Abstract
OBJECTIVE
To assess the incidence of remission of type 2 diabetes in routine care settings.
RESEARCH DESIGN AND METHODS
People with type 2 diabetes (HbA1c ≥48 mmol/mol [6.5%] or <48 mmol/mol [6.5%] with a prescription for glucose-lowering medications) alive on 1 April 2018 were identified from a national collation of health records in England and followed until 31 December 2019. Remission was defined as two HbA1c measurements of <48 mmol/mol (6.5%) at least 182 days apart, with no prescription for glucose-lowering medications 90 days before these measurements.
RESULTS
In 2,297,700 people with type 2 diabetes, the overall incidence of remission per 1,000 person-years was 9.7 (95% CI 9.6–9.8) and 44.9 (95% CI 44.0–45.7) in 75,610 (3.3%) people who were diagnosed <1 year. In addition to shorter duration of diagnosis, baseline factors associated with higher odds of remission were no prescription for glucose-lowering medication, lower HbA1c and BMI, BMI reduction, White ethnicity, female sex, and lower socioeconomic deprivation. Among 8,940 (0.4%) with characteristics associated with remission (diagnosed <2 years, HbA1c <53 mmol/mol [7.0%], prescribed metformin alone or no glucose-lowering medications, BMI reduction of ≥10%), incidence of remission per 1,000 person-years was 83.2 (95% CI 78.7–87.9).
CONCLUSIONS
Remission of type 2 diabetes was generally infrequent in routine care settings but may be a reasonable goal for a subset of people who lose a significant amount of weight shortly after diagnosis. Policies that encourage intentional remission of type 2 diabetes should seek to reduce the ethnic and socioeconomic inequalities identified.
Introduction
The prevalence of diagnosed and undiagnosed diabetes in adults in England is estimated to be 8.6% and is projected to rise to 9.7% by 2035 (1). Type 2 diabetes accounts for 90–95% of people with diabetes (2). Historically, type 2 diabetes was considered to be a lifelong progressive condition, but there is now clear evidence that remission of type 2 diabetes is feasible following intensive lifestyle interventions (3–5) or bariatric surgery (6–9). However, successful lifestyle interventions are of high intensity and have, as yet, unclear long-term sustainability and scalability in the real-world setting. Similarly, bariatric surgery is only indicated for a small proportion of people with type 2 diabetes.
We are not aware of any population-based studies that have assessed the incidence of remission of type 2 diabetes in routine clinical care, and the recent consensus report on the definition and interpretation of remission in type 2 diabetes highlights the need for evidence on the frequency and duration of remission (10). It remains unclear what the incidence of remission is for people diagnosed with type 2 diabetes in routine care settings. As one of the few national registries of type 2 diabetes, the National Diabetes Audit (NDA) provides an opportunity to quantify the incidence of diabetes remission in routine care. The aim of this study was to determine the incidence of remission over a follow-up period of 21 months and which characteristics predict remission from type 2 diabetes in the English population.
Research Design and Methods
Data Sources
The NDA has collated data on people with diagnosed diabetes registered with a primary or specialist health care provider in England since 2003 (11). Individuals are included if they have a valid diagnostic code for diabetes (excluding gestational diabetes mellitus) in their electronic health record. Demographic and clinical data are extracted from general practice electronic clinical systems using the General Practice Extraction Service (a national centralized data collection service for England) supplemented by data submitted by specialist diabetes services. The data collated by the NDA from primary care and specialist services includes all HbA1c measurements and prescriptions for glucose-lowering medications. Data from the NDA are linked using unique National Health Service (NHS) numbers to Hospital Episode Statistics (HES), which records all hospital admissions in England and death registrations collated by the Office for National Statistics. In 2017/2018, the NDA collected data from 98.2% of general practices in England (12).
Study Population and Observation Period
The study population was people with a documented diagnosis of type 2 diabetes registered with a health care provider in England and included in the 2017/2018 NDA data collection and still alive on 1 April 2018. The cohort was followed to 31 December 2019. Those who did not have a valid HbA1c measurement between 1 April 2017 and 31 March 2018 (n = 207,295, 7.3%) were excluded from the cohort. People with an HbA1c measurement between 1 April 2017 and 31 March 2018 <48 mmol/mol (6.5%) with no prescriptions for glucose-lowering medication over the same period were also excluded from the cohort (n = 339,440, 12.9%) because they may have already been in remission from type 2 diabetes and therefore could not become incident cases over the period of observation. The resulting cohort was people with type 2 diabetes who did not meet the criteria for remission at the start of the follow-up period (1 April 2018) with either an HbA1c of ≥48 mmol/mol (6.5%) irrespective of prescription for glucose-lowering medications or an HbA1c <48 mmol/mol (6.5%) and one or more prescriptions for glucose-lowering medications in the 90 days before HbA1c measurement at the start of follow-up.
The latest recorded type of diabetes was assumed to be most accurate. Where data for an individual were provided by both primary care and a specialist diabetes service, the type of diabetes identified by the specialist service was assumed to be more accurate (13). For example, an individual with a diagnostic code for type 2 diabetes in general practice but a code for type 1 diabetes from a specialist provider in the same year would be considered to have type 1 diabetes and excluded from the cohort. People who had a hospital admission for cystic fibrosis (ICD-10 code E84, n = 610) between 1 January 2003 and 31 December 2019 and those who had a hospital admission for pancreatic disease (ICD-10 codes K85–86, n = 21,165) between 1 January 2003 and date of diagnosis of diabetes were excluded because they may have been inaccurately categorized as having type 2 diabetes. Five individuals whose sex was not recorded as male or female were also excluded.
Outcome
Remission was defined as at least two HbA1c measurements <48 mmol/mol (6.5%) at least 26 weeks apart (without any intervening HbA1c measurements of ≥48 mmol/mol [6.5%]) and no record of prescription for glucose-lowering medications between these measurements and at least 90 days before each measurement (14). The date of remission was taken as the date of the second relevant HbA1c. A sensitivity analysis was undertaken using a single HbA1c measurement <48 mmol/mol (6.5%) in the absence of a prescription for glucose-lowering medications in the preceding 90 days as the definition of remission. Where people met the criteria for remission of type 2 diabetes, HbA1c measurements after entering remission were grouped into those who continued to meet the criteria for remission and those who were subsequently in the diabetes range (≥48 mmol/mol [6.5%], <48 mmol/mol [6.5%] with a prescription for glucose-lowering medications in the previous 90 days). Systemized Nomenclature of Medicine codes 703136005 (diabetes mellitus in remission) and 703138006 (type 2 diabetes mellitus in remission) were identified as being coded as in remission in order to assess the number and proportion of people meeting the definition of remission described above who had a record of remission in their records.
