Abstract
Introduction:
Compared with docetaxel, the phase-III trial, ULTIMATE, showed a significant improvement of progression-free survival (PFS) with paclitaxel–bevacizumab combination (PB) as second- or third-line treatment in advanced non-small cell lung cancer (NSCLC). With the increase of immunotherapy treatment in first-line settings, the optimal treatment after first-line failure must be redefined.
Methods:
This multicentric retrospective study identified all advanced NSCLC patients treated with PB as second-line therapy and beyond. The main efficacy outcomes assessed were objective response rate (ORR), disease control rate (DCR), PFS, and overall survival (OS). The adverse events were reported according to Common Terminology Criteria for Adverse Events (CTCAE).
Results:
From January 2010 to February 2020, 314 patients in 16 centers received the PB combination. Most patients were male (55%), with a median age of 60 years (19–82), 95% had adenocarcinoma, 27% had a performance status ⩾2, 45% had brain metastases at the time of inclusion. They mostly received the PB combination either in second (20%) or in third-line (39%), and 28% were treated just after ICI failure. ORR and DCR were 40% and 77%, respectively; median PFS and OS were 5.7 [interquartile range (IQR): 3.2–9.6] and 10.8 [IQR: 5.3–19.6] months, respectively. All grade adverse events concerned 82% of patients, including 53% asthenia and 39% neurotoxicity, and 25% of patients continued monotherapy (mostly with bevacizumab) alone due to toxicity. Median PFS for patients treated after ICI failure (ICI+) was significantly superior compared with those not previously treated with ICI (ICI−): 7.0 [IQR: 4.2–11.0] versus 5.2 [IQR: 2.9–8.8] months, p = 0.01, without statistically significant difference for OS between these two groups. In multivariate analysis, factors associated with superior PFS were previous ICI treatment and performance status of 0–1. Only a performance status of 0–1 was associated with superior OS.
Conclusion:
PB combination as second-line treatment or beyond for advanced non-squamous NSCLC had acceptable toxicity and a clinically relevant efficacy and is an option as salvage treatment for these patients, more particularly after ICI progression.
Keywords: bevacizumab, chemotherapy, immunotherapy, non-small cell lung cancer, NSCLC, paclitaxel
Highlights
This real-life study has evaluated paclitaxel–bevacizumab (PB) combination in pretreated advanced non-squamous non-small cell lung cancer (NSCLC).
The results confirmed the efficiency of PB with an objective response rate (ORR) of 40% and a median progression-free survival (PFS) of 5.7 months
PB was particularly efficient following progression on immune checkpoint inhibitors.
Introduction
Lung cancer has become the most widespread cancer and the main cause of cancer deaths worldwide, accounting for nearly a quarter of all cancer deaths, with the most common histological form being non-squamous non-small cell lung cancer (NSCLC). 1 Despite recent progress and an increase in the number of patients who are candidates for second-line treatment or more, 2 prognostic remains poor. 1
Until 2015 when immunotherapy was implemented in NSCLC, the second-line reference chemotherapies were docetaxel or pemetrexed monotherapy yielding poor results with an objective response rate (ORR) between 5% and 10%, median progression-free survival (PFS) of 1.5–3 months, and median overall survival (OS) of 4.6–12.8 months.3 –6 Alternative strategies combining mono-chemotherapy with anti-angiogenic agents have been studied to improve treatment beyond failure of platinum-based chemotherapy. Following promising results in phase-II and several retrospective studies,7 –9 phase-III studies were conducted with the association of docetaxel with ramucirumab 10 and nintedanib, 11 leading to a European authorization for these combinations.
The combination of paclitaxel and bevacizumab (PB) was evaluated in three retrospective studies with limited numbers of patients but with encouraging results. The response rate ranged from 33–44%, median PFS from 4.6–6.4 months, and median OS reported in one study was 9.6 months.12 –14 Based on these promising results, the phase-III trial IFCT-1103 ULTIMATE evaluated the efficacy of the PB combination compared with standard docetaxel as second- or third-line treatment in 166 patients with advanced non-squamous NSCLC. 15 Compared with docetaxel, PB combination yielded a significant increase of PFS (5.4 versus 3.9 months, p = 0.005) without difference in OS, although this may be due to the crossover that was allowed in this study. Adverse events were different between arms with an increase in hematological toxicity with docetaxel compared with PB. These results supported the use of the PB doublet in second line and beyond, and since 2016 it has been included as an option for the management of advanced non-squamous NSCLC in French guidelines. 16
Among recent therapeutic advances, immunotherapy has been a turning point in the management of NSCLC especially more recently in first-line treatment. As monotherapy for patients with a PDL1 tumor expression ⩾ 50% or in combination with chemotherapy, anti-PD1 agents are now part of the first line for most patients.17,18 Although the first-line treatment has changed, the options for second-line treatment (for those who received chemo-immunotherapy combination) or third-line treatment (for those who received immunotherapy then platinum-based chemotherapy) remain the same, while pursuit or rechallenge with immunotherapy has not yet proven efficacy.19,20 Thus, chemotherapy is still the standard of care in this setting and the PB doublet could be an option.
While data on the efficacy of chemotherapy administered after progression under immunotherapy seemed particularly interesting,21–23 the efficacy of the combination of chemotherapy (docetaxel) with an anti-angiogenic molecule (nintedanib or ramucirumab) in post-immunotherapy setting has been the subject of a few studies.24–32
The objective of this study was to evaluate the efficacy and toxicity of the PB combination in clinical practice in second line and beyond in advanced non-squamous NSCLC.
Methods
Study design and patients
We conducted a multicenter retrospective study among 16 hospitals including all advanced NSCLC patients treated with PB as second-line therapy and beyond in routine practice, between January 2010 and January 2020. Patients should have received at least one dose of the combination. Patients who received PB with another treatment drug were excluded.
The primary objective was to describe the median real-world PFS (rwPFS) in patients treated by PB. The secondary objectives included comparison of PFS between patients who previously received immunotherapy and those who did not, objective response rate (ORR) according to RECIST 1.1, disease control rate (DCR), OS, and toxicity. ICI+ subgroup was defined by patients treated with PB immediately after progression under immune checkpoint inhibitors (ICI) therapy, regardless of the line of treatment with ICI.
ICI− subgroup was defined by patients treated with PB without previous ICI therapy just before. Those patients could have received ICI in their previous line of treatment, but not at the line just before PB.
As one of our main objectives was to describe the efficacy of ICI followed immediately by PB, and as we still do not know the accurate duration of the effect of ICI after its discontinuation, this exact sequence of treatment was important to constitute a homogeneous group of patients to avoid bias in the analyses.
