Abstract
Immunotherapy with immune checkpoint inhibitor (ICI) drugs is gradually becoming a hot topic in cancer treatment. To comprehensively evaluate the safety and efficacy of ICI drugs, we employed the Bayesian model and conducted a network meta-analysis in terms of progression-free survival (PFS), overall survival (OS) and severe adverse events (AEs). Our study found that treatment with ipilimumab was significantly worse than standard therapies in terms of PFS, whereas treatment with cemiplimab significantly improved PFS. The results also indicated that cemiplimab was the best choice for PFS. Treatment with nivolumab, pembrolizumab and nivolumab plus ipilimumab significantly improved OS compared to standard therapies. In terms of OS, cemiplimab was found to be the best choice, whereas avelumab was the worst. In terms of severe AEs, atezolizumab, avelumab, durvalumab, nivolumab, and pembrolizumab all significantly reduced the risk of grade 3 or higher AEs compared to standard therapy. The least likely to be associated with severe AEs were as follows: cemiplimab, avelumab, nivolumab, atezolizumab, and camrelizumab, with nivolumab plus ipilimumab to be the worst. Therefore, different ICI drug therapies may pose different risks in terms of PFS, OS and severe AEs. Our study may provide new insights and strategies for the clinical practice of ICI drugs.
Keywords: immune checkpoint inhibitor, cancer immunotherapy, programmed death-1 (PD-1), programmed death-ligand-1 (PD-L1), cytotoxic T lymphocyte antigen-4 (CTLA-4)
1 Introduction
Immunotherapy has become one of the most important breakthroughs in the treatment of cancer in recent years, and its development has promoted changes in many cancer treatment methods. As a series of co-inhibitory and co-stimulatory receptors and ligands, immune checkpoint inhibitors (ICI) drugs can block negative regulatory factors expressed by immune or tumor cells to enhance their immune function against cancer cells, mainly programmed death-1 (PD-1), programmed death-ligand-1 (PD-L1) and cytotoxic T lymphocyte antigen-4 (CTLA-4) (Rosenberg et al., 2004). In 2011, the CTLA-4 inhibitor ipilimumab was approved by the US Food and Drug Administration for the treatment of advanced melanoma (Hodi et al., 2010). Subsequently, several ICI drugs were also approved for the treatment of cancer (Topalian et al., 2012; Gong et al., 2018). Since then, interest for immunotherapy with ICI drugs has been increasing. Many studies focused on the prognosis and treatment for different cancers (Wu et al., 2015).
Chemotherapy is the first-line treatment for advanced cancer, and patients undergoing chemotherapy often experience severe adverse events (AEs). Although ICI drugs have achieved good anticancer effects in the treatment of many solid tumors, they may still cause severe treatment-related or drug-related AEs. Progression-free survival (PFS) and overall survival (OS) are usually efficacy end-points. In terms of PFS and OS, the therapeutic effects of ICI drugs remain unclear compared with standard therapies. Due to the limitations of randomized clinical trials, the overall safety evaluation of different ICI drugs for cancer treatment is not comprehensive, especially in terms of PFS and OS.
We conducted a systematic review and network meta-analysis of the therapeutic effects of ICI drugs targeting PD-1, PD-L1, and CTLA-4, focusing on PFS, OS and treatment-related severe AEs in patients receiving ICI drug monotherapy, combination therapies and standard therapies (chemotherapy, targeted therapies and their combination therapies included). This study comprehensively evaluated the safety and efficacy of different ICI drugs and their combination therapies, aiming to provide better guidance for the clinical application of various ICI drugs.
2 Methods
2.1 Search Methods and Study Selection
We searched PubMed, Embase, and Cochrane Library for English-language studies between January 2000 and September 2021, using keywords such as ipilimumab, tremelimumab, pembrolizumab, nivolumab, cemiplimab, camrelizumab, toripalimab, tislelizumab, spartalizumab, atezolizumab, avelumab, durvalumab, PD-1, PD-L1, and CTLA-4. The search strategy was described in Supplementary Table S1. The study search, selection and data extraction were independently conducted by two reviewers (ZX and ZZ), and discrepancies were evaluated by an independent reviewer (JL). The three authors (ZX, JL and ZZ) reviewed and discussed the full text of studies that may be eligible, and differences of opinions were resolved by consensus.
Only high-quality head-to-head phase 2 and 3 randomized controlled trials (RCTs) comparing two or more treatments including ICI drug monotherapy, ICI drug combination therapies and standard therapies were included. Some RCTs only presented interim results, as insufficient information may affect the final analysis, we selected the most recent results as much as possible. Data provided include at least one of the following: hazard ratios (HRs) of PFS, OS and treatment-related severe AEs. We excluded reviews, conference abstracts and posters. The study was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline (Hutton et al., 2015; Wang et al., 2021). This study was approved by International prospective register of systematic reviews (PROSPERO) (registered ID: CRD42021278158).
2.2 Data Extraction
The authors (ZX and ZZ) independently extracted data according to the PRISMA guidelines. The first author, year of publication, national clinical trial identification number, trial name, phase, number of patients, type of cancer, drug used, follow-up time, number of severe AEs, HRs, and confidence interval (CI) of PFS and OS were summarized in standardized Tables 1–3.
TABLE 1.
List of the studies involving PFS in this meta-analysis.
| First author | Year | NCT | Trial name | Total number | Phase | Canner type | Treatment 1 | Patient number | Treatment 2 | Patient number | Follow-up time | PFS HR | PFS CI lower limit | PFS CI upper limit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fehrenbacher L et al. (Fehrenbacher et al., 2018) | 2018 | NCT02008227 | OAK | 1225 | 3 | Non small cell lung cancer | Atezolizumab | 613 | Docetaxel | 612 | 21 | 0.96 | 0.85 | 1.08 |
| McDermott DF et al. (McDermott et al., 2018) | 2018 | NCT01984242 | IMmotion150 | 204 | 2 | Renal cell carcinoma | Atezolizumab | 103 | Sunitinib | 101 | 20.7 | 1.19 | 0.82 | 1.71 |
| Powles T et al. (Powles et al., 2018) | 2018 | NCT02302807 | IMvigor211 | 234 | 3 | Urothelial carcinoma | Atezolizumab | 116 | Chemotherapy | 118 | 17.3 | 1.01 | 0.75 | 1.34 |
| Eng C et al. (Eng et al., 2019) | 2019 | NCT02788279 | IMblaze370 | 180 | 3 | Colorectal cancer | Atezolizumab | 90 | Regorafenib | 90 | 7.3 | 1.39 | 1.00 | 1.94 |
| Pujol JL et al. (Pujol et al., 2019) | 2019 | NCT03059667 | IFCT-1603 | 73 | 2 | Small Cell Lung Cancer | Atezolizumab | 49 | Chemotherapy | 24 | 13.7 | 2.26 | 1.3 | 3.93 |
| Herbst RS et al. (Herbst et al., 2020) | 2020 | NCT02409342 | IMpower110 | 554 | 3 | Non small cell lung cancer | Atezolizumab | 277 | Chemotherapy | 277 | 13.4 | 0.77 | 0.63 | 0.94 |
| Bang YJ et al. (Bang et al., 2018) | 2018 | NCT02625623 | JAVELIN Gastric 300 | 371 | 3 | Gastric/gastrooesophageal junction cancer | Avelumab | 185 | Chemotherapy | 186 | 10.6 | 1.73 | 1.4 | 2.2 |
| Barlesi F et al. (Barlesi et al., 2018) | 2018 | NCT02395172 | JAVELIN Lung 200 | 529 | 3 | Non small cell lung cancer | Avelumab | 264 | Docetaxel | 265 | T1:18.9 | 1.01 | 0.80 | 1.28 |
| T2:17.8 | ||||||||||||||
| Pujade-Lauraine E et al. (Pujade-Lauraine et al., 2021) | 2021 | NCT02580058 | JAVELIN Ovarian 200 | 378 | 3 | Ovarian cancer | Avelumab | 188 | Pegylated liposomal doxorubicin | 190 | T1:18.2 | 1.68 | 1.32 | 2.60 |
| T2:17.4 | ||||||||||||||
| Huang J et al. (Huang et al., 2020) | 2020 | NCT03099382 | ESCORT | 448 | 3 | Squamous cell carcinoma | Camrelizumab | 228 | Chemotherapy | 220 | 8.3 | 0.69 | 0.56 | 0.86 |
| Sezer A et al. (Sezer et al., 2021) | 2021 | NCT03088540 | EMPOWER-Lung 1 | 563 | 3 | Non small cell lung cancer | Cemiplimab | 283 | Chemotherapy | 280 | T1:10.8 | 0.54 | 0.43 | 0.68 |
| T2:10.9 | ||||||||||||||
| Siu LL et al. (Siu et al., 2019) | 2019 | NCT02319044 | CONDOR | 267 | 2 | Squamous cell carcinoma | Durvalumab+ | 133 | Durvalumab | 67 | T1:6.5 | 1.13 | 0.82 | 1.56 |
| Tremelimumab | T2:6.0 | |||||||||||||
| 2 | Squamous cell carcinoma | Durvalumab+ | 133 | Tremelimumab | 67 | T1:6.5 | 0.73 | 0.53 | 1.01 | |||||
| Tremelimumab | T2:5.2 | |||||||||||||
| Ferris RL et al. (Ferris et al., 2020) | 2020 | NCT02369874 | EAGLE | 736 | 3 | Squamous cell carcinoma | Durvalumab | 240 | Standard of care | 249 | T1:7.6 | 1.02 | 0.84 | 1.25 |
| T2:7.8 | ||||||||||||||
| 3 | Squamous cell carcinoma | Durvalumab+ | 247 | Standard of care | 249 | T1:6.3 | 1.09 | 0.90 | 1.33 | |||||
| Tremelimumab | T2:7.8 | |||||||||||||
