Abstract
Although androgens are widely studied in the context of aggression, androgenic influences on prosocial behaviours have been less explored. We examined testosterone's (T) influence on prosocial and aggressive responses in a positively valenced social context (interacting with a pairbond partner) and a negatively valenced context (interacting with an intruder) in socially monogamous Mongolian gerbils. T increased and decreased prosocial responses in the same individuals towards a pairbond partner and an intruder, respectively, both within 30 min, but did not affect aggression. T also had persistent effects on prosocial behaviour; males in which T initially increased prosocial responses towards a partner continued to exhibit elevated prosocial responses towards an intruder male days later until a second T injection rapidly eliminated those responses. Thus, T surges can rapidly match behaviour to current social context, as well as prime animals for positive social interactions in the future. Neuroanatomically, T rapidly increased hypothalamic oxytocin, but not vasopressin, cellular responses during interactions with a partner. Together, our results indicate that T can facilitate and inhibit prosocial behaviours depending on social context, that it can influence prosocial responses across rapid and prolonged time scales, and that it affects oxytocin signalling mechanisms that could mediate its context-dependent behavioural influences.
Keywords: testosterone, social behaviour, aggression, oxytocin, steroids
1. Introduction
The ability to shift behaviour to rapidly adapt to dynamic social environments is crucial for the survival and fitness of most organisms [1]. While there are several mechanisms that contribute to an animal's ability to shift social behaviour in a context-dependent manner, there are two hormone systems in the brain that are of particular interest—sex steroids and nonapeptides.
Sex steroids can be peripherally derived or produced in the brain and may act through genomic (transcription dependent) and non-genomic (transcription independent) mechanisms on neural circuits to modulate behaviour [2,3]. Rapid effects of sex steroids via non-genomic mechanisms have most often been studied in relation to sexual behaviour and social challenges [1,4,5]. Testosterone (T), in particular, which often increases in response to sexual and/or aggressive stimuli [6], can rapidly stimulate sexual and aggressive behaviours in males, though many of those effects depend on the activation of oestrogen receptors following its conversion to oestradiol in the brain [5,7,8]. Although human studies indicate T can promote positive social responses in some contexts, particularly when such responses can promote social status [9–11], T's influence on nonsexual, prosocial behaviours have not been clearly demonstrated in non-human animals. Further, it has not been demonstrated in any species that T can promote and inhibit prosocial responses in the same individual across different social contexts.
The nonapeptides (i.e. vasopressin (VP) and oxytocin (OT)) are evolutionarily conserved peptides that play important roles in a variety of social behaviours ranging from affiliation to anxiety and aggression [12]. Nonapeptides function in a multi-modal manner such that they can modulate neural signalling in a diffusive, slow and global manner, as well as through a targeted axonal, fast and focal manner [13], the latter of which can allow for the execution of rapid responses to external stimuli. The nonapeptide cell groups of the paraventricular nucleus of the hypothalamus (PVN) represent the largest VP and OT neuronal populations in the brain and play prominent roles in social regulation. Both cell groups send axonal projections throughout the brain, as well as to the pituitary [14]. Because OT can serve to facilitate the stress response as well as enhance prosocial behaviour, it has been proposed that OT generally serves to enhance social saliency and shift an animal's attention towards social cues [15]. This characteristic of OT signalling suggests that this peptide may play a particularly important role in the exhibition of context-appropriate behaviour.
Taken together, steroids and nonapeptides exhibit properties that can facilitate rapid effects on behaviour, and both systems modulate social and aggressive behaviours. Whether these two systems interact to produce context-appropriate behaviour is not well understood. Neurosteroid biosynthesis is regulated by VP and OT in frogs and birds [16], and, conversely, hypothalamic VP and OT release is modulated by neurosteroids in rats [17]. Additionally, VP neuronal populations and receptor densities are sensitive to gonadal steroids in rodents and humans [18–20]. However, if and how these systems interact to rapidly modulate social behaviour has not been determined.
In the present study, we first sought to determine the rapid influences of T on prosocial behaviours across different social contexts, including those in which prosocial behaviours are most apparent and presumably adaptive (e.g. interacting with a pairbond partner) and those in which antisocial/aggressive behaviours are most apparent (e.g. a resident intruder). We hypothesized that T would decrease prosocial behaviours across contexts if its primary role is to promote antisocial/aggressive responses, independent of the current social context. However, if T promotes context-appropriate responses, be they prosocial or antisocial/aggressive, we predicted it would increase prosocial responses towards a partner but decrease prosocial behaviour, and increase aggression, towards a conspecific intruder. Further, if any such influences depend on the activation of non-genomic mechanisms, those influences should occur rapidly (within 30 min). We hypothesized that T would influence PVN OT and/or VP cellular responses to those social cues if steroid—nonapeptide interactions underlie any such effects. To test these hypotheses, we used the socially monogamous Mongolian gerbil (Meriones unguiculatus), a territorial rodent in which adult males and females form stable pair bonds and share a nest in burrows with a few litters of offspring [21,22]. Although Mongolian gerbils are affiliative in reproductive contexts with mates and offspring, they exhibit aggression during interactions with novel, same-sex individuals and reliably attack an intruder [23–26], making it possible to examine how T and neuropeptides may promote context-appropriate behavioural responses.
2. Material and methods
(a) . Animals
All Mongolian gerbils were obtained as young adults (postnatal day (PND) 50–65) from Charles River Laboratories and tested between PND 80–100. Gerbils become sexually mature by PND 60 [27]. Gerbils were group housed (2–4) with same-sex littermates in standard rat polycarbonate cages (40.64 × 20.32 × 20.32 cm) prior to the establishment of male-female pairs. All cages were lined with Sani-Chips bedding and included nesting material, chewing blocks and shepherd shacks. Animals were able to obtain food and water ad libitum and were kept on a 14 L : 10 D cycle. Ambient temperatures were maintained at 24 ± 2°C. All procedures were approved by the Institutional Animal Care and Use Committee of Emory University (protocol 202000131).
(b) . Intraperitoneal injections
T (Steraloids) was dissolved in beta-cyclodextrin (Sigma Aldrich) and diluted to 50ug kg−1 in sterile isotonic saline and injected intraperitoneally (IP). T in beta-cyclodextrin quickly delivers T to the bloodstream for rapid metabolization, resulting in circulating T that resembles natural T pulses [28,29]. We first validated a dose of T that elevated plasma T within the range of values previously reported in gerbils after pair bonding [30] (see the electronic supplementary material, figure S1).
(c) . Behavioural testing
Fourteen adult males were randomly assigned to either a saline group or testosterone (T) group. We used a within-subjects design and tested subjects in 2 pair interaction and 2 resident-intruder tests. This allowed us to obtain baseline behaviour in the absence of an acute T injection. Therefore, males in the saline group received saline for both tests 1 and 2, whereas males in the T group received saline for test 1 and T for test 2. We did not counterbalance treatment delivery to avoid potential carryover effects of an acute T injection through long-term genomic mechanisms.
