Key Points
Question
What are the associations between physician adenoma detection rates and patients’ risk of postcolonoscopy colorectal cancer across a broad range of adenoma detection rate values?
Findings
In a retrospective cohort study of 735 396 patients with 852 624 colonoscopies without detection of cancer, higher physician adenoma detection rates were significantly associated with a lower risk of postcolonoscopy colorectal cancer (hazard ratio, 0.97 per 1% absolute adenoma detection rate increase) across a broad range of adenoma detection rate values.
Meaning
The inverse associations between adenoma detection rates and postcolonoscopy colorectal cancers extend to higher adenoma detection rate values than commonly reported; these findings, with other factors, can inform targets for colonoscopy quality.
Abstract
Importance
Although colonoscopy is frequently performed in the United States, there is limited evidence to support threshold values for physician adenoma detection rate as a quality metric.
Objective
To evaluate the association between physician adenoma detection rate values and risks of postcolonoscopy colorectal cancer and related deaths.
Design, Setting, and Participants
Retrospective cohort study in 3 large integrated health care systems (Kaiser Permanente Northern California, Kaiser Permanente Southern California, and Kaiser Permanente Washington) with 43 endoscopy centers, 383 eligible physicians, and 735 396 patients aged 50 to 75 years who received a colonoscopy that did not detect cancer (negative colonoscopy) between January 2011 and June 2017, with patient follow-up through December 2017.
Exposures
The adenoma detection rate of each patient’s physician based on screening examinations in the calendar year prior to the patient’s negative colonoscopy. Adenoma detection rate was defined as a continuous variable in statistical analyses and was also dichotomized as at or above vs below the median for descriptive analyses.
Main Outcomes and Measures
The primary outcome (postcolonoscopy colorectal cancer) was tumor registry–verified colorectal adenocarcinoma diagnosed at least 6 months after any negative colonoscopy (all indications). The secondary outcomes included death from postcolonoscopy colorectal cancer.
Results
Among 735 396 patients who had 852 624 negative colonoscopies, 440 352 (51.6%) were performed on female patients, median patient age was 61.4 years (IQR, 55.5-67.2 years), median follow-up per patient was 3.25 years (IQR, 1.56-5.01 years), and there were 619 postcolonoscopy colorectal cancers and 36 related deaths during more than 2.4 million person-years of follow-up. The patients of physicians with higher adenoma detection rates had significantly lower risks for postcolonoscopy colorectal cancer (hazard ratio [HR], 0.97 per 1% absolute adenoma detection rate increase [95% CI, 0.96-0.98]) and death from postcolonoscopy colorectal cancer (HR, 0.95 per 1% absolute adenoma detection rate increase [95% CI, 0.92-0.99]) across a broad range of adenoma detection rate values, with no interaction by sex (P value for interaction = .18). Compared with adenoma detection rates below the median of 28.3%, detection rates at or above the median were significantly associated with a lower risk of postcolonoscopy colorectal cancer (1.79 vs 3.10 cases per 10 000 person-years; absolute difference in 7-year risk, −12.2 per 10 000 negative colonoscopies [95% CI, −10.3 to −13.4]; HR, 0.61 [95% CI, 0.52-0.73]) and related deaths (0.05 vs 0.22 cases per 10 000 person-years; absolute difference in 7-year risk, −1.2 per 10 000 negative colonoscopies [95%, CI, −0.80 to −1.69]; HR, 0.26 [95% CI, 0.11-0.65]).
Conclusions and Relevance
Within 3 large community-based settings, colonoscopies by physicians with higher adenoma detection rates were significantly associated with lower risks of postcolonoscopy colorectal cancer across a broad range of adenoma detection rate values. These findings may help inform recommended targets for colonoscopy quality measures.
This retrospective cohort study evaluates the association between physician adenoma detection rate values and risks of postcolonoscopy colorectal cancer and related deaths among 735 396 patients aged 50 to 75 years with negative colonoscopy results.
Introduction
Colorectal cancer is the third leading cause of cancer death in the United States.1 Colonoscopy screening reduces colorectal cancer incidence and deaths by removing precancerous adenomatous polyps (adenomas) and detecting cancers at an earlier, more treatable stage.2 However, failing to detect adenomas is associated with an increased risk of postcolonoscopy colorectal cancer (PCCRC),3,4,5 a cancer diagnosed after a colonoscopy in which no cancer was detected.6
Physician adenoma detection rate is the percent of colonoscopies (typically screening examinations) that detect at least 1 adenoma.7 Physician adenoma detection rates vary widely in practice,8,9,10,11,12 and studies have reported inverse associations between physician adenoma detection rates and the risk of PCCRC, which influences screening effectiveness.3,4,5 Current guidelines recommend physicians have adenoma detection rates of at least 30% for average-risk men and at least 20% for average-risk women.13 However, these metrics were based on studies from time periods when physician adenoma detection rates were lower. These studies also lacked sufficient precision to evaluate detection rates separately for men and women and across a broad range of values, including those above which there may be minimal further benefit in reducing cancer risk prior to rescreening.
This study investigated the relationship between physician adenoma detection rate and the risks of PCCRC and related deaths across multiple regions in large community-based populations with reliable pathologic review and cancer diagnosis.
Methods
Study Oversight and Setting
The study was approved with waivers of informed consent by each region’s institutional review board.
Three regions contributed data to the study: Kaiser Permanente Northern California (KPNC), Kaiser Permanente Southern California (KPSC), and Kaiser Permanente Washington (KPWA).14 KPNC and KPSC are separate integrated health care systems that collectively include 41 endoscopy centers. KPWA is a mixed-model health care system with 2 system-directed endoscopy centers. KPNC and KPSC implement colonoscopy screening by request, and for those not up to date, annual mailing of fecal immunochemical test kits.15 KPWA patients receive an annual reminder letter for colonoscopy or a fecal immunochemical test screening at a KPWA-owned or contracted facility.
Study Eligibility Criteria
The study population included patients aged 50 to 75 years at the time of a colorectal cancer–negative colonoscopy completed between January 2011 and June 2017, defined as a colonoscopy in which colorectal cancer was not detected within 6 months after the examination.6 Individuals with a prior colorectal cancer diagnosis or lower gastrointestinal tract surgery (eg, colectomy) were excluded. Individuals who were included had at least 1 year of health plan enrollment prior to their first recorded negative colonoscopy, for ascertaining history data; their physicians had to have performed at least 100 total colonoscopy examinations, including at least 25 screening examinations, during the calendar year prior to the negative colonoscopy date. This excluded physicians who infrequently performed colonoscopies (eg, surgeons).
