Abstract
OBJECTIVE
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study reported a 13.9% prevalence of diabetic retinopathy (DR) in youth with mean ± SD type 2 diabetes duration of 4.9 ± 1.5 years. After 7 years of additional follow-up, we report the risk factors for progression of DR in the TODAY cohort.
RESEARCH DESIGN AND METHODS
Retinal photographs (n = 517) were obtained in 2010–2011 and again in 2017–2018 (n = 420) with standard stereoscopic seven-field digital fundus photography. Photographs were graded centrally using the Early Treatment Diabetic Retinopathy Study (ETDRS) scale. A total of 367 patients with gradable fundus photographs in at least one eye at both assessments were included in analyses of progression of DR, defined as an increase of three or more steps on the ETDRS scale.
RESULTS
With mean ± SD age of 25.4 ± 2.5 years and diabetes duration of 12.0 ± 1.5 years, there was a 49% prevalence of any DR among participants. Prevalence by DR stage was as follows: 39% for very mild or mild nonproliferative DR (NPDR), 6% moderate to severe NPDR, and 3.8% proliferative DR. Compared with nonprogressors, participants who progressed three or more steps had significantly lower BMI, higher HbA1c, higher blood pressure, increased triglycerides, decreased C-peptide, and higher prevalence of other comorbidities. Multivariate analysis demonstrated that HbA1c was the dominant factor impacting DR progression.
CONCLUSIONS
Poor glycemic control of youth-onset type 2 diabetes imparts a high risk for progression of DR, including advanced, sight-threatening disease by young adulthood.
Introduction
Diabetic retinopathy (DR) remains the leading cause of blindness in working-age adults and the fifth most common cause of preventable blindness (1). Prevention of DR relies on effective management of hyperglycemia with the goals of attaining near-normal glycemia as soon as possible after diagnosis and continuing to achieve target range HbA1c over time (2–4). Clinical trial data suggest that β-cell decline occurs more rapidly and failure to achieve glycemic targets using oral agents is more common and occurs sooner in patients diagnosed with youth-onset type 2 diabetes compared with those with onset of type 2 diabetes later in life (5,6). The challenge of managing youth with type 2 diabetes effectively is compounded by the social and economic burdens of these youth, largely representing underserved, racial and ethnic minorities (7). Thus, physiology and socioeconomic barriers combine to place youth with type 2 diabetes at very high risk for rapid worsening of glycemic control and potentially more rapid progression of diabetes-related complications, including DR.
In the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study and its follow-up observational study, TODAY2, participants were monitored with longitudinal assessments of diabetes management and DR progression from 2004 to 2020. Two rounds of standard stereoscopic seven-field digital fundus photography were performed in 2010–2011 and ∼7 years later in 2017–2018. Although with only a brief mean duration of diabetes at the time of the first fundus photography (mean of 4.9 years, range 2–8), TODAY participants had a 13.9% prevalence of DR (8). The TODAY/TODAY2 study investigators recen tly published the longitudinal prevalence of complications over a mean follow-up period of 10 years, reporting that the prevalence of all complications had risen and DR prevalence increased to 49% (9). With the DR prevalence rising markedly and the presence of sight-threatening lesions, understanding the modifiable risk factors driving progression of DR during the transition from youth to young adult with type 2 diabetes is critical to preserve vision long-term, which is essential to future physical functioning, financial employment opportunities, and overall quality of life. We now report the risk factors associated with DR progression in youth-onset type 2 diabetes.
Research Design and Methods
Study Design
The study design and results of the TODAY study have previously been published (10,11). Briefly, a total of 699 participants enrolled in the TODAY clinical trial over the course of 4.5 years and were followed for 2.0–6.5 years. After the TODAY trial ended in 2011, 572 participants enrolled in the TODAY2 observational follow-up study. In the last year of TODAY (2010–2011), stereoscopic color fundus photographs were collected from 517 participants, with results previously reported (8). During the TODAY2 observational follow-up study, fundus photographs were collected in 2017–2018 from 423 participants. A subset of 367 participants had gradable photographs in at least one eye at both assessments.
