Abstract
At Chocolate Pots Hot Springs in Yellowstone National Park the source waters have a pH near neutral, contain high concentrations of reduced iron, and lack sulfide. An iron formation that is associated with cyanobacterial mats is actively deposited. The uptake of [14C]bicarbonate was used to assess the impact of ferrous iron on photosynthesis in this environment. Photoautotrophy in some of the mats was stimulated by ferrous iron (1.0 mM). Microelectrodes were used to determine the impact of photosynthetic activity on the oxygen content and the pH in the mat and sediment microenvironments. Photosynthesis increased the oxygen concentration to 200% of air saturation levels in the top millimeter of the mats. The oxygen concentration decreased with depth and in the dark. Light-dependent increases in pH were observed. The penetration of light in the mats and in the sediments was determined. Visible radiation was rapidly attenuated in the top 2 mm of the iron-rich mats. Near-infrared radiation penetrated deeper. Iron was totally oxidized in the top few millimeters, but reduced iron was detected at greater depths. By increasing the pH and the oxygen concentration in the surface sediments, the cyanobacteria could potentially increase the rate of iron oxidation in situ. This high-iron-content hot spring provides a suitable model for studying the interactions of microbial photosynthesis and iron deposition and the role of photosynthesis in microbial iron cycling. This model may help clarify the potential role of photosynthesis in the deposition of Precambrian banded iron formations.
Phototrophic mats at Chocolate Pots Hot Springs in Yellowstone National Park form an important boundary layer at the interface of the iron sediment surface and the flowing spring water, which contains about 100 μM ferrous iron at the source (55). We analyzed the structure of these mats and identified four different types of mats composed predominantly of cyanobacteria, although the anoxygenic phototroph Chloroflexus sp. appeared to be an important constituent of the mats at the highest temperature (55). The major pigments in most of the mats are chlorophyll a, phycocyanin, and carotenoids. Bacteriochlorophyll a is present in some sediments, and both bacteriochlorophyll a and bacteriochlorophyll c are present in Synechococcus mats that contain Chloroflexus sp. (55). A colorful iron formation is being deposited at these hot springs (1). The microbial mats, which are composed chiefly of filamentous gliding phototrophs, may play a substantial role in stabilizing oxidized iron, thereby enhancing accumulation of soft sediments that can be compacted to form the iron deposits (55).
The evolution of photosynthesis in the Precambrian is tightly linked to major changes in the oxidation states of the atmosphere, hydrosphere, and lithosphere. The increase in the atmospheric oxygen content around 2,000 million years ago was probably due to the widespread development of oxygenic photosynthetic cyanobacteria (33, 60). Production of oxygen as a photosynthetic waste product resulting from the oxidation of water is a physiological capability that must have arisen in the cyanobacterial ancestors much earlier than this and perhaps occurred as early as 3,500 million years ago (60). Ancient Archean banded iron formations (BIFs) containing various amounts of oxidized iron have been used as evidence that early localized production of oxygen by cyanobacterial photosynthesis occurred (7, 8, 10).
Other workers have proposed that the activity of anoxygenic phototrophs capable of directly oxidizing ferrous iron may account for the relatively low levels of oxidized iron in BIFs without invoking the presence of oxygen (19, 20, 30, 36, 47, 50, 53, 66, 68). Cohen has suggested (12, 13) that cyanobacteria may also be capable of directly oxidizing iron without producing oxygen. Pierson and Olson (50) have postulated that iron-dependent photosynthesis may have been an important step in the evolution of oxygenic photosynthesis in ancestral cyanobacteria. The various redox potentials of reduced iron which are influenced by environmental pH and the presence of complexing ions (15, 25) make reduced iron a potential electron donor for photosystem I (PSI) and PSII types of reaction centers (RC1 and RC2, respectively). The widespread abundance of reduced iron on the early Earth prior to the appearance of oxygen would have made it particularly suitable as an electron donor for photosynthesis. Pierson et al. (52) have also pointed out that the oxidized iron products of this type of photosynthesis could have provided substantial protection from UV radiation for surface-dwelling phototrophs prior to the development of an ozone shield.
Chocolate Pots Hot Springs is a hydrothermal system in which we are investigating the interaction of phototrophs with reduced and oxidized iron in a setting in which an iron formation is actively being deposited. While it is not representative of the deep-ocean models of Precambrian iron deposition (34), the Chocolate Pots iron formation provides a suitable system in which to study the potential impact of photosynthesis on iron oxidation and mineralization processes and to explore hypotheses regarding the association of phototrophic prokaryotes with iron. The association of well-developed contemporary microbial mats with an iron-depositing environment is a model that has been missing for the microfossiliferous stromatolites of the Gunflint Iron Formation (9, 10, 35). Chocolate Pots Hot Springs provides such a model.
At first, a shallow subaerial hot spring in a contemporary oxic atmosphere may not seem to be a suitable model for studying the mechanisms of iron oxidation in the anoxic Precambrian world. One might suppose that all of the reduced iron in the source waters is immediately oxidized by the abundant oxygen in the atmosphere, leaving a ferrous iron-depleted system in which to try to study microbial processes. Chemical oxidation rates are relatively low compared to enzymatically based metabolism, however, (21, 22, 31, 32), and such rates are greatly influenced by other factors, such as pH (25, 26, 37). The water flows quickly down steep slopes at Chocolate Pots, and much reduced iron is still in solution at the base of the thermal gradient (see Table 1). Water also flows through microchannels or interstitial voids within biofilms, not just over the surface (38). It is known that even in very active oxygenic cyanobacterial mats the oxygen is depleted within a few millimeters or less of the surface (6, 17, 57, 58, 64). Furthermore, in the absence of oxygen, anaerobic microbial activity can reduce Fe(III) to Fe(II) (18, 39, 44, 45, 59, 65). Consequently, just beneath the surface of the microbial mat-water interface, the environment can be quite different, anoxic, and rich in Fe(II). The processes that occur in this area may well represent processes that occurred on a more extensive scale in an ancient anoxic world.
TABLE 1.
Water chemistry of Chocolate Pots Hot Springs
| Location along drainway | Time of day | Fe(II) concn (mg/liter) | O2 concn (mg/liter) | pH | Temp (°C) |
|---|---|---|---|---|---|
| Top | Early morning | 9.5 | 1.2 | 5.8 | 51.8 |
| Late afternoon | 8.2 | 1.0 | 6.0 | 51.5 | |
| Middle | Early morning | 3.4 | 5.2 | 6.3 | 39.8 |
| Late afternoon | 4.7 | 4.6 | 7.0 | 40.7 | |
| Bottom | Early morning | 2.3 | 5.8 | 6.3 | 37.3 |
| Late afternoon | 4.0 | 6.0 | 7.0 | 37.2 |
In a related study we characterized the specific phototrophs, the mat structure, and the physical associations of the organisms with the accumulating iron deposit (55). We found that filamentous phototrophs are intimately associated with the iron mineral grains and that the motility and orientation of the filaments may be important for trapping and stabilizing the sediments to produce the iron formation. We found that these characteristics are most evident in an olive mat consisting of a narrow Oscillatoria sp., which is the major mat that dominates the iron formation studied (55). Mats dominated by Oscillatoria cf. princeps are much more limited in distribution. At the highest hot spring temperatures (50 to 54°C) the mats were composed primarily of Pseudanabaena sp. in some places and of Synechococcus and Chloroflexus spp. in other places.
In this study, our overall objective was to assess the potential impact of microbial photosynthesis on the deposition of an iron formation. We investigated the photosynthetic activity in microbial mats by using microelectrodes in order to measure the potential impact of this activity on oxygen and pH, which can influence iron transformations in mats. We also investigated the impact of iron on the physiology of photosynthesis and the impact of iron deposition on the availability of light in the photosynthetic microenvironment. Although we conducted experiments with all four types of photosynthetic mats present, as well as with non-mat-covered sediments at Chocolate Pots, our emphasis was on the most pervasive olive cyanobacterial mat.
MATERIALS AND METHODS
Microelectrode studies.