Data Sources
Data on colonoscopy procedures, cancer diagnoses and deaths, and patient and physician characteristics were obtained from clinical and administrative databases. Colonoscopy procedures were identified using Current Procedural Terminology codes, International Classification of Disease procedure codes, Healthcare Common Procedure Coding System codes, and site-specific codes.16 All sites linked to cancer registries that report to the Surveillance, Epidemiology, and End Results (SEER) program and used SEER methods. Colorectal cancer was defined as an adenocarcinoma within the colon or rectum using registry cancer site group codes (21040 and 21050) and International Classification of Diseases for Oncology, Third Edition site (topography) codes (C18.0, C18.2-C18.9, C19.9, and C20.9) and histology codes (morphology) (8000, 8010, 8020, 8021, 8140, 8141, 8143, 8144, 8147, 8200, 8210, 8211, 8215, 8220, 8221, 8230, 8255, 8260-8263, 8323, 8410, 8430, 8440, 8470, 8480, 8481, 8490, 8510, 8560, 8570-8574, 8576) for adenocarcinoma. Data on cancer diagnoses and deaths through the end of the study period (December 31, 2017) were obtained from California and Washington State cancer registries; however, patients were censored (and discontinued follow-up for cancer diagnoses and deaths) on the date of health plan disenrollment.
Adenoma detection, histology, and location were ascertained using manually validated methods including systematized nomenclature of medicine (SNOMED) coding in electronic pathology databases (KPNC and KPSC)3 and natural language processing of pathology reports (KPWA). Internal manual validation demonstrated that SNOMED classified sessile serrated adenomas as adenomas. The KPWA natural language processing did not include sessile serrated adenomas as adenomas. For KPNC and KPSC, colonoscopy indication used a modified validated algorithm that incorporates administrative and clinical data.17,18 KPWA used natural language processing of colonoscopy reports to determine the indication for colonoscopy. Screening colonoscopies excluded surveillance and diagnostic colonoscopies (positive fecal tests including fecal immunochemical tests were considered diagnostic) and the small number of examinations with an unclassified indication.
Exposure
The primary exposure was physician adenoma detection rate calculated annually as the percentage of screening colonoscopies in which at least 1 adenoma was detected.3 All negative colonoscopies, regardless of indication, were assigned the physician’s adenoma detection rate based on screening examinations from the prior calendar year (eg, negative colonoscopies performed in 2011 and followed for PCCRC were assigned the endoscopist’s screening adenoma detection rate from 2010).3 Physician adenoma detection rates were also calculated separately for adenomas detected in the distal colon (ie, splenic flexure, descending colon, sigmoid colon, or rectum) and proximal colon (ie, cecum, ascending colon, hepatic flexure, or transverse colon) using KPNC and KPSC data only; adenoma location was not available for KPWA.
Outcomes
Consistent with World Endoscopy Organization PCCRC definitions,6 the primary outcome was a colorectal adenocarcinoma occurring at least 6 months after a negative colonoscopy; cancers diagnosed less than 6 months after the colonoscopy were considered cancers detected by that colonoscopy (eg, by a repeat colonoscopy for an inadequate bowel preparation).
Secondary outcomes were PCCRCs by location (proximal or distal colon), stage (early stage or advanced stage), stratified by patient demographics (sex and self-reported race and ethnicity), and PCCRC-related deaths. Cancer stage was defined using SEER summary staging (early stage: in situ, localized, or regional with direct extension only; advanced stage: regional or distant).
For each eligible colonoscopy, PCCRC follow-up ended at the earliest of the following reasons: (1) subsequent negative colonoscopy; (2) health plan disenrollment; (3) PCCRC diagnosis; (4) death; or (5) end of the study period (December 31, 2017, the latest cancer registry data available at analysis). For PCCRC-related deaths, follow-up ended at the earliest of the following reasons: (1) health plan disenrollment; (2) death; or (3) the end of the study period. Patients with a subsequent negative colonoscopy were monitored as a separate observation from the subsequent negative colonoscopy date.
Statistical Analyses
For associations with PCCRC and PCCRC-related deaths, adenoma detection rate was evaluated as a continuous variable, a dichotomous variable (ie, less than vs equal to or greater than the median), and to investigate potential threshold associations (by detection rates of <20%, 20%-24.9%, 25%-29.9%, 30%-34.9%, 35%-39.9%, 40%-44.9%, 45%-49.9%, and ≥50%). For other secondary outcomes, adenoma detection rate was evaluated as a continuous variable.
Summary statistics describe demographic and colonoscopy characteristics by adenoma detection rate group. Adenoma detection rate/PCCRC cumulative incidence curves used the Kaplan-Meier method.19
Cox proportional hazards regression was used to estimate adjusted hazard ratios (HRs) and 95% CIs for outcomes by adenoma detection rate group using the lowest (eg, <20%) as the referent. The HR was assumed to estimate risk. We accounted for clustering of patients within physicians using the robust sandwich variance estimator.20 Model covariates included patient sex, self-reported race and ethnicity, age, body mass index (calculated as weight in kilograms divided by height in meters squared; <25.0, 25.0-29.9, ≥30.0), Charlson comorbidity score (0, 1, ≥2), health system site (to adjust for differences in equipment or bowel preparation), colonoscopy year (to adjust for temporal trends, including cancer incidence), and colonoscopy indication (screening, nonscreening). Comparative absolute difference calculations in 7-year risk describe the number of excess PCCRC cases per 10 000 negative colonoscopies using the Kaplan-Meier cumulative incidence in the referent adenoma detection rate category and the relevant comparator covariate-adjusted HR for other adenoma detection rate categories.21
Adenoma detection rate/PCCRC associations were evaluated by sex (men, women) and by self-reported race and ethnicity (non-Hispanic Asian or Pacific Islander [Asian or Pacific Islander], non-Hispanic Black [Black], Hispanic, other [Native American, Alaska Native, multiracial or other race or ethnicity, or not otherwise specified], and non-Hispanic White [White]), consistent with the National Institutes of Health policy on inclusion of women and minorities.22
Effect modification of the association between adenoma detection rate as a continuous variable and PCCRC was evaluated using cross-product terms in the multivariable models. Heterogeneity in the associations between dichotomized adenoma detection rate (ie, <28.3% and ≥28.3%] and PCCRC by location and cancer stage was assessed using a joint Cox proportional hazards regression method proposed for analyzing competing risk events.23
The proportionality assumption was tested using the absence of statistically significant interaction between time, and each model covariate and was found to not be violated. Variables with missing values used a separate category of unknown.
