic biopsy in detecting CSC [32]. In summary, recent data from large and high-quality prospective studies consistently demonstrated the superiority of pre-biopsy MRI with MRI-targeted biopsy over standard TRUS-guided biopsy in detecting CSC (Fig. 1B), which potentially reduced unnecessary biopsies in biopsy-naïve patients with clinical suspicion of PCa. These results may support the current paradigm shift in the diagnostic strategies for PCa.
Table 1. Comparison of Diagnostic Performance between MRI-Targeted and Standard TRUS-Guided Biopsy in the Biopsy Naïve Patient.
| Study (Year) | Study Design | MRI Interpretation | Population (Male) | Cancer Detection Rate (%) | |||
|---|---|---|---|---|---|---|---|
| MRI-Targeted Biopsy | Standard Biopsy | ||||||
| Overall PCa | CSC | Overall PCa | CSC | ||||
| Panebianco et al.* (2015)[25] | Prospective | PI-RADS v1 | 1140 | 73 | N/A | 38 | N/A |
| Tonttila et al.* (2016) [26] | Prospective | Likert scale | 113 | 64 | 55 | 57 | 45 |
| Baco et al.* (2016) [27] | Prospective | PI-RADS v1 | 175 | 51 | 44 | 48 | 49 |
| Porpiglia et al. (2017) [28] | Prospective | PI-RADS v1 | 212 | 51 | 44 | 30 | 18 |
| Kasivisvanathan et al. (2018) [31] | Prospective | PI-RADS v2 | 500 | 47 | 38 | 48 | 26 |
| van der Leest et al.* (2019) [29] | Prospective | PI-RADS v2 | 626 | 39 | 25 | 48 | 23 |
| Rouviere et al.* (2019) [32] | Prospective | Likert scale | 251 | 64 | 3 | 52 | 30 |
*These studies included systematic biopsies in the MRI-targeted biopsy process. CSC = clinically significant cancer, MRI = magnetic resonance imaging, N/A = not applicable, PCa = prostate cancer, PI-RADS = Prostate Imaging Reporting and Data System, TRUS = transrectal ultrasonography
Interpretation of Pre-Biopsy MRI and Indication of MRI-Targeted Biopsy
Precise and standardized interpretation of prostate MRI is essential for utilizing MRI as a triage system in the initial assessment of patients with clinically suspected PCa. In 2014, PI-RADS was initially proposed by the European Society of Urogenital Radiology (ESUR); in 2015, it was updated to its second version by the ESUR and American Urologic Association (AUA) [12,33]. In the updated version, the PI-RADS was further simplified to improve CSC detection. This system defines each category according to the probability of CSC. PI-RADS category 4 or 5 was assigned if CSC was likely or highly likely to be present, and category 3 if a lesion was equivalent to the probability of CSC. The guidelines distinctly described that biopsy should be considered for category 4 or 5, but not for category 1 or 2. For category 3, the PI-RADS ambiguously described that biopsy may or may not be appropriate, depending on non-imaging factors. This is because the PI-RADS was developed and modified based on the consensus of the expert committee; therefore, it encourages researchers to validate these guidelines.
Many studies have reported either biopsy or surgical pathological findings in each of the PI-RADS version 2 categories. A study reported that CSC detection rates on MRI-targeted biopsy were 44%–49% for category 4 lesions, and 72%–74% for category 5 lesions [34]. In this study, 11% of the category 3 lesions were CSCs. In the PRECISION trial, CSCs were identified in 12% of category 3 lesions, 60% of category 4 lesions, and 83% of category 5 lesions. In a prospective study by van der Leest et al. [29], CSCs were identified in 15%–18% of category 3 lesions, 25%–40% of category 4 lesions, and 68%–70% of category 5 lesions. In a multicenter study of 3349 male from 26 different centers, Westphalen et al. [35] reported that the PCa detection rates were 15% for category 3 lesions, 39% for category 4 lesions, and 72% for category 5 lesions; moreover, the overall PCa detection rates were 35% for PI-RADS scores greater than or equal to 3 and 49% for PI-RADS scores greater than or equal to 4.
Considering the reported data, category 4 or 5 lesions should be targeted during biopsy because of the high probability of CSC, as recommended by the PI-RADS. However, routine inclusion of category 3 lesions in targeted biopsy may still be controversial because both the number of biopsy avoidances and the detection rate of CSC can be increased by omitting biopsies for category 3 lesions. If category 3 lesions were excluded from targeted biopsy, biopsy avoidance would increase from 28% to 48%, with a higher detection rate of CSC (from 28% to 71%) in the PRECISION trial. Similarly, biopsy avoidance increased from 49% to 56%, with an increased detection rate of CSC (from 25% to 55%) in the study by van der Leest et al. [29]. These findings are associated with a relatively low rate of CSC detection in category 3 compared with that in category 4 or 5. However, the absolute number of missed CSCs would increase if targeted biopsy was omitted for category 3 lesions. Table 2 summarizes the literature reporting PCa detection rates in patients with a PI-RADS version 2 score of 3 on prostate MRI. Tan et al. [36] reported that 3 of 31 (9.7%) PI-RADS category 3 lesions were confirmed as CSCs in their analysis. In a retrospective analysis by Sheridan et al. [37], 19 of 111 (17.1%) PI-RADS category 3 lesions were CSCs on MRI-TRUS fusion biopsy. In another study, 26 of 156 patients (16.7%) with PI-RADS category 3 lesions showed CSCs on targeted biopsy [38]. Therefore, a targeted biopsy is required to increase the absolute number and sensitivity of CSC detection, even though the detection rate and specificity may decrease. Almost all recent multicenter prospective trials have included equivocal lesions (i.e., score 3 on a Likert scale or category 3 in the PI-RADS) in MRI-targeted biopsy to prevent under-diagnosis of PCa [29,30,31,32,39,40].