| Planchard D et al. (Planchard et al., 2020) | 2020 | NCT02352948 | ARCTIC | 595 | 3 | Non small cell lung cancer | Durvalumab | 62 | Standard of care | 64 | 9.1 | 0.71 | 0.49 | 1.04 |
| 3 | Non small cell lung cancer | Durvalumab+ | 174 | Standard of care | 118 | 9.1 | 0.77 | 0.59 | 1.01 | |||||
| Tremelimumab | ||||||||||||||
| 3 | Non small cell lung cancer | Durvalumab | 117 | Standard of care | 118 | 9.1 | 0.87 | 0.65 | 1.16 | |||||
| 3 | Non small cell lung cancer | Tremelimumab | 60 | Standard of care | 118 | 9.1 | 1.25 | 0.88 | 1.77 | |||||
| Rizvi NA et al. (Rizvi et al., 2020) | 2020 | NCT02453282 | MYSTIC | 488 | 3 | Non small cell lung cancer | Durvalumab | 163 | Chemotherapy | 162 | 10.6 | 0.87 | 0.59 | 1.29 |
| 3 | Non small cell lung cancer | Durvalumab+ | 163 | Chemotherapy | 162 | 10.6 | 1.05 | 0.72 | 1.53 | |||||
| Tremelimumab | ||||||||||||||
| Bachelot T et al. (Bachelot et al., 2021) | 2021 | NCT02299999 | SAFIR02-BREAST IMMUNO | 199 | 2 | Breast cancer | Durvalumab | 68 | Chemotherapy | 131 | 19.7 | 1.40 | 1.00 | 1.96 |
| Bang YJ et al. (Bang et al., 2017) | 2017 | NCT01585987 | NA | 108 | 2 | Gastric/gastrooesophageal junction cancer | Ipilimumab | 57 | Best supportive care | 51 | 24 | 1.44 | 1.09 | 1.91 |
| Borghaei H et al. (Borghaei et al., 2015) | 2015 | NCT01673867 | CheckMate 057 | 582 | 3 | Non small cell lung cancer | Nivolumab | 292 | Docetaxel | 290 | 13.2 | 0.92 | 0.77 | 1.11 |
| Brahmer J et al. (Brahmer et al., 2015) | 2015 | NCT01642004 | CheckMate 017 | 272 | 3 | Non small cell lung cancer | Nivolumab | 135 | Docetaxel | 137 | 11 | 0.62 | 0.47 | 0.81 |
| Motzer RJ et al. (Motzer et al., 2015) | 2015 | NCT01668784 | CheckMate 025 | 821 | 3 | Renal cell carcinoma | Nivolumab | 410 | Everolimus | 411 | 14 | 0.88 | 0.75 | 1.03 |
| Ferris RL et al. (Ferris et al., 2016) | 2016 | NCT02105636 | CheckMate 141 | 361 | 3 | Squamous cell carcinoma | Nivolumab | 240 | Standard therapy | 121 | 5.1 | 0.89 | 0.70 | 1.13 |
| Hodi FS et al. (Hodi et al., 2016) | 2016 | NCT01927419 | CheckMate 069 | 142 | 2 | Melanoma | Nivolumab+ | 95 | Ipilimumab | 47 | 24.5 | 0·36 | 0.22 | 0.56 |
| Ipilimumab | ||||||||||||||
| Carbone DP et al. (Carbone et al., 2017) | 2017 | NCT02041533 | CheckMate 026 | 541 | 3 | Non small cell lung cancer | Nivolumab | 271 | Chemotherapy | 270 | 13.5 | 1.19 | 0.97 | 1.46 |
| Hodi FS et al. (Hodi et al., 2018) | 2018 | NCT01844505 | CheckMate 067 | 945 | 3 | Melanoma | Nivolumab+ | 314 | Ipilimumab | 315 | T1:46.9 | 0.42 | 0.35 | 0.51 |
| Ipilimumab | T2:18.6 | |||||||||||||
| 3 | Melanoma | Nivolumab | 316 | Ipilimumab | 315 | T1:18.6 | 0.53 | 0.44 | 0.64 | |||||
| T2:36 | ||||||||||||||
| Larkin J et al. (Larkin et al., 2018) | 2018 | NCT01721746 | CheckMate 037 | 405 | 3 | Melanoma | Nivolumab | 272 | Chemotherapy | 133 | 24 | 1.00 | 0.78 | 1.44 |
| Hellmann MD et al. (Hellmann et al., 2019) | 2019 | NCT02477826 | CheckMate 227 | 299 | 3 | Non small cell lung cancer | Nivolumab+ | 139 | Chemotherapy | 160 | 11.2 | 0.58 | 0.41 | 0.81 |
| Ipilimumab | ||||||||||||||
| Kato K et al. (Kato et al., 2019) | 2019 | NCT02569242 | ATTRACTION-3 | 419 | 3 | Squamous cell carcinoma | Nivolumab | 210 | Chemotherapy | 209 | 17.6 | 1.08 | 0.87 | 1.34 |
| Wu YL et al. (Wu et al., 2019) | 2019 | NCT02613507 | CheckMate 078 | 504 | 3 | Non small cell lung cancer | Nivolumab | 338 | Docetaxel | 166 | 8.8 | 0.77 | 0.62 | 0.95 |
| Motzer RJ et al. (Motzer et al., 2020) | 2020 | NCT02231749 | CheckMate 214 | 1096 | 3 | Renal cell carcinoma | Nivolumab+ | 550 | Sunitinib | 546 | 42 | 0.88 | 0.75 | 1.04 |
| Ipilimumab | ||||||||||||||
| Reardon DA et al. (Reardon et al., 2020) | 2020 | NCT02017717 | CheckMate 143 | 369 | 3 | Glioblastoma | Nivolumab | 184 | Bevacizumab | 185 | 9.5 | 1.97 | 1.57 | 2.48 |
| Robert C et al. (Robert et al., 2020) | 2020 | NCT01721772 | CheckMate 066 | 418 | 3 | Melanoma | Nivolumab | 210 | Dacarbazine | 208 | 60 | 0.40 | 0.33 | 0.54 |
| Zamarin D et al. (Zamarin et al., 2020) | 2020 | NCT02498600 | NRG GY003 | 100 | 2 | Ovarian Cancer | Nivolumab+ | 51 | Nivolumab | 49 | NA | 0.53 | 0.34 | 0.82 |
| Ipilimumab | ||||||||||||||
| Baas P et al. (Baas et al., 2021) | 2021 | NCT02899299 | CheckMate 743 | 605 | 3 | Malignant pleural mesothelioma | Nivolumab+ | 303 | Chemotherapy | 302 | 29.7 | 1.00 | 0.82 | 1.21 |
| Ipilimumab | ||||||||||||||
| Spigel DR et al. (Spigel et al., 2021) | 2021 | NCT02481830 | CheckMate 331 | 569 | 3 | Small cell lung cancer | Nivolumab | 284 | Chemotherapy | 285 | 15.8 | 1.41 | 1.18 | 1.69 |
| Tannir NM et al. (Tannir et al., 2021) | 2021 | NA | CheckMate 214 | 139 | 3 | Renal cell carcinoma | Nivolumab+ | 74 | Sunitinib | 65 | 42 | 0.54 | 0.33 | 0.86 |
| Ipilimumab | ||||||||||||||
| Herbst RS et al. (Herbst et al., 2016) | 2016 | NCT01905657 | KEYNOTE-010 | 687 | 2/3 | Non small cell lung cancer | Pembrolizumab | 344 | Docetaxel | 343 | 13.1 | 0.88 | 0.74 | 1.05 |
| Hamid O et al. (Hamid et al., 2017) | 2017 | NCT01704287 | KEYNOTE-002 | 359 | 2 | Melanoma | Pembrolizumab | 180 | Chemotherapy | 179 | 28 | 0.58 | 0.46 | 0.73 |
| Shitara K et al. (Shitara et al., 2018) | 2018 | NCT02370498 | KEYNOTE-061 | 395 | 3 | Gastric/gastrooesophageal junction cancer | Pembrolizumab | 196 | Paclitaxel | 199 | 8.5 | 1.27 | 1.03 | 1.57 |
| Cohen EEW et al. (Cohen et al., 2019) | 2019 | NCT02252042 | KEYNOTE-040 | 495 | 3 | Ssquamous cell carcinoma | Pembrolizumab | 247 | Standard of care | 248 | T1:7.5 | 0.96 | 0.79 | 1.16 |
| T2:7.1 | ||||||||||||||
| Fradet Y et al. (Fradet et al., 2019) | 2019 | NCT02256436 | KEYNOTE-045 | 542 | 3 | Urothelial cancer | Pembrolizumab | 270 | Chemotherapy | 272 | 27.7 | 0.96 | 0.79 | 1.16 |
| Mok TSK et al. (Mok et al., 2019) | 2019 | NCT02220894 | KEYNOTE-042 | 1274 | 3 | Non small cell lung cancer | Pembrolizumab | 637 | Chemotherapy | 637 | 12.8 | 1.07 | 0.94 | 1.21 |
| Reck M et al. (Reck et al., 2019) | 2019 | NCT02142738 | KEYNOTE-024 | 305 | 3 | Non small cell lung cancer | Pembrolizumab | 154 | Chemotherapy | 151 | 11.2 | 0.50 | 0.37 | 0.68 |
| Robert C et al. (Robert et al., 2019) | 2019 | NCT01866319 | KEYNOTE-006 | 834 | 3 | Melanoma | Pembrolizumab | 556 | Ipilimumab | 278 | 57.7 | 0.57 | 0.48 | 0.67 |
| André T et al. (André et al., 2020) | 2020 | NCT02563002 | KEYNOTE-177 | 307 | 3 | Colorectal cancer | Pembrolizumab | 153 | Chemotherapy | 154 | 32.4 | 0.60 | 0.45 | 0.80 |
| Kojima T et al. (Kojima et al., 2020) | 2020 | NCT02564263 | KEYNOTE-181 | 628 | 3 | Esophageal Cancer | Pembrolizumab | 314 | Chemotherapy | 314 | T1:7.1 | 1.11 | 0.94 | 1.31 |
| T2:6.9 | ||||||||||||||
| Popat S et al. (Popat et al., 2020) | 2020 | NCT02991482 | ETOP 9-15 | 144 | 3 | Malignant pleural mesothelioma | Pembrolizumab | 73 | Chemotherapy | 71 | 17.5 | 1.06 | 0.73 | 1.53 |
| Shitara K et al. (Shitara et al., 2020) | 2020 | NCT02494583 | KEYNOTE-062 | 506 | 3 | Gastric/gastrooesophageal junction cancer | Pembrolizumab | 256 | Chemotherapy | 250 | 29.4 | 1.66 | 1.37 | 2.01 |
| Kuruvilla J et al. (Kuruvilla et al., 2021) | 2021 | NCT02684292 | KEYNOTE-204 | 304 | 3 | Hodgkin lymphoma | Pembrolizumab | 151 | Brentuximab vedotin | 153 | 24 | 0.65 | 0.48 | 0.88 |
PFS = Progression-free survival. HR = Hazard ratio. CI = Confidence interval.
TABLE 2.
List of the studies involving OS in this meta-analysis.
| First author | Year | NCT | Trial name | Total number | Phase | Canner type | Treatment 1 | Patient number | Treatment 2 | Patient number | Follow-up time | OS HR | OS CI lower limit | OS CI upper limit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fehrenbacher L et al. (Fehrenbacher et al., 2016) | 2016 | NCT01903993 | POPLAR | 287 | 2 | Non small cell lung cancer | Atezolizumab | 144 | Docetaxel | 143 | 13 | 0.73 | 0.53 | 0.99 |
| Fehrenbacher L et al. (Fehrenbacher et al., 2018) | 2018 | NCT02008227 | OAK | 1225 | 3 | Non small cell lung cancer | Atezolizumab | 613 | Docetaxel | 612 | 26 | 0.80 | 0.70 | 0.92 |
| Powles T et al. (Powles et al., 2018) | 2017 | NCT02302807 | IMvigor211 | 234 | 3 | Urothelial carcinoma | Atezolizumab | 116 | Chemotherapy | 118 | 17.3 | 0.87 | 0.63 | 1.21 |
| Eng C et al. (Eng et al., 2019) | 2019 | NCT02788279 | IMblaze370 | 180 | 3 | Colorectal cancer | Atezolizumab | 90 | Regorafenib | 90 | 7.3 | 1.19 | 0.83 | 1.71 |
| Pujol JL et al. (Pujol et al., 2019) | 2018 | NCT03059667 | IFCT-1603 | 73 | 2 | Small cell lung cancer | Atezolizumab | 49 | Chemotherapy | 24 | 13.7 | 0.84 | 0.45 | 1.58 |
| Herbst RS et al. (Herbst et al., 2020) | 2020 | NCT02409342 | IMpower110 | 554 | 3 | Non small cell lung cancer | Atezolizumab | 277 | Chemotherapy | 277 | 13.4 | 0.83 | 0.65 | 1.07 |
| Bang YJ er al (Bang et al., 2018) | 2018 | NCT02625623 | JAVELIN Gastric 300 | 371 | 3 | Gastric/gastrooesophageal junction cancer | Avelumab | 185 | Chemotherapy | 186 | 10.6 | 1.1 | 0.9 | 1.4 |
| Park K et al. (Park et al., 2021) | 2021 | NCT02395172 | JAVELIN Lung 200 | 529 | 3 | Non small cell lung cancer | Avelumab | 264 | Docetaxel | 265 | 24 | 0.87 | 0.71 | 1.05 |
