Abstract
Background
Bone alterations have been observed in the course of HIV infection, characterized by a marked decrease in bone mineral density (BMD) and an increase in the frequency of fractures as a result of fragility. We aim to evaluate early changes in bone metabolic profile and the possible association with tenofovir and other nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs) in treatment-naïve HIV patients.
Methods
We conducted a prospective study in naïve HIV-infected adults (under 50 years), separated into three groups according to NRTI therapy: tenofovir disoproxil fumarate (TDF); tenofovir alafenamide (TAF) and abacavir (ABC). BMD and epidemiological, immunological and metabolic bone parameters were evaluated. Bone markers were analyzed in plasma at baseline, 12 and 48 weeks after initiating treatment.
Results
Average age of patients was 34.8 years (± 9.6). 92.4% of them with CD4 count > 200 cel/μL. At week 12 after starting treatment, both TDF [increase in PN1P (31.7%, p = 0.004), TRAP (11.1%, p = 0.003), OPN (19.3%, p = 0.045) and OC (38.6%, p = 0.001); decrease in OPG (-23.4%, p = 0.003)] and TAF [increase in 42.6% for CTX (p = 0.011), 27.3% for OC (p = 0.001) and 21% for TRAP (p = 0.008); decrease in OPG (-28.8%, p = 0.049)] presented a deep resorption profile compared to ABC, these differences in bone molecular markers, a tendency to equalize at week 48, where no significant differences were observed. Patients treated with TDF showed the greatest decrease in Z-score in both lumbar spine (LS) and femoral neck (FN) at week 48 without statistically significant differences.
Conclusion
Treatment-naïve HIV patients have a high prevalence of low bone density. Treatment with TDF is associated with greater bone deterioration at 12 and 48 weeks. TAF seems to present similar early bone deterioration at 12 weeks which disappears at 48 weeks.
Keywords: HIV, tenofovir, abacavir, osteopenia, osteoporosis, bone markers
1. INTRODUCTION
The emergence and development of combination antiretroviral treatment (cART) have increased not only the life expectancy of people living with Human Immunodeficiency Virus (PLHIV), but also associated comorbidities, the so-called serious non-AIDS events (SNAEs) [1, 2]. HIV infection affects tissues and organs such as the heart, kidney and liver, as well as the central nervous system and the musculoskeletal system, with changes similar to those observed in aging [3]. In recent years, the incidence of bone alterations during the course of HIV infection has increased, characterized by a notable decrease in bone mineral density (BMD) in the lumbar spine (LS) and femoral neck (FN), and an increase in the frequency of fractures [4]. Both the risk factors associated with HIV infection (malnutrition, low body weight, high tobacco and alcohol consumption, and low levels of vitamin D) and the disease itself induce bone remodeling through a complex interaction of T cells with osteoclasts and osteoblasts. In adults [3, 5-8], a decrease in BMD has also been associated with prolonged antiretroviral treatment, principally tenofovir disoproxil fumarate (TDF), indicating that the initial loss of BMD after beginning cART may be due to effects of the medication [5, 6]. However, several studies have shown bone damage in therapy-naïve HIV-infected patients [3, 9, 10], cases in which the main guidelines do not specifically recommend evaluating BMD [11-13].
Nucleoside and nucleotide reverse-transcriptase inhibitors (NRTIs) are included in most of the recommended regimens for treating HIV infection. Among these, TDF has been widely used for many years based on its established efficacy and tolerance, as demonstrated in clinical trials and real-life studies [14-17]. It is widely used as first-line treatment of HIV infection, for pre- and post-exposure therapy, and for the treatment of hepatitis B virus infection. Adverse effects of TDF include proximal tubular kidney dysfunction, Fanconi syndrome and renal insufficiency [18, 19], in addition to the bone damage mentioned anteriorly in patients taking TDF for years [20, 21]. Recently, new drugs with an improved metabolic profile that apparently does not affect bone remodeling, such as tenofovir alafenamide (TAF), have been developed as an alternative to TDF [18, 19, 22].
Many clinical trials have demonstrated a tenofovir-associated bone loss in HIV patients [14, 15, 17, 20-24], both in therapy-naïve patients and in patients who have formerly received other antiretrovirals. These studies provide evidence for a direct role of tenofovir in BMD loss, as well as changes in bone remodeling biomarkers: C-terminal telopeptide of collagen type 1 (CTX), osteocalcin and procollagen type 1 N propeptide (P1NP), osteoprotegerin (OPG), sclerostin (SOST gene) and receptor activator for NF-κB ligand (RANKL) [25] but most of them show changes after 48, 96 or more weeks of treatment [26-29].
The aim of this study is to evaluate the early changes in bone remodeling in HIV patients without prior treatment during the first 12 and 48 weeks of treatment, analyzing a complete panel of bone profile markers and the changes in BMD observed by DXA.
2. MATERIALS AND METHODS
2.1. Subject and Design Inclusion and Exclusion criteria and Ethical Aspects
From May 2016 to September 2017, 110 treatment-naïve male patients under 50 years with a new diagnosis of HIV infection were included in the study. Before enrollment, the treatment was chosen by their physicians, in accordance with the standard of care based on current national and international guidelines.