Table 2. PCa Detection Rate in the Subjects with PI-RADS v2 Score 3 on MRI.
| Study (Year) | Study Design | MRI Scanner | Subject No. | Subject No. with Score 3 (%) | Cancer Detection Rate (%) | |
|---|---|---|---|---|---|---|
| Overall PCa | CSC | |||||
| Tan et al. (2017) [36] | Retrospective | 3T | 134 lesions | 31 lesions (23) | 19 | 10 |
| Venderink et al.* (2018) [38] | Retrospective | 3T | 1057 male | 156 male (15) | 35 | 17 |
| Rosenkrantz et al. (2017) [34] | Retrospective | 3T | 343 lesions | 79 lesions (23) | 28 | 11 |
| Kasivisvanathan et al. (2018) [31] | Prospective | 1.5 or 3T | 252 male | 51 male (20) | 33 | 12 |
| Sheridan et al. (2018) [37] | Retrospective | 3T | 474 lesions | 111 lesions (23) | 27 | 17 |
| van der Leest et al. (2019) [29] | Prospective | 3T | 317 male | 40 male (13) | 35 | 18 |
| Wegelin et al. (2019) [39] | Prospective | 3T | 665 male | 64 male (10) | 25 | 17 |
| Westphalen et al. (2020) [35] | Retrospective | 1.5 or 3T | 5082 lesions | 1490 lesions (29) | 30 | 15 |
*This study utilized both PI-RADS v1 and v2. CSC = clinically significant cancer, MRI = magnetic resonance imaging, PCa = prostate cancer, PI-RADS = Prostate Imaging Reporting and Data System
In the recently modified PI-RADS version 2.1, there were some changes, especially in the definitions of Categories 2 and 3 in the transition zone [41]. Rosenkrantz et al. [34] reported a relatively wide discrepancy in the frequency of scoring category 3 in PI-RADS version 2 among radiologists. This tendency resulted in discrepancies in the overall PCa and CSC detection rates for category 3 lesions. On the basis of these findings, the authors proposed several adjustments to PI-RADS version 2 for more concordant and better interpretations. Several recent studies reported slightly improved diagnostic performance of PI-RADS version 2.1 compared with the previous version, in both transitional and peripheral zone cancer [42,43,44]. The recent change in the PI-RADS might influence the frequency of scoring category 3 in MRI interpretation; accordingly, the results of targeted biopsy might be slightly different from the reported range according to the previous PI-RADS version.
In summary, a targeted biopsy should be performed for lesions of category 3 or higher on mpMRI, according to the latest PI-RADS version. Furthermore, more data should be collected and analyzed in future PI-RADS versions to validate the effectiveness and appropriateness of MRI-targeted biopsies for category 3 lesions.
Techniques for MRI-Targeted Biopsy
There are three different technical strategies for MRI-targeted biopsies: in-bore MRI biopsy, MRI-TRUS fusion biopsy, and cognitive registration TRUS biopsy [45]. In-bore MRI biopsy is the first technique developed for targeted prostate biopsy under MRI guidance; it allows direct and precise sampling of suspicious lesions on MRI [46]. Several studies have reported PCa detection rates ranging from 15% to 52% by adopting this technique in patients with prior negative systematic biopsy [46,47,48,49,50,51,52,53]. However, this technique requires specialized MRI-compatible equipment. Furthermore, systematic biopsy is not affordable because each sampling takes a substantial amount of time compared to TRUS biopsy; and is therefore costly. Conversely, cognitive registration for TRUS biopsy requires no additional software or equipment. The operator reviews the lesion and anatomy of the prostate gland on MRI and then estimates the target using real-time TRUS imaging. Both targeted and systematic biopsies can be sequentially performed under TRUS guidance. Therefore, this biopsy technique has advantages over in-bore MRI biopsies in terms of time and cost. It has been proven to be a better technique for detecting CSC than non-targeted systematic biopsy [54,55,56,57,58,59,60,61,62]. However, a disadvantage is that the processes of cognitive fusion and visual registration are operator-dependent. Furthermore, visual discrepancies between parallel axial images and fanwise-acquired TRUS images may result in incorrect registration, especially for lesions located in the far apex or base of the prostate gland [63]. Instead of cognitive registration, MRI-TRUS fusion biopsy utilizes software-based platforms for fusion during biopsy to minimize operator errors. Therefore, MRI-TRUS fusion biopsy yields a moderate position regarding cost, procedure time, and technical availability, compared with the other biopsy techniques.
