Abstract
Colour signals of many animals are surrounded by a high-contrast achromatic background, but little is known about the possible function of this arrangement. For both humans and non-human animals, the background colour surrounding a colour stimulus affects the perception of that stimulus, an effect that can influence detection and discrimination of colour signals. Specifically, high colour contrast between the background and two given colour stimuli makes discrimination more difficult. However, it remains unclear how achromatic background contrast affects signal discrimination in non-human animals. Here, we test whether achromatic contrast between signal-relevant colours and an achromatic background affects the ability of zebra finches to discriminate between those colours. Using an odd-one-out paradigm and generalized linear mixed models, we found that higher achromatic contrast with the background, whether positive or negative, decreases the ability of zebra finches to discriminate between target and non-target stimuli. This effect is particularly strong when colour distances are small (less than 4 ΔS) and Michelson achromatic contrast with the background is high (greater than 0.5). We suggest that researchers should consider focal colour patches and their backgrounds as collectively comprising a signal, rather than focusing on solely the focal colour patch itself.
Keywords: chromatic, background, model, contrast, distance, frequency
1. Introduction
Information collected and processed via sensory systems is central to the expression and modulation of animal behaviour. Signals are an especially important source of information. Signals are defined as structures or behaviours that have evolved to modify the behaviour of receivers [47] and that reliably convey information benefiting, on average, both the signal sender and the signal receiver [20,44]. Signals are used in many contexts of animal and plant communication including, but not limited to, mate choice [41], intraspecific conflict [51], species recognition [56], predator avoidance [8,46] and mutualistic interactions [27]. Although signals can be transmitted in many sensory modalities (e.g. vision, olfaction and audition), they are only effective if they can be accurately perceived and assessed by the receiver.
Colour signals are particularly interesting because colour perception is unusually malleable, depending not only on the visual physiology of the receiver, but also on environmental contextual factors such as illumination, transmission medium (e.g. air versus water) and the visual background against which a signal is displayed [41]. Although the effects of illumination and transmission medium are well studied [41], fewer studies have addressed the effect of visual background on the perception of colour signals, and those that do have focused primarily on coloured backgrounds (e.g. [28,39]). This work has demonstrated that high colour contrast between the background and target stimuli (e.g. discriminating between two red stimuli on a green background) increases colour discrimination thresholds in zebra finches (Taeniopygia guttata) [28] and goldfish (Cassius auratus) [57], and decreases the certainty with which chickens (Gallus gallus) make discrimination decisions [39].
The above studies have generally considered the impact of a coloured background on colour perception, but backgrounds in many taxa are achromatic. For example, many animals have skin, scales, fur or feathers that span the achromatic gamut from extremely black (e.g. [12,32]) to bright white (e.g. [48]) and that may surround a patch of colour serving as a potential signal [14,15,34] (figure 1). Although considerable work has been done on the effects of a high-contrast achromatic background around a functionally important colour signal on attention and learning, particularly in the context of aposematism [1,19,42], little is known about the effects of high-contrast patterns on colour discrimination in other functional contexts.
Figure 1.
Colourful signals with achromatic backgrounds in birds. Many species of birds have colourful signals or ornaments displayed against largely achromatic surrounding backgrounds that may affect detection and discrimination of the signal. (a) Painted finch (Emblema pictum), (b) European robin (Erithacus rubecula), (c) red-bellied woodpecker (Melanerpes carolinus), (d) red-winged blackbird (Agelaius phoeniceus), (e) zebra finch (Taeniopygia guttata) and (f) tufted titmouse (Baeolophus bicolor). All images are from Wikimedia Commons. (Online version in colour.)
Studies of human perception of colour generate predictions about how we expect non-human animal receivers' responses to be shaped by achromatic, high-contrast backgrounds. In humans, brightness discrimination between achromatic stimuli is easiest when the brightness of the background is similar to the stimuli being discriminated between. This effect becomes even more pronounced when the brightness of the background is intermediate between that of the two stimuli being discriminated [54] (figure 2). A similar effect occurs with chromatic discrimination of coloured stimuli. In this case, colour discrimination between the two stimuli is easiest when the stimuli have a low chromatic contrast with the background (e.g. discriminating between two orange stimuli on a red background is easier than discriminating between the same stimuli on a green background) [49]. It is possible that these two effects interact, where achromatic contrast between two stimuli and the background has an effect on colour discrimination. Evidence from humans suggests that colour discrimination between two stimuli depends on the achromatic contrast of both stimuli with the background [37].
Figure 2.