| Pujade-Lauraine E et al. (Pujade-Lauraine et al., 2021) | 2021 | NCT02580058 | JAVELIN Ovarian 200 | 378 | 3 | Ovarian cancer | Avelumab | 188 | Pegylated liposomal doxorubicin | 190 | T1:18.2 | 1.14 | 0.95 | 1.58 |
| T2:17.4 | ||||||||||||||
| Huang J et al. (Huang et al., 2020) | 2020 | NCT03099382 | ESCORT | 448 | 3 | Squamous cell carcinoma | Camrelizumab | 228 | Chemotherapy | 220 | 8.3 | 0.71 | 0.57 | 0.87 |
| Sezer A et al. (Sezer et al., 2021) | 2021 | NCT03088540 | EMPOWER-Lung 1 | 563 | 3 | Non small cell lung cancer | Cemiplimab | 283 | Chemotherapy | 280 | T1:10.8 | 0.57 | 0.42 | 0.77 |
| T2:10.9 | ||||||||||||||
| Siu LL et al. (Siu et al., 2019) | 2019 | NCT02319044 | CONDOR | 267 | 2 | Squamous cell carcinoma | Durvalumab+ | 133 | Durvalumab | 67 | T1:6.5 | 0.99 | 0.69 | 1.43 |
| Tremelimumab | T2:6.0 | |||||||||||||
| 2 | Squamous cell carcinoma | Durvalumab+ | 133 | Tremelimumab | 67 | T1:6.5 | 0.72 | 0.51 | 1.03 | |||||
| Tremelimumab | T2:5.2 | |||||||||||||
| Ferris RL et al. (Ferris et al., 2020) | 2020 | NCT02369874 | EAGLE | 736 | 3 | Squamous cell carcinoma | Durvalumab | 240 | Standard of care | 249 | T1:7.6 T2:7.8 | 0.88 | 0.72 | 1.08 |
| 3 | Squamous cell carcinoma | Durvalumab+ | 247 | Standard of care | 249 | T1:6.3 | 1.04 | 0.85 | 1.26 | |||||
| Tremelimumab | T2:7.8 | |||||||||||||
| Planchard D et al. (Planchard et al., 2020) | 2020 | NCT02352948 | ARCTIC | 595 | 3 | Non small cell lung cancer | Durvalumab | 62 | Standard of care | 64 | 9.1 | 0.63 | 0.42 | 0.93 |
| 3 | Non small cell lung cancer | Durvalumab+ Tremelimumab | 174 | Standard of care | 118 | 9.1 | 0.80 | 0.61 | 1.05 | |||||
| 3 | Non small cell lung cancer | Durvalumab | 117 | Standard of care | 118 | 9.1 | 0.80 | 0.59 | 1.08 | |||||
| 3 | Non small cell lung cancer | Tremelimumab | 60 | Standard of care | 118 | 9.1 | 1.02 | 0.71 | 1.46 | |||||
| Powles T et al. (Powles et al., 2020) | 2020 | NCT02516241 | DANUBE | 1032 | 3 | Urothelial carcinoma | Durvalumab | 346 | Chemotherapy | 344 | 41.2 | 0.99 | 0.83 | 1.17 |
| 3 | Urothelial carcinoma | Durvalumab+ | 342 | Chemotherapy | 344 | 41.2 | 0.85 | 0.72 | 1.02 | |||||
| Tremelimumab | ||||||||||||||
| Rizvi NA et al. (Rizvi et al., 2020) | 2020 | NCT02453282 | MYSTIC | 488 | 3 | Non small cell lung cancer | Durvalumab | 163 | Chemotherapy | 162 | 30.2 | 0.76 | 0.56 | 1.02 |
| 3 | Non small cell lung cancer | Durvalumab+ | 163 | Chemotherapy | 162 | 30.2 | 0.85 | 0.61 | 1.17 | |||||
| Tremelimumab | ||||||||||||||
| Bachelot T et al. (Bachelot et al., 2021) | 2021 | NCT02299999 | SAFIR02-BREAST IMMUNO | 199 | 2 | Breast cancer | Durvalumab | 68 | Chemotherapy | 131 | 19.7 | 0.84 | 0.54 | 1.29 |
| Hodi FS et al. (Hodi et al., 2010) | 2010 | NCT00094653 | MDX010-20 | 273 | 3 | Melanoma | Ipilimumab | 137 | Gp100 | 136 | T1:27.8 T2:17.2 | 0.66 | 0.51 | 0.87 |
| Tarhini AA et al. (Tarhini et al., 2020) | 2020 | NA | E1609 | 1159 | 3 | Melanoma | Ipilimumab | 523 | Interferon Alfa-2b | 636 | 57.4 | 0.78 | 0.61 | 0.99 |
| Borghaei H et al. (Borghaei et al., 2015) | 2015 | NCT01673867 | CheckMate 057 | 582 | 3 | Non small cell lung cancer | Nivolumab | 292 | Docetaxel | 290 | 13.2 | 0.73 | 0.59 | 0.89 |
| Brahmer J et al. (Brahmer et al., 2015) | 2015 | NCT01642004 | CheckMate 017 | 272 | 3 | Non small cell lung cancer | Nivolumab | 135 | Docetaxel | 137 | 11 | 0.59 | 0.44 | 0.79 |
| Motzer RJ et al. (Motzer et al., 2015) | 2015 | NCT01668784 | CheckMate 025 | 821 | 3 | Renal cell carcinoma | Nivolumab | 410 | Everolimus | 411 | 14 | 0.73 | 0.57 | 0.93 |
| Ferris RL et al. (Ferris et al., 2016) | 2016 | NCT02105636 | CheckMate 141 | 361 | 3 | Squamous cell carcinoma | Nivolumab | 240 | Standard therapy | 121 | 5.1 | 0.70 | 0.51 | 0.96 |
| Hodi FS et al. (Hodi et al., 2016) | 2016 | NCT01927419 | CheckMate 069 | 142 | 2 | Melanoma | Nivolumab+ | 95 | Ipilimumab | 47 | 24.5 | 0.74 | 0.43 | 1.26 |
| Ipilimumab | ||||||||||||||
| Carbone DP et al. (Carbone et al., 2017) | 2017 | NCT02041533 | CheckMate 026 | 541 | 3 | Non small cell lung cancer | Nivolumab | 271 | Chemotherapy | 270 | 13.5 | 1.08 | 0.87 | 1.34 |
| Hodi FS et al. (Hodi et al., 2018) | 2018 | NCT01844505 | CheckMate 067 | 945 | 3 | Melanoma | Nivolumab+ Ipilimumab | 314 | Ipilimumab | 315 | T1:46.9 T2:18.6 | 0.54 | 0.44 | 0.67 |
| 3 | Melanoma | Nivolumab | 316 | Ipilimumab | 315 | T1: 36 T2:18.6 | 0.65 | 0.53 | 0.79 | |||||
| Larkin J et al. (Larkin et al., 2018) | 2018 | NCT01721746 | CheckMate 037 | 405 | 3 | Melanoma | Nivolumab | 272 | Chemotherapy | 133 | 24 | 0.95 | 0.73 | 1.24 |
| Hellmann MD et al. (Hellmann et al., 2019) | 2019 | NCT02477826 | CheckMate 227 | 1166 | 3 | Non small cell lung cancer | Nivolumab+ Ipilimumab | 583 | Chemotherapy | 583 | 29.3 | 0.73 | 0.64 | 0.84 |
| Kato K et al. (Kato et al., 2019) | 2019 | NCT02569242 | ATTRACTION-3 | 419 | 3 | Squamous cell carcinoma | Nivolumab | 210 | Chemotherapy | 209 | 17.6 | 0.77 | 0.62 | 0.96 |
| Wu YL et al. (Wu et al., 2019) | 2019 | NCT02613507 | CheckMate 078 | 504 | 3 | Non small cell lung cancer | Nivolumab | 338 | Docetaxel | 166 | 8.8 | 0.68 | 0.52 | 0.90 |
| Motzer RJ et al. (Motzer et al., 2020) | 2020 | NCT02231749 | CheckMate 214 | 1096 | 3 | Renal cell carcinoma | Nivolumab+ | 550 | Sunitinib | 546 | 42 | 0.72 | 0.61 | 0.86 |
| Ipilimumab | ||||||||||||||
| Reardon DA et al. (Reardon et al., 2020) | 2020 | NCT02017717 | CheckMate 143 | 369 | 3 | Glioblastoma | Nivolumab | 184 | Bevacizumab | 185 | 9.5 | 1.04 | 0.83 | 1.3 |
| Robert C et al. (Robert et al., 2020) | 2020 | NCT01721772 | CheckMate 066 | 418 | 3 | Melanoma | Nivolumab | 210 | Dacarbazine | 208 | 60 | 0.50 | 0.40 | 0.63 |
| Zamarin D et al. (Zamarin et al., 2020) | 2020 | NCT02498600 | NRG GY003 | 100 | 2 | Ovarian cancer | Nivolumab+ | 51 | Nivolumab | 49 | NA | 0.79 | 0.44 | 1.42 |
| Ipilimumab | ||||||||||||||
| Baas P et al. (Baas et al., 2021) | 2021 | NCT02899299 | CheckMate 743 | 605 | 3 | Malignant pleural mesothelioma | Nivolumab+ | 303 | Chemotherapy | 302 | 29.7 | 0.74 | 0.60 | 0.91 |
| Ipilimumab | ||||||||||||||
| Spigel DR et al. (Spigel et al., 2021) | 2021 | NCT02481830 | CheckMate 331 | 569 | 3 | Small cell lung cancer | Nivolumab | 284 | Chemotherapy | 285 | 15.8 | 0.86 | 0.72 | 1.04 |
| Tannir NM et al. (Tannir et al., 2021) | 2021 | NA | CheckMate 214 | 139 | 3 | Renal cell carcinoma | Nivolumab+ | 74 | Sunitinib | 65 | 42 | 0.45 | 0.30 | 0.70 |
| Ipilimumab | ||||||||||||||
| Ribas A et al. (Ribas et al., 2013) | 2013 | NCT00257205 | NA | 655 | 3 | Melanoma | Tremelimumab | 328 | Chemotherapy | 327 | NA | 0.88 | NA | NA |
| Herbst RS et al. (Herbst et al., 2016) | 2016 | NCT01905657 | KEYNOTE-010 | 687 | 2/3 | Non small cell lung cancer | Pembrolizumab | 344 | Docetaxel | 343 | 13.1 | 0.71 | 0.58 | 0.88 |
| Hamid O et al. (Hamid et al., 2017) | 2017 | NCT01704287 | KEYNOTE-002 | 359 | 2 | Melanoma | Pembrolizumab | 180 | Chemotherapy | 179 | 28 | 0.86 | 0.67 | 1.10 |
| Shitara K et al. (Shitara et al., 2018) | 2018 | NCT02370498 | KEYNOTE-061 | 395 | 3 | Gastric/gastrooesophageal junction cancer | Pembrolizumab | 196 | Paclitaxel | 199 | 8.5 | 0.82 | 0.66 | 1.03 |
| Cohen EEW et al. (Cohen et al., 2019) | 2019 | NCT02252042 | KEYNOTE-040 | 495 | 3 | Squamous cell carcinoma | Pembrolizumab | 247 | Standard of care | 248 | 7.5 | 0.80 | 0.65 | 0.98 |
| Fradet Y et al. (Fradet et al., 2019) | 2019 | NCT02256436 | KEYNOTE-045 | 542 | 3 | Urothelial cancer | Pembrolizumab | 270 | Chemotherapy | 272 | 27.7 | 0.70 | 0.57 | 0.85 |
| Mok TSK et al. (Mok et al., 2019) | 2019 | NCT02220894 | KEYNOTE-042 | 1274 | 3 | Non small cell lung cancer | Pembrolizumab | 637 | Chemotherapy | 637 | 12.8 | 0.81 | 0.71 | 0.93 |
| Reck M et al. (Reck et al., 2019) | 2019 | NCT02142738 | KEYNOTE-024 | 305 | 3 | Non small cell lung cancer | Pembrolizumab | 154 | Chemotherapy | 151 | 25.2 | 0.63 | 0.47 | 0.86 |
| Robert C et al. (Robert et al., 2019) | 2019 | NCT01866319 | KEYNOTE-006 | 834 | 3 | Melanoma | Pembrolizumab | 556 | Ipilimumab | 278 | 57.7 | 0.73 | 0.61 | 0.88 |
| Kojima T et al. (Kojima et al., 2020) | 2020 | NCT02564263 | KEYNOTE-181 | 628 | 3 | Esophageal cancer | Pembrolizumab | 314 | Chemotherapy | 314 | 7.1 | 0.89 | 0.75 | 1.05 |
| Popat S et al. (Popat et al., 2020) | 2020 | NCT02991482 | ETOP 9-15 | 144 | 3 | Malignant pleural mesothelioma | Pembrolizumab | 73 | Chemotherapy | 71 | 17.5 | 1.04 | 0.66 | 1.67 |
| Shitara K et al. (Shitara et al., 2020) | 2020 | NCT02494583 | KEYNOTE-062 | 506 | 3 | Gastric/gastrooesophageal junction cancer | Pembrolizumab | 256 | Chemotherapy | 250 | 29.4 | 0.91 | 0.69 | 1.18 |
| Powles T et al. (Powles et al., 2021) | 2021 | NCT02853305 | KEYNOTE-361 | 659 | 3 | Urothelial carcinoma | Pembrolizumab | 307 | Chemotherapy | 352 | 31.7 | 0.92 | 0.77 | 1.11 |
| Winer EP et al. (Winer et al., 2021) | 2021 | NCT02555657 | KEYNOTE-119 | 622 | 3 | Breast cancer | Pembrolizumab | 312 | Chemotherapy | 310 | 31.4 | 0.97 | 0.82 | 1.15 |
OS = Overall survival. HR = Hazard ratio. CI = Confidence interval.
TABLE 3.