Several studies have reported the diagnostic performance of each MRI-targeted biopsy method; however, only a few studies have directly compared the results of each technique (Table 3). Initial studies compared the diagnostic performance between MRI-TRUS fusion biopsy and cognitive registration TRUS biopsy, but could not demonstrate a significant superiority of MRI-TRUS fusion biopsy in detecting PCa [64,65,66]. Arsov et al. [67] found no significant difference between in-bore MRI biopsy and MRI-TRUS fusion biopsy in detecting both overall PCa (37% vs. 39%) and CSC (29% vs. 32%). Yaxley et al. [68] also reported no advantage of in-bore MRI biopsy over cognitive registration TRUS biopsy in detecting overall PCa and CSC. In a prospective trial by Hamid et al. [69], neither the overall PCa nor CSC detection rates were significantly different between cognitive registration and MRI-TRUS fusion techniques. However, Kaufmann et al. [70] found a significant advantage of in-bore MRI or MRI-TRUS fusion biopsy over cognitive registration for overall PCa detection, although they also failed to find superiority of any technique in detecting CSC. Similarly, a recent meta-analysis reported that in-bore MRI biopsy showed superior diagnostic performance in overall PCa detection compared with cognitive registration TRUS biopsy [71]. However, MRI-TRUS fusion biopsy showed a similar performance to in-bore MRI biopsy in detecting overall PCa and CSC, and there was no significant difference between any single biopsy technique, in detecting CSC. According to a multicenter randomized controlled trial (FUTURE trial), the detection rates of overall PCa and CSC were not significantly different among the three techniques in a repeat biopsy setting in patients with prior negative biopsies [39]. In the trial, the detection rates of PCa and CSC respectively were 55% and 33% by in-bore MRI biopsy, 49% and 34% by MRI-TRUS fusion biopsy, and 44% and 33% by cognitive registration TRUS biopsy (all p > 0.05). These results suggest that the software or equipment for MRI-TRUS fusion or in-bore MRI biopsy is not mandatory for MRI-targeted biopsy, as cognitive registration TRUS biopsy has shown a similar diagnostic performance, especially in detecting CSC. Nonetheless, we must perceive a potential bias in the results because the majority of procedures in the literature might be performed by experienced operators. The outcome of cognitive registration for TRUS biopsy can be influenced by the skill and experience of the operator. Therefore, the use of in-bore MRI and MRI-TRUS fusion biopsy is recommended if they are available, as they may enable a more standardized and uniform fusion process than cognitive registration TRUS biopsy, especially if the lesions are small or invisible on TRUS.
Table 3. Comparison of Diagnostic Performance among MRI-Targeted Biopsy Techniques.
| Study (Year) | Study Design | MRI Interpretation | Subject No. | Cancer Detection Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cognitive Registration | MRI-TRUS Fusion | In-Bore MRI | |||||||
| Overall Pca | CSC | Overall PCa | CSC | Overall PCa | CSC | ||||
| Puech et al. (2013) [64] | Prospective | Likert scale | 79 lesions | 47 | N/A | 53 | N/A | N/A | N/A |
| Wysock et al. (2014) [65] | Prospective | PI-RADS v1 | 172 lesions | 27 | 15 | 32 | 20 | N/A | N/A |
| Arsov et al. (2015) [67] | Prospective | PI-RADS v1 | 201 male | N/A | N/A | 39 | 32 | 37 | 29 |
| Lee et al. (2016) [66] | Prospective | Likert scale | 396 lesions | 33 | 23 | 37 | 21 | N/A | N/A |
| Yaxley et al. (2017) [68] | Retrospective | PI-RADS v1 | 595 lesions | 75 | 68 | N/A | N/A | 74 | 66 |
| Kaufmann et al. (2018) [70] | Retrospective | PI-RADS v2 | 156 male | 29 | 24 | 52 | 36 | 51 | 40 |
| Hamid et al. (2019) [69] | Prospective | PI-RADS v1 | 129 male | 66 | 53 | 69 | 53 | N/A | N/A |
| Wegelin et al. (2019) [39] | Prospective | PI-RADS v2 | 665 male | 44 | 33 | 49 | 34 | 55 | 33 |
CSC = clinically significant cancer, MRI = magnetic resonance imaging, N/A = not applicable, PCa = prostate cancer, PI-RADS = Prostate Imaging Reporting and Data System, TRUS = transrectal ultrasonography
Optimal Number of Biopsy Cores Per Lesion during MRI-Targeted Biopsy
The ideal number of targeted biopsies per lesion was not determined because of a lack of accumulated data. A recent study reported that increasing the number of biopsy core samples from one to three and three to five per target lesion increased the detection rate of CSC by 6.4% and 2.4%, respectively [72]. The authors also described that increasing the number of samples to more than five per lesion would be ineffective because it would diminish the incremental detection rate of CSC. These results can be explained in terms of the characteristics of the Gleason score for PCa grading. The final Gleason score is calculated as the sum of the first half of the score, based on the dominant morphological pattern, and the second half, based on the non-dominant pattern of the highest grade [73,74]. Accordingly, undersampling can lead to underestimation of the Gleason score, and this phenomenon occurs more frequently in low-volume tumors [75]. Several studies have demonstrated that upto 60% of clinically insignificant PCa determined on biopsy changed to CSC among prostatectomy specimens [76,77,78]. Therefore, multiple cores obtained from a target lesion may lead to the detection of CSC, which could be falsely determined on a single biopsy core, as clinically insignificant PCa. This is especially crucial in determining the eligibility for active surveillance (AS) because a Gleason score of 6 on biopsy is one of the most common and important inclusion criteria for AS [79,80]. While it is reasonable to obtain multiple cores during targeted biopsies for the detection of CSC, there may be a risk of oversampling or increased detection of clinically insignificant cancer. The current consensus statement of the AUA and Society of Abdominal Radiology also recommends at least two cores per target lesion [81]. Nevertheless, the operators cannot neglect the increasing cost and potential complication rate associated with the number of biopsy cores. Therefore, the definitive number of biopsy cores should be determined by each operator during biopsy, considering individual confidence in targeting and lesion characteristics, such as size, location, and visibility during biopsy.
