Abstract
Purpose
The aim of this study was to quantify the initial decline and subsequent rebound in breast cancer screening metrics throughout the coronavirus disease 2019 (COVID-19) pandemic.
Methods
Screening and diagnostic mammographic examinations, biopsies performed, and cancer diagnoses were extracted from the ACR National Mammography Database from March 1, 2019, through May 31, 2021. Patient (race and age) and facility (regional location, community type, and facility type) demographics were collected. Three time periods were used for analysis: pre-COVID-19 (March 1, 2019, to May 31, 2019), peak COVID-19 (March 1, 2020, to May 31, 2020), and COVID-19 recovery (March 1, 2021, to May 31, 2021). Analysis was performed at the facility level and overall between time periods.
Results
In total, 5,633,783 screening mammographic studies, 1,282,374 diagnostic mammographic studies, 231,390 biopsies, and 69,657 cancer diagnoses were analyzed. All peak COVID-19 metrics were less than pre-COVID-19 volumes: 36.3% of pre-COVID-19 for screening mammography, 57.9% for diagnostic mammography, 47.3% for biopsies, and 48.7% for cancer diagnoses. There was some rebound during COVID-19 recovery as a percentage of pre-COVID-19 volumes: 85.3% of pre-COVID-19 for screening mammography, 97.8% for diagnostic mammography, 91.5% for biopsies, and 92.0% for cancer diagnoses. Across various metrics, there was a disproportionate negative impact on older women, Asian women, facilities in the Northeast, and facilities affiliated with academic medical centers.
Conclusions
COVID-19 had the greatest impact on screening mammography volumes, which have not returned to pre-COVID-19 levels. Cancer diagnoses declined significantly in the acute phase and have not fully rebounded, emphasizing the need to increase outreach efforts directed at specific patient population and facility types.
Key Words: COVID-19, screening mammography, diagnostic mammography, breast biopsies, breast cancer
Credits awarded for this enduring activity are designated “SA-CME” by the American Board of Radiology (ABR) and qualify toward fulfilling requirements for Maintenance of Certification (MOC) Part II: Lifelong Learning and Self-assessment. To access the SA-CME activity visit https://cortex.acr.org/Presenters/CaseScript/CaseView?Info=fTHuFtw7Oc%2fz5rY2qFnR7ROBl3P%2bhKo5leeSIT4Hk6E%253d. SA-CME credit for this article expires July 31, 2025. All SA-CME articles can be found on JACR.org by navigating to SA-CME on the dropdown menu.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic had a profound impact on health care delivery in the United States due to mandatary stay-at-home orders and patient fears about visiting health care facilities [1]. During the peak of the pandemic in early 2020, CMS recommended that individuals “consider postponing service” for “preventive care visit/screening” [2]. This led to the near complete cessation of many cancer screening services, including screening mammography [3, 4, 5, 6, 7, 8, 9]. As the public and health care organizations began to adapt, guidelines on a safe return to imaging were released by the Society of Breast Imaging in May 2020, followed by the ACR in July 2020 [10,11]. These guidelines advocated for strategies to shift the risk/benefit ratio for patients to facilitate a safe return to screening practices. In response, practices adopted a wide variety of strategies to encourage patients to return, including expanding hours, switching to electronic intake forms, improved cleaning and sanitation practices, and rearranging the workflows of clinics [12]. Several publications using regional and limited national data sets reported that in the months after the pandemic peak, screening mammography volumes began to rebound, but rates had not yet returned to prepandemic baseline [2,5,6]. However, the intermediate-term impact of COVID-19 on the use of screening and diagnostic mammography, demonstrated on a larger scale that is more representative of the national population and includes subgroup analysis across pertinent patient demographics and analysis at a facility level, has not been well studied.
The purpose of this study was to quantify the decrease and subsequent rebound in breast cancer screening and diagnostic metrics during the COVID-19 pandemic using the National Mammography Database (NMD).
Methods
The NMD
The NMD was established by the ACR in 2008 to facilitate quality improvement and research efforts for screening mammography practices in the United States [13]. The NMD represents the broadest coverage of all national and regional breast imaging databases in the United States and allows subgroup analysis to assess for disproportionate impacts on specific patient and facility demographics. The NMD includes the results of more than 31 million mammographic examinations, representing 690 facilities in 45 states [14]. All NMD data are HIPAA compliant, anonymized, and deidentified before analysis by non-NMD investigators, who do not have access to any patient-, physician-, or facility-identifying information.
Study Population
The 377 distinct facilities that submitted data to the NMD from March 1, 2019, through May 31, 2021, were included for analysis. This duration was chosen to span 1 year before the peak of the COVID-19 pandemic in spring 2020 (March, April, and May) as well as 1 year after the peak. Patient demographics included age and race (Asian; black; Native American, Native Hawaiian, or Pacific Islander; white; or unknown). Facility demographics included regional location (Northeast, South, Midwest, or West), community type (metropolitan [>100,000 persons], suburban [50,000-100,000 persons], or rural [<50,000 persons]), and facility type (academic or university, community hospital, freestanding imaging center, or multispecialty clinic).
Outcomes Measures and Analysis
To assess for changes in the use of breast cancer screening services before, during, and after the height of the COVID-19 pandemic, we compared the number of occurrences per facility for screening mammography, diagnostic mammography, biopsies performed, and cancer diagnoses at all sites that contributed to the NMD across three time periods: pre-COVID-19 (March 1, 2019, to May 31, 2019), peak COVID-19 (March 1, 2020, to May 31, 2020), and COVID-19 recovery (March 1, 2021, to May 31, 2021). Because health care encounters tend to have “seasonality” (eg, patients often seek the bulk of their preventive care at the beginning or end of the year), we matched the date ranges in each of our three time periods [15]. Matched date ranges in each year help mitigate any skewness that would otherwise occur had we widened our ranges to entire calendar years for 2020 and portions of calendar years for 2019 and 2021.
Because the numbers of encounters per facility in each of the four encounter categories listed previously were not normally distributed, we assessed the median number of encounters and compared these medians between each possible pairing of time periods using nonparametric, one-sided Wilcoxon’s rank-sum tests, resulting in three separate measures (ie, pre-COVID-19 vs peak COVID-19, peak COVID-19 vs COVID-19 recovery, and pre-COVID-19 vs COVID-19 recovery). We anticipated seeing significantly fewer encounters from pre-COVID-19 to peak COVID-19 and significantly more encounters from peak COVID-19 to COVID-19 recovery with no statistically significant difference from pre-COVID-19 to COVID-19 recovery. To avoid potentially “artificial” or overstated statistically significant findings with such large encounter numbers, any comparison with more than 10,000 total encounters for both groups used a more conservative α value of 0.01 for statistical significance; comparisons with fewer than 10,000 encounters used an α value of 0.05. Alpha was not further adjusted to account for the number of comparisons. Results are presented as numbers, medians, and interquartile ranges.
Results
Overall Study Population and Metrics
There were 5,633,783 screening mammographic studies, 1,282,374 diagnostic mammographic studies, 231,390 biopsies, and 69,657 cancer diagnoses reported from March 1, 2019, through May 31, 2021. A breakdown by patient and facility demographics over the entire study period is reported in Table 1 . The greatest decrease in volume during the peak COVID-19 period was for screening mammography (36.3% of pre-COVID-19), and the smallest decrease in volumes was for diagnostic mammography (57.9% of pre-COVID-19), followed by biopsies (47.3% of pre-COVID-19) and cancer diagnoses (48.7% of pre-COVID-19). Similarly, the rebound during the COVID-19 recovery period was weakest for screening mammography (85.3% of pre-COVID-19) and greatest for diagnostic mammography (97.8% of pre-COVID-19), followed by biopsies (91.5% of pre-COVID-19) and cancer diagnoses (92.0% of pre-COVID-19). A graphical representation of the outcome metrics over time is shown in Figures 1 and 2 .
