Abstract
Lactic acid bacteria (LAB) are Gram-positive bacteria which are considered for use as adjuvant therapeutics in management of various disease ailments, including obesity, irritable bowel syndrome, lactose intolerance and cancer. To investigate the possible use of Lactococcus lactis strains from our collection in treatment of gastrointestinal cancer, we tested them for the ability to arrest proliferation of human colorectal adenocarcinoma cells (Caco-2). Results of the BrdU assay showed that the anti-proliferative activity of L. lactis cells is strain-specific. We found that particularly, two strains, L. lactis IBB109 and L. lactis IBB417, exhibited the most potent inhibitory effect. Moreover, both strains triggered interleukin 18 gene expression, normally inhibited in Caco-2 (cancer) cells. To examine the probiotic potential of the two strains, we tested them for bile salts and acid tolerance, as well as adhesion properties. Both isolates exhibited probiotic potential—they survived in the presence of 0.3% bile salts and tolerated exposure to low pH and osmotic stress. Notably, we found that L. lactis IBB417 displayed better adherence to mucus and Caco-2 cells than L. lactis IBB109. Additionally, by microdilution tests we confirmed that both strains are sensitive to all nine antibiotics of human and veterinary importance listed by the European Food Safety Authority. Finally, by in silico investigations of whole genome sequencing data, we revealed the genetic features of L. lactis IBB109 and L. lactis IBB417 that can be associated with functional (e.g., adhesion and carbohydrate metabolic genes) and safety (e.g., virulence and antibiotic resistance) aspects of the strains, confirming their health-promoting potential.
Keywords: whole genome sequencing, probiotic bacteria, Lactococcus lactis, colorectal cancer, proliferation inhibition, adhesion, interleukin 18
Introduction
Lactic acid bacteria (LAB) are a diverse group of GRAS (generally regarded as safe) Gram-positive microorganisms used in the food industry, especially for production of dairy foods. Certain LAB strains carry probiotic properties and are documented to exert a positive effect on human and animal health. Due to these features, they are considered for use in therapeutic interventions. The majority of probiotics belong to lactobacilli and bifidobacteria, but include also Lactococcus, Streptococcus, Pediococcus strains, among others (Fijan, 2014; Markowiak and Śliżewska, 2017). Some of these bacteria (e.g., Lacticaseibacillus rhamnosus GG—basonym Lactobacillus rhamnosus GG, Lacticaseibacillus casei Shirota—basonyn Lactobacillus casei, and Lactobacillus acidophilus LA-1) are marketed as probiotic preparations for use as health supplements (Sniffen et al., 2018; Zielińska and Kolożyn-Krajewska, 2018).
Strains with probiotic properties are isolated from various natural sources, such as traditional dairy products (e.g., kefir, oscypek, Iranian Spar), fermented food (e.g., sausages, cabbage, kimchi), raw milk of various origin (e.g., human, cow, goat), and human or animal intestinal microbiota (Zielińska and Kolożyn-Krajewska, 2018). Regardless of the source, the characteristic traits defining probiotic strains include adhesion to intestinal mucosa and/or epithelial layer, resistance to low pH, osmotic stress and bile salts, and the ability to degrade complex carbohydrates (Salminen et al., 2005; Ventura et al., 2012; Markowiak and Śliżewska, 2017).
Among the recognized health-promoting effects of probiotic LAB strains are anti-inflammatory properties, stimulation of the host immune system, competition for nutrients and ecological niches with pathogenic bacteria, inhibition of toxic substance activity, and reduction in lactose intolerance (Saxelin et al., 2005; Markowiak and Śliżewska, 2017). The above-mentioned effects are attained by various pathways/mechanisms and effector molecules. Depending on the site of action, these molecules can be grouped as intracellular factors, such as enzymes (lactase, bile salts hydrolases), metabolites (lactate, short chain fatty acids—SCFAs, bacteriocins), DNA with unmethylated CpG motifs, or surface located determinants (e.g., peptidoglycan, (lipo)teichoic acids, pili, exopolysaccharides, Ser/Thr-rich proteins, S-layer proteins) (Saxelin et al., 2005; Lee et al., 2013; Kim et al., 2017; Wang et al., 2019).
Experimental studies show the beneficial influence of selected LAB strains on the course of certain illnesses related with dysbiosis of the gut microbiota, including irritable bowel syndrome, diarrhoeas, inflammatory bowel disease or vaginal infections (Reid and Bocking, 2003; Markowiak and Śliżewska, 2017). Restoration of microbial balance is often successfully supported by probiotic LAB strains, which produce a plethora of metabolites and bioproducts that maintain gut homeostasis. LAB, provided as dietary supplements, were shown to confer protection against the imbalance of gut microbiota, stimulate the immune defense mechanisms of the host and increase nutrient bioavailability (Zitvogel et al., 2017; Vamanu and Gatea, 2020). Specifically, administration of Lb. plantarum DSM 9843 to patients with irritable bowel syndrome reduced amounts of enterococci in fecal samples and provided symptomatic relief (Nobaek et al., 2000). Intake of probiotic strains (Lb. rhamnosus GG, Lb. rhamnosus Lc705, Propionibacterium freudenreichii ssp. shermanii JS and Bifidobacterium animalis ssp. lactis Bb12) in patients with irritable bowel syndrome was found to stabilize intestinal microbiota (Kajander et al., 2008). A plethora of LAB strains are described to induce immune responses in the gut and activate secretion of proinflammatory and regulatory cytokines (e.g., IFN-ƴ, IL-10, IL-12, TNF-α, IL-1β; Matsuzaki and Chin, 2000; Zhong et al., 2014). In a recent study (Hradicka et al., 2020), a mix of lactobacilli strains were shown to increase the level of interleukin 18 in colorectal cancer (CRC) cells, which is recognized as an important cytokine for homeostasis of the gut epithelial cells and prevention of CRC progression (Mager et al., 2016). In turn, LAB strains (Lb. fermentum B4655, Lb. plantarum B4495, Lb. casei B1922, Lb. bulgaricus CFR2028 and Lb. acidophilus B4496) were described to improve mineral bioavailability during soymilk fermentation, reduce the levels of phytic acid and increase the amount of bioactive isoflavones (Rekha and Vijayalakshmi, 2010).
There is growing evidence on the health-promoting effect of LAB strains in respect to CRC, the third most common malignancy affecting the global human population (Baghbani-Arani et al., 2020; Settanni et al., 2020). Epidemiological studies on cancer patients and populations at increased risk have revealed that consumption of cultured dairy products has an inverse correlation with the risk of CRC (Pala et al., 2011; Zhang et al., 2019). Numerous studies report on the beneficial role of probiotics in treatment of CRC in animals and in preventive or post-operative interventions in humans (Serban, 2014). The anticancer activity of some LAB strains, mainly lactobacilli, is documented also by in vitro assays. Cell-free cultures of vaginal isolates, Lb. acidophilus 36YL and Enterococcus faecalis, displayed anticancer activity against four different human cancer cell lines, including gastric (AGS) and colon (HT-29) cells (Nami et al., 2014a,b). Also, Lb. paracasei IMPC2.1 and Lb. rhamnosus GG inhibited the proliferation of colon (DLD-1) and gastric (HGC-27) cell lines (Orlando et al., 2012). Notably, data concerning the anti-proliferative effect of L. lactis strains are limited. Soluble cell extract of a L. lactis ssp. lactis strain (L.lac CF) was shown to have an antiproliferative effect on the human stomach cancer cell line SNU-1 (Kim et al., 2009). A similar effect was elicited on CRC cells SW480 by the cell wall and cytoplasmic extract of L. lactis PTCC 1336 strain (Hosseini et al., 2020).
In light of these studies, LAB strains with confirmed antitumor effects are widely pursued as novel drugs/food supplements for the modulation of gut microbiota in prevention or supplementary treatment of CRC. Yet, application of LAB of unknown genome content may lead, especially in immunocompromised patients, to the development of opportunistic strain or even bacteremia. The intended therapeutic use of LAB strains necessitates confirmation of their biosafety, both at the physiological and genetic level. The evolving era of probiogenomics allows for an in-depth screening of determinants that stand behind the probiotic potential of selected strains, including adhesion to host mucin layer, tolerance to bile salts and acidic conditions, inhibition of pathogens, production of bioactive compounds, ability to metabolize complex carbohydrates.
Our screening for L. lactis strains with antiproliferative effects on CRC cells showed that the inhibitory activity is strain-dependent. Particularly, two strains (L. lactis IBB109 and L. lactis IBB417) exhibited a high inhibitory effect against Caco-2 cells and increased IL-18 production in these cells. We evaluated the resistance of IBB109 and IBB417 to low pH, bile salts and osmolytes, and characterized their adhesive properties as well as sugar catabolic potential. To identify the genetic factors associated with the physiological properties and to determine the biosafety of both strains at sequence level, we performed whole-genome sequencing (WGS). The presence of putative adhesins suggests possible adherence of L. lactis IBB109 and IBB417 to mucins, extracellular matrix (ECM) and host epithelial cells. Potential stress resistance pathways and genes that are likely to help them survive in the harsh conditions of the gastrointestinal tract (GIT) were detected. We also identified genes encoding proteins that could stimulate the production of immune cells and whole metabolic pathways that might provide prohealth properties. By confirming the lack of virulence, toxic metabolite and transmissible antibiotic resistance genes, we evaluated the biosafety of both strains. Results of our study support future industrial/therapeutic applications of L. lactis IBB109 and L. lactis IBB417 strains.
Materials and Methods
Bacteria Strains and Growth Conditions
Lactococcus lactis strains used in the study derived from the bacterial strain collection of IBB PAS (COLIBB, Poland). All of the tested L. lactis strains in this collection were initially isolated from various natural dairy sources (non-commercial products), such as artisanal, home-made products or raw milk and were previously determined to have different RAPD (randomly amplified polymorphic DNA) profiles. Particularly, IBB109 was isolated from raw cow milk and IBB417 from traditionally produced bryndza cheese. All L. lactis strains taken for analyses are listed in Table 1. Cells were grown in liquid M17 media (Oxoid) supplemented with 0.5% glucose (GM17) or GM17 solid agar (1.5%) plates at 30°C under aerobic conditions.
Table 1.
Lactococcus lactis strains used in the study.
| L. lactis strain | Relevant features | Source or references |
|---|---|---|
| IBB109 | Raw milk isolate | GenBank accession no. CP087600-CP087605 |
| IBB417 | Traditional bryndza cheese isolate | GenBank accession no. CP087699-CP087704 |
| IL1403 | L. lactis subsp. lactis wild-type strain | Chopin et al., 1984 |
| IL6288 | Prophage-free derivative of L. lactis IL1403 strain | Bolotin et al., 2019 |
| TIL448 | L. lactis subsp. lactis NCDO2110, isolated from peas | Le et al., 2013 |
| IBB477 | L. lactis subsp. cremoris, wild-type strain | Radziwiłł-Bieńkowska et al., 2016 |
Cell Cultures and Growth Conditions
Human colorectal adenocarcinoma-derived cell lines Caco-2 (ATCC®HTB-37TM) were cultured in conditions recommended by the American Type Culture Collection i.e., at 37°C, 5% CO2, 95% humidity in MEM (Minimal Essential Medium; Gibco, Waltham, MA, United States), supplemented with 10% FBS (fetal bovine serum, Sigma-Aldrich, St. Louis, MO, United States), NEAA (1X; non-essential amino acid solution, Sigma-Aldrich, St. Louis, MO, United States), 1 mM sodium pyruvate (Sigma-Aldrich, St. Louis, MO, United States) and addition of penicillin (100 U ml−1), and streptomycin (100 μg ml−1) (Sigma-Aldrich, St. Louis, MO, United States) in flasks to obtain appropriate density. Cells were then detached by trypsin (Sigma-Aldrich, St. Louis, MO, United States), and their number was counted in the Thoma cell counting chamber. Next, cells were diluted in cell medium to obtain 105 cells ml−1. Cells were seeded on 96-well plates at 104 cells per cell and incubated for 24 h. Immediately before application of bacterial cell suspensions, the medium was removed.
Cultivation of Bacteria for Cell Proliferation Assay
Overnight (o/n) bacterial cultures grown in liquid GM17 were centrifuged (5,500 × g, 4°C, 10 min.) and washed twice with phosphate-buffered saline (PBS pH 7.4; BioShop, Burlington, Canada). To determine the concentration of bacterial cell suspensions, serial dilutions were plated on GM17 solid agar and counted after o/n incubation at 30°C. Until that time, the remaining part of bacterial cell suspensions were kept at 4°C o/n. Next, they were centrifuged (5,500 × g, 4°C, 10 min.) and resuspended in supplemented MEM without antibiotics to a final concentration of 108 colony forming units (CFUs) ml−1. Samples of 100 μl were applied on 96-well plates coated with Caco-2 cells.