Necessity of Routine Systematic Biopsy in Conjunction with MRI-Targeted Biopsy
Although the PRECISION trial demonstrated the superiority of pre-biopsy MRI with or without the targeted biopsy pathway, the results regarding whether systematic biopsy should be performed in conjunction with targeted biopsy remain unclear. A recent prospective multicenter study (MRI-FIRST trial) compared CSC detection rates between targeted, systematic, and targeted systematic biopsies [32]. The CSC detection rate in the biopsy-naïve cohort was higher in the combined biopsy group (37.5% for ISUP grade ≥ 2; 21.1% for ISUP grade ≥ 3) than in either the systematic (29.9% for ISUP grade ≥ 2; 15.1% for ISUP grade ≥ 3) or targeted biopsy groups (32.3% for ISUP grade ≥ 2; 19.9% for ISUP grade ≥ 3). In a recent study by Kim et al. [82], combined targeted and systematic biopsy yielded an increased detection rate of 5.6%, compared to targeted biopsy alone. In another prospective study, the underdetection rate of CSC was higher in targeted biopsy only (9%) than in the combination of targeted and systematic biopsies (2%) [29]. In the repeated biopsy cohort, combined targeted and systematic biopsies increased the detection rate of ISUP grade ≥ 2 and ≥ 3 PCa by approximately 40% and 50%, respectively [83]. Therefore, targeted biopsy should be accompanied by systematic biopsy to increase the detection rate of CSC for the initial assessment of biopsy-naïve patients as well as repeated biopsy patients. The disadvantages of systematic biopsy include the cost and potential increase in biopsy-related complications. However, there are no results from large prospective trials that directly compare the complication rates between targeted biopsy only and targeted biopsy with systematic biopsy. In a systematic review of prostate biopsy complications, more biopsy cores were somewhat related to minor complications associated with pain, bleeding, infection, hematospermia, and erectile dysfunction, although there were substantial controversies among the results of these studies [84]. However, no previous studies have demonstrated a definite relationship between the number of biopsy cores and fatal complications. Therefore, the addition of systematic biopsy may not inflict a significant disadvantage in the management of patients, considering the diagnostic benefit.
Systematic Biopsy in Patients without Any Target Lesion on MRI
Several studies have reported that some PCa can be missed on MRI by analyzing preoperative prostate MRI with surgical pathological data or systematic TRUS biopsy. According to a systematic review and meta-analysis, the median negative predictive value (NPV) of mpMRI was 82.4% for overall PCa, and 88.1% for CSC in 48 studies (median disease prevalence rate, 50.4% and 32.9% for overall PCa and CSC, respectively) [85]. In conclusion, this study emphasized the variation in the NPV of MRI depending on the prevalence of PCa, definition of CSC, and interpretation of positive MRI findings (i.e., Likert scale or PI-RADS version 1). In a recent retrospective study that adopted PI-RADS version 2 for MRI interpretation by Kim et al. [86], cancer-negative findings on pre-biopsy MRI yielded a missed detection rate of 12.6% for PCa, including 3.9% for CSC (disease prevalence rate, 25% and 8.9% for overall PCa and CSC, respectively). In the PROMIS trial, 158 of 576 (27.4%) biopsy-naïve patients showed no target lesion on MRI, of which 17 (10.8%) had CSC on template prostate mapping biopsy (disease prevalence rate, 71% and 40% for overall PCa and CSC, respectively). Although the NPV and false-negative value of MRI are variable depending on the study design, omitting a biopsy on the basis of negative MRI findings may result in missed PCa, including CSC. This is because small-volume PCa, especially of less than 1.0 cm3, can be invisible on mpMRI [87,88,89].
Performing fewer biopsies may have the advantage of avoiding cost- and procedure-related problems, at the expense of missing cancer. Therefore, it is difficult to determine the diagnostic risk and economic benefits of MRI for determining prostate biopsy. The following issues should be thoroughly considered to conclude whether omitting a biopsy based on negative MRI findings is clinically justifiable. First, the exact epidemiology of PCa should be understood at institutional and national levels. Furthermore, a cost-effectiveness analysis should be conducted to assess the economic benefit of omitting a biopsy and economic loss for MRI under the national medical environment. Faria et al. [90] attempted to optimize PCa diagnosis in terms of effectiveness and cost-effectiveness, according to the PROMIS trial. They concluded that the MRI-first strategy was effective and cost-effective for CSC diagnosis under the circumstances of the UK National Health Service. Second, the interpretation of MRI findings should be standardized and quality-controlled. The results of published studies may be acquired from imaging and biopsy data handled by experienced radiologists or urologists. False-negative MRI findings can result from MRI reading errors in addition to the technical limitations of mpMRI [91]. Therefore, radiologists should use the most recently updated version of PI-RADS. Furthermore, quality control may be mandatory in terms of the imaging protocols and equipment for mpMRI. Finally, patient stratification can be useful in determining candidates who are more eligible to skip biopsy based on negative MRI findings. Panebianco et al. [92] concluded that systematic biopsy should be recommended in younger patients with high or increasing PSA levels despite negative MRI findings. Omitting a biopsy can be relatively effective in low-risk patients, in whom the NPV of MRI may be high owing to the low prevalence of PCa. Meanwhile, systematic biopsy may be necessary in high-risk patients because omitting a biopsy may be at the expense of substantial CSC under-detection.
In patients with negative MRI findings, serum tumor markers can be useful as supplementary indicators for active monitoring or intervention. Washino et al. [93] reported a threshold PSA density of < 0.15 ng/mL2, which may avoid unnecessary biopsy in conjunction with a PI-RADS version 2 score of ≤ 3 on MRI. In addition to PSA density, the prostate health index outperformed PSA, free PSA, and free-to-total PSA levels in predicting PCa; accordingly, the results suggest its potential as a biomarker to triage patients with negative MRI findings [94]. However, the levels of tumor markers used to stratify patients can be affected by the methodology of MRI interpretation and the definition of negative MRI findings. Therefore, further data are needed to determine the threshold values of these novel biomarkers to stratify patients with negative MRI findings, which can be properly interpreted according to the latest version of the PI-RADS.