Table 1.
Total volume of screening mammographic examinations, diagnostic mammographic examinations, breast biopsies, and cancer diagnoses by patient and facility demographics from March 1, 2019, through May 31, 2021
| Variable | Screening Mammography |
Diagnostic Mammography |
Biopsies |
Cancer Diagnoses |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Age | ||||||||
| <40 y | 62,295 | 1.1 | 130,899 | 10.2 | 14,292 | 6.2 | 1,854 | 2.7 |
| 40-49 y | 1,175,114 | 20.9 | 323,706 | 25.2 | 57,021 | 24.6 | 8,980 | 12.9 |
| 50-59 y | 1,551,265 | 27.5 | 304,685 | 23.8 | 55,606 | 24.0 | 14,998 | 21.5 |
| 60-69 y | 1,631,623 | 29.0 | 286,704 | 22.4 | 57,257 | 24.7 | 21,662 | 31.1 |
| 70-79 y | 999,124 | 17.7 | 186,031 | 14.5 | 37,559 | 16.2 | 16,621 | 23.9 |
| ≥80 y | 214,362 | 3.8 | 50,349 | 3.9 | 9,655 | 4.2 | 5,542 | 8.0 |
| Race | ||||||||
| Asian | 109,497 | 1.9 | 20,671 | 1.6 | 5,268 | 2.3 | 1,427 | 2.0 |
| Black | 246,026 | 4.4 | 43,924 | 3.4 | 8,856 | 3.8 | 2,472 | 3.5 |
| Native American, Native Hawaiian, or Pacific Islander | 38,932 | 0.7 | 6,046 | 0.5 | 1,260 | 0.5 | 363 | 0.5 |
| White | 1,923,641 | 34.1 | 367,601 | 28.7 | 75,510 | 32.6 | 24,027 | 34.5 |
| Unknown | 3,315,683 | 58.9 | 844,130 | 65.8 | 140,496 | 60.7 | 41,368 | 59.4 |
| Community type | ||||||||
| Academic/university | 599,772 | 11.0 | 169,674 | 13.5 | 33,856 | 15.0 | 10,251 | 15.0 |
| Community hospital | 2,301,231 | 42.1 | 514,308 | 40.9 | 95,234 | 42.1 | 28,676 | 41.9 |
| Multispecialty clinic | 391,185 | 7.2 | 52,402 | 4.2 | 12,306 | 5.4 | 3,793 | 5.5 |
| Freestanding imaging center | 2,178,651 | 39.8 | 522,098 | 41.5 | 84,699 | 37.5 | 25,660 | 37.5 |
| Facility type | ||||||||
| Metropolitan (>100,000 persons) | 3,268,582 | 58.0 | 828,716 | 64.6 | 142,980 | 61.8 | 44,231 | 63.5 |
| Suburban/small (50,000-100,000 persons) | 1,872,684 | 33.2 | 345,009 | 26.9 | 70,173 | 30.3 | 20,341 | 29.2 |
| Rural (<50,000 persons) | 492,521 | 8.7 | 108,650 | 8.5 | 18,237 | 7.9 | 5,085 | 7.3 |
| Region | ||||||||
| Northeast | 1,348,398 | 23.9 | 286,831 | 22.4 | 53,606 | 23.2 | 14,932 | 21.4 |
| Midwest | 1,391,629 | 24.7 | 257,868 | 20.1 | 51,927 | 22.4 | 17,816 | 25.6 |
| South | 1,404,760 | 24.9 | 328,844 | 25.6 | 56,220 | 24.3 | 15,850 | 22.8 |
| West | 1,489,000 | 26.4 | 408,832 | 31.9 | 69,637 | 30.1 | 21,059 | 30.2 |
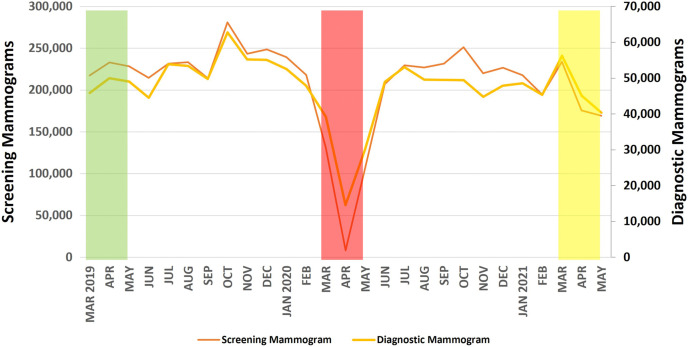
Fig. 1.
Monthly volume of screening and diagnostic mammographic examinations from the National Mammography Database from March 1, 2019, through May 31, 2021. The green, red, and yellow boxes refer to the 3-month-long pre-COVID-19, peak COVID-19, and COVID-19 recovery periods, respectively, used for analysis. COVID-19 = coronavirus disease 2019.
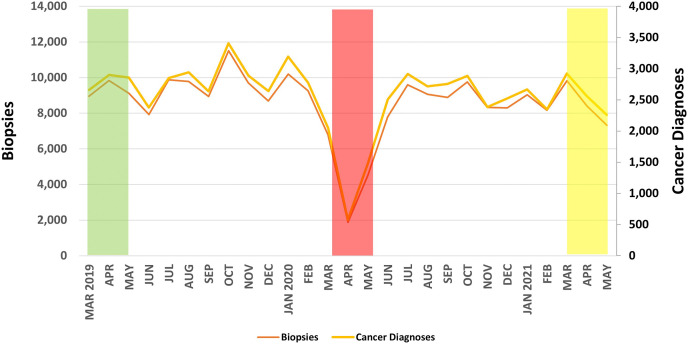
Fig. 2.
Monthly volume of biopsies performed and cancer diagnoses from the National Mammography Database from March 1, 2019, through May 31, 2021. The green, red, and yellow boxes refer to the 3-month-long pre-COVID-19, peak COVID-19, and COVID-19 recovery periods, respectively, used for analysis. COVID-19 = coronavirus disease 2019.
Screening Mammography
The volume of screening mammographic examinations during the peak COVID-19 period (246,610 studies) was 36.3% of the pre-COVID-19 period (678,890 studies; Figure 1). During the nadir of the peak COVID-19 period in April 2020, screening mammography volume was 3.7% (8,403 studies) of the monthly average during the pre-COVID-19 period (226,297 studies). As shown in Table 2 , the greatest decreases were seen for women aged 80 years or older (32.2% of pre-COVID-19), Asian women (28.4% of pre-COVID-19), and facilities in suburban communities (34.3% of pre-COVID-19), with academic or university affiliations (30.4% of pre-COVID-19), and in the Northeast (31.0% of pre-COVID-19). At the facility level, there were statistically significant decreases (P < .01) for all demographics studied.
Table 2.
