Abstract
OBJECTIVE
Data related to diabetic neuropathy in youth with type 2 diabetes are limited. We examined the relationship of glycemic control, sex, race/ethnicity, BMI, and other type 2 diabetes-associated factors with the development of diabetic peripheral neuropathy (DPN) in youth with type 2 diabetes enrolled in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study.
RESEARCH DESIGN AND METHODS
The Michigan Neuropathy Screening Instrument (MNSI) and a 10-g monofilament exam were performed annually. DPN was defined as a score (>2) on the MNSI-exam or combined MNSI-exam and MNSI-survey scores (exam >2 and/or survey ≥4), or monofilament exam (<8 of 10 correct responses) at two or more consecutive visits. Multivariable time-to-event models assessed the association of risk factors evaluated longitudinally with DPN events.
RESULTS
A total of 674 participants (35% male), with a mean age of 14 years and diabetes duration <2 years at study entry, were evaluated annually over an average of 10.2 years. Male subjects had a significantly higher cumulative incidence of DPN than female subjects (38.5% vs. 27.2% via MNSI-exam, P = 0.002; 14.0% vs. 5.1% via monofilament exam, P = 0.01). Rates did not differ by race/ethnicity. Higher HbA1c and BMI were associated with higher DPN, by both MNSI and the monofilament test. In multivariable models, male sex, older age, and higher BMI were associated with MNSI-exam DPN risk.
CONCLUSIONS
DPN was evident early in the course of youth-onset type 2 diabetes and increased over time. It was higher in male subjects and related to glycemic control. These findings raise concern for long-term development of neuropathy-related morbidity in youth with type 2 diabetes and the need to achieve improved glycemic control.
Introduction
The risk of developing retinopathy and nephropathy in youth-onset type 2 diabetes may be higher than in type 1 diabetes diagnosed at a similar age and may occur earlier in the course of the disease than in adult-onset type 2 diabetes (1–5). The early onset and longer lifelong duration of these complications could be associated with increased morbidity and possible early mortality in these patients. Data related to diabetic neuropathy risk in youth-onset type 2 diabetes using the gold standard of nerve conduction velocity studies are needed. We previously reported a 32.4% 15-year cumulative incidence for nerve disease as determined by the Michigan Neuropathy Screening Instrument (MNSI) exam and monofilament exam among participants enrolled in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) observational follow-up (TODAY2) study (6). A similar prevalence (21–25%) has been reported in youth-onset type 2 diabetes in other studies (7,8). However, little is known about risk factors associated with diabetic peripheral neuropathy (DPN). In the SEARCH for Diabetes in Youth (SEARCH) registry, risk factors for DPN (assessed by MNSI-exam) in youth-onset type 2 diabetes (mean age 22 years) were older age, male sex, longer diabetes duration, smoking, and lower HDL cholesterol (8). In this report, we provide longitudinal in-depth analyses of risk factors for DPN in the TODAY study participants followed for an average of 10.2 years.
The aims of this report are to determine 1) the association of glycemic control (as defined by HbA1c) on the risk of DPN over time, 2) the relationship of sex and race/ethnicity on the risk of DPN, and 3) the impact of metabolic and nonmetabolic risk factors and type 2 diabetes–related complications on the risk of DPN. We hypothesized that higher HbA1c during the TODAY trial would be associated with increased risk of DPN.
Research Design and Methods
Study Design and Risk Factor Assessment
The design of the TODAY study has been previously described (www.ClinicalTrials.gov NCT00081328) (9). In brief, 699 participants with type 2 diabetes (American Diabetes Association 2002 criteria) diagnosed before the age of 18, with duration of diabetes <2 years, BMI >85 percentile for age and sex, negative islet cell antibodies, and C-peptide >0.6 ng/mL were randomized to receive metformin alone, metformin plus rosiglitazone, or metformin plus an intensive lifestyle intervention program at 15 participating diabetes centers. The primary outcome of the TODAY study (2004–2011) was to evaluate the effects of the three treatment arms on time to treatment failure, defined as loss of glycemic control (HbA1c ≥8% for 6 consecutive months or failure to wean from temporary insulin after acute metabolic decompensation). The primary outcome (10) and secondary outcomes of the TODAY study have been published (4–6,11,12). Participants in TODAY were followed for an average of 3.9 years (range 2–6).
In 2011, 572 TODAY participants (82%) enrolled in the TODAY2 postintervention follow-up study. Between 2011 and 2014, participants no longer received randomized treatment but continued to receive care from the TODAY staff at 3-month intervals and were treated with metformin and/or insulin as indicated. From 2014 to 2020, 518 TODAY participants (74% of original cohort) transitioned to community care and continued to be followed by the TODAY study group for annual observational visits. Characteristics of the cohort were nearly identical across all study phases.
