Abstract
OBJECTIVE
Semaglutide, a glucagon-like peptide 1 receptor agonist, reduced major adverse cardiovascular events (MACE) in people with type 2 diabetes (T2D) at high risk of cardiovascular disease (CVD) in a post hoc analysis of pooled data from Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN) 6 and Peptide Innovation for Early Diabetes Treatment (PIONEER) 6. We estimated the benefit of adding semaglutide to standard of care (SoC) on life-years free of new/recurrent CVD events in people with T2D at high risk of CVD.
RESEARCH DESIGN AND METHODS
The Diabetes Lifetime-perspective prediction (DIAL) competing risk–adjusted lifetime CVD risk model for people with T2D was developed previously. Baseline characteristics of the pooled cohort from SUSTAIN 6 and PIONEER 6 (POOLED cohort) (N = 6,480) were used to estimate individual life expectancy free of CVD for patients in the POOLED cohort. The hazard ratio of MACE from adding semaglutide to SoC was derived from the POOLED cohort (hazard ratio [HR] 0.76 [95% CI 0.62–0.92]) and combined with an individual’s risk to estimate their CVD benefit.
RESULTS
Adding semaglutide to SoC was associated with a wide distribution in life-years free of CVD gained, with a mean increase of 1.7 (95% CI 0.5–2.9) life-years. Estimated life-years free of CVD gained with semaglutide was dependent on baseline risk (life-years free of CVD gained in individuals with established CVD vs. those with cardiovascular risk factors only: 2.0 vs. 0.2) and age at treatment initiation.
CONCLUSIONS
Adding semaglutide to SoC was associated with a gain in life-years free of CVD events that was dependent on baseline CVD risk and age at treatment initiation. This study helps contextualize the results of semaglutide clinical trials.
Introduction
Cardiovascular disease (CVD) is the main cause of disability and death in people with type 2 diabetes (T2D) (1) and affects ∼32% of people with T2D (2). On average, the risk of CVD is twofold higher in people with T2D than in people without, independent of other risk factors (3). CVD is associated with reduced quality of life (4,5) and high health care costs (6,7). Consequently, the prevention of CVD in patients with T2D is of high importance.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are a class of highly effective blood glucose–lowering medications that provide cardiovascular benefit alongside improved glycemic control and weight loss (8). GLP-1 RAs are recommended for people with T2D and high cardiovascular risk in several recent guidelines (9–12). Semaglutide is a GLP-1 RA that was shown to improve cardiovascular outcomes in people with T2D at high risk of CVD in a pooled analysis of two cardiovascular outcomes trials (13), Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN) 6 (14) and Peptide Innovation for Early Diabetes Treatment (PIONEER) 6 (15). In the pooled analysis, reduction in CVD risk with semaglutide treatment was expressed as the relative risk, based on the average patient from the entire population of both trials. However, the benefit an individual patient may gain with treatment varies based on their baseline CVD risk in combination with their life expectancy.
Patients have reported having trouble in understanding their own CVD risk and potential for CVD risk reduction (16). Better prediction tools may motivate patients to take meaningful actions to reduce their risk, including changing their lifestyle and initiating or adhering to medication (17). Short-term risk models—for example, 10-year risk models such as the ADVANCE model (18)—fail to appreciate the long-term risk and potential high lifetime benefit that a young person with T2D may gain. Competing risk–adjusted lifetime risk models estimate lifetime risk and life expectancy and can be combined with hazard ratios (HRs) from trials to estimate the absolute life expectancy free of new/recurrent CVD events gained with an intervention. Such models can be used at an individual level to estimate the absolute benefit that an individual may gain, based on their characteristics. This absolute benefit in life expectancy free of new/recurrent CVD events that a patient may gain from lifetime treatment can then be discussed with the patient and explained in terms of a return on investment. Understanding and communicating this estimated individual benefit improves shared decision-making.
The Diabetes Lifetime-perspective prediction (DIAL) risk model, which has been developed recently, is currently the only lifetime risk prediction tool specifically for people with T2D and is freely available online (www.u-prevent.com) (19). This model has been recommended for use by the European Society of Cardiology (20). In the current study, we used the DIAL model to estimate the effect of adding semaglutide to standard of care (SoC) on life-years free of new/recurrent CVD events in people with T2D, using pooled data from SUSTAIN 6 and PIONEER 6.
