Abstract
OBJECTIVE
Obesity is a key risk factor for type 2 diabetes; however, up to 20% of patients are normal weight. Our aim was to identify metabolite patterns reproducibly predictive of BMI and subsequently to test whether lean individuals who carry an obese metabolome are at hidden high risk of obesity-related diseases, such as type 2 diabetes.
RESEARCH DESIGN AND METHODS
Levels of 108 metabolites were measured in plasma samples of 7,663 individuals from two Swedish and one Italian population-based cohort. Ridge regression was used to predict BMI using the metabolites. Individuals with a predicted BMI either >5 kg/m2 higher (overestimated) or lower (underestimated) than their actual BMI were characterized as outliers and further investigated for obesity-related risk factors and future risk of type 2 diabetes and mortality.
RESULTS
The metabolome could predict BMI in all cohorts (r2 = 0.48, 0.26, and 0.19). The overestimated group had a BMI similar to individuals correctly predicted as normal weight, had a similar waist circumference, were not more likely to change weight over time, but had a two times higher risk of future type 2 diabetes and an 80% increased risk of all-cause mortality. These associations remained after adjustments for obesity-related risk factors and lifestyle parameters.
CONCLUSIONS
We found that lean individuals with an obesity-related metabolome have an increased risk for type 2 diabetes and all-cause mortality compared with lean individuals with a healthy metabolome. Metabolomics may be used to identify hidden high-risk individuals to initiate lifestyle and pharmacological interventions.
Introduction
The epidemic of obesity is a global health burden, resulting in 2.8 million deaths each year (1) and co-occurring with a rapid increased prevalence of type 2 diabetes. Even if obesity is the key modifiable risk factor for type 2 diabetes, up to 20% of patients with type 2 diabetes of normal weight (2). Obesity is defined by BMI, an imprecise measure that may not accurately describe the associations between obesity and its comorbidities (3). In this light, there are individuals with a hidden increased risk for obesity-related health issues despite having a normal BMI. Conversely, some individuals may have a high BMI but remain more resistant to common co-occurring pathologies, frequently referred to as metabolically healthy obesity (MHO) (4).
Metabolomics has appeared as a useful discipline in characterizing the human metabolism (5). Several studies have found associations between the plasma metabolome and obesity (6,7), highlighting potential obesity-related metabolic dysregulation. Such obesity-related metabolites include amino acids and metabolites of amino acid catabolism, lipids, and nucleotides. Several of these plasma metabolites have also shown BMI-independent associations with future risk of type 2 diabetes (8–11), cardiovascular disease (12–14), and mortality (15,16) in several prospective studies, indicating that these metabolites may have the potential to refine the definition of obesity beyond anthropometrics. Cirulli et al. (17) found that a pattern of 49 plasma metabolites was strongly associated with BMI and applied this pattern to classify individuals as obese or normal weight. Individuals who were classified as obese according to the metabolome had a twice as high risk for future cardiovascular events and more pronounced insulin resistance than BMI-matched individuals who were classified as normal weight by the metabolome (17). These findings indicate that a portion of the normal weight population is at an increased risk of obesity-related diseases, despite being characterized as normal weight according to anthropometrics.
We applied plasma metabolomics in 7,663 individuals from three population-based cohorts to robustly identify two separate strata of the population: normal weight individuals with an obese metabolome and obese individuals with a normal weight metabolome. Hypothesizing that these metabolome alterations are associated with obesity-related pathologies, we investigated whether the risk of future type 2 diabetes and all-cause mortality was different in these strata compared with normal weight and obese counterparts.
Research Design and Methods
Study Samples
Plasma levels of metabolites were measured in 7,663 individuals from two Swedish and one Italian population-based cohort. Two were cross-sectional cohorts (Malmö Offspring Study [MOS] and Cilento in Aging Outcomes Study [CIAO]) and the other a prospective cohort (Malmö Diet and Cancer Study [MDC]). The ethics committee of Lund University approved the study protocols for MOS (DNR 2012/594) and MDC (DNR 2009/633), and the Regional Board of Ethics Azienda Sanitaria Locale Napoli Sud (20171220; Naples, Italy) approved the protocols for CIAO, and all participants provided written informed consent. Participants who were diagnosed with diabetes or a BMI <18 kg/m2 were excluded from the analyses. An overview of the three cohorts and excluded participants can be found in Supplementary Fig. 1.