Microelectrode profiles were determined in situ and with excised mat samples. Because of the steep and rocky nature of the site, it was very difficult to obtain the in situ profiles. Therefore, most of the profiles were determined with excised mat samples within 5 h of collection from the hot spring. Mat samples were maintained at the in situ temperatures in a water bath while electrode profiles were obtained. The entire apparatus was placed under a grounded Faraday cage in order to reduce electrical noise and to regulate the light intensity. The average light intensity under the cage for most profiles was 600 to 700 W m−2. Neutral-density screens were placed on the Faraday cage in order to reduce the light intensity when necessary. Dark conditions were produced by draping black cloths over the cage. The irradiance in the darkened cage was 0 to 4 W m−2. Mats were preincubated in spring water in the light or in the dark for 1 to 2 h at the in situ temperatures before electrode measurements were obtained. All profiles were completed in 20 min. Most mat samples were 2 to 4 mm thick. The thinner mats were supported on an agar base when their profiles were obtained. Light intensities were measured with a pyranometer sensor (model LI-185B; LI-COR, Lincoln, Nebr.), and temperatures within the mats were measured with a model 52 K/J thermometer and bead probe (Fluke, Everett, Wash.).
A needle-encased oxygen microelectrode (model 760; Diamond General, Ann Arbor, Mich.) was used with a reference electrode (model 334; Diamond General) and a polarizing voltage of −0.75 V. Current was measured with an autoranging picoammeter (model 485; Keithly, Cleveland, Ohio). Air saturation values for oxygen were determined by using compressed air to saturate spring water at the recording temperature. Zero oxygen was measured by using zero pO2 ampoules (type S4150; Radiometer, Copenhagen, Denmark).
A pH microelectrode (model 818; Diamond General) was connected to a battery-operated portable pH meter (Jenco Instruments, San Diego, Calif.).
During the light intensity shift experiments, oxygen readings were recorded every 30 s for about 5 min at each intensity until they began to level off. When the light intensity reached zero, the mat was kept in the dark for 10 min and allowed to stabilize before the light intensity was increased again.
The effect of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) (DuPont, Wilmington, Del.) on the olive mat was determined by first recording light and dark profiles without DCMU and then flooding the mat with DCMU diluted with source water (final DCMU concentration, 50 μM). A concentration that was 10 times the normal concentration was used because of the diffusional resistance of the intact mat. The mat was incubated in the presence of DCMU for 1.75 h before dark and light treatments were repeated.
Water chemistry.
The concentration of Fe(II) in the spring water was measured by the ferrozine assay (21, 62). A 0.01-ml sample removed directly from the spring was immediately added to 2.49 ml of a ferrozine solution (0.01% ferrozine in 50 mM HEPES) (Sigma Chemical Co. St. Louis, Mo.), and the preparation was mixed and returned to the lab, where the absorbance at 562 nm was read within 2 h.
Oxygen levels in the flowing water were measured by using a model 51B oxygen meter and a model 5739 probe (Yellow Springs Instrument Co., Yellow Springs, Ohio) with direct temperature, altitude, and salinity compensation. The pH was measured by using ColorpHast pH papers 4.0–7.0 and 6.5–10.0 (EM Science, Gibbstown, N.J.) and a pH meter and probe (model 6009; Jenco, San Diego, Calif.). Temperatures were measured with the model 52 K/J thermometer and a bead probe (Fluke).
14C uptake experiments.
All [14C]bicarbonate uptake experiments were performed in the lab by using excised mats as previously described (54). The mats were examined microscopically to ensure that one type of cyanobacterium was dominant, and an even cell suspension was prepared by using a glass tissue homogenizer and source water. 14C-labeled NaHCO3 was added to a final concentration of 0.2 μCi/ml. DCMU was added to a final concentration of 5 μM when necessary. A stock solution of ferrous chloride was prepared and added anoxically to final concentrations of 0.5, 1.0, 2.5, 5, 10, 25, and 50 mM. All solutions and vials were sparged with argon to prevent oxidation of Fe(II). All preparations were incubated in 2-ml vials filled to the neck.
The vials were incubated horizontally to maximize light exposure in petri dishes blackened on the sides and bottoms, and layers of neutral-density screen were used to vary the light intensity as described previously (54). Dark controls were incubated in totally blackened dishes. Samples were incubated in a water bath at the temperature of the original mat. For most experiments the light intensity was 150 to 300 W m−2. Photosynthesis was stopped by adding 0.1 ml of formalin, the samples were filtered onto membrane filters (diameter, 25 mm; pore size, 0.45 μm; Gelman Metricel, Ann Arbor, Mich.), and the radioactivity was counted by using Ultima Gold LSC cocktail (Packard, Meriden, Conn.) as previously described (54).
Spectral irradiance.
Light penetration through the microbial mats was measured with a model LI-1800 spectroradiometer (LI-COR) by layering the mats on a remote cosine receptor (model 1800-11) (51). All measurements were made in June and July in full sun at an elevation of 2,100 m.
Quantitative pigment analysis.
Chlorophyll concentrations in the suspensions during the 14C uptake studies were measured as previously described (49, 54, 55). Spectra were recorded with a model UV-1601 spectrophotometer (Shimadzu, Columbia, Md.).
Iron distribution in mats.
Ferrous iron contents were measured by using the ferrozine assay. Total iron contents were determined by reducing samples with 0.25 M hydroxylamine in 0.25 M HCl before ferrous iron contents were measured by the ferrozine assay as described previously (21, 62). The ferric iron content was calculated by subtracting the ferrous iron content from the total iron content.
RESULTS
Impact of cyanobacteria on water chemistry.
Measurements were obtained at the following three sites along the hot spring outflow: the source, midway down the channel (about 7 m from the source), and at the end of the drainway where the spring flowed into a river (about 15 m from the source). The concentration of Fe(II) in the water in the main drainway of the hot spring decreased from the source down the outflow channel, while the concentration of oxygen in the water and the pH increased (Table 1). The oxygen content reached saturation values before the drainway ended, but considerable Fe(II) still remained in solution. Measurements obtained in the early morning were compared with measurements obtained late in the afternoon. We observed few changes in the oxygen and Fe(II) contents of the flowing water that could be attributed to the biological activity of the mat phototrophs (Table 1). The pH downstream from the source was higher in the late afternoon than in the early morning.
Impact of cyanobacterial photosynthesis on the iron sediment microenvironment.
The cyanobacteria in Chocolate Pots Hot Springs formed conspicuous green mats among the iron sediments. The surface millimeter of the mats contained the densest accumulations of cyanobacteria, which were closely associated with granules of iron oxides. Beneath the surface layer, the number of phototrophs decreased, and conspicuous orange iron oxide deposits were present. The sediments were often sufficiently consolidated by the microbial masses to form a cohesive layered mat several millimeters thick (55).
When we assessed the potential impact of the phototrophs on the oxygen levels in the sediment environment by using needle-encased microelectrodes, we found that photosynthetic activity substantially altered the prevailing conditions in the sediments. During four field seasons (1995 through 1998) we obtained more than 100 depth profiles for oxygen and pH by using more than 50 excised or in situ mat and sediment samples. Although lateral heterogeneity was observed within mats and there were variations among the different types of mats and sediments, the depth profiles were fundamentally similar and maximum oxygen contents usually occurred in the top millimeter (Fig. 1A through C).
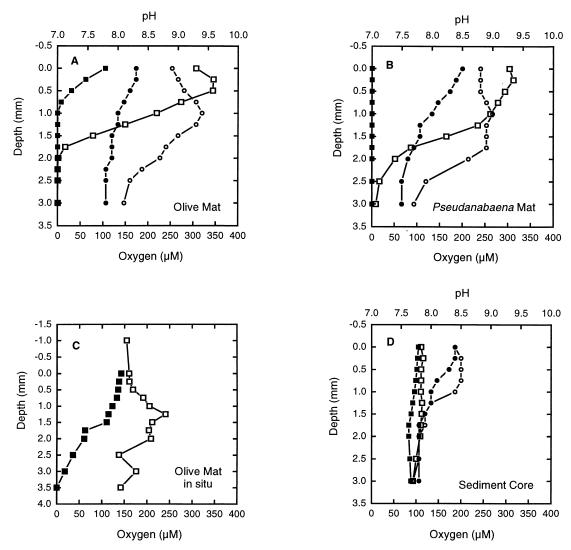
FIG. 1.