List of the studies involving serious AEs in this meta-analysis.
| First author | Year | NCT number | Trail name | Total number | Cancer type | Trial phase | Treatment | Patient number | Total number surveyed | Grade 3 or higher AEs |
|---|---|---|---|---|---|---|---|---|---|---|
| Fehrenbacher L et al. (Fehrenbacher et al., 2016) | 2016 | NCT01903993 | POPLAR | 287 | Non small cell lung cancer | 2 | Atezolizumab | 144 | 142 | 17 |
| 2 | Standard | 143 | 135 | 55 | ||||||
| Fehrenbacher L et al. (Fehrenbacher et al., 2018) | 2018 | NCT02008227 | OAK | 1225 | Non small cell lung cancer | 3 | Atezolizumab | 613 | 609 | 91 |
| 3 | Standard | 612 | 578 | 246 | ||||||
| McDermott DF et al. (McDermott et al., 2018) | 2018 | NCT01984242 | IMmotion150 | 204 | Renal cell carcinoma | 2 | Atezolizumab | 103 | 103 | 17 |
| 2 | Standard | 101 | 100 | 57 | ||||||
| Powles T et al. (Powles et al., 2018) | 2018 | NCT02302807 | IMvigor211 | 234 | Urothelial carcinoma | 3 | Atezolizumab | 116 | 114 | 11 |
| 3 | Standard | 118 | 112 | 43 | ||||||
| Eng C et al. (Eng et al., 2019) | 2019 | NCT02788279 | IMblaze370 | 180 | Colorectal cancer | 3 | Atezolizumab | 90 | 90 | 28 |
| 3 | Standard | 90 | 80 | 46 | ||||||
| Herbst RS et al. (Herbst et al., 2020) | 2020 | NCT02409342 | IMpower110 | 554 | Non small cell lung cancer | 3 | Atezolizumab | 277 | 286 | 97 |
| 3 | Standard | 277 | 263 | 149 | ||||||
| Bang YJ et al. (Bang et al., 2018) | 2018 | NCT02625623 | JAVELIN Gastric 300 | 371 | Gastric/gastrooesophageal junction cancer | 3 | Avelumab | 185 | 184 | 17 |
| 3 | Standard | 186 | 177 | 56 | ||||||
| Park K et al. (Park et al., 2021) | 2021 | NCT02395172 | JAVELIN Lung 200 | 529 | Non small cell lung cancer | 3 | Avelumab | 264 | 393 | 41 |
| 3 | Standard | 265 | 365 | 180 | ||||||
| Pujade-Lauraine E et al. (Pujade-Lauraine et al., 2021) | 2021 | NCT02580058 | JAVELIN Ovarian 200 | 378 | Ovarian cancer | 3 | Avelumab | 188 | 187 | 30 |
| 3 | Standard | 190 | 177 | 56 | ||||||
| Huang J et al. (Huang et al., 2020) | 2020 | NCT03099382 | ESCORT | 448 | Squamous cell carcinoma | 3 | Camrelizumab | 228 | 228 | 44 |
| 3 | Standard | 220 | 220 | 87 | ||||||
| Sezer A et al. (Sezer et al., 2021) | 2021 | NCT03088540 | EMPOWER-Lung 1 | 563 | Non small cell lung cancer | 3 | Cemiplimab | 283 | 355 | 50 |
| 3 | Standard | 280 | 342 | 134 | ||||||
| O'Reilly EM et al. (O'Reilly et al., 2019) | 2019 | NCT02558894 | NA | 65 | Pancreatic ductal adenocarcinoma | 2 | Durvalumab+Tremelimumab | 32 | 32 | 7 |
| 2 | Durvalumab | 33 | 32 | 2 | ||||||
| Siu LL et al. (Siu et al., 2019) | 2019 | NCT02319044 | CONDOR | 267 | Squamous Cell Carcinoma | 2 | Durvalumab+Tremelimumab | 133 | 133 | 21 |
| 2 | Durvalumab | 67 | 65 | 8 | ||||||
| 2 | Tremelimumab | 67 | 65 | 11 | ||||||
| Ferris RL et al. (Ferris et al., 2020) | 2020 | NCT02369874 | EAGLE | 736 | Squamous cell carcinoma | 3 | Durvalumab | 240 | 237 | 24 |
| 3 | Durvalumab+Tremelimumab | 247 | 246 | 40 | ||||||
| 3 | Standard | 249 | 240 | 58 | ||||||
| Planchard D et al. (Planchard et al., 2020) | 2020 | NCT02352948 | ARCTIC | 595 | Non small cell lung cancer | 3 | Durvalumab | 62 | 62 | 6 |
| 3 | Durvalumab+Tremelimumab | 174 | 173 | 38 | ||||||
| 3 | Durvalumab | 117 | 117 | 14 | ||||||
| 3 | Tremelimumab | 60 | 60 | 14 | ||||||
| 3 | Standard | 64 | 63 | 28 | ||||||
| 3 | Standard | 118 | 110 | 40 | ||||||
| Powles T et al. (Powles et al., 2020) | 2020 | NCT02516241 | DANUBE | 1032 | Urothelial carcinoma | 3 | Durvalumab | 346 | 345 | 49 |
| 3 | Durvalumab+Tremelimumab | 342 | 340 | 95 | ||||||
| 3 | Standard | 344 | 313 | 189 | ||||||
| Rizvi NA et al. (Rizvi et al., 2020) | 2020 | NCT02453282 | MYSTIC | 488 | Non small cell lung cancer | 3 | Durvalumab | 163 | 369 | 55 |
| 3 | Durvalumab+Tremelimumab | 163 | 371 | 85 | ||||||
| 3 | Standard | 162 | 352 | 119 | ||||||
| Bachelot T et al. (Bachelot et al., 2021) | 2021 | NCT02299999 | SAFIR02-BREAST IMMUNO | 199 | Breast cancer | 2 | Durvalumab | 68 | 63 | 10 |
| 2 | Standard | 131 | 129 | 17 | ||||||
| Hodi FS et al. (Hodi et al., 2010) | 2010 | NCT00094653 | MDX010-20 | 273 | Melanoma | 3 | Ipilimumab | 137 | 131 | 30 |
| 3 | Standard | 136 | 132 | 15 | ||||||
| Bang YJ et al. (Bang et al., 2017) | 2017 | NCT01585987 | NA | 108 | Gastric/gastrooesophageal junction cancer | 2 | Ipilimumab | 57 | 57 | 13 |
| 2 | Standard | 51 | 45 | 4 | ||||||
| Borghaei H et al. (Borghaei et al., 2015) | 2015 | NCT01673867 | CheckMate 057 | 582 | Non small cell lung cancer | 3 | Nivolumab | 292 | 287 | 30 |
| 3 | Standard | 290 | 268 | 144 | ||||||
| Brahmer J et al. (Brahmer et al., 2015) | 2015 | NCT01642004 | CheckMate 017 | 272 | Non small cell lung cancer | 3 | Nivolumab | 135 | 131 | 9 |
| 3 | Standard | 137 | 129 | 71 | ||||||
| Motzer RJ et al. (Motzer et al., 2015) | 2015 | NCT01668784 | CheckMate 025 | 821 | Renal cell carcinoma | 3 | Nivolumab | 410 | 406 | 76 |
| 3 | Standard | 411 | 397 | 145 | ||||||
| Ferris RL et al. (Ferris et al., 2016) | 2016 | NCT02105636 | CheckMate 141 | 361 | Squamous cell carcinoma | 3 | Nivolumab | 240 | 236 | 31 |
| 3 | Standard | 121 | 111 | 39 | ||||||
| Hodi FS et al. (Hodi et al., 2016) | 2016 | NCT01927419 | CheckMate 069 | 142 | Melanoma | 2 | Nivolumab+Ipilimumab | 95 | 94 | 51 |
| 2 | Ipilimumab | 47 | 46 | 9 | ||||||
| Carbone DP et al. (Carbone et al., 2017) | 2017 | NCT02041533 | CheckMate 026 | 541 | Non small cell lung cancer | 3 | Nivolumab | 271 | 267 | 47 |
| 3 | Standard | 270 | 263 | 133 | ||||||
| Weber J et al. (Weber et al., 2017) | 2017 | NCT02388906 | CheckMate 238 | 906 | Melanoma | 3 | Nivolumab | 453 | 452 | 65 |
| 3 | Ipilimumab | 453 | 453 | 208 | ||||||
| Amaria RN et al. (Amaria et al., 2018) | 2018 | NCT02519322 | NA | 23 | Melanoma | 2 | Nivolumab | 12 | 12 | 1 |
| 2 | Nivolumab+Ipilimumab | 11 | 11 | 8 | ||||||
| Hodi FS et al. (Hodi et al., 2018) | 2018 | NCT01844505 | CheckMate 067 | 945 | Melanoma | 3 | Nivolumab+Ipilimumab | 314 | 313 | 185 |
| 3 | Ipilimumab | 315 | 311 | 86 | ||||||
| 3 | Nivolumab | 316 | 313 | 70 | ||||||
| Larkin J et al. (Larkin et al., 2018) | 2018 | NCT01721746 | CheckMate 037 | 405 | Melanoma | 3 | Nivolumab | 272 | 268 | 37 |
| 3 | Standard | 133 | 102 | 84 | ||||||
| Ascierto PA et al. (Ascierto et al., 2019) | 2019 | NCT01721772 | CheckMate 066 | 418 | Melanoma | 3 | Nivolumab | 210 | 206 | 31 |
| 3 | Standard | 208 | 205 | 36 | ||||||
| Hellmann MD et al. (Hellmann et al., 2019) | 2019 | NCT02477826 | CheckMate 227 | 1166 | Non small cell lung cancer | 3 | Nivolumab+Ipilimumab | 583 | 576 | 189 |
| 3 | Standard | 583 | 570 | 205 | ||||||
| Kato K et al. (Kato et al., 2019) | 2019 | NCT02569242 | ATTRACTION-3 | 419 | Squamous cell carcinoma | 3 | Nivolumab | 210 | 209 | 38 |
| 3 | Standard | 209 | 208 | 133 | ||||||
| Scherpereel A et al. (Scherpereel et al., 2019) | 2019 | NCT02716272 | IFCT-1501 MAPS2 | 125 | Malignant pleural mesothelioma | 2 | Nivolumab | 63 | 63 | 9 |
| 2 | Nivolumab+Ipilimumab | 62 | 61 | 16 | ||||||
| Wu YL et al. (Wu et al., 2019) | 2019 | NCT02613507 | CheckMate 078 | 504 | Non small cell lung cancer | 3 | Nivolumab | 338 | 337 | 35 |
| 3 | Standard | 166 | 156 | 74 | ||||||
| Motzer RJ et al. (Motzer et al., 2020) | 2020 | NCT02231749 | CheckMate 214 | 1096 | Renal cell carcinoma | 3 | Nivolumab+Ipilimumab | 550 | 547 | 259 |
| 3 | Standard | 546 | 535 | 343 | ||||||
| Reardon DA et al. (Reardon et al., 2020) | 2020 | NCT02017717 | CheckMate 143 | 369 | Glioblastoma | 3 | Nivolumab | 184 | 182 | 33 |
| 3 | Standard | 185 | 165 | 25 | ||||||
| Zimmer L et al. (Zimmer et al., 2020) | 2020 | NCT02523313 | IMMUNED | 115 | Melanoma | 2 | Nivolumab+Ipilimumab | 56 | 55 | 39 |
| 2 | Nivolumab | 59 | 56 | 15 | ||||||
| Baas P et al. (Baas et al., 2021) | 2021 | NCT02899299 | CheckMate 743 | 605 | Malignant pleural mesothelioma | 3 | Nivolumab+Ipilimumab | 303 | 300 | 91 |
| 3 | Standard | 302 | 284 | 91 | ||||||
| Owonikoko TK et al. (Owonikoko et al., 2021) | 2021 | NCT02538666 | CheckMate 451 | 559 | Small cell lung cancer | 3 | Nivolumab+Ipilimumab | 279 | 278 | 145 |
| 3 | Nivolumab | 280 | 279 | 32 | ||||||
| Spigel DR et al. (Spigel et al., 2021) | 2021 | NCT02481830 | CheckMate 331 | 569 | Small cell lung cancer | 3 | Nivolumab | 284 | 282 | 39 |
| 3 | Standard | 285 | 265 | 194 | ||||||
| Tannir NM et al. (Tannir et al., 2021) | 2021 | NA | CheckMate 214 | 139 | Renal cell carcinoma | 3 | Nivolumab+Ipilimumab | 74 | 73 | 36 |
| 3 | Standard | 65 | 65 | 29 | ||||||
| Ribas A et al. (Ribas et al., 2013) | 2013 | NCT00257205 | NA | 655 | Melanoma | 3 | Tremelimumab | 328 | 325 | 192 |
| 3 | Standard | 327 | 319 | 132 | ||||||
| Herbst RS et al. (Herbst et al., 2016) | 2016 | NCT01905657 | KEYNOTE-010 | 687 | Non small cell lung cancer | 2/3 | Pembrolizumab | 344 | 339 | 43 |
| 2/3 | Standard | 343 | 309 | 109 | ||||||
| Hamid O et al. (Hamid et al., 2017) | 2017 | NCT01704287 | KEYNOTE-002 | 359 | Melanoma | 2 | Pembrolizumab | 180 | 178 | 24 |
| 2 | Standard | 179 | 171 | 45 | ||||||
| Shitara K et al. (Shitara et al., 2018) | 2018 | NCT02370498 | KEYNOTE-061 | 395 | Gastric/gastrooesophageal junction cancer | 3 | Pembrolizumab | 196 | 294 | 42 |
| 3 | Standard | 199 | 276 | 96 | ||||||
| Cohen EEW et al. (Cohen et al., 2019) | 2019 | NCT02252042 | KEYNOTE-040 | 495 | Squamous cell carcinoma | 3 | Pembrolizumab | 247 | 246 | 33 |
| 3 | Standard | 248 | 234 | 85 | ||||||
| Fradet Y et al. (Fradet et al., 2019) | 2019 | NCT02256436 | KEYNOTE-045 | 542 | Urothelial cancer | 3 | Pembrolizumab | 270 | 266 | 44 |
| 3 | Standard | 272 | 255 | 128 | ||||||
| Mok TSK et al. (Mok et al., 2019) | 2019 | NCT02220894 | KEYNOTE-042 | 1274 | Non-small cell lung cancer | 3 | Pembrolizumab | 637 | 636 | 113 |
| 3 | Standard | 637 | 615 | 252 | ||||||
| Reck M et al. (Reck et al., 2019) | 2019 | NCT02142738 | KEYNOTE-024 | 305 | Non-small cell lung cancer | 3 | Pembrolizumab | 154 | 154 | 48 |
| 3 | Standard | 151 | 150 | 80 | ||||||
| Robert C et al. (Robert et al., 2019) | 2019 | NCT01866319 | KEYNOTE-006 | 834 | Melanoma | 3 | Pembrolizumab | 556 | 555 | 103 |
| 3 | Ipilimumab | 278 | 256 | 54 | ||||||
| André T et al. (André et al., 2020) | 2020 | NCT02563002 | KEYNOTE-177 | 307 | Colorectal cancer | 3 | Pembrolizumab | 153 | 153 | 86 |
| 3 | Standard | 154 | 143 | 111 | ||||||
| Kojima T et al. (Kojima et al., 2020) | 2020 | NCT02564263 | KEYNOTE-181 | 628 | Esophageal cancer | 3 | Pembrolizumab | 314 | 314 | 57 |
| 3 | Standard | 314 | 296 | 121 | ||||||
| Popat S et al. (Popat et al., 2020) | 2020 | NCT02991482 | ETOP 9-15 | 144 | Malignant pleural mesothelioma | 3 | Pembrolizumab | 73 | 72 | 14 |
| 3 | Standard | 71 | 70 | 18 | ||||||
| Kuruvilla J et al. (Kuruvilla et al., 2021) | 2021 | NCT02684292 | KEYNOTE-204 | 304 | Hodgkin lymphoma | 3 | Pembrolizumab | 151 | 148 | 29 |
| 3 | Standard | 153 | 152 | 38 |
AEs = Adverse events.