Standard Seven-Field Fundus Exams
All photographs were graded centrally at the University of Wisconsin-Madison Fundus Photography Reading Center by graders masked to treatment, age, duration of diabetes, glycemic control, and other clinical characteristics, using the final Early Treatment Diabetic Retinopathy Study (ETDRS) grading scale (12). Retinopathy progression was defined as the occurrence of a 3-step or more progression in the ETDRS grading scale from the level of retinopathy at the end of TODAY to the repeat fundus photographs at follow-up, representing a reproducible measure of clinically important worsening as previously described (13,14). All outcomes are reported as participant-level retinopathy severity (i.e., severity of the eye with more advanced disease determines the specific retinopathy grade). Clinically significant macular edema (CSME) was graded with color fundus photography and categorized as absent, definite, questionable, or ungradable.
Grading Based on ETDRS Scale
In addition to the 3-step progression, a condensed numeric retinopathy score was assigned at the University of Wisconsin-Madison Fundus Photography Reading Center. The condensed grading is obtained by collapsing the patient-level ETDRS scale into 8 levels: 1 = “no definitive diabetic retinopathy,” 2–3 = “very mild NPDR,” 4–5 = “mild NPDR,” 6–7 = “moderate NPDR,” 8–9 = “moderately severe NPDR,” 10–11 = “severe NPDR,” 12–15 = “early or stable, treated PDR,” and 16–23 = “high risk PDR,” where NPDR is nonproliferative DR and PDR is proliferative DR.
Risk Factors
During the randomized trial phase of the TODAY study, participants were seen every 2 months for the first year after randomization and quarterly thereafter. During TODAY2 (2011–2020), participants were seen every 3 months for 3 years and annually for 6 years thereafter until the end of the study. Demographic, detailed medical history, self-reported medication usage, physical examination, and fasting laboratory studies were collected as previously described (9,11). Blood and spot urine samples were obtained after a 10- to 14-h overnight fast and processed and analyzed immediately at the TODAY central biochemistry laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle, WA). Hypertension and indices of nephropathy (urine albumin-to-creatinine ratio [ACR] ≥30 mg/g, ACR ≥300 mg/g) were evaluated longitudinally (9). Participants self-reported cigarette smoking, categorized as either “yes” (used within the past month) or “no” (never used/not used within the past month).
Statistical Analyses
All participants with gradable fundus exams in at least one eye at both exams were included in the analysis. All descriptive data are presented as mean ± SD, median [IQR], or n (%). Cohort characteristics are presented at both baseline and the final follow-up fundus exam. The final follow-up exam characteristics are summarized using time-weighted means up to the time of the exam for continuous values, and time-dependent categorical characteristics (comorbidities, medication usage, and smoking) were considered present if they had been at or before the time of the follow-up fundus exam. The same approach is used to present baseline characteristics for the full TODAY cohort. The number and percentage of participants in each of the condensed ETDRS classifications are presented for the cohort with images at both occasions and for the cohort with images only at follow-up. Participants with images at both occasions were categorized based on whether they had progressed at least 3 steps based on the ETDRS classifications. Participant characteristics were compared between the two groups with use of the Fisher exact test for categorical variables and Student t test for continuous variables. Skewed variables were normalized by log-transformation prior to testing. Risk factors for at least a 3-step progression were analyzed using univariate and multivariate logistic regression. Age and any statistically significant (P < 0.05) risk factors from the univariate model were selected as variables for the multivariate analysis. Results for the risk factor analysis are presented as odds ratios and 95% CIs with associated P values for the logistic regression coefficients. Analyses were performed using R (version 4.0.2) and considered exploratory, with statistical significance defined as P < 0.05.
Results
A total of 367 participants completed both assessments and were included in the subsequent risk factor analyses. At the time of the second assessment, participants were on average (mean ± SD) 25.4 ± 2.5 years of age with diabetes duration 12.0 ± 1.5 years, HbA1c 7.9 ± 1.9%, and BMI 36.1 kg/m2 (Table 1). Approximately 60% of participants had hypertension, 58% had moderate or severe albuminuria, and 30% had been treated with lipid-lowering medications (15) (Table 1). Baseline characteristics at the start of the TODAY study in 2004 among the 367 participants with repeat fundus examinations were similar to those of the original full TODAY cohort (Supplementary Table 1).