Depth profiles for oxygen (squares) and pH (circles), as determined with needle-encased microelectrodes in mat and sediment samples. Open symbols, data obtained in the light; solid symbols, data obtained in the dark. (A) Olive mat incubated at 36°C and 690 W m−2. (B) Pseudanabaena mat incubated at 42°C and 660 W m−2. (C) In situ depth profile for oxygen in an olive mat under 1.0 cm of flowing spring water at 43.9°C. The incident light intensity was 910 W m−2, and the pH of the water above the mat was 8.0. (D) Sediment core with no mat incubated at 33°C and 690 W m−2.
In the light, the oxygen concentrations in the top millimeter of cyanobacterial mats were usually greater than the air saturation value (approximately 150 μM at an elevation of 1,950 to 2,100 m and 37 to 47°C). The maximum oxygen concentrations in the light most often occurred between the mat surface and a depth of 500 μm and ranged from 150 to more than 300 μM. The oxygen levels in the light decreased with depth, approaching zero at depths between 1.0 and 2.0 mm in most cases (Fig. 1A and B). In some excised mat samples (data not shown) and in an in situ sample (Fig. 1C), the maximum oxygen concentration regions in the light were broader and occurred as deep as 1.0 to 2.0 mm. These deep oxygen concentrations decreased to zero at depths near 3 mm or persisted at near-saturation levels to the base of the mat (Fig. 1C). In all of the mats which we assayed, the oxygen concentrations were substantially greater in the light and nearly always were greater than the concentrations obtained with air saturation alone. The oxygen levels in the Synechococcus-Chloroflexus mat were lower (data not shown).
In the dark, the maximum oxygen concentrations occurred at the mat surface and were less than the maximum oxygen concentrations in the light (Fig. 1A through C). The surface concentrations were usually less than the saturation concentration and often were less than 100 μM (65% saturated); the concentration usually decreased to zero at depths of 2.0 mm or less. However, a considerable range of oxygen concentrations was observed in the dark when several different mat samples were examined (Fig. 1A through C). In some of the mats, oxygen persisted with depth at levels well below the saturation level, and the oxygen concentration was zero at a depth of 3.0 mm or more (Fig. 1C). In other mats (Fig. 1B) anoxia was much more pervasive, and occasionally the surface was anoxic in the dark.
The oxygen levels in the light in the in situ olive mat in flowing water (Fig. 1C) were similar to the light-enhanced oxygen levels in excised mats (Fig. 1A and B). As in most of the mats which we studied, the levels of oxygen measured in the light in situ were greater than the air saturation value in the flowing water above the mat (150 μM). Although the broad maximum oxygen concentration (200 to 250 μM) occurred deeper in this mat (at depths of 0.5 to 2.5 mm) than in most of the excised mat samples (Fig. 1A and B), it was still within the range of values obtained for other excised mats. High oxygen levels were observed throughout this mat in the light, and the level at a depth of 3.5 mm was the air saturation level (150 μM). Similar persistence of oxygen to the base of the mat was observed with some excised olive mat samples (Fig. 2A). When the mat was darkened in situ, the oxygen level at the surface remained near the air saturation values, but the oxygen level decreased fairly steadily with increasing depth. The mat was anoxic in the dark at a depth of 3.0 to 3.5 mm. The light intensities in situ (900 to 1,100 W m−2) were much higher than the light intensities under the Faraday cage (700 W m−2), and these higher light intensities resulted in photoinhibition near the surface of the mat. After complete profiles were obtained, the electrode was positioned at a fixed depth of 0.25 mm in the mat (Fig. 1C). When the surface light intensity was 910 W m−2, the oxygen concentration was 161 μM. When the surface light intensity was decreased to 540 W m−2, the oxygen concentration increased to 202 μM.
FIG. 2.
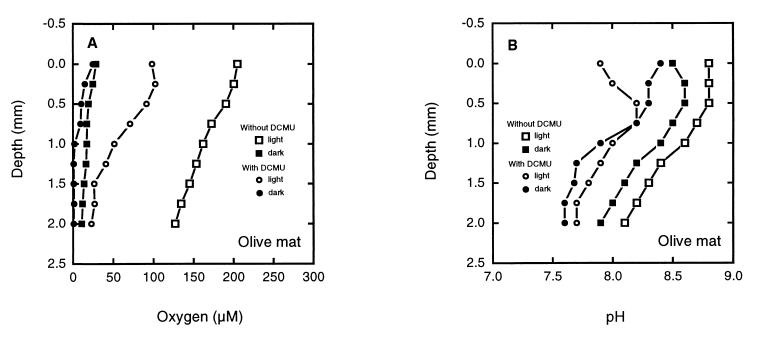
Effects of DCMU on photosynthesis in an olive mat. Oxygen concentration (A) and pH (B) profiles were obtained for an olive mat sample at 35°C in the dark and in the light (600 to 690 W m−2). The mat was then immersed in DCMU (50 μM) and was preincubated for 1.75 h before additional profiles were obtained in the presence of DCMU.
The pH values in the excised cyanobacterium-iron mats were also affected by photosynthetic activity. The pH values at all depths were usually between 7.5 and 8.5 in the dark and sometimes were as high as 8.5 or higher in the light (Fig. 1A and B). The Synechococcus mat (data not shown) had lower pH values than the olive and Pseudanabaena mats (Fig. 1A and B). Although the trends were similar to the oxygen trends, the changes in pH were not as consistent, as dramatic, or as rapidly detected as the changes in oxygen concentration. The maximum pH ranges were usually broader than the maximum oxygen concentration ranges (Fig. 1A and B). Although we were not able to obtain a pH depth profile in situ, we did obtain several measurements in various olive mats at depths between the surface and 1.0 mm in situ in the light and found that the pH values were 8.0 to 8.5.
The pH and oxygen depth profiles obtained for excised iron sediment samples devoid of green cyanobacterial mats were quite variable. They consistently lacked the dramatic light-induced changes in oxygen concentration seen in the photosynthetic mats (Fig. 1D). In both the light and the dark, the oxygen concentration was around 100 μM (65% saturated) from the surface down to a depth of 3.0 mm. In this sediment profile (Fig. 1D), the pH increased in the presence of light at depths ranging from 0.25 to 1.25 mm. Similar increases were not observed in other nonmat sediment profiles.
Profiles for oxygen concentration and pH were obtained in the light and in the dark for an olive mat sample (consisting primarily of narrow Oscillatoria filaments) with and without DCMU (50 μM). DCMU substantially decreased the light-dependent oxygen levels and pH values in the mat (Fig. 2A and B). The maximum oxygen concentration in the light without DCMU was 200 μM at the surface, and the oxygen concentration decreased to 120 μM at a depth of 2.0 mm. In the presence of DCMU, the oxygen concentration at the mat surface was 100 μM, and the oxygen concentration at a depth of 2.0 mm was 25 μM. In the dark, the oxygen concentrations were similar (ca. 25 μM) with and without DCMU at the surface and decreased slightly with depth. DCMU reduced the surface pH value from 8.75 to nearly 8.0 in the light and had less effect on pH in the dark.
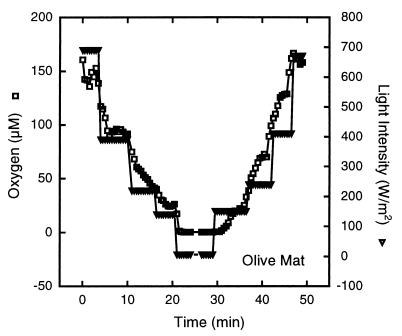
When the olive mat was subjected to sudden changes in light intensity or was shifted to or from dark conditions, the oxygen levels in the mat responded rapidly. The time courses for the changes in oxygen concentration in response to both decreased and increased light intensities were determined at a depth of 0.5 mm (the depth where maximum oxygen production occurred) (Fig. 3). The mat was first adapted to full light (690 W m−2 at the surface) and then exposed to decreasing light intensities over a 20-min period. It then remained in the dark for 10 min and was subsequently exposed to increasing light intensities for another 20 min. Changes in the oxygen levels were detected within seconds of each change in light intensity (Fig. 3). The oxygen concentrations leveled out and stabilized within minutes of each shift. Similar, although less dramatic, changes were observed for pH levels in response to changes in light intensity (data not shown).
FIG. 3.
Effect of changes in light intensity on the oxygen concentration over time at the depth where maximal oxygen production occurred (0.5 mm) in the olive mat at 39°C.