Quality Control for Prostate MRI and Imaging Interpretation
Accurate and standardized imaging interpretations based on quality-controlled mpMRI are preconditions for using MRI as a new diagnostic strategy for PCa. Although the PI-RADS has standardized the process of prostate imaging and interpretation, subjectivity in imaging interpretation owing to the intrinsic limitations of the system still remains. Furthermore, the system does not guarantee the quality of the acquired images or the performance of individual radiologists in practice. The ESUR and the European Urological Association Section of Urologic Imaging recently provided a consensus statement on recommendations for controlling image quality and interpretation performance [95]. Furthermore, a new scoring system called the Prostate Imaging Quality was suggested according to the PRECISION trial [96]. Although these attempts are currently incipient, the accumulation of consensus statements and guidelines for quality standards may impel preparation for national or international certifications in the near future. Radiologists need to consider not only the technical aspects of PI-RADS, but also the efforts for quality control in prostate imaging and interpretation.
CONCLUSION
Pre-biopsy MRI with subsequent targeted biopsy has added value in diagnosing CSC in both biopsy-naïve patients and those with prior negative biopsy results. The accumulated data seems to be sufficient for a paradigm shift in diagnosing PCa because recent prospective studies have consistently demonstrated the superiority of the MRI-first strategy over the conventional diagnostic pathway. Cognitive registration TRUS biopsy is the most cost-effective method for targeted biopsy without significant limitations in CSC detection rate, although in-bore MRI or MRI-TRUS fusion biopsy is recommended if available. During targeted biopsy, systematic biopsy seems to be necessary in both biopsy-naïve and repeated biopsy patients, especially in those at high risk for CSC. However, whether a systematic biopsy can be omitted in patients without a target lesion on MRI remains controversial. Risk stratification and a stepwise strategy can be effective, although further data are necessary to address this issue. Quality control of imaging and interpretation is an important precondition for these above issues.
Acknowledgments
We thank the Editage (www.editage.co.kr) for English language editing.
Footnotes
Conflicts of Interest: Chan Kyo Kim who is on the editorial board of the Korean Journal of Radiology was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
- Conceptualization: all authors.
- Data curation: Jung Jae Park.
- Investigation: all authors.
- Methodology: all authors.
- Project administration: Chan Kyo Kim.
- Supervision: Chan Kyo Kim.
- Validation: all authors.
- Writing—original draft: all authors.
- Writing—review & editing: all authors.
Funding Statement: None
Availability of Data and Material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020;52:335–350. doi: 10.4143/crt.2020.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 4.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–1290. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 5.Dähnert WF, Hamper UM, Eggleston JC, Walsh PC, Sanders RC. Prostatic evaluation by transrectal sonography with histopathologic correlation: the echopenic appearance of early carcinoma. Radiology. 1986;158:97–102. doi: 10.1148/radiology.158.1.3510032. [DOI] [PubMed] [Google Scholar]
- 6.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 7.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 8.Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 9.Matoso A, Epstein JI. Defining clinically significant prostate cancer on the basis of pathological findings. Histopathology. 2019;74:135–145. doi: 10.1111/his.13712. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 11.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA Grading Committee. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 12.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167:2435–2439. [PubMed] [Google Scholar]
- 14.Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001;166:1679–1683. [PubMed] [Google Scholar]
- 15.Stewart CS, Leibovich BC, Weaver AL, Lieber MM. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol. 2001;166:86–91. discussion 91-92. [PubMed] [Google Scholar]
- 16.Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520–527. doi: 10.1016/j.juro.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Portalez D, Mozer P, Cornud F, Renard-Penna R, Misrai V, Thoulouzan M, et al. Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multiparametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol. 2012;62:986–996. doi: 10.1016/j.eururo.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Costa DN, Bloch BN, Yao DF, Sanda MG, Ngo L, Genega EM, et al. Diagnosis of relevant prostate cancer using supplementary cores from magnetic resonance imaging-prompted areas following multiple failed biopsies. Magn Reson Imaging. 2013;31:947–952. doi: 10.1016/j.mri.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438–450. doi: 10.1016/j.eururo.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management. nice.org.uk Web site. [Published 2019]. [Accessed November 23, 2021]. https://www.nice.org.uk/guidance/ng131 .