Table 1.
Characteristics of participants with type 2 diabetes (n = 367) at time of first (TODAY) and second (TODAY2) fundus exam
| Characteristic | TODAY (2010–2011) | TODAY2 (2017–2018) |
|---|---|---|
| Female sex | 236 (64.3) | |
| Race/ethnicity | ||
| Hispanic | 146 (39.7) | |
| Non-Hispanic Black | 126 (34.3) | |
| Non-Hispanic White | 71 (19.3) | |
| Other | 24 (6.5) | |
| Age (years) | ||
| At baseline | 13.7 ± 2.0 | |
| At Fundus exam | 18.4 ± 2.5 | 25.4 ± 2.5 |
| Diabetes duration (years) | 4.9 ± 1.5 | 12.0 ± 1.5 |
| BMI (kg/m2) | 35.8 ± 8.0 | 36.1 ± 7.8 |
| HbA1c (%) | 7.1 ± 1.7 | 7.9 ± 1.9 |
| Blood pressure | ||
| Systolic (mmHg) | 115 ± 9.1 | 116.7 ± 8.9 |
| Diastolic (mmHg) | 68.4 ± 6.7 | 70.5 ± 6.8 |
| Cholesterol | ||
| LDL (mg/dL) | 89.7 ± 23.9 | 94.8 ± 23.6 |
| HDL (mg/dL) | 40.9 ± 8.6 | 42.3 ± 9 |
| Triglycerides (mg/dL) | 103.7 [32.7, 748.6] | 113 [36.0, 907.2] |
| Fasting C-peptide (ng/mL) | 3.2 [0.7, 9.6] | 2.9 [0.5, 10.1] |
| Fasting glucose (mg/dL) | 129.7 [76.8, 249.8] | 149.3 [80.4, 295.1] |
| Comorbidities (%) | ||
| Hypertension | 162 (44.1) | 224 (61.0) |
| ACR ≥30 mg/g | 74 (20.1) | 166 (45.2) |
| ACR ≥300 mg/g | 16 (4.4) | 48 (13.1) |
| Medications | ||
| History of any hypertensive medication | 125 (34.0) | 216 (58.8) |
| History of any lipid-lowering medication | 47 (12.8) | 118 (32.2) |
| Ever smoked | 48 (12.8) | 161 (43.8) |
Data are means ± SD, median [IQR], or n (%).
Progression of DR and CSME
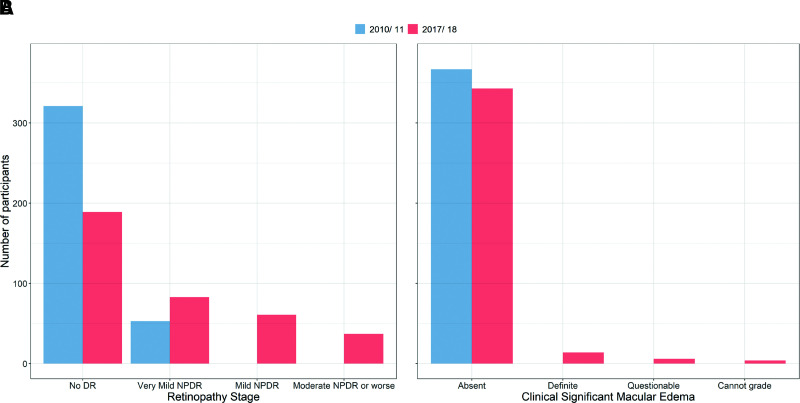
Among participants with repeated fundus photography, 315 (85.8%) participants had no signs of DR at the first exam with mean diabetes duration of 4.9 years, (Fig. 1 and Supplementary Table 2), and 52 (13.9%) participants had very mild NPDR (3). No participant had mild NPDR or worse. Seven years later, the percentage of participants with no retinopathy had decreased to 51% (n = 187). Among those who progressed, 5 (1.4%) had severe NPDR, 10 (2.7%) had early or stable treated PDR, and 4 (1%) had high risk PDR (Fig. 1 and Supplementary Table 2). CSME was not present in the original cohort. Seven years later, 14 participants (3.8%) had developed CSME affecting the center subfield (Fig. 1 and Supplementary Table 2).