The MDC is a population-based prospective cohort consisting of 28,449 individuals. The cardiovascular cohort of MDC was designed to study the epidemiology of carotid artery disease, with participants being enrolled between 1991 and 1996 (18). Among the 5,405 participants with fasted blood samples, citrate plasma was available for 3,833. After applying exclusion criteria, 3,579 samples were submitted for metabolite analysis. During an average follow-up time of 18.2 years, 491 participants developed type 2 diabetes, and within 19.7 years, 967 participants died. Participants from the cardiovascular cohort of MDC were also invited to a follow-up examination between 2007 and 2012. Anthropometric measurements from the re-examination (n = 1,416) have been used to calculate longitudinal weight change in MDC.
MOS is an ongoing population-based cohort study where adult (>18 years old) children and grandchildren from the MDC study are recruited (19). Participants were invited through letter and visited the research clinic where overnight fasting EDTA plasma samples were collected and anthropometric measurements performed. Plasma samples were available for 3,430 participants, and metabolomic analysis was performed on 3,263 samples after application of exclusion criteria.
CIAO is a population-based cohort from the area of Cilento in the Campania region of South Italy. A random sample of middle-aged (50–67 years old) individuals from Cilento were invited through their local primary health care providers (20). A total of 935 individuals had overnight-fasted EDTA plasma samples available, and metabolomic analysis was performed on 821 samples after application of exclusion criteria.
End Point Definitions, Biochemical Measurements, and Lifestyle Assessments
End point retrieval was performed through record linkage of the personal identification number of each Swedish citizen with Swedish local or national registries (21). Type 2 diabetes was defined as a fasting plasma glucose of >7.0 mmol/L or a history of physician diagnosis of type 2 diabetes, being on antidiabetic medication, or having been registered in local or national Swedish diabetes registries (22). Methods for biochemical measurements and assessment of dietary intakes and physical activity are found in the Supplementary Material.
Analytical Procedure
Profiling of plasma metabolites was performed using a ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry system (1290 LC, 6550 MS; Agilent Technologies, Santa Clara, CA) and has previously been described in detail (23). Briefly, plasma samples stored at −80°C were thawed and extracted by the addition of 6 volumes of extraction solution. The extraction solution consisted of 80:20 methanol/water. Extracted samples were separated on an ACQUITY UPLC BEH Amide Column (1.7 μm, 2.1 ∗ 100 mm; Waters Corporation, Milford, MA). Information about quality control, normalization, metabolite annotation, missing values, and measures of technical variation is found in the Supplementary Material.
Statistical Analysis
In each cohort, metabolite levels were mean centered and unit variance scaled. Outliers were defined as >5 SD units away from the mean and were imputed as either −5 or 5 (1.7% in total). BMI was modeled using ridge regression with the R package glmnet (version 3.0-2) (24). Metabolome-predicted BMI is referred to hereafter as metabolic BMI (mBMI). The λ-parameter was optimized using cv.glmnet, minimizing the mean squared error, varying λ between 1,000 and 0.01 (Supplementary Fig. 2). Model training was performed in 80% randomly selected participants from MOS, and validation was performed in the remaining 20%. The model was replicated in MDC and CIAO. Prediction of obesity and overweight was assessed using the receiver operating characteristic area under the curve (AUC). BMI was mean centered, and unit variance was scaled for ridge regression modeling. Participants whose BMI could be predicted within an error margin of 5 kg/m2 (mBMI-BMI −5 kg/m2 to 5 kg/m2) were characterized according to their BMI as normal weight (NW) (BMI <25 kg/m2), overweight (OW) (BMI 25–30 kg/m2), or obese (OB) (BMI >30 kg/m2). Participants whose BMI was not predicted within the error margin were either classified as overestimated (OE) (mBMI-BMI >5 kg/m2) or underestimated (UE) (mBMI-BMI less than −5 kg/m2). Levels for traditional risk factors and of all metabolites were compared among BMI classes using ANOVA and Tukey post hoc test. Prospective analyses of weight change, type 2 diabetes, and all-cause mortality was performed in MDC. The association between BMI class and weight change was analyzed using ANOVA, while the associations between BMI class and incident type 2 diabetes and all-cause mortality were analyzed using Cox proportional hazards regression models. Regression model 1 was adjusted for age and sex, and model 2 was additionally adjusted for fasting levels of glucose, triglycerides, and HDL cholesterol and smoking status. Differences in dietary intakes and physical activity levels between BMI classes were analyzed using ANOVA and linear regression. All statistical analyses were performed using R version 3.6.1 software. ANOVA was performed using aov, Tukey post hoc tests using TukeysHSD, AUC using pROC, regression modeling using survival, and data visualizations using ggplot2. Scripts are publicly available at https://github.com/immu-flo/metabolome_BMI.