After assignment to the saline or T groups, male subjects and 14 females were moved from same-sex sibling housing into single housing and primed for 1 day by having their future pairbond partner's soiled bedding added to their cage. The day after priming, males were moved into the females' cages and pairs were given 5 days to establish a pair bond. Gerbils that do not accept pairing fight. Pairs were monitored for 48 h after pairing. All pairs exhibited consistent huddling behaviour and no aggression within 48 h after pairing, and thus the pairing was considered to be accepted. Pairs then underwent pair interaction testing over a 3 day period. Gerbils are diurnally active and were tested during the day [31]. On the first day, all male subjects received an IP injection of saline and were then tested in a pair interaction test. The next day pairs were not tested. The following day, male subjects were re-tested in the pair interaction test after receiving either saline or T. For the next 4 days, pairs were not tested, serving as a washout period to avoid carryover effects of T treatment. Males then underwent a resident-intruder test over a 3 day period. Again, on the first day, all male subjects received an IP injection of saline and were then tested in a resident-intruder test. The next day subjects were not tested. The following day, male subjects were re-tested in the resident-intruder test after receiving either saline or T. For the next 5 days, pairs were not tested, serving as a washout period to avoid carryover effects of T treatment through potential genomic mechanisms. Males were then tested in a pair interaction immediate early gene (IEG) test and perfused for subsequent histological analysis (figure 1) for a timeline of testing.
Figure 1.
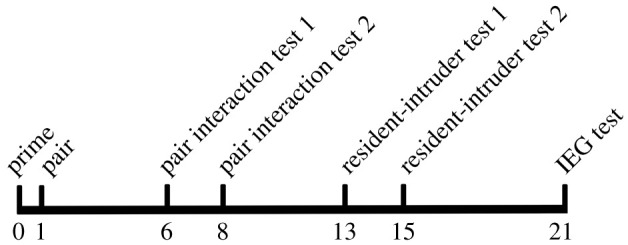
Testing timeline. Male subjects were primed for pairbonding on day 1. On day 6, subjects underwent the first pair interaction test in which both groups received a saline injection. On day 8, subjects were tested in a second pair interaction test after either a saline injection (saline group) or a testosterone injection (T group). On day 13, subjects underwent the first resident-intruder test in which both groups received a saline injection. On day 15, subjects were tested in a second resident-intruder test after either a saline injection (saline group) or a T injection (T group). On day 21, subjects were perfused immediately after an IEG test where subjects interacted with their pairbond partner.
(d) . Pair interaction test
Food and water were temporarily removed from the homecage. Male subjects were then administered an IP injection of either saline or T and were then immediately placed back in the homecage with their pairbond partner. Pairs were video recorded for 30 min and videos were scored for the time spent engaging in prosocial (positive investigation, positive side-by-side contact, huddling, allogrooming) and aggressive (chasing, lunging, pinning, aggressive side-by-side contact) behaviours using behavioural observation research interactive software (BORIS; [32]). Behaviours for each functional category (i.e. prosocial or aggressive) were summed owing to low frequencies of distinct behaviours for some animals because of natural variation in behaviour. Behaviour was analysed in three 10 min intervals for the 30 min pair interaction because previous studies have found that IP T injections begin to influence behaviour around 20–30 min after injection and thus we hypothesized that any behavioural effects of the T injection would be observable in the 20–30 min post-injection period [33,34]. No aggressive behaviour was observed in any pair interaction test, and thus no data on aggression during the pair interaction tests is reported.
(e) . Resident intruder test
Food and water were temporarily removed from the homecage. Female partners were also temporarily removed from the homecage and placed in holding cages. Male subjects were then administered an IP injection of either saline or T and were immediately placed back in the homecage for 15 min. A novel, age-matched, same-sex conspecific (i.e. an intruder) was then placed into the male's homecage and behaviour was video recorded for 15 min. Male intruders were group housed in same-sex sibling groups and removed directly from their homecage; intruders were allowed 3 h to rest before being used a second time as a stimulus animal. A resident-intruder test was immediately terminated if an injury occurred to any animal and veterinary care was sought. A total of two resident-intruder tests were terminated only on the second day of testing and only in animals in the T group. Behavioural videos were scored for prosocial and aggressive behaviour as described above. Because the resident-intruder test was shorter than the pair interaction test, behaviour was analysed in three 5 min intervals for the 15 min test. Two hours after the end of the resident-intruder test, female partners were returned to the homecage. Pairs were monitored for 15 min for aggression, none of which was observed.
(f) . Immediate early gene study design
Using the same animals from the tests described above, the IEG study aimed to assess behavioural and neural responses of males injected with saline or T during an interaction with their pairbond partner. Subjects underwent a modified pair interaction test. The female partner was temporarily removed from the homecage and placed in a holding cage. The male subject then received an IP injection of either saline or T and was immediately returned to the homecage. Ten minutes later, female partners were returned to the homecage and the 90 min IEG began. Females remained into the homecage with males for 30 min and were allowed to freely interact. After 30 min, females were removed and males remained in the homecage for an additional 60 min prior to undergoing a perfusion. Pair interactions were video recorded. Prosocial and aggressive behaviours were scored as described above in 10 min intervals for the pair interaction. No aggressive behaviour was observed in any pair, and thus no data on aggression during the IEG test is reported.
(g) . Histology and immunohistochemistry
At the end of the IEG test, male subjects were immediately euthanized by isoflurane overdose and were transcardially perfused with 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde dissolved in 0.1 M borate buffer (pH 9.5). Brains were extracted, post-fixed overnight in 4% paraformaldehyde dissolved in 0.1 M borate buffer (pH 9.5) before cryoprotection in 30% sucrose dissolved in PBS for 48 h. Brains were then frozen in Tissue-Tek O.C.T. Compound and stored at −80°C. Prior to immunohistochemical processing, brains were thawed and sectioned coronally at 40 μm using a Leica cryostat, with every third section being saved for use in the present study. Female partners were euthanized; all females were pregnant at the time of euthanasia. Postmortem analysis indicated that fetuses for all female partners were between embryonic day 16–21, suggesting all pair-bonded couples became pregnant within 1 week of pairing. Tissue sections were immunofluorescently stained for VP, OT, and Fos (the protein of the immediate early gene cFos) as previously described [35] (see the electronic supplementary material, figure S2). All females were in late stages of pregnancy; this is consistent with the typical latency to copulation after initial pairing (i.e. typically within 2 days) and a gestation period of 21 days [36].
(h) . Neural quantification
Photomicrographs were obtained using a Zeiss AxioImager II microscope (see the electronic supplemental materials for more information). For PVN cell counts, we quantified the total number of OT-immunoreactive (-ir) and VP-ir neurons and the number of OT-ir and VP-ir neurons that expressed Fos-ir in the PVN at rostral and caudal levels. A percentage of the number of nonapeptide-ir neurons expressing Fos-ir was used to account for individual differences in nonapeptide cell number. No significant differences between rostral and caudal levels were observed, so an average of the two levels was used for analyses.