Research Design and Methods
Study Populations
SUSTAIN 6 (NCT01720446) and PIONEER 6 (NCT02692716) were randomized, double-blind, placebo-controlled trials where inve-stigators evaluated the cardiovascular effects of once-weekly subcutaneous semaglutide (0.5 mg or 1.0 mg) and once-daily oral semaglutide (target dose 14 mg), respectively, in people with T2D and high CVD risk. High CVD risk was defined based on age ≥50 years with established CVD or age ≥60 years with at least one cardiovascular risk factor in addition to T2D; further information on the criteria for CVD risk can be found in Supplementary Material. In the individual trials, semaglutide was superior to (14) or noninferior to (15) placebo, respectively. SUSTAIN 6 included 3,297 people with T2D, of whom 2,735 had established CVD and 562 had CVD risk factors only. PIONEER 6 included 3,183 people with T2D, of whom 2,695 had established CVD and 488 had CVD risk factors only. The median follow-up in SUSTAIN 6 and PIONEER 6 was 2.1 and 1.3 years, and full information on the methodology and countries included can be found in the works by Marso et al. (14) and Husain et al. (15), respectively. In this study, pooled data from SUSTAIN 6 and PIONEER 6 (POOLED cohort) (N = 6,480) were used.
Outcomes
Major adverse cardiovascular events (MACE) were defined as cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction. The HR for the effect of adding semaglutide to SoC on MACE was calculated in a post hoc analysis of data from SUSTAIN 6 and PIONEER 6, with use of a stratified Cox proportional hazards model with treatment as a categorical fixed factor (HR 0.76 [95% CI 0.62–0.92]) (13). Key outcomes in the current analyses were absolute gain in life-years free of new/recurrent CVD events, defined as life-years free of new or recurrent MACE, and 10-year CVD risk, defined as 10-year risk of new or recurrent MACE.
DIAL Model Overview
The DIAL model is an externally validated, competing risk–adjusted model for life expectancy free of new/recurrent CVD events with incorporation of lifetime predictions of MACE and non-cardiovascular-related mortality in people with T2D. The DIAL model was developed using data from 389,366 people with T2D in the Swedish National Diabetes Register and externally validated across geographical regions. The model was derived by fitting two Fine and Gray competing risk Cox proportional hazards models using left truncation and right censoring, thereby using age as a timescale. One model was derived for the prediction of MACE with non-CVD mortality as a competing outcome and the other model was derived for the prediction of non-CVD mortality with MACE as a competing outcome. The coefficients from these models were combined with baseline hazards with 1-year intervals to predict individual 10-year and lifetime risk of CVD using previously validated lifetable methods (21). Predictors included in the model were age, sex, smoking status, non-HDL cholesterol, systolic blood pressure, glycated hemoglobin (HbA1c), estimated glomerular filtration rate, albuminuria (defined as none, microalbuminuria, or macroalbuminuria), use of insulin, duration of T2D, and established CVD. Full details of the development and validation of the DIAL model, as well as individual risk calculations, are reported by Berkelmans et al. (19). The DIAL model is freely available and can be accessed at www.u-prevent.com.
Prediction of Individual Patient Outcomes
We estimated life expectancy free of new/recurrent CVD events and 10-year CVD risk for every individual in the POOLED cohort using the yearly patient-level risk of MACE and noncardiovascular mortality, derived from the DIAL model. Life-years free of new/recurrent CVD events were then calculated as the difference between baseline age and the age at which the estimated cumulative probability of survival free from MACE first falls below 50%. Ten-year CVD risk was calculated as the sum of the yearly risk of MACE over 10 years from patients’ current age, conditional on survival.
To estimate individual treatment benefit from adding semaglutide to SoC, the HR for MACE of 0.76 from SUSTAIN 6 and PIONEER 6 was applied to the yearly risk of MACE on an individual patient level. It was assumed that there was no effect of interaction between semaglutide treatment and established CVD and/or chronic kidney disease (CKD) on MACE because no interactions were seen in SUSTAIN 6 and PIONEER 6 (P = 0.944) (13–15). The HR for MACE was therefore assumed to be suitable for the whole population, regardless of the presence of CVD or CKD at baseline. Finally, no treatment effect from adding semaglutide to SoC on nonvascular mortality was applied because no treatment effect was shown in earlier analyses (13).
Model Validation
For details on the validation of the DIAL model, see the work by Berkelmans et al. (19). For calibration of the model, adjustments for differences in the observed versus predicted rate of MACE in the SoC-only group were applied. We then assessed the validity of the use of the DIAL model with pooled data from SUSTAIN 6 and PIONEER 6 using the C statistic for discrimination and calibration plots comparing the predicted versus observed 1-year risk of MACE according to risk quintile.