Exposures
Age on 31 March 2018 was calculated based on date of birth. Self-reported ethnicity was grouped into White, mixed, Asian, Black, other, and missing. Socioeconomic deprivation was measured using the Indices of Multiple Deprivation 2019 (15) on the basis of home postcode and stratified into quintiles. Duration of diagnosed diabetes on 31 March 2018 was calculated on the basis of the date of diagnosis recorded in the electronic health record. The lowest HbA1c and BMI recorded between 1 April 2017 and 31 March 2018 were identified and defined as baseline measurements. For individuals who entered remission, change in BMI was calculated as the difference between baseline BMI and the measurement within 90 days closest to the date of entering remission (the second HbA1c measurement <48 mmol/mol [6.5%]). Change in BMI for people who did not enter remission was calculated as the difference between baseline BMI and the latest measurement before 31 December 2019.
NHS hospital admission for comorbidities associated with unintentional weight loss (heart failure [ICD-10 code I50], cancer [ICD-10 codes C01–C99], chronic obstructive pulmonary disease [COPD] [ICD-10 codes J40–J44], and dementia [ICD-10 codes F00–F03]) between 1 April 2015 and 31 March 2019 were identified using HES, a database of all NHS hospital activity in England. People who had undergone bariatric surgery in the NHS (see Supplementary Table 1 for definition) between 1 April 2017 and 31 March 2019 were also identified using HES.
Statistical Methods
Remission rates per 1,000 person-years with CIs were calculated using the Byar method (16). Person-years were calculated as the time from 1 April 2018 to the earliest of date of remission, date of death, or 31 December 2019. A multivariable logistic regression model was constructed for all individuals in the cohort to calculate the adjusted odds ratios (ORs) of entering remission associated with age, sex, deprivation, ethnic group, duration of diagnosis, baseline HbA1c, baseline BMI, and change in BMI. All variables were treated as categorical variables and included a category for missing data, meaning that individuals were not excluded from the regression model because of incomplete data and that the potentially different outcomes of those with missing data could be identified. A further multivariable logistic regression model in the subgroup of people with early-stage type 2 diabetes (a baseline HbA1c <53 mmol/mol [7.0%], duration of diagnosed diabetes of ≤2 years and prescribed only metformin or no glucose-lowering medication at baseline) and no history of heart failure, COPD, cancer, or dementia was performed using the same variables. Statistical significance was defined as P < 0.05, and CIs were set at 95%. Statistical calculations were performed using SAS Enterprise Guide 7.1.
Information Governance
The legal basis for the NDA data collection and linkage is a direction from NHS England to NHS Digital according to section 254 of the Health and Social Care Act 2012. All numbers taken from the NDA are rounded to the nearest 5 to protect confidentiality.
Results
Between 1 April 2007 and 31 March 2018, there were 2,297,700 people with type 2 diabetes defined as a baseline HbA1c of ≥48 mmol/mol (6.5%) or <48 mmol/mol (6.5%) with one or more prescriptions for glucose-lowering medications in the 90 days before measurement. Of these, 38,530 (1.7%) met the criteria for remission in the 21 months between 1 April 2018 and 31 December 2019 (median follow-up 91 weeks) (Table 1). People who entered remission were more likely to be older, female, living in less socioeconomically deprived areas, and of White ethnicity compared with those who did not meet the criteria for remission. They also had a shorter duration of diagnosis, lower baseline HbA1c, and lower baseline BMI (Table 1) compared with those without remission. Baseline and follow-up BMIs were recorded for 1,746,940 people (76.0%). Among those entering remission, the mean reduction in BMI from baseline was 2.5% (SD 10.2%) compared with a reduction of 0.2% (SD 8.1%) in those with no remission. At cohort entry, 48.6% of people who later entered remission were not prescribed any glucose-lowering medication, 40.7% were prescribed metformin only, 10.0% were prescribed other noninsulin glucose-lowering medication (with or without metformin), and 0.7% were prescribed insulin.
Table 1.
Characteristics of people who were alive on 1 April 2018 who did and did not achieve remission of diagnosed type 2 diabetes before 31 December 2019
| Not in remission | In remission | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All in remission | Without comorbidity* | With comorbidity† | ||||||||
| n | % | n | % | n | % | n | % | |||
| People, n | 2,259,170 | 38,530 | 30,465 | 8,065 | ||||||
| Sex | ||||||||||
| Female | 980,380 | 43.4 | 18,620 | 48.3 | 14,795 | 48.6 | 3,825 | 47.4 | ||
| Male | 1,278,795 | 56.6 | 19,910 | 51.7 | 15,670 | 51.4 | 4,240 | 52.6 | ||
