Abstract
The emerging entity, long COVID -19 is characterised by long-lasting dyspnoea, fatigue, cognitive dysfunction and other symptoms. Cardiac involvement manifested as conduction abnormalities, left ventricle mechanical dyssynchrony, dyspnoea, palpitation and postural orthostatic tachycardia syndrome (POTS) are common in long COVID-19. The direct viral damage to the myocardium or immune-mediated inflammation are postulated mechanisms. A woman in her forties presented with a 2-month history of chest pain, functional dyspnoea, palpitation and an episode of syncope after having been home-isolated for mild COVID infection. During clinical workup, a clustering of ECG and echocardiographic abnormalities including left bundle branch block, septal flash, and presystolic wave on spectral Doppler echocardiography, and POTS were detected. The echocardiographic findings together with POTS and persistent dyspnoea indicated the presence of a long COVID-19 state. The prevalence and clinical significance of these finding, as well as the impact on long-term prognosis, should be investigated in future studies.
Keywords: Arrhythmias, COVID-19, Radiology
Background
The clinical spectrum of Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) ranges from asymptomatic infection to severe multisystem disease with high risk of fatal outcome.1 COVID-19 predominantly involves the respiratory system but can also affect the cardiovascular system leading to a spectrum of cardiovascular complications during initial hospitalisation or recovery.1 2 After acute infection, patients may suffer a variety of long-lasting symptoms, referred to as long COVID-19. While long COVID-19 is not well understood, a proposed definition includes long-lasting (≥2 months) cognitive dysfunction, fatigue, dyspnoea and other symptoms persisting beyond 3 months after acute infection.3 Research has documented thoroughly cardiovascular complications in hospitalised patients with COVID-19, but paid less attention to sequela in milder, home-isolated cases. These patients may develop clinical or subclinical myocardial dysfunction assessed by either strain imaging derived by speckle tracking echocardiography, or cardiac magnetic resonance imaging (CMR).4 5 Other common persistent symptoms and findings are dyspnoea, exercise intolerance, elevated resting heart rate and blood pressure (BP), and electrocardiogram (ECG) abnormalities
Case presentation
A previously healthy woman in her early 40s presented to the emergency department of our hospital with a 2-month history of chest pain, functional dyspnoea, palpitations and an episode of syncope. She had suffered mild COVID-19 seven months earlier and recovered following self-isolation at home, without need for hospitalisation (figure 1).
Figure 1.
Patient’s history on a timeline. This Figure was created by the authors for the scope of this manuscript and has not been published elsewhere.
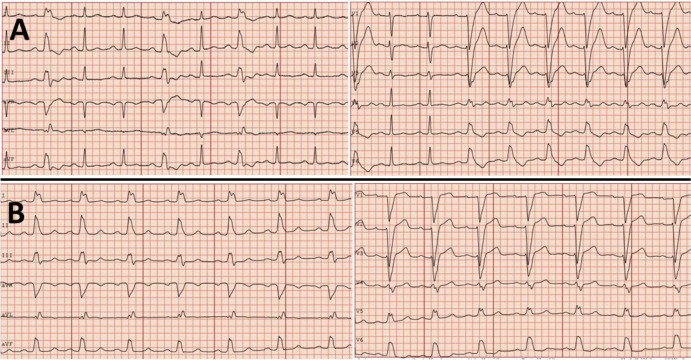
Three months after the recovery, she had presented to the emergency department with the same symptoms and was discharged a few hours later. The ECG had shown an intermittent left bundle branch block (LBBB) (figure 2A).
Figure 2.
ECG at initial presentation (A) showing sinus rhythm and intermittent left bundle branch block (LBBB); and ECG recorded during the index hospitalisation (B) demonstrating persistent LBBB.
The patient had consistently been complaining of orthostatic intolerance and palpitations after recovery from COVID-19 infection. At presentation, her BP was 142/80 mm Hg, oxygen saturation 100% at room air, respiration rate 20 breaths/min and temperature 37°C. The ECG on admission showed sinus rhythm with a rate of 96 beats/min and LBBB (figure 2B).
She experienced repeated episodes of sinus tachycardia at 140 beats/min with changing posture, without associated orthostatic hypotension (table 1).
Table 1.