Data collection
Patients’ electronic medical records, in each center, were reviewed for collecting data. Details are provided in the flow chart.
At the time of PB prescription, variables related to characteristics of patients, lung cancer (age, ECOG-PS, histology subtype, metastatic site), and previous lines of treatment were recorded. Line of treatment of PB combination, dosing of both drugs, number of cycles, toxicity, and reason for discontinuing were recorded.
Objective response criteria, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), were evaluated according to the RECIST 1.1 guidelines (very few according to RECIST 1.0). Similarly, DCR was calculated by adding CR, PR, and SD. The rhythm of the scanner’s evaluation varied from center to center. Tolerance was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, assessed by reviewing the patients’ medical histories and laboratory records.
Statistical analysis
Data were expressed as number (and percentage) for qualitative variables and median (and (IQR) for quantitative variables and were using chi-square (or Fisher-exact) or Mann–Whitney tests, as appropriate.
The rwPFS was defined as the time from the first dose of treatment with PB to the first occurrence of disease progression or death from any cause during the study. The date of cancer progression was defined as the date of the first source evidence for progression referenced by the clinician – for example, radiology report, pathology, or exam – or the clinician note date when no evidence sources were documented. Similarly, OS was calculated from the start of PB therapy to death from any cause. The date of data cutoff was 1 August 2020. Survival curves were obtained using the Kaplan–Meier method and compared using log-rank testing. Sensitivity analyses were performed in the following subgroups of patients: patients previously treated with immunotherapy (ICI+), patients with oncogenic addiction and patients previously treated with taxanes or anti-angiogenic agents.
Univariate and multivariate analyses for survival outcomes were analyzed using the Cox proportional hazards model. All clinically significant variables were entered in the multivariate model if they were not collinear. Missing data were indicated in the population description and imputed elsewhere by the variable median value (for quantitative variables) or the most common level (for qualitative variables) in the Cox models.
All tests were two-sided, and p-values < 0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient’s characteristics at the initiation of PB
From the 16 participating centers, 314 non-squamous advanced NSCLC patients were included. Patients’ characteristics at the time of PB initiation are listed in Table 1.
Table 1.
Patients’ characteristics at the beginning of paclitaxel–bevacizumab (PB).
| Total patients (n = 314) | ICI−, 226 (72%) | ICI+, 88 (28%) | p-value | |
|---|---|---|---|---|
| Patients’ characteristics | ||||
| Age, years | 60 (54–68) | 59 (54–67) | 62 (56–69) | 0.08 |
| Male sex | 141 (55%) | 132 (58) | 41 (47) | 0.06 |
| Smoker (MD = 8) | 249 (81%) | 175 (80) | 74 (86) | 0.19 |
| ECOG PS score (MD = 12) | 0.13 | |||
| 0 | 63 (21%) | 44 (20) | 19 (22) | |
| 1 | 156 (52%) | 105 (49) | 51 (59) | |
| ⩾2 | 83 (27%) | 66 (31) | 17 (20) | |
| Cancer characteristics | ||||
| Histology | 1 | |||
| Adenocarcinoma | 299 (95%) | 215 (95) | 84 (95) | |
| Others | 15 (5%) | 22 (5) | 4 (5) | |
| Brain metastasis | 140 (45%) | 107 (47) | 33 (38) | 0.12 |
| Molecular status | <10−4 | |||
| KRAS mutation | 98 (31%) | 58 (26) | 40 (45) | |
| EGFR mutation | 46 (15%) | 42 (19) | 4 (5) | |
| ALK rearrangement | 9 (3%) | 7 (3) | 1 (2) | |
| Wild type | 161 (51%) | 119 (53) | 42 (48) | |
| Treatment characteristics | ||||
| Treatment previously received | ||||
| Taxane-based chemotherapy | 97 (31%) | 89 (39) | 8 (9) | <10−4 |
| Bevacizumab | 91 (29%) | 61 (27) | 30 (34) | 0.21 |
| Line of treatment of PB combination | <10−4 | |||
| Second line | 63 (21%) | 62 (27) | 1 (1) | |
| Third line | 124 (39%) | 62 (27) | 62 (70) | |
| Fourth line | 66 (21%) | 48 (22) | 17 (19) | |
| Fifth line or more | 61 (19%) | 53 (23) | 8 (9) | |
ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitors; MD, missing data; PB, paclitaxel and bevacizumab.
ICI+ subgroup is defined by patients treated with PB immediately after progression under immune checkpoint inhibitors (ICI) therapy.
ICI− subgroup is defined by patients treated with PB without previous ICI therapy just before.
Qualitative variables are expressed as n (%) and quantitative variables as median (IQR: 25–75%).
The median age was 60 years (range 19–82); 141 (45%) were male; 219 (72%) had an ECOG PS score of 0–1; 63 (21%) patients received the combination as second-line treatment, 124 (39%) patients as third-line, and 127 (40%) patients as fourth-line and above. Ninety-eight patients (31%) harbored a KRAS mutation and 46 (15%) patients had an EGFR mutation (29 had an activating mutation, that is, deletion in exon 19 or L858R in exon 21; 13 had another known activating or non-activating mutation; and 4 had an EGFR mutation with no details found). At the time of initiation of the PB, 140 (45%) patients had brain metastasis and 82 (26%) liver metastasis (Table 1).
The median duration of follow-up since initiation of PB therapy was 15.5 months (IQR: 25–75%, 9.3–30.5). At the end of the follow-up period, 50 (16%) patients were alive and 3 (1%) patients were still treated with PB.
PB administration
The treatment regimen received was in the majority of cases paclitaxel (90 mg/m2 on days 1, 8, and 15) and bevacizumab (10 mg/kg on days 1 and 15), of each 28-day cycle (Appendix 1). The median duration of paclitaxel was 3.5 months (IQR: 25–75%, 2.1–5.3) and the median duration of bevacizumab was 4.2 months (IQR: 25–75%, 2.1–8.1) as shown in Appendix 2.