Changes in screening mammography quarterly volumes in the pre-COVID-19, peak COVID-19, and COVID-19 recovery periods by patient and facility demographics
| Variable | Pre-COVID-19 |
Peak COVID-19 |
COVID-19 Recovery |
Pre-COVID-19 vs Peak COVID-19 |
Peak COVID-19 vs COVID-19 Recovery |
Pre-COVID-19 vs COVID-19 Recovery |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Facility Median (IQR) | Total n | Facility Median (IQR) | Total n | Facility Median (IQR) | Total n | Facility P Value | Total Change % | Facility P Value | Total Change % | Facility P Value | Total Change % | |
| Age group | ||||||||||||
| <40 y | 14 (4-25) | 7,922 | 5 (2-10) | 2,936 | 11 (4-24) | 7,055 | <0.001 | 37.1 | <0.001 | 240.3 | 0.172 | 89.1 |
| 40-49 y | 247 (110-468) | 142,207 | 88 (39-181) | 52,377 | 242 (110-409) | 128,369 | <0.001 | 36.8 | <0.001 | 245.1 | 0.131 | 90.3 |
| 50-59 y | 329 (144-607) | 189,226 | 119 (48-225) | 68,741 | 272 (128-496) | 158,123 | <0.001 | 36.3 | <0.001 | 230.0 | 0.017 | 83.6 |
| 60-69 y | 334 (149-642) | 194,679 | 109 (53-237) | 70,686 | 279 (135-537) | 163,760 | <0.001 | 36.3 | <0.001 | 231.7 | 0.017 | 84.1 |
| 70-79 y | 192 (79-393) | 117,886 | 67 (27-144) | 43,181 | 164 (72-330) | 101,003 | <0.001 | 36.6 | <0.001 | 233.9 | 0.021 | 85.7 |
| ≥80 y | 43 (17-89) | 26,969 | 11 (5-29) | 8,689 | 30 (13-67) | 20,526 | <0.001 | 32.2 | <0.001 | 236.2 | 0.001 | 76.1 |
| Race | ||||||||||||
| Asian | 0 (0-26) | 14,705 | 0 (0-7) | 4,181 | 0 (0-20) | 10,664 | 0.002 | 28.4 | 0.018 | 255.1 | 0.2145 | 72.5 |
| Black | 2 (0-58) | 32,163 | 0 (0-18) | 10,627 | 1 (0-43) | 24,717 | 0.001 | 33.0 | 0.021 | 232.6 | 0.157 | 76.8 |
| Native American, Native Hawaiian or Pacific Islander | 0 (0-4) | 3,405 | 0 (0-1) | 2,479 | 0 (0-4) | 4,538 | <0.001 | 72.8 | <0.001 | 183.1 | 0.421 | 133.3 |
| White | 152 (0-862) | 243,520 | 44 (0-262) | 83,897 | 76 (0-663) | 191,245 | <0.001 | 34.5 | 0.002 | 228.0 | 0.123 | 78.5 |
| Unknown | 422 (84-1,209) | 385,095 | 141 (23-478) | 145,426 | 420 (71-1,079) | 347,672 | <0.001 | 37.8 | <0.001 | 239.1 | 0.189 | 90.3 |
| Community type | ||||||||||||
| Metropolitan (>100,000 persons) | 1,839 (805-3,218) | 397,037 | 646 (222-1,235) | 144,170 | 1,539 (729-2,904) | 347,318 | <0.001 | 36.3 | <0.001 | 240.9 | 0.124 | 87.5 |
| Suburban/small (50,000-100,000 persons) | 1,213 (766-1,905) | 226,200 | 432 (234-694) | 77,598 | 990 (589-1,544) | 180,933 | <0.001 | 34.3 | <0.001 | 233.2 | 0.010 | 80.0 |
| Rural (<50,000 persons) | 391 (139-939) | 55,653 | 167 (71-423) | 24,842 | 323 (160-748) | 50,585 | <0.001 | 44.6 | <0.001 | 203.6 | 0.454 | 90.9 |
| Facility type | ||||||||||||
| Academic/university | 1,475 (719-3,841) | 73,164 | 510 (243-1,287) | 22,231 | 1,221 (667-2,507) | 58,027 | <0.001 | 30.4 | 0.002 | 261.0 | 0.188 | 79.3 |
| Community hospital | 1,290 (402-2,227) | 286,548 | 410 (133-796) | 102,767 | 820 (239-1,881) | 231,914 | <0.001 | 35.9 | <0.001 | 225.7 | 0.028 | 80.9 |
| Multispecialty clinic | 835 (450-2,022) | 46,341 | 264 (135-657) | 15,954 | 781 (492-1,504) | 43,143 | <0.001 | 34.4 | <0.001 | 270.4 | 0.451 | 93.1 |
| Freestanding imaging center | 1,183 (627-2,093) | 252,863 | 457 (213-778) | 97,591 | 1,148 (677-1,794) | 224,701 | <0.001 | 38.6 | <0.001 | 230.2 | 0.233 | 88.9 |
| Region | ||||||||||||
| Northeast | 1,209 (516-1,780) | 162,705 | 378 (167-601) | 50,486 | 955 (446-1,575) | 138,814 | <0.001 | 31.0 | <0.001 | 275.0 | 0.076 | 85.3 |
| Midwest | 997 (584-2,185) | 172,423 | 342 (175-691) | 59,834 | 852 (465-1,769) | 140,220 | <0.001 | 34.7 | <0.001 | 234.3 | 0.084 | 81.3 |
| South | 1,260 (563-2,379) | 160,976 | 602 (221-987) | 67,588 | 1,193 (602-2,009) | 137,475 | <0.001 | 42.0 | <0.001 | 203.4 | 0.254 | 85.4 |
| West | 1,281 (462-2,888) | 182,786 | 488 (151-1,192) | 68,702 | 1,164 (324-2,271) | 162,327 | <0.001 | 37.6 | <0.001 | 236.3 | 0.222 | 88.8 |
Note: Facility refers to the median and IQR for facilities. Total refers to the total reported at all facilities. Facility P value compares the metrics at the facility level. Statistical significance was defined as a P value of .01 for encounters greater than 10,000 and .05 for encounters less than 10,000. Statistically significant values are in boldface type. Total change refers to the second comparator group divided by the first comparator group as a percentage. COVID-19 = coronavirus disease 2019; IQR = interquartile range.
During the COVID-19 recovery period, screening mammography volume (578,836 studies) rebounded to 85.3% of the pre-COVID-19 level (678,890 studies; Figure 1). The smallest rebounds were for women aged 80 years or older (76.1% of pre-COVID-19, P = .001), Asian women (72.5% of pre-COVID-19), and facilities in suburban communities (80.0% of pre-COVID-19), with academic or university affiliations (79.3% of pre-COVID-19), and in the Midwest (81.3% of pre-COVID-19).
Diagnostic Mammography
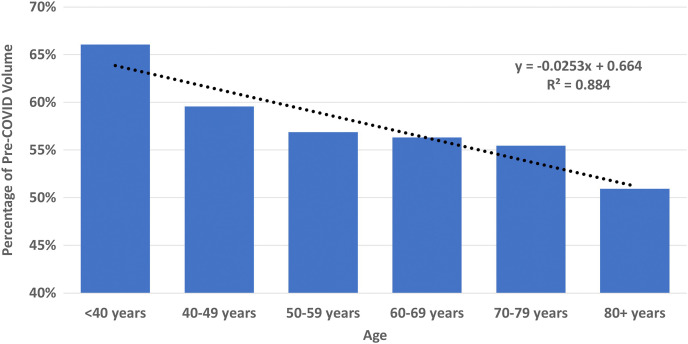
The volume of diagnostic mammographic examinations during the peak COVID-19 period (83,915 studies) was 57.9% of the pre-COVID-19 period (144,855 studies, Figure 1). During the nadir of the peak COVID-19 period in April 2020, diagnostic mammography volume was 30.2% (14,586 studies) of the monthly average in the pre-COVID-19 period (48,285 studies). As shown in Table 3 , the greatest decreases were seen for women aged 80 years and older (50.9% of pre-COVID-19), Asian women (45.1% of pre-COVID-19), and facilities in metropolitan communities (53.2% of pre-COVID-19), with academic or university affiliations (55.1% of pre-COVID-19), and in the West (54.2% of pre-COVID-19). At the facility level, there were statistically significant decreases for all age groups 50 years and older (P < .01 for all); Native American, Native Hawaiian, or Pacific Islander women (P = .014); and facilities in metropolitan communities (P = .009), as shown in Table 2. There was a significant linear decrease in diagnostic mammographic examinations with increasing age decade (R 2 = 0.88, P = .005; Figure 3 ).