Figure 1.
Results from the cohort (N = 367) with standard seven-field fundus exams during TODAY (2010–2011) and TODAY2 (2017–2018) for DR (A) and CSME (B).
Risk Factors for Progression of DR
Twenty-five percent (n = 93) of participants progressed ≥3 steps on the ETDRS scale (Table 2). Participants who progressed had significantly lower mean BMI and mean C-peptide as well as significantly higher mean HbA1c, mean blood pressure, mean concentration of triglycerides, mean fasting glucose, and prevalence of comorbidities over the course of TODAY and TODAY2 (Table 2). In univariate analyses, a 1-unit increase in HbA1c (e.g., from 7 to 8%) increased the probability of retinopathy progression by 2.3-fold. The presence of other comorbidities such as hypertension and kidney disease was associated with a two- to fourfold increased likelihood of retinopathy progression. Other factors associated with the probability of progression of retinopathy, in descending order of odds ratio, were diastolic blood pressure, fasting glucose, and mean triglycerides. A 5 kg/m2 increase of BMI reduced the probability of progression by ∼18 ± 8% (Table 3) (all P ≤ 0.02). Sex, HDL, and LDL were not significantly associated with retinopathy progression. In the multivariate analyses, only HbA1c had a significant impact on the progression of DR (Table 3) (P < 0.001).
Table 2.
Participant characteristics at follow-up fundus exam (2017–2018) by status of 3-step progression of retinopathy (N = 367)
| <3 steps progression* | ≥3 steps progression* | P † | |
|---|---|---|---|
| N | 274 | 93 | |
| Female sex | 183 (66.7) | 53 (56.9) | 0.10 |
| Race/ethnicity | 0.17 | ||
| Hispanic | 107 (39.1) | 39 (41.9) | |
| Non-Hispanic Black | 89 (32.5) | 37 (39.8) | |
| Non-Hispanic White | 60 (21.9) | 11 (11.8) | |
| Other | 18 (6.5) | 6 (6.5) | |
| Age (years) | |||
| At baseline | 13.7 ± 2.0 | 13.9 ± 2.1 | 0.31 |
| At exam | 25.3 ± 2.4 | 25.7 ± 2.6 | 0.18 |
| Diabetes duration (years) | 11.9 ± 1.5 | 12.1 ± 1.5 | 0.25 |
| BMI (kg/m2) | 36.8 ± 8 | 34.6 ± 6.7 | 0.01 |
| HbA1c (%) | 7.7 ± 1.9 | 10.3 ± 1.4 | <0.0001 |
| Blood pressure | |||
| Systolic (mmHg) | 116.5 ± 8.6 | 119.5 ± 9.9 | 0.01 |
| Diastolic (mmHg) | 70.4 ± 6.6 | 74.6 ± 7.4 | <0.0001 |
| Cholesterol | |||
| HDL (mg/dL) | 42.5 ± 9.3 | 42 ± 9.2 | 0.60 |
| LDL (mg/dL) | 94.6 ± 24.1 | 94.4 ± 21.4 | 0.93 |
| Triglycerides (mg/dL)§ | 102.2 [38.3, 771.6] | 128.5 [41.3, 1,211.2] | 0.001 |
| Fasting C-peptide (ng/mL)§ | 3 [0.5, 11.0] | 2.5 [0.5, 6.8] | 0.0004 |
| Fasting glucose (mg/dL)§ | 129.7 [79.3, 286.7] | 189.8 [87.2, 340.2] | <0.0001 |
| Comorbidities | |||
| Hypertension | 154 (56.2) | 70 (75.3) | 0.001 |
| ACR ≥30 mg/g | 103 (37.6) | 63 (67.7) | <0.0001 |
| ACR ≥300 mg/g | 21 (7.7) | 27 (29.0) | <0.0001 |
| Medications | |||
| History of any hypertensive medication | 151 (55.1) | 65 (69.9) | 0.01 |
| History of any lipid-lowering medication | 84 (30.7) | 34 (36.5) | 0.30 |
| Ever smoked | 39 (14.2) | 9 (9.7) | 0.72 |
Data are mean ± SD, median [IQR], or n (%) unless otherwise indicated. BP, blood pressure.