Results
There were several differences among the three investigated cohorts. Most notably, MOS comprised participants from the age of 18 to 70 years, while the participants in both MDC and CIAO were middle-aged (50–65 years old). There were also differences in several metabolic risk factors among the cohorts, such as BMI and fasting glucose levels (Table 1).
Table 1.
Characteristics of the participants in the MOS, MDC, and CIAO
| MOS (n = 3,263) | MDC (n = 3,579) | CIAO (n = 821) | P | |
|---|---|---|---|---|
| Age (years) | 41.59 (14.11) | 57.59 (6.00) | 57.76 (4.53) | <0.001 |
| Female sex (%) | 52.4 | 59.3 | 56.8 | <0.001 |
| BMI (kg/m2) | 26.02 (4.63) | 25.56 (3.72) | 28.08 (5.39) | <0.001 |
| Waist circumference (cm) | 89.94 (13.69) | 82.97 (12.32) | 96.46 (69.58) | <0.001 |
| Glucose (mmol/L) | 5.32 (0.57) | 4.96 (0.52) | 5.58 (0.65) | <0.001 |
| HDL (mmol/L) | 1.62 (0.48) | 1.40 (0.37) | 1.56 (0.39) | <0.001 |
| Triglycerides (mmol/L) | 1.12 (0.67) | 1.28 (0.61) | 1.35 (0.78) | <0.001 |
| Current smoker (%) | 14.0 | 27.5 | 23.9 | <0.001 |
Data are mean (SD) unless otherwise indicated.
A Wide Range of Metabolites Is Associated With Obesity
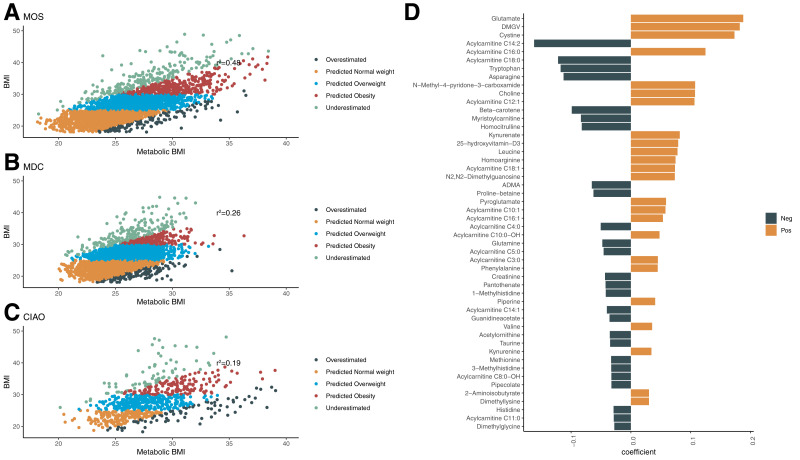
We used 108 plasma metabolites to find associations with BMI in the 7,663 participants from the three investigated cohorts. A ridge regression model was trained in a subset of 80% randomly selected participants from MOS (n = 2,611, BMI 26.0 ± 4.6 kg/m2). In the validation set, comprising the remaining 20% (n = 652, BMI 26.0 ± 4.7 kg/m2), the ridge regression model could explain approximately one-half of the variation of BMI (r2 = 0.476) (Fig. 1A). The model predicted both OB (AUC 0.936) and OW (AUC 0.803) compared with NW. The model was validated in MDC and CIAO and was able to predict BMI with good accuracy (MDC: r2 = 0.256; CIAO: r2 = 0.196) (Fig. 1B and C). In both MDC and CIAO, the model could predict OB (AUC 0.86 and 0.86, respectively) and OW (AUC 0.71 and 0.70, respectively) compared with NW.
Figure 1.
Metabolite prediction of BMI. Correlation between ridge regression–based prediction of BMI (mBMI) and BMI in MOS (n = 3,263) (A), MDC (n = 3,579) (B) and CIAO (n = 821) (C). The outlier groups were defined as having an mBMI >5 kg/m2 higher than BMI (OE) or an mBMI <5 kg/m2 lower than BMI (UE). The remaining participants were categorized according to their BMI as NW (<25 kg/m2), OW (25–30 kg/m2), and OB (>30 kg/m2). The coefficients of the top 30 metabolites in the ridge regression model are shown (D). Neg, negative; Pos, positive.
Metabolites of different biochemical classes contributed to the OB-predictive model, such as amino acids (glutamate, cystine, and tryptophan), acylcarnitines (C14:2 and C18:0), nucleotides (N2,N2-dimethylguanosine), and food-derived metabolites (β-carotene and proline betaine) (Fig. 1D). The metabolites that contributed most to the model in a positive direction were dimethylguanidino valerate (DMGV) and glutamate and in the negative direction, acylcarnitines C14:2 and C18:2.