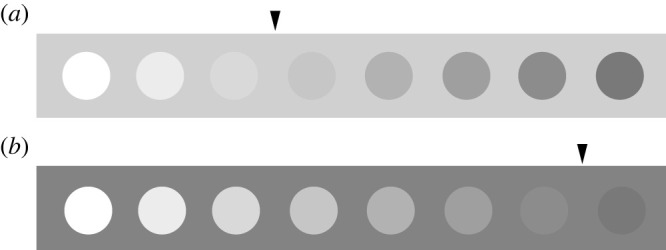
Effect of achromatic background contrast on brightness discrimination. Evidence from humans suggests that stimuli are easiest to discriminate when placed on a background with low brightness contrast [37,54]. We can demonstrate this effect using equally spaced achromatic stimuli on a light grey (a) and dark grey (b) background. The arrows mark the brightness of the background relative to the series of stimuli. Stimuli that are closer in brightness to each background are more easily discriminable, and this effect is most pronounced when the background has an intermediate brightness to the two stimuli. This effect is apparent with colour stimuli, as well, but because digital displays are highly variable, we opt here to show only the achromatic version of this effect.
Understanding the effects of the contrast between the background and the signal (referred to hereafter as ‘background contrast’) in animals is critical for understanding signalling behaviour and signal evolution. Researchers generally consider colour signals in isolation from their backgrounds. But if the impact of the colour immediately surrounding a focal colour patch on an animal (the local background) shapes perception of the patch, considering only the patch itself paints an incomplete picture of the signalling interaction. Instead, it is more appropriate to think of the signal as being comprised both the colour patch and its local background. Similarly, the larger environmental background in which signalling occurs can affect the perception of a signal and can be manipulated by signallers through means such as constructing bowers [17], clearing display courts [50] or changing signalling locations to locate a suitable background [18,24]. By isolating the effects of local background contrast on the colour perception of signal-relevant colours with behavioural experiments, we can determine to what extent backgrounds may influence the perception and assessment of colour signals.
Here, we examine whether the brightness of achromatic backgrounds affects colour discrimination in the orange-red range using zebra finches as a model. Zebra finches offer a useful system to explore the effects of backgrounds in the context of signalling because they use an orange–red sexual signal [5,33], have largely achromatic feathers surrounding their colourful beaks and cheek patches, and have established visual sensitivity data [6]. Furthermore, previous work on zebra finches provides a validated experimental design to answer questions about their colour discrimination capabilities (e.g. [9,10,59]).
2. Methods
(a) . Experimental subjects
The birds used in this study were female zebra finches (n = 10; age 2–26 months) obtained from a colony maintained by R. Mooney at Duke University (IACUC A227-17-09). Birds were housed in single cages (46 × 23 × 23 cm) and provided with a cuttlebone and two wooden perches. The room was kept on a 15 h : 9 h light : dark cycle and the birds were given birdseed (Kaytee Supreme Finch Food) and water ad libitum. Testing was done under Duke IACUC protocol A260-19-12.
(b) . Colour stimuli
Colour stimuli were made using Munsell colour paper (Pantone Corporation, Grand Rapids, MI, USA). We used a subset (colours 3–8) of the orange and red Munsell colours used in the Caves et al. [9] study, which have been shown previously to represent the range of beak coloration in zebra finch males [4,7,11] and that are roughly evenly spaced in chromatic distance as perceived by zebra finches [9]. This subset was chosen because colours 3–8 represent the full range of Munsell brightness levels used by Caves et al. [9] (see electronic supplementary material, table S1 for Munsell classifications). To remain consistent with prior work, we refer to the colours used in this study by the same numbers used previously (for full list of stimulus numbers and corresponding Munsell colours see electronic supplementary material, table S1). To create the stimuli, 2.5 cm diameter circles of Munsell colour paper were glued to card stock and covered with a clear epoxy disc. Then, we attached small vinyl discs to the back of the card stock that fit in the well of the foraging grid (see below). In total, we created six sets of colour stimuli corresponding to colours 3 (the most red as viewed by humans) through 8 (the most orange).
(c) . Foraging grids
To test whether achromatic contrast with the background affects colour discrimination, we created three sets of foraging grids: white, grey and black. All grids consisted of two blocks made from plastic home construction material (13.5 × 19 cm2) with six evenly spaced wells per block. The top two rows of each block were used to display stimuli (figure 3). Each block was covered with a 3 mm thick piece of matte PVC foam (Acme Plastics Inc, Woodland Park, NJ, USA) that was white, grey or black. The average reflectances across the human-visible range (400–700 nm) for the white, grey and black PVC foam pieces were 82%, 26% and 6.9%, respectively. While zebra finches are sensitive to ultraviolet (UV) light, the lighting used in our experimental room produces very little UV light, so we consider only the human-visible spectrum [9,58].
Figure 3.