In their previous lines of treatment, 97 (31%) patients were already treated with taxane-containing chemotherapy, and 91 (29%) patients already received bevacizumab as shown in Table 1. Also, 114 (36%) patients received ICI therapy in their previous lines of treatment. Among them, 88 (28%) patients were treated by PB immediately after ICI progression (ICI+ subgroup): 2 (1%) patients in second line after ICI in first line (ICI monotherapy), 62 (70%) patients in third line after ICI in second line (all ICI monotherapy), 25 (28%) patients in fourth line or more after ICI immediately before (all ICI monotherapy). The median duration of ICI treatment before PB (ICI+ subgroup) was 4.2 months (range 0.5–19) and the median duration between the last infusion of ICI and the first infusion of PB was 27.6 days (range 1–150). Among the EGFR+ mut population with activating mutation, all but one received targeted anti-EGFR therapy, and all were treated with chemotherapy other than PB in their different treatment lines, each time before PB. Four EGFRmut+ patients were in the ICI+ group, that is, treated with ICI just before PB.
Data on possible treatment with radiotherapy were not available.
Efficacy
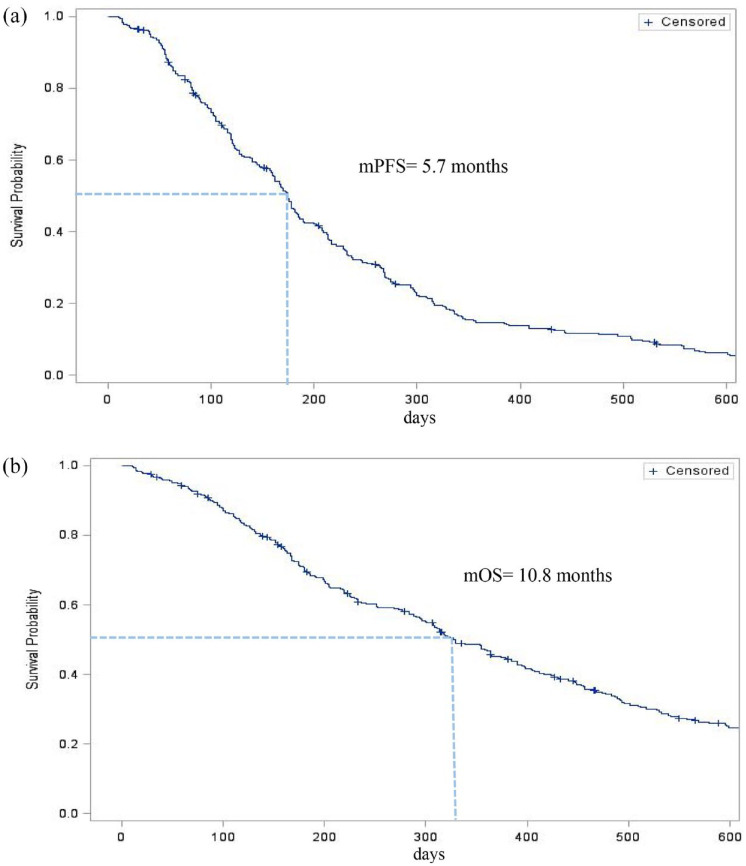
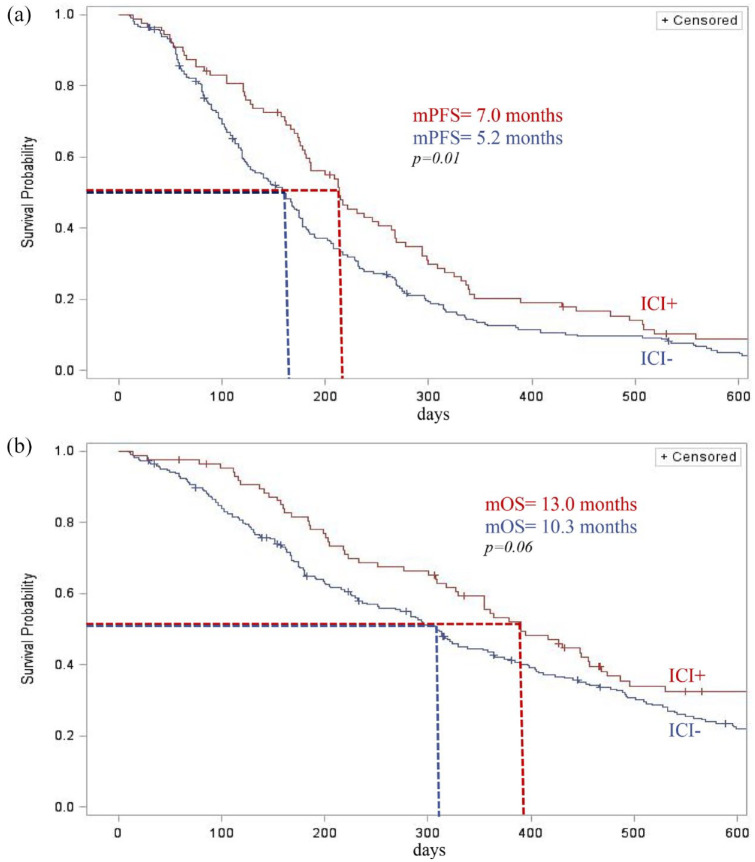
For the 314 patients included, the median PFS was 5.7 months (IQR: 25–75% 3.2–9.6), 7.0 months (IQR: 25–75% 4.2–11.0) in the ICI+ group versus 5.2 months (IQR 25–75% 2.9–8.8) in the ICI− group, p-value = 0.01, and hazard ration (HR) = 0.74 [95% confidence interval (CI): 0.56–0.94]. Median PFS was 5.2 months (IQR: 2.9–9.5) for patients harboring EGFR pathway mutation, 5.5 months (IQR: 3.3–9.7) for patients previously treated with taxane-based chemotherapy, and 5.3 months (IQR: 2.7–8.7) for patients previously treated with bevacizumab (Figures 1 and 2).
Figure 1.
Kaplan–Meier estimates of the (a) progression-free survival (PFS) and (b) overall survival (OS) of patients with advanced non-small cell lung cancer receiving paclitaxel–bevacizumab treatment.
Figure 2.
Kaplan–Meier estimates of the (a) progression-free survival (PFS) and (b) overall survival (OS) of patients according to prior treatment with immunotherapy (ICI).
The median OS was 10.8 months (IQR: 25–75% 5.3–19.6), 13.0 months (IQR: 25–75% 6.5–25.3) in the ICI+ group and 10.3 months in the ICI− group (IQR: 25–75% 4.9–18.0), p-value = 0.06. In subgroup analysis, median OS was 12.8 months (IQR: 5.6–19.5) for patients harboring EGFR pathway mutation, 10.3 months (IQR: 5.5–21.3) for patients previously treated with taxane-based chemotherapy, and 11.6 months (IQR: 5.9–20.2) for patients previously treated with bevacizumab (Figures 1 and 2).