PFS is considered to be the primary endpoint of randomized clinical trials evaluating patients with solid tumors (Korn and Crowley, 2013). OS is defined as the time from the start of treatment to death or the last follow-up. The HRs of PFS and OS represent HRs between treatment 1 and 2. In assessing AEs, we chose treatment-related or drug-related AEs as the main results. If there were no treatment or drug-related AEs in studies, we included all AEs. The classification of AEs is often used to evaluate the type and severity of AEs in clinical trials. According to AE classification, grade 3 or higher AEs are considered as severe AEs. The risk of severe AEs is the focus of the evaluation of therapeutic effectiveness, so the number of AEs surveyed and severe AEs were both extracted (Xu et al., 2018).
2.3 Data Synthesis and Statistical Analysis
2.3.1 Adverse Events Analysis
We used gemtc and pcnetmeta packages in R v4.0.3 and called JAGS v4.3.0 to perform statistical analysis in a Bayesian framework based on Markov Chain Monte Carlo (MCMC) methods, and generated the graph depicting the network geometry (Wang et al., 2019).
Firstly, we made a rough comparison between the fit of the consistent model with the inconsistent model. Secondly, the inconsistency on the specific comparison was tested by node splitting analysis. p < 0.05 was considered as indicating a significant inconsistency. Outstanding consistency is the key to robust results, as evidenced by the consistency between direct and indirect results. We compared the results of network meta-analysis (indirect results) with those of pairwise analysis (direct results) to explore the sources of inconsistency. Additionally, if there existed significant heterogeneity, we used the random-effect model. Otherwise, we used the fixed-effect model (Dias et al., 2011). We used non-information prior distributions and overdispersed initial values (scaling 2.5) in 3 chains to fit the model. 56 independent randomized controlled experiments yielded 100,000 iterations (including 20,000 optimization iterations) with 10 refinement intervals for each chain. This method was used to generate a posterior distribution of model parameters. The convergence of iterations was evaluated by using the Gelman-Rubin-Brooks statistics, all of which converge near 1. Based on the odds ratio (OR) advantage ratio and posterior probability, we ranked probabilities of each treatment as the safest, followed by the second, third and so on.
2.4 Progression-free Survival and Overall Survival Analysis
For the consistency and heterogeneity analysis of PFS and OS, we chose to use R’s netmeta package in the Frequentist framework to make a preliminary judgment using the traditional frequency method, avoiding the artificial bias caused by complex prior settings, settings of dummy variables and variance-covariance matrices of regression models in Bayesian statistics, which would simplify the operator’s parameter setting. The I2 test was used to evaluate the heterogeneity between studies, with the significance level set as p < 0.05. I2 greater than 25, 50 or 75% indicated low, medium and high heterogeneity respectively. If significant heterogeneity exists, the random-effect model was used. Otherwise, we employed the fixed-effect model (Higgins and Thompson, 2002).
Since Bayesian statistics are more accurate and the results are highly consistent with those in the frequency model, we subsequently chose the Bayesian framework by using the MCMC method in WinBUGS v1.4.3 for network meta-analysis. We used the consistency model (due to I2 < 25) to calculate HRs and 95% CIs. We simulated 3 different chains, each with 45,000 built-in samples, resulting in 15 iterations with a refinement rate of 15 (3 different chains with 15,000 iterations and 45,000 burn-in samples and 50 thinning rates). The model fitting was further determined according to the deviation information criterion. The output was a posterior distribution of relative effect size, and we got the estimated average of HR and 95% CI (95% CI as the 2.5th and 97.5th percentiles) (van Valkenhoef et al., 2012). The ranking probability distribution was calculated, ranking the probabilities of each treatment as the safest, followed by the second, third and so on.
3 Results
3.1 Literature Search and Study Characteristics
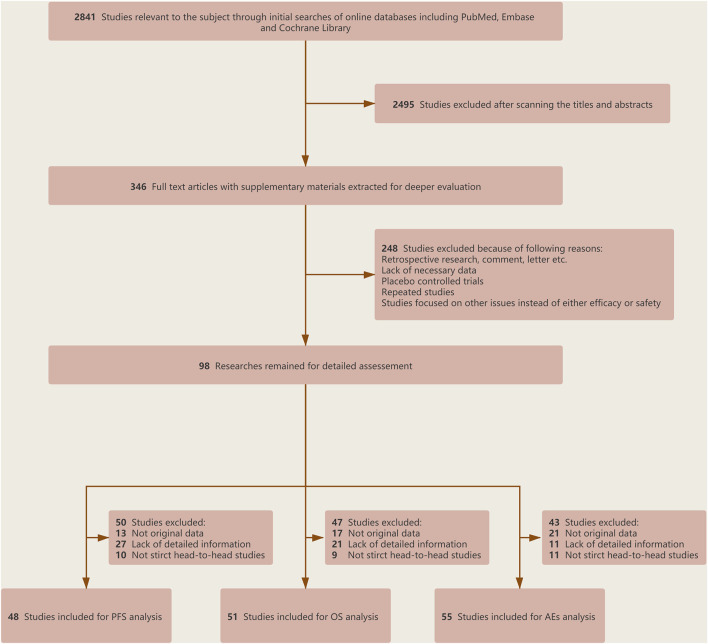
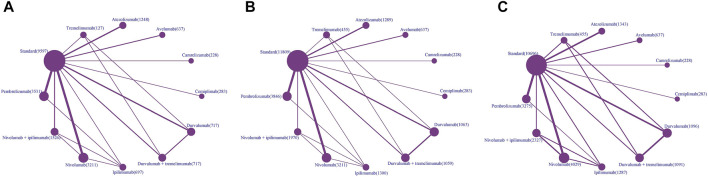
After a preliminary search, a total of 2,841 related articles were identified. After the screening of the title and abstract, 2,495 studies were excluded because they did not meet the corresponding standards. We carefully reviewed the remaining studies and then incorporated 63 RCTs for final analysis (2,14–75). The literature selection flowchart is shown in Figure 1. Of these, 48 RCTs involving 22,519 patients were analyzed for HRs of PFS, 51 RCTs involving 27,150 patients were analyzed for HRs of OS, and 55 RCTs involving 26,747 patients were analyzed for severe AEs (Figure 2).
FIGURE 1.
Flowchart of selection criteria and study design.
FIGURE 2.
Network plots of comparisons for PFS (A), OS (B) and AEs (C) of different types of treatment-based network meta-analysis. Each node represents a treatment. The size of the circle is in proportion to the number of patients. The line width is in proportion to the number of patients included in the direct comparison of two treatments.
In terms of PFS, ICI drugs included nivolumab (n = 13), pembrolizumab (n = 13), atezolizumab (n = 6), durvalumab (n = 6), ipilimumab (n = 5), avelumab (n = 3), tremelimumab (n = 2), camrelizumab (n = 1), cemiplimab (n = 1), nivolumab plus ipilimumab (n = 7), durvalumab plus tremelimumab (n = 5). Cancer types tested in these studies include lung cancer (n = 16), melanoma (n = 6), squamous cell carcinoma (n = 6), gastric/gastrooesophageal junction cancer (n = 4), renal cell carcinoma (n = 4), colorectal cancer (n = 2), malignant pleural mesothelioma (n = 2), ovarian cancer (n = 2), urothelial cancer (n = 2), breast cancer (n = 1), esophageal cancer (n = 1), glioblastoma (n = 1), Hodgkin lymphoma (n = 1) (Figure 2A).
In terms of OS, ICI drugs included nivolumab (n = 13), pembrolizumab (n = 13), durvalumab (n = 7), atezolizumab (n = 6), ipilimumab (n = 6), avelumab (n = 3), tremelimumab (n = 3), camrelizumab (n = 1), cemiplimab (n = 1), nivolumab plus ipilimumab (n = 7), durvalumab plus tremelimumab (n = 6). Cancer types tested in these studies include lung cancer (n = 17), melanoma (n = 9), squamous cell carcinoma (n = 6), urothelial cancer (n = 4), gastric/gastrooesophageal junction cancer (n = 3), renal cell carcinoma (n = 3), breast cancer (n = 2), colorectal cancer (n = 1), malignant pleural mesothelioma (n = 2), ovarian cancer (n = 2), esophageal cancer (n = 1), glioblastoma (n = 1) (Figure 2B).
In terms of severe AEs, ICI drugs included nivolumab (n = 17), pembrolizumab (n = 12), durvalumab (n = 8), atezolizumab (n = 6), ipilimumab (n = 6), avelumab (n = 3), tremelimumab (n = 3), camrelizumab (n = 1), cemiplimab (n = 1), nivolumab plus ipilimumab (n = 11), durvalumab plus tremelimumab (n = 7). Cancer types tested in these studies include lung cancer (n = 17), melanoma (n = 11), squamous cell carcinoma (n = 6), renal cell carcinoma (n = 4), gastric/gastrooesophageal junction cancer (n = 3), malignant pleural mesothelioma (n = 3), urothelial cancer (n = 3), colorectal cancer (n = 2), breast cancer (n = 1), esophageal cancer (n = 1), glioblastoma (n = 1), hodgkin lymphoma (n = 1), ovarian cancer (n = 1), pancreatic ductal adenocarcinoma (n = 1) (Figure 2C).