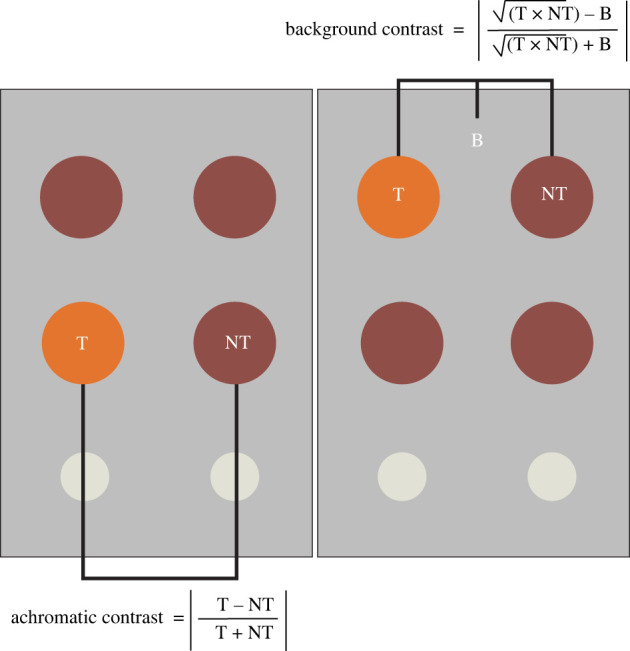
Diagram of a foraging grid. Birds were presented with a foraging grid containing eight coloured stimuli. The two target stimuli (T) were a different colour than the six non-target stimuli (NT). All trials were repeated on three different backgrounds (B): black, white and grey. To calculate background contrast for our analyses, we took the geometric mean double cone catch from the target and non-target stimuli (given as the square root of the product of the two double cone catches) and used it and the double cone catch of the background to calculate Michelson contrast. The second value used in our models was the achromatic Michelson contrast between the target and non-target stimuli. (Online version in colour.)
(d) . Experimental protocol
We tested the birds’ ability to discriminate between colours along a red-orange spectrum using an ‘odd-one-out’ testing paradigm. The birds were tasked with uncovering the foraging grid wells beneath two target stimuli before uncovering any non-target stimuli, in exchange for a food reward of millet (for an image of the experimental set-up, see electronic supplementary material, figure S1). The two target stimuli were the same colour as each other, but they differed in colour from the six non-target stimuli, all of the same colour. A trial was considered successful if both target wells were uncovered before any non-target wells within 2 min. We trained the birds to uncover the target wells in four phases. First, birds were presented with foraging grids that had millet in two wells, with target coloured discs (colour 3 or colour 8) placed next to the wells. Then, once the birds would reliably eat out of the target wells, we began to partially cover wells with the discs. In the third phase, wells were fully covered by the discs. Finally, we presented birds with the same two target discs (either colour 3 or colour 8) surrounded by six disks from the opposite end of the colour spectrum (e.g. colour 8 if the target colour was 3). In all training and experimental trials, the locations of the target wells were selected randomly. Food was removed from the cage approximately 5 h prior to testing to ensure individuals were motivated to participate.
In our testing, each of the 10 birds completed 10 trials per background (i.e. foraging grid colour), per colour combination. In total, we had 15 combinations of target and non-target colours that were one, two, three, four or five colour steps apart. On each experimental day, the birds were presented with one refresher trial, a comparison between colour 3 (the most red) and colour 8 (the most orange), followed by five experimental trials. With the exception of the refresher trial, only one colour combination was tested per day. We first tested birds on the easiest discrimination tasks (five colour steps apart or approximately 14 just-noticeable differences, hereafter abbreviated as JNDs) and progressively moved to the hardest tasks (one colour step apart or approx. 2.5 JNDs). Following Caves et al. [9], JNDs were calculated with the receptor noise-limited (RNL) model [52] using photon catches calculated from the spectral sensitivity of the zebra finch visual system [28]. All trials for a given background were completed before changing the background. Once the birds had completed 10 trials per colour combination on a given background, we repeated the training and experimental phases for the new background. All birds were tested first on grey, then black, then white.
(e) . Statistical analysis
To determine whether achromatic contrast between the background and colour stimuli had an effect on colour discrimination, we used the lme4 [3] package in R [60] to create generalized linear mixed (GLM) effect models considering different combinations of three fixed effects: (i) chromatic distance between the target and non-target stimuli in JNDs, (ii) the Michelson achromatic contrast [35] between the target and non-target stimuli and (iii) the Michelson achromatic contrast between the geometric mean of the target stimuli and non-target stimuli and the background (background contrast). We chose to use geometric means because brightness perception is proportional (e.g. doubling the number of photons reflected increases the perceived brightness by the same amount regardless of the absolute number of photons) [25]. Brightness values were calculated using the photon catches from zebra finch double cones, which are implicated in brightness perception [40]. Bird ID was included as a random effect in all models to account for individual variation in task performance. Our proxy for discrimination ability was the pass frequency for a given experimental comparison, where pass frequency is the percentage of trials that a bird correctly selected the two target stimuli before any non-target stimuli. We then compared a selected set of models using the Akaike information criterion (ΔAIC) to select the best model.