Blood pressure and heart rate measurements during orthostatic test
| Position | Blood pressure (mm Hg) | Heart rate (beats/min) |
| Supine | 141/83 | 86 |
| Standing 1 min | 145/98 | 140 |
| Standing 3 min | 138/77 | 92 |
| Standing 5 min | 136/81 | 92 |
| Standing 7 min | 129/80 | 89 |
Investigations
The blood tests were normal including cardiac troponins and pro-brain natriuretic peptide. The LBBB on resting ECG was not related to higher ventricular rates. Pulmonary embolism was ruled out by a normal CT scan of the pulmonary arteries, and a coronary CT angiography demonstrated normal coronary arteries.
During hospitalisation, the patient was monitored by a cardiac telemetry for 48 hours, which showed frequent short runs of sinus tachycardia induced by the change of posture, but no other arrhythmias.
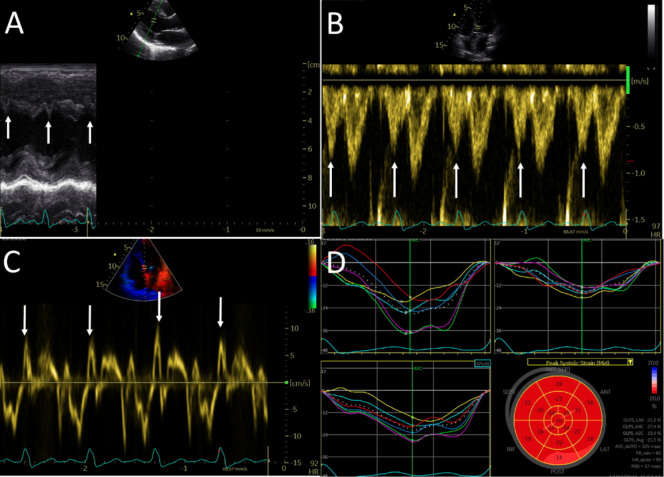
After a thorough investigation, including exercise stress test, telemetry and normal thyroid function tests, her sinus tachycardia was deemed attributable to postural orthostatic tachycardia syndrome (POTS). An echocardiogram revealed a normal ejection fraction, dyssynchronous septum with a typical septal flash feature (figure 3A, arrows), a presystolic wave on pulsed-wave Doppler through the left ventricle (LV) outflow tract (figure 3B, arrows) and prominent isovolumetric contraction time on tissue Doppler of the septal mitral annulus, corresponding with the QRS complex on ECG (figure 3C), and a LBBB strain pattern (figure 3D).
Figure 3.
Echocardiographic images showing dyssynchronous septum with a typical septal flash (2A, arrows), a presystolic wave on pulsed-wave Doppler through the left ventricular outflow tract (2B, arrows) and prominent isovolumetric contraction time on tissue Doppler of the septal mitral annulus, corresponding with the QRS complex on ECG (2C), and a LBBB strain pattern (2D).
Outcome and follow-up
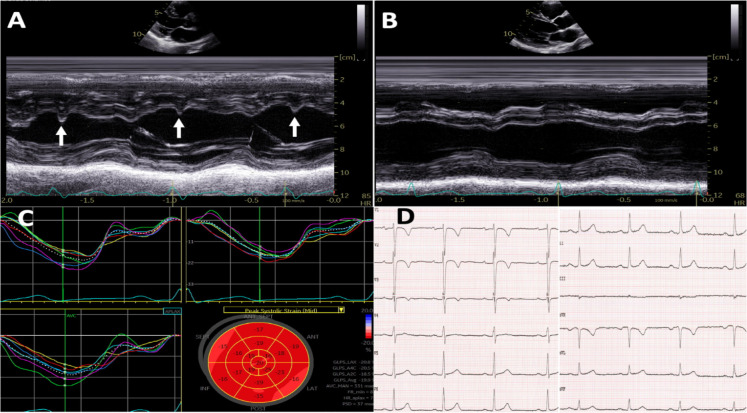
She was put on a low dose betablocker (metoprolol) and was discharged. A cardiac magnetic resonance (CMR) at 4-week follow-up after discharge did not reveal any signs of myocardial injury, inflammation or oedema (data not shown). The LBBB was alternating during the echocardiographic examination, while it was present in the beginning (figure 4A), later during the same examination (figure 4B, C) and on a repeated ECG at the end of the consultation, it spontaneously terminated and was absent (figure 4D). A 24-hour Holter at the outpatient clinic, 6 months after discharge showed significant improvement of the sinus tachycardia episodes, with an average 24-hour heart rate of 76 beats/min.