(i) . Statistical analysis
Behavioural data were analysed using repeated measures general linear models (RM GLMs) and Mann-Whitney U-tests. Test (baseline, test 1; manipulation, test 2) was used as a within-subjects factor and group (saline; T) was used as a fixed factor. If T rapidly affected the behavioural responses, we predicted there would be a significant interaction between test and group, such that responses would not change in animals given saline both test days, but would increase (or decrease) in animals given T for test 2. Three time intervals were analysed separately, per the rationale above for the predicted time course for rapid, non-genomic T effects; 0–10, 10–20, 20–30 min post injection for the pair interaction test because previous studies have found that IP T injections begin to influence behaviour around 20–30 min after injection via non-genomic mechanisms [33,34]. Resident-intruder tests were limited to 15 min to avoid injury, so they were broken down into three 5 min intervals. For consistency of T effects on behaviour across behavioural tests, we also combined the last two 5 min intervals for analysis because together this period reflected 20–30 min post-injection when rapid T effects are expected to occur. Analysis of behaviour in the IEG test used an RM GLM with group as a fixed between-subjects factor and time interval as a within-subjects factor; because there can be only one behaviour test in an IEG design, analysing the time intervals of the single test aimed to capture increases or decreases in prosocial behaviour associated with a T injection. All post-hoc pairwise comparisons were adjusted using the Sidak correction. All data were analysed using SPSS 27 (IBM Analytics, USA) and graphs were made using Prism 8 (GraphPad, USA).
3. Results
(a) . Testosterone increases prosocial behaviour during a pair interaction
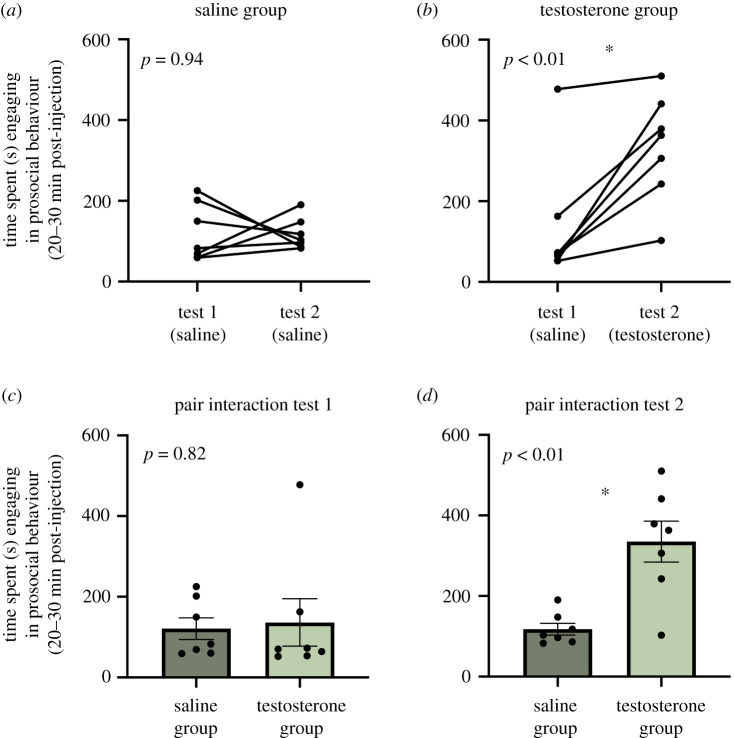
An RM GLM with group as a fixed between-subject factor and test (i.e. pair interaction test 1 or pair interaction test 2) as a within-subjects factor revealed no main effects or significant interactions for the 0–10 min and 10–20 min intervals (all p > 0.40). However, as predicted we did find a significant interaction of group X test for the 20–30 min interval (F1,12 = 11.42; p < 0.01). Sidak-corrected post-hoc comparisons showed that within the saline group prosocial behaviour did not differ between tests 1 and 2 (p = 0.94; figure 2a), but within the T group prosocial behaviour was significantly higher in test 2 following a T injection (p < 0.01; figure 2b). Further, prosocial behaviour for test 1 did not significantly differ between the two groups when both groups had been injected with saline (p = 0.82; figure 2c), but did differ for test 2, such that T-injected males spent significantly more time engaging in prosocial behaviour compared to saline-injected males (p < 0.01; figure 2d).
Figure 2.
Pair interaction test. (a) Males in the saline group show no difference in prosocial behaviour from test 1 to test 2. (b) Males in the testosterone (T) group show an increase in prosocial behaviour from test 1 to test 2 after receiving a T injection. (c) For test 1, the amount of prosocial behaviour exhibited by the saline and T groups did not differ. (d) For test 2, males in the T group were significantly more prosocial than males in the saline group. An asterisk indicates statistical significance (p < 0.05).
(b) . Testosterone decreases prosocial behaviour in a resident-intruder assay
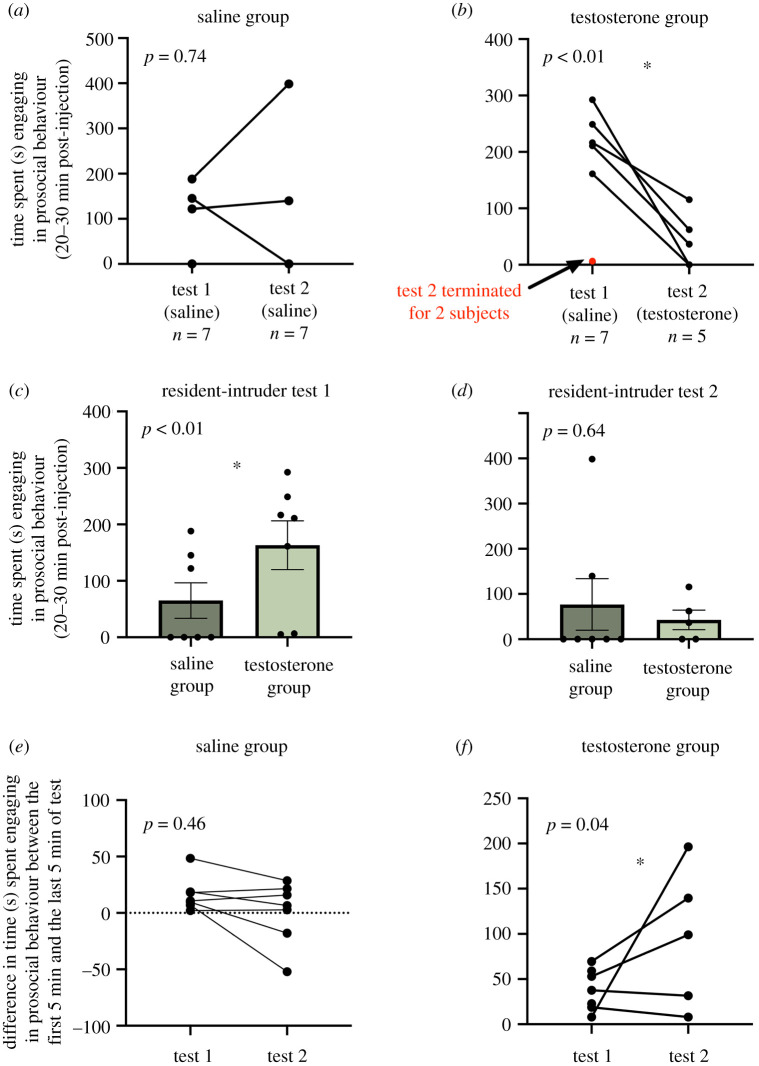
An RM GLM with group as a fixed between-subject factor and test (i.e. resident-intruder test 1 or resident-intruder test 2) as a within-subjects factor revealed no main effects or significant interactions for the 0–5 min interval (all p > 0.08). However, we did find a significant interaction of group X test for the 5–10 min (F1,10 = 20.58; p < 0.01) and 10–15 min (F1,10 = 6.17; p = 0.03) intervals. For consistency in behavioural windows examined across tests, we also analysed the last two intervals combined to capture the 20–30 min time period post-injection, which likewise yielded a significant interaction of group X test (F1,10 = 13.19; p < 0.01). Sidak-corrected post-hoc comparisons showed that within the saline group prosocial behaviour did not differ between tests 1 and 2 (p = 0.74; figure 3a), but within the T group prosocial behaviour was significantly lower in test 2 following a T injection (p < 0.01; figure 3b). Additionally, for test 1, T-injected males exhibited significantly more prosocial behaviour than saline-injected males (p < 0.01; figure 3c), whereas prosocial behaviour did not differ between groups in test 2 (p = 0.64; figure 3d). Two subjects lack data for test 2 because the stimulus animals sustained a minor injury and the resident-intruder tests were immediately terminated (datapoints are highlighted in red). Notably, tests were only terminated for subjects in the T group for test 2. Because analyses used a repeated-measures design, these two animals were dropped from the analysis owing to a lack of data for test 2.