Results
Study Population
Patients in the POOLED cohort had a mean age of 65.4 years and a mean diabetes duration of 14.4 years, and 35.5% were female. Mean HbA1c was 68 mmol/mol (8.4%), and mean BMI was 32.5 kg/m2. Patients were categorized according to whether they had established CVD (72.8%) or whether they had no prior CVD but had CKD (estimated glomerular filtration rate <60 mL/min/1.73 m2 [estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation]) (10.9%) or only cardiovascular risk factors (16.3%). Patients with established CVD were younger than those with CKD or cardiovascular risk factors only, and a greater proportion were male. Baseline characteristics of the POOLED cohort can be found in Table 1.
Table 1.
Baseline characteristics, demographics, and treatment of all patients in the POOLED cohort
| Established CVD | CKD only* or CV risk factors only | POOLED cohort† | |
|---|---|---|---|
| N (%) | 4,720 (72.8) | CKD only 1,050 (16.2), CV risk factors only 710 (11.0) | 6,480 (100) |
| Age, years | 64.8 (7.6) | 66.9 (6.2) | 65.4 (7.3) |
| Sex, female, n (%) | 1,431 (30.3) | 871 (49.5) | 2,302 (35.5) |
| Diabetes duration, years | 14.2 (8.4) | 14.8 (8.1) | 14.4 (8.3) |
| Current smoker, n (%) | 604 (12.8) | 151 (8.6) | 755 (11.7) |
| BMI, kg/m2 | 32.4 (6.2) | 32.9 (6.7) | 32.5 (6.4) |
| HbA1c, mmol/mol | 68 (18) | 69 (16) | 68 (18) |
| HbA1c, % | 8.4 (1.6) | 8.5 (1.5) | 8.4 (1.6) |
| SBP, mmHg | 135 (17) | 137 (18) | 136 (17) |
| DBP, mmHg | 76 (10) | 77 (10) | 77 (10) |
| Total cholesterol, mmol/L, geometric mean (CoV) | 4.06 (28.10) | 4.35 (25.04) | 4.13 (27.41) |
| LDL cholesterol, mmol/L | 2.20 (0.92) | 2.40 (0.91) | 2.25 (0.92) |
| Non-HDL cholesterol, mmol/L | 3.10 (1.13) | 3.30 (1.09) | 3.15 (1.12) |
| Triglycerides, mmol/L | 2.07 (1.60) | 2.07 (1.56) | 2.06 (1.57) |
| eGFR, mL/min/1.73 m2‡ | 77 (21) | 70 (23) | 75 (22) |
Data are means (SD) unless otherwise indicated. CoV, coefficient of variation; CV, cardiovascular; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
eGFR <60 mL/min/1.73 m2 (estimated using the CKD-EPI creatinine equation) and no established CVD.
In total, 5,041 patients were treated with antithrombotic medication at baseline and 638 patients initiated treatment with antithrombotic medication during SUSTAIN 6 and PIONEER 6.
Estimated using the CKD-EPI creatinine equation.
Validation of the DIAL Model in the POOLED Cohort
Adjustments of the predicted rate of MACE were based on the observed 1-year rate of MACE in the SoC-only group in the POOLED cohort, which was 4.09 per 100 exposure years, and the unadjusted predicted 1-year rate of MACE, which was 4.16 per 100 exposure years. The C statistic for the predicted 2-year rate of MACE was 0.64 (95% CI 0.61–0.68) (Supplementary Fig. 1), and the plot of predicted versus observed 1-year risk of CVD according to risk quintile is shown in Supplementary Fig. 2.