| Age (years) | ||||||||||
| <40 | 65,555 | 2.9 | 1,270 | 3.3 | 1,240 | 4.1 | 25 | 0.3 | ||
| 40–49 | 200,890 | 8.9 | 2,790 | 7.2 | 2,700 | 8.9 | 95 | 1.2 | ||
| 50–59 | 452,150 | 20.0 | 5,810 | 15.1 | 5,350 | 17.6 | 460 | 5.7 | ||
| 60–69 | 598,680 | 26.5 | 8,895 | 23.1 | 7,500 | 24.6 | 1,395 | 17.3 | ||
| 70–79 | 585,785 | 25.9 | 11,070 | 28.7 | 8,085 | 26.5 | 2,980 | 36.9 | ||
| ≥80 | 356,110 | 15.8 | 8,695 | 22.6 | 5,590 | 18.3 | 3,110 | 38.6 | ||
| Mean (SD) | 66.2 (13.1) | 68.6 (13.8) | 66.6 (14) | 76.1 (10.2) | ||||||
| Quintiles of deprivation index | ||||||||||
| Most deprived | 554,495 | 24.5 | 8,075 | 21.0 | 6,260 | 20.5 | 1,810 | 22.4 | ||
| Second most deprived | 504,825 | 22.3 | 8,185 | 21.2 | 6,450 | 21.2 | 1,735 | 21.5 | ||
| Third most deprived | 460,745 | 20.4 | 8,155 | 21.2 | 6,450 | 21.2 | 1,705 | 21.1 | ||
| Second least deprived | 406,645 | 18.0 | 7,595 | 19.7 | 6,040 | 19.8 | 1,550 | 19.2 | ||
| Least deprived | 331,810 | 14.7 | 6,510 | 16.9 | 5,255 | 17.2 | 1,260 | 15.6 | ||
| Missing | 650 | 0.03 | 10 | 0.03 | 5 | 0.02 | 5 | 0.06 | ||
| Ethnicity | ||||||||||
| Asian | 261,060 | 11.6 | 2,785 | 7.2 | 2,465 | 8.1 | 295 | 3.7 | ||
| Black | 108,385 | 4.8 | 1,535 | 4.0 | 1,325 | 4.3 | 200 | 2.5 | ||
| Mixed | 23,345 | 1.0 | 330 | 0.9 | 285 | 0.9 | 45 | 0.6 | ||
| White | 1,511,220 | 66.9 | 27,990 | 72.6 | 21,320 | 70.0 | 6,490 | 80.5 | ||
| Other | 107,060 | 4.7 | 1,220 | 3.2 | 1,090 | 3.6 | 120 | 1.5 | ||
| Missing | 247,855 | 11.0 | 4,920 | 12.8 | 3,980 | 13.1 | 915 | 11.3 | ||
| Duration (years) | ||||||||||
| <1 | 141,620 | 6.3 | 11,690 | 30.3 | 9,910 | 32.5 | 1,780 | 22.1 | ||
| 1–2 | 267,195 | 11.8 | 7,050 | 18.3 | 5,765 | 18.9 | 1,285 | 15.9 | ||
| 3–5 | 255,040 | 11.3 | 4,740 | 12.3 | 3,770 | 12.4 | 970 | 12.0 | ||
| 5–9 | 608,535 | 26.9 | 8,125 | 21.1 | 6,215 | 20.4 | 1,910 | 23.7 | ||
| 10–14 | 507,925 | 22.5 | 4,530 | 11.8 | 3,210 | 10.5 | 1,320 | 16.4 | ||
| ≥15 | 478,505 | 21.2 | 2,385 | 6.2 | 1,590 | 5.2 | 795 | 9.9 | ||
| Mean (SD) | 9.9 (7.6) | 5.1 (5.9) | 4.8 (5.7) | 6.6 (6.6) | ||||||
| HbA1c (mmol/mol) | ||||||||||
| Mean (SD) in 2017/2018 | 57.3 (15.1) | 45.9 (8.1) | 46 (8.2) | 45.5 (7.6) | ||||||
| Mean change (SD) | 0.2 (11.8) | −4.1 (7.8) | −4.2 (7.9) | −3.8 (7.4) | ||||||
| BMI (kg/m2) | ||||||||||
| Mean (SD) in 2017/2018 | 31 (6.5) | 30.6 (6.6) | 30.9 (6.5) | 29.5 (6.5) | ||||||
| Change in BMI (%) | ||||||||||
| ≥10% reduction | 106,315 | 4.7 | 3,870 | 10.0 | 3,590 | 11.8 | 875 | 10.8 | ||
| 5–9.9% reduction | 220,725 | 9.8 | 4,130 | 10.7 | 3,930 | 12.9 | 755 | 9.4 | ||
| 0–4.9% reduction | 639,835 | 28.3 | 6,935 | 18.0 | 6,635 | 21.8 | 1,235 | 15.3 | ||
| 0.1–4.9% increase | 479,820 | 21.2 | 4,575 | 11.9 | 4,320 | 14.2 | 865 | 10.7 | ||
| ≥5% increase | 179,805 | 8.0 | 2,125 | 5.5 | 1,965 | 6.5 | 455 | 5.6 | ||
| Missing | 632,675 | 28.0 | 16,895 | 43.8 | 15,975 | 52.4 | 3,880 | 48.1 | ||
| Mean (SD) change 2017/2018 to 2018/2019 | −0.2 (8.1) | −2.5 (10.2) | −2.5 (10) | −2.2 (11) | ||||||
| Comorbidities | ||||||||||
| Heart failure | 143,105 | 6.3 | 2,585 | 6.7 | — | — | 2,585 | 32.1 | ||
| Cancer | 162,090 | 7.2 | 3,180 | 8.3 | — | — | 3,180 | 39.4 | ||
| COPD | 150,135 | 6.6 | 3,115 | 8.1 | — | — | 3,115 | 38.6 | ||
| Dementia | 59,135 | 2.6 | 1,220 | 3.2 | — | — | 1,220 | 15.1 | ||
| Any comorbidity | 408,440 | 18.1 | 8,065 | 20.9 | — | — | 8,065 | 100.0 | ||
| Bariatric surgery | 1,020 | 0.05 | 370 | 0.96 | 315 | 1.03 | 55 | 0.7 | ||
| Treatment in 2017/18 | ||||||||||
| None | 179,250 | 7.9 | 18,735 | 48.6 | 15,060 | 49.4 | 3,675 | 45.6 | ||
| Metformin only | 816,900 | 36.2 | 15,675 | 40.7 | 12,430 | 40.8 | 3,240 | 40.2 | ||
| Other noninsulin glucose-lowering agents with and without metformin | 1,164,340 | 51.5 | 3,850 | 10.0 | 2,750 | 9.0 | 1,100 | 13.6 | ||
| Insulin with or without other glucose-lowering agents | 98,680 | 4.4 | 270 | 0.7 | 220 | 0.7 | 50 | 0.6 | ||
See text for definition of diabetes remission.
No history of a hospital admission for heart failure, cancer, COPD, or dementia between 1 April 2015 and 31 March 2019.
History of a hospital admission for heart failure, cancer, COPD, or dementia between 1 April 2015 and 31 March 2019.
The overall incidence of remission was 9.7 (95% CI 9.6–9.8) per 1,000 person-years. In people with comorbidities that may induce weight loss, incidence of remission was higher (11.8 [95% CI 11.5–12.0] per 1,000 person-years) compared with those without such a comorbidity (9.2 [95% CI 9.1–9.3] per 1,000 person-years). A history of bariatric surgery in the NHS was rare (0.06% of all people included in the analysis). In those who had bariatric surgery but no comorbidity that may induce weight loss, the rate of remission was 205.8 (95% CI 183.8–229.8) per 1,000 person-years, falling to 82.5 (95% CI 61.8–107.9) per 1,000 person-years for those with a comorbidity associated with unintentional weight loss who underwent bariatric surgery.