Rapid responses of oxygen concentrations to changes in light intensity were also observed in olive mats in situ in the flowing springs. The electrode was embedded at a depth of 0.5 mm in a mat exposed to full sun (1,100 W m−2 at the mat surface), and the oxygen concentration was 238 μM (155% of saturation) under these conditions. When the light intensity at the surface was suddenly decreased to 140 W m−2, the oxygen concentration decreased to 201 μM in 10 s and to 181 μM in less than 1 min. When the light intensity was returned to 1,100 W m−2, the oxygen concentration rose to 228 μM in less than 30 s.
Impact of iron on cyanobacterial photosynthesis.
The impact of ferrous iron on photosynthesis in the cyanobacterial mat populations was assessed by measuring the fixation of 14C-labeled bicarbonate under different conditions. Uptake experiments were performed with dilute suspensions of the mat communities incubated under moderate to low light intensities to avoid photoinhibition.
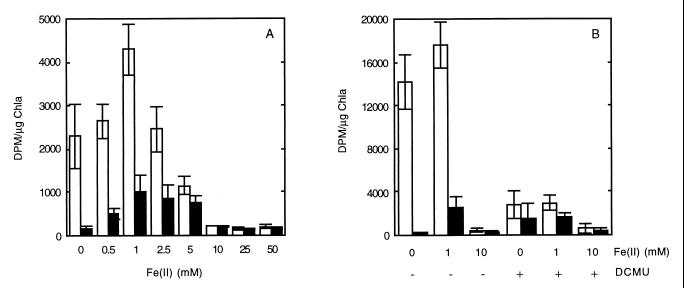
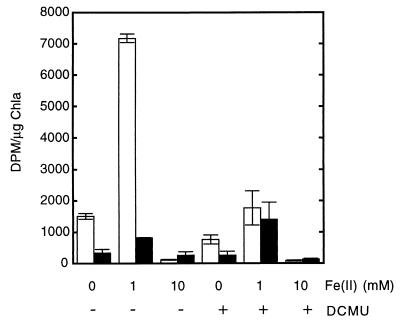
Both in the light and in the dark, ferrous iron stimulated bicarbonate uptake in the olive mat, and maximal stimulation occurred at a concentration of 1.0 mM (Fig. 4A). Ferrous iron at a concentration of 5 mM inhibited photosynthesis (although not uptake in the dark), and higher concentrations of iron eliminated photosynthetic activity and depressed activity in the dark (Fig. 4A). The iron enhancement of dark-corrected uptake in the light was substantial in this experiment, and the levels were as high as 175% of the non-iron-containing control levels. In other experiments, however, the stimulation was much less substantial and perhaps not significant (Fig. 4B). No evidence of inhibition of photosynthesis by 1.0 mM ferrous iron was ever observed in the olive mat, although this concentration of ferrous iron did inhibit Oscillatoria cf. princeps mat suspensions (data not shown). Photosynthesis in Pseudanabaena spp. was not consistently stimulated by ferrous iron (1.0 mM) (data not shown). Ferrous iron (1.0 mM) strongly stimulated light-dependent uptake of bicarbonate (500% of the no-iron control value) but not uptake of bicarbonate by the Synechococcus mat suspensions in the dark (Fig. 5). A high level of stimulation was observed consistently in four different experiments with Synechococcus mat suspensions. DCMU inhibited photosynthetic activity both in the presence and in the absence of iron in all mat suspensions (Fig. 4B and 5). In all of the mat suspensions, 10 mM ferrous iron strongly inhibited uptake both in the light and in the dark (Fig. 4 and 5).
FIG. 4.
Effects of ferrous iron and DCMU (5.0 μM) on uptake of [14C]bicarbonate in the light (open bars) and in the dark (solid bars) by suspensions of thin filaments obtained from surface phototrophic layers of an olive mat. Data are means ± standard deviations based on triplicate samples. (A) Uptake of bicarbonate in the light and in the dark in the absence of DCMU as a function of ferrous iron concentration. Maximal stimulation occurred with 1.0 mM ferrous iron at 41°C and 100 to 160 W m−2. (B) Effects of ferrous iron (1.0 and 10.0 mM) and DCMU on uptake of bicarbonate in the light and in the dark by a suspension of olive mat thin filaments incubated at 40°C. Chla, chlorophyll a.
FIG. 5.
Effects of ferrous iron (1.0 and 10.0 mM) and DCMU (5.0 μM) on uptake of bicarbonate by Synechococcus mat suspensions incubated at 49°C in the light and in the dark. For details see the legend to Fig. 4.
Nature of the light environment in mats and in iron sediments.
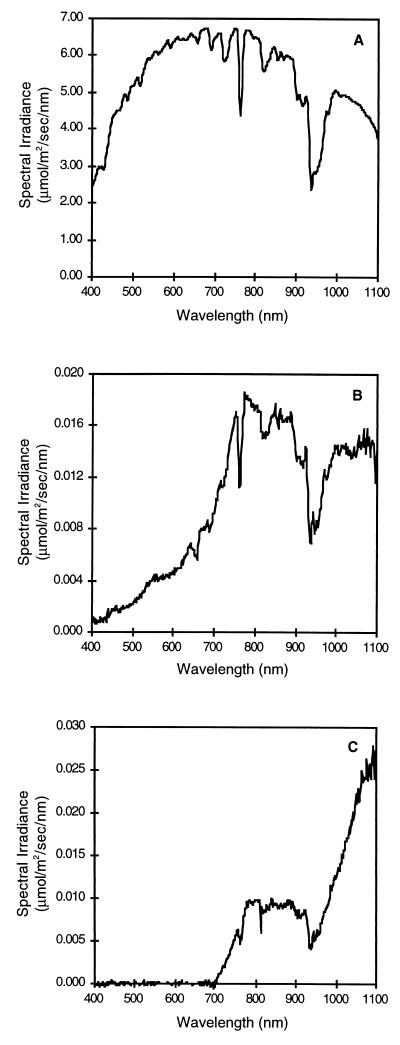
The downward spectral irradiance penetrating the top 1 mm of packed iron sediments devoid of cyanobacterial mats lacked specific pigment absorption signatures (Fig. 6B). The sediments transmitted relatively little visible radiation (wavelengths, 400 to 700 nm) due to attenuation from scattering and absorption by the iron minerals (Fig. 6B). However, they transmitted considerable red and near-infrared (NIR) radiation and retained most of the solar spectral characteristics at the NIR wavelengths (700 to 900 nm) (Fig. 6A and B).
FIG. 6.
Spectral irradiance in olive mats and plain iron sediments at Chocolate Pots Hot Springs measured by layering mats and sediments on top of the remote cosine receptor of a spectroradiometer. (A) Solar spectral irradiance measured at the surface of a mat or sediment. (B) Spectral irradiance transmitted through a 1.0-mm-thick packed iron oxide sediment core from the hot spring. (C) Spectral irradiance at a depth of 2.0 mm beneath the surface of the olive mat.
Penetration of radiation through the dense olive cyanobacterial iron mat produced a distinctive pigmentation signature due to visible absorption by chlorophyll a and phycocyanin (Fig. 6C). At a depth of 2.0 mm beneath the surface of the mat, all of the visible radiation (wavelengths, 400 to 700 nm) was attenuated by these pigments and iron. However, some NIR radiation (wavelengths, 700 to 900 nm) was still available (Fig. 6C). The spectral irradiance in the NIR region (Fig. 6C) did not retain the solar spectral characteristics (Fig. 6A) but appeared to be altered by specific absorption by bacteriochlorophylls.
Iron distribution with depth.
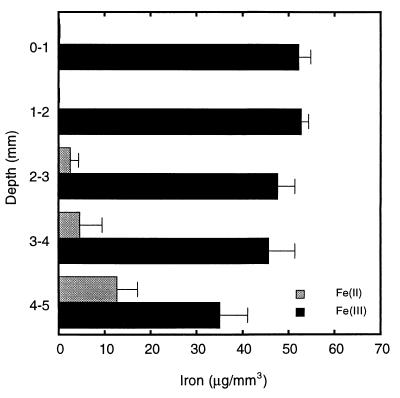
The concentrations of ferrous iron increased and the concentrations of ferric iron decreased in the mat sediments as the depth increased (Fig. 7). The total iron concentration remained constant at around 50 μg mm of wet sediment−3.