On the 308 of the 314 evaluable patients, 122 (40%) achieved a PR, 115 (37%) had SD, and 71 (23%) progressed at first follow-up. None of the patients had a CR. The ORR was, therefore, 40% and DCR 77%. In the subgroup of ICI+ patients, the ORR was 51% versus 35% in the ICI− group (225/314) as shown in Table 2.
Table 2.
Efficacy of the paclitaxel–bevacizumab combination.
| Patients evaluable for therapeutic response | N = 314 | ICI− 226 (72%) |
ICI+ 88 (28%) |
p-value |
|---|---|---|---|---|
| For patients with evaluable response | 308 | |||
| Best overall response | 0.03 | |||
| Partial response | 122 (40%) | 77 (35) | 45 (51) | |
| Stable disease | 115 (37%) | 88 (40) | 27 (31) | |
| Progressive disease | 71 (23%) | 55 (25) | 16 (18) | |
| Overall response rate | 122 (40%) | 77 (35) | 45 (51) | 9 × 10−3 |
| Disease control rate | 237 (77%) | 165 (75) | 72 (82) | 0.20 |
ICI, immune checkpoint inhibitors.
ICI+ subgroup is defined by patients treated with PB immediately after progression under immune checkpoint inhibitors (ICI) therapy.
ICI− subgroup is defined by patients treated with PB without previous ICI therapy just before.
Qualitative variables are expressed as n (%).
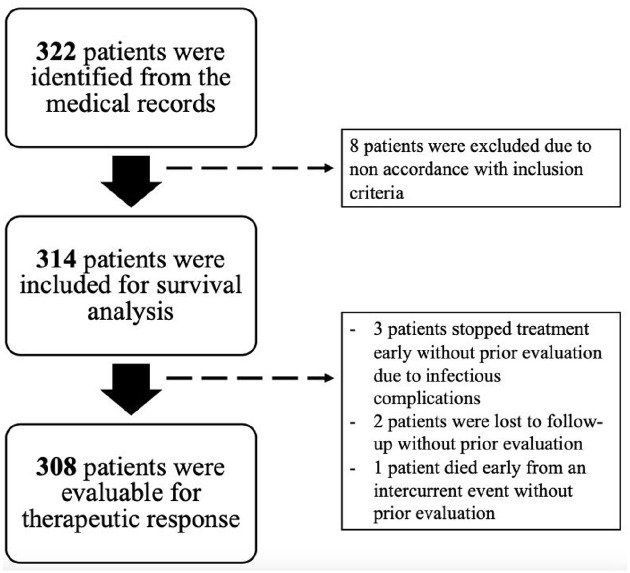
Of the 314 patients included, 308 were evaluable for therapeutic response: 3 patients stopped treatment early without prior evaluation due to infectious complications, 2 patients were lost to follow-up without prior evaluation, and 1 patient died early from an intercurrent event without prior evaluation.
A total of 193 (63%) patients were treated with subsequent treatment after progression with PB (8 missing data): 51% (n = 97) with immunotherapy, 26% (n = 49) with PB rechallenge, 23% (n = 45) with chemotherapy, and 1% (n = 1) with tyrosine kinase inhibitor.
Multivariate analysis demonstrated that the only independent factors positively impacting PFS were ICI+ treatment and an ECOG PS score of 0–1. For the OS, the only independent factor was an ECOG PS score of 0–1 (Tables 3 and 4).
Table 3.
Univariate and multivariate analysis of factors associated with PFS from the initiation of paclitaxel–bevacizumab therapy.
| Median PFS (IQR, 25–75%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| in months | HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Patients’ characteristics | |||||
| Sex | 0.72 | 0.89 | |||
| Male (n = 141) | 5.8 (3.4–9.7) | 1 | 1 | ||
| Female (n = 173) | 5.4 (2.8–8.9) | 10.43 [0.83–1.31] | 1.02 [0.80–1.30] | ||
| Age | 0.71 | 0.43 | |||
| < 68 years (n = 220) | 5.7 (0.9–9.6) | 1 | 1 | ||
| ⩾ 68 years (n = 94) | 5.8 (3.9–8.6) | 0.95 [0.73–1.23] | 1.12 [0.84–1.49] | ||
| ECOG PS score | 10−3 | 5 × 10−3 | |||
| 0 (n = 63) | 5.8 (3.6–9.0) | 1 | 1 | ||
| 1 (n = 156) | 6.5 (3.9–10.1) | 0.99 [0.73–1.33] | 1.01 [0.74–1.39] | ||
| ⩾ 2 (n = 83) | 3.8 (1.8–7.6) | 1.625 [1.153–2.291] | 1.58 [1.12–2.24] | ||
| Cancer characteristics | |||||
| Histology | 0.71 | – | |||
| Adenocarcinoma | 5.8 (3.2–9.2) | 1 | |||
| Other | 4.3 (2.9–12.7) | 1.10 [0.66–1.86] | |||
| Brain metastasis | 0.09 | 0.23 | |||
| No | 6.0 (3.7–10.1) | 1 | 1 | ||
| Yes | 5.0 (2.8–8.7) | 1.22 [0.97–1.54] | 1.16 [0.91–1.49] | ||
| Molecular status | 0.05 | 0.02 | |||
| Wild type | 6.5 (3.4–10.3) | 1 | 1 | ||
| KRAS mutation | 5.3 (2.8–8.6) | 1.62 [0.96–1.80] | 1.47 [1.12–1.94] | ||
| EGFR/ALK | 5.2 (2.9–8.8) | 1.34 [1.03–1.94] | 1.23 [0.90–1.69] | ||
| Anticancer treatment characteristics | |||||
| Line of treatment of the PB combination | 0.82 | 0.86 | |||
| Second line | 5.4 (2.6–9.9) | 1 | 1 | ||
| Third line | 5.8 (3.4–9.7) | 0.94 [0.69–1.29] | 1.15 [0.81–1.64] | ||
| Fourth line | 5.7 (2.9–8.7) | 1.10 [0.77–1.57] | 1.11 [0.77–1.61] | ||
| Fifth line or more | 6 (3.5–8.9) | 0.99 [0.69–1.42] | 1.03 [0.71–1.48] | ||
| Treatment previously received | |||||
| Taxane | 0.67 | – | |||
| No | 5.7 (3.1–8.8) | 1 | |||
| Yes | 5.5 (3.3–9.7) | 0.95 [0.74–1.21] | |||
| Bevacizumab | 0.53 | – | |||
| No | 5.8 (3.3–9.7) | 1 | |||
| Yes | 5.3 (2.7–8.7) | 1.084 [0.84–1.40] | |||
| ICI | 0.01 | 6 × 10−3 | |||
| No | 5.2 (2.8–8.8) | 1 | 1 | ||
| Yes | 7.0 (4.2–11.0) | 0.74 [0.56–0.94] | 0.65 [0.48–0.89] | ||
ALK, anaplastic lymphoma kinase; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; HR, hazard ratio; ICI, immune checkpoint inhibitors; IQR, interquartile range; PB, paclitaxel–bevacizumab; PFS, progression-free survival.