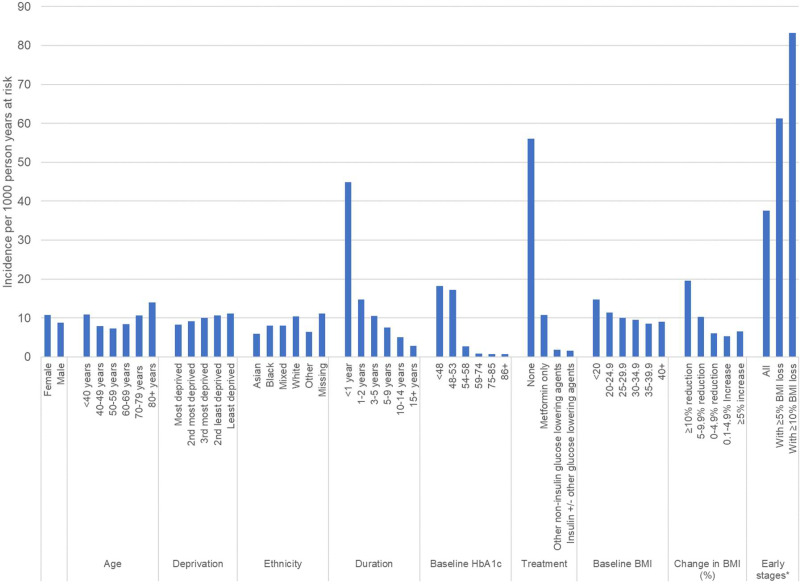
Overall rates of remission were higher in women, older people, those living in less socioeconomically deprived areas, those from White ethnic groups, and those with a shorter duration of diagnosed diabetes than their respective comparison groups (see Fig. 1). For the subgroup of 208,260 people (9.1% of the whole cohort) who had a diagnosis of diabetes for <2 years, had a baseline HbA1c of <53 mmol/mol (7.0%), were prescribed metformin alone or no glucose-lowering drugs, and had no history of cancer, heart failure, COPD, or dementia, the overall rate of remission was 37.6 (95% CI 37.0–38.3) per 1,000 person-years. In an additional subgroup of 8,940 people (0.4% of the total cohort) who had a diagnosis of diabetes of <2 years, had a baseline HbA1c of <53 mmol/mol (7.0%), and achieved a reduction in BMI of ≥10% from baseline, the incidence of remission was 83.2 (95% CI 78.7–87.9) per 1,000 person-years.
Figure 1.
Incidence of remission of type 2 diabetes per 1,000 person-years. *Early stages of type 2 diabetes defined as diagnosed <2 years previous, baseline HbA1c ≤53 mmol/mol (7.0%), prescribed metformin only or no glucose-lowering drugs, and no history of heart failure, cancer, COPD, or dementia.
Incidence of remission also varied by medications prescribed at baseline. Remission rates were higher among people who were not prescribed any glucose-lowering medications at baseline (58.4 [95% CI 57.6–59.3] per 1,000 person-years) compared with those prescribed metformin alone (11.1 [95% CI 10.9–11.3] per 1,000 person-years). In people prescribed noninsulin, nonmetformin glucose-lowering medications at cohort entry remission were rare, and incidence was significantly lower (2.0 [95% CI 1.9–2.0] per 1,000 person-years), as was the case in those prescribed insulin (1.6 [95% CI 1.4–1.8] per 1,000 person-years).
After adjusting for other characteristics, men had a lower odds of entering remission than women (OR 0.96 [95% CI 0.94–0.98]), while those from all non-White ethnic groups had a lower odds of entering remission than those from White ethnic groups. There was a significant deprivation gradient, with people living in the least-deprived quintile of areas having an OR of entering remission of 1.13 (95% CI 1.09–1.18) compared with those in the most-deprived areas.
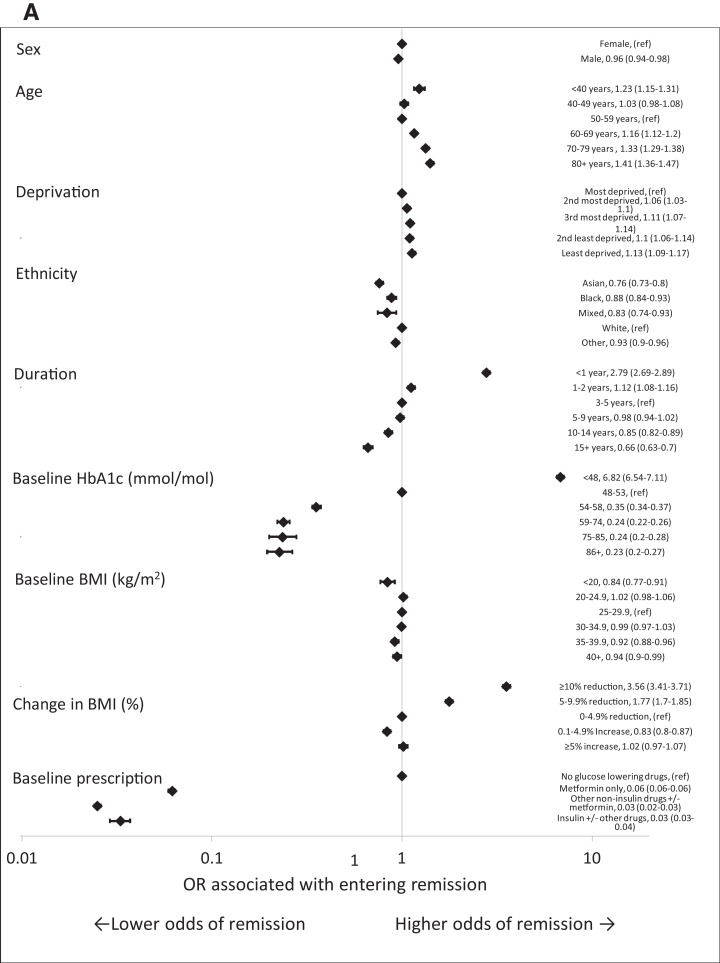
People who were diagnosed with type 2 diabetes for <2 years had a higher odds of entering remission (OR 2.78 [95% CI 2.68–2.88] for those diagnosed <1 year and 1.12 [95% CI 1.08–1.17] for those diagnosed 1–2 years compared with those diagnosed 3–5 years). Baseline HbA1c of <48 mmol/mol (6.5%) was associated with a greater odds of entering remission (OR 6.84 [95% CI 6.55–7.15]) compared with baseline HbA1c of 48–53 mmol/mol (6.5–7.0%). Reduction in BMI of ≥10% and 5–9.9% was associated with a greater odds of entering remission (OR 3.57 [95% CI 3.42–3.72] and 1.78 [95% CI 1.71–1.85], respectively) compared with a reduction in BMI of 0–4.9% (see Fig. 2A).
Figure 2.