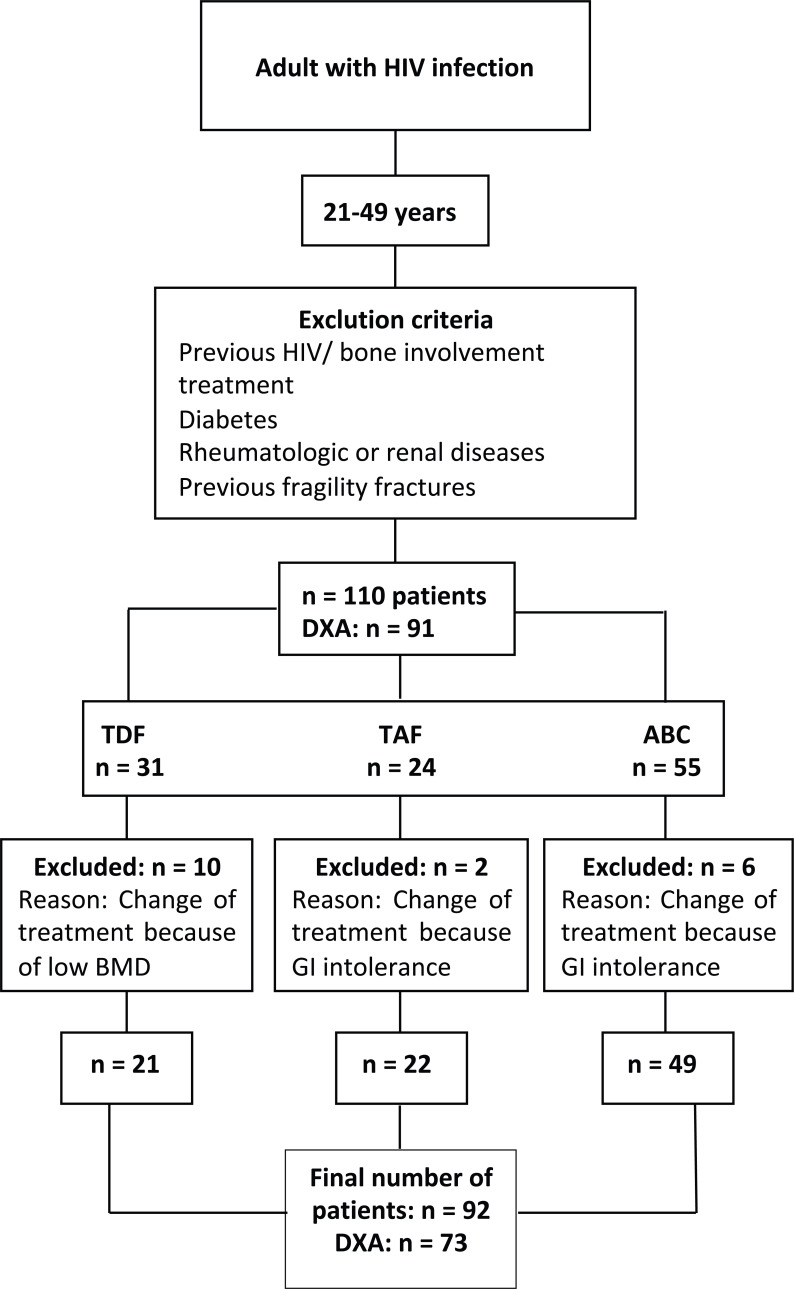
Of the 110 therapy-naïve patients originally enrolled, 18 patients were switched to different regimens due to gastrointestinal intolerance or bone involvement and were excluded from the analysis (Fig. 1). The 92 remaining patients were treated with TDF based regimen (elvitegravir/cobicistat/TDF/emtricitabine [Stribild®] available in hospital in 2016), TAF-based regimen (elvitegravir/cobicistat/TAF/emtricitabine [Genvoya®] available in hospital in 2017) and ABC based regimen (dolutegravir/ABC/lamivudine [Triumeq®]). Patients were followed for up to 12 months (48 weeks) after starting treatment. Immunological and bone turnover markers were evaluated at 0, 12 and 48 weeks after treatment initiation. DXA was done baseline and after 48 weeks from starting antiretroviral treatment.
Fig. (1).
Diagram of patients included in the study based on the antiretroviral treatments applied. GI (gastrointestinal), HIV (human immunodeficiency virus), TDF (tenofovir disoproxile fumarate), TAF (tenofovir alafenamide), ABC (abacavir).
Patients with the following criteria were excluded from the study: age over 50 years, previous antiretroviral treatment, bone-targeting treatment (denosumab, vitamin D), diabetes, treatment with corticosteroids, rheumatic diseases, renal failure, thyrotoxicosis, advanced liver disease, malabsorption syndrome, or neoplasia; based on a bone remodeling probably affected by these conditions.
Ethics committee approval was obtained from the institutional review board (PIC 155/16). All research was performed according to the right to privacy, guaranteed as stipulated in organic Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016 on data protection (GDPR), on the protection of personal data, and the Declaration of Helsinki. All patients provided signed informed consent before being included in the study.
2.2. Measurements and Reference Values
Epidemiological aspects, and lifestyle habits, including exercise, calcium intake, and toxic habits (alcohol, tobacco, and drug consumption), were registered using a physician-administered questionnaire. Tobacco use was defined as any previous or current history of smoking, alcohol abuse as an intake of more than 30 grams of alcohol per day, and drug abuse as consumption of recreational drugs once a week or more. The exercise was defined as physical activity three or more times a week. A calcium-rich diet was defined as at least three rations per day of milk, yogurt, cheese, and/or vegetables [30]. The patients’ height and weight were measured at the DXA appointment, and their body mass index (BMI) was calculated, with low weight defined as BMI < 20 kg/m2 [31, 32].
A fasting blood test was ordered before starting treatment (baseline), and at 12 and 48 weeks after treatment initiation. Hematologic parameters related to phospho-calcium metabolism, such as 25-OH-Vitamin D (30-50 ng/ml), parathyroid hormone (PTH) (10-70 pg/ml) [33, 34], thyroid hormone (TSH) (0.35-5.5μUI/ml), and renal function were measured using the Advia 2400 system (Siemens®, Munich, Germany). Immunovirological parameters, such as CD4 and CD8 were measured by flow cytometry, and the HIV-1 viral load was measured by PCR (Roche, Basel, Switzerland). Other parameters related to bone and calcium metabolism were measured, including osteocalcin (OC) (14-46 ng/dl) (measured by luminescence immunoassay), carboxy-terminal telopeptide of type 1 collagen (CTX) (0.064-0.5 ng/ml), procollagen type 1 N-terminal propeptide (P1NP) (10.4-62 ng/dl) (both were quantified by Electrochemiluminescence immunoassay (ECLIA) and tartrate-resistant acid phosphatase (TRAP) (<4.6 U/L) (analyzed by kinetic assay). All of them were performed in the Department of Clinical Analysis and Biochemistry at Hospital Fundación Jiménez Díaz.
Hip and spine bone DXA (Dual X-ray absorptiometry) scanning (HOLOGIC QDR 4500C, Marlborough, MA, USA) was carried out at baseline and after 48 weeks from starting antiretroviral treatment. BMD was measured at the LS (L2-L4), FN and right TH (total hip). As participants were all under 50 years of age, the Z-score was considered, estimating values below -2.0 as a low BMD for their age [35, 36].
2.3. Bone Marker Measurement by Luminex
Luminex multiplex assays are designed to simultaneously detect and quantitate multiple secreted proteins (e.g., cytokines and growth factors), and produce results comparable to conventional ELISA with greater efficiency and throughput [37]. Millipore`s MILLIPLEX MAP Human Bone Magnetic Bead Multiplex kit and MILLIPLEX MAP Human RANKL Magnetic Bead-Single Plex kit (Merk Millipore, Billerica, MA, USA) were used to analyze the different bone markers studied in plasma samples of patients. After centrifugation (15 min at 2500× g), plasma aliquots were kept at -80°C for a short period until used. ELISA multiplex was performed following the manufacturer’s protocol, including bead preparation and standard and quality controls. Each sample was analyzed in duplicate by LUMINEX MAGPIX® technology (Luminex Corp., Austin, TX, USA). The limits of detection were as follows: DKK-1 (Dickkopf-1), 5 pg/mL; OPG (Osteoprotegerin), 7 pg/mL; OPN (Osteopontin), 98 pg/mL; SOST (Sclerostin), 24 pg/mL; and RANKL, 4.88 pg/mL. Intra-assay coefficients of variation for the plasma bone markers studied were less than 10%; inter-assay coefficients of variation were less than 15% for both multiplex kits.