3.2 Progression-free Survival
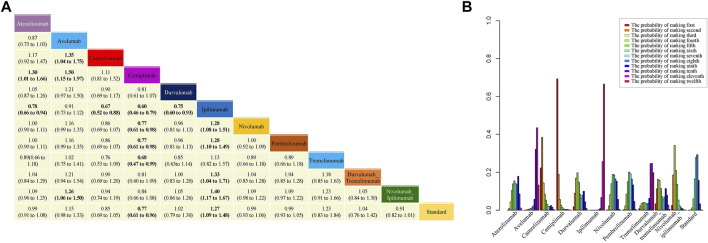
In analyzing PFS, no significant heterogeneity (I2 = 19%) or inconsistency was observed (p = 0.97) (Supplementary Table S2). Therefore, the Bayesian fixed-effect model was used. HRs and 95% CI from the network meta-analysis are shown in Figure 3A. Treatment with ipilimumab was significantly worse in terms of PFS than standard therapies, whereas treatment with cemiplimab significantly improved PFS. According to the probability ranking diagram, the results showed that cemiplimab was the best choice in terms of PFS, camrelizumab ranked the second safest and nivolumab plus ipilimumab ranked the third safest, whereas ipilimumab was the worst (Figure 3B). Additionally, treatment with ipilimumab was significantly worse than most other treatments in terms of PFS. Interestingly, nivolumab plus ipilimumab significantly improved PFS compared to ipilimumab, which suggested that treatment with combinations of ICI drugs may benefit PFS compared to monotherapy. The results calculated according to the frequency model were highly consistent with the results of the Bayesian fixed-effect model.
FIGURE 3.
Results of network meta-analysis (NMA), safety profile (A) and probability ranking diagram (B) in the Bayesian model. In the safety profile, efficacy of treatment for progression-free survival (PFS) is represented as hazard ratios (HRs) with 95% confidence intervals. All comparisons are made as column versus row. Statistically significant results are in bold. Probability ranking diagram shows the probability of the safety of different therapies ranking the first to the last for PFS.
Additionally, we performed subgroup analyses based on treatment of different cancer types, particularly lung cancer and melanoma. The safety profile and probability ranking diagram for lung cancer and melanoma are shown in Supplementary Figures S1, S4 in the Supplement respectively. Cemiplimab was also the best choice in terms of PFS in treating lung cancer, and nivolumab plus ipilimumab ranked the second safest. Compared with standard therapies, HR (95% CI) for cemiplimab was 0.77 (0.61–0.96). Treatment with cemiplimab also significantly improved PFS compared to nivolumab. Tremelimumab was considered the worst choice in terms of PFS in treating lung cancer. In terms of melanoma, our results showed that nivolumab plus ipilimumab was the best choice for PFS. HR (95% CI) for nivolumab plus ipilimumab was 0.69 (0.52–0.92) compared with standard therapies. In addition, HRs (95% CI) for nivolumab and pembrolizumab were 0.78 (0.65–0.94) and 0.80 (0.64–0.99) respectively. The probability ranking diagram of melanoma indicated that ipilimumab was the worst choice for PFS.
3.3 Overall Survival
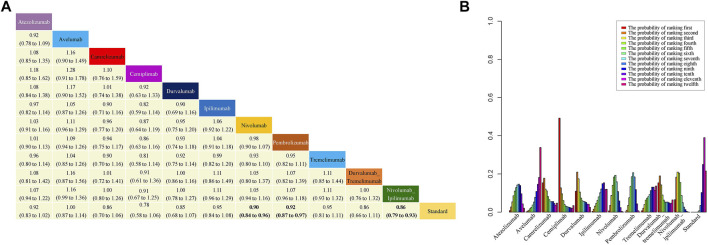
In analyzing OS, no consistency (I2 = 0%) or inconsistency (p = 0.60) (Supplementary Table S2) was observed, and so the Bayesian fixed-effects model was applied. HRs and 95% CI are shown in Figure 4A. Treatment with nivolumab, pembrolizumab and nivolumab plus ipilimumab significantly improved OS compared to standard therapies. According to the probability ranking diagram, cemiplimab was the best choice in terms of OS, and durvalumab ranked the second safest, whereas avelumab was the worst (Figure 4B). Of note, nivolumab plus ipilimumab may improve OS compared with nivolumab and ipilimumab monotherapy, which was similar to durvalumab plus tremelimumab compared with durvalumab and tremelimumab monotherapy. The results calculated based on the frequency model were also highly similar to the results of the Bayesian fixed-effect model.
FIGURE 4.
Results of NMA, safety profile (A) and probability ranking diagram (B). In the safety profile, efficacy of treatment for overall survival (OS) is represented as HRs with 95% confidence intervals. All comparisons are made as column versus row. Statistically significant results are in bold. Probability ranking diagram shows the probability of the safety of different therapies ranking the first to the last for OS.
We also conducted subgroup analyses for lung cancer and melanoma. The safety profiles and probability ranking diagrams of lung cancer and melanoma are shown in Supplementary Figures S5, S8 in the Supplement. For lung cancer treatment, cemiplimab was the best option for OS and durvalumab ranked the second safest. Safety profile of lung cancer suggested that compared with standard therapies, HRs (95% CI) for nivolumab, nivolumab plus ipilimumab and pembrolizumab were 0.91 (0.82–0.99), 0.87 (0.76–0.99), and 0.89 (0.80–0.99) respectively. Standard therapies were considered to be the worst option for lung cancer in terms of OS whereas. For melanoma treatment, nivolumab plus ipilimumab was the best option. Safety profiles showed that HR (95% CI) for nivolumab was 0.84 (0.71–0.99) compared with standard therapies. Our results also indicated that standard therapies were the worst choice for melanoma in terms of OS.
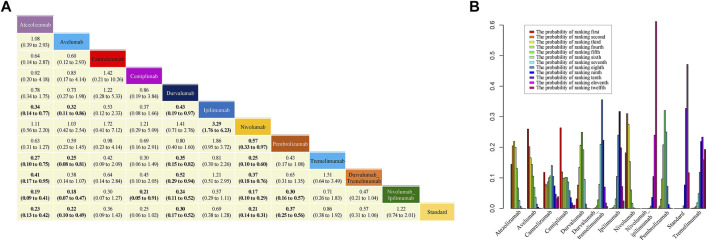
3.4 Severe Adverse Events
In the network meta-analyses of severe AEs, high heterogeneity was found (Supplememntary Table S3), and the random-effect model was employed. Safety profile in the consistency model is shown in Figure 5A. Atezolizumab, avelumab, durvalumab, nivolumab, and pembrolizumab all significantly reduced the risk of grade 3 or higher AEs compared to standard therapies. Compared with standard therapies, ORs (95% CI) for atezolizumab, avelumab, durvalumab, nivolumab, and pembrolizumab were 0.23 (0.13–0.42), 0.22 (0.10–0.49), 0.30 (0.17–0.52), 0.21 (0.14–0.31), and 0.37 (0.25–0.56) respectively. It is worth noting that there was no direct evidence that durvalumab plus tremelimumab could reduce the risk of severe AEs compared to durvalumab and tremelimumab monotherapy. Similarly, there was no evidence that the combination of nivolumab and ipilimumab could significantly reduce the risk of AEs compared with nivolumab and ipilimumab monotherapy. Even combination therapies increased the risk of severe AEs (durvalumab vs. durvalumab plus tremelimumab: [OR], 0.52%; 95% CI, 0.29–0.94; nivolumab vs. nivolumab plus ipilimumab: [OR], 0.17%; 95% CI, 0.10–0.29).
FIGURE 5.
Results of NMA, safety profile (A) and probability ranking diagram (B). In the safety profile, efficacy of treatment for grade 3–5 adverse events is represented as odd ratios (ORs) with 95% confidence intervals. All comparisons are made as column versus row. Statistically significant results are in bold. Probability ranking diagram shows the probability of the safety of different therapies ranking the first to the last for severe adverse events.
Figure 5B shows the probability ranking diagram of 12 interventions. The probabilities of becoming the safest choice for severe AEs were as follows: cemiplimab (26.3%), avelumab (25.9%), nivolumab (18.2%), atezolizumab (14.5%), and camrelizumab (11.8%). The remaining interventions were all less than 5% likely to be the safest option, with nivolumab plus ipilimumab appearing to be the worst choice.
Through node splitting analysis, significant inconsistency could not be detected for most comparisons (Supplementary Table S4). There was significant inconsistency between ipilimumab and nivolumab plus ipilimumab, and ipilimumab and standard therapies (p < 0.05). The comparison between nivolumab plus ipilimumab and standard therapies also showed a degree of inconsistency (p = 0.08). In the direct comparisons, patients receiving nivolumab plus ipilimumab were more likely to have severe AEs than those receiving ipilimumab, and patients receiving ipilimumab were more likely to have severe AEs than those receiving standard therapies. However, patients receiving standard therapies were more likely to have severe AEs than those receiving nivolumab plus ipilimumab. The comparison between the above three groups may be the main reason for the inconsistency.
We performed subgroup analyses of lung cancer, melanoma, and squamous cell carcinoma treatment. The respective safety profiles and probability ranking diagram are shown in Supplementary Figures S9, S14 in the Supplement. Interestingly, the results suggested that nivolumab was the joint best choice for lung cancer, melanoma and squamous cell carcinoma. Standard therapies, based on the probability ranking diagram, were considered to be the worst for lung cancer and squamous cell carcinoma treatment, and nivolumab plus ipilimumab was the worst for melanoma treatment.
4 Discussion
In order to comprehensively evaluate the safety and efficacy of ICI drug monotherapy and combination therapies, we conducted a network meta-analysis combining HRs of PFS and OS, and the risk of severe AEs, and performed subgroup analyses particularly for lung cancer and melanoma. The application of bioinformatics is often used to analyze published data (Wu et al., 2017). To our knowledge, this study is the first comprehensive report comparing PFS HRs, OS HRs, and corresponding treatment-related severe AEs among ICI drug monotherapy, combination therapies and standard therapies.
In terms of PFS and OS, we first tested the heterogeneity and consistency of network meta-analysis based on the frequency method. No significant heterogeneity and consistency were found, indicating that this network meta-analysis was consistent in PFS and OS. We used the frequency model and the Bayesian model separately. The results of the frequency model and the Bayesian model agree well. In view of the greater accuracy of the Bayesian model, our final results were presented by the Bayesian model.
In terms of PFS, treatment with ipilimumab was significantly worse than standard therapies, whereas treatment with cemiplimab significantly improved PFS. The results also indicated that cemiplimab was the best choice for PFS. Treatment with nivolumab, pembrolizumab and nivolumab plus ipilimumab significantly improved OS compared to standard therapies. For OS, cemiplimab was considered to be the best choice, whereas avelumab was the worst. Since few studies compared cemiplimab and camrelizumab with other therapies, nivolumab plus ipilimumab ranked the third safest in PFS and durvalumab ranked the second safest in terms of OS. In comparing ICI drug combination therapies with monotherapy, we found that nivolumab plus ipilimumab significantly improved PFS compared to ipilimumab. Additionally, nivolumab plus ipilimumab may improve OS compared with nivolumab and ipilimumab monotherapy, similar to durvalumab plus tremelimumab compared with durvalumab, and tremelimumab monotherapy. However, further studies are needed to compare the safety and efficacy of ICI drug combination treatment with monotherapy. Although abundant included studies may lead to low significance of the overall results in terms of PFS and OS, we thought that the results would be more reliable and further performed subgroup analysis.
Severe AEs were examined as the measure of the toxicity of different ICI drug therapies and standard therapies. In terms of severe AEs, there was a large inconsistency in the comparison between the above three groups, which is similar to inconsistency reported by Chen et al (Xu et al., 2018), but the degree of inconsistency was more obvious in the comparison between ipilimumab and standard therapies in this study. We considered the main reasons as follows: In spite that the inclusion and exclusion criteria in our study are similar to those of Chen et al, eligible studies with inconsistent results were relatively abundant, which may lead to higher inconsistency. Of note, Chen et al combined durvalumab plus tremelimumab and nivolumab plus ipilimumab as the two ICI drug group, however, we grouped them in our study to assess the safety and efficacy more precisely.
4.1 Strengths and Limitations
The main strengths of our studies are as follows: we used the Bayesian model to conduct network meta-analysis and then employed the frequency model for inconsistency test and result verification in terms of PFS and OS. We found that the results of the frequency model were highly consistent with those of the Bayesian model, and we represented our final results from the Bayesian model, which greatly enhanced the reliability of conclusions. To comprehensively investigate the safety and efficacy of various ICI drugs, we analyzed three different indicators PFS, OS and severe AEs. Of note, we included enough studies to ensure the accuracy of the results. Despite that some studies represent the data of the same RCTs at different times, we chose the most recent results as much as possible.