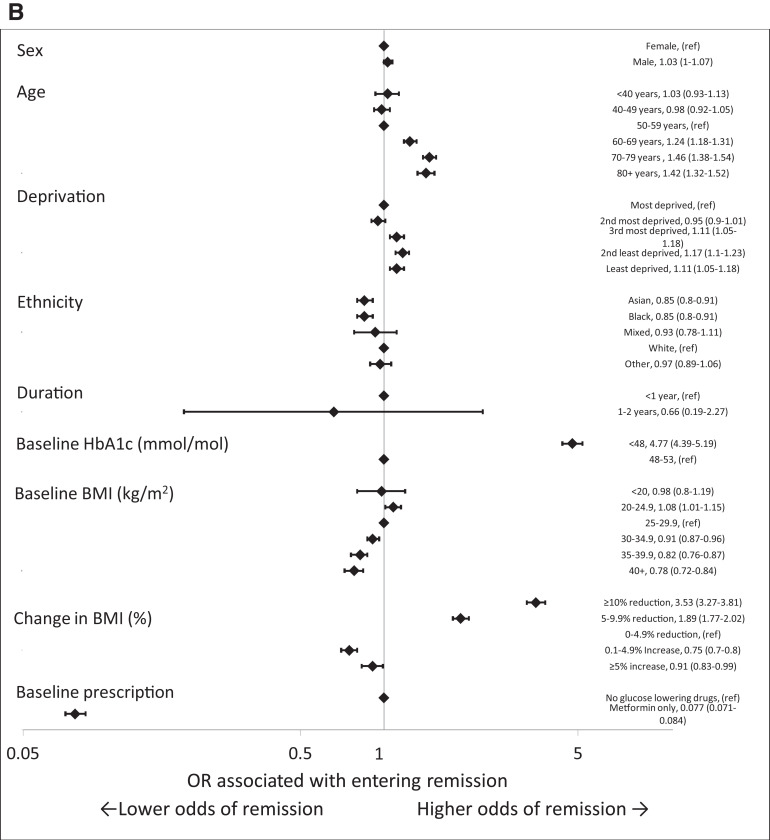
Forest plots of ORs from a multivariable model including all factors for demographic and clinical characteristics associated with remission of type 2 diabetes in the whole cohort (A) and in people with early-stage type 2 diabetes (baseline HbA1c ≤53 mmol/mol [7.0%], duration of diagnosed diabetes of <2 years previous, and prescribed only metformin or no glucose-lowering medication at baseline) (B). ref, reference.
Among the 208,260 people (9.1% of the cohort) in the early stages of type 2 diabetes (defined as diagnosed for <2 years, a baseline HbA1c <53 mmol/mol [7.0%], and prescribed metformin alone or no glucose-lowering medications at baseline) with no history of heart failure, COPD, cancer, or dementia, higher odds of remission were associated with older age and living in less-deprived areas. In this early-stage cohort, people from Asian and Black ethnic groups had lower odds of remission than White ethnic groups (Table 2). The multivariable-adjusted odds of remission across BMI categories were greatest in people with a baseline BMI of 20–24.9 kg/m2 (OR 1.08 [95% CI 1.01–1.15]) compared with those with a baseline BMI of 25–29.9 kg/m2. Increasing baseline BMI >30 kg/m2 was associated with a lower odds of entering remission (OR 0.91 [95% CI 0.87–0.96] for 30–34.9 kg/m2, 0.82 [95% CI 0.77–0.88] for 35–39.9 kg/m2, 0.78 [95% CI 0.72–0.84] for ≥40 kg/m2) compared with 25–29.9 kg/m2. Compared with a 0–4.9% reduction in BMI, a reduction of 5–9.9% and ≥10% was associated with ORs of entering remission of 1.89 (95% CI 1.77–2.02) and 3.54 (95% CI 3.28–3.82), respectively. Having a baseline HbA1c of <48 mmol/mol (6.5%) vs. 48–53 mmol/mol (6.5–7.0%) was associated with an OR of entering remission of 4.78 (95% CI 4.39–5.2) (see Fig. 2B).
Table 2.
Characteristics of people who entered remission by remission status at the end of follow-up
| Stayed in remission | Returned to diabetic hyperglycemia | No follow-up HbA1c | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| People, n | 9,175 | 3,420 | 25,935 | |||
| Sex | ||||||
| Female | 4,460 | 48.6 | 1,600 | 46.8 | 12,560 | 48.4 |
| Male | 4,715 | 51.4 | 1,815 | 53.1 | 13,380 | 51.6 |
| Age (years) | ||||||
| <40 | 265 | 2.9 | 80 | 2.3 | 925 | 3.6 |
| 40–49 | 635 | 6.9 | 225 | 6.6 | 1,935 | 7.5 |
| 50–59 | 1,390 | 15.1 | 505 | 14.8 | 3,915 | 15.1 |
| 60–69 | 2,225 | 24.3 | 815 | 23.8 | 5,850 | 22.6 |
| 70–79 | 2,700 | 29.4 | 1,095 | 32.0 | 7,275 | 28.1 |
| ≥80 | 1,960 | 21.4 | 700 | 20.5 | 6,035 | 23.3 |
| Mean (SD) | 68.5 (13.4) | 68.9 (12.9) | 68.6 (14.1) | |||
| Deprivation | ||||||
| Most deprived | 1,935 | 21.1 | 635 | 18.6 | 5,505 | 21.2 |
| Second most deprived | 1,935 | 21.1 | 660 | 19.3 | 5,590 | 21.6 |
| Third most deprived | 1,970 | 21.5 | 780 | 22.8 | 5,405 | 20.8 |
| Second least deprived | 1,805 | 19.7 | 740 | 21.6 | 5,050 | 19.5 |
| Least deprived | 1,530 | 16.7 | 600 | 17.5 | 4,380 | 16.9 |
| Missing | — | 0.00 | — | 0.00 | 10 | 0.04 |
| Ethnicity | ||||||
| Asian | 600 | 6.5 | 280 | 8.2 | 1,880 | 7.2 |
| Black | 345 | 3.8 | 115 | 3.4 | 1,065 | 4.1 |
| Mixed | 65 | 0.7 | 25 | 0.7 | 235 | 0.9 |
| White | 6,795 | 74.1 | 2,435 | 71.2 | 18,585 | 71.7 |
| Other | 255 | 2.8 | 100 | 2.9 | 855 | 3.3 |
| Missing | 1,110 | 12.1 | 470 | 13.7 | 3,315 | 12.8 |
| Duration (years) | ||||||
| <1 | 3,125 | 34.1 | 1,300 | 38.0 | 7,265 | 28.0 |
| 1–2 | 1,645 | 17.9 | 620 | 18.1 | 4,785 | 18.4 |
| 3–5 | 1,065 | 11.6 | 410 | 12.0 | 3,270 | 12.6 |
| 5–9 | 1,845 | 20.1 | 600 | 17.5 | 5,680 | 21.9 |
| 10–14 | 980 | 10.7 | 315 | 9.2 | 3,235 | 12.5 |
| ≥15 | 515 | 5.6 | 180 | 5.3 | 1,690 | 6.5 |
| Mean (SD) | 4.8 (5.8) | 4.3 (5.2) | 5.4 (6.1) | |||
| HbA1c (mmol/mol) in 2017/2018 | ||||||
| <48 | 4,250 | 46.3 | 930 | 27.2 | 11,695 | 45.1 |
| 48–53 | 4,360 | 47.5 | 2,225 | 65.1 | 12,215 | 47.1 |
| 54–58 | 335 | 3.7 | 185 | 5.4 | 1,200 | 4.6 |
| 59–74 | 165 | 1.8 | 65 | 1.9 | 580 | 2.2 |
| 75–85 | 35 | 0.4 | 5 | 0.1 | 105 | 0.4 |