During TODAY, participants were seen every 2 months during the first year after randomization and quarterly thereafter. During the TODAY2 study, participants were seen every 3 months in the first phase and annually during the second phase. Demographic, history, medications, including prescribed insulin and antihypertensive, primarily ACE inhibitors (ACEi) or angiotensin receptor blockers (ARBs), physical examination, and fasting laboratory data were collected as previously described (9). Participants self-reported cigarette smoking categorized as “yes” (used within the past month) or “no” (never used/not used within the past month). Vitamin B12 levels ≤298 pg/mL were considered borderline low, whereas levels >298 pg/mL were considered normal. Two-hour oral glucose tolerance tests were performed as previously described (12). Results were used to assess insulin sensitivity (1/fasting insulin [1/IF]), C-peptide index, a measure of insulin secretion, defined as the increment in C-peptide values over the first 30 min, divided by the increment in glucose over 30 min (ΔC30/ΔG30), and the oral disposition index (oDI), a measure of insulin sensitivity relative to β-cell function (1/IF × ΔC30/ΔG30) (12). All blood and urine samples were processed centrally at the TODAY Central Biochemistry Laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle WA) (9). Hypertension, dyslipidemia, and indices of nephropathy (microalbuminuria [urine albumin-to-creatinine ratio ≥30 mg/g], estimated glomerular filtration rate [eGFR], and hyperfiltration [eGFR ≥135 mL/min/1.73 m2]) were evaluated longitudinally as previously described (6,10). Retinopathy was assessed 7 years apart via fundus photography, and vascular stiffness and heart rate variability were measured 5 years apart using the SphygmoCor device, as previously described (6,10). History of erectile dysfunction was assessed annually (2014–2020) and due to low frequency, was recorded as present if reported on any visit.
TODAY and TODAY2 were approved by the institutional review boards at all 15 centers, and all participants and guardians provided written informed assent and/or consent as appropriate for age and local guidelines.
DPN Outcomes
The MNSI and monofilament evaluations were administered at the randomization visit and at each annual visit thereafter. The MNSI, a validated screening tool for DPN (13), consists of a 15-item self-administered questionnaire (MNSI-survey) (Supplementary Table 1) and a 4-item examination (MNSI-exam) (Supplementary Table 2) during which a health professional examines each foot and looks for presence of deformities, dry skin/calluses, fissures, infections, ulcers, and lack of vibratory sensation and reflexes. Vibratory sensation was assessed using a 128-Hz tuning fork applied to the dorsal surface of the great toe. Scores were derived, and results from the MNSI-exam and MNSI-survey were reported separately. The MNSI-survey result was considered normal if the score was <4 (14), and the MNSI-exam result was considered normal if the score (across both feet) was ≤2 (13). A combined abnormal MNSI score was defined as an MNSI-survey score ≥4 or an MNSI-exam score >2, or both. Sustained abnormal MNSI scores, defined as abnormal scores at two or more consecutive visits, were used for analyses.
The monofilament examination consisted of applying a Semmes-Weinstein 5.07 10-g monofilament to the dorsum of the great toe of each foot 10 times (15). Correct identification of at least 8 of the 10 applications on each foot was considered normal. Analyses were based on sustained abnormal monofilament exam scores (<8 of 10 correct responses), defined as abnormal scores at two or more consecutive visits.
Statistical Methods
Baseline demographic, metabolic, and nonmetabolic characteristics of the participants with and without a sustained abnormal MNSI-exam or monofilament exam score during the study were compared using the Student t test for quantitative variables and the χ2 test for categorical variables. Variables with a skewed distribution were log-transformed prior to testing. The Kaplan-Meier method was used to estimate the cumulative incidence of the first occurrence of any sustained DPN event (abnormal MNSI-exam score, abnormal combined MNSI-exam and/or MNSI-survey score, or abnormal monofilament test score), and the log-rank test was used to compare event-free survival between sex, race/ethnicity, and loss of glycemic control (i.e., reached the primary outcome during TODAY) groups. Separate univariable and multivariable Cox proportional hazards regression models were used to estimate the effects of HbA1c, and other covariates (e.g., blood pressure, BMI, vitamin B12, hypertension, microalbuminuria, HDL cholesterol, LDL cholesterol, and smoking) on the risk of incident DPN. Participants with the event at baseline (n = 7 with an abnormal MNSI-exam score and n = 0 with an abnormal monofilament test score) were excluded from all time-to-event analyses. Covariates were included in the Cox models as fixed and/or time varying as appropriate. For those with a missing covariate value at a visit, the prior observed value was carried forward. For some variables, the time-weighted arithmetic mean values (computed by weighting each value by the interval between measurements to account for the varying frequencies of covariate measurement during the study) were calculated to assess the cumulative exposure effect of the covariates on the risk of DPN. Generalized estimating equations were used to evaluate the association between HbA1c and abnormal DPN over repeated time points. The χ2 and t tests were used to evaluate the cross-sectional association between DPN and the presence of erectile dysfunction collapsed across all TODAY2 visits, and with retinopathy, heart rate variability, and vascular stiffness. Analyses were performed using SAS 9.4 for Windows (SAS Institute, Cary, NC) and considered exploratory, with statistical significance defined as P < 0.05.
Results
This analysis included 674 of the 699 TODAY participants. Excluded were 22 participants subsequently found to have monogenic diabetes mutations and 3 participants with missing baseline data. Analyses included all available data collected at study visits during TODAY and TODAY2 for up to 15 years (mean 10.2 ± 4.5) of follow-up. Participants followed for the entire follow-up period did not differ from those who were followed for shorter periods by sex, race/ethnicity, maternal history of diabetes, or baseline factors (age, BMI, blood pressure, and HbA1c). They did, however, have a slightly longer duration of diabetes at baseline compared with those followed for a shorter period (9.4 months vs. 7.2 months; P = 0.0001). Supplementary Table 3 shows baseline demographics and characteristics stratified according to sustained abnormal MNSI-exam status (n = 146 [21.7%]) or sustained abnormal monofilament exam status (n = 31 [4.6%]) compared with those without sustained abnormal scores. Participants with sustained abnormal scores on the MNSI-exam were more likely to be male (P = 0.007), have slightly older age (P = 0.03), and have higher BMI and BMI-z score (P < 0.0001 for both), at study entry compared with those participants without sustained abnormal scores. Participants with sustained abnormal monofilament exam scores were more likely to be male (P = 0.02) compared with those with normal monofilament exam scores. No other difference among baseline demographics or characteristics was found (Supplementary Table 3). The original treatment assignment in TODAY did not impact DPN.