CVD Benefit With Semaglutide
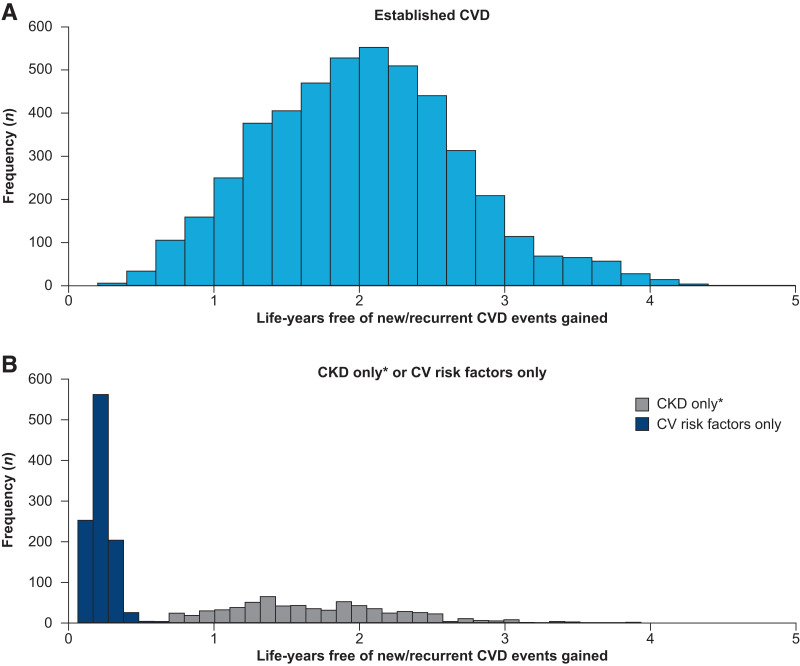
Using the DIAL model, we showed that the addition of semaglutide to SoC was associated with a wide distribution in life-years free of new/recurrent CVD events gained in the POOLED cohort (Fig. 1 and Table 2), with a mean increase of 1.7 (95% CI 0.5–2.9) life-years. In the quartile of people with T2D who were found to benefit the least, the mean estimated gain in life-years free of new/recurrent CVD events with semaglutide was 0.4. In the quartile of people with T2D who were found to benefit the most, the mean estimated gain in life-years free of new/recurrent CVD events with semaglutide was 2.8 (further details of these quartiles of patients can be found in Supplementary Table 1). Mean absolute reduction in 10-year CVD risk was 6.0% (95% CI 1.9–10.0), from 33.3% with SoC alone to 27.3% with the addition of semaglutide, corresponding to a number needed to treat of 16.6. The mean relative risk reduction (RRR) was 19.9%.
Figure 1.
Distribution of life-years free of new/recurrent CVD events gained by adding semaglutide to SoC in patients with established CVD (A) and CKD only or cardiovascular risk factors only (B). *Estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (estimated using the CKD-EPI creatinine equation) and no established CVD. CV, cardiovascular.
Table 2.
Baseline characteristics of patients in the POOLED cohort with established CVD, with stratification by the number of life-years free of new/recurrent CVD events gained by adding semaglutide to SoC
| Life-years free of new/recurrent CVD events gained | |||||
|---|---|---|---|---|---|
| 0 to <1 | 1 to <2 | 2 to <3 | 3 to <4 | 4 to <5 | |
| N | 307 | 2,031 | 2,028 | 334 | 20 |
| Age, years | 78.5 (4.1) | 69.2 (4.7) | 60.0 (4.8) | 56.3 (3.4) | 51.9 (1.2) |
| Sex, female, n (%) | 40.0 (13.0) | 431.0 (21.2) | 617.0 (30.4) | 323.0 (96.7) | 20.0 (100.0) |
| Diabetes duration, years | 20.5 (10.6) | 16.1 (8.6) | 12.0 (7.0) | 10.6 (6.8) | 10.1 (7.9) |
| Current smoker, n (%) | 24.0 (7.8) | 263.0 (12.9) | 287.0 (14.2) | 30.0 (9.0) | 0.0 (0) |
| BMI, kg/m2 | 30.9 (6.8) | 32.3 (6.4) | 32.5 (6.0) | 33.5 (5.8) | 33.8 (4.8) |
| HbA1c, mmol/mol | 66 (13) | 67 (16) | 69 (18) | 73 (19) | 71 (16) |
| HbA1c, % | 8.2 (1.2) | 8.3 (1.5) | 8.5 (1.6) | 8.8 (1.7) | 8.6 (1.5) |
| SBP, mmHg | 137.5 (20.8) | 136.3 (17.6) | 133.5 (16.2) | 132.5 (15.6) | 134.3 (12.2) |
| DBP, mmHg | 72.4 (10.6) | 75.0 (10.2) | 77.9 (9.8) | 78.7 (9.7) | 79.6 (9.5) |
| Total cholesterol, mmol/L, geometric mean (CoV) | 3.92 (24.50) | 3.94 (26.66) | 4.10 (28.60) | 4.61 (29.92) | 4.81 (28.32) |
| LDL cholesterol, mmol/L | 2.10 (0.74) | 2.10 (0.86) | 2.24 (0.93) | 2.59 (1.13) | 2.91 (1.26) |
| Non-HDL cholesterol, mmol/L | 2.91 (0.96) | 2.97 (1.05) | 3.16 (1.16) | 3.58 (1.40) | 3.77 (1.40) |
| Triglycerides, mmol/L | 1.80 (1.08) | 1.99 (1.49) | 2.15 (1.73) | 2.30 (1.86) | 1.90 (0.74) |
| eGFR, mL/min/1.73 m2* | 49.8 (16.8) | 69.3 (19.6) | 85.9 (17.1) | 95.3 (13.2) | 102.8 (9.1) |
Data are means (SD) unless otherwise indicated. CoV, coefficient of variation; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Estimated using the CKD-EPI creatinine equation.