| ≥86 | 25 | 0.3 | 10 | 0.3 | 140 | 0.5 |
| Mean (SD) | 45.3 (7.6) | 47.8 (6) | 45.9 (8.4) | |||
| Mean (SD) change 2017/2018 to 2018/2019 | −4.1 (7.4) | −4.1 (6) | −4.1 (8.1) | |||
| BMI (kg/m2) in 2017/2018 | ||||||
| <20 | 140 | 1.5 | 50 | 1.5 | 445 | 1.7 |
| 20–24.9 | 1,260 | 13.7 | 445 | 13.0 | 3,490 | 13.5 |
| 25–29.9 | 2,690 | 29.3 | 1,070 | 31.3 | 7,140 | 27.5 |
| 30–34.9 | 2,180 | 23.8 | 850 | 24.9 | 5,795 | 22.3 |
| 35–39.9 | 940 | 10.2 | 350 | 10.2 | 2,660 | 10.3 |
| ≥40 | 640 | 7.0 | 190 | 5.6 | 1,850 | 7.1 |
| Missing | 1,325 | 14.4 | 465 | 13.6 | 4,555 | 17.6 |
| Mean (SD) | 30.5 (6.5) | 30.3 (6) | 30.6 (6.7) | |||
| Change in BMI | ||||||
| ≥10% reduction | 970 | 10.6 | 140 | 4.1 | 2,760 | 10.6 |
| 5–9.9% reduction | 945 | 10.3 | 345 | 10.1 | 2,840 | 11.0 |
| 0–4.9% reduction | 1,695 | 18.5 | 780 | 22.8 | 4,460 | 17.2 |
| 0.1–4.9% increase | 1,080 | 11.8 | 500 | 14.6 | 2,995 | 11.5 |
| ≥5% increase | 475 | 5.2 | 190 | 5.6 | 1,460 | 5.6 |
| Missing | 4,010 | 43.7 | 1,460 | 42.7 | 11,425 | 44.1 |
| Mean (SD) change 2017/2018 to 2018/2019 | −2.6 (9.9) | −0.6 (9.6) | −2.7 (10.4) | |||
| Comorbidities | ||||||
| Heart failure | 570 | 6.2 | 215 | 6.3 | 1,800 | 6.9 |
| Cancer | 735 | 8.0 | 280 | 8.2 | 2,165 | 8.3 |
| COPD | 725 | 7.9 | 265 | 7.7 | 2,125 | 8.2 |
| Dementia | 215 | 2.3 | 70 | 2.0 | 935 | 3.6 |
| Any comorbidity | 1,830 | 19.9 | 685 | 20.0 | 5,545 | 21.4 |
| Bariatric surgery | 135 | 1.47 | 10 | 0.29 | 225 | 0.87 |
| Treatment in 2017/2018 | ||||||
| None | 4,435 | 48.3 | 2,280 | 66.7 | 12,020 | 46.3 |
| Metformin only | 3,790 | 41.3 | 890 | 26.0 | 10,990 | 42.4 |
| Other noninsulin glucose-lowering agents with or without metformin | 875 | 9.5 | 230 | 6.7 | 2,750 | 10.6 |
| Insulin with or without other glucose-lowering agents | 75 | 0.8 | 20 | 0.6 | 180 | 0.7 |
Overall, 3,420 (8.9%) of the 38,530 of people who entered remission subsequently had an HbA1c measurement of ≥48 mmol/mol (6.5%), suggesting a return to hyperglycemia in the diabetes range. Median time from entering remission of type 2 diabetes to this measurement was 190 days (interquartile range [IQR] 144–247); 9,175 (238 per 1,000) people in remission had an HbA1c measurement that indicated that they continued to meet the definition of remission of type 2 diabetes (median follow-up 294 days, IQR 222–361), and 25,940 (673 per 1,000) people did not have any further HbA1c measurements within the study follow-up period (median follow-up time 148 days, IQR 77–235). Compared with those who stayed in remission, people who returned to diabetic hyperglycemia had a lower mean reduction in BMI (−0.6% vs. −2.6% for those that stayed in remission and −2.7% for those that did not have a further HbA1c measurement) and were more likely to not be prescribed any glucose-lowering medication at baseline (66.7% vs. 48.3% for those who stayed in remission and 46.3% for those who did not have a further HbA1c measurement). Of those meeting the criteria for remission, only 2,110 (5.5%) had a diagnosis code for remission recorded in their electronic health record. There were no consistent differences in the characteristics of people with and without a diagnosis code for diabetes remission, but there was considerable geographical variation in coding for remission by the 160 Clinical Commission Groups (health organizations with responsibility for commissioning health care for their local population), varying from 25% to <1%.
In sensitivity analysis, defining remission based on only one HbA1c measurement <48 mmol/mol (6.5%) in the absence of a prescription for glucose-lowering medications in the preceding 90 days increased the number of people identified as in remission from 3,420 (9.7 [95% CI 9.6–9.8] per 1,000 person-years) to 91,405 (23.5 [95% CI 23.3–23.7] per 1,000 person-years). The pattern of incidence rates by demographic and clinical characteristics were similar to the primary analysis (Supplementary Table 3).