Table 3.
Changes in diagnostic mammography quarterly volumes in the pre-COVID-19, peak COVID-19, and COVID-19 recovery periods by patient and facility demographics
| Variable | Pre-COVID-19 |
Peak COVID-19 |
COVID-19 Recovery |
Pre-COVID-19 vs Peak COVID-19 |
Peak COVID-19 vs COVID-19 Recovery |
Pre-COVID-19 vs COVID-19 Recovery |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Facility Median (IQR) | Total n | Facility Median (IQR) | Total n | Facility Median (IQR) | Total n | Facility P Value | Total Change % | Facility P Value | Total Change % | Facility P Value | Total Change % | |
| Age group | ||||||||||||
| <40 y | 7 (0-44) | 15,062 | 6 (0-34) | 9,952 | 11 (0-45) | 15,056 | 0.107 | 66.1 | 0.019 | 151.3 | 0.236 | 100.0 |
| 40-49 y | 30 (1-104) | 35,981 | 20 (0-75) | 21,434 | 35 (1-118) | 37,240 | 0.023 | 59.6 | 0.002 | 173.7 | 0.214 | 103.5 |
| 50-59 y | 30 (1-95) | 34,765 | 19 (0-60) | 19,772 | 35 (1-96) | 33,087 | 0.007 | 56.9 | 0.003 | 167.3 | 0.411 | 95.2 |
| 60-69 y | 28 (0-91) | 32,216 | 17 (0-58) | 18,145 | 32 (2-93) | 30,647 | 0.009 | 56.3 | 0.001 | 168.9 | 0.296 | 95.1 |
| 70-79 y | 17 (0-64) | 20,964 | 10 (0-38) | 11,624 | 18 (1-57) | 20,042 | 0.004 | 55.4 | 0.001 | 172.4 | 0.409 | 95.6 |
| ≥80 y | 4 (0-17) | 5,866 | 2 (0-9) | 2,988 | 5 (0-16) | 5,615 | 0.001 | 50.9 | <0.001 | 187.9 | 0.339 | 95.7 |
| Race | ||||||||||||
| Asian | 0 (0-0) | 2,563 | 0 (0-0) | 1,155 | 0 (0-0) | 2,176 | 0.265 | 45.1 | 0.411 | 188.4 | 0.342 | 84.9 |
| Black | 0 (0-4) | 5,241 | 0 (0-2) | 2,848 | 0 (0-4) | 4,496 | 0.202 | 54.3 | 0.141 | 157.9 | 0.406 | 85.8 |
| Native American, Native Hawaiian, or Pacific Islander | 0 (0-0) | 598 | 0 (0-0) | 518 | 0 (0-0) | 653 | 0.014 | 86.6 | 0.180 | 126.1 | 0.105 | 109.2 |
| White | 0 (0-113) | 43,546 | 0 (0-57) | 24,621 | 0 (0-81) | 36,543 | 0.125 | 56.5 | 0.325 | 148.4 | 0.246 | 83.9 |
| Unknown | 35 (0-197) | 92,907 | 21 (0-156) | 54,773 | 40 (0-245) | 97,818 | 0.041 | 59.0 | 0.008 | 178.6 | 0.233 | 105.3 |
| Community type | ||||||||||||
| Metropolitan (>100,000 persons) | 276 (9-874) | 99,039 | 178 (4-526) | 52,650 | 303 (58-864) | 94,817 | 0.009 | 53.2 | 0.009 | 180.1 | 0.483 | 95.7 |
| Suburban/small (50,000-100,000 persons) | 97 (0-256) | 35,065 | 70 (0-180) | 23,219 | 125 (0-285) | 36,169 | 0.078 | 66.2 | 0.027 | 155.8 | 0.301 | 103.1 |
| Rural (<50,000 persons) | 40 (15-171) | 10,751 | 36 (13-138) | 8,046 | 54 (11-167) | 10,701 | 0.258 | 74.8 | 0.156 | 133.0 | 0.352 | 99.5 |
| Facility type | ||||||||||||
| Academic/university | 275 (0-1,267) | 18,618 | 223 (0-660) | 10,256 | 350 (0-988) | 17,277 | 0.142 | 55.1 | 0.179 | 168.5 | 0.470 | 92.8 |
| Community hospital | 150 (30-385) | 56,929 | 97 (25-271) | 34,632 | 155 (39-389) | 57,305 | 0.049 | 60.8 | 0.007 | 165.5 | 0.248 | 100.7 |
| Multispecialty clinic | 1 (0-182) | 5,098 | 1 (0-153) | 4,193 | 0 (0-117) | 5,832 | 0.365 | 82.2 | 0.377 | 139.1 | 0.251 | 114.4 |
| Freestanding imaging center | 155 (0-376) | 60,146 | 90 (0-260) | 33,950 | 176 (0-407) | 59,554 | 0.050 | 56.4 | 0.024 | 175.4 | 0.389 | 99.0 |
| Region | ||||||||||||
| Northeast | 137 (0-368) | 31,526 | 65 (0-181) | 17,194 | 174 (20-399) | 32,166 | 0.067 | 54.5 | 0.003 | 187.1 | 0.172 | 102.0 |
| Midwest | 86 (0-255) | 26,799 | 48 (0-178) | 17,579 | 72 (0-254) | 25,637 | 0.118 | 65.6 | 0.183 | 145.8 | 0.402 | 95.7 |
| South | 172 (2-594) | 38,841 | 157 (1-406) | 23,280 | 213 (1-603) | 34,729 | 0.122 | 59.9 | 0.108 | 149.2 | 0.472 | 89.4 |
| West | 157 (24-590) | 47,689 | 111 (28-431) | 25,862 | 218 (37-606) | 49,155 | 0.185 | 54.2 | 0.108 | 190.1 | 0.381 | 103.1 |
Note: Facility refers to the median and IQR for facilities. Total refers to the total reported at all facilities. Facility P value compares the metrics at the facility level. Statistical significance was defined as a P value of .01 for encounters greater than 10,000 and .05 for encounters less than 10,000. Statistically significant values are in boldface type. Total change refers to the second comparator group divided by the first comparator group as a percentage. COVID-19 = coronavirus disease 2019; IQR = interquartile range.
Fig. 3.
Percentage of diagnostic mammographic examinations during the peak COVID-19 versus the pre-COVID-19 period by age decade with fitted trend line. COVID-19 = coronavirus disease 2019.
During the COVID-19 recovery, diagnostic mammography volume (141,687 studies) rebounded to 97.8% of the pre-COVID-19 level (144,855 studies; Figure 1). The smallest rebounds were for women aged 60 to 69 years (95.1% of pre-COVID-19), white women (83.9% of pre-COVID-19), and facilities in metropolitan communities (95.7% of pre-COVID-19), with academic or university affiliations (92.8% of pre-COVID-19), and in the South (89.4% of pre-COVID-19). There were no significant differences in the pre-COVID-19 versus COVID-19 recovery period diagnostic volumes for the demographics studied.