Effect of Baseline Risk on CVD Benefit With Semaglutide
Baseline risk had a large effect on the number of life-years free of new/recurrent CVD events gained with the addition of semaglutide. Patients with established CVD had a higher baseline risk of cardiovascular events and, consequently, saw larger gains in life-years free of new/recurrent CVD events with the addition of semaglutide to SoC (2.0 years) (Fig. 1A and Table 2) compared with patients with cardiovascular risk factors only (0.2 years) (Fig. 1B); both subgroups had wide distributions in life-years free of new/recurrent CVD events gained. Patients with CKD only had a gain in life-years free of new/recurrent CVD events of 1.7 years with a similarly wide distribution.
Mean absolute reduction in 10-year CVD risk was 6.8% (RRR 19.4%) in patients with established CVD, 0.8% (RRR 23.7%) in patients with cardiovascular risk factors only, and 8.5% (RRR 17.1%) in patients with CKD only.
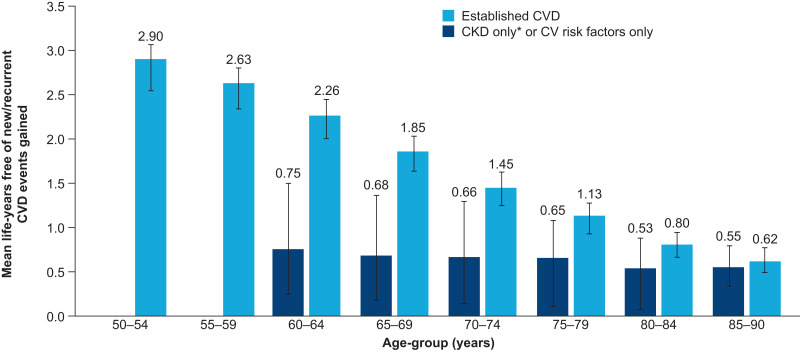
Effect of Age on CVD Benefit With Semaglutide
Age at treatment initiation also had a large effect on the number of life-years free of new/recurrent CVD events gained in patients with established CVD. In this subgroup, the mean number of life-years free of new/recurrent CVD events gained with the addition of semaglutide to SoC decreased with increasing age, from 2.9 years in patients aged 50–54 years to 0.6 years in patients aged 85–90 years (Fig. 2). A similar trend was seen in the subgroup with cardiovascular risk factors only or CKD only, although to a lesser extent.
Figure 2.
Life-years free of new/recurrent CVD events gained by adding semaglutide to SoC, by age-group. *Estimated glomerular filtration rate <60 mL/min/1.73 m2 (estimated using the CKD-EPI creatinine equation) and no established CVD. Error bars show interquartile range. CV, cardiovascular.
CVD Benefit With Semaglutide for Hypothetical Individuals With T2D
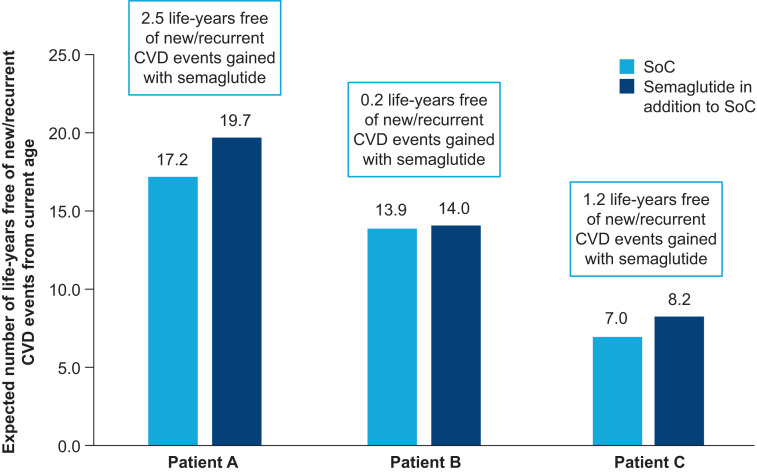
For illustration of the effect of an individual’s characteristics on the absolute benefit that they may gain with semaglutide, the number of life-years free of new/recurrent CVD events gained with semaglutide was estimated for three hypothetical individuals with T2D with different demographic and clinical characteristics. The characteristics of these patients and the number of life-years free of new/recurrent CVD events they are expected to gain with the addition of semaglutide to SoC are shown in Table 3 and Fig. 3, respectively.