At study year 14 (mean diabetes duration 15 years), the most common abnormality on the MNSI-exam was an abnormal ankle reflex (44.0%), followed by dry skin/callus (26.9%), and reduction/absence of vibration at the great toe (29.1%). At the same time, the most common symptom reported on the self-report MNSI-survey was cramps in the legs and/or feet (36.0%), followed by prickling feelings in the legs and/or feet (26.9%) (Supplementary Table 4).
Prevalence of DPN During the Study
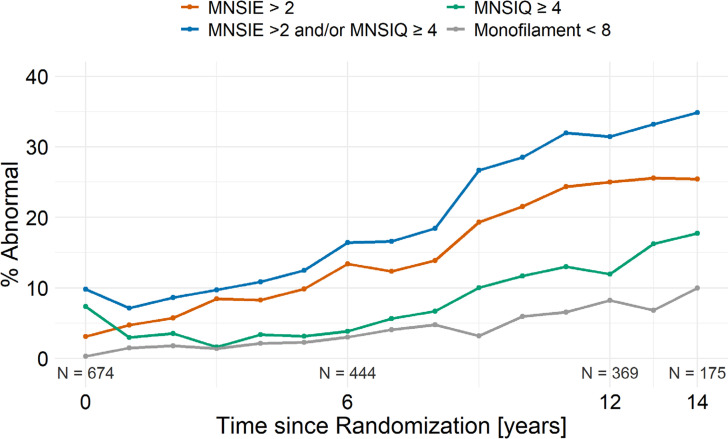
At baseline, 3.1% of participants had an abnormal score via the MNSI-exam (>2), 7.3% had an abnormal score via the MNSI-survey (≥4), 9.8% had an abnormal score via the MNSI-exam and/or MNSI-survey, and 0.3% had an abnormal score via the monofilament exam (Fig. 1). The prevalence of DPN rose steadily during the study for all of the DPN assessment methods: 25.4% via MNSI-exam, 17.7% via MNSI-survey, 34.9% via both, and 10.0% via the monofilament exam at year 14 (Fig. 1).
Figure 1.
Prevalence of abnormal DPN scores during the study via the MNSI and/or the monofilament exam.
Cumulative Incidence of DPN by Sex and Race/Ethnicity
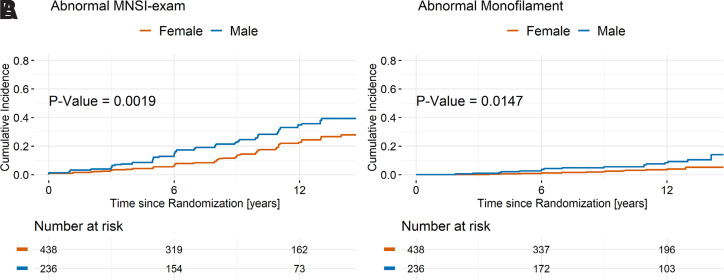
The cumulative incidence of DPN via the MNSI-exam and monofilament exam was higher in male than in female participants (38.5% vs. 27.2%, P = 0.002 for MNSI-exam, and 14.0% vs. 5.1%, P = 0.01 for monofilament exam) (Fig. 2A and B) at end of study. Similar results were obtained for DPN, defined as a sustained abnormal score on the MNSI-exam and/or MNSI-survey (48.2% in males vs. 38.1% in females, P = 0.03) (Supplementary Fig. 1). No difference by race/ethnicity in the cumulative incidence of DPN was found (data not shown).
Figure 2.
Cumulative incidence of abnormal result on the MNSI-exam (A) and monofilament exam (B) by sex during the study.
Impact of HbA1c and Other Risk Factors for DPN
No difference in baseline HbA1c was found between participants who did and did not have DPN during the study (Supplementary Table 3); however, there was a 15% increase in the odds of having an abnormal MNSI-exam score (odds ratio 1.15, 95% CI 1.08–1.22, P < 0.0001) over time per 1-unit increase in HbA1c (e.g., 7.0% to 8.0%). Similarly, there was a 22% increase in the odds of having an abnormal monofilament test result (odds ratio 1.22, 95% CI 1.09–1.35, P = 0.0004) over time per 1-unit increase in HbA1c. In addition to time-weighted mean HbA1c, male sex, older age, and higher BMI, plasminogen activator inhibitor 1 (PAI-1), hs-CRP, time-weighted mean systolic blood pressure (SBP), hypertension, and prescribed insulin and ACEi/ARB use were associated with the risk of DPN, defined as sustained abnormal scores on the MNSI-exam (Table 1). Male sex, higher time-weighted mean HbA1c, prescribed insulin and ACEi/ARB use, and lower β-cell function (assessed by the C-peptide index and oDI) were associated with higher risk of DPN defined as sustained abnormal scores on the monofilament exam. Maternal diabetes, smoking in the past month, HDL and LDL cholesterol, dyslipidemia, urine albumin-to-creatinine ratio, microalbuminuria, and vitamin B12 levels were not associated with the risk of DPN. Higher eGFR and presence of hyperfiltration were associated with a borderline significant (both P = 0.05) increased risk of DPN based on the MNSI-exam (Table 1). Similar results were found when the univariable risk factors were evaluated in relation to the risk of DPN, defined as a sustained combined abnormal MNSI-exam and/or MNSI-survey result (data not shown).