- 22.Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1119–1134. doi: 10.1016/j.annonc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–262. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 24.Coakley FV, Oto A, Alexander LF, Allen BC, Davis BJ, Froemming AT, et al. ACR Appropriateness Criteria® prostate cancer-pretreatment detection, surveillance, and staging. J Am Coll Radiol. 2017;14(5S):S245–S257. doi: 10.1016/j.jacr.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Panebianco V, Barchetti F, Sciarra A, Ciardi A, Indino EL, Papalia R, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol. 2015;33:17.e1–17.e7. doi: 10.1016/j.urolonc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Tonttila PP, Lantto J, Pääkkö E, Piippo U, Kauppila S, Lammentausta E, et al. Prebiopsy multiparametric magnetic resonance imaging for prostate cancer diagnosis in biopsy-naive men with suspected prostate cancer based on elevated prostate-specific antigen values: results from a randomized prospective blinded controlled trial. Eur Urol. 2016;69:419–425. doi: 10.1016/j.eururo.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Baco E, Rud E, Eri LM, Moen G, Vlatkovic L, Svindland A, et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol. 2016;69:149–156. doi: 10.1016/j.eururo.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 28.Porpiglia F, Manfredi M, Mele F, Cossu M, Bollito E, Veltri A, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naïve patients with suspected prostate cancer. Eur Urol. 2017;72:282–288. doi: 10.1016/j.eururo.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 29.van der Leest M, Cornel E, Israël B, Hendriks R, Padhani AR, Hoogenboom M, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol. 2019;75:570–578. doi: 10.1016/j.eururo.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–822. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 31.Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–1777. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouvière O, Puech P, Renard-Penna R, Claudon M, Roy C, Mège-Lechevallier F, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20:100–109. doi: 10.1016/S1470-2045(18)30569-2. [DOI] [PubMed] [Google Scholar]
- 33.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenkrantz AB, Babb JS, Taneja SS, Ream JM. Proposed adjustments to PI-RADS version 2 decision rules: impact on prostate cancer detection. Radiology. 2017;283:119–129. doi: 10.1148/radiol.2016161124. [DOI] [PubMed] [Google Scholar]
- 35.Westphalen AC, McCulloch CE, Anaokar JM, Arora S, Barashi NS, Barentsz JO, et al. Variability of the positive predictive value of PI-RADS for prostate MRI across 26 centers: experience of the Society of Abdominal Radiology Prostate Cancer Disease-focused Panel. Radiology. 2020;296:76–84. doi: 10.1148/radiol.2020190646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan N, Lin WC, Khoshnoodi P, Asvadi NH, Yoshida J, Margolis DJ, et al. In-bore 3-T MR-guided transrectal targeted prostate biopsy: prostate imaging reporting and data system version 2–based diagnostic performance for detection of prostate cancer. Radiology. 2017;283:130–139. doi: 10.1148/radiol.2016152827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheridan AD, Nath SK, Syed JS, Aneja S, Sprenkle PC, Weinreb JC, et al. Risk of clinically significant prostate cancer associated with prostate imaging reporting and data system category 3 (equivocal) lesions identified on multiparametric prostate MRI. AJR Am J Roentgenol. 2018;210:347–357. doi: 10.2214/AJR.17.18516. [DOI] [PubMed] [Google Scholar]
- 38.Venderink W, van Luijtelaar A, Bomers JGR, van der Leest M, Hulsbergen-van de Kaa C, Barentsz JO, et al. Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur Urol. 2018;73:353–360. doi: 10.1016/j.eururo.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Wegelin O, Exterkate L, van der Leest M, Kummer JA, Vreuls W, de Bruin PC, et al. The FUTURE trial: a multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate cancer in patients with prior negative biopsies. Eur Urol. 2019;75:582–590. doi: 10.1016/j.eururo.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 40.Elkhoury FF, Felker ER, Kwan L, Sisk AE, Delfin M, Natarajan S, et al. Comparison of targeted vs systematic prostate biopsy in men who are biopsy naive: the prospective assessment of image registration in the diagnosis of prostate cancer (PAIREDCAP) study. JAMA Surg. 2019;154:811–818. doi: 10.1001/jamasurg.2019.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–351. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 42.Tamada T, Kido A, Takeuchi M, Yamamoto A, Miyaji Y, Kanomata N, et al. Comparison of PI-RADS version 2 and PI-RADS version 2.1 for the detection of transition zone prostate cancer. Eur J Radiol. 2019;121:108704. doi: 10.1016/j.ejrad.2019.108704. [DOI] [PubMed] [Google Scholar]
- 43.Byun J, Park KJ, Kim MH, Kim JK. Direct comparison of PI-RADS version 2 and 2.1 in transition zone lesions for detection of prostate cancer: preliminary experience. J Magn Reson Imaging. 2020;52:577–586. doi: 10.1002/jmri.27080. [DOI] [PubMed] [Google Scholar]
- 44.Kim HS, Kwon GY, Kim MJ, Park SY. Prostate imaging-reporting and data system: comparison of the diagnostic performance between version 2.0 and 2.1 for prostatic peripheral zone. Korean J Radiol. 2021;22:1100–1109. doi: 10.3348/kjr.2020.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das CJ, Netaji A, Razik A, Verma S. MRI-targeted prostate biopsy: what radiologists should know. Korean J Radiol. 2020;21:1087–1094. doi: 10.3348/kjr.2019.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, et al. Three-tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol. 2012;62:902–909. doi: 10.1016/j.eururo.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 47.Franiel T, Stephan C, Erbersdobler A, Dietz E, Maxeiner A, Hell N, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding--multiparametric MR imaging for detection and biopsy planning. Radiology. 2011;259:162–172. doi: 10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]