Table 3.
Baseline characteristics of hypothetical individuals with T2D
| Patient A | Patient B | Patient C | |
|---|---|---|---|
| Age, years | 60 | 70 | 75 |
| Sex | Male | Female | Male |
| Diabetes duration, years | 10 | 12 | 18 |
| Current smoker | No | Yes | No |
| BMI, kg/m2 | 33 | 28 | 30 |
| HbA1c, mmol/mol | 52 | 48 | 56 |
| SBP, mmHg | 137 | 125 | 140 |
| Non-HDL cholesterol, mmol/L | 3 | 2.5 | 3.5 |
| eGFR, mL/min/1.73 m2* | 75 | 70 | 82 |
| Microalbumin | No | Yes | No |
| Macroalbumin | No | No | No |
| History of CVD | Yes | No | Yes |
| Use of insulin | No | No | Yes |
| 10-year CVD risk, % | 25.4 | 2.9 | 53.2 |
| 10-year CVD risk with the addition of semaglutide to SoC, % | 19.9 | 2.2 | 44.2 |
eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Estimated using the CKD-EPI creatinine equation.
Figure 3.
CVD benefit with semaglutide for hypothetical individuals with T2D.
Conclusions
This study is the first with use of the validated competing risk–adjusted lifetime DIAL model to contextualize the results of cardiovascular outcomes trials in people with T2D. We report that the addition of semaglutide to SoC was associated with an important gain in life-years free of new/recurrent CVD events and a de-crease in 10-year CVD risk. The distribution of estimated life-years free of new/recurrent CVD events gained with semaglutide was wide, reflecting variation in absolute benefit gained with semaglutide between patients, with those with higher baseline risk and who were younger at treatment initiation experiencing the greatest absolute benefit.
Clinical trials generate HRs showing reduction in risk with an intervention; however, they are challenging to interpret because they are based on the average patient, only show the reduction in risk over a short period of time, and do not show the absolute benefit gained. Lifetime models, such as the DIAL model, can be combined with HRs from clinical trials to help contextualize these results with estimation of the absolute benefit that an individual patient may gain from an intervention, based on the patient’s characteristics. They go beyond simple subgroup analyses, taking into account multiple patient characteristics and the risk of dying from non-CVD causes, while focusing on the lifetime perspective.
In our study, 99.8% of patients in the POOLED cohort were categorized as having very high cardiovascular risk and were therefore eligible for treatment with glucose-lowering medication with proven cardiovascular benefits, as per the current 2021 European Society of Cardiology guidelines (20). However, despite these patients all having very high cardiovascular risk and being eligible for semaglutide, there was a wide distribution in the estimated number of life-years free of new/recurrent CVD events gained with the addition of semaglutide to SoC. Younger patients are among those expected to gain the greatest lifetime benefit with the addition of semaglutide to SoC. By contrast, older patients, who have a relatively high 10-year CVD risk, often by virtue of being older, may gain less lifetime benefit from semaglutide because they are at high risk of dying from non-CVD causes and have less time to benefit. Patients with a short diabetes duration also benefitted more from the addition of semaglutide to SoC than patients with a long diabetes duration. This is likely because diabetes duration is strongly correlated with age, with patients with a shorter duration of diabetes younger than those with a longer diabetes duration. These factors are not taken into consideration in simple 10-year risk models, and, therefore, lifetime models can help in shared decision-making in discussing semaglutide as a potential intervention. What is deemed an acceptable return on investment, in terms of life-years free of new/recurrent CVD events gained over a certain number of remaining life-years, differs between patients and doctors (22) and may also be dependent on the type of intervention and expected side effects.
In this study, we found that patients with established CVD who had the greatest gain in life-years free of new/recurrent CVD events with the addition of semaglutide to SoC had a higher BMI and higher levels of HbA1c than patients who had less benefit. This highlights that the patients with highest baseline risk experienced the greatest absolute benefit with the addition of semaglutide to SoC. In this study, we looked at the lifetime CVD benefit from adding semaglutide to SoC; however, first-line treatment for the prevention of CVD in clinical practice typically includes multiple therapies to lower elevated cardiovascular risk factors. The lifetime benefit that a patient may gain from optimal cardiovascular risk management would be of interest for future analyses.