ICI+ subgroup is defined by patients treated with PB immediately after progression under immune checkpoint inhibitors (ICI) therapy.
ICI− subgroup is defined by patients treated with PB without previous ICI therapy just before.
Table 4.
Univariate and multivariate analysis of factors associated with OS from the initiation of paclitaxel–bevacizumab therapy.
| Median OS (IQR, 25–75%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| in months | HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Patients’ characteristics | |||||
| Sex | 0.50 | 0.47 | |||
| Male | 11.6 (5.5–19.5) | 1 | 1 | ||
| Female | 10.4 (5.3–20.1) | 0.92 [0.72–1.17] | 0.91 [0.70–1.18] | ||
| Age | 0.73 | 0.28 | |||
| <68 years | 11.1 (5.3–19.5) | 1 | 1 | ||
| ⩾68 years | 10.4 (5.5–21.1) | 1.05 [0.90–1.38] | 1.17 [0.88–1.57] | ||
| ECOG PS score | <10−4 | <10−4 | |||
| 0 | 13.0 (6.3–24.2) | 1 | 1 | ||
| 1 | 12.6 (6.6–23.2) | 1.17 [0.84–1.63] | 1.22 [0.87–1.71] | ||
| ⩾2 | 6.1 (2.8–15.0) | 2.14 [1.48–3.10] | |||
| Cancer characteristics | |||||
| Histology | 0.90 | – | |||
| Adenocarcinoma | 10.8 (5.3–19.5) | 1 | |||
| Other | 11.9 (5.5–24.3) | 0.96 [0.54–1.72] | |||
| Brain metastasis | 0.05 | 0.04 | |||
| No | 12.0 (5.5–22.6) | 1 | 1 | ||
| Yes | 9.7 (5.2–16.7) | 1.27 [1.00–1.62] | 1.31 [1.00–1.71] | ||
| Molecular status | 0.21 | 0.02 | |||
| Wild type | 11.6 (5.3–23.2) | 1 | 1 | ||
| KRAS mutation | 9.6 (5.3–15.0) | 1.28 [0.97–1.69] | 1.49 [1.11–1.98] | ||
| EGFR/ALK | 12.8 (5.6–19.5) | 1.11 [0.79–1.54] | 1.01 [0.74–1.42] | ||
| Anticancer treatment characteristics | |||||
| Line of treatment of the PB combination | 0.92 | 0.95 | |||
| Second line | 10.1 (4.5–21.5) | 1 | 1 | ||
| Third line | 11.8 (5.3–21.1) | 0.92 [0.66–1.28] | 1.11 [0.76–1.60] | ||
| Fourth line | 10.4 (4.9–17.8) | 1.02 [0.71–1.48] | 1.02 [0.69–1.50] | ||
| Fifth line or more | 10.4 (5.9–17.4) | 0.96 [0.66–1.40] | 1.07 [0.72–1.57] | ||
| Treatment previously received | |||||
| Taxane | 0.57 | – | |||
| No | 11.6 (5.5–21.3) | 1 | |||
| Yes | 10.3 (5.1–18.0) | 1.08 [0.83–1.40] | |||
| Bevacizumab | 0.55 | – | |||
| No | 10.6 (5.3–19.6) | 1 | |||
| Yes | 11.6 (5.9–20.2) | 0.92 [0.70–1.21] | |||
| ICI | 0.03 | 0.02 | |||
| No | 10.1 (4.9–18.3) | 1 | 1 | ||
| Yes | 12.8 (6.6–25.7) | 0.74 [0.56–0.97] | 0.68 [0.49–0.94] | ||
ALK, anaplastic lymphoma kinase; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; HR, hazard ratio; ICI, immune checkpoint inhibitors; IQR, interquartile range; OS, overall survival; PB, paclitaxel and bevacizumab.
ICI+ subgroup is defined by patients treated with PB immediately after progression under immune checkpoint inhibitors (ICI) therapy.
ICI− subgroup is defined by patients treated with PB without previous ICI therapy just before.
Duration of treatment and toxicity
A total of 257 (82%) patients experienced adverse events of all grades during the administration of PB. The most common adverse events were asthenia (53%), neurotoxicity (39%), gastrointestinal toxicity (21%), hematotoxicity (18%), proteinuria (17%), bleeding events (15%), hypertension (11%), and ungueal toxicity (9%) as shown in Table 5.
Table 5.
Tolerance of the paclitaxel–bevacizumab combination.
| Total patients (n = 314) | |
|---|---|
| Adverse events of any grade | 257 (82%) |
| Asthenia | 165 (53%) |
| Neurological disorders | 121 (39%) |
| Gastrointestinal disorders | 66 (21%) |
| Blood disorders | 55 (18%) |
| Proteinuria | 52 (17%) |
| Bleeding events | 48 (15%) |
| Hypertension | 33 (11%) |
| Ungueal disorders | 28 (9%) |
Qualitative variables are expressed as n (%).
One hundred and four patients (33%) discontinued the combination for monotherapy with either molecule from whom 77 patients (25%) due to toxicity: paclitaxel was discontinued in 30 (10%) patients due to neurological toxicity, bevacizumab in 11 (4%) patients due to toxicity, particularly hemorrhagic toxicity (Appendix 2).
No treatment-related deaths were observed.
Discussion
In this large real-world analysis of non-squamous advanced NSCLC patients treated with the PB combination, median PFS was 5.7 months and ORR was 40%. These results compare favorably with regimens of chemotherapy usually used following failure of platinum-based chemotherapy,3–6 in France being docetaxel or pemetrexed. The results of AVATAX are in line with those found in the phase-III ULTIMATE, 15 REVEL 10 and LUME-Lung 111 studies. However, in contrast to the above-mentioned studies, our population better reflects real-world practice, with 27% of patients with an ECOG PS score ⩾ 2, and 40% treated in fourth line or more. The reported toxicities are also very acceptable and consistent with the known safety profile of this combination, the majority of treatment discontinuation being related to fatigue or limiting neurotoxicity. Adverse events were, however, less frequent and severe than in the studies that evaluated the association docetaxel and nintedanib or ramucirumab in the same indication, which may be due to the retrospective design that may lead to underreporting of adverse events.11,12,24–32 Interestingly, we found that patients previously treated with bevacizumab still benefit from PB combination, in contrast with previous results of the ULTIMATE study. 15 However, bevacizumab tends to be less and less given in first-line regimens, as most chemo-immunotherapy regimens do not include it. Another interesting finding from our results is the effectiveness of the PB combination in patients with EGFR mutation.