Conclusions
In this nationally representative population-based study of >2.2 million people with type 2 diabetes, the incidence of remission over a maximum of 21 months was low at ∼1% per year overall and ∼4.5% per year in those within the first year of diagnosis. However, there was also considerable variation in incidence by baseline and change in BMI, by ethnicity, and by duration of diagnosed diabetes. Among the subgroup of people who had been diagnosed for <2 years, with a baseline HbA1c of <53 mmol/mol (7.0%), and who also reduced their BMI by ≥10%, who formed 5.2% of the cohort, the incidence of remission rose to 8% per year, implying that the opportunity to achieve remission is greatest in the period immediately after diagnosis, particularly in the context of intensive lifestyle interventions (3).
Greater remission rates were consistently associated with weight loss and degree of weight loss. The higher incidence of remission in people with a very low baseline BMI may reflect underlying comorbidities and consequent unintentional weight loss. Bariatric surgery was associated with a very high incidence of entering remission (169.5 [95% CI 152.7–187.7] per 1,000 person-years). However, the absolute number of people with a record of undergoing bariatric surgery in the NHS during the study period was small (n = 1,390, 0.06%), and the characteristics and motivations of these people may have differed, which increases the likelihood of achieving remission.
Not being prescribed any glucose-lowering medications in the baseline period was associated with an incidence of remission approximately five times higher than in those prescribed metformin as their only glucose-lowering medication, while remission was rare in those who were prescribed noninsulin, nonmetformin glucose-lowering medications, or insulin in the baseline period. These findings are likely to reflect difficulty in controlling hyperglycemia rather than any remission-inhibiting effect of the medications or that people on more than one drug will have more advanced diabetes. People living in the least-deprived areas and those from White ethnic groups were more likely to enter remission than those in the respective comparison groups. This may be explained by people from non-White ethnic groups having higher HbA1c at diagnosis and poorer HbA1c trajectories following diagnosis of type 2 diabetes (17,18). Our findings are in contrast to a U.S. study where higher adjusted incidence of remission occurred in those from Black ethnic groups (19). Further exploration of physical, psychological, social, and health care factors that may affect awareness, motivation, and capacity for entering remission among people of varying ethnic backgrounds in England is warranted.
We found men to have slightly lower odds of remission overall, perhaps because of their generally higher levels of HbA1c. However, this finding was reversed and not significant in the subset with <2 years since diagnosis and warrants further investigation. People in older age-groups had a higher incidence of remission of type 2 diabetes than those in younger age-groups, which could be attributable to lower average BMI. (It is well known that average BMI at diabetes diagnosis is lower in older people, and therefore, the weight loss needed to move into remission is less [20]). In addition, older people may have a higher prevalence of comorbidities that may be associated with unintentional weight loss. Policies focusing on lifestyle interventions to increase rates of remission of type 2 diabetes should take into account these inequalities and incorporate measures that aim to address them.
Only 5.5% of people meeting the criteria for remission had a diagnosis code related to remission of diabetes. Given that some definitions of remission of type 2 diabetes require a longer period of glycemia below the diabetic range than used in this analysis, the low proportion of coded remissions may be due to the relatively short follow-up period. However, it may also reflect a lack of awareness of the concept of remission of diabetes, with clinicians having traditionally considered type 2 diabetes to be a chronic, progressive disease. Additionally, it may simply be lack of awareness of such codes. There may also be concerns about the impact of these codes on routine diabetes monitoring and screening for complications, incentive payments, or ongoing care arrangements. Interventions are needed to raise awareness and encourage the appropriate use of these codes, particularly as intensive lifestyle interventions, new weight loss medications, and bariatric surgery are more widely used. However, in the meantime, our findings suggest that codes for remission should not be relied on for research purposes.
Intensive lifestyle intervention studies in the U.K. have shown that approximately one-third of selected trial participants undergoing a very-low-calorie diet and large weight loss enter, and remain in, remission of type 2 diabetes over a period of 2–5 years (3,4,21). The Look AHEAD (Action for Health in Diabetes) study showed an incidence of remission of type 2 diabetes of 11.5% at 1 year and 7.3% at 4 years in the intensive lifestyle intervention group and 2.0% at both 1 and 4 years in the group who received three group sessions per year (5). These rates are higher than found in the current analysis but are likely to reflect the impact of intensive interventions in selected trial populations. However, we sought to assess the incidence of remission in a real-world community setting where evidence is more sparse and incidence is considerably lower. While this cohort may have included a small number of people enrolled in specific trials focusing on remission, the majority will not have received the intensive support that goes alongside specific research studies. The overall incidence of remission found is similar to a community-based study in the U.S. that found a 7-year cumulative incidence of remission of 1.6%, rising to 4.6% in those whose diabetes was diagnosed <2 years previously (19).
In 2020, the NHS in England established a low-calorie diet pilot program to test the effectiveness of intensive lifestyle interventions within live NHS environments for people within 6 years of diagnosis with type 2 diabetes (22). The results from this current study can be used as a baseline for monitoring the incidence and characteristics of remission following the possible rollout of this program in England and suggest that codes for remission of diabetes are currently of limited value.
For this analysis, remission was defined as two consecutive HbA1c measurements at least 26 weeks apart that were <48 mmol/mol (6.5%) with no prescription for glucose-lowering medication between these measurements and at least 90 days before each HbA1c measurement. The recent consensus statement on the definition and interpretation of remission in type 2 diabetes defines remission depending on the route by which it is achieved (pharmacotherapy, bariatric surgery, or lifestyle interventions) (10). In each case, remission is defined as an HbA1c measurement of <48 mmol/mol (6.5%) at least 3 months after the cessation of glucose-lowering medications and at least 3 months after bariatric surgery or 6 months after the start of lifestyle interventions. However, routinely collated data record the date prescriptions are issued, and it is difficult to ascertain the exact date at which the individual ceased to take the prescribed medications. Furthermore, our data set (like most routine health care records) did not include data on lifestyle interventions. These factors mean that there are some differences between the definition in the consensus statement and the criteria used to identify remission in this study.
In this analysis, follow-up was limited to a maximum period of 21 months, during which 8.9% of people meeting the criteria for remission went on to have an HbA1c measurement indicating a return to diabetic hyperglycemia. Those who returned to hyperglycemia in the diabetic range during the follow-up period of this study had a smaller mean reduction in BMI and were more likely to not be prescribed glucose-lowering medication at baseline than those who remained in remission or did not have subsequent HbA1c measurements. This highlights the potentially transient nature of remission in the context of routine traditional care and emphasizes the need for longer-term follow-up of these individuals.