All statistical tests were 2-sided; a P value of less than .05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, analyses of secondary end points should be interpreted as exploratory. Analyses used SAS/STAT software, version 9.4 (SAS Institute Inc).
Results
Cohort Characteristics
Among 1 545 601 colonoscopies performed in 2010-2017 by 4572 physicians, 1 251 623 were colonoscopies in 2011-2017 by 383 eligible physicians who performed at least 100 total colonoscopies and at least 25 screening colonoscopies per year (eFigure 1 in the Supplement). After excluding 398 999 ineligible procedures, the final analytic sample for outcome assessment included 852 624 negative colonoscopies performed by 383 physicians among 735 396 patients, which included 440 352 examinations among female patients (51.6%), 412 237 among male patients (48.4%), and 35 among patients of unknown sex (Table).
Table. Characteristics of Study Participants and Colorectal Cancer–Negative Colonoscopy Procedures.
| Colonoscopy proceduresa | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Adenoma detection rate, % | ||||||||
| <20 | 20-24.9 | 25-29.9 | 30-34.9 | 35-39.9 | 40-44.9 | 45-49.9 | ≥50 | ||
| All, No. | 852 624 | 166 650 | 153 253 | 156 944 | 132 983 | 99 414 | 70 809 | 43 759 | 28 812 |
| Region | |||||||||
| Northern California | 43.1 | 30.2 | 32.7 | 45.6 | 43.9 | 50.6 | 56.7 | 64.0 | 66.2 |
| Southern California | 53.6 | 67.7 | 64.2 | 51.1 | 53.0 | 45.0 | 39.0 | 33.0 | 30.4 |
| Washington State | 3.2 | 2.2 | 3.1 | 3.3 | 3.1 | 4.3 | 4.2 | 3.1 | 3.4 |
| Participant characteristics | |||||||||
| Age at colonoscopy, y | |||||||||
| Median (IQR) | 61.4 (55.5-67.2) | 61.0 (55.2-66.9) | 61.1 (55.3-67.0) | 61.5 (55.6-67.3) | 61.4 (55.5-67.3) | 61.6 (55.6-67.3) | 61.8 (55.8-67.6) | 61.7 (55.7-67.6) | 62.1 (56.1-67.9) |
| 50-59 | 43.7 | 45.4 | 44.8 | 43.2 | 43.6 | 43.1 | 41.9 | 42.4 | 41.0 |
| 60-69 | 41.9 | 41.0 | 41.4 | 42.3 | 41.9 | 42.2 | 42.8 | 42.4 | 42.3 |
| 70-75 | 14.4 | 13.6 | 13.8 | 14.5 | 14.5 | 14.7 | 15.3 | 15.3 | 16.7 |
| Sex, No.b | 852 589 | 166 644 | 153 245 | 156 939 | 132 979 | 99 409 | 70 806 | 43 758 | 28 809 |
| Men | 48.4 | 48.4 | 48.2 | 48.3 | 48.2 | 48.3 | 48.1 | 49.1 | 50.1 |
| Women | 51.6 | 51.6 | 51.8 | 51.7 | 51.8 | 51.7 | 51.9 | 50.9 | 49.9 |
| Race and ethnicity, No.c | 835 909 | 163 411 | 150 471 | 153 812 | 130 317 | 97 471 | 69 393 | 42 821 | 28 213 |
| Asian or Pacific Islander | 14.3 | 13.1 | 13.3 | 14.3 | 14.0 | 15.5 | 15.8 | 16.9 | 15.7 |
| Black | 9.6 | 9.1 | 9.5 | 10.9 | 11.2 | 10.1 | 7.7 | 6.2 | 6.6 |
| Hispanic | 21.6 | 23.0 | 25.1 | 20.0 | 21.4 | 22.5 | 18.4 | 18.0 | 16.2 |
| White | 54.2 | 54.5 | 51.8 | 54.6 | 53.2 | 51.6 | 57.8 | 58.6 | 61.1 |
| Otherd | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Body mass index, No.e | 704 543 | 139 737 | 128 503 | 1 306 68 | 108 402 | 81 807 | 57 302 | 35 124 | 23 000 |
| <25 | 26.5 | 25.8 | 26.0 | 26.9 | 26.3 | 26.7 | 27.5 | 26.9 | 27.2 |
| 25-29.9 | 36.7 | 36.8 | 36.9 | 36.6 | 36.5 | 36.6 | 36.6 | 36.5 | 36.5 |
| ≥30 | 36.9 | 37.4 | 37.1 | 36.5 | 37.2 | 36.7 | 35.9 | 36.6 | 36.3 |
| Charlson comorbidity score, No.f | 724 395 | 147 194 | 134 228 | 135 427 | 111 415 | 83 205 | 56 851 | 34 441 | 21 634 |
| 0 | 60.4 | 61.2 | 61.1 | 60.3 | 60.4 | 59.7 | 59.4 | 59.0 | 59.3 |
| 1 | 18.7 | 18.4 | 18.4 | 18.8 | 18.8 | 18.9 | 19.1 | 19.6 | 18.7 |
| 2-19 | 20.9 | 20.4 | 20.5 | 20.9 | 20.9 | 21.4 | 21.6 | 21.5 | 22.0 |
| Cancer-negative colonoscopy indication, No. | 851 357 | 166 535 | 153 058 | 156 738 | 132 770 | 99 186 | 70 620 | 43 699 | 28 751 |
| Screening | 29.9 | 33.1 | 33.0 | 29.7 | 29.4 | 28.1 | 25.5 | 24.0 | 22.6 |
| Biopsy sent to pathology | 45.3 | 30.6 | 38.6 | 45.5 | 50.5 | 55.3 | 60.9 | 64.8 | 70.2 |
| Adenoma detected | 29.1 | 17.4 | 24.0 | 28.5 | 32.9 | 37.1 | 42.5 | 45.8 | 52.2 |
| Diagnosticg | 54.7 | 53.6 | 52.7 | 54.7 | 54.4 | 55.6 | 57.3 | 58.4 | 57.0 |
| Biopsy sent to pathology | 59.0 | 44.2 | 52.4 | 58.9 | 62.4 | 66.9 | 71.0 | 73.6 | 78.0 |
| Adenoma detected | 39.3 | 26.1 | 33.6 | 38.3 | 41.9 | 46.7 | 50.6 | 52.8 | 58.3 |
| Surveillance | 15.5 | 13.3 | 14.2 | 15.6 | 16.2 | 16.3 | 17.1 | 17.6 | 20.4 |
| Biopsy sent to pathology | 65.0 | 49.7 | 57.2 | 63.5 | 67.9 | 72.9 | 76.4 | 78.4 | 83.9 |
| Adenoma detected | 47.4 | 31.9 | 39.8 | 45.3 | 50.2 | 54.6 | 60.0 | 61.9 | 68.6 |
| Follow-up time, median (IQR), y | |||||||||
| For PCCRC cases | 2.5 (1.0-4.3) | 3.4 (1.6-5.1) | 2.9 (1.4-4.7) | 2.7 (1.3-4.5) | 2.2 (0.9-3.8) | 2.4 (1.0-3.9) | 1.7 (0.7-3.3) | 1.4 (0.6-2.8) | 1.1 (0.5-2.6) |
| For PCCRC-related deaths | 2.7 (1.1-4.4) | 3.6 (1.7-5.2) | 3.1 (1.5-4.8) | 2.9 (1.3-4.6) | 2.3 (0.9-3.9) | 2.5 (1.1-4.0) | 1.7 (0.7-3.3) | 1.5 (0.6-2.9) | 1.1 (0.5-2.6) |
Abbreviation: PCCRC, postcolonoscopy colorectal cancer.