Cell Proliferation Assay
Ninety six-well plates covered with the human colorectal adenocarcinoma-derived cell line Caco-2 were co-incubated with bacterial suspensions (107 CFU per well) for 72 h in optimal conditions for cell culture growth (37°C, 5% CO2, 95% humidity). Proliferation of Caco-2 was determined by colorimetric method using the Cell Proliferation Kit, BrdU (Roche) according to manufacturer’s instruction. The assay was performed in 10 technical repeats. Cell cultures incubated without bacteria served as a control.
Sugar Fermentation Profiles
The sugar catabolic potential was assessed using the API® 50 CHL kit (BioMerieux, France) containing strips with 49 different carbon sources. Strains were prepared and assayed according to the manufacturer’s recommendations. The resulting sugar fermentation pattern was established after 48-h incubation at 37°C in aerobic conditions. Full change in color of the indicator medium was interpreted as a partial sugar utilization. The experiment was performed in triplicate. L. lactis IL6288 strain—the prophage-free derivative of the model IL403 strain, served as a control for comparison.
Adhesion Assays to Polystyrene and Mucin
The ability of the tested bacteria to adhere to abiotic (bare polystyrene) and biotic surfaces (polystyrene plates coated with mucin) was investigated according to Radziwiłł-Bieńkowska et al. (2016). Mucin-covered 96-well plates (Thermo Scientific, Waltham, MA, United States) were prepared by coating each well with type III mucin from porcine stomach (PGM; Sigma-Aldrich, St. Louis, MO, United States) dissolved to a final concentration of 10 mg ml−1 in PBS pH 7.4. After the removal of mucin plates were washed twice with PBS (BioShop, Burlington, Canada). Then, 100 μl of bacterial cell suspension at optical density of 1 at 600 nm (OD600 1) was placed in each well of bare or mucin-covered plates and incubated aerobically at 30°C for 3 h. Next, the plates were washed with PBS buffer and stained with 0.22 μm-membrane filtered crystal violet (Scharlau, Sentmenat, Spain). The adhesion was assessed by spectrophotometric measurements of stained cells at OD583. Strains with confirmed high adhesion properties to polystyrene (IBB477; Radziwiłł-Bieńkowska et al., 2016) and to mucins (TIL448; Le et al., 2013) were used as positive controls. Each experiment was made in three independent biological replicates with eight technical repetitions.
Adhesion to Caco-2 Cells
The ability of the tested bacteria to adhere to the Caco-2 cell layer was investigated according to the previously published method (Turpin et al., 2012) with minor modifications. For this purpose, Caco-2 cells were cultured in supplemented MEM with antibiotics (as specified above) on 12-well tissue plates for 14 days at 37°C, 5% CO2, 95% humidity. The cell culture MEM medium was changed every 3 days; the last change was for medium without antibiotics. Bacterial cells were grown in GM17 liquid medium for 16 h, then harvested by centrifugation (5,500 × g, 4°C, 10 min.), washed twice with cold PBS buffer and resuspended in supplemented MEM without antibiotics. Next, bacterial suspensions were added to each well at a ratio of 10:1 (10 CFU of bacteria per 1 Caco-2 cell). Plates were incubated for 3 h at optimal conditions and then washed three times with PBS to remove unbound bacteria. Caco-2 cells with attached bacteria were gently lysed in PBS with 0.1% TRITON X100 (Sigma-Aldrich, St. Louis, MO, United States). Serial dilutions of recovered bacterial cells (adherent bacteria) were plated on GM17 agar and incubated at 30°C for 48 h for viable count enumeration. The adhesion was expressed as the number of viable adherent bacterial cells (CFU) per 1 Caco-2 cell. Results were compared with those obtained for the control strains—L. lactis IL1403 non-adherent strain (negative control) and adherent TIL448 strain. Each experiment was made at least in three independent biological replicates with triplicate technical repetitions.
Bile Salt, Osmolytes, and Acid Tolerance Assays
Simulated conditions of the gut involving acid stress, bile salts resistance and osmotolerance were examined as previously described (Radziwiłł-Bieńkowska et al., 2017; Aleksandrzak-Piekarczyk et al., 2019) with minor modifications. For bile salt tolerance assay, bacterial suspensions at OD600 0.5 were prepared in 1 ml of PBS pH 7.4 (control), PBS pH 7.4 containing 0.1% (w/v) or 0.3% (w/v) bile salts (Sigma cat. no. B8756) and incubated for 1.5, 3, or 6 h at 37°C. To determine resistance toward acidic stress, bacterial suspensions at OD600 0.5 were prepared in 1 ml of saline solution pH 7.0 (control), 4.0 and 2.5. Next, bacterial cells were centrifuged (6,000 g, 5 min, RT) and re-suspended in respective control solutions. Serial dilutions were plated on GM17 solid medium and incubated for 48 h at 30°C. After that time, viable CFUs were determined (per ml) in comparison with control conditions. Osmotic shock characteristics were examined by spot assay. For this o/n, bacterial cultures were plated on GM17 solid plates supplemented with 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0% (w/v) NaCl (Sigma-Aldrich, United States) and incubated at 30°C for 48 h. The results were read by visual inspection of colony growth in comparison with cells plated on GM17 agar lacking NaCl. Each experiment was carried out in three independent biological experiments at two repetitions.
Antibiotic Susceptibility
The antibiotic susceptibility expressed as a minimal inhibitory concentration (MIC) was determined for L. lactis IBB109 and IBB417 using the microdilution method in accordance with the International Organization for Standardization (2010) standard.1 The selected antibiotics, namely ampicillin (AM), vancomycin (VA), gentamicin (GM), kanamycin (KM), streptomycin (SM), erythromycin (EM), clindamycin (CM), tetracycline (TC), and chloramphenicol (CL) were recommended for testing of bacterial strains intended for use as feed additives by the European Food Safety Authority (EFSA, 2012).
Effect of Lactococcus lactis IBB109 and IBB417 on Cytokine Gene Expression in Mammalian Cells
Influence of L. lactis IBB109 and IBB417 on cytokine (IL-1a, IL-18, and TNFɑ) gene expression levels was evaluated in adenocarcinoma colorectal cell line. For this purpose, 24-h cultures of Caco-2 cell lines were incubated with live bacterial cells from the late stationary growth phase. Both human and bacterial cells were cultured as described earlier in the text. Total RNA was extracted using the Universal RNA/miRNA Purification Kit (EURx, Gdańsk, Poland) as three independent biological replicates with three technical repetitions. The level of expression was defined by RT-qPCR method using specific primer pairs: IL-1aF (5′ cgccaatgactcagaggaaga 3′) and IL-1aR (5′ agggcgtcattcaggatgaa 3′) for IL-1a, IL-18F (5′ atcgcttcctctcgcaacaa 3′) and IL-18R (5′ cttctactggttcagcagccatct 3′) for IL-18 and TNF-F (5′ ctcttctgcctgctgcactttg 3′) and TNF-R (5′ atgggctacaggcttgtcactc 3′) for TNFɑ. Results of the assay were normalized against the reference host gene HPRT using specific primer pair: HPRTF (5′ tatggcgacccgcagccct 3′) and HPRTR (5′ catctcgagcaagacgttcag 3′). Reverse transcription was performed with RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, United States) in conditions recommended by the producer, with oligo d(T)18 primers using 0.9 μg of total RNA for each reaction. The qPCR reaction was performed with the LightCycler 480 system (Roche, Basel, Switzerland) and LightCycler 480 SYBR Green I Master (Roche, Basel, Switzerland) by applying 95°C for 10 min, followed by 45 cycles at 95°C for 15 s, 58°C for 15 s, and 72°C for 15 s. Each reaction was performed in three technical repetitions. After completion of the reaction, its specificity was verified by generating melting curves in a single step at 95°C for 5 s and next from 65°C to 97°C with the plate reading at increments of 0.5°C after a 5-s residence time at each temperature. The relative gene expression was calculated using the 2-ΔΔCT method.
Isolation and Genomic DNA Sequencing
Total DNA of L. lactis IBB109 and IBB417 strain was extracted using a Genomic Maxi AX kit (A&A Biotechnology, Gdańsk, Poland) according to the manufacturer’s recommendations. Isolation was preceded by the incubation of the cell pellet with TES (25 mM TRIS pH 8, 10 mM EDTA, 50 mM saccharose) with lysozyme (8 mg ml−1) for 15–30 min. DNA quality control was performed by measuring the absorbance at 260/230 nm, template concentration was determined using Qubit fluorometer (Thermo Fisher Scientific, Waltham, United States), and DNA integrity was analyzed by 0.8% agarose gel electrophoresis and by PFGE using BioRad CHEF-III instrument (BioRad, Hercules, United States). Paired-end sequencing library was constructed using the NEB Ultra II FS Preparation Kit (New England Biolabs, Beverly, United States) according to the manufacturer’s instructions. Library was sequenced using an Illumina MiSeq platform (Illumina, San Diego, United States) with 2 × 300 paired-end reads. Sequence quality metrics were assessed using FASTQC (v0.11.9) (Andrews, 2010) and quality trimmed using fastp (v0.20.0) (Chen et al., 2018). Long reads were obtained using the GridION sequencer (Oxford Nanopore Technologies, Oxford, United Kingdom). Prior to long-read library preparation, genomic DNA was sheared into 30 kb fragments using a 26G needle followed by size selection using Short Read Eliminator kit (Circulomics, United States). Recovered DNA (5 μg) was taken for 1D library construction using SQK-LSK109 kit, and 0.5 μg of final library was loaded into R9.4.1 flowcell and sequenced on the MinION sequencer.
Genome Assembly
Raw nanopore data were base-called using Guppy v3.2.2 (Oxford Nanopore Technologies, Oxford, UK). After quality filtering using NanoFilt (De Coster et al., 2018) and residual adapter removal using Porechop,2 the obtained dataset was quality checked using NanoPlot (De Coster et al., 2018). Long nanopore reads were then assembled in hybrid mode using Unicycler (Wick et al., 2017). The remaining ambiguities in the genome assemblies were verified by PCR amplification of DNA fragments, followed by Sanger sequencing with an ABI3730xl Genetic Analyzer (Life Technologies) using BigDye Terminator Mix v. 3.1 chemistry (Life Technologies). All of the possible sequence errors and mis-assemblies were manually corrected using Seqman software (DNAStar) to obtain the complete nucleotide sequence of bacterial genomes.
Bioinformatic Analyses
Annotation of coding sequences (CDS) and non-coding RNAs was done using the RAST server (Aziz et al., 2008) and checked by BLAST analysis when needed. Prophage loci in the bacterial genomes were identified using the web-based Phaster tool (Zhou et al., 2011; Arndt et al., 2016). Predicted sequence outputs were classified as intact, questionable or incomplete based on their scores: >90, 70–90, or <70, respectively. Plasmid sequences were compared at the nucleotide level using the Circoletto tool (Darzentas, 2010). KEGG database (Kanehisa et al., 2021) and BlastKOALA automatic annotation server (Kanehisa et al., 2016) were used for functional assignment of the identified genes. Search for bacteriocin-encoding genes was performed using the BAGEL4 web server (de Jong et al., 2006). CRISPRCasFinder was used to search for putative CRISPR arrays within the genome sequences of both strains (Couvin et al., 2018). Subcellular localization of proteins was predicted using PSORTb version 3.0 (Yu et al., 2010). The putative adhesion domains in the encoded amino acid sequences were found based on Pfam database (Mistry et al., 2021) using HmmerWeb version 2.41.1 (Potter et al., 2018). Genome sequences were analyzed for the presence of virulence determinants using the VirulenceFinder 2.0 Server version 2.0.3. The database system was designed to detect homologous sequences for the virulence genes related to Escherichia coli, Listeria, Staphylococcus aureus and Enterococcus in WGS data (Malberg Tetzschner et al., 2020). The antibiotic resistance genes (ARGs) were searched using the ResFinder 4.1 Server (Bortolaia et al., 2020), CARD 3.1.3 and RGI 5.2.0 (Jia et al., 2017; Alcock et al., 2020) as well as KEGG database (release 100.0).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, CA, United States) using the Student’s t-test. A value <0.05 was considered statistically significant. Normal distribution was checked with the Shapiro–Wilk test.