Figure 3.
Resident-intruder test. (a) Males in the saline group show no difference in prosocial behaviour from test 1 to test 2. (b) Males in the testosterone (T) group show a decrease in prosocial behaviour from test 1 to test 2 after receiving a T injection. (c) For test 1, males in the T group were significantly more prosocial than males in the saline group. (d) For test 2, prosocial behaviour did not differ between the saline and T groups. (e) The magnitude of the decrease in prosocial behaviour within a resident-intruder test does not significantly differ between tests 1 and 2 in saline males. (f) The magnitude of the decrease in prosocial behaviour within a test is significantly greater in test 2 (post T-injection) in T group males. An asterisk indicates statistical significance (p < 0.05). (Online version in colour.)
To better understand the potential influence of T on prosocial behaviour in the resident-intruder assay, which was complicated by the carryover effect of T that results in starting group differences, we examined the progression of prosocial behaviour within the resident-intruder test. Regardless of manipulation, prosocial behaviour exhibited by a resident gerbil decreased during the resident-intruder test over time, probably owing to the animal calibrating to the experience of having an intruder in their homecage that is not leaving their territory. We refer to this natural decrease in prosocial behaviour within a test as a magnitude of change. To determine whether the magnitude of the decrease in prosocial behaviour within a test was different between test 1 and test 2, and therefore may have been enhanced by a T injection, we compared the magnitude of the decrease, operationally defined as prosocial behaviour in the first 5 min of the test minus the last 5 min of the test. We predicted the magnitude would be greatest for test 2 after T injections if T hastened the decline; thus, even though animals that had previously been injected with T started with higher levels of prosocial responses for test 1, when all animals were injected with saline, the rate of decline would be faster during test 2, after T injections. An RM GLM with group as a fixed factor and difference score as a within-subjects factor revealed a significant interaction of group X difference score (F1,10 = 5.34; p = 0.04). As predicted, T group males exhibited a significantly greater magnitude in the decrease in prosocial behaviour from the beginning of the test to the end of the test in test 2 (i.e. after a T injection) compared to test 1 (p = 0.04; figure 3f). The difference scores for test 1 and test 2 were not significantly different for the saline group males (p = 0.46; figure 3e). Therefore, there was a greater magnitude of within-test change in prosocial behaviour between test 1 and test 2 only for T group males.
An RM GLM revealed no main effects or significant interactions for aggression at any 5 min interval, including the last two 5 min intervals combined (all p > 0.07; electronic supplementary material, figure S3).
(c) . Testosterone increases paraventricular nucleus of the hypothalamus oxytocin neural responses during a pair interaction
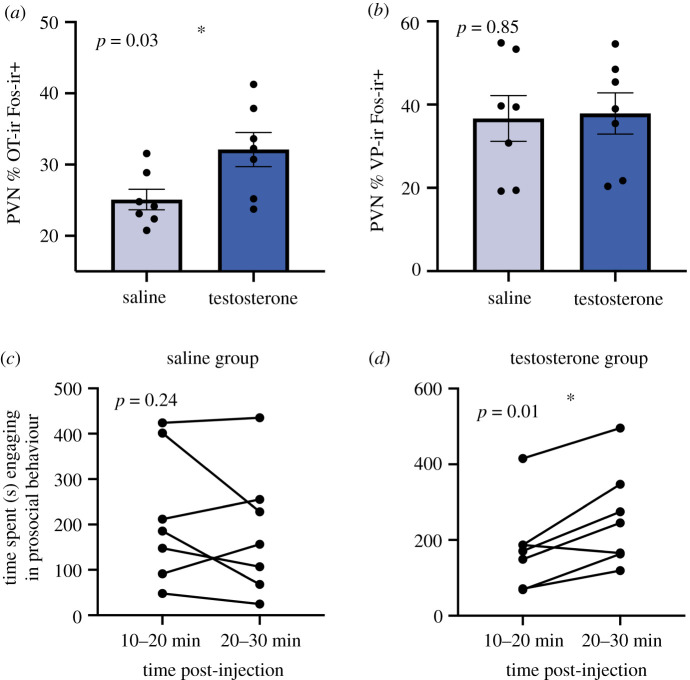
A Mann-Whitney U-test revealed a significant difference in PVN OT-Fos colocalization, with males that received a T injection exhibiting a higher percentage of colocalized cells compared to males that received a saline injection (z = −2.24; p = 0.03; figure 4a). However, we observed no significant difference between groups for PVN VP-Fos colocalization (z = −0.19; p = 0.85; figure 4b).
Figure 4.
PVN neural responses and prosocial behaviour in the IEG test. (a) Males in the testosterone (T) group exhibited significantly greater levels of PVN OT-Fos colocalization than males in the saline group. (b) The amount of PVN VP-Fos colocalization did not significantly differ between saline and T groups. (c) Males in the saline group exhibited no change in prosocial behaviour over time during the IEG test. (d) Males in the T group exhibited a significant increase in prosocial behaviour over time in the IEG test. An asterisk indicates statistical significance (p < 0.05). (Online version in colour.)
Because an IEG test cannot include repeated tests of the same subject to control for individual differences in behaviour, we compared time intervals from the interactions within each subject to see if behaviour increased across the time points in T-injected animals consistent with the time course established in the first round of pair interaction testing. An RM GLM with group as a fixed factor and time interval as a within-subjects factor yielded a significant interaction of group X time interval (F1,12 = 8.68; p = 0.01). Sidak-corrected post-hoc pairwise comparisons showed that within the saline group prosocial behaviour did not differ between the 10–20 min and 20–30 min post-injection time intervals (p = 0.24; figure 4c), however, within the T group prosocial behaviour increased over time (p = 0.01; figure 4d). We observed no between-group differences at either time interval (all p > 0.31).