Biopsies
The volume of biopsies during the peak COVID-19 period (13,191 biopsies) was 47.3% of the pre-COVID-19 period (27,907 biopsies; Figure 2). During the nadir of the peak COVID-19 period in April 2020, the biopsy volume was 20.1% (1,876 biopsies) of the monthly average in the pre-COVID-19 period (9,302 biopsies). As shown in Table 4 , the largest declines were seen for women aged 50 to 59 and 60 to 69 years (45.2% of pre-COVID-19 for both), Asian women (43.2% of pre-COVID-19), and facilities in suburban communities (44.0% of pre-COVID-19), with academic or university affiliations (45.1% of pre-COVID-19), and in the Northeast (38.9% of pre-COVID-19). At the facility level, there were statistically significant decreases (P < .01) for all demographics except for facilities in rural communities (P = .122) and in the West (P = .095).
Table 4.
Changes in biopsy quarterly volumes in the pre-COVID-19, peak COVID-19, and COVID-19 recovery periods by patient and facility demographics
| Variable | Pre-COVID-19 |
Peak COVID-19 |
COVID-19 Recovery |
Pre-COVID-19 vs Peak COVID-19 |
Peak COVID-19 vs COVID-19 Recovery |
Pre-COVID-19 vs COVID-19 Recovery |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Facility Median (IQR) | Total n | Facility Median (IQR) | Total n | Facility Median (IQR) | Total n | Facility P Value | Total Change % | Facility P Value | Total Change % | Facility P Value | Total Change % | |
| Age | ||||||||||||
| <40 y | 1 (0-4) | 1,676 | 0 (0-3) | 1,148 | 1 (0-4) | 1,611 | 0.024 | 68.5 | 0.021 | 140.3 | 0.469 | 96.1 |
| 40-49 y | 6 (1-20) | 6,715 | 3 (0-11) | 3,198 | 7 (1-19) | 6,527 | <0.001 | 47.6 | <0.001 | 204.1 | 0.477 | 97.2 |
| 50-59 y | 6 (1-21) | 6,950 | 3 (0-10) | 3,143 | 6 (1-16) | 6,061 | <0.001 | 45.2 | <0.001 | 192.8 | 0.158 | 87.2 |
| 60-69 y | 7 (2-23) | 6,936 | 3 (0-10) | 3,133 | 7 (2-18) | 6,245 | <0.001 | 45.2 | <0.001 | 199.3 | 0.279 | 90.0 |
| 70-79 y | 4 (0-14) | 4,462 | 2 (0-6) | 2,021 | 4 (0-12) | 4,056 | <0.001 | 45.3 | <0.001 | 200.7 | 0.143 | 90.9 |
| ≥80+ y | 1 (0-4) | 1,168 | 0 (0-2) | 548 | 1 (0-3) | 1,047 | <0.001 | 46.9 | <0.001 | 191.1 | 0.076 | 89.6 |
| Race | ||||||||||||
| Asian | 0 (0-0) | 662 | 0 (0-0) | 286 | 0 (0-0) | 537 | 0.006 | 43.2 | 0.011 | 187.8 | 0.405 | 81.1 |
| Black | 0 (0-1) | 1,078 | 0 (0-0) | 573 | 0 (0-1) | 930 | 0.001 | 53.2 | 0.009 | 162.3 | 0.261 | 86.3 |
| Native American, Native Hawaiian, or Pacific Islander | 0 (0-0) | 122 | 0 (0-0) | 84 | 0 (0-0) | 143 | 0.003 | 68.9 | 0.002 | 170.2 | 0.439 | 117.2 |
| White | 2 (0-28) | 9,736 | 0 (0-10) | 4,247 | 2 (0-18) | 7,350 | 0.002 | 43.6 | 0.017 | 173.1 | 0.208 | 75.5 |
| Unknown | 9 (1-37) | 16,309 | 4 (0-20) | 8,001 | 9 (0-41) | 16,587 | <0.001 | 49.1 | <0.001 | 207.3 | 0.360 | 101.7 |
| Community type | ||||||||||||
| Metropolitan (>100,000 persons) | 54 (13-155) | 17,393 | 24 (5-79) | 8,262 | 48 (14-146) | 16,382 | <0.001 | 47.5 | <0.001 | 198.3 | 0.362 | 94.2 |
| Suburban/small (50,000-100,000 persons) | 29 (7-60) | 8,414 | 11 (2-30) | 3,698 | 26 (9-58) | 7,446 | <0.001 | 44.0 | <0.001 | 201.4 | 0.426 | 88.5 |
| Rural (<50,000 persons) | 5 (0-26) | 2,100 | 4 (0-16) | 1,231 | 6 (0-24) | 1,719 | 0.122 | 58.6 | 0.218 | 139.6 | 0.340 | 81.9 |
| Facility type | ||||||||||||
| Academic/university | 91 (17-260) | 4,095 | 43 (5-111) | 1,847 | 56 (9-205) | 3,231 | 0.012 | 45.1 | 0.094 | 174.9 | 0.147 | 78.9 |
| Community hospital | 30 (4-73) | 11,248 | 15 (2-39) | 5,564 | 24 (4-68) | 10,348 | 0.002 | 49.5 | 0.015 | 186.0 | 0.226 | 92.0 |
| Multispecialty clinic | 10 (2-51) | 1,344 | 1 (0-34) | 676 | 10 (4-40) | 1,554 | 0.030 | 50.3 | 0.021 | 229.9 | 0.356 | 115.6 |
| Freestanding imaging center | 26 (8-74) | 10,396 | 11 (4-34) | 4,853 | 26 (10-71) | 9,830 | <0.001 | 46.7 | <0.001 | 202.6 | 0.375 | 94.6 |
| Region | ||||||||||||
| Northeast | 24 (4-76) | 6,809 | 9 (1-30) | 2,649 | 24 (9-59) | 5,550 | 0.003 | 38.9 | <0.001 | 209.5 | 0.495 | 81.5 |
| Midwest | 19 (7-71) | 6,277 | 9 (1-27) | 2,783 | 19 (6-51) | 5,450 | <0.001 | 44.3 | <0.001 | 195.8 | 0.186 | 86.8 |
| South | 28 (8-107) | 6,860 | 11 (3-63) | 3,444 | 32 (8-96) | 6,192 | 0.008 | 50.2 | 0.014 | 179.8 | 0.458 | 90.3 |
| West | 37 (8-116) | 7,961 | 27 (7-66) | 4,315 | 36 (7-118) | 8,355 | 0.095 | 54.2 | 0.105 | 193.6 | 0.486 | 104.9 |
Note: Facility refers to the median and IQR for facilities. Total refers to the total reported at all facilities. Facility P value compares the metrics at the facility level. Statistical significance was defined as a P value of .01 for encounters greater than 10,000 and .05 for encounters less than 10,000. Statistically significant values are in boldface type. Total change refers to the second comparator group divided by the first comparator group as a percentage. COVID-19 = coronavirus disease 2019; IQR = interquartile range.
During the COVID-19 recovery, biopsy volume (25,547 biopsies) rebounded to 91.5% of the pre-COVID-19 level (27,907 biopsies; Figure 1). The smallest rebounds were for women aged 60 to 69 years (90.0% of pre-COVID-19), white women (75.5% of pre-COVID-19), and facilities in rural communities (81.9% of pre-COVID-19), with academic or university affiliations (78.9% of pre-COVID-19), and in the Northeast (81.5% of pre-COVID-19). There were no significant differences in the pre-COVID-19 versus COVID-19 recovery period diagnostic volumes for the demographics studied.