Results
Screening of Lactococcus lactis Strains for Their Effect on Colorectal Cancer Cell Proliferation
Seventy Lactococcus sp. strains were tested for their anti-proliferation activity against the Caco-2 CRC cell line. Proliferation of the cultured cells was shown to be affected in a strain-dependent manner (Figure 1; Supplementary Table S1). Stimulation of Caco-2 cell proliferation was observed for three stains. Seven strains had a weak or no adverse effect on Caco-2 proliferation (80%–100% proliferation level compared to untreated cell culture). Semi-moderate (60%–80% proliferation level) and moderate (40%–60% proliferation level) antiproliferative effect was found for 11 and 19 strains, respectively. Majority of the strains (24 strains) negatively affected Caco-2 cells by inhibition of proliferation to the level of 20%–40%. Finally, strong reduction in Caco-2 cell proliferation to the level of ≤20% was observed for six strains. Among them, two strains—IBB109 and IBB417, displayed the most profound antiproliferative effect. Incubation of Caco-2 cells individually with either IBB109 or IBB417 inhibited almost completely the proliferation of the Caco-2 cell line as detected by the BrdU assay. Based on these observations, IBB109 and IBB417 strains seemed to be of particular interest in potential future anti-cancer prophylactic and therapeutic applications and were selected to be analyzed for their probiotic and biosafety potential, including whole-genome sequencing.
Figure 1.
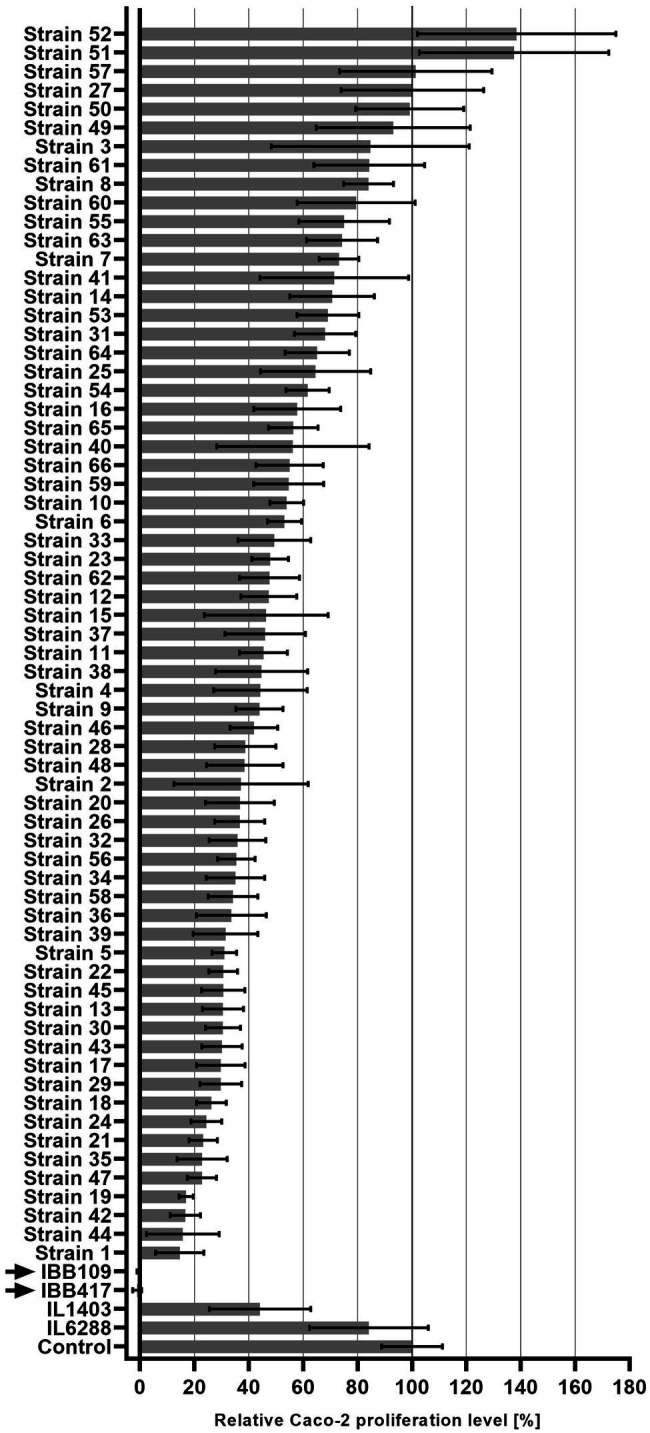
Inhibitory effect of Lactococcus lactis strains on Caco-2 cell proliferation. Proliferation of Caco-2 cells incubated with L. lactis strains was measured in reference to bacteria-free Caco-2 cell culture (100%) using the BrdU colorimetric assay.
Bacterial Viability Under Acidic, Bile Salts, and Osmolytic Stress Conditions
Survival and persistence in the harsh conditions of the GIT are important properties defining probiotic bacteria intended for oral ingestion. For the assessment of this probiotic trait, L. lactis IBB109 and IBB417 strains were tested for their tolerance against bile salts, low pH and osmotic stress as well as the ability to adhere to abiotic (polystyrene) and biotic (mucin and Caco-2 cells) surfaces.
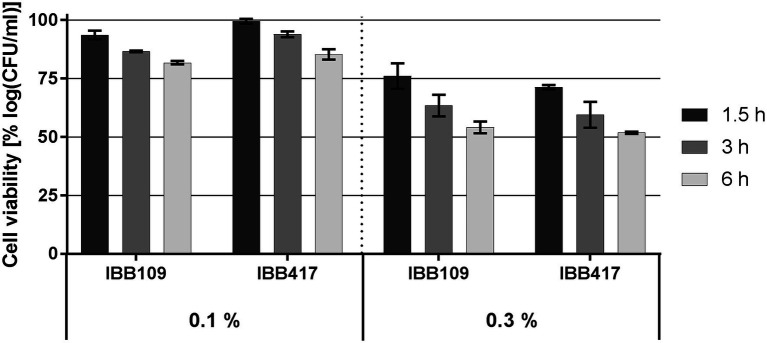
Survival of IBB109 and IBB417 was inversely correlated with bile salts concentrations and time of exposure (Figure 2; Supplementary Table S2). The best tolerance rate for both strains was noted in 0.1% (w/v) bile salts after a 1.5-h incubation compared to control conditions (incubation without bile salts). Extended exposure to bile stress up to 3 h resulted in the decrease in cell count for both strains and reached at 6 h to an overall relative growth reduction by 18.2% (for IBB109) and 14.7% (for IBB417). Increased bile salts concentration (0.3% w/v) had a more pronounced impact on cell survival. After 6 h, the relative cell viability for both strains declined by approx. 50%.
Figure 2.
Bile salts tolerance of L. lactis strains. Cell survival of L. lactis strains was assayed by plate counting after exposure to 0.1% and 0.3% (w/v) bile salts for 1.5, 3, and 6 h. Results were normalized against cell viability under control conditions (without bile salts) and expressed as percent of log CFU ml−1.
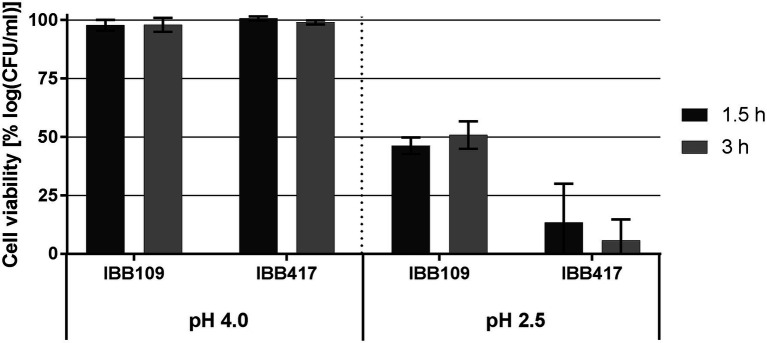
At pH 4, the viability of IBB109 and IBB417 was barely affected compared to control conditions (incubation at pH 7; Figure 3; Supplementary Table S3). In turn, exposure to pH 2.5 was highly lethal for IBB417 (13.4% and 5.8% of survival after 1.5 and 3 h). IBB109 tolerated better the same conditions, presenting approx. 50% relative cell survival after 1.5-hour incubation which remained stable at this level over 3 h.
Figure 3.
Tolerance to acidic conditions of L. lactis strains. Cell survival of L. lactis strains was assayed by plate counting after exposure to pH 4 and pH 2.5 for 1.5 and 3 h. Results were normalized against cell viability under control conditions (pH 7) and expressed as percent of log CFU ml−1.
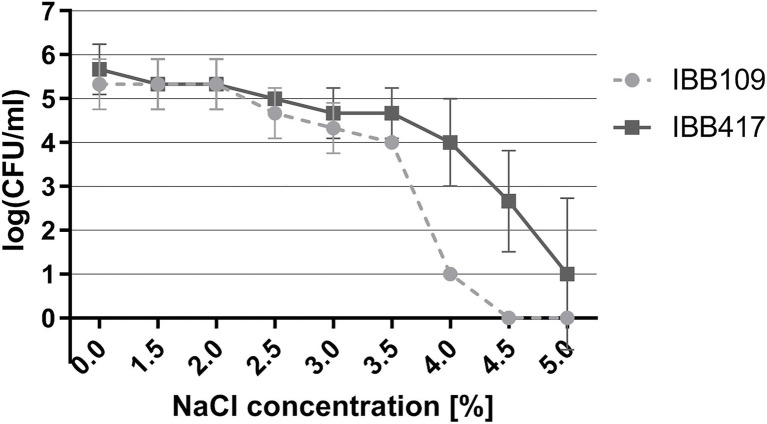
Under osmotic stress, the growth of both strains was essentially unaltered in the range of 0.5%–2% NaCl (Figure 4; Supplementary Table S4). The IBB417 strain showed good tolerance to the rising NaCl concentrations and displayed a 1 log decrease in CFU ml−1 at 4% NaCl vs. 4.5 log declination of IBB109 viability under the same conditions. Osmotic stress was sustained by IBB417 up to 5% NaCl, albeit with a 4.5 log decline in cell growth. In contrast, IBB109 growth was completely inhibited at 4.5% NaCl. In conclusion, IBB417 was determined as more resistant to conditions of osmotic stress compared to IBB109.
Figure 4.
Tolerance of L. lactis strains to osmotic stress. Strains were exposed to NaCl (0%–5%; w/v) by spot assay tests. Cell viability is presented as changes in log CFU ml−1 values at increasing NaCl concentrations.
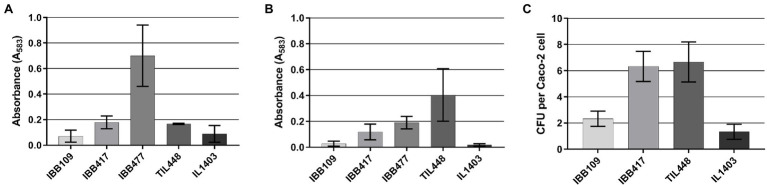
In further assays, we determined that IBB109 and IBB417 exhibit different potential to adhere to the tested abiotic (polystyrene) and biotic (mucin) surfaces (Figures 5A,B). The IBB417 strain demonstrated moderate adherence to both polystyrene and mucin as judged by comparison with adherent control strains, i.e., L. lactis IBB477 (polystyrene) and TIL448 (mucin). The adhesive properties of IBB109 were weaker than of IBB417, yet still higher than that of the non-adherent control strain (L. lactis IL1403).
Figure 5.
In vitro adhesion of L. lactis IBB109 and IBB417 strains to (A) abiotic and (B) biotic surfaces and to (C) Caco-2 cells. Adhesion to bare polystyren (abiotic) and mucin-coated polystyrene (biotic) microplates is expressed as optical density (OD583 nm) of stained cells. Adhesion to Caco-2 cells is expressed as the number of viable adherent bacterial cells (CFU) per single Caco-2 cell. The mean values ± SD from three independent experiments are shown. Control L. lactis strains: IL1403 non-adherent strain (negative control); TIL448 adherent strain to mucin and IBB477 adherent strain to bare polystyrene (positive controls).
Adherence of Lactococcus lactis Strains to the Caco-2 Human Colonic Cells
Results of the proliferation study implied direct interaction of L. lactis IBB109 and IBB417 with the human colonic cells. To test this hypothesis, both strains were investigated for the ability to adhere to Caco-2 (Figure 5C). Results of this assay showed differences in adherence between IBB109 and IBB417 cells. IBB417 demonstrated good adherence to the differentiated Caco-2 monolayer with an approx. 6:1 ratio (6 CFU to 1 Caco-2 cell) and was comparable to values determined for the highly adhesive TIL448 reference strain. This strongly suggests that IBB417 has good adhesive properties to colonic cells. IBB109 displayed moderate adherence to Caco-2, which was at around 2 CFU per 1 Caco-2 cell (2:1 ratio). Still, both strains could adhere to the Caco-2 cell surface at higher levels than that determined for the non-adherent control strain—IL1403.