2.4. Statistical Analysis
Qualitative variables were summarized by frequencies and percentages, and quantitative by the mean and standard deviation or by median and quartiles, depending on the skewness of the data distribution. To study the potential relations between variables, Chi-squared test or Fisher’s exact test was used for qualitative variables, and Spearman’s rank correlation coefficient was used for quantitative variables. Relative changes in metabolic parameters were calculated by the expression 100x (follow-up visit - baseline visit)/baseline visit. For densitometries, due to the presence of zeroes, absolute changes were calculated by the expression (follow-up visit-baseline visit). Statistical significance of changes in each treatment group was evaluated by the Wilcoxon signed-rank test. To compare the changes between treatments, Wilcoxon rank-sum test was used. In order to study if changes in densitometries could be affected by changes in BMI, ANCOVA models were used to compare treatments adjusting by changes in BMI.
Finally, two-way ANOVA models were performed for densitometries and biomarkers, including time, treatment and its interaction. Statistical analysis was carried out using R, software version 3.6.0 (R Core team (2020); R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Cohort Analysis and Lifestyle
All of 92 patients included in the study 100% were male. The main characteristics of subjects are shown in Table 1. The average age was 34.8 years (± 9.6). 69% were European and 29% Latin American. All of them had acquired HIV infection through sexual intercourse with other men. At the time of diagnosis, 45.6% were in CDC stage 1 [38] (6.5% with CD4 <200 cell/µL). Regarding the NRTIs-based regimen, 21 subjects received TDF-based regimen, 22 TAF-based regimen and 49 ABC-based regimen.
Table 1.
Baseline demographics and disease characteristics.
| Variables | N | % |
|---|---|---|
| - | Age | - |
| - | 34.8 ± 9.6 | - |
| - | Gender | - |
| Male | 92 | 100 |
| - | Region of Birth | - |
| Europe | 63 | 68.5 |
| Latin American | 27 | 29.3 |
| Africa | 1 | 1.1 |
| Asia | 1 | 1.1 |
| - | Physical Activity | - |
| + 3 times per week | 46 | 59.7 |
| - 3 times per weeks | 31 | 40.3 |
| - | BMI (kg/m2) | - |
| - | 23.6 ± 3.0 | - |
| - | < 20 | 7.6 |
| - | Tobacco | - |
| Yes | 49 | 57 |
| No | 34 | 39.5 |
| Former smoker | 3 | 3.5 |
| - | Alcohol | - |
| Yes | 4 | 4.7 |
| No | 81 | 95.3 |
| - | Drugs | - |
| Yes | 30 | 34.9 |
| No | 55 | 64.0 |
| Former drug user | 1 | 1.2 |
| - | HIV Adquisiton | - |
| Homosexual | 92 | 100 |
| - | HIV Infection Stage Based on CD4+ T-lymphocyte Count | - |
| Stage 1 | 42 | 45.6 |
| Stage 2 | 43 | 46.3 |
| Stage 3 | 7 | 7.6 |
| - | CD4 Median | - |
| - | 505.73 cells/μl | - |
| - | CD4/CD8 Ratio | - |
| < 0.4 | 37 | 40.2 |
| - | Viral Load >100.000 | - |
| - | 18 | 19.5 |
| - | Vitamin D | - |
| < 30ng/ml | 78 | 85 |
| - | HBV | - |
| HBV Ag B- | 91 | 98.9 |
| HBV Ag B+ | 1 | 1.1 |
| - | HCV | - |
| IgG - | 90 | 97.8 |
| IgG+ | 2 | 2.2 |
| - | Baseline Z-score | - |
| Normal | 74 | 81.3 |
| Low BMD for age | 17 | 18.6 |
| - | 48-week Z-score | - |
| Normal | 59 | 80.2 |
| Low BMD for age | 14 | 19.1 |
Regarding other life habits and risk factors associated with a lower BMD, 48.9% consumed dairy products on a regular basis, and only 2.17% used steroids for bodybuilding.
We do not see significant changes between the treatment groups in the baseline sample for the measured parameters (Table 2), having a similar bone deterioration at baseline, regardless of the treatment group.
Table 2.
Comparison of baseline data of the biomarkers studied by treatment group. Data are presented as median and quartiles (percentiles of 25% and 75%) and were compared using the Wilcoxon rank test. Statistical significance was considered for p <0.05. p: TDF vs TAF vs ABC. p1: TDF vs TAF; p2: TDF vs ABC; p3: TAF vs ABC).
| Variables | TDF | TAF | ABC | P | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
| Ca | 9.40 (9.02, 9.5) | 9.30 (9.00, 9.6) | 9.5 (9.20, 9.8) | 0.132 | 0.733 | 0.076 | 0.152 |
| P | 3.50 (3.10, 4.00) | 3.20 (2.70, 3.60) | 3.20 (2.85, 3.55) | 0.361 | 0.241 | 0.190 | 0.858 |
| 25 OH Vit D | 22.5 (19.2, 26.8) | 15.8 (12.7, 23.6) | 20.2 (15.7, 24.0) | 0.083 | 0.049 | 0.136 | 0.175 |
| CD4 | 550 (340, 630) | 472 (376, 667) | 465 (334, 595) | 0.701 | 0.709 | 0.419 | 0.682 |
| CD8 | 1122 (792, 1225) | 893 (808, 1116) | 986 (769, 1276) | 0.692 | 0.356 | 0.553 | 0.795 |
| CD4/CD8 ratio | 0.47 (0.31, 0.72) | 0.48 (0.34, 0.66) | 0.48 (0.36, 0.67) | 0.957 | 0.903 | 0.804 | 0.844 |
| Cholesterol | 157 (139, 194) | 164 (147, 176) | 147 (128, 173) | 0.371 | 0.601 | 0.338 | 0.222 |
| TG | 106 (90.0, 158) | 88.0 (68.5, 111) | 96.5 (72.5, 143) | 0.204 | 0.063 | 0.268 | 0.393 |
| CTX | 0.28 (0.20, 0.38) | 0.33 (0.21, 0.46) | 0.27 (0.22, 0.37) | 0.420 | 0.473 | 0.604 | 0.201 |
| P1NP | 46.3 (31.0, 56.6) | 44.8 (37.3, 50.4) | 41.5 (31.1, 51.9) | 0.936 | 0.767 | 0.751 | 0.929 |
| OC | 15.8 (13.2, 21.4) | 14.2 (11.2, 20.6) | 15.9 (13.0, 20.4) | 0.774 | 0.450 | 0.716 | 0.682 |
| TRAP | 3.00 (2.40, 3.50) | 3.05 (2.52, 3.75) | 2.95 (2.50, 3.73) | 0.851 | 0.543 | 0.686 | 0.894 |
| PTH | 31.9 (22.2, 41.3) | 36.4 (25.5, 56.3) | 39.5 (27.0, 49.6) | 0.556 | 0.315 | 0.456 | 0.604 |
| DKK1 | 436 (261, 670) | 324 (249, 565) | 454 (276, 650) | 0.574 | 0.545 | 0.777 | 0.302 |
| OPG | 216 (119, 334) | 235 (121, 303) | 236 (157, 316) | 0.882 | 0.989 | 0.681 | 0.704 |
| OPN | 11025 (4435, 18313) | 12158 (7897, 17304) | 14171 (10203, 19242) | 0.229 | 0.737 | 0.150 | 0.202 |