3. Results
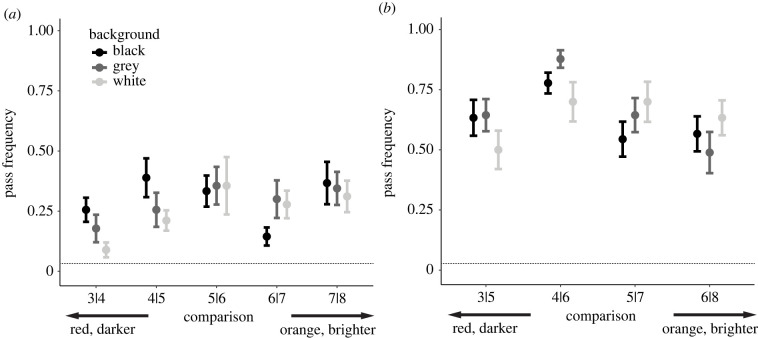
Mean pass frequencies for one-apart trials (e.g. 3|4, 5|6) were between 8.9% and 40% (figure 4a). The pass rates for two-apart comparisons were higher than for one-apart comparisons and ranged from 49% to 79% (figure 4b). Generally, for both the one- and two-apart trials, the highest pass frequencies for a given comparison were on backgrounds closest in brightness to the target and non-target stimuli (e.g. dark red colours were better discriminated on black backgrounds, when ‘background contrast’ was low, than on white backgrounds, see below). Trials that were three or more colour steps apart all had pass frequencies greater than 80% with little differentiation between backgrounds suggesting that these tasks were sufficiently easy for the birds to overcome any potential effect of achromatic contrast with the background.
Figure 4.
Discrimination test results. Mean (+/− s.d.) pass frequency over 10 one-apart (a) and two-apart (b) discrimination trials per bird. Results are segmented by the background and, generally, pass frequencies for darker colours are lower on brighter backgrounds, and vice-versa. The dotted line on both graphs represents the expected pass frequency if birds chose stimuli randomly (1 in 24).
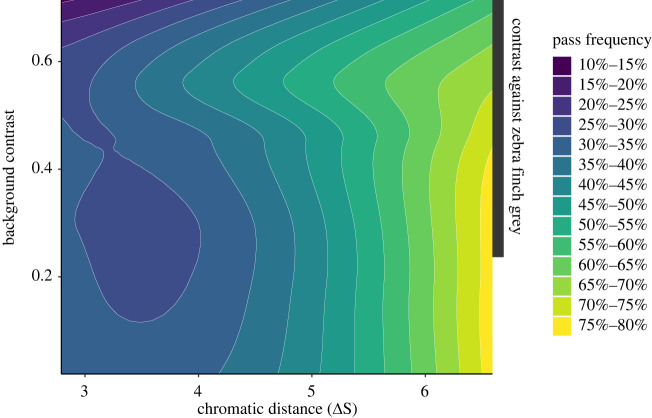
To determine whether achromatic contrast between the target and non-target colour stimuli with an achromatic background affected colour discrimination in the zebra finches, we built GLM models with different combinations of chromatic distance between the colour stimuli, Michelson achromatic contrast between the two colour stimuli and the background, and the Michelson achromatic contrast between the target and non-target stimuli (table 1). Only the one-apart and two-apart data were used for the models because of the 80–100% pass rate for larger colour distances. We found the model that best predicted pass frequency to be an additive model with chromatic distance (ΔS) and background contrast as fixed effects. There were three other models with 0 < ΔAIC < 3, including two that had achromatic contrast between the target and non-target stimuli as a fixed effect. Because our model with the lowest AIC was also the most parsimonious of those with meaningful support, we chose that as our best model. Further supporting our lowest AIC model as the best model, a weighted model yields an almost identical estimate for the effect of background contrast on pass frequency (−0.616 versus −0.6; electronic supplementary material, table S2). Chromatic distance has a stronger effect on pass frequency than background contrast, consistent with the findings of Miyahara et al. [37] that demonstrate achromatic background contrast is a second-order contributor to colour discrimination thresholds. Miyahara et al. [37] found that changes in background brightness can increase colour discrimination thresholds by as much as 0.4 JNDs in their experimental paradigm. Our model predicts that pass frequency declines by 6% for every 0.1 unit increase in Michelson achromatic contrast between the two colour stimuli and the background. Finally, we used a local regression (LOESS) model to interpolate pass frequency as a function of the chromatic distance between non-target and target stimuli (ΔS) and the achromatic Michelson contrast between colour stimuli and the background. Using this local regression, we found that reduced discrimination occurs primarily in contexts of high-contrast backgrounds (background contrast greater than 0.5) and small chromatic distances between the colour stimuli (figure 5) table 2.
Table 1.