Table 1.
Univariable Cox proportional hazard models predicting DPN during the study via the MNSI-exam and monofilament exam
| Abnormal MNSI-exam result | Abnormal monofilament exam result | |||||
|---|---|---|---|---|---|---|
| Characteristics (reference group or unit change) | HR | 95% CI | P value | HR | 95% CI | P value |
| Male sex (male vs. female) | 1.68 | 1.21, 2.35 | 0.002 | 2.35 | 1.16, 4.76 | 0.02 |
| Race/ethnicity (vs. non-Hispanic White) | ||||||
| Non-Hispanic Black | 1.15 | 0.70, 1.88 | 0.57 | 2.03 | 0.67, 6.16 | 0.21 |
| Hispanic | 1.30 | 0.81, 2.09 | 0.28 | 1.43 | 0.45, 4.49 | 0.54 |
| Age (per year) | 1.14 | 1.05, 1.24 | 0.002 | 1.09 | 0.91, 1.30 | 0.36 |
| Type 2 diabetes duration (per month) | 0.98 | 0.95, 1.01 | 0.15 | 0.99 | 0.93, 1.05 | 0.78 |
| Maternal diabetes (yes vs. no) | 1.17 | 0.83, 1.65 | 0.37 | 1.53 | 0.75, 3.14 | 0.24 |
| Smoking in past month (yes vs. no) | 0.99 | 0.59, 1.67 | 0.97 | 1.02 | 0.33, 3.17 | 0.97 |
| Treatment group (vs. metformin only) | ||||||
| Metformin + rosiglitazone | 0.89 | 0.60, 1.33 | 0.57 | 0.88 | 0.41, 1.90 | 0.74 |
| Metformin + intensive lifestyle | 0.82 | 0.55, 1.24 | 0.35 | 0.39 | 0.12, 1.00 | 0.05 |
| BMI (per 5 kg/m2) | 1.24 | 1.13, 1.36 | <0.0001 | 0.91 | 0.73, 1.14 | 0.42 |
| Mean HbA1c (per %)† | 1.19 | 1.10, 1.29 | <0.0001 | 1.37 | 1.15, 1.63 | 0.0004 |
| Prescribed insulin medication (yes vs. no) | 1.60 | 1.13, 2.27 | 0.008 | 5.34 | 2.03, 14.1 | 0.0007 |
| Mean SBP (per 10 mmHg)† | 1.31 | 1.10, 1.57 | 0.003 | 1.34 | 0.91, 1.95 | 0.13 |
| Mean DBP (per 10 mmHg)† | 1.25 | 0.98, 1.59 | 0.07 | 1.21 | 0.72, 2.02 | 0.47 |
| Antihypertensive medication use (yes vs. no) | 1.49 | 1.06, 2.10 | 0.02 | 2.25 | 1.11, 4.56 | 0.02 |
| Hypertension (yes vs. no) | 1.51 | 1.07, 2.14 | 0.02 | 1.16 | 0.56, 2.38 | 0.69 |
| Mean LDL cholesterol (per mg/dL)† | 1.00 | 0.99, 1.01 | 0.81 | 1.00 | 0.99, 1.02 | 0.63 |
| Mean HDL cholesterol (per mg/dL)† | 1.01 | 0.99, 1.03 | 0.35 | 0.99 | 0.96, 1.04 | 0.88 |
| Dyslipidemia (yes vs. no) | 1.08 | 0.70, 1.65 | 0.73 | 0.80 | 0.30, 2.10 | 0.65 |
| Log UACR (per SD) | 1.14 | 0.96, 1.35 | 0.14 | 1.12 | 0.79, 1.59 | 0.52 |
| Microalbuminuria (yes vs. no) | 1.38 | 0.97, 1.97 | 0.07 | 1.65 | 0.79, 3.42 | 0.18 |
| eGFR (per 10 mL/min/1.73 m2) | 1.07 | 0.99, 1.14 | 0.05 | 1.06 | 0.92, 1.22 | 0.40 |
| Hyperfiltration (yes vs. no) | 1.41 | 0.99, 1.99 | 0.05 | 1.28 | 0.63, 2.63 | 0.49 |
| Vitamin B12 level (normal [>298 pg/mL] vs. borderline low/low [≤298 pg/mL]) | 1.69 | 0.38, 7.52 | 0.49 | 0.40 | 0.04, 4.39 | 0.45 |
| Log insulin sensitivity (per SD) | 0.96 | 0.82, 1.12 | 0.61 | 0.90 | 0.65, 1.25 | 0.53 |
| Log C-peptide index (per SD) | 0.90 | 0.77, 1.05 | 0.19 | 0.63 | 0.48, 0.82 | 0.0007 |
| Log C-peptide oDI (per SD) | 0.88 | 0.76, 1.03 | 0.10 | 0.66 | 0.49, 0.88 | 0.005 |
| Log PAI-1 (per SD) | 1.26 | 1.07, 1.49 | 0.005 | 1.12 | 0.79, 1.57 | 0.53 |
| Log hs-CRP (per SD) | 1.34 | 1.12, 1.62 | 0.002 | 0.92 | 0.64, 1.34 | 0.67 |
HR, 95% CIs, and P values per reference group or unit change (as indicated) from separate univariable Cox proportional hazards model predicting the risk of abnormal MNSI-exam result or monofilament exam result during the study. Factors are entered as fixed (sex, race/ethnicity, maternal diabetes, randomized treatment group), baseline (age, diabetes duration), or as time-dependent (all other factors, assessed or measured at or at the most recent visit up to the particular time of the event or right censoring time) covariates in each of the models. DBP, diastolic blood pressure; UACR, urine albumin-to-creatinine ratio.