Sugar Catabolic Potential of IBB109 and IBB417
Results of the carbohydrate fermentation profiles are summarized in Supplementary Table S5. Overall, IBB109 and IBB417 presented a similar sugar catabolic spectrum. Each strain was able to fully metabolize 14 out of 49 carbohydrates tested (D-ribose, D-galactose, D-glucose, D-fructose, D-mannose, N-acetylglucosamine, arbutin, esculin ferric citrate, salicin, D-cellobiose, D-maltose, D-lactose, D-saccharose and D-trehalose) and partially utilize starch/amidon (amylolytic activity) and gentiobiose (slight change in medium color). Additionally, a partial fermentation of amygdalin was noted for IBB417. Both strains presented a wider spectrum of sugar metabolism compared to the control model laboratory strain and were in this respect unique in utilizing lactose, saccharose, and arbutin. Neither of the strains was found to ferment D-raffinose or inulin, which are known to have a prebiotic effect. Nonetheless, the obtained results indicate good potential of metabolizing a wide variety of plant-derived sugars or milk sugars that may be encountered in the gut.
Antibiotic Susceptibility
The inhibitory effect of nine antibiotic substances was tested against using the microdilution method to determine the level of resistance of L. lactis IBB109 and IBB417 strains. Based on the obtained results, both strains were determined to have highly similar antibiotic susceptibility profiles (Table 2). The minimal inhibitory values (MICs) against all of the assayed antibiotics were below the break-point values specified by the International Organization for Standardization (2010) standard (see footnote 1) for L. lactis strains. As the influence of different antibiotics is species-specific, the obtained data were compared with the antibiotic resistance profile of the ATCC 19435 L. lactis control strain lacking antibiotic resistance genes. Both IBB109 and IBB417 exhibited lower MICs than ATCC 19435 and were considered susceptible to all tested antibiotics.
Table 2.
Antibiotic resistance of L. lactis IBB109 and IBB417 strains.
| Antibiotic | Minimal inhibitory concentration (MIC)* | Break-point values for L. lactis strains according to EFSA | ||
|---|---|---|---|---|
| IBB109 | IBB417 | ATCC 19435 (L. lactis AbR-free control strain) | ||
| Gentamicin | 1 | 1–2 | 0.5–4 | 32 |
| Kanamycin | 8–16 | 8 | 2–8 | 64 |
| Streptomycin | 8 | 8–16 | 2–16 | 32 |
| Tetracycline | 0.5 | 0.25–0.5 | 0.5–2 | 4 |
| Erythromycin | 0.063 | 0.032–0.063 | 0.12–0.5 | 1 |
| Clindamycin | 0.032–0.063 | 0.032 | 0.25–0.5 | 1 |
| Chloramphenicol | 4 | 4 | 4–16 | 8 |
| Ampicillin | 0.25–0.5 | 0.5 | 0.5–1 | 2 |
| Vancomycin | 0.25 | 0.25 | 0.25–1 | 4 |
MIC values are given in μg ml−1.
In vitro Effect of IBB109 and IBB417 on Cytokine Production
Probiotic LAB are known to render various immunomodulatory effects in the host organism (Taverniti and Guglielmetti, 2011; Cristofori et al., 2021). To establish whether L. lactis IBB109 and IBB417 can influence host responses, the strains were examined for their ability to induce cytokine production (TNFɑ, IL1a, IL-18) in colorectal adenocarcinoma Caco-2 cells (Supplementary Table S6). Cells treated with IBB109 or IBB417 exhibited significant increase in interleukin-18 gene expression compared to cells without bacteria supplementation (Figure 6). The observed effect was determined to be strain-specific and was not noted for L. lactis IL6288 which served as a control. Notably, the gene expression levels for the other tested cytokines (TNFα and IL-1a) were not significantly affected by IBB109 and IBB417 (Supplementary Table S6).
Figure 6.
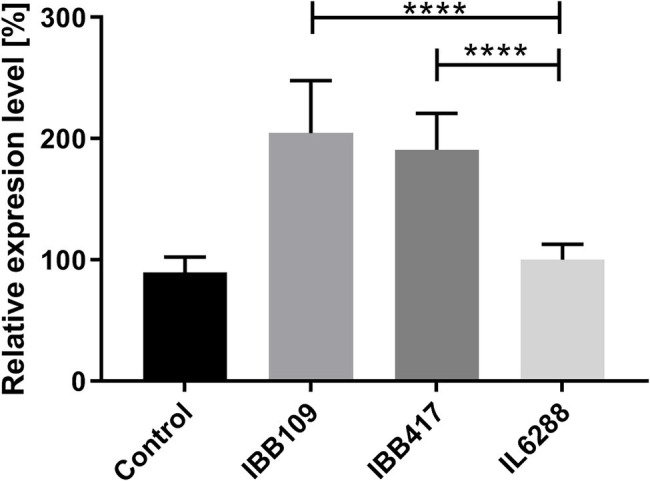
Effect of L. lactis IBB109 and IBB417 strains on interleukin 18 gene expression in Caco-2 cells. L. lactis IL6288 served as a control strain. Gene expression levels were quantified by real-time PCR (RT-qPCR). Relative gene expression levels were normalized against the HPRT host gene. Error bars represent the SE. For statistical analysis, Student’s t-test was used, ****p < 0.0001.
Analysis of Whole Genome Sequencing Data From IBB109 and IBB417 Strains
Lactococcus lactis IBB109 and IBB417 strains, presenting the most prominent activity against human colonic cells, were subjected to whole genome sequencing. Genome sequence analyses of both strains indicated that they belong to the L. lactis subsp. lactis group. The complete genome of each strain contains a circular double-stranded (ds) DNA chromosome of 2,344,660 bp for IBB109 and 2,380,344 bp for IBB417 with an overall 35.3% GC content typical for this bacterial species (Table 3). The number of chromosomal coding DNA sequences (CDS) determined for IBB109 was 2,388 and 2,424 for IBB 417, where approx. 30% encoded hypothetical products of unknown function in both strains.
Table 3.
Main features and annotation data for L. lactis IBB109 and IBB417 genomes.
| Strain | Chromo-some size (bp) | GC content (%) | Number of coding sequences (CDS) in chDNA | Number of CDS in chDNA encoding hypothetical products | tRNA genes | Pro-phage regions | Plasmids |
|---|---|---|---|---|---|---|---|
| IBB 109 | 2,344,660 | 35.3 | 2,388 | 690 | 83 | 9 | pIBB109_AgSa_1 (109 kb) pIBB109_AgSa_2 (55 kb) pIBB109_AgSa_3 (52 kb) pIBB109_AgSa_4 (37 kb) pIBB109_AgSa_5 (35 kb) |
| IBB 417 | 2,380,344 | 35.3 | 2,424 | 704 | 82 | 10 | pIBB417_PrSa_1 (67 kb) pIBB417_PrSa_2 (38 kb) pIBB417_PrSa_3 (9 kb) pIBB417_PrSa_4 (7 kb) pIBB417_PrSa_5 (7 kb) |
IBB109 and IBB417 genomes were searched for the presence of prophage sequences (Supplementary Table S7). Our investigation revealed nine prophage-carrying regions for IBB109 (with three intact prophages, six incomplete and one questionable prophage), and ten regions for IBB417 (with two intact, six incomplete and two questionable prophages). The identified intact prophages exhibited the highest sequence similarity to prophages found in the genome of L. lactis IL1403 (bIL286, bIL311, bIL312) and L. lactis SMQ-86 strain (phage 28201). No confirmed CRISPR/Cas arrays could be detected within the genomes of L. lactis IBB417 and IBB109.
Whole genome sequences of both strains were deposited in GenBank under accession no. CP087600-CP087605 (IBB109) and CP087699-CP087704 (IBB417).
Plasmid Content
IBB109 and IBB417 were found to carry five distinct plasmids each, ranging in size from 7 to 109 kb. All plasmids showed highest similarity to plasmids of other L. lactis strains deposited in the GenBank database (Supplementary Figure S1). Each of the plasmids, except for the 109-kb pIBB109_AgSa_1 mega plasmid, carried at least one gene encoding a replication protein having the Rep_3, L_lactis_RepB_C, RepA_N domains. The presence of mega plasmids in L. lactis is not a typical feature—out of 140 deposited plasmids in the NCBI database (up to 27 Oct, 2021), only four are larger than 100 kb. Examination of the plasmidic gene content in both strains revealed the presence of genes encoding, among others, transporter proteins, genes involved in metabolic pathways of protein (e.g., casein) degradation or sugar (e.g., lactose) catabolism, exopolysaccharide (EPS) synthesis, resistance to heavy metals, restriction–modification systems, biosynthesis of vitamins and amino acids, and DNA repair. Comparison of plasmid sequences between both strains revealed that two pairs of plasmids, pIBB109_AgSa_2 and pIBB417_PrSa_1 as well as pIBB109_AgSa_4 and pIBB417_PrSa_2, are highly conserved at the nucleotide sequence level (respectively, 99% identity, 100% coverage, and 99% identity, 99% coverage) and in terms of gene arrangement (Supplementary Figure S2). These two pairs of plasmids showed the highest sequence similarity also to other lactococcal plasmids present in databases, whereas pIBB109_AgSa_3 and pIBB417_PrSa_3 seemed to be the most unique.
Functional Characterization of IBB109 and IBB417 Genomes
Characterization of IBB109 and IBB417 genomes (Supplementary Tables S8, S9) with BlastKOALA revealed that both strains individually encode 199 metabolic pathways. The pathway modules of the two strains (Supplementary Table S10) showed a high degree of similarity. Particularly, no differences in complete pathway modules between the strains were observed. Comparison of complete pathway modules to the IL1403 strain in the KEGG database revealed the lack of five modules present in IBB109 and IBB417. The exclusive metabolic features absent in IL1403 were the complete pathways for glycogen biosynthesis (KEGG entry: M00854), beta-oxidation (Fatty acid metabolism; KEGG entry: M00086), leucine biosynthesis (KEGG entry: M00432), menaquinone (vitamin K2) biosynthesis (KEGG entry: M00116), and the multidrug resistance efflux pump—MepA (KEGG entry: M00705). In regard to vitamins, IBB109 and IBB417, both have complete pathways for biosynthesis of thiamin (vitamin B1), riboflavin (vitamin B2), tetrahydrofolate (vitamin B9), and menaquinone (vitamin K2). Both strains encode enzymes that are dedicated to sugar internalization, through the ATP-binding cassette (ABC) transporters (galactose oligomer/maltooligosaccharide, ribose/autoinducer 2/D-xylose), phosphotransferase systems (sucrose, β-glucoside, mannitol, lactose, cellobiose/diacetylchitobiose), and phosphoenolpyruvate phosphotransferase systems (mannose, fructose). After internalization, sugars can be further utilized by the various carbohydrate metabolic pathways, including at the first step: glycolysis, pentose phosphate pathway and galactose degradation, Leloir pathway.
Genetic Determinants Encoding Adhesive and Mucoadhesive Properties
The search for extracellular proteins or proteins attached to the cell wall encoded in the L. lactis IBB109 genome using PSORTb indicated 57 chromosomal and 11 plasmidic proteins. Similar analysis for IBB417 indicated 60 chromosomally- encoded and four plasmid-encoded proteins of this type. Both genomes have been further searched for the presence of putative domains involved in adhesion to mucus, ECM or to epithelial cells. The following domains have been detected in both strains: MucBP domains [PF06458]; Mub B2-like domain (Mub_B2) [PF17966]; bacterial Ig-like domain—group 3 (Big_3) [PF07523]; collagen binding domain (Collagen-bind) [PF05737]; BspA-type Leucine-rich repeat region (LRR_5) [PF13306]; fibronectin-binding domain (fibronectin-binding protein A N-terminus (FbpA) [PF05833]; Cna protein B-type domain (Cna_B) [PF05738]; bacterial lectin domains (Bact_lectin) [PF18483]; WxL domain surface cell wall-binding (WxL) [PF13731]; clostridial hydrophobic W domain (ChW) [PF07538] and type II secretory pathway pseudopilin (PulG) [PF11773]. The list of all extracellular or attached to cell wall proteins as well as putative adhesins of other localization with domains detected using the Pfam database that are encoded in the genome of IBB109 and IBB417 strain is provided in Supplementary Tables S11, S12, respectively. Based on these analyses, we identified genes encoding putative adhesins which can be selected for further functional studies. Bacterial adhesion to mammalian cells can also be mediated by non-proteinaceous molecules, such as (lipo)teichoic acids. Similar function could possibly be performed by rhamnose-containing cell wall polysaccharides (RhaCWP; Mistou et al., 2016). A set of genes involved in biosynthesis of teichoic acids and rhamnose-containing glycans were found in genomes of both, IBB109 and IBB417.