Cancer Diagnoses
The volume of cancer diagnoses during the peak COVID-19 period (4,101 cancers) was 48.7% of the pre-COVID-19 period (8,413 cancers; Figure 2). During the nadir of the peak COVID-19 period in April 2020, the cancer diagnosis volume was 20.5% (576 cancers) of the monthly average in the pre-COVID-19 period (2,804 cancers). As shown in Table 5 , the greatest decreases were seen for women aged 60 to 69 years (46.3% of pre-COVID-19), Asian women (35.5% of pre-COVID-19), and facilities in suburban communities (45.2% of pre-COVID-19), with academic or university affiliations (45.4% of pre-COVID-19), and in the Northeast (39.7% of pre-COVID-19). At the facility level, there were statistically significant decreases (P < .05) for all demographics except age < 40 years (P = .245); Asian race (P = .069); Native American, Native Hawaiian, or Pacific Islander race (P = .154); rural location (P = .054); and location in the West (P = .095).
Table 5.
Changes in cancer quarterly volumes in the pre-COVID-19, peak COVID-19, and COVID-19 recovery periods by patient and facility demographics
| Variable | Pre-COVID-19 |
Peak COVID-19 |
COVID-19 Recovery |
Pre-COVID-19 vs Peak COVID-19 |
Peak COVID-19 vs COVID-19 Recovery |
Pre-COVID-19 vs COVID-19 Recovery |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Facility Median (IQR) | Total n | Facility Median (IQR) | Total n | Facility Median (IQR) | Total n | Facility P Value | Total Change % | Facility P Value | Total Change % | Facility P Value | Total Change % | |
| Age | ||||||||||||
| <40 y | 0 (0-0) | 199 | 0 (0-0) | 189 | 0 (0-0) | 200 | 0.245 | 95.0 | 0.424 | 105.8 | 0.315 | 100.5 |
| 40-49 y | 1 (0-3) | 1,079 | 0 (0-2) | 558 | 1 (0-3) | 1,047 | <0.001 | 51.7 | <0.001 | 187.6 | 0.397 | 97.0 |
| 50-59 y | 2 (0-5) | 1,888 | 1 (0-3) | 894 | 1 (0-5) | 1,614 | <0.001 | 47.4 | 0.001 | 180.5 | 0.103 | 85.5 |
| 60-69 y | 3 (0-9) | 2,633 | 1 (0-4) | 1,219 | 2 (0-8) | 2,428 | <0.001 | 46.3 | <0.001 | 199.2 | 0.193 | 92.2 |
| 70-79 y | 2 (0-6) | 1,958 | 1 (0-3) | 929 | 1 (0-5) | 1,868 | <0.001 | 47.4 | <0.001 | 201.1 | 0.126 | 95.4 |
| ≥80 y | 0 (0-2) | 656 | 0 (0-1) | 312 | 0 (0-2) | 583 | <0.001 | 47.6 | 0.001 | 186.9 | 0.118 | 88.9 |
| Race | ||||||||||||
| Asian | 0 (0-0) | 211 | 0 (0-0) | 75 | 0 (0-0) | 152 | 0.069 | 35.5 | 0.344 | 202.7 | 0.144 | 72.0 |
| Black | 0 (0-0) | 303 | 0 (0-0) | 176 | 0 (0-0) | 257 | 0.018 | 58.1 | 0.098 | 146.0 | 0.211 | 84.8 |
| Native American, Native Hawaiian, or Pacific Islander | 0 (0-0) | 34 | 0 (0-0) | 20 | 0 (0-0) | 34 | 0.154 | 58.8 | 0.015 | 170.0 | 0.121 | 100.0 |
| White | 0 (0-9) | 3,103 | 0 (0-3) | 1,354 | 0 (0-5) | 2,496 | <0.001 | 43.6 | 0.011 | 184.3 | 0.107 | 80.4 |
| Unknown | 3 (0-12) | 4,762 | 1 (0-6) | 2,476 | 2 (0-12) | 4,801 | <0.001 | 52.0 | <0.001 | 193.9 | 0.277 | 100.8 |
| Community type | ||||||||||||
| Metropolitan (>100,000 persons) | 17 (5-48) | 5,277 | 8 (1-27) | 2,653 | 12 (4-48) | 5,011 | <0.001 | 50.3 | <0.001 | 188.9 | 0.297 | 95.0 |
| Suburban/small (50,000-100,000 persons) | 9 (3-20) | 2,503 | 3 (0-9) | 1,131 | 9 (2-19) | 2,228 | <0.001 | 45.2 | <0.001 | 197.0 | 0.270 | 89.0 |
| Rural (<50,000 persons) | 2 (0-11) | 633 | 1 (0-4) | 317 | 1 (0-8) | 501 | 0.054 | 50.1 | 0.243 | 158.0 | 0.205 | 79.1 |
| Facility type | ||||||||||||
| Academic/university | 22 (6-78) | 1,256 | 13 (2-31) | 570 | 16 (3-55) | 1,035 | 0.032 | 45.4 | 0.147 | 181.6 | 0.248 | 82.4 |
| Community hospital | 10 (1-27) | 3,422 | 4 (0-14) | 1,681 | 8 (1-25) | 3,101 | <0.001 | 49.1 | 0.019 | 184.5 | 0.158 | 90.6 |
| Multispecialty clinic | 3 (0-17) | 420 | 0 (0-8) | 212 | 4 (1-11) | 533 | 0.014 | 50.5 | 0.009 | 251.4 | 0.402 | 126.9 |
| Freestanding imaging center | 8 (2-22) | 3,110 | 3 (1-11) | 1,565 | 9 (2-21) | 2,970 | <0.001 | 50.3 | <0.001 | 189.8 | 0.334 | 95.5 |
| Region | ||||||||||||
| Northeast | 9 (1-20) | 1,943 | 2 (0-9) | 771 | 7 (1-16) | 1,494 | <0.001 | 39.7 | 0.004 | 193.8 | 0.228 | 76.9 |
| Midwest | 8 (2-26) | 2,217 | 3 (0-10) | 988 | 6 (2-23) | 1,969 | <0.001 | 44.6 | <0.001 | 199.3 | 0.192 | 88.8 |
| South | 9 (2-25) | 1,911 | 4 (1-20) | 1,019 | 9 (1-35) | 1,736 | 0.013 | 53.3 | 0.025 | 170.4 | 0.447 | 90.8 |
| West | 8 (2-41) | 2,342 | 5 (1-23) | 1,323 | 11 (0-42) | 2,541 | 0.095 | 56.5 | 0.149 | 192.1 | 0.437 | 108.5 |
Note: Facility refers to the median and IQR for facilities. Total refers to the total reported at all facilities. Facility P value compares the metrics at the facility level. Statistical significance was defined as a P value of .01 for encounters greater than 10,000 and .05 for encounters less than 10,000. Statistically significant values are in boldface type. Total change refers to the second comparator group divided by the first comparator group as a percentage. COVID-19 = coronavirus disease 2019; IQR = interquartile range.