GLM model results. We created GLM models using different combinations of chromatic distance, background contrast and brightness ratio. Of our six models, the model with the lowest AIC was an additive model including chromatic distance and background contrast as fixed effects. Three other models had some support (0 < ΔAIC < 3) and all of them included background contrast as a fixed effect.
| model | Akaike weight | ΔAIC |
|---|---|---|
| chromatic distance + background contrast | 0.35 | — |
| chromatic distance + background contrast × stimulus contrast | 0.26 | 0.6 |
| chromatic distance + background contrast + stimulus contrast | 0.17 | 1.5 |
| chromatic distance × background contrast | 0.13 | 2.0 |
| chromatic distance × background contrast + stimulus contrast | 0.06 | 3.5 |
| chromatic distance | 0.01 | 6.7 |
Figure 5.
Model predictions of pass frequency. Using a LOESS model [23] to predict pass frequency as a function of background contrast (the achromatic Michelson contrast between the target stimuli and background) and chromatic distance between the colour stimuli, we see that high background contrast (greater than 0.6) reduces predicted pass frequency for a given chromatic distance, particularly when the chromatic distance is small. The dark grey box represents the range of background contrast values that the Munsell colours used in this experiment would produce against the grey colour of male zebra finches. (Online version in colour.)
Table 2.
Summary of most parsimonious model. Model estimates and 95% confidence intervals for the effects of chromatic distance and achromatic Michelson contrast between the stimuli and background. Increasing chromatic distance makes discrimination easier (i.e. a higher pass frequency in our experiments), while increasing background contrast makes discrimination more difficult (i.e. a lower pass frequency). We also include the marginal pseudo-R2 calculated using the MuMIn package in R [2].
| predictor | point estimate | 95% CI lower bound | 95% CI upper bound |
|---|---|---|---|
| chromatic distance*** | 0.538 | 0.478 | 0.598 |
| background contrast** | −0.616 | −1.025 | −0.207 |
**p < 0.01; ***p < 0.001; marginal pseudo-R2 = 0.161.
4. Discussion
Here we demonstrate that the brightness of achromatic backgrounds can affect colour discrimination in zebra finches in the orange–red range. Specifically, high achromatic contrast between the colour stimuli and the background impedes colour discrimination when the chromatic distance between two stimuli is small (less than 4 ΔS). In essence, colour discrimination thresholds are dictated not only by the relative colour distance of the two stimuli (Weber's law), but also their achromatic contrast with an achromatic background. To our knowledge, this is the first demonstration of the brightness of achromatic backgrounds affecting colour discrimination in a non-human animal.
Our findings are consistent with results from humans showing that colour discrimination thresholds can change with achromatic background contrast [37] and support a growing body of research suggesting that chromatic and achromatic channels are not entirely separate in birds [26,29,36,38]. Although visual models such as the RNL model assume zero achromatic contrast between a stimulus and background in their formulation [52], in practice, visual models are used to study colour vision in more natural contexts where the brightness of the background can vary dramatically relative to the brightness of the signal [30,31,45]. Our results for achromatic backgrounds, and previous experiments on the effects of background colour on discrimination [28,39,57], support more conservative usage of visual models when studying interactions that take place outside of the laboratory, where several assumptions of these models are violated. They also support continued work to behaviourally test the assumption results of such models (as advocated for elsewhere [38]).
In addition to supporting conservative use of visual models, the effect of background achromatic contrast on colour discrimination has important implications for understanding signalling systems. Two primary features of effective signals are detectability and discriminability [22]. Detectability describes how easily a signal is perceived; signals that are poorly detected by receivers are less likely to evolve. Once a signal is detected, it may also need to be discriminated from signals of another type, or, in the case of assessment signals, signals of the same type [55]. As detectability is generally enhanced by increasing visual contrast with the background, and discriminability is enhanced by minimizing contrast with the background [1,16,28,43,53], there is a potential trade-off between the two. This trade-off may lead to adaptations in coloration and signalling behaviour such as more variable coloration of signals in animals where the signal contrasts strongly with the background [13], binary threshold responses to circumvent the need for fine-scale discrimination [21], or additional modes of assessment in animals with high-contrast signals, such as courtship dances in ultra-black birds of paradise [32]. Furthermore, this trade-off may also be influenced by the visual physiology of the receiver(s). For example, differences in visual sensitivity between conspecifics and predators may constrain colour signals to be less detectable to predators, lowering the optimal contrast between the signal and background, thus making the signal more discriminable.
Our results on achromatic backgrounds combined with previous work on coloured backgrounds prompt further study into the evolution of coloration in birds. Instead of thinking of colour signals as solely the focal colour patch, we suggest that signals be considered as the combination of the focal colour patch and the local background, even when the local background is achromatic. Local background colours and brightnesses can evolve in concert with focal patches or offer an additional axis of diversification in the evolution of signal form. By studying signals under this framework, we may reveal diversity in bird colour signals that arise as signals begin to diverge between different populations or species.