Nowadays, immunotherapy can be used in all lines of treatment in advanced NSCLC and several retrospective studies reported an improved efficacy of rescue chemotherapy after failure of ICI.21–23 In published studies, the combination of docetaxel and an antiangiogenic agent as salvage treatment directly after ICI yielded an ORR of 18–60%, a DCR of 80–90%, a median PFS of 3.2–6.8 months and a median OS of 8.8–20.9 months.24–32 In line with these results, our study shows a longer PFS on PB for patients who previously received immunotherapy compared with those who did not.
The hypothesis of possible chemosensitization after prior exposure to immunotherapy is supported by synergistic activity in both preclinical and clinical models.33,34 Also, mechanisms positively enhancing the immunomodulatory effect of chemotherapy include the elimination of suppressor myeloid cells (MDSCs) and regulatory T cells (Tregs), increased antigen-specific immune response, improved antigen presentation, increased death receptors in tumor cells, and increased PD1–PDL1 expression.35,36 Finally, it has also been reported that the therapeutic level of anti-PD1/PDL1 immune checkpoint inhibitors was maintained during chemotherapy administration due to the long half-life of the antibody, 37 producing a residual effect on immune memory cells, which could be stimulated by successive chemotherapy with its immunological effects. Although the positive effects of PD1/PDL1 inhibitors can affect the initial response to salvage treatment, these effects are likely not persistent.
Parallel to all this, it is known that antiangiogenic agents stimulate the immune system. The VEGF plays a crucial role in modulating the tumor immune microenvironment with an immunosuppressive role. Inhibition of VEGF significantly increases infiltration of CD4-positive and CD8-positive T cells and reduces the number of Tregs and MDSCs in tumor tissue, facilitates dendritic cells maturation, and reduces tumoral expression of PDL1.38–40 Some recent studies indicated that ICI resistance could be alleviated with a combination of ICI with anti-angiogenesis treatment by reprograming the tumor immune microenvironment that was the cause of immunotherapy failure. Interestingly, Tozuka et al. 31 showed in patients with advanced NSCLC that the addition of ramucirumab enhanced docetaxel efficacy only in patients previously treated with ICI, with no difference in the case of absence of ICI pretreatment, suggesting a real synergy between antiangiogenic agents and immunotherapy.
With the growing role of immunotherapy and antiangiogenics agents from the first line in advanced NSCLC, one important question remains the optimal treatment sequencing strategies and the choice of the optimal treatment when initial therapy failed.
A growing body of evidence suggests the importance of the use of ICI and antiangiogenic therapy in the therapeutic pathway, leading to the best chance of survival. 41 Since the first current treatment line has changed compared with when studies such as ULTIMATE, LUME-Lung 1, and REVEL were designed, the place of the PB combination remains to be defined in the new treatment sequence used nowadays. To date, there have been no prospective, randomized controlled trials demonstrating the optimal treatment choice for patients with advanced NSCLC following failure of ICI treatment. Thus, analyses of real-world data from clinical practice are essential to help physicians’ choices. While pursuit or rechallenge with immunotherapy has not yet proved efficacy,19,20 our results suggest the efficacy of the PB combination following failure of immunotherapy.
Our study has several limitations. First, the design is retrospective, with possibly some underreporting of potential side effects and a lack of central review of the radiological response. Second, patients treated with PB in the ICI+ group were mostly treated in third-line therapy after immunotherapy failure (n = 62/88) and, therefore, may have a better clinical outcome compared with the ICI− group who received PB later (n = 101/226 treated with PB in fourth line or more). Our results with PB treatment should be interpreted with caution in patients treated in first line with immunotherapy alone as this population was not represented in our study. Fourth, we cannot exclude that some patients in the ICI+ group were treated with PB after ICI cessation for another reason that progression, for example due to toxicity, even if it represents a minority of the case. Finally, as the treatment sequence studied in our cohort was PB following ICI monotherapy, effectiveness after combination ICI and chemotherapy today use in first-line remains unclear.
However, these observations supported that immunotherapy followed by PB is a promising sequential strategy, and further studies are warranted to evaluate our observations. In the case of progression following ICI therapy or combination of ICI chemotherapy, patients should however be encouraged to participate in clinical trials evaluating novel agents.
Conclusion
PB combination in patients with advanced non-squamous NSCLC in second line and beyond was efficient and well-tolerated. Prospective trials are needed to address the question of the best chemotherapy association after first-line ICI combined or not with chemotherapy.
Appendix
Appendix 1.
Schedule of the paclitaxel–bevacizumab combination.
| Total patients (n = 314) | |
|---|---|
| Initial treatment dose of paclitaxel, MD = 17 | |
| 90 mg/m2, D1 D8 D15, D1 = D28 | 150 (51%) |
| 80 mg/m2, D1 D8 D15, D1 = D21 | 108 (36%) |
| 90 mg/m2, D1 D8, D1 = D21 | 39 (13%) |
| Initial treatment dose of bevacizumab, MD = 31 | |
| 10 mg/kg, D1 D15, D1 = D28 | 212 (75%) |
| 15 mg/kg, D1 D21, D1 = D21 | 65 (23%) |
| 7.5 mg/kg, D1 D21, D1 = D21 | 6 (2%) |
MD, missing data.
Appendix 2.
Tolerance of the paclitaxel-bevacizumab combination.
| Total patients (n = 314) | |
|---|---|
| Administration of PB | |
| Duration of paclitaxel (in days) | 105 (63–161) |
| Duration of bevacizumab (in days) | 127 (63–245) |
| Discontinuation of one of the drugs (MD = 4) | 104 (34%) |
| Reason of discontinuation of one of the drugs (n = 104) | |
| Neuropathy | 30 (29%) |
| Asthenia | 17 (16%) |
| Bleeding | 7 (7%) |
| Hematological reason | 5 (5%) |
| Other | 45 (43%) |
| Reason of final discontinuation (n = 311) | |
| Progression | 230 (74%) |
| Toxicity | 34 (11%) |
| Therapeutic window | 23 (7%) |
| Death | 13 (4%) |
| Other | 11 (4%) |
MD, missing data; PB, paclitaxel and bevacizumab.