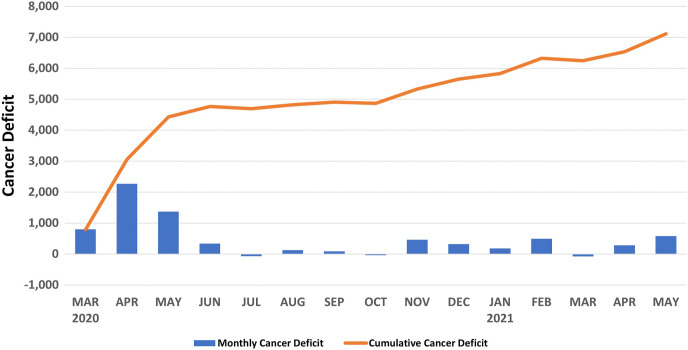
During the COVID-19 recovery, cancer diagnoses (7,740 cancers) rebounded to 92.0% of the pre-COVID-19 level (8,413 cancers; Figure 2). The smallest rebounds were for women aged 50 to 59 years (85.5% of pre-COVID-19), Asian women (72.0% of pre-COVID-19), and facilities in rural communities (79.1% of pre-COVID-19), with academic or university affiliations (82.4% of pre-COVID-19), and in the Northeast (76.9% of pre-COVID-19). There were no significant differences in the pre-COVID-19 versus COVID-19 recovery period diagnostic volumes for the demographics studied. The monthly average of breast cancers diagnosed from March 2019 to February 2020 was 2,843 cancers. The cumulative cancer deficit (ie, monthly average from preceding year minus cancer diagnoses per month in current year) accumulated from the peak COVID-19 to the COVID-19 recovery period was 7,113 cancers, as shown in Figure 4 .
Fig. 4.
Monthly and cumulative cancer deficits from March 2020 to May 2021 on the basis of the average monthly cancer diagnoses from March 2019 to February 2020.
Discussion
These results from the NMD provide the broadest and largest analysis in the United States of the impact of the COVID-19 pandemic on breast cancer screening outcomes both during the peak of the pandemic and in the subsequent (rebound) year. The COVID-19 pandemic resulted in acute and pronounced declines for all breast imaging metrics studied during the peak COVID-19 period, but the effect was greatest for screening mammography (36.3% of pre-COVID-19). The decrease in screening mammography followed federal guidelines to postpone screening services, and similar results have been reported using regional and smaller national data sets [2,5,6]. However, there is a paucity of longer follow-up data to document changes in patient behaviors and the seasonality associated with health maintenance examinations [5,6,16]. Our data collected 1 year after the acute phase of the pandemic, during the COVID-19 recovery period, demonstrate that screening mammography volumes (85.3% of pre-COVID-19) continued to lag behind all other breast imaging metrics studied (range, 91.5%-97.8% of pre-COVID-19), and this was exaggerated for women aged 80 years and older (76.1% of pre-COVID-19). The persistent failure to return to regular screening intervals has also been reproduced in colon and cervical cancer screening [16]. If volumes do not normalize, screening mammography will remain underused among asymptomatic women. Strategies to facilitate safe breast imaging have been developed, but radiologists will need to develop outreach efforts, especially at the local level, directed toward patients and ordering providers on the importance of screening mammography in order to improve utilization rates [10,11].
As expected, there was an acute decrease in breast cancer diagnoses during the peak COVID-19 period. But although cancer diagnoses largely rebounded (92.0% of pre-COVID-19). This is especially worrying as cancer diagnoses have not reached pre-COVID-19 levels, and the cumulative breast cancer deficit since the start of the pandemic continues to grow (Fig. 4). This rebound represents a mix of cancers not diagnosed during the peak COVID-19 period as well as the normal cancers detected through routine practice. There are major concerns that in the near future, depending on the lead time of breast cancer, there will be an increase in cancer diagnoses and that a larger proportion of breast cancers will be diagnosed at a higher stage and thus have a worse prognosis. Analysis of Breast Cancer Surveillance Consortium data demonstrates that decreases in cancer diagnoses were due largely to fewer screen-detected cancers [3]. The NMD unfortunately does not include tumor staging information to assess clinical outcomes and prognosis. To date, one study from Italy has demonstrated that a 2-month pause in mammographic screening resulted in an 11% increase in node-positive breast cancer and a 10% increase in stage III breast cancer [17]. Similarly, an increase in stage III breast cancer (8.4% pre-COVID-19 vs 23.8% post-COVID-19) and systemic first-line chemotherapy (23.0% pre-COVID-19 vs 36.5% post-COVID-19) have been reported in South Korea [18]. Longer term modeling studies all report an increase in poor outcomes, including life years lost, excess breast cancer deaths, and treatment morbidity [4,19,20]. Although there are differences in the modeling estimates on the basis of assumptions around screening practices (ie, annual vs biennial, screening utilization rates), a key unknown is how quickly screening practices will normalize. Our results indicate that screening practices may take much longer to normalize than previously hypothesized and that models will need to be adjusted for the persistent decline. Given the latency of breast cancer diagnosis and mortality as well as the persistent reduction in breast cancer screening practices currently, it may be several years before these results are fully realized.
There were notable differences in the reported breast cancer metrics on the basis of patient demographics. Compared with all other racial groups, Asian women had the largest decrease in screening mammography, diagnostic mammography, and cancer diagnoses during peak COVID-19 and experienced the smallest rebound in screening mammography and cancer diagnoses during COVID-19 recovery. Published literature examining the impact of the pandemic on breast cancer screening for different racial and ethnic groups is very mixed; however, there are examples of the disproportionate impact on Asian women, with studies demonstrating large decreases in screening mammography volumes [5,7,21] and fewer breast cancer diagnoses, similar to our findings [3]. Similar patterns for Asian women have been noted for other screening services, including cervical cancer [7]. Much of the focus on race/ethnicity in breast cancer screening outreach efforts unrelated to the pandemic has been directed toward black women because of their high rates of breast cancer and generally worse outcomes [22]. But our results suggest that outreach efforts directed toward the Asian community are important as well. These efforts could include engaging with local community organizations, disaggregating the impact of COVID-19 on specific Asian populations, especially immigrant groups, and identifying any underlying cultural barriers to returning for routine care [23]. Finally, there were greater decreases and more limited recovery across most breast cancer metrics for elderly women. COVID-19 had a disproportionate impact on elderly patients, especially minority elderly patients, with higher rates of death and severe infection from COVID-19 as well as an increased risk for neglect, loss of social support networks, and economic hardships [24, 25, 26]. Outreach efforts and reestablishing support networks are critical to meet the needs of this patient population, who are at the highest risk for developing breast cancer.
The early phase of the COVID-19 pandemic had an unequal geographic distribution, which is reflected in the outcome differences by facility demographics [27]. Facilities in the Northeast had the largest decrease in all metrics during the peak COVID-19 period and continued to have the smallest rebound in breast biopsies and cancer diagnoses during the COVID-19 recovery period. This may reflect the very large numbers of COVID-19 cases in metropolitan areas such as New York City [27]. The impact of breast imaging metrics on community type is mixed. Suburban and metropolitan facilities had larger decreases across all metrics during the peak COVID-19 period, which likely reflects the initial spread of disease in denser population centers. However, there were notably smaller rebounds in biopsies and cancer diagnoses in rural facilities during the COVID-19 recovery period, which likely reflects shifts in the spread of disease from urban to rural facilities [28,29]. Facilities affiliated with academic practices had the largest declines during peak COVID-19 and the smallest rebound during the COVID-19 recovery period for every breast cancer metric in our database. This may be due to a fear that academic centers were also treating many patients with COVID-19 and may be confounded by the higher concentration of academic facilities in metropolitan and suburban locations.