- 48.Zangos S, Eichler K, Engelmann K, Ahmed M, Dettmer S, Herzog C, et al. MR-guided transgluteal biopsies with an open low-field system in patients with clinically suspected prostate cancer: technique and preliminary results. Eur Radiol. 2005;15:174–182. doi: 10.1007/s00330-004-2458-2. [DOI] [PubMed] [Google Scholar]
- 49.Engelhard K, Hollenbach HP, Kiefer B, Winkel A, Goeb K, Engehausen D. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol. 2006;16:1237–1243. doi: 10.1007/s00330-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 50.Hambrock T, Fütterer JJ, Huisman HJ, Hulsbergen-vandeKaa C, van Basten JP, van Oort I, et al. Thirty-two-channel coil 3T magnetic resonance-guided biopsies of prostate tumor suspicious regions identified on multimodality 3T magnetic resonance imaging: technique and feasibility. Invest Radiol. 2008;43:686–694. doi: 10.1097/RLI.0b013e31817d0506. [DOI] [PubMed] [Google Scholar]
- 51.Zangos S, Melzer A, Eichler K, Sadighi C, Thalhammer A, Bodelle B, et al. MR-compatible assistance system for biopsy in a high-field-strength system: initial results in patients with suspicious prostate lesions. Radiology. 2011;259:903–910. doi: 10.1148/radiol.11101559. [DOI] [PubMed] [Google Scholar]
- 52.Roethke M, Anastasiadis AG, Lichy M, Werner M, Wagner P, Kruck S, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2012;30:213–218. doi: 10.1007/s00345-011-0675-2. [DOI] [PubMed] [Google Scholar]
- 53.Engehausen DG, Engelhard K, Schwab SA, Uder M, Wach S, Wullich B, et al. Magnetic resonance image-guided biopsies with a high detection rate of prostate cancer. ScientificWorldJournal. 2012;2012:975971. doi: 10.1100/2012/975971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lichy MP, Anastasiadis AG, Aschoff P, Sotlar K, Eschmann SM, Pfannenberg C, et al. Morphologic, functional, and metabolic magnetic resonance imaging-guided prostate biopsy in a patient with prior negative transrectal ultrasound-guided biopsies and persistently elevated prostate-specific antigen levels. Urology. 2007;69:1208.E5–1208.E8. doi: 10.1016/j.urology.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Lattouf JB, Grubb RL, 3rd, Lee SJ, Bjurlin MA, Albert P, Singh AK, et al. Magnetic resonance imaging-directed transrectal ultrasonography-guided biopsies in patients at risk of prostate cancer. BJU Int. 2007;99:1041–1046. doi: 10.1111/j.1464-410X.2006.06690.x. [DOI] [PubMed] [Google Scholar]
- 56.Park BK, Lee HM, Kim CK, Choi HY, Park JW. Lesion localization in patients with a previous negative transrectal ultrasound biopsy and persistently elevated prostate specific antigen level using diffusion-weighted imaging at three tesla before rebiopsy. Invest Radiol. 2008;43:789–793. doi: 10.1097/RLI.0b013e318183725e. [DOI] [PubMed] [Google Scholar]
- 57.Labanaris AP, Engelhard K, Zugor V, Nützel R, Kühn R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis. 2010;13:65–70. doi: 10.1038/pcan.2009.41. [DOI] [PubMed] [Google Scholar]
- 58.Haffner J, Lemaitre L, Puech P, Haber GP, Leroy X, Jones JS, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108(8 Pt 2):E171–E178. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 59.Pinto PA, Chung PH, Rastinehad AR, Baccala AA, Jr, Kruecker J, Benjamin CJ, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi MS, Choi YS, Yoon BI, Kim SJ, Cho HJ, Hong SH, et al. The clinical value of performing an MRI before prostate biopsy. Korean J Urol. 2011;52:572–577. doi: 10.4111/kju.2011.52.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watanabe Y, Terai A, Araki T, Nagayama M, Okumura A, Amoh Y, et al. Detection and localization of prostate cancer with the targeted biopsy strategy based on ADC map: a prospective large-scale cohort study. J Magn Reson Imaging. 2012;35:1414–1421. doi: 10.1002/jmri.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shigemura K, Motoyama S, Yamashita M. Do additional cores from MRI cancer-suspicious lesions to systematic 12-core transrectal prostate biopsy give better cancer detection? Urol Int. 2012;88:145–149. doi: 10.1159/000334375. [DOI] [PubMed] [Google Scholar]
- 63.Kwak JT, Hong CW, Pinto PA, Williams M, Xu S, Kruecker J, et al. Is visual registration equivalent to semiautomated registration in prostate biopsy? Biomed Res Int. 2015;2015:394742. doi: 10.1155/2015/394742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puech P, Rouvière O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology. 2013;268:461–469. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 65.Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66:343–351. doi: 10.1016/j.eururo.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 66.Lee DJ, Recabal P, Sjoberg DD, Thong A, Lee JK, Eastham JA, et al. Comparative effectiveness of targeted prostate biopsy using magnetic resonance imaging ultrasound fusion software and visual targeting: a prospective study. J Urol. 2016;196:697–702. doi: 10.1016/j.juro.2016.03.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arsov C, Rabenalt R, Blondin D, Quentin M, Hiester A, Godehardt E, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol. 2015;68:713–720. doi: 10.1016/j.eururo.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Yaxley AJ, Yaxley JW, Thangasamy IA, Ballard E, Pokorny MR. Comparison between target magnetic resonance imaging (MRI) in-gantry and cognitively directed transperineal or transrectal-guided prostate biopsies for prostate imaging-reporting and data system (PI-RADS) 3-5 MRI lesions. BJU Int. 2017;120 Suppl 3:43–50. doi: 10.1111/bju.13971. [DOI] [PubMed] [Google Scholar]
- 69.Hamid S, Donaldson IA, Hu Y, Rodell R, Villarini B, Bonmati E, et al. The SmartTarget biopsy trial: a prospective, within-person randomised, blinded trial comparing the accuracy of visual-registration and magnetic resonance imaging/ultrasound image-fusion targeted biopsies for prostate cancer risk stratification. Eur Urol. 2019;75:733–740. doi: 10.1016/j.eururo.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaufmann S, Russo GI, Bamberg F, Löwe L, Morgia G, Nikolaou K, et al. Prostate cancer detection in patients with prior negative biopsy undergoing cognitive-, robotic- or in-bore MRI target biopsy. World J Urol. 2018;36:761–768. doi: 10.1007/s00345-018-2189-7. [DOI] [PubMed] [Google Scholar]