Numeric values are presented as percents unless otherwise indicated.
Numbers and percentages sum to those with known reported sex (not known for 35 patients).
Numbers and percentages sum to those with known reported race and ethnicity.
Other includes Native American, Alaska Native, those who reported multiple race and ethnicity, and those who reported race and ethnicity as other, not otherwise specified.
Body mass index (calculated as weight in kilograms divided by height in meters squared) was calculated from measures recorded as closely as possible to the start of the calendar year of the colonoscopy date.
Charlson comorbidity score (range, 0-19; 0 equates to no comorbidities) was calculated for the 365 days preceding the calendar quarter of the colonoscopy date.
Diagnostic indication included colonoscopy performed as follow-up to a positive fecal test (eg, a fecal immunochemical test).
The median patient age was 61.4 years (IQR, 55.5-67.2). Across adenoma detection rate groups, screening examinations comprised 22.6% to 33.1% of all negative colonoscopies with a known indication, and diagnostic examinations comprised 52.7% to 58.4%. eTable 1 in the Supplement summarizes the distribution of physicians and the sex and age of their patients whose screening colonoscopies were used as the basis for adenoma detection rate calculations across groups.
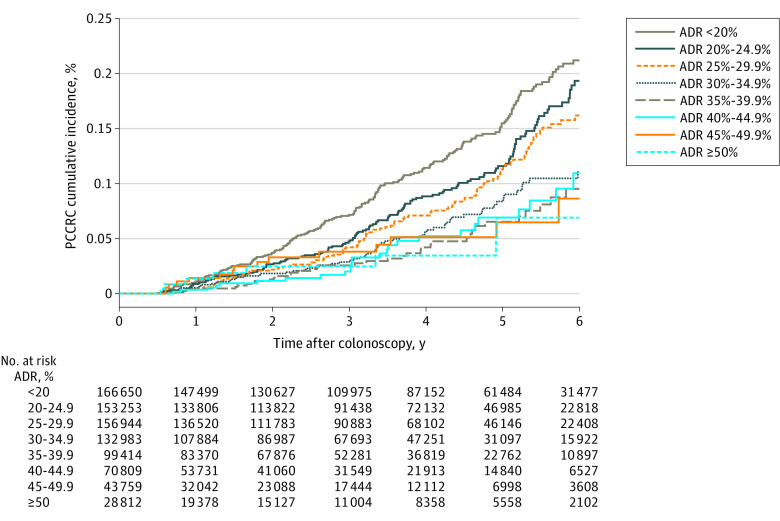
Among all negative colonoscopies (independent of indication), 619 PCCRC cases were detected after 2 435 707 person-years of follow-up, and 36 PCCRC-related deaths were detected after 2 436 995 person-years of follow-up (eTable 2 in the Supplement). Across adenoma detection rate groups (Table), PCCRC diagnoses ranged from 8 to 209 with follow-up durations of 49 430 to 579 842 person-years (median 2.5 years [IQR, 1.0-4.3]), and PCCRC-related deaths ranged from 0 to 13 with follow-up durations of 49 445 to 580 306 person-years (median 2.7 years [IQR, 1.1-4.4]). See Figure 1, eFigure 2, and eFigure 3 in the Supplement for PCCRC cumulative incidence curves for the 8 adenoma detection rate groups overall and by screening and diagnostic indications.
Figure 1. Postcolonoscopy Colorectal Cancer Cumulative Incidence Stratified by Physician Adenoma Detection Rate Group.
ADR indicates adenoma detection rate; PCCRC, postcolonoscopy colorectal cancer.
Adenoma Detection Rate and Risk of PCCRC (Primary Outcome)
Higher physician adenoma detection rates as a continuous measure were significantly associated with lower risks of PCCRC (HR per 1% absolute adenoma detection rate increase, 0.97 [95% CI, 0.96-0.98]; eTable 2 in the Supplement).
Significant associations for adenoma detection rate as a continuous measure were present across all sites (for KPNC, HR per 1% absolute adenoma detection rate increase, 0.97 [95% CI, 0.96-0.98]; for KPSC, HR per 1% absolute adenoma detection rate increase, 0.97 [95% CI, 0.96-0.99]; and for KPWA, HR per 1% absolute adenoma detection rate increase, 0.96 [95% CI, 0.93-0.99]).
Statistically significant associations were seen across most adenoma detection rate categories, compared with detection rates of lower than 20%; for the higher detection rate categories of 45% to 49.9%, and 50% or higher, the CIs were wider and overlapped 1.0 (Figure 2A; eTable 2 in the Supplement).
Figure 2. Associations Between Physician Adenoma Detection Rate and Risk of Postcolonoscopy Colorectal Cancer and Related Deaths.