Time-weighted mean.
No association was found between DPN (abnormal result on the MNSI-exam or monofilament exam) and the presence of diabetic retinopathy (Supplementary Table 5), nor was there a significant association between an abnormal MNSI-exam score and retinopathy progression. However, 47.6% of participants who had an abnormal monofilament exam score during the study had a three-step progression in retinopathy versus 24.8% of those with a normal monofilament exam score (P = 0.03). Additionally, participants with an abnormal MNSI-exam or monofilament exam score during the study had significantly worse vascular stiffness and heart rate variability compared with participants without any sustained abnormal scores (Supplementary Table 5).
Those who reached the primary end point of TODAY (sustained HbA1c ≥8.0%) had higher risk of an abnormal MNSI-exam result (cumulative incidence 37.4% vs. 25.6%; P = 0.0008) and abnormal monofilament exam score (cumulative incidence 13.4% vs. 2.9%; P = 0.0008) compared with participants who did not reach the end point during TODAY (Supplementary Fig. 2A and B). Similar results were obtained for DPN, defined as a sustained abnormal score on the MNSI-exam and/or MNSI-survey (P = 0.0003) (Supplementary Fig. 2C). These differences remained significant after adjustment for randomized treatment group.
In a multivariable Cox model (Table 2) controlling for sex, age, randomized treatment group, BMI, time-weighted mean HbA1c and SBP, C-peptide oDI, and hs-CRP, only time-weighted mean HbA1c (hazard ratio [HR] 1.26 per 1-unit increase, 95% CI 1.14–1.40, P < 0.0001), male sex (HR 1.81, 95% CI 1.25–2.62, P = 0.002), age (HR 1.11 per year increase, 95% CI 1.01–1.21, P = 0.03), and higher BMI (HR 1.28 per 5-kg/m2 increase, 95% CI 1.15–1.43, P < 0.0001) were associated with risk of DPN via the MNSI-exam. Higher time-weighted mean HbA1c levels (HR 1.32 per 1-unit increase, 95% CI 1.07–1.63, P = 0.01) remained the only significant factor associated with the risk of an abnormal monofilament exam result in the multivariable Cox model. There were no interactions between HbA1c with sex, race/ethnicity, or randomized treatment group identified in any of the Cox regression models.
Table 2.
Multivariable Cox proportional hazard models predicting DPN via the MNSI-exam and via the monofilament exam
| Abnormal MNSI-exam result | Abnormal monofilament exam result | |||||
|---|---|---|---|---|---|---|
| Characteristics (reference group or unit change) | HR | 95% CI | P value | HR | 95% CI | P value |
| Male (male vs. female) | 1.81 | 1.25, 2.62 | 0.002 | 2.06 | 0.94, 4.53 | 0.07 |
| Age (per year) | 1.11 | 1.01, 1.21 | 0.03 | 1.08 | 0.89, 1.30 | 0.44 |
| Treatment group (vs. metformin only) | ||||||
| Metformin + rosiglitazone | 0.88 | 0.59, 1.32 | 0.54 | 0.94 | 0.43, 2.05 | 0.87 |
| Metformin + intensive lifestyle | 0.97 | 0.64, 1.46 | 0.88 | 0.40 | 0.14, 1.14 | 0.09 |
| BMI (per 5 kg/m2) | 1.28 | 1.15, 1.43 | <0.0001 | 0.96 | 0.74, 1.23 | 0.73 |
| Mean HbA1c (per %)† | 1.26 | 1.14, 1.40 | <0.0001 | 1.32 | 1.07, 1.63 | 0.01 |
| Mean SBP (per 10 mmHg)† | 0.90 | 0.72, 1.11 | 0.32 | 0.99 | 0.64, 1.54 | 0.97 |
| Log C-peptide oDI (per SD) | 1.09 | 0.89, 1.33 | 0.42 | 0.86 | 0.59, 1.25 | 0.42 |
| Log hs-CRP (per SD) | 1.13 | 0.91, 1.40 | 0.28 | 0.92 | 0.59, 1.44 | 0.72 |
HRs, 95% CIs, and P values per reference group or unit change (as indicated) from a multivariable Cox proportional hazards model predicting the risk of abnormal MNSI-exam result or monofilament exam result during the study. Factors are entered as fixed (sex, randomized treatment group), baseline (age), or as time-dependent (all other factors, assessed or measured at or at the most recent visit up to the particular time of the event or right censoring time) covariates in the models.
Time-weighted mean.