P values from Fisher exact test for categorical variables and Student t test for continuous variables.
Log-transformed for testing.
Based on original ETDRS scale on patient level (18 steps).
Table 3.
Predictors of ≥3 steps progression of retinopathy based on logistic regression models (N = 367)
| Predictor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Sex (male vs. female) | 1.52 (0.94, 2.46) | 0.09 | ||
| Race/ethnicity | ||||
| Non-Hispanic Black vs. Hispanic | 1.14 (0.67, 1.94) | 0.63 | ||
| Non-Hispanic White vs. Hispanic | 0.50 (0.24, 1.05) | 0.069 | ||
| Age (years) | ||||
| At baseline | 1.06 (0.95, 1.20) | 0.30 | ||
| At exam | 1.07 (0.97, 1.18) | 0.17 | 1.10 (0.96, 1.25) | 0.17 |
| Diabetes duration (per year) | 1.10 (0.94,1.29) | 0.24 | ||
| BMI (per 5 kg/m2 increase)* | 0.82 (0.69, 0.97) | 0.02 | 0.83 (0.64, 1.07) | 0.15 |
| HbA1c (per %)* | 2.30 (1.90, 2.78) | <0.0001 | 1.93 (1.49, 2.50) | <0.0001 |
| Blood pressure | ||||
| Diastolic (per 10 mmHg increase)* | 2.31 (1.63, 3.27) | <0.0001 | 1.62 (0.78, 3.38) | 0.20 |
| Systolic (per 10 mmHg increase)* | 1.44 (1.11, 1.87) | 0.006 | 0.94 (0.52, 1.73) | 0.85 |
| Cholesterol | ||||
| Mean HDL (per 10 mg/dL increase)* | 0.93 (0.72, 1.21) | 0.60 | ||
| Mean LDL (per 10 mg/dL increase)* | 1.00 (0.90, 1.10) | 0.93 | ||
| Mean triglycerides (per 10 mg/dL increase)* | 1.03 (1.01, 1.05) | 0.0006 | 1.01 (0.99, 1.04) | 0.20 |
| Fasting C-peptide (ng/mL) * | 0.69 (0.56, 0.85) | 0.0004 | 0.92 (0.68, 1.25) | 0.60 |
| Fasting glucose (per 10 mg/dL increase) * | 1.28 (1.21, 1.37) | <0.0001 | 1.06 (0.97, 1.15) | 0.20 |
| Comorbidities (%) | ||||
| Hypertension | 1.89 (1.14, 3.13) | 0.01 | ||
| ACR ≥30 mg/g | 3.49 (2.12, 5.74) | <0.0001 | 1.09 (0.52, 2.30) | 0.82 |
| ACR ≥300 mg/g | 4.93 (2.62, 9.27) | <0.0001 | 2.00 (0.80, 4.97) | 0.14 |
| Medications (%) | ||||
| History of any hypertensive medication | 2.37 (1.40, 4.02) | 0.001 | 0.75 (0.35, 1.64) | 0.48 |
| History of any lipid-lowering medication | 1.30 (0.80, 2.14) | 0.29 | ||
| Smoking (ever vs. never)* | 0.90 (1.08, 1.85) | 0.66 | ||
Based on cumulative exposure until follow-up Fundus exam.
Conclusions
After ∼10 years of follow-up and an average diabetes duration of 12 years, nearly one-half of the TODAY study participants developed DR. In the 7 years between retinal assessments, participants progressed from at most very mild NPDR on initial assessment to more advanced stages of DR, including 5% of participants progressing to severe NPDR or PDR despite being, on average, only 25 years of age. CSME, not detected on the initial assessment, was present in 3.8% of participants on fundus photography 7 years later.