Quantifying both the environmental background and the local background formed by integumentary structures immediately surrounding colour signals in future work will provide valuable data to test the predictions made here and assess the effects of background contrast on natural signalling behaviours. Additionally, testing the effects of background contrast in signalling interactions will allow researchers to determine the relative contributions of chromatic contrast, achromatic contrast, colour memory and higher-level perceptual mechanisms, furthering our understanding of signal form and evolution.
Acknowledgements
We thank Drs Eleanor Caves and Patrick Green for helpful discussions when designing the experiments; D. Adrussier and J. Sokoloff for assistance with trials; and R. Mooney for providing access to zebra finches from his colony.
Data accessibility
The datasets supporting this article have been provided in the electronic supplementary material [61].
Authors' contributions
A.D.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing—original draft and writing—review and editing; M.N.Z.: conceptualization, formal analysis and writing—review and editing; D.D.: conceptualization, investigation and writing—review and editing; S.P.: conceptualization, investigation, methodology, project administration, resources, supervision and writing—review and editing; S.N.: conceptualization, funding acquisition, methodology, project administration, supervision and writing—review and editing; S.J.: conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, validation and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
A.D. conducted this research with Government support under and awarded by DoD, Army Research Office, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a.
References
- 1.Aronsson M, Gamberale-Stille G. 2008. Domestic chicks primarily attend to colour, not pattern, when learning an aposematic coloration. Anim. Behav. 75, 417-423. ( 10.1016/j.anbehav.2007.05.006) [DOI] [Google Scholar]
- 2.Barton K. 2009. MuMIn: multi-model inference, R package version 0.12. 0. See http://r-forge.r-project.org/projects/mumin/.
- 3.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 4.Birkhead TR, Fletcher F, Pellatt EJ. 1998. Sexual selection in the zebra finch Taeniopygia guttata: condition, sex traits and immune capacity. Behav. Ecol. Sociobiol. 44, 179-191. ( 10.1007/s002650050530) [DOI] [Google Scholar]
- 5.Blount JD, Metcalfe NB, Birkhead TR, Surai PF. 2003. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300, 125-127. ( 10.1126/science.1082142) [DOI] [PubMed] [Google Scholar]
- 6.Bowmaker JK, Heath LA, Wilkie SE, Hunt DM. 1997. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vis. Res. 37, 2183-2194. ( 10.1016/S0042-6989(97)00026-6) [DOI] [PubMed] [Google Scholar]
- 7.Burley N, Coopersmith CB. 1987. Bill color preferences of zebra finches. Ethology 76, 133-151. ( 10.1111/j.1439-0310.1987.tb00679.x) [DOI] [Google Scholar]
- 8.Caro T, Ruxton G. 2019. Aposematism: unpacking the defences. Trends Ecol. Evol. 34, 595-604. ( 10.1016/j.tree.2019.02.015) [DOI] [PubMed] [Google Scholar]
- 9.Caves EM, Green PA, Zipple MN, Peters S, Johnsen S, Nowicki S. 2018. Categorical perception of colour signals in a songbird. Nature 560, 365-367. ( 10.1038/s41586-018-0377-7) [DOI] [PubMed] [Google Scholar]
- 10.Caves EM, Green PA, Zipple MN, Bharath D, Peters S, Johnsen S, Nowicki S. 2021. Comparison of categorical color perception in two Estrildid finches. Am. Nat. 197, 190-202. ( 10.1086/712379) [DOI] [PubMed] [Google Scholar]
- 11.Collins SA, Hubbard C, Houtman AM. 1994. Female mate choice in the zebra finch—the effect of male beak colour and male song. Behav. Ecol. Sociobiol. 35, 21-25. ( 10.1007/BF00167055) [DOI] [Google Scholar]
- 12.Davis AL, Nijhout HF, Johnsen S. 2020. Diverse nanostructures underlie thin ultra-black scales in butterflies. Nat. Comm. 11, 1-7. ( 10.1038/s41467-020-15033-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delhey K, Szecsenyi B, Nakagawa S, Peters A. 2017. Conspicuous plumage colours are highly variable. Proc. R. Soc. B 284, 20162593. ( 10.1098/rspb.2016.2593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doucet SM, Meadows MG. 2009. Iridescence: a functional perspective. J. R. Soc. Int. 6, 115-132. ( 10.1098/rsif.2008.0395.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doucet SM, Mennill DJ, Hill GE. 2007. The evolution of signal design in manakin plumage ornaments. Am. Nat. 169, 62-80. ( 10.1086/510162) [DOI] [PubMed] [Google Scholar]