Of the 171 males still enrolled in the study during TODAY2, 39 (22.8%) reported erectile dysfunction on at least one visit. Erectile dysfunction was significantly associated with lower (i.e., worse) heart rate variability (P = 0.003; data not shown). Males who had DPN via the MNSI-exam during the study reported higher proportions of erectile dysfunction during TODAY2 (32.8%) versus those who did not have DPN (17.3%, P = 0.02). Similarly, males who had DPN via the monofilament exam during the study reported higher proportions of erectile dysfunction during TODAY2 (52.9%) versus those who did not have DPN (19.5%, P = 0.004).
Conclusions
These findings from the TODAY study indicate that youth with type 2 diabetes show evidence of DPN on MNSI screening early in the course of diabetes and that this complication increases over time. Baseline monofilament testing results were normal in most participants, but abnormalities in testing also increased over time. Sex differences were seen, with males having a higher cumulative incidence of DPN compared with females on both MNSI and monofilament exams after up to 15 years of follow-up. Glycemic control, as defined by HbA1c, was a significant risk factor for DPN on both MNSI and monofilament exams. In addition, older age, higher BMI, PAI-1, hs-CRP, time-weighted mean SBP, hypertension, and prescribed insulin and ACEi/ARB use were associated with a sustained abnormal score on the MNSI-exam, and prescribed insulin and ACEi/ARB use and lower β-cell function were associated with a sustained abnormal score on the monofilament exam. Rates of DPN were not significantly different by race/ethnicity.
The 25.4% overall prevalence of DPN in TODAY is similar to the prevalence of DPN reported by other studies in youth with type 2 diabetes. In an Australian cohort of youth with median age of 15.3 years and recent-onset type 2 diabetes, peripheral neuropathy (via thermal and vibration threshold) was found in 21% of participants (7). A preliminary cross-sectional report from the SEARCH study reported a DPN prevalence by MNSI-exam of 25.7% (16).
Findings from the current study add to the understanding associated with morbidity associated with type 2 versus type 1 diabetes. Although one study in a Canadian cohort found that youth with type 2 diabetes showed an earlier diagnosis of neuropathy than youth with type 1 diabetes (17), reports of prevalence of DPN in pediatric populations with type 1 diabetes have varied widely. A report from the Pittsburgh Epidemiology of Diabetes Complications Study indicated that DPN was very uncommon in participants <18 years of age (18). In contrast, prevalence of subclinical peripheral neuropathy in type 1 diabetes assessed by nerve conduction studies was 68.4% in a small population of Chinese children and 57% in a larger population of Swedish youth (19,20). A Finish study indicated that peroneal motor conduction velocity was abnormal in 30% of youth with type 1 diabetes compared with control subjects (21). In a later report from SEARCH, DPN prevalence evaluated by MNSI was 22% in youth with type 2 diabetes with a mean diabetes duration of 7.9 years, a higher prevalence than reported in youth-onset type 1 diabetes (8).
Poor glycemic control is an established risk factor for diabetic neuropathy in longitudinal studies in adults (22,23), but reports in pediatric studies are more limited. In the TODAY cohort, higher risk of DPN was related to glycemic control, as defined by HbA1c. We found that during an average of 10.2 ± 4.5 years of follow-up, a 1-unit increase in HbA1c (e.g., from 7.0 to 8.0%) resulted in a 15% increase in the odds of having an abnormal MNSI-exam score and a 22% increase in the odds of having an abnormal monofilament exam result. In SEARCH, glycemic control over time was a risk factor for DPN in pediatric type 1 diabetes but not in youth with type 2 diabetes; this may be attributed in part to a lack of power (i.e., small sample size), because the association was in the same direction as that of the group with type 1 diabetes but did not reach statistical significance (8). In the Australian youth cohort, type 2 diabetes prevalence of peripheral and autonomic neuropathy was similar to that of the cohort with type 1 diabetes despite shorter diabetes duration (1.3 years vs. 6.8 years) and lower HbA1c (7.3% vs. 8.5%) (7).
Indeed, previous reports in type 1 diabetes have shown an association of neuropathy with glycemic control. In youth, duration of diabetes, age, and diabetes control each had significant and independent effects on the prevalence of delayed nerve conduction (24). The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Group reported that in 1,184 adults with type 1 diabetes and diabetes duration of 26 years (14), in whom clinical neuropathy was confirmed via nerve conduction studies and neurological exam, 33% had an MNSI-exam score >2. Bao et al. (19) reported that higher HbA1c was a risk factor for the development of subclinical peripheral neuropathy in their pediatric population with type 1 diabetes. There is evidence from the Look Action for Health in Diabetes (AHEAD) study that weight loss among adult participants with type 2 diabetes in an intensive lifestyle intervention resulted in a significant decrease in HbA1c and MNSI-survey scores (25). However, a recent report on the observational long-term follow-up of the TODAY participants observed that median BMI remained in the narrow range of 35.0–37.5 kg/m2, irrespective of original treatment group assignment (6). In this current report, although higher BMI was associated with an increased risk of DPN, we cannot conclude that weight loss would reduce the risk of neuropathy.