4. Discussion
Our findings demonstrate that T can, within 30 min, increase prosocial behaviour in a positively valenced social context (e.g. interacting with a pairbond partner). Further, those effects persist over time, in what appears to be a carryover effect, leading to initial elevations of prosocial responses towards an intruder on subsequent tests. At that time, our data suggest that T may again adjust social responses to the new social context by rapidly decreasing prosocial behaviours, although the starting differences in prosociality at the onset of those tests make that conclusion tentative (see further discussion below). Thus, acute elevations of T can rapidly increase and decrease prosocial responses, depending on social context, through a presumed non-genomic mechanism, as well as produce lasting increases, potentially through genomic mechanisms and/or as a consequence of the initial elevations of positive social interactions. We also found that T rapidly increased prosocial behaviour within the same time frame in the IEG test and that PVN OT neural responses, but not VP responses, were greater in the T-injected animals compared to the saline-injected animals. Together, these results indicate that T can rapidly enhance context-appropriate behaviour and may do so via influences on OT circuitry that enhances attention to salient social cues. Further, T primes animals for future social encounters that would typically be expected based on current social contexts.
(a) . Testosterone facilitates context-appropriate behaviour
Most studies examining the role of T on social behaviour examine anti-social behaviours associated with the Challenge Hypothesis (i.e. rapid T responses facilitate aggressive responses to a challenge) and the Biosocial Model of Status (i.e. T advances social status by promoting aggression, lack of empathy and selfishness) [37–40]. Although studies in non-human animals overwhelmingly examine overt aggression, studies in humans typically do not, and instead examine more nuanced aspects of social interactions or indirect measures of aggression. Such human studies, which thus move beyond simple measures of overt aggression, have begun to suggest that T may not only promote aggressive responses but also foster prosocial behaviours, particularly when there is an advantage of gaining social status. For example, recent studies have demonstrated that T increases altruistic behaviour when being watched (the audience effect) [11] and that higher T levels are associated with higher prosocial conformity after observing a peer act prosocially [9]. Similarly, higher T levels are associated with increased ingroup cooperation during an intergroup competition [10]. Thus, T is not limited to the promotion of aggressive or antisocial behaviours but can also promote prosocial or altruistic behaviours in appropriate contexts, at least in humans.
We likewise found that T can increase prosocial behaviours during social interactions in a non-human animal. Importantly, the prosocial behaviour towards gerbils' partners that was enhanced by T was not overtly sexual and probably was not related to courtship because we did not observe any copulations and pairbonds were already established. Exogenous T can facilitate copulatory behaviour in male gerbils within 15 min in appropriate contexts (i.e. when a female is in oestrus) [41], but, in rodents mating typically does not occur when females are pregnant [42], and all female partners in our study were pregnant at the time of testing. Of course, it remains possible that courtship behaviours such as scent marking or ultrasonic vocalizations, which our experiment was not designed to detect, were exhibited and possibly enhanced by T. Regardless, and further supporting our argument that T promoted nonsexual, prosocial responses, we did not observe any mounting, lordosis or male foot-stomping, which is exhibited when a female gerbil is sexually receptive [43]. Thus, the increase in prosocial behaviour observed probably reflected pair maintenance behaviours critical for future reproductive success. Mechanistically, T may do this by amplifying the social cues used to assess social context and/or enhancing the motivational state most appropriate for a given context, in this case, strengthening an existing pairbond with a partner. Indeed, studies have demonstrated that androgens can rapidly enhance the reinforcing value of contextual stimuli associated with social interactions [44,45]. Consistent with the possibility that T can rapidly adjust internal states that promote alternate behavioural responses in different social contexts, T also appeared to rapidly decreased prosocial responses towards an intruder in the same animals in which it had previously increased prosocial responses towards the partner, though we discuss the limitations for that interpretation below. To our knowledge, this is the first demonstration of pro- and anti-social effects of testosterone in the same individuals tested in different social contexts. Notably, although our measures of aggressive behaviour were not quantitatively significant in the resident-intruder test, tests that required early termination owing to intense aggression only occurred in animals that had received a T injection, suggesting that T may qualitatively enhance aggression in ways that were difficult to detect in our test paradigm. Consistent with that possibility, T elevations have been found to rapidly increase overt aggression and approach responses to intruders that probably reflect increased territorial vigilance in other species [46–48]. It may have been difficult to detect T effects on aggression in our study because aggression typically increases in monogamous male rodents when their mate becomes pregnant [49], so levels of aggression, as captured by our dependent variables, may have already been elevated in both groups of our animals, thereby making it difficult to detect extra elevations as a result of our exogenous T administration. Indeed, the increased aggression during a partner's pregnancy may be caused by the increased levels of endogenous T that occur in male gerbils at parturition [50] and which presumably occurred in males in both groups of animals in our experiment.
Our interpretation that T rapidly decreased prosocial responses towards the intruder must be considered cautiously because of the carryover effects of T on prosocial behaviour in the T group animals. T group males, who previously exhibited an increase in prosocial responses following a T injection in the pair interaction test, exhibited more prosocial behaviour than saline group males in the first resident-intruder test. Thus, the rapid decrease in T group males' prosocial behaviour during the second resident-intruder test, after T injections, could have simply been the result of increased experience in an agonistic context, but which could not be observed in saline group animals because they showed very little prosocial behaviour to begin with. Indeed, in the first resident-intruder test when all subjects received a saline injection, prosocial behaviour decreased from the beginning of the resident-intruder towards the end of the test in all animals, suggesting that experience during the test provides context-relevant information and may drive a decrease in prosocial behaviour. However, we note that of the three animals in the saline group that began with appreciable amounts of prosocial responses on the first resident-intruder test day, there was not a uniform decrease on the subsequent test day like that observed in all the T-injected animals. Further, and more convincingly, when we compared the magnitude of the prosocial decline during the first resident-intruder test with the magnitude during the second resident-intruder test, we found that the decrease in prosocial behaviour was significantly greater on the second day in animals injected with T, but not in those injected with saline. It is worth considering that the two subjects in the T group that had their test 2 results dropped because of extreme aggression also had very low prosocial behaviour in test 1. This could have led to an overestimation of T's inhibition of prosocial behaviour in test 2 because those animals would have not been able to show a substantial decrease in prosocial behaviour. To address this, we ran follow-up analyses in which we included the two subjects and assigned either a score of 0 s prosocial behaviour during test 2 or a score identical to test 1 (5 and 6 s, respectively). We chose these hypothetical scores because neither animal exhibited any prosocial behaviour during test 2 prior to the aggression that terminated the test. Further, it is highly unlikely that a gerbil would exhibit prosocial behaviour in a resident-intruder context following intense aggression. These analyses still yielded significance on all tests (see the electronic supplementary material, figure S4), and thus it is unlikely that the exclusion of these two subjects biased our findings that T decreased prosocial behaviour during the resident-intruder test. Nonetheless, future tests will still be necessary to verify that T rapidly facilitates shifts from behaviours appropriate to a previous social context towards responses that better match the current context.