Outliers of Metabolome-Predicted BMI Have Different Levels of Circulating Lipids
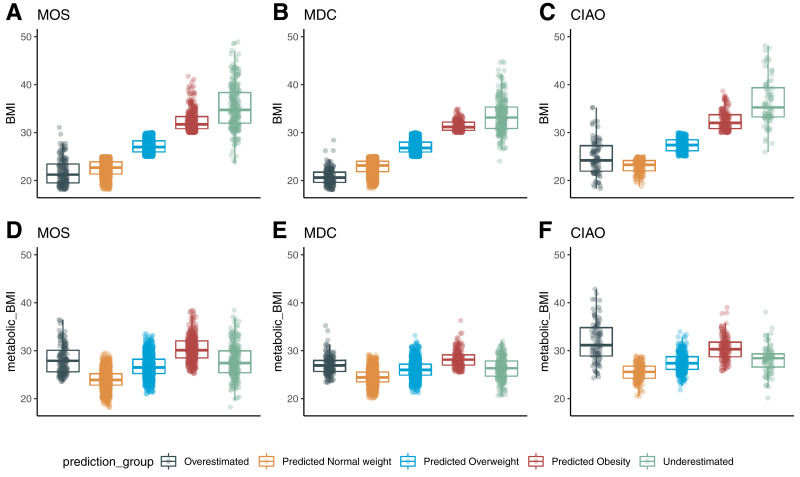
Outliers of the BMI prediction (mBMI), with an error margin >5 kg/m2, were either classified as OE (mBMI > BMI) or UE (mBMI < BMI). In MOS, 88.7% (n = 2,894) had a BMI predicted within the error margin, 4.1% were classified as OE (n = 133), and 7.2% were classified as UE (n = 236). A similar proportion of participants were predicted within the error margin in MDC (90.1%), while 3.8% were characterized as OE (n = 132) and 6.1% as UE (n = 210). In CIAO, the proportion of participants predicted within the error margin was slightly lower (81.6%), while 9.7% were OE (n = 80) and 8.6% were UE (n = 71).
The average BMI in the OE groups (MOS 21.8 kg/m2, MDC 20.8 kg/m2, CIAO 24.9 kg/m2) was similar to NW in all three cohorts, while the average BMI of the UE groups (MOS 35.4 kg/m2, MDC 33.6 kg/m2, CIAO 37.9 kg/m2) was similar to OB. Despite this large difference in BMI between OE and UE, the mBMI in OE (MOS 27.0 kg/m2, MDC 28.1 kg/m2, CIAO 32.0 kg/m2) and UE (MOS 27.7 kg/m2, MDC 26.2 kg/m2, CIAO 28.2 kg/m2) was similar to each other and more similar to OW or OB (Fig. 2A–F). There were no differences in waist circumference between OE and NW in either MOS (P = 0.49) or MDC (P = 0.17). In CIAO, the waist circumference in OE was 8 cm larger than in NW (P < 0.001). Comparing the waist circumference of UE and OB, there were no differences in MDC (P = 0.83), and in both MOS (difference 2.6 cm, P = 0.003) and CIAO (difference 5 cm, P = 0.003), the waist circumference in UE was slightly larger than in OB (Supplementary Fig. 3).
Figure 2.
Differences in BMI (kg/m2) (A–C) and mBMI (kg/m2) (D–F) between mBMI outlier groups. Results from the MOS (n = 3,263) are shown in A and D, the MDC (n = 3,579) in B and E, and CIAO (n = 821) in C and F.
Plasma triglycerides were significantly higher in OE than NW and lower in UE than OB in all three investigated cohorts (Supplementary Fig. 3). For HDL cholesterol, levels were significantly lower in OE than NW and higher in UE than OB in both MOS and CIAO, but no differences were observed in MDC (Supplementary Fig. 4). No differences were seen in fasting glucose levels between OE and NW in any cohort, and slightly lower levels in UE than OB were seen only in CIAO (Supplementary Fig. 4). In MOS and MDC, OE had higher proportions of smokers than NW (Pdiff < 0.001). The proportion of smokers in OE was 24.6, 47.2, and 26.0% compared with 13.3, 30.9, and 24.7% in NW in MOS, MDC, and CIAO, respectively. Group-wise differences are found in Supplementary Tables 1–3.