FIG. 7.
Distribution of ferrous iron and ferric iron with depth in a microbial mineral mat.
DISCUSSION
We observed novel stimulation of photosynthetic bicarbonate uptake by Fe(II) in suspensions of phototrophic mats obtained from Chocolate Pots Hot Springs and analyzed the microbial ecology of cyanobacterial mats in this high-iron-concentration thermal environment. The iron stimulation of photosynthesis which we observed is particularly interesting and is discussed below, as is our interpretation of the ecological data and its potential relevance to the microbial geochemistry of iron formations.
Impact of iron on photosynthesis in cyanobacterial mats.
Iron [Fe(II)] stimulated [14C]bicarbonate uptake in the olive mat, and it did so most consistently in the Synechococcus mat suspensions. In the olive mat suspensions, the maximal dark-corrected stimulation of photosynthesis which we observed was 175% of the control value, which occurred in the presence of 1.0 mM Fe(II). In Synechococcus mat suspensions, the stimulation was very consistent and considerably greater than the stimulation in olive mat suspensions; the values were as high as 500 to 600% of the control values. There are three reasonable explanations for the stimulation of photosynthesis observed in the presence of iron. One hypothesis is that the Fe(II) increases the photosynthetic rate by lowering the redox potential or reducing the amount of free oxygen that builds up during oxygenic photosynthesis, which can potentially become inhibitory (43, 48, 67). However, when we tested an alternative reductant (thioglycolate), which was used by Weller et al. (67) to lower the redox potentials in suspensions of hot spring cyanobacteria, stimulation of photosynthesis was not observed (data not shown).
The second hypothesis is that Fe(II) functions as an electron donor during cyanobacterial photosynthesis in some of the Chocolate Pots mats. Some mat-forming cyanobacteria, such as marine Microcoleus spp. and some Oscillatoria species from hot springs (11), are known to use sulfide to sustain anoxygenic photosynthesis. The magnitude of the iron stimulation of photosynthesis which we observed in the olive mat suspensions was comparable to the magnitude reported for sulfide stimulation in cyanobacteria (11). The iron stimulation which we observed in the Synechococcus mat suspensions was greater than the sulfide stimulation observed in cyanobacterial cultures (11). Iron actually inhibited photosynthesis in the Oscillatoria cf. princeps mat suspensions, however. Cohen et al. (11) likewise showed that although sulfide stimulated photosynthesis in some cyanobacteria, it strongly inhibited photosynthesis in others. Although we have not eliminated the possibility that iron stimulates photosynthesis in some cyanobacteria by lowering the redox potential or consuming oxygen, our data suggest that iron may directly support photosynthesis in some of these organisms; these findings are similar to the sulfide findings of Cohen et al. (11).
Cohen explored the possibility that Fe(II) is an electron donor for cyanobacterial photosynthesis and found evidence which supported this possibility (12, 13). It is not clear from Cohen’s early findings whether cyanobacteria use iron as an electron donor for PSI or PSII (12, 13). Sulfide appears to donate electrons exclusively to PSI in cyanobacteria. However, both sulfide and Fe(II) can donate electrons to PSII-related RC2 reaction centers in purple bacteria (19, 20, 68). Therefore, it seems at least plausible that Fe(II) can donate electrons to cyanobacterial PSII. The lower pH values of source waters in which Synechococcus-Chloroflexus mats are found could increase the redox potential of the Fe(III)-Fe(II) couple, bringing it closer to the potential of the PSII reaction center chlorophyll a (P680). It is not unreasonable to postulate that such a mechanism was part of an evolutionary process leading to water-oxidizing PSII in cyanobacteria (47, 50).
The third hypothesis is that Fe(II) stimulates photosynthesis in anoxygenic phototrophs present in some of the cyanobacterial mat suspensions rather than in the cyanobacteria themselves. These putative photoferrotrophs could use mechanisms of photosynthetic iron oxidation similar to mechanisms used by the iron-dependent purple bacteria recently described by Ehrenreich and Widdel (19, 20) and Widdel et al. (68). The Synechococcus mat which exhibited the highest level of iron stimulation also contained abundant Chloroflexus filaments. Members of the genus Chloroflexus contain bacteriochlorophylls and a reaction center similar to that of the purple bacteria, so it is possible that some Chloroflexus strains may indeed be able to oxidize Fe(II) to support photoautotrophy. Thermophilic mat-forming Chloroflexus strains have been shown to use sulfide for photosynthetic CO2 fixation in hot springs in the Mammoth area of Yellowstone National Park (27).
The DCMU data set some limits on the possible ways to explain iron stimulation of photosynthesis. Cohen et al. (11) showed that sulfide-dependent anoxygenic photosynthesis in cyanobacteria was not sensitive to DCMU and therefore was a function of PSI. In our experiments performed with iron, all photosynthetic activity [with and without Fe(II)] was inhibited by DCMU. This observation suggests that direct reduction of the reaction center in PSI (which is not sensitive to DCMU) by Fe(II) does not occur in the cyanobacteria examined. Our results are consistent with the interpretation that if Fe(II) donates electrons for photosynthesis in some of these cyanobacteria, it donates them to a PSII type of reaction center, as suggested by Cohen et al. (11). Cyanobacteria with this iron-oxidizing ability would not necessarily be distributed widely. They would exist only in environments such as Chocolate Pots Hot Springs, which contain abundant Fe(II) that is not consumed immediately by iron-oxidizing chemotrophs or directly by oxygen. The Fe(II) might inhibit water-oxidizing activity and then be an electron donor in the same RC2 reaction center, or it might donate electrons to another RC2-like reaction center specialized for iron oxidation in order to augment CO2 fixation.
Generally, Chloroflexus strains containing a PSII type of reaction center (RC2) have been shown to be photosynthetically insensitive to DCMU (3, 67). It is possible, however, that an anoxygenic RC2 reaction center capable of oxidizing iron would be sensitive to DCMU. Apparently, it is not known yet whether the iron-oxidizing photosynthetic purple bacteria are sensitive to DCMU (19, 20, 68). Isolation of the Chocolate Pots mat phototrophs and experiments performed with pure cultures are needed to distinguish among the proposed novel physiological activities.
Impact of cyanobacterial mats on water chemistry.
The ferrous iron and oxygen contents of the spring water flowing over the mats changed little between early morning and late afternoon, and based on this information we concluded that cyanobacterial photosynthetic activity had little impact on either parameter. The springs were shallow; the oxygen concentrations in the flowing water reached near air-saturated levels quickly, and the water was never supersaturated. Consequently, the major source of oxygen in the flowing spring water was mixing with air rather than photosynthesis in the mats below.
It was assumed that most of the iron in the water flowing over the mats was oxidized by the air-saturated water. Some of the resulting oxidized ferric precipitates settled out in quieter areas on the mound, while the finer precipitates were carried into the river by the rapidly flowing water. Although the Fe(II) levels in the water decreased down the drainway as the oxygen content increased, Fe(II) was still detected where the effluent flowed into the river.
High rates of photosynthesis in the mats may have contributed to the increase in pH in water downstream from the source. This increase was substantially higher in the late afternoon than in the early morning. Revsbech and Ward (57) observed that in Octopus Spring mats and in the overlying water the highest pH occurred in late afternoon. The Chocolate Pots spring waters appeared to be affected similarly.
Impact of cyanobacterial mats on the chemistry of the sediment environments.
In contrast to the flowing water, the microenvironment consisting of the mat-containing sediments was affected substantially by the photosynthetic activity of the cyanobacterial mats. Several experiments confirmed that cyanobacterial photosynthesis played a role in oxygenating the sediments. (i) Microelectrode depth profiles revealed that the oxygen levels in the mats in the light exceeded the air saturation levels characteristic of the flowing water by more than 200%, while the values in the dark never exceeded the air saturation levels and were often much less. (ii) In the places where sediments were devoid of cyanobacterial mats, the oxygen concentration was not elevated in the light, and the values never exceeded the values obtained from air saturation alone. (iii) The oxygen concentrations in the mats changed rapidly in response to changes in light intensity, as observed by Revsbech and Ward (57) for Octopus Spring mats. (iv) DCMU reduced the oxygen levels in mats by shutting down PSII. (v) The in situ oxygen profiles showed that local cyanobacterial oxygen production rather than the aerated flowing spring water was the critical factor in determining oxygenation of sediments. When the area being measured with the electrode was darkened, the oxygen levels decreased steeply despite the fact that photosynthesis was still occurring in mats located immediately upstream from the electrode.