The prevalence rates of DR in younger patients with type 2 diabetes have been reported from other cross-sectional studies across various populations and have ranged from 4 to 37% (16–19). Notably, these studies included wide age ranges for diabetes diagnosis, spanned longer diabetes durations, and involved varied methods to detect DR. Our results rigorously confirm the presence of, and progression to, advanced retinal pathology over only 7–8 years in youth-onset type 2 diabetes. Of clinical concern, the prevalence of DR in our cohort is nearly twice the 28.5% prevalence reported for adults with type 2 diabetes aged 40 years and older with an average diabetes duration of 15 years as previously reported by National Health and Nutrition Examination Survey (NHANES) (20). Perhaps the elevated prevalence and accelerated progression of DR in youth-onset T2D or type 2 diabetes compared with adult-onset type 2 diabetes reflect the challenge of attaining and maintaining euglycemia in youth. The high rates of early treatment failure (i.e., defined as HbA1c ≥8% for 6 months or sustained metabolic decompensation requiring insulin) observed in the TODAY trial suggested that youth were less responsive to oral therapies used and experienced a more rapid loss of endogenous insulin production by β-cells compared with adults (21).
Indeed, even with an average diabetes duration of only approximately one decade, those who experienced a 3-step progression or more in DR grading had many of the well-recognized risk factors for DR from studies of adults with diabetes: higher HbA1c, blood pressure, and triglycerides as well as the presence of diabetic kidney disease (4,22). Not surprisingly, as in adults with short-duration type 2 diabetes, multivariate analyses identified glycemic control as the predominant risk factor for the development and progression of DR (23). Unlike in studies of adult-onset type 2 diabetes, however, males in our cohort were not more likely than females to experience progression of DR (19). As this cohort ages, one might hypothesize that hypertension and hyperlipidemia will play more significant roles, heralding concern for the proposed association of early retinal vascular changes with later cardiovascular disease risk (24). Future studies might include more detailed retinal vascular imaging to investigate the retina-heart connection in patients with youth-onset type 2 diabetes (25,26).
This is the first comprehensive report on the risk factors for progression of DR in youth-onset type 2 diabetes. The study strengths include the longitudinal study design and the systematic analysis of fundus photos performed by masked graders using the ETDRS scale in the TODAY/TODAY2 study. Although not all TODAY participants completed the two retinal assessments, the participants studied are a representative cohort, with no significant differences in clinical characteristics at TODAY baseline between those with repeat fundus examinations and the full TODAY cohort (Supplementary Table 1). Although the initial retinal assessment was performed early in the course of youth-onset type 2 diabetes, it is not a true baseline retinal assessment at the time of diabetes diagnosis. Yet, because only a minority of participants (13.9%) had developed retinopathy no more severe than very mild NPDR at the time of the initial assessment, this is a convincing baseline for analysis of further disease development and progression.
Given the accelerated decline of β-cell function documented among patients with youth-onset type 2 diabetes and the predominant role of glycemia in the progression of DR (19), screening and identification of at-risk youth with obesity who belong to historically marginalized populations for type 2 diabetes must occur routinely in clinical care and investigations to identify additional treatment options must continue. For youth with type 2 diabetes, aggressive management of glycemia from the time of diagnosis accompanied by the recommended annual screening exams for the development and progression of DR, with additional, more intensive monitoring as warranted by disease state, is critical to preserve vision into adulthood.
Article Information
Acknowledgments. The authors gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective Tribes or the Indian Health Service.
Funding. This work was completed with funding from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Institutes of Health (NIH) Office of the Director through grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The NIDDK project office was involved in all aspects of the study, including study design and conduct; collection, management, analysis, and interpretation of data; review and approval of the manuscript; and decision to submit the manuscript for publication.
Duality of Interest. The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, LifeScan, Inc., Pfizer, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.G.K. wrote the manuscript. D.U. conducted the statistical analyses and wrote sections of the manuscript. I.L., K.L.D., B.A.B., L.L., L.L.L., M.M., S.M.W., N.H.W., and P.Z. wrote sections of the manuscript and reviewed and edited the manuscript. D.U. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
TODAY Study Group Writing Committee. Rose Gubitosi-Klug (Rainbow Babies and Children’s Hospital and Case Western Reserve University School of Medicine, Cleveland, OH), Ingrid Libman (UPMC-Children’s Hospital of Pittsburgh, Pittsburgh, PA), Kimberly L. Drews (The Biostatistics Center, George Washington University, Rockville, MD), Diane Uschner (The Biostatistics Center, George Washington University, Rockville, MD), Barbara A. Blodi (University of Wisconsin, School of Medicine, Madison, WI), Lori Laffel (Joslin Diabetes Center, Boston, MA), Lynne L. Levitsky (Massachusetts General Hospital, Boston, MA), Mihai Mititelu (University of Wisconsin, School of Medicine, Madison, WI), Steven M. Willi (Children’s Hospital of Philadelphia, Philadelphia, PA), Neil H. White (Washington University in St. Louis School of Medicine, St. Louis, MO), and Phil Zeitler (University of Colorado Anschutz Medical Center, Aurora, CO).