Stress Resistance-Related Genes
Probiotics face stress conditions along their transit across the GIT, such as acid, bile, and osmotic stresses. The genome sequences of L. lactis IBB109 and IBB417 were screened for genes previously shown to be differentially expressed in cells cultivated under low and optimum pH in L. lactis subsp. cremoris MG1363 (Carvalho et al., 2013). Upregulated under low pH stress-related genes (such as dnaK and groEL), H+-ATPase subunits, glycolytic genes as well as genes involved in catabolism of amino acids were identified in both analyzed genomes. Additionally, both genomes were searched for genes differentially regulated by bile exposure in Lacticaseibacillus paracasei L9 (Ma et al., 2018). The presence of genes of the malolactic enzyme (MLE) pathway as well several other genes upregulated under bile stress—genes involved in various biological processes, including carbon source utilization (mannose/fructose-inducible phosphotransferase system, alpha-glucosidase, and phosphoglycerate mutase), amino acids and peptide metabolism (ABC oligopeptide transport system and biosynthesis of L-lysine), transmembrane transport (multidrug ABC transport system), transcription factors (Xre family), and membrane proteins (phage holin protein, and hemolysin III protein) were confirmed in IBB109 and IBB417 genomes. The in silico analysis of genes involved in mechanism for salt tolerance found in genomes of Lactiplantibacillus plantarum D31 and T9 strains (Yao et al., 2020) was performed in the tested L. lactis strains. Both genomes contained genes involved in mechanisms associated with: (i) the balancing/conservation of intracellular ionic concentrations (Na+/H+ antiporter and K+ transport systems); (ii) absorption, accumulation or synthesis of compatible solutes (glycine betaine ABC transporter OpuAA/OpuAB and choline-betaine ABC transporter BusAA/BusAB, proline synthesis); (iii) transcriptional or response regulators (GntR, Crp/Fnr, LysR families; RNA polymerase sigma factor, S-adenosylmethionine synthetase, DNA-directed RNA polymerase subunit beta, and amino acid permease); and (iv) universal stress response (DnaK, DnaJ, GroES, GroEL). Most of the genes previously found to be involved in acid/bile/osmotic stresses were also identified in the genome of IBB109 and IBB417; however, the resistance to stress must be further explored using transcriptomics analyses to determine the expression rates of the described genes.
Prohealth-Related Genes
Probiotic bacteria encode a plethora of factors that render health beneficial effects. One of them is an extracellular protein, GroEL, secreted by a range of LAB strains, which was shown to be implicated in adhesion to and immunostimulation of epithelial cells (Bergonzelli et al., 2006; Izquierdo et al., 2009; Gilad et al., 2011). Analysis of the IBB109 and IBB417 genomes revealed that they both encode identical GroEL-like proteins (locus reference: 1_1535829_1534201 for IBB109; locus reference: 1_1528557_1526929 for IBB417), with 100% of identity to the GroEL chaperonin of L. cremoris SMQ562 strain. The presence of the GroEL-encoding gene in IBB109 and IBB417 genomes was suspected to contribute to both adherence and increased cytokine gene expression levels in Caco-2 cells.
Antioxidant enzymes, such as superoxide dismutase (SOD), play a significant role in oxidant stress regulation by detoxifying reactive oxygen species. Moreover, SODs produced by LAB strains were shown to decrease gut inflammation and reduce tumor development in mice (Nishikawa et al., 2004; LeBlanc et al., 2011) as well as influence the host immune response by regulating cytokine production (Carroll et al., 2007; LeBlanc et al., 2011). Both of the studied strains were found to encode for manganese superoxide dismutase (MnSOD) genes in their chromosomes (locus reference: 1_1522447_1521827 for IBB109; locus reference: 1_1515175_1514555 for IBB417). Additionally, for IBB417, a second SOD-encoding gene localized on a plasmid (pIBB417_PrSa_3; locus reference: 3_16496_16299) was determined. In both terms, the SOD-encoding genes could be responsible for the observed in vitro effects of IBB109 and IBB417 on Caco-2 cells.
Due to the antimicrobial and antagonistic activity, which involves production of various antimicrobial compounds (e.g., organic acids, SCFAs, diacetyl, acetoin, bacteriocins), probiotic bacteria can inhibit the growth of other bacteria, including pathogenic species. Both L. lactis strains IBB109 and IBB417 were found to possess complete metabolic pathways to produce lactate, SCFAs (acetate, formate), diacetyl and acetoin (Supplementary Tables S8, S9). Screening for bacteriocins using the BAGEL4 web server revealed that neither strain carried the respective genes.
Anticancer effects have also been associated with production of exopolysaccharides (EPS)–extracellular biopolymers produced by bacteria that form a capsule around the cell or connected loosely as a slime layer (Nwodo et al., 2012; Wu et al., 2021). EPS genes were found only in the IBB109 genome (loci references: 6_15440_14754; 6_16226_15462; 6_16976_16281; Supplementary Table S8).
Virulence Genes
The safety, in respect to the presence of virulence genes, of IBB109 and IBB417 strains was evaluated in silico by comparison of their whole-genome sequences with known virulence genes of E. coli, Enterococcus, Listeria, and S. aureus. The virulence factors included E. coli Shiga toxin gene and S. aureus exoenzyme genes, host immune alteration or evasion genes and toxin genes. Results showed no virulence factors for L. lactis IBB109 and IBB417, allowing the conclusion that the genomic sequences of both strains do not include toxic or pathogenic genes related to these four well-known pathogens.
Antibiotic Resistance Genes
Safety assessment of IBB109 and IBB417 was also evaluated in terms of antibiotic resistance. The results of ARG analysis based on genome sequences data using ResFinder showed that neither of the strains contains antibiotic resistance genes. Similar analysis using CARD and RGI demonstrated that only one gene in IBB417 was homologous to ARG encoding LmrD—a chromosomally encoded efflux pump that confers resistance to lincosamides in Streptomyces lincolnensis and L. lactis and is responsible for intrinsic resistance (Flórez et al., 2006). The functional analysis performed using KEGG database indicated the presence in both strains of only one, chromosomally encoded, non-transferable ARG—multidrug efflux pump (MepA). These findings, together with results of our antibiotic susceptibility test, indicated that no acquired antibiotic resistance genes were present, and that L. lactis IBB109 and IBB417 strains could be recommended as safe.
Discussion
Probiotic bacteria beneficially influence human health via a plethora of mechanisms of action, including modulation of host innate and adaptive immune responses, pathogen exclusion, production of bioactive metabolites or reinforcing gut barrier functions (Guarner et al., 2012).
In the current study, we identified two L. strains, IBB109 and IBB417, which exhibit potent antiproliferative activity and stimulate the production of proinflammatory IL-18 cytokine in the colorectal adenocarcinoma cell line (CRC), Caco-2. Results of our work suggest a specific, beneficial effect of both strains in preventing CRC development. By in silico mining approach, we screened the genomes of IBB109 and IBB417 strains for genes encoding various proteins and synthesis pathways that have been reported elsewhere to play a role in probiotic function (e.g., molecular chaperones; stress proteins; adhesion proteins; teichoic acids, rhamnose-containing glycans and EPS biosynthesis pathways; glycogen and vitamins biosynthesis pathways, etc.).
Among the important attributes of probiotic strains is their tolerance and viability under the harsh intestinal conditions. Successful passage and persistence of bacterial cells in the GIT is dictated by their ability to withstand low pH (2–4), high bile salts (up to 0.3% w/v) concentrations and high osmolarity (up to 0.3 M; Chowdhury et al., 1996; Prete et al., 2020). In our assays, both IBB109 and IBB417 were fairly resistant to bile salts, low pH and osmotic stress, indicating the potential to survive the conditions of the GIT.
The mechanism used by L. lactis species to cope with acid stress is to maintain an optimal intracellular pH by using membrane ATPase FoF1 and the generation of alkaline substances through the catabolism of amino acids (Oliveira et al., 2017). Another acid stress response mechanism is related to proteins that play a key role in general stress response, such as small heat shock proteins, chaperonins, and universal stress proteins. All of these genes have been identified in both analyzed genomes.
The process by which probiotic bacteria could survive bile stress is complex and still unclear. Based on previous investigations, the ability of probiotic strains to survive in the presence of bile salts is linked with production specific enzymes, bile salts hydrolyses (BSH), which deconjugate the bile acids making them accessible for other cellular processes and reducing their toxic effect (Floch et al., 1972; Gilliland and Speck, 1977; Tannock et al., 1989). Genes encoding BSH proteins were not found in the IBB109 and IBB417 genomes, suggesting that the observed tolerance is related to other factors, possibly the presence of the complete glycogen biosynthesis pathway or the malolactic enzyme (MLE) pathway which was shown to be the primary bile tolerance mechanism in L. paracasei L9, enhancing resistance through cytoplasm alkalinization (Ma et al., 2018). Among LAB, complete glycogen metabolic pathways are present only in selected species predominantly associated with mammalian hosts or natural environments. In vitro and in vivo studies established the significance of glycogen biosynthesis not only on bile tolerance but also on growth and sugar utilization as well as the competitive retention of L. acidophilus in the mouse intestinal tract, demonstrating that the ability to synthesize intracellular glycogen contributes to gut fitness and retention among probiotic microorganisms (Goh and Klaenhammer, 2014).
Bacterial cells encounter osmotic stress in various settings, including the natural environment, industrial locations or the upper part of the small intestine (Le Marrec, 2011). Both IBB109 and IBB417 displayed relatively good viability in the presence of NaCl (up to 3.5%) and were found to carry putative genes that based on the literature data (Yao et al., 2020) might contribute to the strain-specific response to salt stress by making an osmotic upshift, maintaining the cytosolic redox status and regulating intracellular metabolism. Among the factors conditioning osmotic tolerance, are osmoprotectants, small organic compounds that protect cells from increased membrane tension. Their accumulation in the cytoplasm stimulates the growth of LAB strains in hyperosmotic conditions (Piuri et al., 2003; Le Marrec et al., 2007). It has been suggested that the ability to resist osmotic stress at least for LAB is strain-specific and related rather to the uptake of osmoprotectants from the environment than to synthesis by intrinsic enzymatic machinery (Robert et al., 2000). The recognition and uptake of osmolytes are largely influenced by the presence and the efficiency of specific transporter systems. Both IBB109 and IBB417 were found to possess two putative osmoprotectant transport systems annotated as glycine betaine ABC transporter OpuAA/OpuAB and choline-betaine ABC transporter BusAA/BusAB. Glycine betaine (GB) is commonly present in food products and plant-derived material, such as meat, bovine milk whey, sugar beets and is one of the most widely accumulated organic osmolytes in nature that stimulates bacterial growth. Growth improvement under osmotic stress was also reported for certain LAB strains in the presence of choline (Kets et al., 1994; Robert et al., 2000; Baliarda et al., 2003). The compound itself is not an osmoprotectant, but can be converted to betaine through the choline-glycine betaine pathway identified in some LAB (Robert et al., 2000). The activity of OpuA/BusA systems in LAB activity was shown to be induced in highly osmotic conditions and involve uptake of glycine betaine leading to growth stimulation (Bouvier et al., 2000; Obis et al., 2001). According to previous studies, the presence of extracellular choline does not influence the growth of L. lactis strains under hyperosmotic stress (Obis et al., 1999; Uguen et al., 1999), suggesting that in this species choline is not converted to betaine. Therefore, additional experimental studies are needed to examine the functionality and substrate specificity of the identified osmoprotectant uptake system(s) (particularly choline-betaine ABC transporter BusAA/BusAB) in IBB109 and IBB417.