This study also has several limitations. Firstly, enrolled studies showed high heterogeneity. In order to avoid publication bias, we tested the heterogeneity and used different models accordingly. Secondly, the number of RCTs that meet the requirements for inclusion is different among ICI drugs at present, and there is obvious inconsistency in severe AEs, which require more studies for higher-level verification. Thirdly, despite randomization of the eligible studies, there are still characteristic imbalances between the groups in trials.
5 Conclusion
In the present study, the Bayesian model was used to comprehensively assess survival data and the risk of severe AEs for ICI drugs, which showed that different ICI drug therapies may pose different risks in terms of PFS, OS and severe AEs. Our study may provide new insights and strategies for the clinical practice of ICI drugs.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
The authors (ZX, JL and ZZ) contributed equally to this work. ZX and JL contributed to the design of the study, literature search and data analysis. ZX and ZZ identified eligible trials, extracted the data and assessed the quality of clinical trials. CC and WC provided some ideas for this study. BJ and GZ processed the data and generated the tables and figures. YW and YM contributed to the interpretation of the data. BB and JW drafted and critically revised the manuscript.
Funding
This study was supported by the Youth Medical Talent of Jiangsu Province (grant no. QNRC2016475).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.883655/full#supplementary-material
References
- Amaria R. N., Reddy S. M., Tawbi H. A., Davies M. A., Ross M. I., Glitza I. C., et al. (2018). Neoadjuvant Immune Checkpoint Blockade in High-Risk Resectable Melanoma. Nat. Med. 24 (11), 1649–1654. 10.1038/s41591-018-0197-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- André T., Shiu K. K., Kim T. W., Jensen B. V., Jensen L. H., Punt C., et al. (2020). Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 383 (23), 2207–2218. 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- Ascierto P. A., Long G. V., Robert C., Brady B., Dutriaux C., Di Giacomo A. M., et al. (2019). Survival Outcomes in Patients with Previously Untreated BRAF Wild-type Advanced Melanoma Treated with Nivolumab Therapy: Three-Year Follow-Up of a Randomized Phase 3 Trial. JAMA Oncol. 5 (2), 187–194. 10.1001/jamaoncol.2018.4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P., Scherpereel A., Nowak A. K., Fujimoto N., Peters S., Tsao A. S., et al. (2021). First-line Nivolumab Plus Ipilimumab in Unresectable Malignant Pleural Mesothelioma (CheckMate 743): a Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet 397 (10272), 375–386. 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- Bachelot T., Filleron T., Bieche I., Arnedos M., Campone M., Dalenc F., et al. (2021). Durvalumab Compared to Maintenance Chemotherapy in Metastatic Breast Cancer: the Randomized Phase II SAFIR02-BREAST IMMUNO Trial. Nat. Med. 27 (2), 250–255. 10.1038/s41591-020-01189-2 [DOI] [PubMed] [Google Scholar]
- Bang Y. J., Cho J. Y., Kim Y. H., Kim J. W., Di Bartolomeo M., Ajani J. A., et al. (2017). Efficacy of Sequential Ipilimumab Monotherapy versus Best Supportive Care for Unresectable Locally Advanced/Metastatic Gastric or Gastroesophageal Junction Cancer. Clin. Cancer Res. 23 (19), 5671–5678. 10.1158/1078-0432.CCR-17-0025 [DOI] [PubMed] [Google Scholar]
- Bang Y. J., Ruiz E. Y., Van Cutsem E., Lee K. W., Wyrwicz L., Schenker M., et al. (2018). Phase III, Randomised Trial of Avelumab versus Physician's Choice of Chemotherapy as Third-Line Treatment of Patients with Advanced Gastric or Gastro-Oesophageal Junction Cancer: Primary Analysis of JAVELIN Gastric 300. Ann. Oncol. 29 (10), 2052–2060. 10.1093/annonc/mdy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlesi F., Vansteenkiste J., Spigel D., Ishii H., Garassino M., de Marinis F., et al. (2018). Avelumab versus Docetaxel in Patients with Platinum-Treated Advanced Non-small-cell Lung Cancer (JAVELIN Lung 200): an Open-Label, Randomised, Phase 3 Study. Lancet Oncol. 19 (11), 1468–1479. 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D. R., Steins M., Ready N. E., et al. (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (17), 1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K. L., Baas P., Crinò L., Eberhardt W. E., Poddubskaya E., et al. (2015). Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (2), 123–135. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone D. P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., et al. (2017). First-Line Nivolumab in Stage IV or Recurrent Non-small-cell Lung Cancer. N. Engl. J. Med. 376 (25), 2415–2426. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. E. W., Soulières D., Le Tourneau C., Dinis J., Licitra L., Ahn M. J., et al. (2019). Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-And-Neck Squamous Cell Carcinoma (KEYNOTE-040): a Randomised, Open-Label, Phase 3 Study. Lancet 393 (10167), 156–167. 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- Dias S., Welton N. J., Sutton A. J., Ades A. (2011). NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. London: National Institute for Health and Care Excellence. [PubMed] [Google Scholar]
- Eng C., Kim T. W., Bendell J., Argilés G., Tebbutt N. C., Di Bartolomeo M., et al. (2019). Atezolizumab with or without Cobimetinib versus Regorafenib in Previously Treated Metastatic Colorectal Cancer (IMblaze370): a Multicentre, Open-Label, Phase 3, Randomised, Controlled Trial. Lancet Oncol. 20 (6), 849–861. 10.1016/S1470-2045(19)30027-0 [DOI] [PubMed] [Google Scholar]
- Fehrenbacher L., Spira A., Ballinger M., Kowanetz M., Vansteenkiste J., Mazieres J., et al. (2016). Atezolizumab versus Docetaxel for Patients with Previously Treated Non-small-cell Lung Cancer (POPLAR): a Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. Lancet 387 (10030), 1837–1846. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- Fehrenbacher L., von Pawel J., Park K., Rittmeyer A., Gandara D. R., Ponce Aix S., et al. (2018). Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-small Cell Lung Cancer. J. Thorac. Oncol. 13 (8), 1156–1170. 10.1016/j.jtho.2018.04.039 [DOI] [PubMed] [Google Scholar]
- Ferris R. L., Blumenschein G., Fayette J., Guigay J., Colevas A. D., Licitra L., et al. (2016). Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 375 (19), 1856–1867. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris R. L., Haddad R., Even C., Tahara M., Dvorkin M., Ciuleanu T. E., et al. (2020). Durvalumab with or without Tremelimumab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: EAGLE, a Randomized, Open-Label Phase III Study. Ann. Oncol. 31 (7), 942–950. 10.1016/j.annonc.2020.04.001 [DOI] [PubMed] [Google Scholar]
- Fradet Y., Bellmunt J., Vaughn D. J., Lee J. L., Fong L., Vogelzang N. J., et al. (2019). Randomized Phase III KEYNOTE-045 Trial of Pembrolizumab versus Paclitaxel, Docetaxel, or Vinflunine in Recurrent Advanced Urothelial Cancer: Results of >2 Years of Follow-Up. Ann. Oncol. 30 (6), 970–976. 10.1093/annonc/mdz127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Chehrazi-Raffle A., Reddi S., Salgia R. (2018). Development of PD-1 and PD-L1 Inhibitors as a Form of Cancer Immunotherapy: a Comprehensive Review of Registration Trials and Future Considerations. J. Immunother. Cancer 6 (1), 8. 10.1186/s40425-018-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O., Puzanov I., Dummer R., Schachter J., Daud A., Schadendorf D., et al. (2017). Final Analysis of a Randomised Trial Comparing Pembrolizumab versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Advanced Melanoma. Eur. J. Cancer 86, 37–45. 10.1016/j.ejca.2017.07.022 [DOI] [PubMed] [Google Scholar]
- Hellmann M. D., Paz-Ares L., Bernabe Caro R., Zurawski B., Kim S. W., Carcereny Costa E., et al. (2019). Nivolumab Plus Ipilimumab in Advanced Non-small-cell Lung Cancer. N. Engl. J. Med. 381 (21), 2020–2031. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- Herbst R. S., Baas P., Kim D. W., Felip E., Pérez-Gracia J. L., Han J. Y., et al. (2016). Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-small-cell Lung Cancer (KEYNOTE-010): a Randomised Controlled Trial. Lancet 387 (10027), 1540–1550. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- Herbst R. S., Giaccone G., de Marinis F., Reinmuth N., Vergnenegre A., Barrios C. H., et al. (2020). Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 383 (14), 1328–1339. 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G. (2002). Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 21 (11), 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Hodi F. S., Chesney J., Pavlick A. C., Robert C., Grossmann K. F., McDermott D. F., et al. (2016). Combined Nivolumab and Ipilimumab versus Ipilimumab Alone in Patients with Advanced Melanoma: 2-year Overall Survival Outcomes in a Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 17 (11), 1558–1568. 10.1016/S1470-2045(16)30366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F. S., Chiarion-Sileni V., Gonzalez R., Grob J. J., Rutkowski P., Cowey C. L., et al. (2018). Nivolumab Plus Ipilimumab or Nivolumab Alone versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 19 (11), 1480–1492. 10.1016/S1470-2045(18)30700-9 [DOI] [PubMed] [Google Scholar]
- Hodi F. S., O'Day S. J., McDermott D. F., Weber R. W., Sosman J. A., Haanen J. B., et al. (2010). Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 363 (8), 711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Xu J., Chen Y., Zhuang W., Zhang Y., Chen Z., et al. (2020). Camrelizumab versus Investigator's Choice of Chemotherapy as Second-Line Therapy for Advanced or Metastatic Oesophageal Squamous Cell Carcinoma (ESCORT): a Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 21 (6), 832–842. 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- Hutton B., Salanti G., Caldwell D. M., Chaimani A., Schmid C. H., Cameron C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern Med. 162 (11), 777–784. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- Kato K., Cho B. C., Takahashi M., Okada M., Lin C. Y., Chin K., et al. (2019). Nivolumab versus Chemotherapy in Patients with Advanced Oesophageal Squamous Cell Carcinoma Refractory or Intolerant to Previous Chemotherapy (ATTRACTION-3): a Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 20 (11), 1506–1517. 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- Kojima T., Shah M. A., Muro K., Francois E., Adenis A., Hsu C. H., et al. (2020). Randomized Phase III KEYNOTE-181 Study of Pembrolizumab versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. 38 (35), 4138–4148. 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- Korn R. L., Crowley J. J. (2013). Overview: Progression-free Survival as an Endpoint in Clinical Trials with Solid Tumors. Clin. Cancer Res. 19 (10), 2607–2612. 10.1158/1078-0432.CCR-12-2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla J., Ramchandren R., Santoro A., Paszkiewicz-Kozik E., Gasiorowski R., Johnson N. A., et al. (2021). Pembrolizumab versus Brentuximab Vedotin in Relapsed or Refractory Classical Hodgkin Lymphoma (KEYNOTE-204): an Interim Analysis of a Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 22 (4), 512–524. 10.1016/S1470-2045(21)00005-X [DOI] [PubMed] [Google Scholar]