There were limitations to this study. Although the number of breast care encounters in the NMD is very large, which facilitates many subgroup analyses, some subgroups were still small. For example, measurements for women younger than 40 years and for Native American, Native Hawaiian, or Pacific Islander women have much wider confidence intervals. We compensated for this by adjusting our threshold for statistical significance on the basis of sample sizes. Additionally, a majority of patients have their race documented as “unknown” (58.9%), which is a limitation of NMD data entry. Data from the NMD are exported in aggregate, precluding multivariate analysis at the patient level, which would facilitate testing of confounding factors, especially for demographic subgroup analysis. Facilities have several months to report their metrics to the NMD, so data from late 2021 were not available for analysis. The NMD is also not linked with a tumor registry to allow assessment of tumor outcomes.
Take-Home Points
-
▪
There were major decreases in screening mammography, diagnostic mammography, breast biopsies, and cancer diagnoses during the peak COVID-19 period, with a rapid rebound during the COVID-19 recovery period.
-
▪
COVID-19 had the greatest impact on screening mammography, and utilization rates have not returned to baseline, which may have long-term implications for breast cancer staging and outcomes.
-
▪
The acute deficits in breast cancer diagnoses during the peak COVID-19 period continued to increase in the following year.
-
▪
COVID-19 had a disproportionate effect on older women and Asian women for multiple breast cancer screening metrics during the peak COVID-19 and COVID-19 recovery periods.
-
▪
The impact of COVID-19 on facility demographics likely reflects differences in the temporal and geographic distribution of disease.
Acknowledgments
The authors thank ACR staff members for assistance in preparation of the NMD data and acknowledge guidance and input by the NMD Steering Committee for this analysis.
Footnotes
This research was supported by the ACR’s National Radiology Data Registry. The views expressed in this report represent those of the authors and do not necessarily represent the official views of the National Radiology Data Registry or the ACR. The authors state that they have no conflict of interest related to the material discussed in this article. The authors are non-partner/non-partnership track/employees.
References
- 1.Moreland A., Herlihy C., Tynan M.A. Timing of state and territorial COVID-19 stay-at-home orders and changes in population movement—United States, March 1–May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1198–1203. doi: 10.15585/mmwr.mm6935a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae K.T., Tao C., Gurel S., et al. Effect of patient weight and scanning duration on contrast enhancement during pulmonary multidetector CT angiography. Radiology. 2007;242:582–589. doi: 10.1148/radiol.2422052132. [DOI] [PubMed] [Google Scholar]
- 3.Lowry K.P., Bissell M., Miglioretti D.L., et al. Breast biopsy recommendations and breast cancers diagnosed during the COVID-19 pandemic. Radiology. 2022;33:287–294. doi: 10.1148/radiol.2021211808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpless N.E. COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 5.Sprague B.L., Lowry K.P., Miglioretti D.L., et al. Changes in mammography use by women’s characteristics during the first 5 months of the COVID-19 pandemic. J Natl Cancer Inst. 2021;113:1161–1167. doi: 10.1093/jnci/djab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyante S.J., Benefield T.S., Kuzmiak C.M., Earnhardt K., Pritchard M., Henderson L.M. Population-level impact of coronavirus disease 2019 on breast cancer screening and diagnostic procedures. Cancer. 2021;127:2111–2121. doi: 10.1002/cncr.33460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeGroff A., Miller J., Sharma K., et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January-June 2020, in the United States. Prev Med. 2021;151:106559. doi: 10.1016/j.ypmed.2021.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidich J.J., Boltyenkov A., Wang J.J., Chusid J., Hughes D., Sanelli P.C. Impact of the coronavirus disease 2019 (COVID-19) pandemic on imaging case volumes. J Am Coll Radiol. 2020;17:865–872. doi: 10.1016/j.jacr.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen M.P., Norbash A., Kruskal J., Meltzer C.C., Yee J., Thrall J. Impact of coronavirus disease 2019 (COVID-19) on the practice of clinical radiology. J Am Coll Radiol. 2020;17:1096–1100. doi: 10.1016/j.jacr.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davenport M.S., Bruno M.A., Iyer R.S., et al. ACR statement on safe resumption of routine radiology care during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Radiol. 2020;17:839–844. doi: 10.1016/j.jacr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Society of Breast Imaging SBI recommendations for a thoughtful return to caring for patients. https://www.sbi-online.org/Portals/0/Position%20Statements/2020/SBI-recommendations-for-a-thoughtful-return-to-caring-for-patients_April-16-2020.pdf Available at:
- 12.Dodelzon K., Grimm L.J., Tran K., et al. Impact of COVID-19 on breast imaging practice operations and recovery efforts: a North American study. J Breast Imaging. 2021;3:156–167. doi: 10.1093/jbi/wbab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Radiology National Mammography Database. https://www.acr.org/Practice-Management-Quality-Informatics/Registries/National-Mammography-Database Available at:
- 14.Lee C.S., Bhargavan-Chatfield M., Burnside E.S., Nagy P., Sickles E.A. The National Mammography Database: preliminary data. AJR Am J Roentgenol. 2016;206:883–890. doi: 10.2214/AJR.15.14312. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services Measuring healthy days. https://www.cdc.gov/hrqol/pdfs/mhd.pdf Available at: [DOI] [PubMed]
- 16.Mast C., Munoz del Rio A., Heist T. Cancer screenings are still lagging. https://epicresearch.org/articles/cancer-screenings-are-still-lagging Available at:
- 17.Toss A., Isca C., Venturelli M., et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6:100055. doi: 10.1016/j.esmoop.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S.A., Cheun J.H., Yang H.R., et al. Stage migration in newly diagnosed cancer patients during the COVID-19 pandemic era. Lancet. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3675408 Available at:
- 19.Yong J.H., Mainprize J.G., Yaffe M.J., et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2021;28:100–107. doi: 10.1177/0969141320974711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alagoz O., Lowry K.P., Kurian A.W., et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst. 2021;113:1484–1494. doi: 10.1093/jnci/djab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacson R., Shi J., Kapoor N., Eappen S., Boland G.W., Khorasani R. Exacerbation of inequities in use of diagnostic radiology during the early stages of reopening after COVID-19. J Am Coll Radiol. 2021;18:696–703. doi: 10.1016/j.jacr.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monticciolo D.L., Malak S.F., Friedewald S.M., et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280–1288. doi: 10.1016/j.jacr.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Sohn Y.J., Chang C.Y., Miles R.C. Current gaps in breast cancer screening among Asian and Asian American women in the United States. J Am Coll Radiol. 2021;18:1376–1383. doi: 10.1016/j.jacr.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Yanez N.D., Weiss N.S., Romand J.A., Treggiari M.M. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20:1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerrero L.R., Wallace S.P. The impact of COVID-19 on diverse older adults and health equity in the United States. Front Public Health. 2021;9:661592. doi: 10.3389/fpubh.2021.661592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United Nations Policy brief: the impact of COVID-19 on older persons. https://unsdg.un.org/sites/default/files/2020-05/Policy-Brief-The-Impact-of-COVID-19-on-Older-Persons.pdf Available at:
- 27.Centers for Disease Control and Prevention United States COVID-19 cases and deaths by state over time. https://data.cdc.gov/Case-Surveillance/United-States-COVID-19-Cases-and-Deaths-by-State-o/9mfq-cb36 Available at:
- 28.Huang Q., Jackson S., Derakhshan S., et al. Urban-rural differences in COVID-19 exposures and outcomes in the South: a preliminary analysis of South Carolina. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0246548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul R., Arif A.A., Adeyemi O., Ghosh S., Han D. Progression of COVID-19 from urban to rural areas in the United States: a spatiotemporal analysis of prevalence rates. J Rural Health. 2020;36:591–601. doi: 10.1111/jrh.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]