Adherence to epithelial cells is a highly sought feature of bacterial strains with probiotic potential which can serve a protective role against enteropathogens by competition for host cell binding sites (Oliveira et al., 2017). The ability to attach to the host intestinal epithelial layer or mucus may prolong transient colonization and consequently, increase the fitness of probiotic strains in exerting the pro-health effects in the gut. Mucus is among the main components of the gut barrier that serves in protection of the host against e.g., chemical compounds and pathogens. Bacterial cells that adhere to polystyrene usually adhere well to biotic surfaces, including mucus (Aleksandrzak-Piekarczyk et al., 2016). In our assays, L. lactis IBB109 and IBB417 presented from low (IBB109) to moderate (IBB417) adhesion to both mucus and polystyrene, but bound with a fairly high (IBB417) or moderate (IBB109) efficiency to Caco-2 cells. Adhesion of bacterial cells to intestinal surfaces can be mediated by several factors, including proteinases (Radziwiłł-Bieńkowska et al., 2017), mucus-binding proteins (Boekhorst et al., 2006), fibronectin- and collagen-binding proteins (Muscariello et al., 2020) or pili (Meyrand et al., 2013). Our probiogenomics analyses revealed the presence of genes encoding extracellular or attached to cell wall proteins as well as putative adhesins of other localization. These proteins contain domains involved in adhesion to mucus, but also to ECM or to epithelial cells. Results of adhesion assay to Caco-2 cells indicated that most probably adhesion to intestinal epithelial cells does not occur via mucus-binding proteins but is related to other protein domains (or non-proteinaceous factors) detected for L. lactis IBB109 and IBB417 strains.
A genetic factor that has not been detected in our search for determinants encoding adhesive and mucoadhesive properties, but is encoded in the genomes of both strains, is chaperonin GroEL, an intracellular/surface moonlighting protein, which, apart from its role in protein folding, is believed to be involved in bacterial adhesion to mucin and epithelial cells (Bergonzelli et al., 2006; Lippolis et al., 2013 and references within). Such proteins do not contain signal sequences for secretion or known sequence motifs for binding to the cell surface, so in most cases it is not known how these proteins are secreted or how they become attached to the cell surface (Jeffery, 2018). The presence of the GroEL-encoding gene in the genomes of IBB109 and IBB417 could account for adhesive properties that we observed for both strains.
Numerous studies report that wall teichoic acids (WTA) associated with the cell surface of LAB can also play a role in adhesion to intestinal epithelial cells (Op den Camp et al., 1985; Granato et al., 1999). Similar function to WTA is assigned to rhamnose-containing cell wall polysaccharides (RhaCWP; Mistou et al., 2016). Rhamnose-containing glycans were shown to be involved in processes that affect host-pathogen interactions, including adhesion and biofilm formation in fungi (Martinez et al., 2012). Homologues of biosynthesis genes of wall teichoic acids (WTA) and rhamnose-containing glycans were found in both of the analyzed lactococcal genomes.
We have shown that IBB109 and IBB417 exhibit a strong anti-proliferative and significant immunomodulatory effect on colorectal adenocarcinoma Caco-2 cells in vitro. The level of cancer cell inhibition can be due to different factors, e.g., adhesion, SCFAs or exopolysaccharide production (Thirabunyanon and Hongwittayakorn, 2013; Mantzourani et al., 2019; Wu et al., 2021). It has been argued that prolonged duration of LAB strains in the gut due to adherence to intestinal cells promotes the action of their secondary metabolites, including SCFAs. SCFAs were found to counteract the deleterious activity of histone deacetylase (HDCA) implicated in cancer development and progression (Glozak et al., 2005; Zhong et al., 2014). A study by Thirabunyanon and Hongwittayakorn (2013) has shown that LAB strains exhibiting adhesive ability coupled with SCFAs production inhibited Caco-2 cell proliferation. Waldecker et al. (2008) demonstrated that inhibition of colon cell lines is specifically related to butyrate. IBB417 and IBB109 displayed, respectively, strong and moderate adhesion to Caco-2 cells and were determined to encode for the complete pathways for lactate and SCFAs (acetate and formate) synthesis. Both of these features could account for the strong inhibitory effect on CRC cells in vitro. Lactate is a powerful antimicrobial factor that inhibits the growth of pathogens and participates in the trophic chain between microbial communities, because it favors the growth of bacteria that consume lactate and subsequently generate SCFAs (Louis and Flint, 2017). Despite the fact that the complete set of well-characterized key synthesis genes necessary for butyrate production was not identified in the IBB109 and IBB417 genomes, the possibility of butyrate synthesis cannot be excluded via alternative pathways. It is postulated that unknown genes may contribute to the formation of SCFAs (Zhao et al., 2019) as was also in the case of the butyrogenic capability of L. plantarum which is highly dependent on the substrate type and for a long time has not been assigned to any specific metabolic pathway (Botta et al., 2017).
Exposition of bacterial molecular structures (cell-anchored or secreted) known as MAMPs (microbially associated molecular patterns) to epithelial cell receptors induces signaling pathways that lead to immune stimulation (Lebeer et al., 2010). Despite the lack of a direct link between adhesion and immune responses, studies show that bacteria which adhere to intestinal surfaces can affect the host immune system, particularly through the gut-associated lymphoid tissue (GALT). Close contact with the gut epithelial cells allows components anchored in the cell wall or secreted by the probiotic bacteria to induce signaling pathways that stimulate the immune cells. Our studies indicate that L. lactis IBB109 and IBB417 strains may trigger an immune response in CRC cell lines as they were found to significantly increase the level of proinflammatory IL-18 gene expression. Intestinal epithelial cells (IEC) naturally produce IL-18 at a relatively constant level, which is considered a crucial factor in inhibiting CRC tumor development, tumor reduction and maintaining mucosal equilibrium (Mager et al., 2016; Hradicka et al., 2020). In CRC cells, the IL-18 level is significantly reduced or not detectable (Feng et al., 2020). Such phenomenon is postulated to be a form of escape of cancer cells from the host’s immune system. We suggest that the elevated levels of IL-18 gene expression upon treatment of Caco-2 cells with IBB109 and IBB417 may contribute to the stimulation of the immune system, leading to the recognition of cancer cells by the host organism, and thus, early prevention of disease development.
The ability to antagonize or competitively exclude pathogens is a crucial probiotic trait achieved by bacterial cells upon secretion of antimicrobial substances. LAB produce different antimicrobial components, such as organic acids, hydrogen peroxide, carbon peroxide, diacetyl, low molecular weight antimicrobial substances, bacteriocins and adhesion inhibitors. The presence of genes and pathways dedicated to some of these compounds, such as organic acids (lactate, acetate, formate), diacetyl, acetoin, were detected in the analyzed lactococcal genomes.
The fitness of bacteria to particular niches, such as the GIT, is favored by the ability to utilize various carbon sources. In our assays, IBB109 and IBB417 presented essentially the same carbohydrate fermentation pattern. Of particular importance is the ability of both strains to degrade sugars found in ingested foods, such as milk (lactose), cereals (e.g., maltose, esculin), or plant material (e.g., fructose, arbutin, sucrose) (Gänzle and Follador, 2012), which may support their survival in the intestine.
Additional beneficial, prohealth effects may derive from the presence of genes encoding complete synthesis pathways for essential vitamins of the B and K groups. Most B group vitamins are directly involved in energy metabolism (LeBlanc et al., 2017) and play important roles in the maintenance of immune homeostasis (Yoshii et al., 2019). When produced in the GIT, vitamins may be utilized by the host or by intestinal bacteria which are unable to synthesize them. Vitamins are normally stored inside the cells and released by direct diffusion via specific transporters in the cell membrane or cellular lysis, e.g., inside the GIT of the host. Thus, vitamin producing strains are good candidates for in situ delivery of vitamins to consumers.
In summary, we showed that two L. lactis strains, IBB109 and IBB417, exhibit potent a proliferation inhibition and increased interleukin 18 (IL-18) expression in human colon cancer cells (Caco-2). Physiological assays revealed the health-promoting, metabolic and biosafety features of L. lactis IBB109 and IBB417. By whole-genome sequencing and in silico analyses, we evaluated the presence of genes and pathways that may be responsible for their in vitro effects. Our studies underlie the multi-method approach that can be adapted when examining the potential of bacterial strains in developing innovative therapeutic microbial-based products.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials file; further inquiries can be directed to the corresponding author.
Author Contributions
PS performed all experimental works, statistical and part of bioinformatic analyses, prepared figures and tables, and wrote parts of the manuscript. MK performed bioinformatics analyses, prepared figures and tables, and wrote sections of the manuscript. JB co-supervised the work and provided conceptual insights. AS was responsible for the experimental concept, critically reviewed and analyzed all data, prepared figures and tables, and wrote the main text of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was financed by the statutory funds of the Institute of Biochemistry and Biophysics, Polish Academy of Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.822912/full#supplementary-material
References
- Alcock B. P., Raphenya A. R., Lau T. T. Y. (2020). CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Narrative 48, D517–D525. doi: 10.1093/nar/gkz935, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrzak-Piekarczyk T., Koryszewska-Bagińska A., Grynberg M., Nowak A., Cukrowska B., Kozakova H., et al. (2016). Genomic and functional characterization of the unusual pLOCK0919 plasmid harboring the spaCBA pili cluster in Lactobacillus casei LOCK 0919. Genome Biol. Evol. 8, 202–217. doi: 10.1093/gbe/evv247, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrzak-Piekarczyk T., Puzia W., Żylińska J., Cieśla J., Gulewicz K. A., Bardowski J. K., et al. (2019). Potential of Lactobacillus plantarum IBB3036 and Lactobacillus salivarius IBB3154 to persist in chicken after in ovo delivery. Microbiology 8:e00620. doi: 10.1002/mbo3.620, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. (2010). FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. Available at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Arndt D., Grant J., Marcu A., Sajed T., Pon A., Liang Y., et al. (2016). PHASTER: a better, faster version of the PHAST phage search tool. Narrative 44, W16–W21. doi: 10.1093/nar/gkw387, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz R. K., Bartels D., Best A. A., DeJongh M., Disz T., Edwards R. A., et al. (2008). The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghbani-Arani F., Asgary V., Hashemi A. (2020). Cell-free extracts of Lactobacillus acidophilus and Lactobacillus delbrueckii display antiproliferative and antioxidant activities against HT-29 cell line. Nutr. Cancer 72, 1390–1399. doi: 10.1080/01635581.2019.1685674, PMID: [DOI] [PubMed] [Google Scholar]
- Baliarda A., Robert H., Jebbar M., Blanco C., Deschamps A., Le Marrec C. (2003). Potential osmoprotectants for the lactic acid bacteria Pediococcus pentosaceus and Tetragenococcus halophila. Int. J. Food Microbiol. 84, 13–20. doi: 10.1016/s0168-1605(02)00388-4, PMID: [DOI] [PubMed] [Google Scholar]
- Bergonzelli G. E., Granato D., Pridmore R. D., Marvin-Guy L. F., Donnicola D., Corthesy-Theulaz I. E. (2006). GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen, Helicobacter pylori. Infect. Immun. 74, 425–434. doi: 10.1128/IAI.74.1.425-434.2006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhorst J., Helmer Q., Kleerebezem M., Siezen R. J. (2006). Comparative analysis of proteins with a mucus-binding domain found exclusively in lactic acid bacteria. Microbiology 152, 273–280. doi: 10.1099/mic.0.28415-0, PMID: [DOI] [PubMed] [Google Scholar]