| SOST | 1591 (1019, 2225) | 1158 (687, 2298) | 1468 (885, 2369) | 0.495 | 0.239 | 0.746 | 0.361 |
| RANKL | 87.7 (35.9, 121) | 88.2 (4.88, 188) | 52.9 (4.88, 131) | 0.654 | 0.677 | 0.562 | 0.424 |
| OPG/RANKL | 2.27 (0.89, 5.72 | 2.15 (1.07, 22.37) | 3.25 (1.20, 41.78) | 0.512 | 0.562 | 0.319 | 0.461 |
3.2. Comparative Evolution of Bone Metabolism at 12 Weeks After Initiating Treatment
12 weeks after initiating antiretroviral treatment, a significant increase in PN1P (31.7%, p = 0.004), TRAP (11.1%, p = 0.003), OPN (19.3%, p = 0.045) and OC (38.6%, p = 0.001) and a non-significant increase in CTX (62.1%, p = 0.064) were observed in those patients who received TDF when compared to baseline. Patients on TAF regimen presented a significant increase in the following proteins when compared to baseline: 42.6% for CTX (p = 0.011), 27.3% for OC (p = 0.001) and 21% for TRAP (p = 0.008). Patients who received ABC therapy presented a 54.2% increase in CTX (p=0.002), 17.2% in P1NP (p=0.001), 13.9% in OC (p =0.001) and 20% in TRAP (p = 0.002) when compared to baseline. In contrast, there were no significant changes in OPN after 12 weeks of treatment with ABC (Table 3). We observed a significant decrease in OPG both in patients taking TDF (-23.4%, p = 0.003) and TAF (-28.8%, p = 0.049), with non-significant decrease in the ABC group (-11.9%; p = 0.294) when compared with baseline measurements. DKK1 levels decreased significantly in patients treated with ABC (-16.8%, p = 0.007), but presented a non-significant downward trend in both the TDF and TAF groups (-21% with TDF, -15.7% with TAF, p>0.05) (Table 3). To verify treatment-induced changes in bone remodeling, we calculated the OPG/RANKL ratio, which tended to decrease [more with TAF (-33.67%, p = 0.922)] in all three treatment groups by 12 weeks of treatment, thus suggesting an appreciable increase in bone resorption. These and other bone parameters studied are shown in Table 3.
Table 3.
Changes in parameters of plasma samples between TDF, TAF and ABC after 12 weeks of treatment. Data are presented as median and quartiles (percentiles of 25% and 75%) and were compared using the Wilcoxon rank test. Statistical significance was considered for p <0.05. p: 12 weeks vs baseline; p1: TDF vs TAF (12 weeks vs baseline); p2: TDF vs ABC (12 weeks vs baseline); p3: TAF vs ABC (12 weeks vs baseline).
| Parameters Increments Baseline: 12 Weeks After Initiating Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | TDF | TAF | ABC | - | |||||
| Median (Q1, Q3) | p | Median (Q1, Q3) | p | Median (Q1, Q3) | p | p1 | p2 | p3 | |
| Ca | 0.53 (-0.53, 5.53) | 0.286 | 2.06 (-2.59, 5.53) | 0.374 | 0.00 (-3.06, 5.29) | 0.858 | 0.745 | 0.643 | 0.802 |
| P | -2.86 (-21.8, 18.4) | 0.629 | 3.70 (-2.63, 18.4) | 0.182 | 2.94 (-4.18, 25.3) | 0.166 | 0.256 | 0.165 | 0.962 |
| 25 OH Vit. D | -10.8 (-25.9, 156) | 0.980 | 47.8 (1.88, 156) | 0.003 | 2.73 (-28.6, 21.3) | 0.336 | 0.044 | 0.403 | 0.031 |
| CD4 | 26.5 (-4.91, 46.9) | 0.016 | 29.2 (2.00, 46.9) | 0.001 | 43.9 (22.6, 41.0) | <0.001 | 0.961 | 0.521 | 0.518 |
| CD8 | -11.6 (-22.8, 28.6) | 0.744 | 5.24 (-29.1, 28.6) | 0.798 | -3.47 (-21.2, 3.53) | 0.779 | 0.683 | 0.521 | 0.825 |
| CD4/CD8 ratio | 49.3 (15.9, 87.7) | 0.001 | 30.4 (7.58, 87.7) | 0.001 | 43.6 (10.8, 35.3) | <0.001 | 0.635 | 0.476 | 0.975 |
| Cholesterol | -1.90 (-12.0, 39.3) | 1.000 | 27.3 (10.2, 39.3) | <0.001 | 13.5 (1.76, 19.0) | 0.002 | 0.001 | 0.021 | 0.046 |
| Triglycerides | -0.96 (-6.50, 57.7) | 0.404 | 34.9 (10.6, 57.7) | 0.003 | -0.08 (-25.9, 41.2) | 0.224 | 0.124 | 0.823 | 0.138 |
| CTX | 62.1 (-6.56, 73.7) | 0.064 | 42.6 (-0.36, 73.7) | 0.011 | 54.2 (-3.13, 53.0) | 0.002 | 0.635 | 0.723 | 0.523 |
| P1NP | 31.7 (25.7, 38.0) | 0.004 | 3.79 (-15.9, 38.0) | 0.243 | 17.2 (4.16, 35.0) | 0.001 | 0.075 | 0.128 | 0.255 |
| OC | 38.6 (22.2, 62.7) | 0.001 | 27.3 (10.8, 62.7) | 0.001 | 13.9 (0.97, 67.6) | 0.001 | 0.433 | 0.096 | 0.435 |
| TRAP | 11.1 (7.69, 45.5) | 0.003 | 21.0 (-3.45, 45.5) | 0.008 | 20.0 (2.08, 15.5) | 0.002 | 0.564 | 0.939 | 0.824 |
| PTH | -1.88 (-37.4, 27.4) | 1.000 | -5.76 (-18.0, 27.4) | 0.934 | 11.5 (-25.3, 114) | 0.674 | 0.800 | 0.627 | 0.581 |
| DKK1 | -21.0 (-58.0, 25.3) | 0.098 | -15.7 (-60.1, 25.3) | 0.515 | -16.8 (-47.7, 37.6) | 0.007 | 0.707 | 0.736 | 0.766 |
| OPG | -23.4 (-41.5, 2.54) | 0.003 | -28.8 (-40.4, 2.54) | 0.049 | -11.9 (-37.6, 60.7) | 0.294 | 0.683 | 0.114 | 0.292 |
| OPN | 19.3 (-3.53, 45.1) | 0.045 | 13.6 (-6.93, 45.1) | 0.104 | 0.75 (-14.3, 30.5) | 0.670 | 0.552 | 0.107 | 0.269 |
| SOST | -9.8 (-26.3, 15.1) | 0.074 | -30.5 (-47.8, 15.1) | 0.216 | -8.77 (-24.7, 52.5) | 0.646 | 0.880 | 0.268 | 0.227 |
| RANKL | 17.7 (-29.5, 23.2) | 0.610 | 0.00 (-0.19, 23.2) | 0.675 | 0.00 (-12.4, 16.8) | 0.162 | 0.859 | 0.853 | 0.534 |
| OPG/RANKL | -23.08 (-62.23, 23.67) | 0.413 | -33.67 (-79.32, 71.60) | 0.922 | -23.24 (-90.89, 29.21) | 0.165 | 0.875 | 0.625 | 0.437 |
3.3. Comparative Evolution of Bone Metabolism at 48 Weeks After Initiating Treatment
We observed a significant increase in CTX (92.3%, p <0.001), P1NP (81.4%, p <0.001), OC (49.6%, p <0.001) and TRAP (51.1%, p = 0.002) in the group treated with TDF when compared to baseline. These increases were 31.6% (p = 0.027) for CTX, 17.8% (p = 0.017) for P1NP, 1.65% (p = 0.024) for OC and 120% for TRAP (p <0.001) in those who took TAF. Finally, in the ABC group we observed an increase of 45% (p <0.001) for CTX, 21.4% (p <0.001) for P1NP, 20.1% (p =0.001) for OC and 68.1% for TRAP (p <0.001) (Table 4).