Metabolite-Level Differences in Outliers of Metabolic Obesity Are Consistent Among Cohorts
In MOS, the levels of 13 metabolites were significantly different (P < 4.6e-4) among participants classified as OE and NW. These included several of the metabolites that influence mBMI, such as DMGV, glutamate, and β-carotene. All 13 metabolites had at least nominally significant differences, in a consistent direction, between NW and OE in at least two of the three investigated cohorts. For five metabolites (DMGV, glutamate, β-carotene, isoleucine, and kynurenine), significant (P < 0.05) differences were found in all cohorts (Supplementary Fig. 5 and Supplementary Table 4).
Normal Weight Individuals With Metabolic Obesity Are at Higher Risk of Future Diabetes Mortality
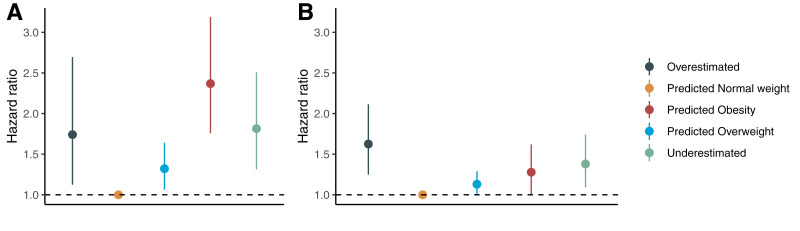
Next, we wanted to investigate whether being an outlier of mBMI is associated with risk of future obesity, diabetes, and death. Prospective analyses of obesity, type 2 diabetes, and mortality were performed in the MDC cohort. During an average follow-up of 15.6 years, no differences in weight change were observed among any of the five investigated groups (Supplementary Fig. 6 and Supplementary Table 5). More specifically, no significant differences in weight change were seen between baseline and follow-up between OE and NW (P = 0.98). However, during an average follow-up time of 18.5 years, participants in OE had more than a twofold higher risk of developing type 2 diabetes (hazard ratio [HR] per SD 2.22, 95% CI 1.38–3.56, P = 0.001) compared with NW. This association was attenuated but remained significant when adjusting for smoking status and fasting levels of glucose, triglycerides, and HDL cholesterol. In the adjusted model, there were no significant differences in risk of future type 2 diabetes between OE and any of the other groups (Fig. 3 and Supplementary Table 6).
Figure 3.
Associations between outlier groups of mBMI and future risk of diabetes (A) and mortality (B) in the MDC (n = 3,579). The HRs are expressed compared with the reference group (predicted NW). Error bars correspond to 95% CIs. HRs are calculated from Cox proportional hazards regression models adjusted for age, sex, smoking status, and fasting glucose, HDL cholesterol, and triglyceride levels.
Participants in OE compared with NW were also at almost twice as high a risk of all-cause mortality within an average follow-up time of 19.7 years (HR 1.85, 95% CI 1.50–2.05, P < 0.001). OE was also associated with an increased risk of all-cause mortality compared with OW (HR 1.72, 95% CI 1.30–2.29, P = 0.0002) and OB (HR 1.56, 95% CI 1.09–2.24, P = 0.015). After additional adjustments for smoking status and levels of glucose, triglycerides, and HDL cholesterol, these associations were attenuated but remained statistically significant with regard to OE versus NW and OW (Fig. 3 and Supplementary Table 6). Participants in OB had a 53% increased risk of type 2 diabetes compared with UE (HR 1.53, 95% CI 1.08–2.20, P = 0.017) but not an increased risk of all-cause mortality (HR 0.84, 95% CI 0.60–1.18, P = 0.32). The association with type 2 diabetes was attenuated after adjustments for confounders (HR 1.31, 95% CI 0.91–1.87, P = 0.14) (Supplementary Table 6).
Lifestyle Factors Associated With Metabolic Obesity
Using dietary assessments in MOS (n = 1,526) and MDC (n = 3,471), we tested whether seven dietary intakes differed among the five BMI prediction groups. In MDC, the diet of OE consisted of significantly (P < 0.05) more saturated fat and fewer fruits and vegetables (Supplementary Fig. 7) and whole grains than NW. No differences in intakes were seen for polyunsaturated fats, fish and shellfish, meat, or added sugar. The diet of UE consisted of higher amounts of fruits and vegetables and lower amounts of polyunsaturated fats than OB (Supplementary Table 7). In MOS, the diet of OE had significantly (P < 0.05) lower amounts of fruits and vegetables than NW (Supplementary Fig. 7), but no differences were seen for any other examined dietary factor. The differences in intakes of fruits and vegetables between NW and OE were significant after adjustments for age, sex, and smoking status in both MDC (β = 6.6, 95% CI 2.9–6.3, P = 4.4e-4) and MOS (β = 8.9, 95% CI 3.6–14.1, P = 1.0e-3). No differences were seen between UE and OB in dietary intakes in MOS (Supplementary Table 7). Self-assessed levels of leisure time physical activity were significantly (P < 0.05) different between OE and NW but not between UE and OB in MOS, but no significant differences were seen in MDC (Supplementary Table 8). In sensitivity analysis in MDC (n = 3,472), the risk for type 2 diabetes and all-cause mortality in OE compared with NW was only marginally changed after adjustments for intakes of fruits and vegetables, whole grains, saturated fats, and leisure time physical activity (Supplementary Table 9).