The depth profiles for oxygen and pH in the light and in the dark in the Chocolate Pots cyanobacterial mats were typical of the profiles obtained for other cyanobacterial mats, including thermal mats in hot springs in which the concentration of iron is low (57, 58). As observed by Revsbech and Ward (57, 58), we found that the changes in pH value were not always as dramatic or as consistent as the changes in oxygen concentration. The actual concentrations of oxygen in the Chocolate Pots mats in the light, although well above the air saturation values, were still considerably lower than the concentrations observed in low-iron-concentration thermal springs (57, 58). The continuous supply of reduced iron in the Chocolate Pots source water could have consumed some of the oxygen.
All of the microelectrode profiles and in situ observations discussed above demonstrated that cyanobacteria create a microenvironment in which it is possible that the rate of oxidation of Fe(II) increases to a value that is greater than the value possible with air exposure alone. Water flows through the microchannels and interstitial voids of mats and biofilms, which enhances the direct interaction of microbes with water chemistry (16, 38). The pore water is clearly affected on a diel basis by the photosynthetic activity of the cyanobacteria and perhaps by the chemotrophic activity of other autotrophs and heterotrophs. Our data confirmed that in the light both the pH and the oxygen concentration of the pore water of mat-dominated sediments are significantly higher than the pH and the oxygen concentration of the flowing spring water above the mat, and thus the rate of oxidation of reduced iron may be increased. Since the microenvironment of the mats becomes anoxic in the dark, Chocolate Pots Hot Springs should provide an excellent model with which to directly test the impact of photosynthesis on iron oxidation and mineralization in situ.
Comparison of Chocolate Pots cyanobacterial mats to other iron-containing systems.
Emerson and Revsbech (21) studied oxygen profiles in the sediments of a cold iron-containing spring that was devoid of cyanobacteria and was dominated by chemotrophic iron bacteria. The oxygen concentrations in the depth profiles which these authors obtained never exceeded the ambient air saturation values. The oxygen concentrations decreased sharply with depth primarily due to respiratory consumption by chemotrophs in the mats containing iron bacteria (22). In contrast, we did not observe rapid depletion of oxygen with depth in plain sediments that lacked cyanobacterial mats (Fig. 1D), which indicated that similar purely chemotrophic iron-oxidizing mats were probably not present in the Chocolate Pots springs.
However, in many sediments containing cyanobacterial mats, we did observe rapid and strong depletion of oxygen with depth in the light and greater depletion in the dark; most mats became anoxic. We attributed this oxygen depletion in the mat-dominated sediments at Chocolate Pots to metabolic consumption. Respiratory activity can occur in phototrophs, as well as in chemoautotrophs and chemoheterotrophs. The fact that oxygen depletion did not occur in the sediments that lacked mats is evidence that purely chemical oxidation of reduced iron is not the major factor that contributes to oxygen depletion in the mats. Thus, we concluded that microbial metabolism in well-developed mats is the major cause of oxygen depletion; Emerson and Revsbech (21, 22) reached a similar conclusion concerning their purely chemotrophic iron mats.
The pH values which we obtained (pH 8 to 9) were much higher than the values reported by Emerson and Revsbech (21) (pH 7.1 to 7.6), probably because of the high rates of autotrophic activity in the photosynthetic mats which we studied. We observed some lower pH values closer to neutral in the dark or deeper in some mats and sediments. Interpretation of changes in pH, however, may be complicated by the presence of other organisms (46) and the effects of abiotic water chemistry. In one sediment sample that lacked a cyanobacterial mat, we observed a light-dependent increase in pH without an accompanying increase in the oxygen concentration at depths of 0.25 to 1.25 mm (Fig. 1D). Since NIR radiation penetrates to this depth (Fig. 6B), this increase in pH could indicate that anoxygenic photoautotrophs were present.
Light penetration in Chocolate Pots Hot Springs mats and sediments.
The presence of oxidized iron minerals in mats and sediments affects the light environment by selectively attenuating short-wavelength visible radiation and transmitting red and NIR radiation. The dominant phototrophs in the mats are cyanobacteria, which also selectively attenuate visible radiation. NIR radiation penetrated below the cyanobacterial layers to depths greater than 2 mm and provided a light environment that could support bacteriochlorophyll-containing anoxygenic phototrophic bacteria. If iron-dependent photoautotrophs, such as those described by Ehrenreich and Widdel (19, 20), were present, they could contribute to direct oxidation of Fe(II) and thus enhance iron mineralization in the sediments. Fe(II)-containing enrichment cultures obtained from some of the sediments have revealed that purple bacteria are present (unpublished data). Although purple photosynthetic bacteria are present in the springs, the distribution and abundance of these organisms are more limited than the distribution and abundance of cyanobacteria. The one mat containing substantial bacteriochlorophyll was the Synechococcus mat which contained Chloroflexus filaments, as in other hot springs in the region (55).
Iron reduction and cycling.
Although we studied only photosynthetic activity in the mats, depth profiles for Fe(II) and Fe(III) (Fig. 7) showed that the Fe(II) fraction of the total iron started to increase with depth at 2 mm. This increase coincided with the depth of anoxia in both the light and the dark in most mat-dominated sediments (but not in plain sediments). Our data suggest that too little short-wavelength light reaches this depth (Fig. 6) to sustain abiotic photoreduction of iron (14, 24, 40, 41). The increase in the Fe(II) concentration with depth, which was accompanied by a corresponding decrease in the Fe(III) concentration, could be evidence that the microbes present exhibited iron-reducing activity. It would not be surprising to observe microbial iron reduction below the oxic zone of the mats since cyanobacteria produce abundant organic substrates that could be sources of carbon and reducing power. Iron-reducing bacteria have been detected in many types of sediments (39, 45).
Chocolate Pots Hot Springs mats provide a unique high-iron-concentration environment in which to study the cycling of iron among iron oxidizers, reducers, and phototrophs. The presence of a rapidly flowing shallow water zone that appears to be primarily abiotic, a 2-mm-thick phototrophic mat in which there are diel shifts in oxic and anoxic conditions, and a permanently anoxic zone that may receive small amounts or NIR radiation provides a compact environment that is less than 1 cm deep in which to study aerobic and anaerobic biotic and abiotic transformations of iron.
Geochemical significance and BIFs.
The geochemical potential of microbial iron transformation was noted in the early studies of chemotrophic iron bacteria (29, 56, 71). There has been speculation about the role of bacterial oxidizing activities in the deposition of the Precambrian BIFs (31, 32), including the role of photosynthesis (7, 36, 66, 68). Although many models for the deposition of iron formations have been proposed, most researchers favor the stratified deep-ocean model; according to this model high concentrations of ferrous iron and silica accumulated in deep anoxic water and precipitated when the anoxic water was mixed with more oxic water from above, and biological activity was not required (4, 34).
Recent studies performed in natural aquatic systems have shown that oxidation of Fe(II) to Fe(III) can proceed by a variety of mechanisms. The rate is influenced by a large number of factors, including pH, the presence of surfaces, the concentration of oxygen, the presence of other oxidants, and biological activity. Photochemically produced hydrogen peroxide can be a significant factor (42, 69, 70). Biological activity has been found to accelerate the rate of iron oxidation in mildly acidic freshwater lake sediments (2) and in a shallow water marine hydrothermal environment (31, 32). Holm (31, 32) favored Simonson’s hydrothermal model (61) for the origin of BIFs but suggested that the low levels of oxygen required could have been provided by early cyanobacteria, as proposed by Cloud (7). Microaerophilic chemolithotrophs could have enhanced the rate of iron oxidation, as suggested by Emerson and Revsbech (21, 22), Emerson and Moyer (23), and Holm (31, 32). Anaerobic oxidation of iron is also possible (28, 63). Whether phototrophs play a direct or indirect role or no role in the oxidation of iron in natural environments has not been determined yet.
Conclusions.