- 16.Endler JA. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat. 139, 125-153. ( 10.1086/285308) [DOI] [Google Scholar]
- 17.Endler JA, Day LB. 2006. Ornament colour selection, visual contrast and the shape of colour preference functions in great bowerbirds, Chlamydera nuchalis. Anim. Behav. 72, 1405-1416. ( 10.1016/j.anbehav.2006.05.005) [DOI] [Google Scholar]
- 18.Endler JA, Thery M. 1996. Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds. Am. Nat. 148, 421-452. ( 10.1086/285934) [DOI] [Google Scholar]
- 19.Gittleman JL, Harvey PH, Greenwood PJ. 1980. The evolution of conspicuous coloration: some experiments in bad taste. Anim. Behav. 28, 897-899. ( 10.1016/S0003-3472(80)80150-3) [DOI] [Google Scholar]
- 20.Grafen A, Johnstone RA. 1993. Why we need ESS signalling theory. Phil. Trans. R. Soc. Lond. B 340, 245-250. ( 10.1098/rstb.1993.0064) [DOI] [PubMed] [Google Scholar]
- 21.Green PA, Brandley NC, Nowicki S. 2020. Categorical perception in animal communication and decision-making. Behav. Ecol. 31, 859-867. ( 10.1093/beheco/araa004) [DOI] [Google Scholar]
- 22.Guilford T, Dawkins MS. 1991. Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1-14. ( 10.1016/S0003-3472(05)80600-1) [DOI] [Google Scholar]
- 23.Harrell FE. 2001. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer. [Google Scholar]
- 24.Heindl M, Winkler H. 2003. Vertical lek placement of forest-dwelling manakin species (Aves, Pipridae) is associated with vertical gradients of ambient light. Biol. J. Linn. Soc. 80, 647-658. ( 10.1111/j.1095-8312.2003.00264.x) [DOI] [Google Scholar]
- 25.Johnsen S. 2012. The optics of life. Princeton, NJ: Princeton University Press. [Google Scholar]
- 26.Jones CD, Osorio D. 2004. Discrimination of oriented visual textures by poultry chicks. Vis. Res. 44, 83-89. ( 10.1016/j.visres.2003.08.014) [DOI] [PubMed] [Google Scholar]
- 27.Koski MH. 2020. The role of sensory drive in floral evolution. New Phytol. 227, 1012-1024. [DOI] [PubMed] [Google Scholar]
- 28.Lind O. 2016. Colour vision and background adaptation in a passerine bird, the zebra finch (Taeniopygia guttata). R. Soc. Open Sci. 3, 160383. ( 10.1098/rsos.160383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lind O, Kelber A. 2011. The spatial tuning of achromatic and chromatic vision in budgerigars. J. Vision 11, 2. ( 10.1167/11.7.2) [DOI] [PubMed] [Google Scholar]
- 30.Manna TJ, Hanley D, Honza M, Capek M, Rutila J, Samaš P, Abolins-Abols M, Hauber ME. 2020. Fitting different visual models to behavioral patterns of parasitic egg rejection along a natural egg color gradient in a cavity-nesting host species. Vis. Res. 167, 54-59. ( 10.1016/j.visres.2019.12.007) [DOI] [PubMed] [Google Scholar]
- 31.Martin M, Badiane A, Le Galliard JF. 2019. The relative importance of body size and UV coloration in influencing male-male competition in a lacertid lizard. Behav. Ecol. Sociobiol. 73, 1-14. ( 10.1007/s00265-018-2618-z) [DOI] [Google Scholar]
- 32.McCoy DE, Feo T, Harvey TA, Prum RO. 2018. Structural absorption by barbule microstructures of super black bird of paradise feathers. Nat. Comm. 9, 1-8. ( 10.1038/s41467-017-02088-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGraw KJ, Ardia DR. 2003. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 162, 704-712. ( 10.1086/378904) [DOI] [PubMed] [Google Scholar]
- 34.McLean CA, Moussalli A, Stuart-Fox D. 2014. Local adaptation and divergence in colour signal conspicuousness between monomorphic and polymorphic lineages in a lizard. J. Evol. Biol. 27, 2654-2664. ( 10.1111/jeb.12521) [DOI] [PubMed] [Google Scholar]
- 35.Michelson AA. 1927. Studies in optics. Chicago, IL: University of Chicago Press. [Google Scholar]
- 36.Mitkus M, Olsson P, Toomey MB, Corbo JC, Kelber A. 2017. Specialized photoreceptor composition in the raptor fovea. J. Comp. Neuro. 525, 2152-2163. ( 10.1002/cne.24190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyahara E, Smith VC, Pokorny J. 1993. How surrounds affect chromaticity discrimination. J. Opt. Soc. Am. 10, 545-553. ( 10.1364/JOSAA.10.000545) [DOI] [PubMed] [Google Scholar]
- 38.Olsson P, Lind O, Kelber A. 2018. Chromatic and achromatic vision: parameter choice and limitations for reliable model predictions. Behav. Ecol. 29, 273-282. ( 10.1093/beheco/arx133) [DOI] [Google Scholar]
- 39.Olsson P, Johnsson RD, Foster JJ, Kirwan JD, Lind O, Kelber A. 2020. Chicken colour discrimination depends on background colour. J. Exp. Biol. 223, jeb209429. ( 10.1242/jeb.209429) [DOI] [PubMed] [Google Scholar]