Conclusions
Using the metabolome of 7,663 individuals, the current study identifies a metabolite fingerprint of BMI (mBMI) and investigates the risk of type 2 diabetes and mortality in individuals whose mBMI differs from their actual BMI. Middle-aged individuals who are normal weight but have an mBMI >5 kg/m2 above their actual BMI have a doubled risk of future type 2 diabetes and all-cause mortality compared with individuals who are normal weight both according to BMI and mBMI. In this study, we attempted to refine the definition of obesity by identifying strata of the population who, based on their metabolome, have different risks of type 2 diabetes and death compared with what their BMI indicates.
The association between the metabolome and obesity has been described in detail in several population-based cohorts (6,17,25,26). Consequently, most metabolites that strongly influence mBMI in this study have documented associations with BMI or waist circumference in previous publications. For instance, these include DMGV (27,28), glutamate (29), and the branched-chain amino acids leucine and valine (25). Moreover, these metabolites have been associated with an increased risk of future type 2 diabetes (10,11,14,27,30). The associations between metabolites and BMI were relatively consistent across the three investigated cohorts. mBMI explained 47% of the variation of BMI in the validation set of MOS. Despite the model being trained in MOS, it was able to explain a large portion of the variation of BMI (19–26%) in MDC and CIAO, indicating that the BMI prediction model is not specific to the population of MOS but, rather, is generalizable to independent cohorts with different characteristics.
The current study was conducted using data from a relatively limited number of 108 metabolites. Applying similar strategies using a larger number of metabolites from a wide range of biochemical classes should improve prediction. For instance, in Cirulli et al. (17), extending a model using the 49 metabolites with the strongest associations with BMI to a model containing 650 metabolites improved the explained variation of BMI from 39 to 49%. Among the 49 metabolites, several were also important predictors of BMI in the current study. Most notably, these included glutamate, asparagine, leucine, N2,N2-dimethylguanosine, and kynurenate. Although it is reasonable that a larger number of metabolites should result in an improved prediction, the explained variation of BMI in the current study ranged from 19 to 47%, which is in line with previous studies reporting a range from 23 to 49% (7,17).
A subset of the participants (4.1–9.7%) were classified as OE (mBMI at least 5 kg/m2 above their actual BMI). The risk for future type 2 diabetes was twice as high in OE compared with NW, despite OE having slightly lower average BMI. In line with previous findings (17), individuals in OE compared with NW had higher levels of triglycerides and lower levels of HDL cholesterol. Fasting glucose levels were, however, not significantly different between the groups. There was still a significant difference in type 2 diabetes risk between OE and NW after adjustments for these potential confounders, although the association was slightly attenuated. Similarly, the risk of all-cause mortality was 80% higher in OE than NW, an association that also remained significant after adjustments for potential confounders. We argue that characterizing this subset of the population may be clinically relevant for two separate reasons. First, this subset of the population is at high risk of type 2 diabetes and premature mortality but may likely be missed by conventional methods because of their normal BMI and nonelevated fasting glucose levels. This is further stressed by our finding that OE does not differ in weight gain over time compared with NW. Thus, the mBMI could help to pinpoint this hidden high-risk subset of the population, motivating lifestyle and pharmacological interventions. Importantly, since mBMI can identify increased risk of type 2 diabetes and mortality up to 20 years before the event, intervention strategies could be implemented early enough to potentially reach a substantial risk reduction. Second, alterations in metabolite levels in OE compared with NW could highlight metabolic pathways involved in the pathological process of type 2 diabetes. In the current study, the five metabolites that consistently differed between OE and NW (i.e., DMGV [27,30], glutamate [31,32], β-carotene [10], isoleucine [11,32], and kynurenine) have previously been associated with a future risk of type 2 diabetes. Studies have indicated a causal link between branched-chain amino acids, such as isoleucine, and diabetes by using Mendelian randomization (9). Moreover, DMGV is considered to be an early marker for cardiometabolic dysfunction and is associated with attenuated metabolic improvements of exercise training (28). If a causal link between the metabolites and either type 2 diabetes or mortality risk can be proven, such metabolites could be potential pharmacological target molecules. This strategy could be particularly efficient for OE, since weight reduction per se is unlikely to be effective.