Qualitative variables are expressed as n (%) and quantitative variables as median (IQR: 25–75%).
Other included: bevacizumab maintenance (n = 19), cessation of bevacizumab due to radiotherapy (n = 5), ungueal disorder (n = 4), gastrointestinal disorder (n = 2), hypertension (n = 2), cessation of bevacizumab due to excavation of pulmonary lesion (n = 2), wound healing problem (n = 2), infection (n = 2), oedematous syndrome (n = 1), pulmonary embolism (n = 1), discovery of another cancer (n = 1), pneumothorax (n = 1), proteinuria (n = 1), MD = 2.
Flow chart of the study population.
Footnotes
Ethics approval and consent to participate: Ethical approval was obtained on 9 October 2019 (Observational Research Protocol Review Committee) of the French Society of Pneumology (SPLF), [Institutional Review Board (IRB) CEPRO 2019-033]. An information letter was sent to each living patient providing him the opportunity to refuse study participation, as per French law.
Author contribution(s): Geoffroy Bilger: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Anne-Claire Toffart: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Supervision; Validation; Visualization; Writing – review & editing.
Marie Darrason: Data curation; Formal analysis; Investigation; Writing – review & editing.
Michaël Duruisseaux: Data curation; Investigation; Writing – review & editing.
Lucie Ulmer: Data curation; Investigation; Writing – review & editing.
Pascal Wang: Data curation; Investigation; Writing – review & editing.
Etienne Giroux Leprieur: Data curation; Investigation; Project administration; Supervision; Writing – review & editing.
Nicolas Girard: Data curation; Investigation; Project administration; Supervision; Writing – review & editing.
Marie Ange Massiani: Data curation; Investigation; Writing – review & editing.
Paul Bore: Data curation; Formal analysis; Investigation; Writing – review & editing.
Renaud Descourt: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Writing – review & editing.
Julian Pinsolle: Data curation; Investigation; Writing – review & editing.
Solene Valery: Data curation; Formal analysis; Investigation; Writing – review & editing.
Isabelle Monnet: Data curation; Formal analysis; Investigation; Writing – review & editing.
Aurélie Swalduz: Data curation; Investigation; Writing – review & editing.
Claire Tissot: Data curation; Investigation; Writing – review & editing.
Pierre Fournel: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – review & editing.
Anne Baranzelli: Data curation; Investigation; Writing – review & editing.
Alexis B. Cortot: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – review & editing.
Chantal Decroisette: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft.
ORCID iD: Geoffroy Bilger  https://orcid.org/0000-0001-6811-0941
https://orcid.org/0000-0001-6811-0941
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Geoffroy Bilger, Centre Hospitalier Universitaire de Grenoble, 38700 Grenoble, France. Oncology, Grenoble University Hospital, Grenoble, France.
Anne-Claire Toffart, Centre Hospitalier Universitaire de Grenoble, Grenoble, FranceOncology, Grenoble University Hospital, Grenoble, France.
Marie Darrason, Service de Pneumologie Aigue Spécialisée et Cancérologie Thoracique, Hôpital Lyon-Sud, CHU Lyon, Pierre-Bénite, France; Department of Pneumology and Thoracic Oncology, University Hospital of Lyon, Pierre-Bénite, France; Institut de Recherches Philosophiques de Lyon, Université Lyon 3, Lyon, France; Lyon Institute of Philosophical Research, Lyon 3 University, Lyon, France.
Michaël Duruisseaux, URCOT, Institut de Cancérologie des Hospices Civils de Lyon, Lyon, France.
Lucie Ulmer, Thoracic Oncology Department, Hospital Albert Calmette, Lille, France.
Pascal Wang, Thoracic Oncology, APHP, Paris, France.
Etienne Giroux Leprieur, APHP Hopital Ambroise Pare and Universite Paris-Saclay, Boulogne-Billancourt, France.
Nicolas Girard, Institut Curie, Paris, France.
Marie Ange Massiani, Oncology Hospital Rene Huguenin, Saint-Cloud, France.
Paul Bore, Thoracic Oncology Department, Hospital Morvan, Brest, France.
Renaud Descourt, Thoracic Oncology Department, Hospital Morvan, Brest, France.
Julian Pinsolle, Unité de pneumologie, Centre Hospitalier Métropole Savoie, Chambéry, France.
Solene Valery, Thoracic Oncology, APHP, Paris, France.
Isabelle Monnet, Pneumology Department, CHI Creteil, Creteil, France.
Aurélie Swalduz, Department of Thoracic Oncology, Centre Léon Bérard, Lyon, France.
Claire Tissot, Pneumology Department, Institut de Cancérologie de la Loire Lucien Neuwirth, Saint-Priest-en-Jarez, France.
Pierre Fournel, Service d’Oncologie Médicale, Institut de Cancérologie, CHU de Saint-Etienne, Saint-Etienne Cedex 2, France.
Anne Baranzelli, Unité de pneumologie, Centre Hospitalier Métropole Savoie, Chambéry, France.
Alexis B. Cortot, Thoracic Oncology Department, Hospital Albert Calmette, Lille, France
Chantal Decroisette, CH Annecy Genevois, Epagny Metz-Tessy, France.