The strength of this study lies in the large population-based cohort that included people registered at 98.2% of general practices in England, including ∼2.3 million people with type 2 diabetes. By combining data on routinely recorded HbA1c measurements and prescriptions for glucose-lowering medications, it provides a reliable assessment of the incidence of remission of type 2 diabetes using the specified definition in people receiving routine current care. The relatively short follow-up time is a weakness, limiting inferences about the durability of remission, but follow-up was limited to 31 December 2019 to avoid altered patterns of behavior and health care delivery due to the coronavirus disease 2019 pandemic. Data on prescriptions for glucose-lowering medications were collected for the first time in the 2017/2018 data collection, and therefore, earlier data sets could not be used. The NDA does not yet collate data on lifestyle interventions; therefore, it is not currently possible to identify the means through which individuals achieved remission, although planned linkage with national data sets related to lifestyle interventions should permit this in future years. However, we include data on remission from bariatric surgery because of linkage of our data with hospital data.
It is possible that some people entered remission of type 2 diabetes following unintentional weight loss as a result of ill health, meaning that their remission may not represent a positive health change. We have tried to compensate for this limitation by using hospital discharge data to identify people with comorbidities that are associated with unintentional weight loss, but we recognize that it is difficult to differentiate intentional versus unintentional weight loss. People who received bariatric surgery outside the NHS will not have had their procedures recorded, and so the numbers of such operations will have been underestimated.
This study has identified that remission of type 2 diabetes does occur within a community setting among a population receiving standard clinical care, but the absolute incidence is low. The characteristics of those who did go into remission will help in the design of lifestyle intervention programs for induction and maintenance of remission. Longer-term follow-up of this cohort of people entering remission to identify their glycemic trajectories and the subsequent risk of macro- and microvascular disease as recommended by the recent consensus statement on the definition and interpretation of remission in type 2 diabetes (10) would be informative.
Article Information
Acknowledgments. The authors thank Liz Coyle, University of Glasgow, for her assistance in the preparation of this article.
Funding. N.H is funded by Diabetes UK and NHS England, and NHS Improvement (grant number 20/0006367). K.K. is supported by the National Institute for Health Research Applied Research Collaboration East Midlands and the National Institute for Health Research Leicester Biomedical Research Centre. N.S. is supported by the British Heart Foundation Research Excellence Award (RE/18/6/34217).
Duality of Interest. K.K., B.Y., and J.V. are members of the NDA Research Advisory Group. K.K. has acted as a consultant, speaker, or received grants for investigator-initiated studies for AstraZeneca, Novartis, Novo Nordisk, Sanofi, Eli Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG/Menarini Group, Janssen, and Napp. J.V. is National Clinical Director for Diabetes and Obesity at NHS England and NHS Improvement. N.S. has received grants from AstraZeneca, Boehringer Ingelheim, and Roche Diagnostics and personal fees from Afimmune, Amgen, AstraZeneca, Eli Lilly, Hanmi Pharmaceuticals, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. N.H. performed the statistical analysis. N.H., S.H.W., K.K., P.K., J.O., C.B., B.Y., N.S., J.V., and E.W.G. reviewed the methods, assisted in writing the manuscript, and reviewed the final manuscript. N.H., S.H.W., K.K., B.Y., N.S., and E.W.G. designed the study. N.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19165148.
References
- 1. Public Health England . Diabetes Prevalence Model 2016. Accessed 17 August 2021. Available from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/612306/Diabetesprevalencemodelbriefing.pdf
- 2. International Diabetes Federation . IDF Diabetes Atlas. 9th ed. 2019. Accessed 27 May 2021. Available from https://diabetesatlas.org/en/
- 3. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391:541–551 [DOI] [PubMed] [Google Scholar]
- 4. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:344–355 [DOI] [PubMed] [Google Scholar]
- 5. Gregg EW, Chen H, Wagenknecht LE, et al.; Look AHEAD Research Group . Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012;308:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia 2016;59:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–2304 [DOI] [PubMed] [Google Scholar]
- 8. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 9. Cummings DE, Cohen RV. Bariatric/metabolic surgery to treat type 2 diabetes in patients with a BMI <35 kg/m2. Diabetes Care 2016;39:924–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care 2021;44:2438–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holman N, Knighton P, Wild SH, et al. Cohort profile: National Diabetes Audit for England and Wales. Diabet Med 2021;38:e14616. [DOI] [PubMed] [Google Scholar]
- 12. NHS Digital . National Diabetes Audit - Report 1 Care Processes and Treatment Targets 2017-18, Full Report. Accessed 12 July 2021. Available from https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/report-1-care-processes-and-treatment-targets-2017-18-full-report
- 13. de Lusignan S, Sadek N, Mulnier H, Tahir A, Russell-Jones D, Khunti K. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med 2012;29:181–189 [DOI] [PubMed] [Google Scholar]
- 14. Nagi D, Hambling C, Taylor R. Remission of type 2 diabetes: a position statement from the Association of British Clinical Diabetologists (ABCD) and the Primary Care Diabetes Society (PCDS). Br J Diabetes 2019;19:73–76 [Google Scholar]
- 15. GOV.UK . English indices of deprivation. 2019. Accessed 22 April 2021. Available from https://www.gov.uk/government/statistics/english-indices- of-deprivation-2019
- 16. Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ 1987;(82):1–406 [PubMed] [Google Scholar]
- 17. Luo M, Tan KHX, Tan CS, Lim WY, Tai E-S, Venkataraman K. Longitudinal trends in HbA1c patterns and association with outcomes: a systematic review. Diabetes Metab Res Rev 2018;34:e3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mostafa SA, Davies MJ, Webb DR, Srinivasan BT, Gray LJ, Khunti K. Independent effect of ethnicity on glycemia in South Asians and White Europeans. Diabetes Care 2012;35:1746–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karter AJ, Nundy S, Parker MM, Moffet HH, Huang ES. Incidence of remission in adults with type 2 diabetes: the diabetes & aging study. Diabetes Care 2014;37:3188–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wright AK, Welsh P, Gill JMR, et al. Age-, sex- and ethnicity-related differences in body weight, blood pressure, HbA1c, and lipid levels at the diagnosis of type 2 diabetes relative to people without diabetes. Diabetologia 2020;63:1542–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dambha-Miller H, Day AJ, Strelitz J, Irving G, Griffin SJ. Behaviour change, weight loss and remission of Type 2 diabetes: a community-based prospective cohort study. Diabet Med 2020;37:681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NHS England . Low calorie diets to treat obesity and type 2 diabetes. 2021. Accessed 8 July 2021. Available from https://www.england.nhs.uk/diabetes/treatment-care/low-calorie-diets/