The DIAL model was developed with use of real-world data (19), increasing the applicability of the results to patients receiving treatment in routine clinical practice. One of the strengths of these analyses was that they were informed by data from many patients: nearly 400,000 patients in the Swedish National Diabetes Register and 6,480 patients receiving semaglutide in SUSTAIN 6 or PIONEER 6 (13–15). The POOLED population of our study, from SUSTAIN 6 and PIONEER 6, in the model serves as an example. It is expected that a similarly wide distribution in the absolute benefit would be seen if a different population were used, although the average benefit would differ based on the CVD risk and the life expectancy of the population. Indeed, nearly all patients in SUSTAIN 6 and PIONEER 6 had a very high risk of CVD and still a wide distribution in absolute benefit was observed. The use of data from SUSTAIN 6 and PIONEER 6 in the DIAL model was validated with comparison of observed and predicted outcomes, which were found to be broadly consistent. Considering the short study length (median 2.1 years in SUSTAIN 6 [14] and 1.3 years in PIONEER 6 [15]), a C statistic of 0.60 from the receiver operating characteristic curve showing predicted risk over a 1-year period is moderate, which we consider to be an acceptable level of validity for the DIAL model in these analyses. The C statistic was comparable with that in previous external validations of the DIAL model (0.64–0.65) for 10-year predictions (19) and for similar CVD prediction models (0.62–0.76) (23,24), with C statistics in this range being a well-known artifact for prediction models dealing with relatively low levels of risk (25). Since the predictions from the DIAL model are intended to inform the prognosis of a patient and support treatment decisions, calibration is superior to discrimination when assessing model performance (25,26). The DIAL model performed well in this regard, showing good agreement between predicted and observed risks. However, it should be acknowledged that the DIAL model has only been validated for up to 10 years and there is a need for validation of longer predictions, including lifetime predictions. However, this is not feasible with the current data from the POOLED cohort due to the restricted follow-up. The methodology underpinning the DIAL model has been validated for up to 17 years, but, nonetheless, the lifetime predictions should be interpreted with this in mind.
There were some limitations in our study. The DIAL model was informed by baseline data from the well-characterized population included in SUSTAIN 6 and PIONEER 6, who were treated as per the guidelines and can be considered an example cohort. Inclusion of a cohort from a different source will invariably show slightly different outcomes; however, the absolute gain in life expectancy free of new/recurrent CVD events with the addition of semaglutide to SoC would undoubtedly have a similarly wide distribution. In addition, ethnicity is associated with CVD risk; however, ethnicity was not included as a predictor in the original DIAL model and so we did not include it in our study. This may have led to some imprecision in the predicted life expectancy free of new/recurrent CVD events. Also, due to the shorter follow-up time of SUSTAIN 6 and PIONEER 6, we were only able to assess external validation of the DIAL model in these cohorts for up to 2 years’ predictions.
Furthermore, the limitations of the DIAL model and, in particular, lifetime predictions should be addressed. The baseline factors used for prediction in this model are subject to change over the course of a patient’s lifetime. Therefore, lifetime predictions should be performed at regular intervals (e.g., every 10 years), and the lifetime predictions should be interpreted considering this. In addition, some assumptions were made for the model, including that the patients receiving semaglutide experienced the same clinical benefit for the remainder of their lives (and therefore the HR remained constant over time). A full list of assumptions of the DIAL model, including with regard to treatment effects, can be found in Supplementary Table 2. Future advancements of lifetime prediction models should lead to more precise individual predictions.
Conclusion
The addition of semaglutide to SoC in people with T2D was associated with a wide distribution in the gain in life-years free of new/recurrent CVD events, with greater absolute benefit seen in younger people and those with established CVD. This study helps to contextualize the results of cardiovascular outcomes trials of use of semaglutide and can be used to aid in clinical decision-making.
Article Information
Acknowledgments. The authors are grateful to Helen Schofield at Oxford PharmaGenesis, Oxford, U.K., for medical writing assistance (funded by Novo Nordisk A/S).
Funding. This work was supported by Novo Nordisk A/S.
Duality of Interest. K.S.M., U.F., and M.L.W. are employees of Novo Nordisk A/S. S.N. was an employee of Novo Nordisk A/S at the time of this analysis. N.S. has consulted for Affimune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi and has also received grant support from Boehringer Ingelheim. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.W., K.S.M., S.N., U.F., M.L.W, H.B.Ø., and F.V. were involved in the design of the analysis, interpreted the results, critically revised the manuscript for important intellectual content, and gave final approval. J.W. and N.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the European Society of Cardiology Congress 2020, 29 August 2020 to 1 September 2020, and the 56th Annual Meeting of the European Association for the Study of Diabetes, 21–25 September 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19146551.