- Bolotin A., Aucouturier A., Sorokin A., Bidnenko E. (2019). Genomic sequence of the prophage-free Lactococcus lactis strain IL6288. Microbiol. Resour. Announc. 8, e01545–e01618. doi: 10.1128/MRA.01545-18, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolaia V., Kaas R. F., Ruppe E., Roberts M. C., Schwarz S., Cattoir V., et al. (2020). ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 75, 3491–3500. doi: 10.1093/jac/dkaa345, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta C., Acquadro A., Greppi A., Barchi L., Bertolino M., Cocolin L., et al. (2017). Genomic assessment in Lactobacillus plantarum links the butyrogenic pathway with glutamine metabolism. Sci. Rep. 7:15975. doi: 10.1038/s41598-017-16186-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J., Bordes P., Romeo Y., Fourçans A., Bouvier I., Gutierrez C. (2000). Characterization of OpuA, a glycine-betaine uptake system of Lactococcus lactis. J. Mol. Microbiol. Biotechnol. 2, 199–205. [PubMed] [Google Scholar]
- Carroll I. M., Andrus J. M., Bruno-Barcena J. M., Klaenhammer T. R., Hassan H. M., Threadgill D. S. (2007). Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G729–G738. doi: 10.1152/ajpgi.00132.2007, PMID: [DOI] [PubMed] [Google Scholar]
- Carvalho A. L., Turner D. L., Fonseca L. L., Solopova A., Catarino T., Kuipers O. P., et al. (2013). Metabolic and transcriptional analysis of acid stress in Lactococcus lactis, with a focus on the kinetics of lactic acid pools. PLoS One 8:e68470. doi: 10.1371/journal.pone.0068470, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhou Y., Chen Y., Gu J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. doi: 10.1093/bioinformatics/bty560, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin A., Chopin M.-C., Moillo-Batt A., Langella P. (1984). Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid 11, 260–263. doi: 10.1016/0147-619X(84)90033-7, PMID: [DOI] [PubMed] [Google Scholar]
- Chowdhury R., Sahu G. K., Das J. (1996). Stress response in pathogenic bacteria. J. Biosci. 21, 149–160. doi: 10.1007/BF02703105 [DOI] [Google Scholar]
- Couvin D., Bernheim A., Toffano-Nioche C., Touchon M., Michalik J., Néron B., et al. (2018). CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrated search for Cas proteins. Narrative 46, W246–W251. doi: 10.1093/nar/gky425, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofori F., Dargenio V. N., Dargenio C., Miniello V. L., Barone M., Francavilla R. (2021). Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front. Immunol. 12:578386. doi: 10.3389/fimmu.2021.578386, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzentas N. (2010). Circoletto: visualizing sequence similarity with Circos. Bioinformatics 26, 2620–2621. doi: 10.1093/bioinformatics/btq484, PMID: [DOI] [PubMed] [Google Scholar]
- De Coster W., D’Hert S., Schultz D. T., Cruts M., Van Broeckhoven C. (2018). NanoPack: visualizing and processing long-read sequencing data. Bioinformatics 34, 2666–2669. doi: 10.1093/bioinformatics/bty149, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A., van Hijum S. A., Bijlsma J. J., Kok J., Kuipers O. P. (2006). BAGEL: a web-based bacteriocin genome mining tool. Narrative 34, W273–W279. doi: 10.1093/nar/gkl237, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2012). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 10:2740. doi: 10.2903/j.efsa.2012.2740 [DOI] [Google Scholar]
- Feng X., Zhang Z., Sun P., Song G., Wang L., Sun Z., et al. (2020). Interleukin-18 is a prognostic marker and plays a tumor suppressive role in colon cancer. Dis. Markers 2020:6439614. doi: 10.1155/2020/6439614, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijan S. (2014). Microorganisms with claimed probiotic properties: an overview of recent literature. Int. J. Environ. Res. Public Health 11, 4745–4767. doi: 10.3390/ijerph110504745, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floch M. H., Binder H. J., Filburn B., Gershengoren W. (1972). The effect of bile acids on intestinal microflora. Am. J. Clin. Nutr. 25, 1418–1426. doi: 10.1093/ajcn/25.12.1418 [DOI] [PubMed] [Google Scholar]
- Flórez A. B., de Los Reyes-Gavilán C. G., Wind A., Mayo B., Margolles A. (2006). Ubiquity and diversity of multidrug resistance genes in Lactococcus lactis strains isolated between 1936 and 1995. FEMS Microbiol. Lett. 263, 21–25. doi: 10.1111/j.1574-6968.2006.00371.x [DOI] [PubMed] [Google Scholar]
- Gänzle M. G., Follador R. (2012). Metabolism of oligosaccharides and starch in lactobacilli: a review. Front. Microbiol. 3:340. doi: 10.3389/fmicb.2012.00340, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad O., Svensson B., Viborg A. H., Stuer-Lauridsen B., Jacobsen S. (2011). The extracellular proteome of Bifidobacterium animalis subsp. lactis BB-12 reveals proteins with putative roles in probiotic effects. Proteomics 11, 2503–2514. doi: 10.1002/pmic.201000716, PMID: [DOI] [PubMed] [Google Scholar]
- Gilliland S. E., Speck M. L. (1977). Deconjugation of bile acids by intestinal lactobacilli. Int. J. Bacteriol. 33, 15–18. doi: 10.1128/aem.33.1.15-18.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozak M. A., Sengupta N., Zhang X., Seto E. (2005). Acetylation and deacetylation of non-histone proteins. Gene 363, 15–23. doi: 10.1016/j.gene.2005.09.010 [DOI] [PubMed] [Google Scholar]
- Goh Y. J., Klaenhammer T. R. (2014). Insights into glycogen metabolism in Lactobacillus acidophilus: impact on carbohydrate metabolism, stress tolerance and gut retention. Microb. Cell Factories 13:94. doi: 10.1186/s12934-014-0094-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato D., Perotti F., Masserey I., Rouvet M., Golliard M., Servin A., et al. (1999). Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells. Appl. Environ. Microbiol. 65, 1071–1077. doi: 10.1128/AEM.65.3.1071-1077.1999, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner F., Khan A. G., Garisch J., Eliakim R., Gangl A., Thomson A., et al. (2012). World gastroenterology organisation global guidelines: probiotics and prebiotics. J. Clin. Gastroenterol. 46, 468–481. doi: 10.1097/MCG.0b013e3182549092 [DOI] [PubMed] [Google Scholar]
- Hosseini S. S., Goudarzi H., Ghalavand Z., Hajikhani B., Rafeieiatani Z., Hakemi-Vala M. (2020). Anti-proliferative effects of cell wall, cytoplasmic extract of Lactococcus lactis and nisin through down-regulation of cyclin D1 on SW480 colorectal cancer cell line. Iran. J. Microbiol. 12, 424–430. doi: 10.18502/ijm.v12i5.4603, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hradicka P., Beal J., Kassayova M., Foey A., Demeckova V. (2020). A novel lactic acid bacteria mixture: macrophage-targeted prophylactic intervention in colorectal cancer management. Microorganisms 8:387. doi: 10.3390/microorganisms8030387, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Organization for Standardization (2010). ISO 10932 [IDF 223:2010]. Milk and milk products—determination ofthe minimal inhibitory concentration (MIC) ofantibiotics applicable to bifidobacteria and non-enterococcal lactic acid bacteria (LAB). International Organization for Standardization, Geneva, Switzerland. Available at: https://www.iso.org/standard/46434.html
- Izquierdo E., Horvatovich P., Marchioni E., Aoude-Werner D., Sanz Y., Ennahar S. (2009). 2-DE and MS analysis of key proteins in the adhesion of Lactobacillus plantarum, a first step toward early selection of probiotics based on bacterial biomarkers. Electrophoresis 30, 949–956. doi: 10.1002/elps.200800399, PMID: [DOI] [PubMed] [Google Scholar]
- Jeffery C. (2018). Intracellular proteins moonlighting as bacterial adhesion factors. AIMS Microbiol. 4, 362–376. doi: 10.3934/microbiol.2018.2.362, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B., Raphenya A. R., Alcock B., Waglechner N., Guo P., Tsang K. K., et al. (2017). CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Narrative 45, D566–D573. doi: 10.1093/nar/gkw1004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajander K., Myllyluoma E., Rajilić-Stojanović M., Kyrönpalo S., Rasmussen M., Järvenpää S., et al. (2008). Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment. Pharmacol. Ther. 27, 48–57. doi: 10.1111/j.1365-2036.2007.03542.x, PMID: [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Sato Y., Kawashima M. (2021). KEGG mapping tools for uncovering hidden features in biological data. Protein Sci. 31, 47–53. doi: 10.1002/pro.4172, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Sato Y., Morishima K. (2016). BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 428, 726–731. doi: 10.1016/j.jmb.2015.11.006, PMID: [DOI] [PubMed] [Google Scholar]
- Kets E. P. W., Galinski E. A., de Bont J. A. M. (1994). Carnitine: a novel compatible solute in Lactobacillus plantarum. Arch. Microbiol. 162, 243–248. doi: 10.1007/BF00301845 [DOI] [Google Scholar]
- Kim K. W., Kang S. S., Woo S. J., Park O.-J., Ahn K. B., Song K.-D., et al. (2017). Lipoteichoic acid of probiotic Lactobacillus plantarum attenuates poly I:C-induced IL-8 production in porcine intestinal epithelial cells. Front. Microbiol. 8:1827. doi: 10.3389/fmicb.2017.01827, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Kim J. E., Lee K. W., Lee H. J. (2009). Lactococcus lactis ssp. lactis inhibits the proliferation of SNU-1 human stomach cancer cells through induction of G0/G1 cell cycle arrest and apoptosis via p53 and p21 expression. Ann. N. Y. Acad. Sci. 1171, 270–275. doi: 10.1111/j.1749-6632.2009.04721.x, PMID: [DOI] [PubMed] [Google Scholar]
- Lebeer S., Vanderleyden J., De Keersmaecker S. C. (2010). Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 8, 171–184. doi: 10.1038/nrmicro2297, PMID: [DOI] [PubMed] [Google Scholar]
- Le Marrec C. (2011). “Responses of lactic acid bacteria to osmotic stress” in Stress Responses of Lactic Acid Bacteria. eds. Tsakalidou E., Papadimitriou K. (Boston, MA: Food Microbiology and Food Safety. Springer; ). [Google Scholar]
- Le Marrec C., Bon E., Lonvaud-Funel A. (2007). Tolerance to high osmolality of the lactic acid bacterium Oenococcus oeni and identification of potential osmoprotectants. Int. J. Food Microbiol. 115, 335–342. doi: 10.1016/j.ijfoodmicro.2006.12.039, PMID: [DOI] [PubMed] [Google Scholar]
- Le D. T., Tran T. L., Duviau M. P., Meyrand M., Guérardel Y., Castelain M., et al. (2013). Unraveling the role of surface mucus-binding protein and pili in muco-adhesion of Lactococcus lactis. PLoS One 8:e79850. doi: 10.1371/journal.pone.0079850, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc J. G., Chain F., Martín R., Bermúdez-Humarán L. G., Courau S., Langella P. (2017). Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 16:79. doi: 10.1186/s12934-017-0691-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc J. G., del Carmen S., Miyoshi A. (2011). Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn's disease in mice. J. Biotechnol. 151, 287–293. doi: 10.1016/j.jbiotec.2010.11.008, PMID: [DOI] [PubMed] [Google Scholar]
- Lee I. C., Tomita S., Kleerebezem M., Bron P. A. (2013). The quest for probiotic effector molecules—unraveling strain specificity at the molecular level. Pharmacol. Res. 69, 61–74. doi: 10.1016/j.phrs.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Lippolis R., Siciliano R. A., Mazzeo M. F., Abbrescia A., Gnoni A., Sardanelli A. M., et al. (2013). Comparative secretome analysis of four isogenic Bacillus clausii probiotic strains. Proteome Sci. 11:28. doi: 10.1186/1477-5956-11-28, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P., Flint H. J. (2017). Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41. doi: 10.1111/1462-2920.13589 [DOI] [PubMed] [Google Scholar]
- Ma X., Wang G., Zhai Z., Zhou P., Hao Y. (2018). Global transcriptomic analysis and function identification of malolactic enzyme pathway of Lactobacillus paracasei L9 in response to bile stress. Front. Microbiol. 9:1978. doi: 10.3389/fmicb.2018.01978, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager L. F., Wasmer M. H., Rau T. T., Krebs P. (2016). Cytokine-induced modulation of colorectal cancer. Front. Oncol. 6:96. doi: 10.3389/fonc.2016.00096, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg Tetzschner A. M., Johnson J. R., Johnston B. D., Lund O., Scheutz F. (2020). In silico genotyping of Escherichia coli isolates for extraintestinal virulence genes by use of whole-genome sequencing data. J. Clin. Microbiol. 58, e01269–e01320. doi: 10.1128/JCM.01269-20, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzourani I., Chondrou P., Bontsidis C., Karolidou K., Terpou A., Alexopoulos A., et al. (2019). Assessment of the probiotic potential of lactic acid bacteria isolated from kefir grains: evaluation of adhesion and antiproliferative properties in in vitro experimental systems. Ann. Microbiol. 69, 751–763. doi: 10.1007/s13213-019-01467-6 [DOI] [Google Scholar]