Footnotes
Clinical trial reg. nos. NCT00081328, NCT01364350, NCT02310724, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.15183393.
Members of the TODAY Study Group Writing Committee are listed in the appendix. A complete list of the TODAY Study Group members can be found in the supplementary material online.
This article is part of a special article collection available at diabetesjournals.org/journals/collection/268/Serious-Later-Risks-Associated.
This article is featured in a podcast available at diabetesjournals.org/journals/pages/diabetes-core-update-podcasts.
Contributor Information
Collaborators: Jeanie B. Tryggestad, Megan M. Kelsey, Kimberly L. Drews, Steven D. Chernausek, Elia N. Escaname, Elvira Isganaitis, Sarah Macleish, Siripoom McKay, Jennifer Sprague, and Steve Willi
References
- 1. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 3. Hainsworth DP, Bebu I, Aiello LP, et al.; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC study. Diabetes Care 2019;42:875–88230833368 [Google Scholar]
- 4. Chew EY, Davis MD, Danis RP, et al.; Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group . The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 2014;121:2443–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. RISE Consortium; RISE Consortium Investigators . Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes 2019;68:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arslanian S, Kim JY, Nasr A, et al. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: who is worse off? Pediatr Diabetes 2018;19:205–211 [DOI] [PubMed] [Google Scholar]
- 7. Copeland KC, Zeitler P, Geffner M, et al.; TODAY Study Group . Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. TODAY Study Group . Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care 2013;36:1772–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bjornstad P, Drews KL, Caprio S, et al.; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeitler P, Epstein L, Grey M, et al.; TODAY Study Group . Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Early Treatment Diabetic Retinopathy Study Research Group . Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl.):823–833 [PubMed] [Google Scholar]
- 13. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial. Arch Ophthalmol 1995;113:36–51 [DOI] [PubMed] [Google Scholar]
- 14. White NH, Sun W, Cleary PA, et al. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol 2008;126:1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levey AS, Eckardt KU, Dorman NM, et al. Nomenclature for kidney function and disease-executive summary and glossary from a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Eur Heart J 2020;41:4592–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 2006;29:1300–1306 [DOI] [PubMed] [Google Scholar]
- 17. Nagi DK, Pettitt DJ, Bennett PH, Klein R, Knowler WC. Diabetic retinopathy assessed by fundus photography in Pima Indians with impaired glucose tolerance and NIDDM. Diabet Med 1997;14:449–456 [DOI] [PubMed] [Google Scholar]
- 18. Scott A, Toomath R, Bouchier D, et al. First national audit of the outcomes of care in young people with diabetes in New Zealand: high prevalence of nephropathy in Maori and Pacific Islanders. N Z Med J 2006;119:U2015. [PubMed] [Google Scholar]
- 19. Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010;304:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. TODAY Study Group . Postintervention effects of varying treatment arms on glycemic failure and β-cell function in the TODAY trial. Diabetes Care 2021;44:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodríguez-Poncelas A, Mundet-Tudurí X, Miravet-Jiménez S, et al. Chronic kidney disease and diabetic retinopathy in patients with type 2 diabetes. PLoS One 2016;11:e0149448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 2001;44:156–163 [DOI] [PubMed] [Google Scholar]
- 24. Shoeibi N, Bonakdaran S. Is there any correlation between diabetic retinopathy and risk of cardiovascular disease? Curr Diabetes Rev 2017;13:81–86 [DOI] [PubMed] [Google Scholar]
- 25. McClintic BR, McClintic JI, Bisognano JD, Block RC. The relationship between retinal microvascular abnormalities and coronary heart disease: a review. Am J Med 2010;123:374.e1–374.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Witt N, Wong TY, Hughes AD, et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension 2006;47:975–981 [DOI] [PubMed] [Google Scholar]