In our IEG study, we again found that T rapidly, from the beginning to the end of the test period, increased prosocial responses towards the partner, and that PVN OT neural responses were greater in males that received a T injection prior to the test compared to males that received a saline injection. A recent study demonstrated that PVN OT is recruited to shift female focus to pups for mothers and novice female mice, endowing the pup stimuli with enhanced saliency and enabling social learning of maternal behaviour for the novice females [51]. This study shows that PVN OT aids in social transmission of context-appropriate behaviour, supporting the social salience hypothesis of OT. Additionally, in male mice, social exposure induces PVN OT neural activation (assessed via two-photo calcium imaging), suggesting that this cell group may act to convey information about social salience; this study also demonstrated that chemogenetic activation of PVN OT increased social investigation [52]. In the light of these studies, as well as evidence that neurosteroids can modulate PVN VP and OT release in mammals [17], it is possible that T rapidly induced context-appropriate social responses in our study by activating OT cells that promote attention to social saliency. However, a limitation of our study is that we cannot know whether the higher PVN OT neural responses in T group males were the result of T directly increasing OT neural activity or a consequence of the increase in prosocial behaviour caused by T. Future studies will aim to disentangle this confound. Further, it is possible that T injections may have long-term effects on nonapeptide neural functioning, and thus the group differences observed were not owing to acute effects of T. This would still be an interesting and novel finding revealing an interaction of steroids and nonapeptides; future studies can be designed to specifically probe this further.
(b) . Genomic and non-genomic testosterone actions on behaviour
Rapid, non-genomic steroid effects on physiology and behaviour have been demonstrated for all major classes of steroids [53,54]. The time course in our study (30 min) within which T-enhanced prosocial behaviour during interactions with a pairbond partner and decreased prosocial responses towards a same-sex intruder is consistent with such a non-genomic mechanism. On the other hand, the prolonged enhancement of prosocial responses that T-injections during the first pairbond test produced on the first resident-intruder test 5 days later, when those animals were only injected with saline, is more consistent with a traditional, transcriptional regulatory mechanism. Similarly, several studies have shown that androgen elevations during social interactions can prime future behaviour [47,55–57]. For example, California mice that win a fight exhibit elevated levels of T and subsequently have an enhanced ability to win future fights [57], an effect that depends upon an interaction of the increased T and the experience of winning the initial fight. We cannot yet say whether the T injections prior to the initial pair interaction test were solely responsible for the carryover effect on the first day of the resident-intruder test, potentially via a transcriptional regulatory mechanism, or whether, as for the ‘winner effect,’ the prolonged effect was the result of an interaction between the T elevations and the increased prosocial interactions with their partners during the test. Indeed, T can rapidly increase reinforcement mechanisms that result in prolonged place preferences [45,58], so T could have increased the rewarding aspects of the initial social interactions in the partner interaction test and, as a result, increased the tendency to act prosocially on the subsequent resident-intruder test, at least initially. It also remains possible that it was simply the T-induced elevations of prosocial responses themselves in the second pair interaction test that produced the starting differences on the first day of the intruder test, in which case it would reflect a mechanism through which T shapes future social behaviours indirectly by modifying the immediate social experiences, which then induce the lasting changes in behaviour. If T did play a direct role, it would indicate that while rapid T mechanisms are critical for matching behaviour to current social context, delayed, presumably genomic mechanisms prime animals for future interactions—in this case and presumably in natural contexts, for future interactions with their pairbonded mate. However, the carryover effect on prosociality in our study persisted even in a resident-intruder test—an inherently different social context than the pair interaction test and one that, in nature, would be less likely to occur than would another interaction with their mate. Yet, upon another injection of T (i.e. resident-intruder test 2), prosocial behaviour rapidly decreased, more quickly than it did during the first day of the resident-intruder test when only saline was administered, suggesting that rapid, non-genomic effects of T corrected a priming error induced by presumed genomic effects of our initial T-injections. Mechanisms that allow T to rapidly adjust behaviour to match affiliative and agonistic contexts are probably operative in nature given that pair bonding and aggressive interactions both increase levels of T in this species [30]. Together, these findings suggest that acute fluctuations of T, and perhaps other steroids, can promote flexible behavioural responses by titrating genomic and non-genomic mechanisms, allowing an animal to prepare for predicable future interactions, yet with the ability to adjust rapidly when less predictable social experiences occur. Future studies aim to determine whether these effects depend on direct androgen receptor activation or on oestrogen receptor activation following its local conversion to oestradiol, and whether the rapid and prolonged influences on prosocial responses depend on the same or different receptor mechanisms.
Here we showed that T promotes prosocial, but not overtly sexual behaviours in gerbils, contributing to a growing body of studies demonstrating that T can facilitate non-aggressive/selfish behaviours that are not directly related to sexual performance. Because T rapidly increased prosocial behaviour with a pairbond partner but decreased prosocial responses towards an intruder, our results also illustrate how T can promote pro- and anti-social responses in the same individuals, depending on social context and through presumed non-genomic mechanisms. Additionally, we show that T can prime future prosocial responses through persistent, presumably non-genomic mechanisms, but that this effect can be rapidly over-ridden by further elevations of T in the new context, suggesting a balance of genomic and non-genomic T-influences on behaviour. Finally, our results suggest that T may interact with the OT system to influence social behaviour and/or enhance social saliency. Future causal studies will be needed to determine whether a mechanism by which steroids modulate complex social behaviour is via regulation of the nonapeptide system.
Acknowledgements
We thank Zia Huballah for assistance with scoring behavioural videos.
Ethics
All procedures were approved by the Institutional Animal Care and Use Committee of Emory University (Protocol 202000131).
Data accessibility
The data associated with this paper are available on Dryad: https://doi.org/10.5061/dryad.4tmpg4fch [59].
The data are provided in the electronic supplementary material [60].
Authors' contributions
A.M.K.: conceptualization, data curation, formal analysis, methodology, project administration, funding acquisition, supervision, writing—original draft; J.A.G.: investigation, methodology; R.R.T.: conceptualization, funding acquisition, methodology, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no competing interests.
Funding
This work was supported by the National Science Foundation (National Science Foundation Award no. 1656596 to R.R.T.).