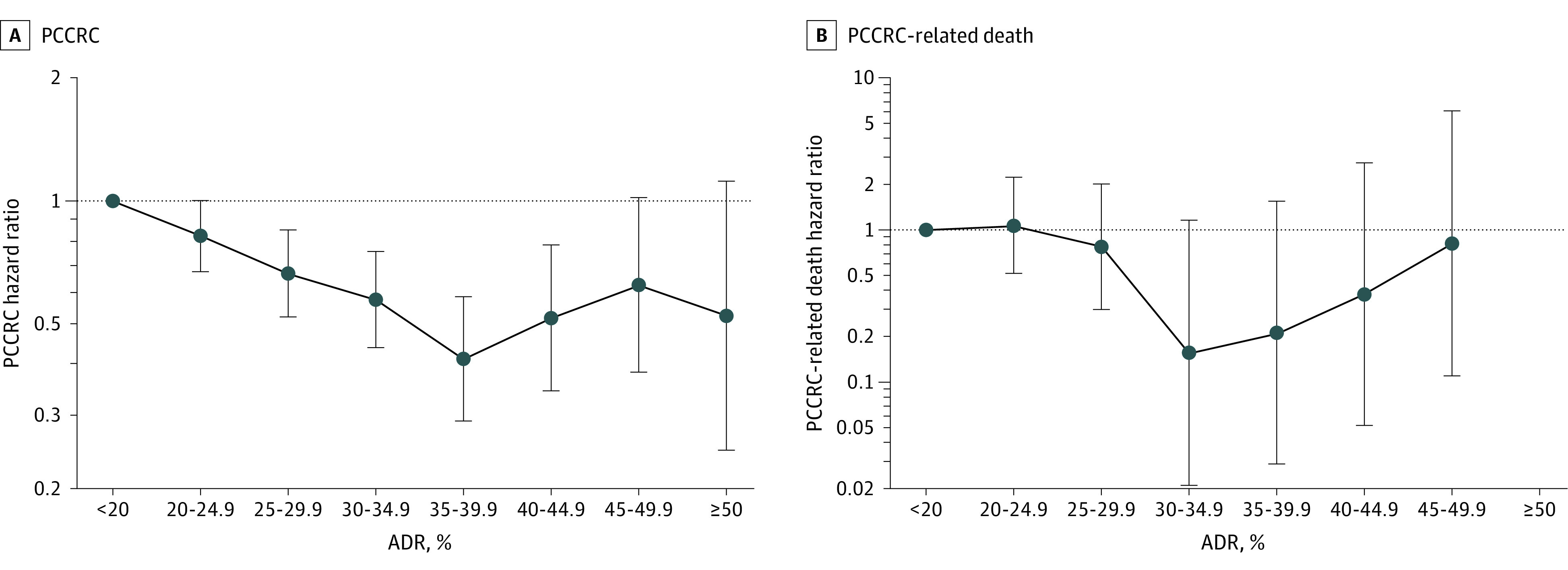
Whiskers indicate 95% CIs. Hazard ratios (HRs) were calculated for 8 adenoma detection rate (ADR) groups (<20% was set as the reference standard [eg, 20%-24.9% equates to 20%-<25%]). HRs were adjusted for patient sex, race and ethnicity, age, body mass index (calculated as weight in kilograms divided by height in meters squared), Charlson comorbidity score, health system site, colonoscopy year, and indication for the colorectal cancer–negative colonoscopy. See eTable 1 in the Supplement for data on the total number of physicians and patients upon which this figure is based. PCCRC indicates postcolonoscopy colorectal cancer.
Compared with adenoma detection rates below the median of 28.3%, detection rates at or above the median were significantly associated with a lower risk of PCCRC (1.79 vs 3.10 cases per 10 000 person-years; absolute difference in 7-year risk, −12.2 per 10 000 negative colonoscopies [95% CI, −10.3 to −13.4]; HR, 0.61; [95% CI, 0.52-0.73]) (eTable2 in the Supplement).
Adenoma Detection Rate and Risk of PCCRC by Sex and by Race and Ethnicity (Secondary Outcome)
Higher physician adenoma detection rates were significantly associated with lower risks of PCCRC in men (HR per 1% absolute adenoma detection rate increase, 0.97 [95% CI, 0.96-0.98]) and women (HR, 0.98 [95% CI, 0.97-0.99]; P value for interaction by sex = .18) (eTable 3 in the Supplement).
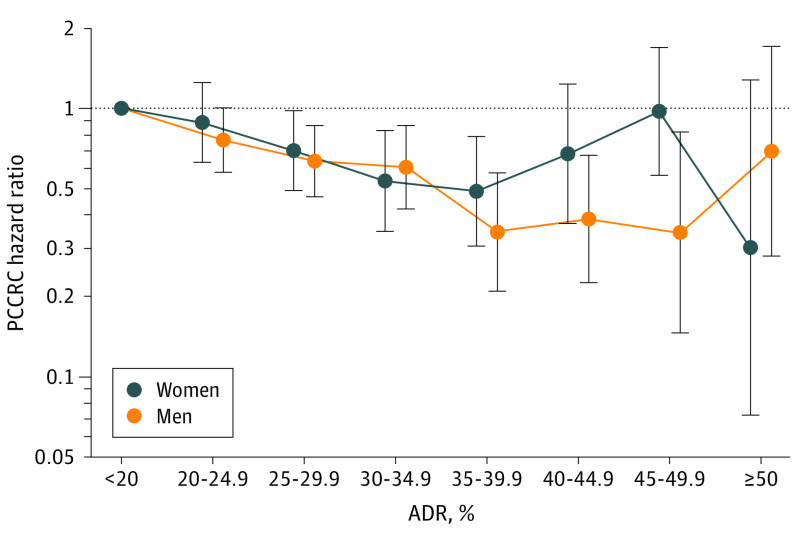
Compared with a detection rate of less than 20%, statistically significant associations with PCCRC were observed for adenoma detection rate categories of 25% to 29.9%, 30% to 34.9%, and 35% to 39.9% for women and 25% to 29.9%, 30% to 34.9%, 35% to 39.9%, 40% to 44.9%, and 45% to 49.9% for men (Figure 3; eTable 3 in the Supplement).
Figure 3. Associations Between Physician Adenoma Detection Rate and Risk of Postcolonoscopy Colorectal Cancer by Patient Sex.
Whiskers indicate 95% CIs. Hazard ratios (HRs) were calculated for 8 adenoma detection rate (ADR) groups (<20% was set as the reference standard [eg, 20%-24.9% equates to 20% to <25%]). HRs were adjusted for patient race and ethnicity, age, body mass index (calculated as weight in kilograms divided by height in meters squared), Charlson comorbidity score, health system site, colonoscopy year, and indication for the colorectal cancer–negative colonoscopy. See eTable 1 in the Supplement for data on the total number of physicians and patients upon which this figure is based. PCCRC indicates postcolonoscopy colorectal cancer.
Risk estimates did not differ significantly by race or ethnicity (P value for interaction = .60) (eTable 4 in the Supplement).
Adenoma Detection Rate and Risk of PCCRC by Location and Stage (Secondary Outcome)
The strength of the association between increasing adenoma detection rate and PCCRC differed between the proximal and distal colon (proximal colon HR per 1% absolute adenoma detection rate increase, 0.98 [98% CI, 0.97-1.00]; P = .007 vs distal colon HR, 0.96 (95% CI, 0.95-0.97]; P value for heterogeneity = .002) (Figure 4A; eTable 5 in the Supplement).