Individuals classified as OE consumed significantly fewer fruits and vegetables and had a higher prevalence of smoking than those classified as NW. These factors themselves do not explain the difference in disease risk between OE and NW, since the associations with type 2 diabetes and mortality remained significant after adjustments. Given that lifestyle factors are strongly correlated with one another, it is possible that the differences between NW and OE in smoking status and dietary intakes of fruits and vegetables may be explained by a generally unhealthy lifestyle, typical for individuals classified as OE.
The proportion of participants classified as UE (mBMI at least 5 kg/m2 below their actual BMI) was between 6.1 and 8.6%. Similar to the comparison between OE and NW, the risk of developing type 2 diabetes was 50% higher in OB than UE, despite that the average BMI was 2–5 kg/m2 higher in UE. This association was attenuated after adjustments for confounders. The phenomenon that a portion of the obese population may be protected from obesity-related pathologies, commonly referred to as MHO, have been discussed extensively elsewhere, and several definitions have been suggested (4,33). Our study suggests that defining MHO on the basis of metabolomics may be an alternative. Levels of type 2 diabetes–related metabolites, such as DMGV, isoleucine, and leucine, were different between UE and OB. This is consistent with a lower risk of type 2 diabetes in MHO (34), suggesting that these metabolites may help to characterize the metabolic difference between MHO and metabolically unhealthy obesity and explain their different clinical prospects.
This study has several limitations. First, although we show that the association between the metabolome and obesity is consistent across different cohorts, major hurdles remain to reach clinical utility, including absolute quantification of metabolite levels, which is needed to determine the mBMI clinically. Second, the outlier classifications were arbitrarily determined as mBMI 5 kg/m2 above or below the actual BMI and may not be the most clinically relevant classification. Third, since metabolites only were measured at one time point, we could not evaluate the stability of the mBMI classifications over time. Finally, prospective analyses could only be performed in one of the investigated cohorts, which calls for replication of the association between mBMI and type 2 diabetes and mortality in other populations to confirm its validity.
Article Information
Acknowledgments. The authors thank the participants in MOS, MDC, and CIAO for making this research possible.
Funding. This work was supported by the Swedish Foundation for Strategic Research (IRC LUDC), Vetenskapsrådet (VR) (Strategic Research Area Excellence in Diabetes Research in Diabetes [ExoDiab]), Artificially Intelligent Use of Registers at Lund University (AIR Lund) research environment (VR grant 2019-61406), Lund University infrastructure grants for population-based cohorts and metabolomics platforms (STYR 2019/2046), European Research Council advance grant 2019-885003, Novo Nordisk Foundation grant NNF200C0063465, VR grant 2018-02760, Swedish Heart and Lung Foundation grant Dnr 20180278, Ernhold Lundstrom Research Foundation, Hulda and E Conrad Mossfelts Foundation, and the Albert Pahlsson Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. F.O. contributed to the study design, acquisition and interpretation of data, and statistical analyses and drafted the manuscript. E.S. and U.E. contributed to the acquisition and interpretation of data. L.B., M.O.-M., S.D.S., P.A., P.M.N., and C.F. contributed to the study concept and design. O.M. contributed to the study concept and design, interpretation of data, and statistical analyses. All authors made intellectual contributions to drafting and/or revising the manuscript and approved the final version. O.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity for the data and the accuracy of the data analysis.
Prior Presentation. A non–peer-reviewed version of this article was submitted to the MedRxiv preprint server (https://www.medrxiv.org/content/10.1101/2021.11.03.21265744v1) on 3 November 2021.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19196900.