References
- 1.Kelly AM, Vitousek MN. 2017. Dynamic modulation of sociality and aggression: an examination of plasticity within endocrine and neuroendocrine systems. Phil. Trans. R. Soc. B 372, 20160243. ( 10.1098/rstb.2016.0243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frye CA. 2009. Neurosteroids' effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinol 34(Suppl 1), S143-S161. ( 10.1016/j.psyneuen.2009.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon NG. 2002. Hormonal processes in the development and expression of aggressive behavior. In Hormones, brain and behavior (eds Pfaff DW, Arnold AP, Etgen AM, Fahrbach SeE, Rubin RT), pp. 339-392. New York, NY: Academic Press. [Google Scholar]
- 4.Remage-Healey L. 2014. Frank Beach Award Winner: Steroids as neuromodulators of brain circuits and behavior. Horm. Behav. 66, 552-560. ( 10.1016/j.yhbeh.2014.07.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soma KK. 2006. Testosterone and aggression: Berthold, birds and beyond. J. Neuroendocrinol. 18, 543-551. ( 10.1111/j.1365-2826.2006.01440.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. 2009. Testosterone release and social context: when it occurs and why. Front Neuroendocrinol. 30, 460-469. ( 10.1016/j.yfrne.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 7.Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. 2006. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm. Behav. 49, 45-67. ( 10.1016/j.yhbeh.2005.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lord LD, Bond J, Thompson RR. 2009. Rapid steroid influences on visually guided sexual behavior in male goldfish. Horm. Behav. 56, 519-526. ( 10.1016/j.yhbeh.2009.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duell N, van Hoorn J, McCormick EM, Prinstein MJ, Telzer EH. 2021. Hormonal and neural correlates of prosocial conformity in adolescents. Dev. Cogn. Neurosci. 48, 100936. ( 10.1016/j.dcn.2021.100936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reimers L, Diekhof EK. 2015. Testosterone is associated with cooperation during intergroup competition by enhancing parochial altruism. Front. Neurosci. 9, 183. ( 10.3389/fnins.2015.00183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Zhang Y, Ou J, Hu Y, Zilioli S. 2020. Exogenous testosterone increases the audience effect in healthy males: evidence for the social status hypothesis. Proc. R. Soc. B 287, 20200976. ( 10.1098/rspb.2020.0976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodson JL, Thompson RR. 2010. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin Neurobiol. 20, 784-794. ( 10.1016/j.conb.2010.08.020) [DOI] [PubMed] [Google Scholar]
- 13.Stoop R. 2012. Neuromodulation by oxytocin and vasopressin. Neuron 76, 142-159. ( 10.1016/j.neuron.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 14.Landgraf R, Neumann ID. 2004. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 25, 150-176. ( 10.1016/j.yfrne.2004.05.001) [DOI] [PubMed] [Google Scholar]
- 15.Shamay-Tsoory SG, Abu-akel A. 2015. The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194-202. ( 10.1016/j.biopsych.2015.07.020) [DOI] [PubMed] [Google Scholar]
- 16.Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. 2009. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 30, 259-301. ( 10.1016/j.yfrne.2009.05.006) [DOI] [PubMed] [Google Scholar]
- 17.Widmer H, Ludwig M, Bancel F, Leng G, Dayanithi G. 2003. Neurosteroid regulation of oxytocin and vasopressin release from the rat supraoptic nucleus. J. Physiol. 548, 233-244. ( 10.1113/jphysiol.2002.036863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caldwell HK. 2017. Oxytocin and vasopressin: powerful regulators of social behavior. Neuroscientist 23, 517-528. ( 10.1177/1073858417708284) [DOI] [PubMed] [Google Scholar]
- 19.De Vries GJ. 2004. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145, 1063-1068. ( 10.1210/en.2003-1504) [DOI] [PubMed] [Google Scholar]
- 20.DeLeon KR, Grimes JM, Melloni RH Jr. 2002. Repeated anabolic-androgenic steroid treatment during adolescence increases vasopressin V(1A) receptor binding in Syrian hamsters: correlation with offensive aggression. Horm. Behav. 42, 182-191. ( 10.1006/hbeh.2002.1802) [DOI] [PubMed] [Google Scholar]
- 21.Agren G. 1976. Social and territorial behaviour in the mongolian gerbil (Meriones unguiculatus) under seminatural conditions. Bio. Behav. 1, 267-285. [Google Scholar]
- 22.Deng K, Liu W, Wang D. 2017. Inter-group associations in Mongolian gerbils: quantitative evidence from social network analysis. Integr. Zool. 12, 446-456. ( 10.1111/1749-4877.12272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gromov VS. 2008. Interactions of partners in family pairs, care of the offspring, and the role of tactile stimulation in formation of parental behavior of the Mongolian gerbil (Meriones unguiculatus) under laboratory conditions. Izvestiya Akademii Nauk, Seriya Biologicheskaya: Zoology 5, 569-579. ( 10.1134/s1062359009050082) [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Wang G, Wang Y, Zhong W, Wan X. 2009. Population ecology of wild Mongolian gerbils (Meriones unguiculatus). J. Mammal. 90, 832-840. ( 10.1644/08-MAMM-A-265.1) [DOI] [Google Scholar]
- 25.Pan Y, Zhu Q, Xu T, Zhang Z, Wang Z. 2020. Aggressive behavior and brain neuronal activation in sexually naive male Mongolian gerbils. Behav. Brain Res. 378, 112276. ( 10.1016/j.bbr.2019.112276) [DOI] [PubMed] [Google Scholar]
- 26.Roper TJ, Polioudakis E. 1977. The behaviour of Mongolian gerbils in a semi-natural environment, with special reference to ventral marking, dominance and sociability. Behaviour 61, 207-237. ( 10.1163/156853977X00351) [DOI] [Google Scholar]
- 27.Pinto-Fochi ME, Negrin AC, Scarano WR, Taboga SR, Goes RM. 2016. Sexual maturation of the Mongolian gerbil (Meriones unguiculatus): a histological, hormonal and spermatic evaluation. Reprod. Fertil. Dev. 28, 815-823. ( 10.1071/RD14074) [DOI] [PubMed] [Google Scholar]
- 28.Fuxjager MJ, Oyegbile TO, Marler CA. 2011. Independent and additive contributions of postvictory testosterone and social experience to the development of the winner effect. Endocrinology 152, 3422-3429. ( 10.1210/en.2011-1099) [DOI] [PubMed] [Google Scholar]
- 29.Taylor GT, Weiss J, Pitha J. 1989. Testosterone in a cyclodextrin-containing formulation: behavioral and physiological effects of episode-like pulses in rats. Pharm. Res. 6, 641-646. ( 10.1023/A:1015922019038) [DOI] [PubMed] [Google Scholar]
- 30.Pina-Andrade S, Ramos G, Cárdenas-León M, Martínez A, Romero-Morales L, Martínez-Torres M, Cedillo-Ildefonso B, Luis J. 2020. Testosterone dependent territorial aggression is modulated by cohabitation with a female in male Mongolian gerbils (Meriones unguiculatus). Horm. Behav. 117, 104611. ( 10.1016/j.yhbeh.2019.104611) [DOI] [PubMed] [Google Scholar]
- 31.Refinetti R, Kenagy GJ. 2018. Diurnally active rodents for laboratory research. Lab. Anim. 52, 577-587. ( 10.1177/0023677218771720) [DOI] [PubMed] [Google Scholar]
- 32.Friard O, Gamba M. 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Meth. Ecol. Evol 7, 1325-1330. ( 10.1111/2041-210X.1284) [DOI] [Google Scholar]