We confirmed that microbial photosynthetic activity has a significant impact on the sedimentary microenvironments in a high-iron-concentration thermal spring. We are currently determining the quantitative impact in real time of photosynthetic microbial activity on the rates of oxidation of iron and the subsequent early mineralization of iron in microbial mat sediments. We have detected iron-dependent stimulation of photosynthesis in some mats and are currently trying to identify the nature of this stimulation.
While deep-sea hydrothermal effluents can obviously be factors that contribute to the deep-ocean model of BIFs, subaerial hot springs cannot. Nevertheless, studies of the iron being deposited at Chocolate Pots Hot Springs provide a unique opportunity to test some of the hypotheses regarding the role of biological activity in iron oxidation and deposition in which the primary source of ferrous iron, silica, and carbonate is a hydrothermal fluid. In this setting, the oxygenic photosynthetic cyanobacteria substantially increase the oxygen concentration and pH compared to the ambient environmental conditions. Thus, Chocolate Pots Hot Springs provides an accessible subaerial iron model in which biogenic effects on reduced iron can be studied. Furthermore, the complex microbial community that could develop at this site within and beneath the steep, fluctuating oxygen and light gradients provides a unique opportunity to study a wide range of direct and indirect microbial interactions during the cycling of iron.
ACKNOWLEDGMENTS
This work was supported by grant NAGW-5090 from the NASA Exobiology Program to B.K.P. During 1997, B.K.P. was supported by a John Lantz Senior Research Fellowship from the University of Puget Sound. Research funds were also provided by the University of Puget Sound Enrichment Committee.
We thank R. W. Castenholz for helpful comments. We thank the National Park Service for permission to conduct research in Yellowstone National Park.
REFERENCES
- 1.Allen E T, Day A L. Hot springs of the Yellowstone National Park. Carnegie Institute of Washington publication 466. Baltimore, Md: Waverly Press; 1935. [Google Scholar]
- 2.Barry R C, Schnoor J L, Sulzberger B, Sigg L, Stumm W. Iron oxidation kinetics in an acidic alpine lake. Water Res. 1994;28:323–333. [Google Scholar]
- 3.Bauld J, Brock T D. Ecological studies of Chloroflexus, a gliding photosynthetic bacterium. Arch Mikrobiol. 1973;92:267–284. [Google Scholar]
- 4.Beukes N J, Klein C. Models for iron-formation deposition. In: Schopf J W, Klein C, editors. The proterozoic biosphere: a multidisciplinary study. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 147–151. [Google Scholar]
- 5.Bridge T A M, Johnson D B. Reduction of soluble iron and reductive dissolution of ferric iron-containing minerals by moderately thermophilic iron-oxidizing bacteria. Appl Environ Microbiol. 1998;64:2181–2186. doi: 10.1128/aem.64.6.2181-2186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canfield D E, Des Marais D J. Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochim Cosmochim Acta. 1993;57:3971–3984. doi: 10.1016/0016-7037(93)90347-y. [DOI] [PubMed] [Google Scholar]
- 7.Cloud P. Paleoecological significance of the banded iron-formation. Econ Geol. 1973;68:1135–1143. [Google Scholar]
- 8.Cloud P. Beginnings of biospheric evolution and their biogeochemical consequences. Paleobiology. 1976;2:351–387. [Google Scholar]
- 9.Cloud P E. Significance of the gunflint (Precambrian) microflora. Science. 1965;148:27–35. doi: 10.1126/science.148.3666.27. [DOI] [PubMed] [Google Scholar]
- 10.Cloud P E, Licari G R. Microbiotas of the banded iron formations. Proc Natl Acad Sci USA. 1968;61:779–786. doi: 10.1073/pnas.61.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen J, Jørgensen B B, Revsbech N P, Poplawski R. Adaptation to hydrogen sulfide of oxygenic and anoxygenic photosynthesis among cyanobacteria. Appl Environ Microbiol. 1986;51:398–407. doi: 10.1128/aem.51.2.398-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen Y. Comparative N and S cycles. In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C.: American Society for Microbiology; 1984. pp. 435–441. [Google Scholar]
- 13.Cohen Y. Photosynthesis in cyanobacterial mats and its relation to the sulfur cycle: a model for microbial sulfur interactions. In: Cohen Y, Rosenberg E, editors. Microbial mats: physiological ecology of benthic microbial communities. Washington, D.C.: American Society for Microbiology; 1989. pp. 22–36. [Google Scholar]
- 14.Collienne R H. Photoreduction of iron in the epilimnion of acidic lakes. Limnol Oceanogr. 1983;28:83–100. [Google Scholar]
- 15.Cornell R M, Schwertmann U. The iron oxides: structure, properties, reactions, occurrence and uses. Weinheim, Germany: VCH Verlagsgesellschaft; 1996. [Google Scholar]
- 16.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 17.Des Marais D J. The biogeochemistry of hypersaline microbial mats. Adv Microb Ecol. 1995;14:251–274. doi: 10.1007/978-1-4684-7724-5_6. [DOI] [PubMed] [Google Scholar]
- 18.Dobbin P S, Warren L H, Cook N J, McEwan A G, Powell A K, Richardson D J. Dissimilatory iron(III) reduction by Rhodobacter capsulatus. Microbiology. 1996;142:765–774. doi: 10.1099/00221287-142-4-765. [DOI] [PubMed] [Google Scholar]
- 19.Ehrenreich A, Widdel F. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl Environ Microbiol. 1994;60:4517–4526. doi: 10.1128/aem.60.12.4517-4526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrenreich A, Widdel F. Phototrophic oxidation of ferrous minerals—a new aspect in the redox microbiology of iron. In: Stal A J, Caumette P, editors. Microbial mats. NATO ASI Series. G35. Berlin, Germany: Springer-Verlag; 1994. pp. 393–402. [Google Scholar]
- 21.Emerson D, Revsbech N P. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: field studies. Appl Environ Microbiol. 1994;60:4022–4031. doi: 10.1128/aem.60.11.4022-4031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emerson D, Revsbech N P. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: laboratory studies. Appl Environ Microbiol. 1994;60:4032–4038. doi: 10.1128/aem.60.11.4032-4038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emerson D, Moyer C. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol. 1997;63:4784–4792. doi: 10.1128/aem.63.12.4784-4792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finden D A S, Tipping E, Jaworski G H M, Reynolds C S. Light-induced reduction of natural iron(III) oxide and its relevance to phytoplankton. Nature. 1984;309:783–784. [Google Scholar]
- 25.Garrels R M, Christ C L. Solutions, minerals, and equilibria. New York, N.Y: Harper & Row; 1965. [Google Scholar]
- 26.Garrels R M. A model for the deposition of the microbanded Precambrian iron formations. Am J Sci. 1987;287:81–106. [Google Scholar]
- 27.Giovannoni S J, Revsbech N P, Ward D M, Castenholz R W. Obligately phototrophic Chloroflexus: primary production in anaerobic hot spring microbial mats. Arch Microbiol. 1987;147:80–87. [Google Scholar]
- 28.Hafenbradl D, Keller M, Dirmeier R, Rachel R, Roßnagel P, Burggraf S, Huber H, Stetter K O. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch Microbiol. 1996;166:308–314. doi: 10.1007/s002030050388. [DOI] [PubMed] [Google Scholar]
- 29.Harder E C. Iron-depositing bacteria and their geologic relations. U.S. Geological Survey professional paper 113. Washington, D.C.: Department of the Interior, Government Printing Office; 1919. [Google Scholar]
- 30.Hartman H. The evolution of photosynthesis and microbial mats: a speculation on the banded iron formations. In: Cohen Y, Castenholz R W, Halvorson H O, editors. Microbial mats: stromatolites. New York, N.Y: Alan R. Liss, Inc.; 1984. pp. 449–453. [Google Scholar]
- 31.Holm N G. Biogenic influences on the geochemistry of certain ferruginous sediments of hydrothermal origin. Chem Geol. 1987;63:45–57. [Google Scholar]