- 40.Osorio D, Miklósi A, Gonda Z. 1999. Visual ecology and perception of coloration patterns by domestic chicks. Evol. Ecol. 13, 673-689. ( 10.1023/A:1011059715610) [DOI] [Google Scholar]
- 41.Price TD. 2017. Sensory drive, color, and color vision. Am. Nat. 190, 157-170. ( 10.1086/692535) [DOI] [PubMed] [Google Scholar]
- 42.Roper TJ, Redston S. 1987. Conspicuousness of distasteful prey affects the strength and durability of one-trial avoidance learning. Anim. Behav. 35, 739-747. ( 10.1016/S0003-3472(87)80110-0) [DOI] [Google Scholar]
- 43.Santiago C, Green NF, Hamilton N, Endler JA, Osorio DC, Marshall NJ, Cheney KL. 2020. Does conspicuousness scale linearly with colour distance? A test using reef fish. Proc. R. Soc. B 287, 20201456. ( 10.1098/rspb.2020.1456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Searcy WA, Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 45.Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K. 2004. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 207, 2471-2485. ( 10.1242/jeb.01047) [DOI] [PubMed] [Google Scholar]
- 46.Smith JM. 1965. The evolution of alarm calls. Am. Nat. 99, 59-63. ( 10.1086/282349) [DOI] [Google Scholar]
- 47.Smith JM, Harper D. 2003. Animal signals. Oxford, UK: Oxford University Press. [Google Scholar]
- 48.Stavenga DG, Stowe S, Siebke K, Zeil J, Arikawa K. 2004. Butterfly wing colours: scale beads make white pierid wings brighter. Proc. R. Soc. Lond. B 271, 1577-1584. ( 10.1098/rspb.2004.2781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takasaki H. 1967. Chromatic changes induced by changes in chromaticity of background of constant lightness. J. Opt. Soc. Am. 57, 93-96. ( 10.1364/JOSA.57.000093) [DOI] [PubMed] [Google Scholar]
- 50.Uy JAC, Endler JA. 2004. Modification of the visual background increases the conspicuousness of golden-collared manakin displays. Behav. Ecol. 15, 1003-1010. ( 10.1093/beheco/arh106) [DOI] [Google Scholar]
- 51.Vilela DS, Tosta TA, Rodrigues RR, Del-Claro K, Guillermo-Ferreira R. 2017. Colours of war: visual signals may influence the outcome of territorial contests in the tiger damselfly, Tigriagrion aurantinigrum. Biol. J. Linn. Soc. 121, 786-795. ( 10.1093/biolinnean/blx024) [DOI] [Google Scholar]
- 52.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351-358. ( 10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward JL. 2018. Conspicuous signal evolution in heterogeneous environments. Behav. Ecol. Sociobiol. 72, 1-6. ( 10.1007/s00265-017-2413-2) [DOI] [Google Scholar]
- 54.Whittle P. 1992. Brightness, discriminability and the ‘crispening effect’. Vis. Res. 32, 1493-1507. ( 10.1016/0042-6989(92)90205-W) [DOI] [PubMed] [Google Scholar]
- 55.Wiley RH. 2006. Signal detection and animal communication. Adv. Study Behav. 36, 217-247. ( 10.1016/S0065-3454(06)36005-6) [DOI] [Google Scholar]
- 56.Winters S, Allen WL, Higham JP. 2020. The structure of species discrimination signals across a primate radiation. Elife 9, 47428. ( 10.7554/eLife.47428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yager D. 1974. Effects of chromatic adaptation on saturation discrimination in goldfish. Vis. Res. 14, 1089-1094. ( 10.1016/0042-6989(74)90205-3) [DOI] [PubMed] [Google Scholar]
- 58.Yokoyama S, Radlwimmer FB, Blow NS. 2000. Ultraviolet pigments in birds evolved from violet pigments by a single amino acid change. Proc. Natl Acad. Sci. USA 97, 7366-7371. ( 10.1073/pnas.97.13.7366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zipple MN, Caves EM, Green PA, Peters S, Johnsen S, Nowicki S. 2019. Categorical colour perception occurs in both signalling and non-signalling colour ranges in a songbird. Proc. R. Soc. B 286, 20190524. ( 10.1098/rspb.2019.0524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.R Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 61.Davis A, Zipple MN, Diaz D, Peters S, Nowicki S, Johnsen S. 2022. Data from: influence of visual background on discrimination of signal-relevant colors in zebra finches (Taeniopygia guttata). FigShare. ( 10.6084/m9.figshare.c.5994911) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Davis A, Zipple MN, Diaz D, Peters S, Nowicki S, Johnsen S. 2022. Data from: influence of visual background on discrimination of signal-relevant colors in zebra finches (Taeniopygia guttata). FigShare. ( 10.6084/m9.figshare.c.5994911) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The datasets supporting this article have been provided in the electronic supplementary material [61].