- Markowiak P., Śliżewska K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9:1021. doi: 10.3390/nu9091021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V., Ingwers M., Smith J., Glushka J., Yang T., Bar-Peled M. (2012). Biosynthesis of UDP-4-keto-6-deoxyglucose and UDP-rhamnose in pathogenic fungi Magnaporthe grisea and Botryotinia fuckeliana. J. Biol. Chem. 287, 879–892. doi: 10.1074/jbc.M111.287367, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki T., Chin J. (2000). Modulating immune responses with probiotic bacteria. Immunol. Cell Biol. 78, 67–73. doi: 10.1046/j.1440-1711.2000.00887.x [DOI] [PubMed] [Google Scholar]
- Meyrand M., Guillot A., Goin M., Furlan S., Armalyte J., Kulakauskas S., et al. (2013). Surface proteome analysis of a natural isolate of Lactococcus lactis reveals the presence of pili able to bind human intestinal epithelial cells. Mol. Cell. Proteomics 12, 3935–3947. doi: 10.1074/mcp.M113.029066, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistou M.-Y., Sutcliffe I. C., van Sorge N. M. (2016). Bacterial glycobiology: rhamnose-containing cell wall polysaccharides in gram-positive bacteria. FEMS Microbiol. Rev. 40, 464–479. doi: 10.1093/femsre/fuw006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry J., Chuguransky S., Williams L. (2021). Pfam: the protein families database in 2021. Narrative 49, D412–D419. doi: 10.1093/nar/gkaa913, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscariello L., De Siena B., Marasco R. (2020). Lactobacillus cell surface proteins involved in interaction with mucus and extracellular matrix components. Curr. Microbiol. 77, 3831–3841. doi: 10.1007/s00284-020-02243-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nami Y., Abdullah N., Haghshenas B., Radiah D., Rosli R., Khosroushahi A. Y. (2014a). Probiotic potential and biotherapeutic effects of newly isolated vaginal Lactobacillus acidophilus 36YL strain on cancer cells. Anaerobe 28, 29–36. doi: 10.1016/j.anaerobe.2014.04.012, PMID: [DOI] [PubMed] [Google Scholar]
- Nami Y., Abdullah N., Haghshenas B., Radiah D., Rosli R., Yari Khosroushahi A. (2014b). A newly isolated probiotic Enterococcus faecalis strain from vagina microbiota enhances apoptosis of human cancer cells. J. Appl. Microbiol. 117, 498–508. doi: 10.1111/jam.12531, PMID: [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Tamada A., Hyoudou K., Umeyama Y., Takahashi Y., Kobayashi Y., et al. (2004). Inhibition of experimental hepatic metastasis by targeted delivery of catalase in mice. Clin. Exp. Metastasis 21, 213–221. doi: 10.1023/b:clin.0000037706.13747.5e, PMID: [DOI] [PubMed] [Google Scholar]
- Nobaek S., Johansson M. L., Molin G., Ahrné S., Jeppsson B. (2000). Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am. J. Gastroenterol. 95, 1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x, PMID: [DOI] [PubMed] [Google Scholar]
- Nwodo U. U., Green E., Okoh A. I. (2012). Bacterial exopolysaccharides: functionality and prospects. Int. J. Mol. Sci. 13, 14002–14015. doi: 10.3390/ijms131114002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obis D., Guillot A., Gripon J. C., Renault P., Bolotin A., Mistou M. Y. (1999). Genetic and biochemical characterization of a high-affinity betaine uptake system (BusA) in Lactococcus lactis reveals a new functional organization within bacterial ABC transporters. J. Bacteriol. 181, 6238–6246. doi: 10.1128/JB.181.20.6238-6246.1999, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obis D., Guillot A., Mistou M. Y. (2001). Tolerance to high osmolality of Lactococcus lactis subsp. lactis and cremoris is related to the activity of a betaine transport system. FEMS Microbiol. Lett. 202, 39–44. doi: 10.1111/j.1574-6968.2001.tb10777.x, PMID: [DOI] [PubMed] [Google Scholar]
- Oliveira L. C., Saraiva T. D. L., Silva W. M., Pereira U. P., Campos B. C., Benevides L. J., et al. (2017). Analyses of the probiotic property and stress resistance-related genes of Lactococcus lactis subsp. lactis NCDO 2118 through comparative genomics and in vitro assays. PLoS One 12:e0175116. doi: 10.1371/journal.pone.0175116, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Camp H. J., Oosterhof A., Veerkamp J. H. (1985). Interaction of bifidobacterial lipoteichoic acid with human intestinal epithelial cells. Infect. Immun. 47, 332–334. doi: 10.1128/iai.47.1.332-334.1985, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando A., Refolo M. G., Messa C., Amati L., Lavermicocca P., Guerra V., et al. (2012). Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 64, 1103–1111. doi: 10.1080/01635581.2012.717676, PMID: [DOI] [PubMed] [Google Scholar]
- Pala V., Sieri S., Berrino F., Vineis P., Sacerdote C., Palli D., et al. (2011). Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 129, 2712–2719. doi: 10.1002/ijc.26193, PMID: [DOI] [PubMed] [Google Scholar]
- Piuri M., Sanchez-Rivas C., Ruzal S. M. (2003). Adaptation to high salt in Lactobacillus: role of peptides and proteolytic enzymes. J. Appl. Microbiol. 95, 372–379. doi: 10.1046/j.1365-2672.2003.01971.x, PMID: [DOI] [PubMed] [Google Scholar]
- Potter S. C., Luciani A., Eddy S. R., Park Y., Lopez R., Finn R. D. (2018). HMMER web server: 2018 update. Narrative 46, W200–W204. doi: 10.1093/nar/gky448, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prete R., Long S. L., Gallardo A. L., Gahan C. G., Corsetti A., Joyce S. A. (2020). Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci. Rep. 10:1165. doi: 10.1038/s41598-020-58069-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwiłł-Bieńkowska J., Le D. T., Szczęsny P., Duviau M. P., Aleksandrzak-Piekarczyk T., Loubière P., et al. (2016). Adhesion of the genome-sequenced Lactococcus lactis subsp. cremoris IBB477 strain is mediated by specific molecular determinants. J. Appl. Microbiol. Biotechnol. 100, 9605–9617. doi: 10.1007/s00253-016-7813-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwiłł-Bieńkowska J. M., Robert V., Drabot K., Chain F., Cherbuy C., Langella P., et al. (2017). Contribution of plasmid-encoded peptidase S8 (PrtP) to adhesion and transit in the gut of Lactococcus lactis IBB477 strain. Appl. Microbiol. Biotechnol. 101, 5709–5721. doi: 10.1007/s00253-017-8334-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Bocking A. (2003). The potential for probiotics to prevent bacterial vaginosis and preterm labor. Am. J. Obstet. Gynecol. 189, 1202–1208. doi: 10.1067/s0002-9378(03)00495-2, PMID: [DOI] [PubMed] [Google Scholar]
- Rekha C. R., Vijayalakshmi G. (2010). Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 109, 1198–1208. doi: 10.1111/j.1365-2672.2010.04745.x, PMID: [DOI] [PubMed] [Google Scholar]
- Robert H., Le Marrec C., Blanco C., Jebbar M. (2000). Glycine betaine, carnitine, and choline enhance salinity tolerance and prevent the accumulation of sodium to a level inhibiting growth of Tetragenococcus halophila. Appl. Environ. Microbiol. 66, 509–517. doi: 10.1128/AEM.66.2.509-517.2000, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S., Nurmi J., Gueimonde M. (2005). The genomics of probiotic intestinal microorganisms. Genome Biol. 6:225. doi: 10.1186/gb-2005-6-7-225xx, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxelin M., Tynkkynen S., Mattila-Sandholm T., de Vos W. M. (2005). Probiotic and other functional microbes: from markets to mechanisms. Curr. Opin. Biotechnol. 16, 204–211. doi: 10.1016/j.copbio.2005.02.003, PMID: [DOI] [PubMed] [Google Scholar]
- Serban D. E. (2014). Gastrointestinal cancers: influence of gut microbiota, probiotics and prebiotics. Cancer Lett. 345, 258–270. doi: 10.1016/j.canlet.2013.08.013 [DOI] [PubMed] [Google Scholar]
- Settanni C. R., Quaranta G., Bibbò S., Gasbarrini A., Cammarota G., Ianiro G. (2020). Oral supplementation with lactobacilli to prevent colorectal cancer in preclinical models. Minerva Gastroenterol. Dietol. 66, 48–69. doi: 10.23736/S1121-421X.19.02631-X, PMID: [DOI] [PubMed] [Google Scholar]
- Sniffen J. C., McFarland L. V., Evans C. T., Goldstein E. J. C. (2018). Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS One 13:e0209205. doi: 10.1371/journal.pone.0209205, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Dashkevitz M. P., Feighner S. D. (1989). Lactobacilli and bile salt hydrolase in the murine intestinal tract. Appl. Environ. Microbiol. 55, 1848–1851. doi: 10.1128/aem.55.7.1848-1851.1989, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverniti V., Guglielmetti S. (2011). The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr. 6, 261–274. doi: 10.1007/s12263-011-0218-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirabunyanon M., Hongwittayakorn P. (2013). Potential probiotic lactic acid bacteria of human origin induce antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl. Biochem. Biotechnol. 169, 511–525. doi: 10.1007/s12010-012-9995-y, PMID: [DOI] [PubMed] [Google Scholar]
- Turpin W., Humblot C., Noordine M.-L., Thomas M., Guyot J.-P. (2012). Lactobacillaceae and cell adhesion: genomic and functional screening. PLoS One 7:e38034. doi: 10.1371/journal.pone.0038034, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uguen P., Hamelin J., Le Pennec J. P., Blanco C. (1999). Influence of osmolarity and the presence of an osmoprotectant on Lactococcus lactis growth and bacteriocin production. Appl. Environ. Microbiol. 65, 291–293. doi: 10.1128/AEM.65.1.291-293.1999, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamanu E., Gatea F. (2020). Correlations between microbiota bioactivity and bioavailability of functional compounds: a mini-review. Biomedicine 8:39. doi: 10.3390/biomedicines8020039, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M., Turroni F., van Sinderen D. (2012). Probiogenomics as a tool to obtain genetic insights into adaptation of probiotic bacteria to the human gut. Bioeng. Bugs 3, 73–79. doi: 10.4161/bbug.18540, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldecker M., Kautenburger T., Daumann H., Veeriah S., Will F., Dietrich H., et al. (2008). Histone-deacetylase inhibition and butyrate formation: fecal slurry incubations with apple pectin and apple juice extracts. Nutrition 24, 366–374. doi: 10.1016/j.nut.2007.12.013, PMID: [DOI] [PubMed] [Google Scholar]
- Wang G., Yu Y., Garcia-Gutierrez E., Jin X., He Y., Wang L., et al. (2019). Lactobacillus acidophilus JCM 1132 strain and its mutant with different bacteriocin-producing behaviour have various in situ effects on the gut microbiota of healthy mice. Microorganisms 8:49. doi: 10.3390/microorganisms8010049, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick R. R., Judd L. M., Gorrie C. L., Holt K. E. (2017). Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13:e1005595. doi: 10.1371/journal.pcbi.1005595, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Zhang Y., Ye L., Wang C. (2021). The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: a review. Carbohydr. Polym. 253:117308. doi: 10.1016/j.carbpol.2020.117308, PMID: [DOI] [PubMed] [Google Scholar]
- Yao W., Yang L., Shao Z., Xie L., Chen L. (2020). Identification of salt tolerance-related genes of Lactobacillus plantarum D31 and T9 strains by genomic analysis. Ann. Microbiol. 70:10. doi: 10.1186/s13213-020-01551-2 [DOI] [Google Scholar]
- Yoshii K., Hosomi K., Sawane K., Kunisawa J. (2019). Metabolism of dietary and microbial vitamin b family in the regulation of host immunity. Front. Nutr. 6:48. doi: 10.3389/fnut.2019.00048, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N. Y., Wagner J. R., Laird M. R., Melli G., Rey S., Lo R., et al. (2010). PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26, 1608–1615. doi: 10.1093/bioinformatics/btq249, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Dai H., Liang W., Zhang L., Deng Z. (2019). Fermented dairy foods intake and risk of cancer. Int. J. Cancer 144, 2099–2108. doi: 10.1002/ijc.31959, PMID: [DOI] [PubMed] [Google Scholar]
- Zhao C., Dong H., Zhang Y., Li Y. (2019). Discovery of potential genes contributing to the biosynthesis of short-chain fatty acids and lactate in gut microbiota from systematic investigation in E. coli. NPJ Biofilms Microbiomes 5:19. doi: 10.1038/s41522-019-0092-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Zhang X., Covasa M. (2014). Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. 20, 7878–7886. doi: 10.3748/wjg.v20.i24.7878, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liang Y., Lynch K. H., Dennis J. J., Wishart D. S. (2011). PHAST: a fast phage search tool. Narrative 39, W347–W352. doi: 10.1093/nar/gkr485, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska D., Kolożyn-Krajewska D. (2018). Food-origin lactic acid bacteria may exhibit probiotic properties: review. Biomed. Res. Int. 2018:5063185. doi: 10.1155/2018/5063185, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L., Daillère R., Roberti M. P., Routy B., Kroemer G. (2017). Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol. 15, 465–478. doi: 10.1038/nrmicro.2017.44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials file; further inquiries can be directed to the corresponding author.