Table 4.
Changes in parameters of plasma samples between TDF, TAF and ABC after 48 weeks of treatment. Data are presented as median and quartiles (percentiles of 25% and 75%) and were compared using the Wilcoxon rank test. Statistical significance was considered for p <0.05. p: 48 weeks vs baseline; p1: TDF vs TAF (48 weeks vs baseline); p2: TDF vs ABC (48 weeks vs baseline); p3: TAF vs ABC (48 weeks vs baseline).
| Parameters Increment Baseline: 48 Weeks After Initiating Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | TDF | TAF | ABC | - | |||||
| Median (Q1, Q3) | p | Median (Q1, Q3) | p | Median (Q1, Q3) | P | p1 | p2 | p3 | |
| Ca | 2.17 (1.06, 4.48) | 0.005 | 2.11 (-1.59, 4.48) | 0.095 | 1.52 (-2.28, 1.05) | 0.182 | 0.367 | 0.030 | 0.278 |
| P | -5.30 (-20.2, 18.2) | 0.404 | -3.03 (-7.69, 18.2) | 0.660 | 0.00 (-6.27, 25.0) | 0.465 | 0.345 | 0.153 | 0.655 |
| 25 OH Vit. D | 29.4 (-9.24, 44.9) | 0.083 | 15.1 (-11.7, 44.9) | 0.041 | 26.8 (-14.8, 42.6) | 0.021 | 0.823 | 0.914 | 0.607 |
| CD4 | 24.4 (-10.1, 98.1) | 0.074 | 29.4 (8.92, 98.1) | 0.001 | 45.6 (26.4, 162) | <0.001 | 0.523 | 0.120 | 0.349 |
| CD8 | -28.8 (-41.3, 20.6) | 0.051 | -0.27 (-44.6, 20.6) | 0.922 | -12.2 (-38.5, 16.0) | 0.516 | 0.367 | 0.117 | 0.987 |
| CD4/CD8 ratio | 102 (26.7, 130) | <0.001 | 75.0 (35.1, 130) | <0.001 | 65.3 (33.4, 170) | <0.001 | 0.756 | 0.732 | 0.912 |
| Cholesterol | 10.7 (0.53, 31.6) | 0.025 | 19.4 (11.1, 31.6) | 0.001 | 19.2 (-0.40, 31.5) | 0.001 | 0.333 | 0.604 | 0.592 |
| Triglycerides | -1.19 (-24.2, 49.3) | 0.860 | 34.8 (-17.7, 49.3) | 0.029 | 6.38 (-19.2, 142) | 0.198 | 0.088 | 0.134 | 0.975 |
| CTX | 92.3 (17.9, 81.8) | <0.001 | 31.6 (10.3, 81.8) | 0.027 | 45.0 (12.8, 122) | <0.001 | 0.139 | 0.615 | 0.166 |
| P1NP | 81.4 (47.5, 53.4) | <0.001 | 17.8 (6.68, 53.4) | 0.017 | 21.4 (2.84, 70.7) | 0.001 | 0.038 | 0.015 | 0.878 |
| OC | 49.6 (42.7, 50.7) | <0.001 | 1.65 (-10.0, 50.7) | 0.024 | 20.1 (-9.34, 126) | 0.011 | 0.033 | 0.083 | 0.275 |
| TRAP | 51.1 (23.1, 244) | 0.002 | 120 (31.5, 244) | <0.001 | 68.1 (23.3, 56.5) | <0.001 | 0.091 | 0.797 | 0.052 |
| PTH | 35.8 (12.3, 54.7) | 0.078 | 47.6 (-7.11, 54.7) | 0.027 | 22.2 (-8.25, 82.8) | 0.055 | 0.817 | 0.469 | 0.468 |
| DKK1 | 67.4 (-18.2, 17.8) | 0.049 | -12.3 (-50.5, 17.8) | 0.241 | -8.08 (-37.8, -17.7) | 0.582 | 0.046 | 0.096 | 0.647 |
| OPG | 20.1 (-12.2, 54.1) | 0.244 | 0.67 (-38.5, 54.1) | 0.623 | -11.8 (-32.5, 67.8) | 0.218 | 0.472 | 0.330 | 0.904 |
| OPN | 63.5 (16.5, 39.5) | 0.013 | 23.4 (-19.0, 39.5) | 0.123 | -1.59 (-27.1, 5.47) | 0.645 | 0.132 | 0.015 | 0.222 |
| SOST | 20.2 (-30.0, 95.5) | 0.497 | 16.9 (-37.3, 95.5) | 0.296 | -12.3 (-20.8, 78.6) | 0.275 | 0.943 | 0.814 | 0.890 |
| RANKL | 980 (588, 1425) | 0.004 | 302 (-72.2, 1425) | 0.164 | 1067 (325, 1619) | 0.001 | 0.278 | 0.769 | 0.258 |
| OPG/RANKL | -84.91 (-91.90, -59.94) | 0.105 | -62.41 (-85.96, 597.6) | 0.750 | -85.42 (-95.92, 31.37) | 0.275 | 0.500 | 0.500 | 0.500 |
Both DKK1 and OPN were significantly increased in TDF-treated patients (67.4%, p = 0.049 and 63.5% (p = 0.013) respectively) when compared to baseline. However, non-significant changes were observed for patients treated with TAF and ABC (Table 4). Regarding OPG changes at 48 weeks, we found non-significant differences in patients with TDF or TAF (increase) and ABC decrease) (Table 4). Finally, RANKL was increased in all three treatment groups, with values 980 times greater than baseline (p = 0.004) in patients taking TDF, and 1067 times greater (p=0.001) in those taking ABC (Table 4), while patients taking TAF presented measurements up to 302 times greater than baseline (p = 0.164) (Table 4). Regarding changes in the OPG/RANKL ratio, the downward trend observed at 12 weeks was still present at 48 weeks from the start of treatment, with the greatest decrease in TDF and ABC groups (Table 4). Other measurements are provided in supplementary (Tables 1 (105.4KB, pdf) -3 (105.4KB, pdf) ).