References
- 1. International Diabetes Foundation . IDF Diabetes Atlas. 7th ed. Brussels, International Diabetes Foundation, 2015 [Google Scholar]
- 2. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 2018;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SRK, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coffey JT, Brandle M, Zhou H, et al. Valuing health-related quality of life in diabetes. Diabetes Care 2002;25:2238–2243 [DOI] [PubMed] [Google Scholar]
- 5. Hayes A, Arima H, Woodward M, et al. Changes in quality of life associated with complications of diabetes: results from the ADVANCE study. Value Health 2016;19:36–41 [DOI] [PubMed] [Google Scholar]
- 6. Nichols GA, Brown JB. The impact of cardiovascular disease on medical care costs in subjects with and without type 2 diabetes. Diabetes Care 2002;25:482–486 [DOI] [PubMed] [Google Scholar]
- 7. Virtanen M, Ervasti J, Mittendorfer-Rutz E, et al. Work disability before and after a major cardiovascular event: a ten-year study using nationwide medical and insurance registers. Sci Rep 2017;7:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bethel MA, Patel RA, Merrill P, et al.; EXSCEL Study Group . Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018;6:105–113 [DOI] [PubMed] [Google Scholar]
- 9. Sattar N, McMurray JJ, Cheng AY. Cardiorenal risk reduction guidance in diabetes: can we reach consensus? Lancet Diabetes Endocrinol 2020;8:357–360 [DOI] [PubMed] [Google Scholar]
- 10. Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group . 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323 [DOI] [PubMed] [Google Scholar]
- 11. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:1117–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Husain M, Bain SC, Jeppesen OK, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab 2020;22:442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 15. Husain M, Birkenfeld AL, Donsmark M, et al.; PIONEER 6 Investigators . Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–851 [DOI] [PubMed] [Google Scholar]
- 16. Nolan T, Dack C, Pal K, et al. Patient reactions to a web-based cardiovascular risk calculator in type 2 diabetes: a qualitative study in primary care. Br J Gen Pract 2015;65:e152–e160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rossello X, Dorresteijn JA, Janssen A, et al.; This Paper Is A Co-Publication Between European Journal Of Preventive Cardiology European Heart Journal Acute Cardiovascular Care And European Journal Of Cardiovascular Nursing . Risk prediction tools in cardiovascular disease prevention: a report from the ESC Prevention of CVD Programme led by the European Association of Preventive Cardiology (EAPC) in collaboration with the Acute Cardiovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP). Eur J Prev Cardiol 2019;26:1534–1544 [DOI] [PubMed] [Google Scholar]
- 18. Dorresteijn JA, Visseren FL, Ridker PM, et al. Estimating treatment effects for individual patients based on the results of randomised clinical trials. BMJ 2011;343:d5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berkelmans GFN, Gudbjörnsdottir S, Visseren FLJ, et al. Prediction of individual life-years gained without cardiovascular events from lipid, blood pressure, glucose, and aspirin treatment based on data of more than 500 000 patients with Type 2 diabetes mellitus. Eur Heart J 2019;40:2899–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Visseren FLJ, Mach F, Smulders YM, et al.; ESC National Cardiac Societies; ESC Scientific Document Group . 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337 [DOI] [PubMed] [Google Scholar]
- 21. Dorresteijn JA, Kaasenbrood L, Cook NR, et al. How to translate clinical trial results into gain in healthy life expectancy for individual patients. BMJ 2016;352:i1548. [DOI] [PubMed] [Google Scholar]
- 22. Jaspers NEM, Visseren FLJ, Numans ME, et al. Variation in minimum desired cardiovascular disease-free longevity benefit from statin and antihypertensive medications: a cross-sectional study of patient and primary care physician perspectives. BMJ Open 2018;8:e021309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaspers NEM, Blaha MJ, Matsushita K, et al. Prediction of individualized lifetime benefit from cholesterol lowering, blood pressure lowering, antithrombotic therapy, and smoking cessation in apparently healthy people. Eur Heart J 2020;41:1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Vries TI, Eikelboom JW, Bosch J, et al. Estimating individual lifetime benefit and bleeding risk of adding rivaroxaban to aspirin for patients with stable cardiovascular disease: results from the COMPASS trial. Eur Heart J 2019;40:3771–3778a [DOI] [PubMed] [Google Scholar]
- 25. Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928–935 [DOI] [PubMed] [Google Scholar]
- 26. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]