The present investigation also examined associations between DPN and numerous other variables of interest. Studies in adults with type 1 diabetes have evaluated the relationship of DPN with other diabetes complications and have demonstrated that elevated urine albumin was a common comorbidity of diabetic neuropathy (26,27). Although Bao et al. (19) previously reported that serum creatinine, urea, urine microalbumin-to-creatinine ratio, and urinary microalbumin excretion rate were significantly associated with the development of subclinical peripheral neuropathy in specific nerves in pediatric type 1 diabetes, these associations were not seen in our study of youth-onset type 2 diabetes. However, retinopathy progression was associated with an abnormal monofilament exam result, and a worsening in heart rate variability (a measure of cardiac autonomic neuropathy) and pulse wave velocity (a marker of vascular disease) were associated with an abnormal MNSI and abnormal monofilament exam result. However, in contrast with studies of adults with type 2 diabetes (28), there was no relation between inflammatory markers, such as PAI-1, tumor necrosis factor-α, and hs-CRP, and DPN. Further, there was no association between vitamin B12 level and DPN, despite the use of metformin, which may lower vitamin B12. We could not ascertain whether lower vitamin B12 levels or low levels for a longer duration could be associated with neuropathy. Among adults with youth-onset type 2 diabetes in the SEARCH study, older age, male sex, longer diabetes duration, smoking, and lower HDL cholesterol were risk factors for DPN (8). However, in addition to HbA1c and BMI, only older age and male sex were found to relate to the risk of DPN in our cohort. Finally, only a small number of males in the current cohort reported erectile dysfunction, but associations between DPN both via abnormal MNSI and monofilament screening and erectile dysfunction were observed. We also found an association of abnormal MNSI and monofilament exam results with cardiac autonomic neuropathy, as measured by heart rate variability. Data from adults in the DCCT suggest that cardiovascular autonomic neuropathy predicts the development of urological complications in men with type 1 diabetes (29). Although abnormal MNSI and monofilament examination results were common and associated with another potential manifestation of neuropathy (erectile dysfunction), we did not find an association of DPN with microalbuminuria.
Our results are limited by the methodologies used to evaluate neuropathy. Motor and sensory nerve conduction studies to assess peripheral nerve function are required for the definitive diagnosis of DPN and have previously been used in pediatrics (19,30). However, nerve conduction velocity studies are infrequently done because they are expensive, uncomfortable for participants, and difficult to standardize in a multicenter study. The MNSI and monofilament exam are widely used screening examinations for diabetic neuropathy. Although they do not provide a conclusive diagnosis of DPN, the MNSI and monofilament exam are frequently used in conjunction, especially in epidemiological studies. In SEARCH, the MNSI-exam was used to define DPN (8). The sensitivity of the MNSI has been reported to be 79%, with a specificity of 65% for symptomatic adult participants (31). However, the Semmes-Weinstein monofilament exam is often recommended for screening due to ease of administration (32). The monofilament exam is not meant to be a sensitive indicator of early neuropathy but has been reported as a good predictor of ulcer formation (33). Other studies have advocated for symptom questionnaires, particularly reports of paresthesias (30,34). Overall, there is not current consensus about the best screening method for DPN.
While the use of newer neuropathy-measuring methods may identify a higher number of participants with DPN compared with established measures, there is difficulty in objectively identifying DPN and the need for better standardization of screening tools. There remains a role for nerve conduction studies in providing confirmation of DPN in future pediatric research studies. Despite the lack of a definitive diagnosis using nerve conduction studies, the annual evaluations of DPN and other risk factors over more than a decade from the time of diagnosis is a distinct strength of our study. Finally, although we were able to ascertain a history of recent (over the past month) smoking, we were not able to document overall smoking exposure over the course of the study period.
In summary, youth with type 2 diabetes demonstrate evidence of DPN on MNSI screening early in the course of diabetes, with increasing prevalence over time. The prevalence of DPN in TODAY is similar to the prevalence of DPN in pediatric type 2 diabetes reported by SEARCH and other studies with comparable diabetes duration. Sex differences were seen in DPN over time, but there were no race/ethnicity differences. Incident DPN was related to glycemic control and BMI. These findings raise concern for long-term development of neuropathy-related morbidity in youth with type 2 diabetes. Screening for diabetic neuropathy in young adults with type 2 diabetes may be beneficial to detect and prevent nerve damages at early stages.
Article Information
Acknowledgments. The authors gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service.
The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribes and the Indian Health Service.
Funding. This work was completed with funding from the National Institutes of Health Office of the Director and the National Institute of Diabetes and Digestive and Kidney Diseases through grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The National Institute of Diabetes and Digestive and Kidney Diseases project office was involved in all aspects of the study, including design and conduct, collection, management, analysis, and interpretation of the data, review and approval of the manuscript, and decision to submit the manuscript for publication.
Duality of Interest. The TODAY Study Group thanks the following companies for donations of materials in support of the study’s efforts: Becton, Dickinson and Company, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, LifeScan, Inc., Pfizer, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. L.E.L.K. and N.H.W. wrote the manuscript. L.E. conducted the statistical analyses and contributed to data interpretation and writing. C.L.C., K.C.C., T.H.L., M.D.M., and P.Z. contributed to study and manuscript design, interpretation, and manuscript edits. L.E. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
TODAY Study Group Writing Committee. Lorraine E. Levitt Katz (Division of Endocrinology & Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA), Neil H. White (Division of Endocrinology & Diabetes, Department of Pediatrics, Washington University in St. Louis School of Medicine, St. Louis, MO), Laure El ghormli (The Biostatistics Center, George Washington University, Rockville, MD), Christine L. Chan (University of Colorado Anschutz Medical Campus, Aurora, CO), Kenneth C. Copeland (University of Oklahoma Health Sciences Center, Oklahoma City, OK), Terri H. Lipman (Division of Endocrinology & Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA), Marsha D. Marcus (Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA), and Philip Zeitler (University of Colorado Anschutz Medical Campus, Aurora, CO).