References
- 1. de Groot PM, Wu CC, Carter BW, et al. The epidemiology of lung cancer. Transl Lung Cancer Res 2018; 7: 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies J, Patel M, Gridelli C, et al. Real-world treatment patterns for patients receiving second-line and third-line treatment for advanced non-small cell lung cancer: a systematic review of recently published studies. PLoS ONE 2017; 12: e0175679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crino `L, Mosconi AM, Scagliotti G, et al. Gemcitabine as second-line treatment for advanced non-small cell lung cancer: a phase II trial. J Clin Oncol 1999; 17: 2081–2085. [DOI] [PubMed] [Google Scholar]
- 4. Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000; 18: 2095–2103. [DOI] [PubMed] [Google Scholar]
- 5. Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 6. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008; 372: 1809–1818. [DOI] [PubMed] [Google Scholar]
- 7. Herbst RS, O’Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007; 25: 4743–4750. [DOI] [PubMed] [Google Scholar]
- 8. Adjei AA, Mandrekar SJ, Dy GK, et al. Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small cell lung cancer: NCCTG and SWOG study N0426. Clin Oncol 2010; 28: 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu B, Zhou X, Liu Y, et al. Comparison of chemotherapy plus bevacizumab vs. chemotherapy alone as third-line treatment or beyond for advanced non-small cell lung cancer: a propensity score-matched analysis. Oncol Lett 2018; 15: 5671–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garon EB, Ciuleanu T-E, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014; 384: 665–673. [DOI] [PubMed] [Google Scholar]
- 11. Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014; 15: 143–155. [DOI] [PubMed] [Google Scholar]
- 12. Le Moulec S, Hadoux J, Gontier E, et al. Combination of paclitaxel and bevacizumab in heavily pre-treated non-small cell lung cancer (NSCLC) patients: a case series study on 15 patients. Bull Cancer 2013; 100: 30–37. [DOI] [PubMed] [Google Scholar]
- 13. Habib S, Delourme J, Dhalluin X, et al. Bevacizumab and weekly paclitaxel for non-squamous non-small cell lung cancer patients: a retrospective study. Lung Cancer 2013; 80: 197–202. [DOI] [PubMed] [Google Scholar]
- 14. Zarza V, Couraud S, Bosc C, et al. Paclitaxel-bevacizumab is a possible alternative as salvage chemotherapy in advanced non-small cell bronchial carcinoma. Rev Mal Respir 2014; 31: 601–607. [DOI] [PubMed] [Google Scholar]
- 15. Cortot AB, Audigier Valette C, Molinier O, et al. Weekly paclitaxel plus bevacizumab versus docetaxel as second- or third-line treatment in advanced non-squamous non-small-cell lung cancer: results of the IFCT-1103 ULTIMATE study. Eur J Cancer 2020; 131: 27–36. [DOI] [PubMed] [Google Scholar]
- 16. Référentiels Auvergne Rhône-Alpes en oncologie thoracique. 12th ed. 2016. http://referentiels-aristot.com/wp-content/uploads/1_CBNPC_2022b_V2DEF.pdf
- 17. Kim J, Cho J, Lee MH, et al. Relative efficacy of checkpoint inhibitors for advanced NSCLC according to programmed death-ligand-1 expression: a systematic review and network meta-analysis. Sci Rep 2018; 8: 11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dafni U, Tsourti Z, Vervita K, et al. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 2019; 134: 127–140. [DOI] [PubMed] [Google Scholar]
- 19. Giaj Levra M, Cotté FE, Corre R, et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: a national data base analysis. Lung Cancer 2020; 140: 99–106. [DOI] [PubMed] [Google Scholar]
- 20. Gobbini E, Toffart AC, Pérol M, et al. Immune checkpoint inhibitors rechallenge efficacy in non–small-cell lung cancer patients. Clin Lung Cancer 2020; 21: e497–e510. [DOI] [PubMed] [Google Scholar]
- 21. Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 2017; 112: 90–95. [DOI] [PubMed] [Google Scholar]
- 22. Park SE, Lee SH, Ahn JS, et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol 2018; 13: 106–111. [DOI] [PubMed] [Google Scholar]
- 23. Costantini A, Corny J, Fallet V, et al. Efficacy of next treatment received after nivolumab progression in patients with advanced nonsmall cell lung cancer. ERJ Open Res 2018; 4: 00120-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grohe C, Blau W, Gleiber W, et al. Nintedanib + docetaxel in lung adenocarcinoma patients (pts) following treatment with immune checkpoint inhibitors (ICIs): updated efficacy and safety results of the ongoing non- interventional study (NIS) VARGADO (NCT02392455). J Clin Oncol 2020; 38(Suppl. 15): 9604. [Google Scholar]
- 25. Brueckl WM, Reck M, Rittmeyer A, et al. Efficacy of docetaxel plus ramucirumab as palliative third-line therapy following second-line immune-checkpoint-inhibitor treatment in patients with non-small-cell lung cancer stage IV. Clin Med Insights Oncol 2020; 14: 1179554920951358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corral J, Majem M, Rodríguez-Abreu D, et al. Efficacy of nintedanib and docetaxel in patients with advanced lung adenocarcinoma treated with first-line chemotherapy and second-line immunotherapy in the nintedanib NPU program. Clin Transl Oncol 2019; 21: 1270–1279. [DOI] [PubMed] [Google Scholar]
- 27. Reck M, Syrigos K, Miliauskas S, et al. Non-interventional LUME-BioNIS study of nintedanib plus docetaxel after chemotherapy in adenocarcinoma non-small cell lung cancer: a subgroup analysis in patients with prior immunotherapy. Lung Cancer 2020; 148: 159–165. [DOI] [PubMed] [Google Scholar]
- 28. Shiono A, Kaira K, Mouri A, et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer 2019; 10: 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harada D, Takata K, Mori S, et al. Previous immune checkpoint inhibitor treatment to increase the efficacy of docetaxel and ramucirumab combination chemotherapy. Anticancer Res 2019; 39: 4987–4993. [DOI] [PubMed] [Google Scholar]
- 30. Yoshimura A, Yamada T, Okuma Y, et al. Retrospective analysis of docetaxel in combination with ramucirumab for previously treated non-small cell lung cancer patients. Transl Lung Cancer Res 2019; 8: 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tozuka T, Kitazono S, Sakamoto H, et al. Addition of ramucirumab enhances docetaxel efficacy in patients who had received anti-PD-1/PD-L1 treatment. Lung Cancer 2020; 144: 71–75. [DOI] [PubMed] [Google Scholar]
- 32. Gawri K, Dawar R, Rodriguez E, et al. Improved outcomes with ramucirumab & docetaxel in metastatic non- small cell lung cancer after failure of immunotherapy. In: 2020 World conference on lung cancer, Singapore, 28–31 January 2021, poster P01.09. [Google Scholar]
- 33. Ramakrishnan R, Antonia S, Gabrilovich DI. Combined modality immunotherapy and chemotherapy: a new perspective. Cancer Immunol Immunother 2008; 57: 1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Radfar S, Wang Y, Khong HT. Activated CD4+ T cells dramatically enhance chemotherapeutic tumor responses in vitro and in vivo. J Immunol 2009; 183: 6800–6807. [DOI] [PubMed] [Google Scholar]
- 35. Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother 2013; 62: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 2015; 3: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li B, Lalani AS, Harding TC, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res 2006; 12: 6808–6816. [DOI] [PubMed] [Google Scholar]
- 39. Shrimali RK, Yu Z, Theoret MR, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010; 70: 6171–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018; 15: 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Molife C, Hess LM, Lin Ciu Z, et al. Sequential therapy with ramucirumab and/or checkpoint inhibitors for non-small-cell lung cancer in routine practice. Future Oncol 2019; 15: 2915–2931. [DOI] [PubMed] [Google Scholar]