Figure 4.
Echocardiographic findings at 4-week follow-up. (A-B) M-mode with left bundle branch block (LBBB) and septal flash (A) and without LBBB and septal flash (B). (C) Strain curves without LBBB; and (D) ECG without LBBB.
Discussion
To our knowledge, the unique clustering of ECG and echocardiographic findings of LBBB, septal flash (both on M-mode and tissue Doppler images) and presystolic wave on spectral Doppler echocardiography in patients with long COVID-19has not been previously reported in the literature. The aforementioned echocardiographic findings alongside POTS and persistent dyspnoea indicated the presence of a long COVID-19 state.
Postacute COVID-19sequelae is emerging as a new syndrome with a combination of debilitating symptoms which can last for weeks or more following a mild illness,6 7 while long COVID-19 describes symptoms lasting beyond 12 weeks.3 7 The United Kingdom National Institute for Health and Care Excellence (NICE),8 and other studies from Northern Europe,9 have defined various symptomatic phases of COVID-19. In long COVID-19, commonly described symptoms include fatigue, cognitive dysfunction, breathlessness and numerous other symptoms, including palpitations, chest discomfort and orthostatic intolerance.10 Tachycardia is commonly reported, and a subgroup of patients develop POTS,10 characterised by autonomic dysfunction associated with a variety of symptoms, including tachycardia following postural change.11–14
In a prospective study of 312 patients with COVID-19 in our region, more than half of the home-isolated patients (136/247) had persistent symptoms at 6-month follow-up.9 Immune response (anti-spike IgG antibodies), comorbidity and severity of initial disease were independently associated with the fatigue and other symptoms at 6 months, suggesting that immune mechanisms might be implicated in the pathogenesis of long COVID-19.9 Other research alludes to some symptoms of long COVID-19 being related to a virus-mediated or immune-mediated disruption to the autonomic nervous system, resulting in transient or long-term orthostatic intolerance syndrome.12–14 It has previously been documented that viral infections can trigger POTS.13 There is evidence suggesting that the underlying pathophysiological mechanism in POTS could be autoimmunity, that is, autoantibodies activating adrenergic and muscarinic receptors; a hyper-adrenergic state; peripheral denervation, similar to taste and smell loss, causing blood pooling in the lower extremities; and reflex tachycardia and deconditioning.14
The presence of LBBB could also be attributed to the inflammatory response caused by the virus. Although LBBB is rarer (approximately 1%) in the general population,15 its coexistence with POTS, dyspnoea and palpitations, all occurring simultaneously following COVID-19, strongly suggests an association with COVID-19 infection. Both innate and adaptive immune responses are involved in injury to the myocardium and conduction system. Clinically, this injury can manifest as conduction block in the atrioventricular (AV) node or in the infranodal system, and reversibility is related to the severity of inflammation. Furthermore, COVID-19 can also directly involve the conduction system of the heart and cause disturbances such as bundle branch blocks (BBBs)and intraventricular conduction delays, with and without myocardial involvement.14 Theoretically, the presence of intermittent LBBB (figure 4), which can persist weeks or months following initial infection, could be explained by a persistent myocardial inflammation.7 As shown by previous studies,16 17 the persistence of significant symptoms in patients with long COVID-19 may well be explained by the higher burden of persistent subclinical myocardial dysfunction detected by echocardiographic strain imaging or myocardial inflammation/oedema on CMR imaging of the heart. However, in our patient a CMR at a follow-up visit did not reveal any signs of myocardial injury, ongoing inflammation or oedema.