When we analyzed absolute changes in bone parameters between 12 or 48 weeks and baseline, we observed changes with statistical significance in CTX and P1NP with time (ANOVA) at 12 weeks of treatment (supplementary Table 4 (105.4KB, pdf) ), and also in CTX at 48 weeks with time and interaction, where we also find significant changes in P1NP, OC, TRAP and RANKL (supplementary Table 5 (105.4KB, pdf) ).
3.4. Comparing Treatment-Related Changes in Z-scores During the 48-week Study
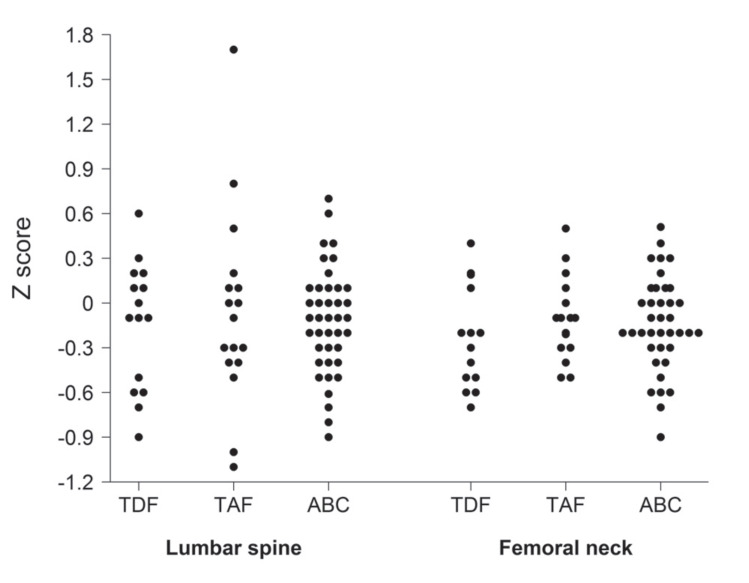
We observed a general deterioration in bone quality in HIV patients after 48 weeks, regardless of the treatment received. Baseline and 48 weeks after treatment DXAs were completed in 73 out of 92 patients (patients lost to follow-up included 6 from the TDF, 5 from the TAF, and 8 from the ABC group). As all our patients were under 50 years old, we calculated the Z-score. We found that 18.6% of our patients had a Z-score below -2 at the beginning of the study, being similar (19.1%) at the end of the study (Table 1). We analyzed the differences in Z-score at the LS and FN sites between baseline and 48 weeks for the three treatments (Table 5). We observed that patients treated with TDF had the greatest decrease in Z-score in both locations (LS and FN), with significant changes compared to baseline. However, there were no statistically significant changes Z- score across different treatment groups (Fig. 2, Table 5). No significant differences were found across different groups and no correlation was found between changes in Z-score and changes in BMI in any group (Supplementary Table 6 (105.4KB, pdf) -7 (105.4KB, pdf) ). Spearman´s rank correlation coefficient analyses showed an association between CD4 and CD4/CD8 ratio with the Z-score at 48 weeks in the total cohort (Supplementary Table 8 (105.4KB, pdf) ). No statistically significant changes were found for Z-score neither on time, treatment or interaction (Supplementary Table 9 (105.4KB, pdf) ). No changes in weight and height between baseline and 48 weeks post-treatment were observed.
Table 5.
Change in Z-score values after 48 weeks of treatment. Data are presented as mean ± SD and were compared using Student’s t-test. Statistical significance was considered as p <0.05. p: 48 weeks vs baseline (paired Student’s t-test); p1: TDF vs TAF (48 weeks vs baseline); p2: TDF vs ABC (48 weeks vs baseline); p3: TAF vs ABC (48 weeks vs baseline) (unpaired Student’s t-test).
| DXA Changes Baseline: 48 Weeks After Initiating Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | TDF | TAF | ABC | - | |||||
| Mean±SD | p | Mean±SD | p | Mean±SD | p | p1 | p2 | p3 | |
| Z-score LS | -0.140 ± 0.427 | 0.225 | -0.059 ± 0.654 | 0.716 | -0.117 ± 0.352 | 0.039 | 0.985 | 0.841 | 0.731 |
| Z-score FN | -0.214 ± 0.343 | 0.030 | -0.095 ± 0.275 | 0.175 | -0.136 ± 0.302 | 0.006 | 0.290 | 0.415 | 0.626 |
Fig. (2).
Changes in Z-score at 48 weeks. Graphs indicate increase or decrease in Z-score values between baseline and 48 weeks DXA scanning in lumbar spine (LS) and femoral neck (FN).
4. DISCUSSION
Our findings corroborate the negative impact of antiretroviral treatment on the bone profile of therapy-naive HIV patients under 50 years, appearing as early as 12 weeks after initiating therapy, and becoming more significant at 48 weeks. To the best of our knowledge, this is one of the first times that a complete panel of bone profile markers has been analyzed in HIV patients under 50 years, in the context of naïve treatment with different most used NRTIs, providing us with an early predictive value that is not considered in the vast majority of studies carried out in HIV patients treated with the same antiretrovirals featured here [14, 18, 19, 22, 39]. A few studies exist on early bone comorbidity in HIV patients treated with TDF or ABC [27, 29, 40, 41], but these are limited to only some bone remodeling markers. Stellbrink et al. [42] found similar findings in a 24 weeks analysis (CTX, P1NP and OC), comparing abacavir/lamivudine and tenofovir/emtricitabine (both in fixed-dose combination daily) plus efavirenz; however, our research contributes with a wide variety of bone turnover markers, such as TRAP, DKK1, OPG, OPN, SOST and RANKL, from as early as 12 weeks after the initiation of cART. Vlot et al. [26] studied BMD using DXA, in addition to measuring several serum bone remodeling markers (CTX, P1NP, OC), with long-term follow-up of patients (60 and 96 weeks), but also with less bone markers that we include.
Bone damage has been fundamentally associated with TDF [41-43] and, because emtricitabine (FTC) and lamivudine (3TC) are very similar molecules, TDF’s standard of comparison for bone damage has classically been abacavir. In recent years, a new tenofovir molecule, tenofovir alafenamide (TAF), has been introduced, widely replacing TDF, with better bone and renal profiles [40, 44]. We have observed that the changes observed in CTX and P1NP between 12 and 48 weeks are greater for TDF than for TAF, and that OC decreases at 48 weeks compared to 12 weeks in patients receiving TAF. In addition, we observed that DKK1 increased for TDF and decreased for TAF after 48 weeks of treatment. These trends in some bone markers might corroborate the reported better profile of TAF regarding bone quality compared to TDF, as DeJesus et al. [45] found in a randomized clinical trial. Seto et al. [46] also demonstrated that TAF is associated with a superior bone safety profile than that of TDF at more than 96 weeks of treatment, even in patients with risk factors associated with bone loss and higher initial FRAX scores. Our study demonstrates that these bone changes are observed even earlier, at the beginning of treatment (12 weeks), and in adult patients younger than 50 years, a population with a high rate of bone affectation even before treatment initiation [8, 47-49]. Therefore, PLHIV of all ages may potentially benefit from avoiding therapies containing TDF, which has repeatedly been shown to cause BMD loss [16, 26, 48, 50-52] .