Footnotes
Clinical trial reg. nos. NCT00081328, NCT01364350, NCT02310724, https://clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.16649851.
Members of the TODAY Study Group Writing Committee are listed in the appendix. A complete list of the TODAY Study Group members can be found in the supplementary material online.
This article is part of a special article collection available at diabetesjournals.org/journals/collection/268/Serious-Later-Risks-Associated.
Contributor Information
Collaborators: Lorraine E. Levitt Katz, Neil H. White, Laure El ghormli, Christine L. Chan, Kenneth C. Copeland, Terri H. Lipman, Marsha D. Marcus, and Philip Zeitler
References
- 1. Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care 2012;35:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 2013;36:3863–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayer-Davis EJ, Davis C, Saadine J, et al.; SEARCH for Diabetes in Youth Study Group . Diabetic retinopathy in the SEARCH for Diabetes in Youth Cohort: a pilot study. Diabet Med 2012;29:1148–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. TODAY Study Group . Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care 2013;36:1772–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. TODAY Study Group . Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013;36:1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. TODAY Study Group; Bjornstad P, Drews KL, Caprio S, et al. Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 2006;29:1300–1306 [DOI] [PubMed] [Google Scholar]
- 8. Jaiswal M, Divers J, Dabelea D, et al. Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 2017;40:1226–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. TODAY Study Group; Zeitler P, Epstein L, Grey M, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. TODAY Study Group; Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. TODAY Study Group . Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013;36:1758–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. TODAY Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 14. Herman WH, Pop-Busui R, Braffett BH, et al.; DCCT/EDIC Research Group . Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar S, Fernando DJ, Veves A, Knowles EA, Young MJ, Boulton AJ. Semmes-Weinstein monofilaments: a simple, effective and inexpensive screening device for identifying diabetic patients at risk of foot ulceration. Diabetes Res Clin Pract 1991;13:63–67 [DOI] [PubMed] [Google Scholar]
- 16. Jaiswal M, Lauer A, Martin CL, et al.; SEARCH for Diabetes in Youth Study Group . Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care 2013;36:3903–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care 2014;37:436–443 [DOI] [PubMed] [Google Scholar]
- 18. Maser RE, Steenkiste AR, Dorman JS, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1989;38:1456–1461 [DOI] [PubMed] [Google Scholar]
- 19. Bao XH, Wong V, Wang Q, Low LC. Prevalence of peripheral neuropathy with insulin-dependent diabetes mellitus. Pediatr Neurol 1999;20:204–209 [DOI] [PubMed] [Google Scholar]
- 20. Hyllienmark L, Brismar T, Ludvigsson J. Subclinical nerve dysfunction in children and adolescents with IDDM. Diabetologia 1995;38:685–692 [DOI] [PubMed] [Google Scholar]
- 21. Käär ML, Saukkonen AL, Pitkänen M, Akerblom HK. Peripheral neuropathy in diabetic children and adolescents. A cross-sectional study. Acta Paediatr Scand 1983;72:373–378 [DOI] [PubMed] [Google Scholar]
- 22. Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ 3rd, O’Brien PC. Risk factors for severity of diabetic polyneuropathy: intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes Care 1999;22:1479–1486 [DOI] [PubMed] [Google Scholar]
- 23. Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care 1997;20:1162–1167 [DOI] [PubMed] [Google Scholar]
- 24. Hoffman WH, Hart ZH, Frank RN. Correlates of delayed motor nerve conduction and retinopathy in juvenile-onset diabetes mellitus. J Pediatr 1983;102:351–356 [DOI] [PubMed] [Google Scholar]
- 25. Look AHEAD Research Group . Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia 2017;60:980–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 27. Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 1996;39:1377–1384 [DOI] [PubMed] [Google Scholar]
- 28. Ge S, Xie J, Zheng L, et al. Associations of serum anti-ganglioside antibodies and inflammatory markers in diabetic peripheral neuropathy. Diabetes Res Clin Pract 2016;115:68–75 [DOI] [PubMed] [Google Scholar]
- 29. Pop-Busui R, Hotaling J, Braffett BH, et al. Cardiovascular autonomic neuropathy, erectile dysfunction and lower urinary tract symptoms in men with type 1 diabetes: findings from the DCCT/EDIC [published correction appears in J Urol 2015;194:855]. J Urol 2015;193:2045–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moser JT, Langdon DR, Finkel RS, et al. The evaluation of peripheral neuropathy in youth with type 1 diabetes. Diabetes Res Clin Pract 2013;100:e3–e6 [DOI] [PubMed] [Google Scholar]
- 31. Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg 2006;108:477–481 [DOI] [PubMed] [Google Scholar]
- 32. Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001;24:250–256 [DOI] [PubMed] [Google Scholar]
- 33. Liniger C, Albeanu A, Bloise D, Assal JP. The tuning fork revisited. Diabet Med 1990;7:859–864 [DOI] [PubMed] [Google Scholar]
- 34. Moser J, Lipman T, Langdon DR, Bevans KB. Development of a youth-report measure of DPN symptoms: conceptualization and content validation. J Clin Transl Endocrinol 2017;9:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]