- 32.Holm N G. Possible biological origin of banded iron-formations from hydrothermal solutions. Origins Life. 1987;17:229–250. [Google Scholar]
- 33.Kasting J F. Earth’s early atmosphere. Science. 1993;259:920–926. doi: 10.1126/science.11536547. [DOI] [PubMed] [Google Scholar]
- 34.Klein C, Beukes N J. Time distribution, stratigraphy, and sedimentologic setting, and geochemistry of Precambrian iron-formations. In: Schopf J W, Klein C, editors. The proterozoic biosphere: a multidisciplinary study. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 139–146. [Google Scholar]
- 35.Knoll A H, Awramik S M. Ancient microbial ecosystems. In: Krumbein W E, editor. Microbial geochemistry. Oxford, United Kingdom: Blackwell Scientific Publications; 1983. pp. 287–315. [Google Scholar]
- 36.Kump L. Bacteria forge a new link. Nature. 1993;362:790–791. [Google Scholar]
- 37.Liang L, McNabb J A, Paulk J M, Gu B, McCarthy J F. Kinetics of Fe(II) oxygenation at low partial pressure of oxygen in the presence of natural organic matter. Environ Sci Technol. 1993;27:1864–1870. [Google Scholar]
- 38.Little B J, Wagner P A, Lewandowski Z. Spatial relationships between bacteria and mineral surfaces. Rev Mineral. 1997;35:123–159. [Google Scholar]
- 39.Lovley D R. Microbial oxidation of organic matter coupled to the reduction of Fe(III) and Mn(IV) oxides. In: Skinner H C W, Fitzpatrick R W, editors. Biomineralization process of iron and manganese: modern and ancient environments. Cremlingen-Destedt, Germany: Catena Verlag; 1992. pp. 101–114. [Google Scholar]
- 40.Madsen E L, Morgan M D, Good R E. Simultaneous photoreduction and microbial oxidation of iron in a stream in the New Jersey pinelands. Limnol Oceanogr. 1986;31:832–838. [Google Scholar]
- 41.McKnight D M, Kimball B A, Bencala K E. Iron photoreduction and oxidation in an acidic mountain stream. Science. 1988;240:637–640. doi: 10.1126/science.240.4852.637. [DOI] [PubMed] [Google Scholar]
- 42.Moffett J W, Zika R G. Reaction kinetics of hydrogen peroxide with copper and iron in seawater. Environ Sci Technol. 1987;8:804–810. doi: 10.1021/es00162a012. [DOI] [PubMed] [Google Scholar]
- 43.Mouget J L, Dakhama A, Lavoie M C, Noüe J D L. Algal growth enhancement by bacteria: is consumption of photosynthetic oxygen involved? FEMS Microbiol Ecol. 1995;18:35–44. [Google Scholar]
- 44.Nealson K H, Saffarini D. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- 45.Nealson K H, Little B. Breathing manganese and iron: solid-state respiration. Adv Appl Microbiol. 1997;45:213–239. [Google Scholar]
- 46.Nold S C, Ward D M. Photosynthate partitioning and fermentation in hot spring microbial mat communities. Appl Environ Microbiol. 1996;62:4598–4607. doi: 10.1128/aem.62.12.4598-4607.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson J M, Pierson B K. Evolution of reaction centers in photosynthetic prokaryotes. Int Rev Cytol. 1987;108:209–248. doi: 10.1016/s0074-7696(08)61439-4. [DOI] [PubMed] [Google Scholar]
- 48.Pearl H W. Microscale physiological and ecological studies of aquatic cyanobacteria: macroscale implications. Microsc Res Technol. 1996;33:47–72. doi: 10.1002/(SICI)1097-0029(199601)33:1<47::AID-JEMT6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 49.Pierson B K, Oesterle A, Murphy G L. Pigments, light penetration and photosynthetic activity in the multilayered microbial mats of Great Sippewissett Salt Marsh, Massachusetts. FEMS Microbiol Ecol. 1987;45:365–376. [Google Scholar]
- 50.Pierson B K, Olson J M. Evolution of photosynthesis in anoxygenic photosynthetic procaryotes. In: Cohen Y, Rosenberg E, editors. Microbial mats: physiological ecology of benthic microbial communities. Washington, D.C.: American Society for Microbiology; 1989. pp. 402–427. [Google Scholar]
- 51.Pierson B K, Sands V M, Frederick J L. Spectral irradiance and distribution of pigments in a highly layered marine microbial mat. Appl Environ Microbiol. 1990;56:2327–2340. doi: 10.1128/aem.56.8.2327-2340.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierson B K, Mitchell H K, Ruff-Roberts A L. Chloroflexus aurantiacus and ultraviolet radiation: implications for archean shallow-water stromatolites. Origins Life Evol Biosphere. 1993;23:243–260. [Google Scholar]
- 53.Pierson B K. The emergence, diversification, and role of photosynthetic eubacteria. Nobel Symp. 1994;84:161–180. [Google Scholar]
- 54.Pierson B K, Valdez D, Larsen M, Morgan E, Mack E E. Chloroflexus-like organisms from marine and hypersaline environments: distribution and diversity. Photosynth Res. 1994;41:35–52. doi: 10.1007/BF02184144. [DOI] [PubMed] [Google Scholar]
- 55.Pierson, B. K., and M. N. Parenteau. Phototrophs in high iron microbial mats: microstructure of mats in iron-depositing hot springs. Submitted for publication. [DOI] [PubMed]
- 56.Pringsheim E G. The filamentous bacteria Sphaerotilus, Leptothrix, Cladothrix, and their relation to iron and manganese. Phil Trans R Soc London B Biol Sci. 1949;233:453–482. [Google Scholar]
- 57.Revsbech N P, Ward D M. Microelectrode studies of interstitial water chemistry and photosynthetic activity in a hot spring microbial mat. Appl Environ Microbiol. 1984;48:270–275. doi: 10.1128/aem.48.2.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Revsbech N P, Ward D M. Microprofiles of dissolved substances and photosynthesis in microbial mats measured with microelectrodes. In: Cohen Y, Castenholz R W, Halvorson H O, editors. Microbial mats: stromatolites. New York, N.Y: Alan R. Liss Inc.; 1984. pp. 171–188. [Google Scholar]
- 59.Roden E E, Zachara J M. Microbial reduction of crystalline iron(III) oxides: influence of oxide surface area and potential for cell growth. Environ Sci Technol. 1996;30:1618–1628. [Google Scholar]
- 60.Schopf J W. Microfossils of the early Archean apex chert: new evidence of the antiquity of life. Science. 1993;260:640–646. doi: 10.1126/science.260.5108.640. [DOI] [PubMed] [Google Scholar]
- 61.Simonson B M. Sedimentological constraints on the origins of Precambrian iron-formations. Geol Soc Am Bull. 1985;96:244–252. [Google Scholar]
- 62.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 63.Straub K L, Benz M, Schink B, Widdel F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol. 1996;62:1458–1460. doi: 10.1128/aem.62.4.1458-1460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Gemerden H, Tughan C S, de Wit R, Herbert R A. Laminated microbial ecosystems on sheltered beaches in Scapa Flow, Orkney Islands. FEMS Microbiol Ecol. 1989;62:87–102. [Google Scholar]
- 65.Vargas M, Kashefi K, Blunt-Harris E L, Lovley D R. Microbiological evidence for Fe(III) reduction on early Earth. Nature. 1998;395:65–67. doi: 10.1038/25720. [DOI] [PubMed] [Google Scholar]
- 66.Walker J C G. Was the Archean biosphere upside down? Nature. 1987;329:710–712. doi: 10.1038/329710a0. [DOI] [PubMed] [Google Scholar]
- 67.Weller D, Doemel W, Brock T D. Requirement of low oxidation-reduction potential for photosynthesis in a blue-green alga (Phormidium sp.) Arch Microbiol. 1975;104:7–13. doi: 10.1007/BF00447293. [DOI] [PubMed] [Google Scholar]
- 68.Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature. 1993;362:834–836. [Google Scholar]
- 69.Wilson C L, Hinman N W, Brown C F. Lunar and Planetary Science Conference XXIX 1998. Houston, Tex: Lunar and Planetary Institute; 1998. Diel cycling of hydrogen peroxide in surface geothermal waters: possible formation from metal redox reactions, abstr. 1406. [Google Scholar]
- 70.Wilson C L, Hinman N W, Sheridan R. Supplement to EOS Transactions, American Geophysical Union 79:F60. (Abstract.) 1998. Hydrogen peroxide formation and decay in thermal waters: possible influence on microbial ecology and evolution. [Google Scholar]
- 71.Winogradsky S. Microbiologie du sol: problèmes et méthods. Paris, France: Libraires de l’Académie de Médecine; 1949. [Google Scholar]