- 33.Aikey JL, Nyby JG, Anmuth DM, James PJ. 2002. Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm. Behav. 42, 448-460. ( 10.1006/hbeh.2002.1838) [DOI] [PubMed] [Google Scholar]
- 34.Onaolapo OJ, Onaolapo AY, Omololu TA, Oludimu AT, Segun-Busari T, Omoleke T. 2016. Exogenous testosterone, aging, and changes in behavioral response of gonadally intact male mice. J. Exp. Neurosci. 10, 59-70. ( 10.4137/JEN.S39042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly AM, Seifert AW. 2021. Distribution of vasopressin and oxytocin neurons in the basal forebrain and midbrain of spiny mice (Acomys cahirinus). Neuroscience 468, 16-28. ( 10.1016/j.neuroscience.2021.05.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roulo RM, Fishburn JD, Alworth L, Hoberman AM, Smith MA. 2013. Producing timed-pregnant Mongolian gerbils for developmental studies. Lab. Anim. (NY) 42, 380-383. ( 10.1038/laban.297) [DOI] [PubMed] [Google Scholar]
- 37.Carre JM, Putnam SK, McCormick CM. 2009. Testosterone responses to competition predict future aggressive behaviour at a cost to reward in men. Psychoneuroendocrinol 34, 561-570. ( 10.1016/j.psyneuen.2008.10.018) [DOI] [PubMed] [Google Scholar]
- 38.Carre JM, Olmstead NA. 2015. Social neuroendocrinology of human aggression: examining the role of competition-induced testosterone dynamics. Neuroscience 286, 171-186. ( 10.1016/j.neuroscience.2014.11.029) [DOI] [PubMed] [Google Scholar]
- 39.Mazur A. 1985. A biosocial model of status in face-to-face primate groups. Soc. Forces 64, 377-402. ( 10.2307/2578647) [DOI] [Google Scholar]
- 40.Mazur A, Booth A. 1998. Testosterone and dominance in men. Behav. Brain Sci. 21, 353-363; discussion 363–397. ( 10.1017/S0140525X98001228) [DOI] [PubMed] [Google Scholar]
- 41.Yahr P, Newman A, Stephens DR. 1979. Sexual behavior and scent marking in male gerbils: comparison of changes after castration and testosterone replacement. Horm. Behav. 13, 175-184. ( 10.1016/0018-506X(79)90056-4) [DOI] [PubMed] [Google Scholar]
- 42.Yoest KE, Cummings JA, Becker JB. 2019. Ovarian hormones mediate changes in adaptive choice and motivation in female rats. Front. Behav. Neurosci. 13, Article 250. ( 10.3389/fnbeh.2019.00250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yahr P. 1981. Scent marking, sexual behavior, and aggression in male gerbils: comparative analysis of endocrine control. Am. Zool. 12, 143-151. ( 10.1093/icb/21.1.143) [DOI] [Google Scholar]
- 44.Sato SM, Johansen JA, Jordan CL, Wood RI. 2010. Membrane androgen receptors may mediate androgen reinforcement. Psychoneuroendocrinol ogy 35, 1063-1073. ( 10.1016/j.psyneuen.2010.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao X, Castelli FR, Wang R, Auger AP, Marler CA. 2020. Testosterone-related behavioral and neural mechanisms associated with location preferences: a model for territorial establishment. Horm. Behav. 121, 104709. ( 10.1016/j.yhbeh.2020.104709) [DOI] [PubMed] [Google Scholar]
- 46.Carre JM, Geniole SN, Ortiz TL, Bird BM, Videto A, Bonin PL. 2017. Exogenous testosterone rapidly increases aggressive behavior in dominant and impulsive men. Biol. Psychiatry 82, 249-256. ( 10.1016/j.biopsych.2016.06.009) [DOI] [PubMed] [Google Scholar]
- 47.Trainor BC, Bird IM, Marler CA. 2004. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm. Behav. 45, 115-121. ( 10.1016/j.yhbeh.2003.09.006) [DOI] [PubMed] [Google Scholar]
- 48.Zhao X, Fuxjager MJ, McLamore Q, Marler CA. 2019. Rapid effects of testosterone on social decision-making in a monogamous California mice (Peromyscus californicus). Horm. Behav. 115, 104544. ( 10.1016/j.yhbeh.2019.06.008) [DOI] [PubMed] [Google Scholar]
- 49.McCracken K, Lewis R, Curtis JT. 2015. Female-paced mating does not affect pair-bond expression by male prairie voles (Microtus ochrogaster). Northeast Nat. 22, 541-550. ( 10.1656/045.022.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juana L, Bárbara VG, Martín MT, Agustín C, Guillermo RB, Guadalupe O. 2010. Neither testosterone levels nor aggression decrease when the male Mongolian gerbil (Meriones unguiculatus) displays paternal behavior. Horm. Behav. 57, 271-275. ( 10.1016/j.yhbeh.2009.12.007) [DOI] [PubMed] [Google Scholar]
- 51.Carcea I, et al. 2021. Oxytocin neurons enable social transmission of maternal behaviour. Nature 596, 553-557. ( 10.1038/s41586-021-03814-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Resendez SL, et al. 2020. Social stimuli induce activation of oxytocin neurons within the paraventricular nucleus of the hypothalamus to promote social behavior in male mice. J. Neurosci. 40, 2282-2295. ( 10.1523/JNEUROSCI.1515-18.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heimovics SA, Trainor BC, Soma KK. 2015. Rapid effects of estradiol on aggression in birds and mice: the fast and the furious. Integr. Comp. Biol. 55, 281-293. ( 10.1093/icb/icv048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wendler A, Baldi E, Harvey BJ, Nadal A, Norman A, Wehling M. 2010. Position paper: rapid responses to steroids: current status and future prospects. Eur. J. Endocrinol. 162, 825-830. ( 10.1530/EJE-09-1072) [DOI] [PubMed] [Google Scholar]
- 55.Carre JM, Campbell JA, Lozoya E, Goetz SM, Welker KM. 2013. Changes in testosterone mediate the effect of winning on subsequent aggressive behaviour. Psychoneuroendocrinol ogy 38, 2034-2041. ( 10.1016/j.psyneuen.2013.03.008) [DOI] [PubMed] [Google Scholar]
- 56.Oliveira GA, Oliveira RF. 2014. Androgen modulation of social decision-making mechanisms in the brain: an integrative and embodied perspective. Front. Neurosci. 8, 209. ( 10.3389/fnins.2014.00209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oyegbile TO, Marler CA. 2005. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 48, 259-267. ( 10.1016/j.yhbeh.2005.04.007) [DOI] [PubMed] [Google Scholar]
- 58.Petric R, Kalcounis-Rueppell MC, Marler CA. 2022. Testosterone pulses paired with a location induce a place preference to the nest of a monogamous mouse under field conditions. Elife 11, e65820. ( 10.7554/eLife.65820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly AM, Gonzalez Abreu JA, Thompson RR. 2022. Data from: Beyond sex and aggression: testosterone rapidly matches behavioural responses to social context and tries to predict the future. Dryad Digital Repository. ( 10.5061/dryad.4mpg4fch) [DOI]
- 60.Kelly AM, Gonzalez Abreu JA, Thompson RR. 2022. Beyond sex and aggression: testosterone rapidly matches behavioural responses to social context and tries to predict the future. Figshare. ( 10.6084/m9.figshare.c.6011742) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kelly AM, Gonzalez Abreu JA, Thompson RR. 2022. Beyond sex and aggression: testosterone rapidly matches behavioural responses to social context and tries to predict the future. Figshare. ( 10.6084/m9.figshare.c.6011742) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data associated with this paper are available on Dryad: https://doi.org/10.5061/dryad.4tmpg4fch [59].
The data are provided in the electronic supplementary material [60].