References
- 1. World Health Organization . Obesity. Accessed 30 August 2021. Available from https://www.who.int/news-room/facts-in-pictures/detail/6- facts-on-obesity
- 2. Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012;308:581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation 2018;137:1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord 2013;14:219–227 [DOI] [PubMed] [Google Scholar]
- 5. Patti GJ, Yanes O, Siuzdak G. Innovation: metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol 2012;13:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ho JE, Larson MG, Ghorbani A, et al. metabolomic profiles of body mass index in the Framingham Heart Study reveal distinct cardiometabolic phenotypes. PLoS One 2016;11:e0148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kliemann N, Viallon V, Murphy N, et al. Metabolic signatures of greater body size and their associations with risk of colorectal and endometrial cancers in the European Prospective Investigation into Cancer and Nutrition. BMC Med 2021;19:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez C, Surma MA, Klose C, et al. Plasma lipidome and prediction of type 2 diabetes in the population-based Malmö Diet and Cancer Cohort. Diabetes Care 2020;43:366–373 [DOI] [PubMed] [Google Scholar]
- 9. Lotta LA, Scott RA, Sharp SJ, et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med 2016;13:e1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ottosson F, Smith E, Gallo W, Fernandez C, Melander O. Purine metabolites and carnitine biosynthesis intermediates are biomarkers for incident type 2 diabetes. J Clin Endocrinol Metab 2019;104:4921–4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ottosson F, Emami Khoonsari P, Gerl MJ, Simons K, Melander O, Fernandez C. A plasma lipid signature predicts incident coronary artery disease. Int J Cardiol 2021;331:249–254 [DOI] [PubMed] [Google Scholar]
- 13. Würtz P, Havulinna AS, Soininen P, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation 2015;131:774–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng Y, Hu FB, Ruiz-Canela M, et al. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvención con DIeta MEDiterránea (PREDIMED) trial. J Am Heart Assoc 2016;5:e003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng S, Larson MG, McCabe EL, et al. Distinct metabolomic signatures are associated with longevity in humans. Nat Commun 2015;6:6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deelen J, Kettunen J, Fischer K, et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat Commun 2019;10:3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cirulli ET, Guo L, Leon Swisher C, et al. Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metab 2019;29:488–500.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosvall M, Janzon L, Berglund G, Engström G, Hedblad B. Incident coronary events and case fatality in relation to common carotid intima-media thickness. J Intern Med 2005;257:430–437 [DOI] [PubMed] [Google Scholar]
- 19. Brunkwall L, Jönsson D, Ericson U, et al. The Malmö Offspring Study (MOS): design, methods and first results. Eur J Epidemiol 2021;36:103–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melander O, Antonini P, Ottosson F, et al. Comparison of cardiovascular disease and cancer prevalence between Mediterranean and north European middle-aged populations (The Cilento on Ageing Outcomes Study and The Malmö Offspring Study). Intern Emerg Med 2021;16:1567–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish National Inpatient Register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Enhörning S, Sjögren M, Hedblad B, Nilsson PM, Struck J, Melander O. Genetic vasopressin 1b receptor variance in overweight and diabetes mellitus. Eur J Endocrinol 2016;174:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ottosson F, Ericson U, Almgren P, et al. Postprandial levels of branch chained and aromatic amino acids associate with fasting glycaemia. J Amino Acids 2016;2016:8576730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010;33:1–22 [PMC free article] [PubMed] [Google Scholar]
- 25. Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ottosson F, Brunkwall L, Ericson U, et al. Connection between BMI-related plasma metabolite profile and gut microbiota. J Clin Endocrinol Metab 2018;103:1491–1501 [DOI] [PubMed] [Google Scholar]
- 27. Ottosson F, Ericson U, Almgren P, et al. Dimethylguanidino valerate: a lifestyle-related metabolite associated with future coronary artery disease and cardiovascular mortality. J Am Heart Assoc 2019;8:e012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robbins JM, Herzig M, Morningstar J, et al. Association of dimethylguanidino valeric acid with partial resistance to metabolic health benefits of regular exercise. JAMA Cardiol 2019;4:636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125:2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Sullivan JF, Morningstar JE, Yang Q, et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest 2017;127:4394–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peddinti G, Cobb J, Yengo L, et al. Early metabolic markers identify potential targets for the prevention of type 2 diabetes. Diabetologia 2017;60:1740–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ottosson F, Smith E, Melander O, Fernandez C. Altered asparagine and glutamate homeostasis precede coronary artery disease and type 2 diabetes. J Clin Endocrinol Metab 2018;103:3060–3069 [DOI] [PubMed] [Google Scholar]
- 33. Korduner J, Bachus E, Jujic A, Magnusson M, Nilsson PM. Metabolically healthy obesity (MHO) in the Malmö diet cancer study - epidemiology and prospective risks. Obes Res Clin Pract 2019;13:548–554 [DOI] [PubMed] [Google Scholar]
- 34. Tajik S, Mirzababaei A, Ghaedi E, Kord-Varkaneh H, Mirzaei K. Risk of type 2 diabetes in metabolically healthy people in different categories of body mass index: an updated network meta-analysis of prospective cohort studies. J Cardiovasc Thorac Res 2019;11:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]