- 71.Wegelin O, van Melick HHE, Hooft L, Bosch JLHR, Reitsma HB, Barentsz JO, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol. 2017;71:517–531. doi: 10.1016/j.eururo.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 72.Zhang M, Milot L, Khalvati F, Sugar L, Downes M, Baig SM, et al. Value of increasing biopsy cores per target with cognitive MRI-targeted transrectal US prostate biopsy. Radiology. 2019;291:83–89. doi: 10.1148/radiol.2019180712. [DOI] [PubMed] [Google Scholar]
- 73.Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50:125–128. [PubMed] [Google Scholar]
- 74.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 75.Corcoran NM, Hovens CM, Hong MK, Pedersen J, Casey RG, Connolly S, et al. Underestimation of Gleason score at prostate biopsy reflects sampling error in lower volume tumours. BJU Int. 2012;109:660–664. doi: 10.1111/j.1464-410X.2011.10543.x. [DOI] [PubMed] [Google Scholar]
- 76.Cohen MS, Hanley RS, Kurteva T, Ruthazer R, Silverman ML, Sorcini A, et al. Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: the Lahey Clinic Medical Center experience and an international meta-analysis. Eur Urol. 2008;54:371–381. doi: 10.1016/j.eururo.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 77.Sved PD, Gomez P, Manoharan M, Kim SS, Soloway MS. Limitations of biopsy Gleason grade: implications for counseling patients with biopsy Gleason score 6 prostate cancer. J Urol. 2004;172:98–102. doi: 10.1097/01.ju.0000132135.18093.d6. [DOI] [PubMed] [Google Scholar]
- 78.Corcoran NM, Hong MK, Casey RG, Hurtado-Coll A, Peters J, Harewood L, et al. Upgrade in Gleason score between prostate biopsies and pathology following radical prostatectomy significantly impacts upon the risk of biochemical recurrence. BJU Int. 2011;108(8 Pt 2):E202–E210. doi: 10.1111/j.1464-410X.2011.10119.x. [DOI] [PubMed] [Google Scholar]
- 79.Briganti A, Fossati N, Catto JWF, Cornford P, Montorsi F, Mottet N, et al. Active surveillance for low-risk prostate cancer: the European Association of Urology position in 2018. Eur Urol. 2018;74:357–368. doi: 10.1016/j.eururo.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 80.Schoots IG, Petrides N, Giganti F, Bokhorst LP, Rannikko A, Klotz L, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015;67:627–636. doi: 10.1016/j.eururo.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 81.Rosenkrantz AB, Verma S, Choyke P, Eberhardt SC, Eggener SE, Gaitonde K, et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol. 2016;196:1613–1618. doi: 10.1016/j.juro.2016.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim CH, Kim CK, Park JJ, Park SY, Yoon YC. Yield of concurrent systemic biopsy during MRI-targeted biopsy according to prostate imaging reporting and data system version 2 in patients with suspected prostate cancer. Eur Radiol. 2021;31:1667–1675. doi: 10.1007/s00330-020-07167-z. [DOI] [PubMed] [Google Scholar]
- 83.Drost FH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019;4:CD012663. doi: 10.1002/14651858.CD012663.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–892. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 85.Moldovan PC, Van den Broeck T, Sylvester R, Marconi L, Bellmunt J, van den Bergh RCN, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol. 2017;72:250–266. doi: 10.1016/j.eururo.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 86.Kim JG, Kim CK, Kim JH. Prebiopsy multiparametric MRI with cancer-negative findings in men with suspected prostate cancer: evaluation using prostate imaging reporting and data system version 2. AJR Am J Roentgenol. 2018;211:121–126. doi: 10.2214/AJR.17.18926. [DOI] [PubMed] [Google Scholar]
- 87.Vargas HA, Akin O, Shukla-Dave A, Zhang J, Zakian KL, Zheng J, et al. Performance characteristics of MR imaging in the evaluation of clinically low-risk prostate cancer: a prospective study. Radiology. 2012;265:478–487. doi: 10.1148/radiol.12120041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Le JD, Tan N, Shkolyar E, Lu DY, Kwan L, Marks LS, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol. 2015;67:569–576. doi: 10.1016/j.eururo.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 89.Vargas HA, Hötker AM, Goldman DA, Moskowitz CS, Gondo T, Matsumoto K, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. 2016;26:1606–1612. doi: 10.1007/s00330-015-4015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Faria R, Soares MO, Spackman E, Ahmed HU, Brown LC, Kaplan R, et al. Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: a cost-effectiveness analysis based on the prostate MR imaging study (PROMIS) Eur Urol. 2018;73:23–30. doi: 10.1016/j.eururo.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muthigi A, George AK, Sidana A, Kongnyuy M, Simon R, Moreno V, et al. Missing the mark: prostate cancer upgrading by systematic biopsy over magnetic resonance imaging/transrectal ultrasound fusion biopsy. J Urol. 2017;197:327–334. doi: 10.1016/j.juro.2016.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panebianco V, Barchetti G, Simone G, Del Monte M, Ciardi A, Grompone MD, et al. Negative multiparametric magnetic resonance imaging for prostate cancer: what’s next? Eur Urol. 2018;74:48–54. doi: 10.1016/j.eururo.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Washino S, Okochi T, Saito K, Konishi T, Hirai M, Kobayashi Y, et al. Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int. 2017;119:225–233. doi: 10.1111/bju.13465. [DOI] [PubMed] [Google Scholar]
- 94.Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol. 2011;185:1650–1655. doi: 10.1016/j.juro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Rooij M, Israël B, Tummers M, Ahmed HU, Barrett T, Giganti F, et al. ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists’ training. Eur Radiol. 2020;30:5404–5416. doi: 10.1007/s00330-020-06929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giganti F, Allen C, Emberton M, Moore CM, Kasivisvanathan V PRECISION Study Group. Prostate imaging quality (PI-QUAL): a new quality control scoring system for multiparametric magnetic resonance imaging of the prostate from the PRECISION trial. Eur Urol Oncol. 2020;3:615–619. doi: 10.1016/j.euo.2020.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.