Figure 4. Associations Between Physician Adenoma Detection Rate and Proximal and Distal Postcolonoscopy Colorectal Cancer.
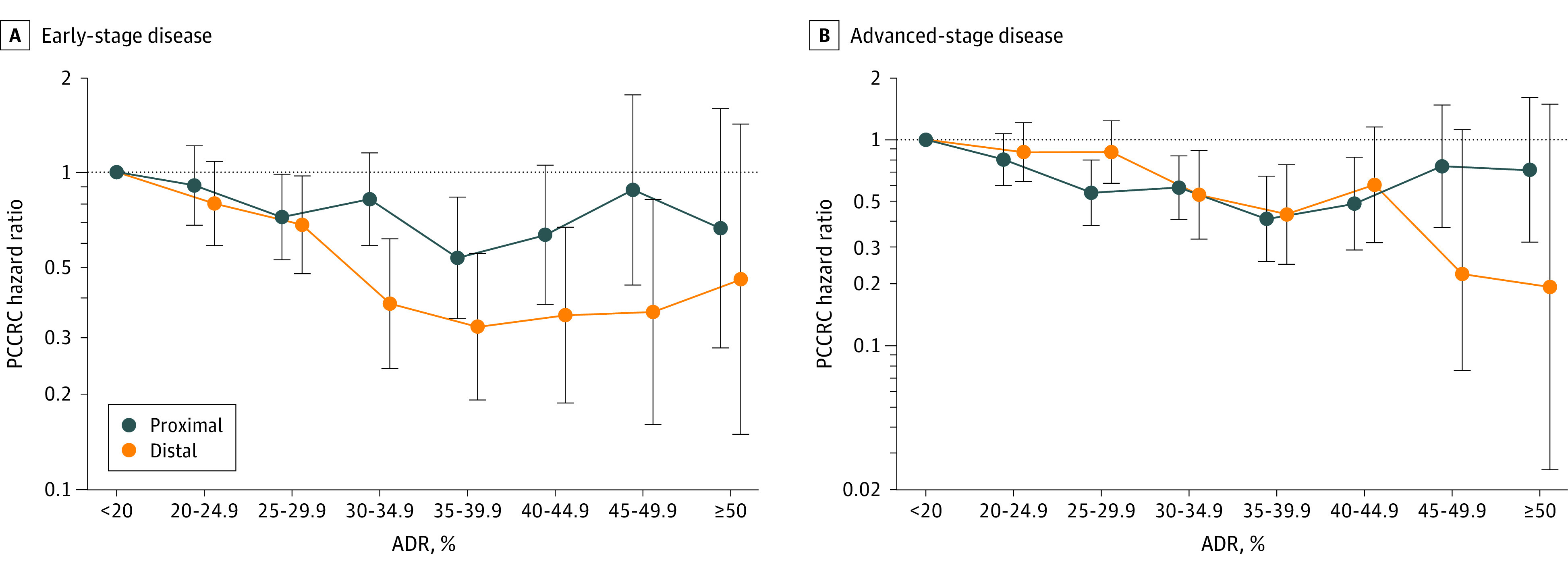
Whiskers indicate 95% CIs. Hazard ratios (HRs) were calculated for 8 adenoma detection rate (ADR) groups (<20% was set as the reference standard [eg, 20%-24.9% equates to 20% to <25%]). HRs were adjusted for patient sex, race and ethnicity, age, body mass index (calculated as weight in kilograms divided by height in meters squared), Charlson comorbidity score, health system site, colonoscopy year, and indication for the colorectal cancer–negative colonoscopy. Proximal location was defined as the cecum, ascending colon, hepatic flexure, and transverse colon. Distal location was defined as the splenic flexure, descending colon, sigmoid colon, and rectum. Colorectal cancer stage was defined using Surveillance, Epidemiology and End Results (SEER) summary staging (early stage: in situ, localized, or regional with direct extension only; advanced stage: regional or distant). See eTable 1 in the Supplement for data on the total number of physicians and patients upon which this figure is based. PCCRC indicates postcolonoscopy colorectal cancer.
We also calculated adenoma detection rates for the proximal and distal colon separately. Higher proximal adenoma detection rates were not significantly associated with a lower risk of PCCRC in the proximal colon (the CI included 1.0; HR for each 1% absolute adenoma detection rate increase, 0.99 [95% CI, 0.97-1.00]; P = .06), whereas higher distal adenoma detection rates were significantly associated with a lower risk of PCCRC in the distal colon (HR, 0.97 [95% CI, 0.95-0.99]).
Performance of colonoscopy by physicians with higher adenoma detection rates was significantly associated with a lower risk of early- and advanced-stage PCCRC (for both outcomes: HR per 1% absolute adenoma detection rate increase, 0.97 [95% CI, 0.96-0.99]), and the risk estimates did not differ significantly (P value for heterogeneity by stage = .30) (Figure 4B; and eTable 6 in the Supplement).
Adenoma Detection Rate and Risk of PCCRC-Related Death (Secondary Outcome)
Performance of colonoscopy by physicians with higher adenoma detection rates was significantly associated with a lower risk of PCCRC-related death (HR for each 1% absolute adenoma detection rate increase, 0.95 [95% CI, 0.92-0.99]; HR for adenoma detection rates of ≥28.3% vs <28.3%, 0.26 [95% CI, 0.11-0.65]; 0.05 vs 0.22 cases per 10 000 person-years; absolute difference in 7-year risk, −1.2 per 10 000 negative colonoscopies [95% CI, −0.80 to −1.69]) (Figure 2B; eTable 2 in the Supplement). Compared with detection rates of lower than 20%, the higher adenoma detection rate categories were not significantly associated with death from PCCRC. The HR point estimates for many adenoma detection rate categories were below 1, but the CI crossed the null.
Discussion
In a cohort of patients enrolled in Kaiser Permanente health plans in Northern and Southern California and Washington State, having a colonoscopy for any indication performed by a physician with a higher adenoma detection rate was significantly associated with a lower risk of PCCRC.