Data exist suggesting bone health improvement in patients with low BMD treated with ABC during 48 weeks, after switching from TDF [25, 27, 53]. Our research detects no short-term differences between treatment groups, suggesting that more specific and comprehensive studies are needed (Tables 2-3). Both the 12 and 48-week OPG/RANKL ratios seem to indicate that changes in bone remodeling, and specifically changes in bone resorption, are stimulated more rapidly with TAF (as shown by a steeper downward trend at 12 weeks of treatment), and are more prolonged in the case of TDF and ABC, with patients in these groups reaching very considerable levels of resorption at 48 weeks of treatment.
The precise mechanism of BMD reduction associated with tenofovir is unclear; however, HIV patients treated with TDF have shown increases in bone turnover markers that suggest a higher activity of both osteoblasts and osteoclasts [28]. Recently, Conesa-Buendía et al. [54] have demonstrated that tenofovir directly promotes osteoclast differentiation and function (increasing the expression of bone markers such as cathepsin K, RANKL and NFATc1), including activation of MAPK and NFkB pathways.
It has been demonstrated that HIV alters -directly or indirectly- both osteoblasts and osteoclasts, affecting both osteoblastogenesis and osteoclastogenesis, and it has also been described that antiretroviral drugs can have an effect on bone cells, through alterations in RANK/RANKL levels, cytokine production, mitochondrial function, phosphate metabolism and vitamin D metabolism [55]. We observed that patients treated with TAF experienced early changes in bone remodeling (OPG/RANKL ratio), with a greater decrease compared to baseline at 12 weeks than in the case of the other treatments. However, although this ratio still tends to decrease at 48 weeks, the greatest resorption is seen in patients treated with TDF and ABC. The OPG / RANKL ratio is a widely used parameter in clinical studies to measure equilibrium in the OPG / RANK / RANKL system [56]. Previous research has confirmed the involvement of OPG / RANK / RANKL system dysregulation in the pathogenesis of reduced bone density among HIV-infected patients [57, 58]. The increase in the bone turnover characteristic of ART initiation in HIV patients seems to be associated with changes in systemic concentrations of OPG and RANKL. In our sample, we found that RANKL increased after initiating ART, with this increase being clearly evident after 48 weeks of treatment, while OPG levels decreased in plasma in the short-term (12 weeks), remaining more or less constant upon measurement at 48 weeks. However, we did not observe major changes in the OPG/RANKL ratio’s downward trend between treatment groups throughout the study. These findings seem to suggest that changes in bone turnover upon ART initiation (regardless of the treatment regimen) may be independent of the OPG / RANK / RANKL system, and that we need to correlate and assess other markers of bone resorption in order to arrive at a definitive conclusion [59-62].
Some studies correlate antiretroviral therapy with changes in bone and inflammatory markers [63-65] and compare TDF and TAF regimens. In our study, changes observed in bone resorption markers at 12 weeks were greater in patients treated with TDF compared with those receiving ABC. However, at 48 weeks, TDF and ABC showed more similar behavior, with TAF being slightly -though not significantly- less resorptive. In addition, we observed that the highest percentage of the average increase in plasma CTX and P1NP occurred in patients treated with TDF, with clinically relevant decreases in BMD of the hip and spine at week 48 with respect to baseline. It is interesting to note that in patients receiving TDF, temporal changes in CTX, a bone resorption marker, compared to changes in bone formation markers, suggest a stage in which bone destruction seems to overcome bone formation. Together, our results support the concept of reduction in systemic exposures to TDF being responsible for the minimal changes in bone renewal and the smaller decreases in BMD observed in patients receiving TAF compared to those receiving TDF [66-69].
Most of the studies and clinical trials focus on longer courses of treatment (starting at 48 weeks) [22, 70]. Our study provides data from the first year of treatment, and could help to understand how the choice of cART can influence the prevention and improvement of cART-derived bone comorbidities from early on. TDF has shown greater bone damage, but despite the fact that TAF and ABC have a better profile, in both, we can observe an increase in bone resorption markers. They can also be reasons for the bone deterioration in the first weeks of antiretroviral treatment, regardless of drugs, due to immune reconstitution or other factors. As recent data has demonstrated cART efficacy when only two drugs are used [71], it could be an option consider to avoid the use of any drug (TDF, TAF and ABC) when possible, with the intention to prevent and reduce bone deterioration as early as possible, while remembering to make the greatest possible emphasis on modifiable risk factors (principally treatment and lifestyle habits).
There are some limitations to our study. First of all, the sample size, which limits the power of some of the comparisons made between the groups. This might explain why no statistically significant changes between treatment groups were found for the DXA results at 12 months. Another reason might be the fact that the size of the ABC group was s more than double that of the TDF or TAF groups. Although we are aware that the limited (48-week) follow-up time could underestimate long-term changes, our data, which shows the presence of osteopenia/osteoporosis as early on as 48 weeks, plausibly indicate future outcomes. A second limitation concerns the lack of a study group consisting of individuals not infected with HIV; nonetheless, our results can be contrasted with well-established findings from studies conducted in the general population. Though our cohort consists of individuals who had lived with HIV infection for a short time, a certain bias may have been introduced in this regard, as we cannot rule out a slight impact of HIV infection on bone metabolism in the first stages of infection. However, the fact that over 90% of patients had CDC stage 1 and 2 of the disease indicates an appropriate degree of homogeneity. Finally, 20% of the patients could not have both DXA studies, due to having a result outside the window protocol. Women were not included due to a very low prevalence of new HIV infection in women in our setting.
Despite these limitations, we believe that our work offers some very novel data to the current literature by describing systemic markers of bone remodeling and, most importantly, evaluating short-term outcomes (12 and 48 weeks). There are not many studies assessing initial (12 weeks from starting treatment) bone involvement in HIV infected adults (under 50 years) [72-75] out of clinical trials, and, moreover, there are scarcely studies that evaluate the changes in a full panel of molecular bone markers.
CONCLUSION
In conclusion, we observe that treatment-naïve HIV patients have a high prevalence of low bone density. Furthermore, treatment with TDF is associated with greater bone deterioration at 12 and 48 weeks, while TAF appears to present similar early bone deterioration at 12 weeks that disappears at 48 weeks. Further studies are necessary to confirm whether our observations are also related to long-term clinical outcomes, for example, the incidence of bone fractures and other associated aspects.
AUTHORS’ CONTRIBUTIONS
AM, ACU and MG designed the experiments, analyzed and interpreted the results, wrote and revised the manuscript. PA was responsible for managing the patient database, clinical visits, treatment distribution, and writing and editing the manuscript. F.M.C.-B. was primarily responsible for carrying out all experimental procedures, analyzed and interpreted the results, and wrote and revised the manuscript. P.Ll.-G collected samples and performed some experimental procedures and edited and revised the manuscript. P. RP-T, BAA, ICA, LPP, RAP, MDC helped to recruit patients, performed clinical visits and revised the manuscript. RL and GH-B interpreted, revised and edited the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Approval of this study was obtained from The Medical Ethics Committee of the Fundación Jiménez Díaz University Hospital (Approval no. PIC155-016) at the Fundación Jiménez Díaz Medical University Hospital Ethics Committee, Community of Madrid, Spain.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All humans research procedures were in accordance with the standards set forth in the Declaration of Helsinki principles of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
The studied participants were informed about the present research, and a written consent form was taken from all of them before their enrollment.