- Larkin J., Minor D., D'Angelo S., Neyns B., Smylie M., Miller W. H., et al. (2018). Overall Survival in Patients with Advanced Melanoma Who Received Nivolumab versus Investigator's Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J. Clin. Oncol. 36 (4), 383–390. 10.1200/JCO.2016.71.8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott D. F., Huseni M. A., Atkins M. B., Motzer R. J., Rini B. I., Escudier B., et al. (2018). Clinical Activity and Molecular Correlates of Response to Atezolizumab Alone or in Combination with Bevacizumab versus Sunitinib in Renal Cell Carcinoma. Nat. Med. 24 (6), 749–757. 10.1038/s41591-018-0053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok T. S. K., Wu Y. L., Kudaba I., Kowalski D. M., Cho B. C., Turna H. Z., et al. (2019). Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-small-cell Lung Cancer (KEYNOTE-042): a Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 393 (10183), 1819–1830. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- Motzer R. J., Escudier B., McDermott D. F., Arén Frontera O., Melichar B., Powles T., et al. (2020). Survival Outcomes and Independent Response Assessment with Nivolumab Plus Ipilimumab versus Sunitinib in Patients with Advanced Renal Cell Carcinoma: 42-month Follow-Up of a Randomized Phase 3 Clinical Trial. J. Immunother. Cancer 8 (2), e000891. 10.1136/jitc-2020-000891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R. J., Escudier B., McDermott D. F., George S., Hammers H. J., Srinivas S., et al. (2015). Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 373 (19), 1803–1813. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly E. M., Oh D. Y., Dhani N., Renouf D. J., Lee M. A., Sun W., et al. (2019). Durvalumab with or without Tremelimumab for Patients with Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 5 (10), 1431–1438. 10.1001/jamaoncol.2019.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owonikoko T. K., Park K., Govindan R., Ready N., Reck M., Peters S., et al. (2021). Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J. Clin. Oncol. 39 (12), 1349–1359. 10.1200/JCO.20.02212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Özgüroğlu M., Vansteenkiste J., Spigel D., Yang J. C. H., Ishii H., et al. (2021). Avelumab versus Docetaxel in Patients with Platinum-Treated Advanced NSCLC: 2-Year Follow-Up from the JAVELIN Lung 200 Phase 3 Trial. J. Thorac. Oncol. 16 (8), 1369–1378. 10.1016/j.jtho.2021.03.009 [DOI] [PubMed] [Google Scholar]
- Planchard D., Reinmuth N., Orlov S., Fischer J. R., Sugawara S., Mandziuk S., et al. (2020). ARCTIC: Durvalumab with or without Tremelimumab as Third-Line or Later Treatment of Metastatic Non-small-cell Lung Cancer. Ann. Oncol. 31 (5), 609–618. 10.1016/j.annonc.2020.02.006 [DOI] [PubMed] [Google Scholar]
- Popat S., Curioni-Fontecedro A., Dafni U., Shah R., O'Brien M., Pope A., et al. (2020). A Multicentre Randomised Phase III Trial Comparing Pembrolizumab versus Single-Agent Chemotherapy for Advanced Pre-treated Malignant Pleural Mesothelioma: the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-Meso Trial. Ann. Oncol. 31 (12), 1734–1745. 10.1016/j.annonc.2020.09.009 [DOI] [PubMed] [Google Scholar]
- Powles T., Csőszi T., Özgüroğlu M., Matsubara N., Géczi L., Cheng S. Y., et al. (2021). Pembrolizumab Alone or Combined with Chemotherapy versus Chemotherapy as First-Line Therapy for Advanced Urothelial Carcinoma (KEYNOTE-361): a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 22 (7), 931–945. 10.1016/S1470-2045(21)00152-2 [DOI] [PubMed] [Google Scholar]
- Powles T., Durán I., van der Heijden M. S., Loriot Y., Vogelzang N. J., De Giorgi U., et al. (2018). Atezolizumab versus Chemotherapy in Patients with Platinum-Treated Locally Advanced or Metastatic Urothelial Carcinoma (IMvigor211): a Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet 391 (10122), 748–757. 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- Powles T., van der Heijden M. S., Castellano D., Galsky M. D., Loriot Y., Petrylak D. P., et al. (2020). Durvalumab Alone and Durvalumab Plus Tremelimumab versus Chemotherapy in Previously Untreated Patients with Unresectable, Locally Advanced or Metastatic Urothelial Carcinoma (DANUBE): a Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol. 21 (12), 1574–1588. 10.1016/S1470-2045(20)30541-6 [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E., Fujiwara K., Ledermann J. A., Oza A. M., Kristeleit R., Ray-Coquard I. L., et al. (2021). Avelumab Alone or in Combination with Chemotherapy versus Chemotherapy Alone in Platinum-Resistant or Platinum-Refractory Ovarian Cancer (JAVELIN Ovarian 200): an Open-Label, Three-Arm, Randomised, Phase 3 Study. Lancet Oncol. 22 (7), 1034–1046. 10.1016/S1470-2045(21)00216-3 [DOI] [PubMed] [Google Scholar]
- Pujol J. L., Greillier L., Audigier-Valette C., Moro-Sibilot D., Uwer L., Hureaux J., et al. (2019). A Randomized Non-comparative Phase II Study of Anti-programmed Cell Death-Ligand 1 Atezolizumab or Chemotherapy as Second-Line Therapy in Patients with Small Cell Lung Cancer: Results from the IFCT-1603 Trial. J. Thorac. Oncol. 14 (5), 903–913. 10.1016/j.jtho.2019.01.008 [DOI] [PubMed] [Google Scholar]
- Reardon D. A., Brandes A. A., Omuro A., Mulholland P., Lim M., Wick A., et al. (2020). Effect of Nivolumab vs Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 6 (7), 1003–1010. 10.1001/jamaoncol.2020.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M., Rodríguez-Abreu D., Robinson A. G., Hui R., Csőszi T., Fülöp A., et al. (2019). Updated Analysis of KEYNOTE-024: Pembrolizumab versus Platinum-Based Chemotherapy for Advanced Non-small-cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 37 (7), 537–546. 10.1200/JCO.18.00149 [DOI] [PubMed] [Google Scholar]
- Ribas A., Kefford R., Marshall M. A., Punt C. J., Haanen J. B., Marmol M., et al. (2013). Phase III Randomized Clinical Trial Comparing Tremelimumab with Standard-Of-Care Chemotherapy in Patients with Advanced Melanoma. J. Clin. Oncol. 31 (5), 616–622. 10.1200/JCO.2012.44.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi N. A., Cho B. C., Reinmuth N., Lee K. H., Luft A., Ahn M. J., et al. (2020). Durvalumab with or without Tremelimumab vs Standard Chemotherapy in First-Line Treatment of Metastatic Non-small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 6 (5), 661–674. 10.1001/jamaoncol.2020.0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Long G. V., Brady B., Dutriaux C., Di Giacomo A. M., Mortier L., et al. (2020). Five-Year Outcomes with Nivolumab in Patients with Wild-type BRAF Advanced Melanoma. J. Clin. Oncol. 38 (33), 3937–3946. 10.1200/JCO.20.00995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Ribas A., Schachter J., Arance A., Grob J. J., Mortier L., et al. (2019). Pembrolizumab versus Ipilimumab in Advanced Melanoma (KEYNOTE-006): Post-hoc 5-year Results from an Open-Label, Multicentre, Randomised, Controlled, Phase 3 Study. Lancet Oncol. 20 (9), 1239–1251. 10.1016/S1470-2045(19)30388-2 [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Yang J. C., Restifo N. P. (2004). Cancer Immunotherapy: Moving beyond Current Vaccines. Nat. Med. 10 (9), 909–915. 10.1038/nm1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherpereel A., Mazieres J., Greillier L., Lantuejoul S., Dô P., Bylicki O., et al. (2019). Nivolumab or Nivolumab Plus Ipilimumab in Patients with Relapsed Malignant Pleural Mesothelioma (IFCT-1501 MAPS2): a Multicentre, Open-Label, Randomised, Non-comparative, Phase 2 Trial. Lancet Oncol. 20 (2), 239–253. 10.1016/S1470-2045(18)30765-4 [DOI] [PubMed] [Google Scholar]
- Sezer A., Kilickap S., Gümüş M., Bondarenko I., Özgüroğlu M., Gogishvili M., et al. (2021). Cemiplimab Monotherapy for First-Line Treatment of Advanced Non-small-cell Lung Cancer with PD-L1 of at Least 50%: a Multicentre, Open-Label, Global, Phase 3, Randomised, Controlled Trial. Lancet 397 (10274), 592–604. 10.1016/S0140-6736(21)00228-2 [DOI] [PubMed] [Google Scholar]
- Shitara K., Özgüroğlu M., Bang Y. J., Di Bartolomeo M., Mandalà M., Ryu M. H., et al. (2018). Pembrolizumab versus Paclitaxel for Previously Treated, Advanced Gastric or Gastro-Oesophageal Junction Cancer (KEYNOTE-061): a Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 392 (10142), 123–133. 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- Shitara K., Van Cutsem E., Bang Y. J., Fuchs C., Wyrwicz L., Lee K. W., et al. (2020). Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients with First-Line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 6 (10), 1571–1580. 10.1001/jamaoncol.2020.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu L. L., Even C., Mesía R., Remenar E., Daste A., Delord J. P., et al. (2019). Safety and Efficacy of Durvalumab with or without Tremelimumab in Patients with PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 5 (2), 195–203. 10.1001/jamaoncol.2018.4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigel D. R., Vicente D., Ciuleanu T. E., Gettinger S., Peters S., Horn L., et al. (2021). Second-line Nivolumab in Relapsed Small-Cell Lung Cancer: CheckMate 331☆. Ann. Oncol. 32 (5), 631–641. 10.1016/j.annonc.2021.01.071 [DOI] [PubMed] [Google Scholar]
- Tannir N. M., Signoretti S., Choueiri T. K., McDermott D. F., Motzer R. J., Flaifel A., et al. (2021). Efficacy and Safety of Nivolumab Plus Ipilimumab versus Sunitinib in First-Line Treatment of Patients with Advanced Sarcomatoid Renal Cell Carcinoma. Clin. Cancer Res. 27 (1), 78–86. 10.1158/1078-0432.CCR-20-2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarhini A. A., Lee S. J., Hodi F. S., Rao U. N. M., Cohen G. I., Hamid O., et al. (2020). Phase III Study of Adjuvant Ipilimumab (3 or 10 Mg/kg) versus High-Dose Interferon Alfa-2b for Resected High-Risk Melanoma: North American Intergroup E1609. J. Clin. Oncol. 38 (6), 567–575. 10.1200/JCO.19.01381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S. L., Hodi F. S., Brahmer J. R., Gettinger S. N., Smith D. C., McDermott D. F., et al. (2012). Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 366 (26), 2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Valkenhoef G., Lu G., de Brock B., Hillege H., Ades A. E., Welton N. J. (2012). Automating Network Meta-Analysis. Res. Synth. Methods 3 (4), 285–299. 10.1002/jrsm.1054 [DOI] [PubMed] [Google Scholar]
- Wang J., Li X., Wu X., Wang Z., Zhang C., Cao G., et al. (2019). Role of Immune Checkpoint Inhibitor-Based Therapies for Metastatic Renal Cell Carcinoma in the First-Line Setting: a Bayesian Network Analysis. EBioMedicine 47, 78–88. 10.1016/j.ebiom.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Yao Q., Chen W., Gao F., Li P., Wu J., et al. (2021). Stem Cell Therapy for Crohn's Disease: Systematic Review and Meta-Analysis of Preclinical and Clinical Studies. Stem Cell Res. Ther. 12 (1), 463. 10.1186/s13287-021-02533-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Mandala M., Del Vecchio M., Gogas H. J., Arance A. M., Cowey C. L., et al. (2017). Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 377 (19), 1824–1835. 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- Winer E. P., Lipatov O., Im S. A., Goncalves A., Muñoz-Couselo E., Lee K. S., et al. (2021). Pembrolizumab versus Investigator-Choice Chemotherapy for Metastatic Triple-Negative Breast Cancer (KEYNOTE-119): a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 22 (4), 499–511. 10.1016/S1470-2045(20)30754-3 [DOI] [PubMed] [Google Scholar]
- Wu J., Chen Z. P., Shang A. Q., Wang W. W., Chen Z. N., Tao Y. J., et al. (2017). Systemic Bioinformatics Analysis of Recurrent Aphthous Stomatitis Gene Expression Profiles. Oncotarget 8 (67), 111064–111072. 10.18632/oncotarget.22347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Lu W. Y., Cui L. L. (2015). Clinical Significance of STAT3 and MAPK Phosphorylation, and the Protein Expression of Cyclin D1 in Skin Squamous Cell Carcinoma Tissues. Mol. Med. Rep. 12 (6), 8129–8134. 10.3892/mmr.2015.4460 [DOI] [PubMed] [Google Scholar]
- Wu Y. L., Lu S., Cheng Y., Zhou C., Wang J., Mok T., et al. (2019). Nivolumab versus Docetaxel in a Predominantly Chinese Patient Population with Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J. Thorac. Oncol. 14 (5), 867–875. 10.1016/j.jtho.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Xu C., Chen Y. P., Du X. J., Liu J. Q., Huang C. L., Chen L., et al. (2018). Comparative Safety of Immune Checkpoint Inhibitors in Cancer: Systematic Review and Network Meta-Analysis. BMJ 363, k4226. 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarin D., Burger R. A., Sill M. W., Powell D. J., Lankes H. A., Feldman M. D., et al. (2020). Randomized Phase II Trial of Nivolumab versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J. Clin. Oncol. 38 (16), 1814–1823. 10.1200/JCO.19.02059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer L., Livingstone E., Hassel J. C., Fluck M., Eigentler T., Loquai C., et al. (2020). Adjuvant Nivolumab Plus Ipilimumab or Nivolumab Monotherapy versus Placebo in Patients with Resected Stage IV Melanoma with No Evidence of Disease (IMMUNED): a Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet 395 (10236), 1558–1568. 10.1016/S0140-6736(20)30417-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.