This work extends the findings of 4 prior studies of adenoma detection rate and PCCRC outcomes in multiple ways. Compared with a 2014 study from KPNC alone, which reported an inverse association between adenoma detection rate and PCCRC, advanced-stage disease, and related deaths,3 the present multiregion study is larger (852 624 vs 314 872 colonoscopies), includes almost 3 times as many physicians (383 vs 136), evaluates and contrasts results across 3 separate health systems in 2 different states, permits more well-powered comparisons between men and women, and includes colonoscopy during a more contemporary time period (2010-2017 vs 1998-2010) with modern bowel preparation techniques and higher adenoma detection rate ranges.3 This allows for a comprehensive re-evaluation of quality metric goals, particularly for higher adenoma detection rate ranges. In a study from Poland with 45 026 screening colonoscopies, 186 endoscopists, and 42 PCCRC cases, analyses were primarily dichotomized into adenoma detection rates of 20% or below vs rates above 20%, and the study did not include detailed evaluations of categories of detection rates above 20%.4 A second study from Poland included 146 860 screening colonoscopies and evaluated physicians who reached or maintained an adenoma detection rate above 24.6% vs lower detection rates, but it did not include detailed evaluations rates above 25%. A US study involving 76 810 screening colonoscopies, 51 physicians, and 78 PCCRC cases also largely dichotomized adenoma detection rates by below 25% vs 25% or above.5 Other metrics have been proposed, such as total adenomas detected, although these have not been clearly demonstrated to be both practical to calculate and better than adenoma detection rate alone with respect to predicting future cancer risk.24
Early US gastroenterology society guidelines recommended adenoma detection rate targets of at least 25% in men and at least 15% in women at average risk for colorectal cancer.7 These were subsequently increased to 30% or above in men and 20% or above in women.13 European guidelines recommend a target of at least 25% for all patients aged 50 years and older.25 Although there are few net downsides to higher detection and removal rates for precancerous polyps, knowledge of associations between quality metrics and outcomes across the full range informs important targets for training, certification, and competence. Adenoma detection rate is associated with other quality metrics, such as completeness of the examination to the cecum and bowel preparation adequacy, given such metrics influence the ultimate goal: the detection of precancerous and cancerous lesions. The current findings can inform discussions regarding whether to increase minimum quality benchmarks for adenoma detection rates for all physicians beyond 20% to 30%, reassessment of whether separate benchmarks should be used for men vs women, and dialogue regarding potential feasible aspirational targets for adenoma detection rates higher than minimum quality benchmarks.24
Interventions to improve physician adenoma detection rates have been attempted, and while a few have reported positive results,5,26,27 many have been unsuccessful or difficult to implement in other settings. Given the strong, consistent associations of higher adenoma detection rates with colonoscopy effectiveness for reducing colorectal cancer incidence and mortality, the current results support more research to identify reliable and readily adoptable methods for increasing adenoma detection rates among physicians with lower values across diverse settings.
Higher adenoma detection rates were significantly associated with lower risks of PCCRC in both the proximal and distal colon, although the magnitude of the association was smaller for the proximal colon. Sessile serrated polyps may contribute to this difference, given their flat appearance impedes easy detection and they occur more frequently in the proximal colon. Internal validation studies indicate that polyps pathologically classified as sessile serrated polyps are captured at KPNC and KPSC as adenomas; however in earlier years, this polyp type was more often pathologically diagnosed as hyperplastic, which would not be included in adenoma detection rate calculations. The temporal increase in both endoscopic identification and removal of sessile serrated polyps, combined with increased pathologic accuracy in classification, would be expected to increase the future strength of the association between increasing adenoma detection rates and lower risks of proximal PCCRC.28 Practice varies regarding whether to include sessile serrated polyps within the adenoma category. Given data suggest similar associations between adenomas and sessile serrated adenomas as regards future cancer risk (assuming complete removal), there is no empirical reason for separating them by histologic type.29,30
Several factors may explain the nonsignificant associations between adenoma detection rates and cancer risk at higher detection rates including random chance (due to less precision of the estimates), fewer person-years of follow-up (given more higher detectors were in later years of the study interval), and total follow-up time (given missed polyps may take many years to develop into cancer). If the additional polyps found between high- and very-high-level detectors are small in size, which is likely, they may not substantially influence PCCRC rates prior to when repeat screening or surveillance at 5 to 10 years provides a subsequent opportunity for precancer polyp identification and removal. Follow-up time may especially influence the nonsignificant associations between higher adenoma detection rates and cancer-related mortality, given high detectors were more common during later study years, and multiple years may pass between cancer diagnosis and death.
Study strengths include the large number of colonoscopy patients, years of follow-up, PCCRC diagnoses, and physicians from several health care settings; the wide variability in physician adenoma detection rates; the evaluation of adenoma detection rates by colon location; the evaluation of multiple outcomes including PCCRC and related deaths; and the ability to adjust for or stratify by patient age, sex, race and ethnicity, body mass index, comorbidity, and procedure indication.
Limitations
This study has several limitations. First, there were relatively few PCCRC-related deaths and few PCCRC cases among select racial and ethnic groups, which decreased precision of risk estimates for subanalyses. Second, although the observed associations between higher adenoma detection rates and PCCRC appear to level off above 35% to 40%, higher adenoma detection rate associations are influenced by lower statistical power given comparatively fewer physicians with higher detection rates, fewer total negative colonoscopies for these physicians, and fewer person-years of follow-up after negative colonoscopies. Third, the results may not be generalizable to physicians with lower procedure volumes or to populations with different underlying adenoma prevalence rates or colorectal cancer-related risk factor distributions.
Conclusions
Within 3 large community-based settings, colonoscopies by physicians with higher adenoma detection rates were significantly associated with lower risks of PCCRC across a broad range of adenoma detection rates. These findings may help inform recommended targets for colonoscopy quality measures.