The patient’s echocardiography presented characteristic findings of dyssynchrony: LBBB, septal flash and apical rocking. Apical rocking is defined as a short rightward motion of the apex caused by the contraction of the septum early in systole and a subsequent longer motion towards the left side during ejection caused by the late lateral contraction caused by LBBB.18 Septal flash is a rapid short leftward septal contraction in early systole that starts and mostly ends before aortic valve opening; corresponding with the systolic isovolumetric time. It is easily detected by M-mode on parasternal long-axis view (figures 2A and 4A). The prevalence of septal flash among patients with LBBB varies substantially (45%–63%) depending on the population studied and the LBBB criteria applied. Septal flash and apical rocking are markers of LV mechanical dyssynchrony due to LBBB and reflect a posterolateral delay in myocardial activation. Both markers strongly associated with mitral regurgitation reduction after cardiac resynchronisation therapy.19 However, the prevalence and clinical significance of septal flash in patients with long COVID-19 are unknown and should be investigated in future studies.
Learning points.
Among the wide range of symptoms associated with long COVID-19, clinicians should be aware of cardiac changes and have low threshold for evaluation by electrocardiography and echocardiography.
Conduction abnormalities and tachyarrhythmias should be considered as differential diagnoses in convalescent individuals, including those who were home-isolated for initial mild COVID-19.
Future studies are needed to explore the pathogenesis and management of these patients.
Acknowledgments
We thank professor dr. Marijana Tadic, University Hospital “Dr. Dragisa Misovic - Dedinje”, Department of Cardiology whose comments improved this manuscript.
Footnotes
Twitter: @bjornblomberg
Contributors: All authors fulfil the authorship criteria. VK, SS contributed to the conception of the case report and treated the patient. BB and TL critically revised the manuscript for important scientific content. All authors approved the final submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021;27:601–15. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahl EH, Mosevoll KA, Cramariuc D, et al. COVID-19 myocarditis and postinfection Bell's palsy. BMJ Case Rep 2021;14:e240095. 10.1136/bcr-2020-240095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102–7. 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saeed S, Tadic M, Larsen TH, et al. Coronavirus disease 2019 and cardiovascular complications: focused clinical review. J Hypertens 2021;39:1282–92. 10.1097/HJH.0000000000002819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeed S, Rajani R. Subclinical myocardial dysfunction in patients following coronavirus disease 2019 infection. J Clin Ultrasound 2022;50:25–7. 10.1002/jcu.23101 [DOI] [PubMed] [Google Scholar]
- 6.Greenhalgh T, Knight M, A'Court C, et al. Management of post-acute covid-19 in primary care. BMJ 2020;370:m3026. 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 7.Chan AT, Drew DA, Nguyen LH, et al. The coronavirus pandemic epidemiology (COPE) Consortium: a call to action. Cancer Epidemiol Biomarkers Prev 2020;29:1283–9. 10.1158/1055-9965.EPI-20-0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence (NICE) . COVID-19 rapid guideline: managing the long-term effects of COVID-19, 2021. Available: https://www.nice.org.uk./ng188 [PubMed]
- 9.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021;27:1607–13. 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson M, Ståhlberg M, Runold M, et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep 2021;3:573–80. 10.1016/j.jaccas.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med 2021;21:e63–7. 10.7861/clinmed.2020-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernino S, Stiles LE. Autoimmunity in postural orthostatic tachycardia syndrome: current understanding. Auton Neurosci 2018;215:78–82. 10.1016/j.autneu.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 13.Ståhlberg M, Reistam U, Fedorowski A, et al. Post-COVID-19 tachycardia syndrome: a distinct phenotype of post-acute COVID-19 syndrome. Am J Med 2021;134:1451–6. 10.1016/j.amjmed.2021.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malekrah A, Fatahian A. A case report of a rare cardiac complication in novel coronavirus disease. Eur Heart J Case Rep 2020;4:1–4. 10.1093/ehjcr/ytaa323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francia P, Balla C, Paneni F, et al. Left bundle-branch block--pathophysiology, prognosis, and clinical management. Clin Cardiol 2007;30:110–5. 10.1002/clc.20034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021;31:100683. 10.1016/j.eclinm.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265. 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calle S, Delens C, Kamoen V, et al. Septal flash: at the heart of cardiac dyssynchrony. Trends Cardiovasc Med 2020;30:115–22. 10.1016/j.tcm.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 19.Michalski B, Stankovic I, Pagourelias E, et al. Relationship of mechanical Dyssynchrony and LV remodeling with improvement of mitral regurgitation after crt. JACC Cardiovasc Imaging 2022;15:S1936-878X(21)00637-9. 10.1016/j.jcmg.2021.08.010 [DOI] [PubMed] [Google Scholar]