eAppendix. Discussion: Statistical Properties of Physician Adenoma Detection Rate and Its Limits as a Predictor of Cancer Outcomes
eTable 1. Distribution of Physicians and the Sex and Age of Their Patients Whose Screening Colonoscopies Were Used as the Basis for ADR Calculations Across ADR Categories in 2010-2016
eTable 2. Associations Between Physician Adenoma Detection Rate and Risk of Postcolonoscopy Colorectal Cancer and Related Death
eTable 3. Associations Between Physician Adenoma Detection Rate and Risk of Postcolonoscopy Colorectal Cancer, by Patient Sex
eTable 4. Associations Between Physician Adenoma Detection Rate and Risk of Postcolonoscopy Colorectal Cancer, by Patient Race and Ethnicity
eTable 5. Associations Between Physician Adenoma Detection Rate and Risk of Proximal and Distal Postcolonoscopy Colorectal Cancer
eTable 6. Associations Between Physician Adenoma Detection Rate and Risk of Early- and Advanced-Stage Disease
eFigure 1. Cohort Eligibility Flow Diagram
eFigure 2. Screening Colonoscopies: Postcolonoscopy Colorectal Cancer Cumulative Incidence Stratified by Physician Adenoma Detection Rate Group
eFigure 3. Diagnostic Colonoscopies: Postcolonoscopy Colorectal Cancer Cumulative Incidence Stratified by Physician Adenoma Detection Rate Group
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564-2575. doi: 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 3.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370(14):1298-1306. doi: 10.1056/NEJMoa1309086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362(19):1795-1803. doi: 10.1056/NEJMoa0907667 [DOI] [PubMed] [Google Scholar]
- 5.Kaminski MF, Wieszczy P, Rupinski M, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology. 2017;153(1):98-105. doi: 10.1053/j.gastro.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 6.Rutter MD, Beintaris I, Valori R, et al. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology. 2018;155(3):909-925.e3. doi: 10.1053/j.gastro.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Petrini JL, Baron TH, et al. ; ASGE/ACG Taskforce on Quality in Endoscopy . Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101(4):873-885. doi: 10.1111/j.1572-0241.2006.00673.x [DOI] [PubMed] [Google Scholar]
- 8.Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? a systematic review of intervention studies. Gastrointest Endosc. 2011;74(3):656-665. doi: 10.1016/j.gie.2011.04.017 [DOI] [PubMed] [Google Scholar]
- 9.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355(24):2533-2541. doi: 10.1056/NEJMoa055498 [DOI] [PubMed] [Google Scholar]
- 10.Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology. 2004;127(2):452-456. doi: 10.1053/j.gastro.2004.05.032 [DOI] [PubMed] [Google Scholar]
- 11.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102(4):856-861. doi: 10.1111/j.1572-0241.2006.01054.x [DOI] [PubMed] [Google Scholar]
- 12.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112(1):24-28. doi: 10.1016/S0016-5085(97)70214-2 [DOI] [PubMed] [Google Scholar]
- 13.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81(1):31-53. doi: 10.1016/j.gie.2014.07.058 [DOI] [PubMed] [Google Scholar]
- 14.Tiro JA, Kamineni A, Levin TR, et al. The colorectal cancer screening process in community settings: a conceptual model for the population-based research optimizing screening through personalized regimens consortium. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1147-1158. doi: 10.1158/1055-9965.EPI-13-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin TR. Colorectal cancer screening: money isn’t everything . . . but it helps! Gastroenterology. 2017;153(5):1181-1183. doi: 10.1053/j.gastro.2017.09.026 [DOI] [PubMed] [Google Scholar]
- 16.Levin TR, Corley DA, Jensen CD, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155(5):1383-1391.e5. doi: 10.1053/j.gastro.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JK, Jensen CD, Lee A, et al. Development and validation of an algorithm for classifying colonoscopy indication. Gastrointest Endosc. 2015;81(3):575-582.e4. doi: 10.1016/j.gie.2014.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnett-Hartman AN, Kamineni A, Corley DA, et al. ; Colonoscopy Indication Algorithm Performance Across Diverse Health Care Systems in the PROSPR Consortium . Colonoscopy indication algorithm performance across diverse health care systems in the PROSPR consortium. EGEMS (Wash DC). 2019;7(1):37. doi: 10.5334/egems.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 20.Lin DY, Wei LJ. The robust inference for the proportional hazards model. J Am Stat Assoc. 1989;84(408):1074-1078. doi: 10.1080/01621459.1989.10478874 [DOI] [Google Scholar]
- 21.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492-1495. doi: 10.1136/bmj.319.7223.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institutes of Health . Inclusion of Women and Minorities as Participants in Research Involving Human Subjects. Accessed January 4, 2022. https://grants.nih.gov/policy/inclusion/women-and-minorities.htm
- 23.Xue X, Kim MY, Gaudet MM, et al. A comparison of the polytomous logistic regression and joint cox proportional hazards models for evaluating multiple disease subtypes in prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2013;22(2):275-285. doi: 10.1158/1055-9965.EPI-12-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedewa SA, Anderson JC, Robinson CM, et al. Prevalence of ‘one and done’ in adenoma detection rates: results from the New Hampshire Colonoscopy Registry. Endosc Int Open. 2019;7(11):E1344-E1354. doi: 10.1055/a-0895-5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaminski MF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49(4):378-397. doi: 10.1055/s-0043-103411 [DOI] [PubMed] [Google Scholar]
- 26.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6(10):1091-1098. doi: 10.1016/j.cgh.2008.04.018 [DOI] [PubMed] [Google Scholar]
- 27.Kaminski MF, Anderson J, Valori R, et al. Leadership training to improve adenoma detection rate in screening colonoscopy: a randomised trial. Gut. 2016;65(4):616-624. doi: 10.1136/gutjnl-2014-307503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li D, Woolfrey J, Jiang SF, et al. Diagnosis and predictors of sessile serrated adenoma after educational training in a large, community-based, integrated healthcare setting. Gastrointest Endosc. 2018;87(3):755-765.e1. doi: 10.1016/j.gie.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 29.Li D, Liu L, Fevrier HB, et al. Increased risk of colorectal cancer in individuals with a history of serrated polyps. Gastroenterology. 2020;159(2):502-511.e2. doi: 10.1053/j.gastro.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 30.Lee JK, Jensen CD, Levin TR, et al. Long-term risk of colorectal cancer and related death after adenoma removal in a large, community-based population. Gastroenterology. 2020;158(4):884-894.e5. doi: 10.1053/j.gastro.2019.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Discussion: Statistical Properties of Physician Adenoma Detection Rate and Its Limits as a Predictor of Cancer Outcomes
eTable 1. Distribution of Physicians and the Sex and Age of Their Patients Whose Screening Colonoscopies Were Used as the Basis for ADR Calculations Across ADR Categories in 2010-2016
eTable 2. Associations Between Physician Adenoma Detection Rate and Risk of Postcolonoscopy Colorectal Cancer and Related Death
eTable 3. Associations Between Physician Adenoma Detection Rate and Risk of Postcolonoscopy Colorectal Cancer, by Patient Sex
eTable 4. Associations Between Physician Adenoma Detection Rate and Risk of Postcolonoscopy Colorectal Cancer, by Patient Race and Ethnicity
eTable 5. Associations Between Physician Adenoma Detection Rate and Risk of Proximal and Distal Postcolonoscopy Colorectal Cancer
eTable 6. Associations Between Physician Adenoma Detection Rate and Risk of Early- and Advanced-Stage Disease
eFigure 1. Cohort Eligibility Flow Diagram
eFigure 2. Screening Colonoscopies: Postcolonoscopy Colorectal Cancer Cumulative Incidence Stratified by Physician Adenoma Detection Rate Group
eFigure 3. Diagnostic Colonoscopies: Postcolonoscopy Colorectal Cancer Cumulative Incidence Stratified by Physician Adenoma Detection Rate Group