STANDARDS OF REPORTING
The study conforms to the STARS (www. stard-statement.org) and TRIPOD guidelines (www. tripod-statement.org).
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the results and findings of this study are available within the article.
FUNDING
This research was financially supported by The Carlos III Health Institute through the “Miguel Servet” program (CP15/00053), co-funded by The European Regional Development Fund and research grants from the Spanish Carlos III Health Institute (PI16/00991 and PI19/00744).
ACKNOWLEDGEMENTS
The authors of the present survey would like to thank all the participants who enrolled in this study. We want to thank Ignacio Mahillo for his help with statistics. We thank Oliver Shaw and Dr. Bernadette Pfang for language editing. We want to thank Dr. Juan Sanchez-Verde Bilbao and Dr. Guido Rodríguez de Lema-Tapetado for the visual abstract.
CONFLICT OF INTEREST
The authors declare the following conflict of interest, financial or otherwise:
AM has filed a patent on the use of adenosine A2AR agonists to prevent prosthesis loosening (pending) and a separate patent on the use of A2AR agonists and agents that increase adenosine levels to promote bone formation/regeneration. PLl-G has filed a patent on the use of Lipocalin-2 as a treatment for abdominal aortic aneurysms, RL and GH-B have filled a patent on the use of 6-shogaol in osteoarthritis. ACU reports grants and personal fees from ViiV Healthcare, personal fees from Gilead, personal fees from Janssen, personal fees from Merck, outside the submitted work. MG reports grants and personal fees from ViiV Healthcare, personal fees from Gilead, personal fees from Janssen, outside the submitted work. BA reports personal fees from Gilead and ViiV Healthcare, outside the submitted work.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
- 1.Deeks S.G., Phillips A.N. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172–a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 2.Hsu D.C., Sereti I. Serious non-AIDS events: therapeutic targets of immune activation and chronic inflammation in HIV infection. Drugs. 2016;76(5):533–549. doi: 10.1007/s40265-016-0546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr. 2013;63:209–215. doi: 10.1097/QAI.0b013e318289bb7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown T.T., Qaqish R.B. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: A meta-analytic review. AIDS. 2006;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 5.Cunha B.M., Mota L.M.H., Pileggi G.S., Safe I.P., Lacerda M.V.G. HIV/AIDS and rheumatoid arthritis. Autoimmun. Rev. 2015;14(5):396–400. doi: 10.1016/j.autrev.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Jiménez B., Sainz T., Díaz L., Mellado M.J., Navarro M.L., Rojo P., González-Tomé M.I., Prieto L., Martínez J., de José M.I., Ramos J.T., Muñoz-Fernandez M.Á. Low bone mineral density in vertically HIV-infected children and adolescents: risk factors and the role of t-cell activation and senescence. Pediatr. Infect. Dis. J. 2017;36(6):578–583. doi: 10.1097/INF.0000000000001506. [DOI] [PubMed] [Google Scholar]
- 7.Atencio P., Cabello A., Conesa-Buendía F.M., Pérez-Tanoira R., Prieto-Pérez L., Carrillo I., Álvarez B., Arboiro-Pinel R., Díaz-Curiel M., Herrero-Beaumont G., Mediero A., Górgolas M. Increased risk factors associated with lower BMD in antiretroviral-therapy-naïve HIV-infected adult male. BMC Infect. Dis. 2021;21(1):542. doi: 10.1186/s12879-021-06263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atencio P, Arboiro-Pinel R, Cabello A, Conesa-Buendía FM, Górgolas M, Mediero A, Díaz-Curiel M. Trabecular bone score and 3D-DXA in young, antiretroviral treatment-naïve patients in Madrid. Arch Med Sci - Civiliz Dis. 2021;6:52–60. doi: 10.5114/amscd.2021.105843. [DOI] [Google Scholar]
- 9.Gill U.S., Zissimopoulos A., Al-Shamma S., Burke K., McPhail M.J.W., Barr D.A., Kallis Y.N., Marley R.T.C., Kooner P., Foster G.R., Kennedy P.T. Assessment of bone mineral density in tenofovir-treated patients with chronic hepatitis B: can the fracture risk assessment tool identify those at greatest risk? J. Infect. Dis. 2015;211(3):374–382. doi: 10.1093/infdis/jiu471. [DOI] [PubMed] [Google Scholar]
- 10.Yin M.T., Shiau S., Rimland D., Gibert C.L., Bedimo R.J., Rodriguez-Barradas M.C., Harwood K., Aschheim J., Justice A.C., Womack J.A. Fracture prediction with modified-FRAX in older HIV-infected and uninfected men. J. Acquir. Immune Defic. Syndr. 2016;72(5):513–520. doi: 10.1097/QAI.0000000000000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinzone M.R., Moreno S., Cacopardo B., Nunnari G. Is there enough evidence to use bisphosphonates in HIV-infected patients? A systematic review and meta-analysis. AIDS Rev. 2014;16(4):213–222. [PubMed] [Google Scholar]
- 12.AIDS Study Group (GeSIDA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Executive summary of the GeSIDA consensus document on control and monitoring of HIV-infected patients. Enferm. Infecc. Microbiol. Clin. 2019;37(7):467–475. doi: 10.1016/j.eimce.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 13.AIDSinfo. How to Cite the Adult and Adolescent Guidelines: Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents Guidelines for the Use of Antiretroviral Agents in HIV. 2009. Available from: https://aidsinfo.nih.gov/ http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 14.Gallant J.E. Efficacy and Safety of Tenofovir DF vs Stavudine in Combination Therapy in Antiretroviral-Naive Patients\textlessSUBTITLE\textgreaterA 3-Year Randomized Trial\textless/SUBTITLE\textgreater. JAMA. 2004;292:191. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 15.Squires K., Pozniak A.L., Pierone G., Jr, Steinhart C.R., Berger D., Bellos N.C., Becker S.L., Wulfsohn M., Miller M.D., Toole J.J., Coakley D.F., Cheng A. Tenofovir disoproxil fumarate in nucleoside-resistant HIV-1 infection: A randomized trial. Ann. Intern. Med. 2003;139(5 Pt 1):313–320. doi: 10.7326/0003-4819-139-5_Part_1-200309020-00006. [DOI] [PubMed] [Google Scholar]
- 16.Ryom L., Boesecke C., Bracchi M., Ambrosioni J., Pozniak A., Arribas J., Behrens G., Mallon P., Puoti M., Rauch A., Miro J.M., Kirk O., Marzolini C., Lundgren J.D., Battegay M. Highlights of the 2017 European AIDS Clinical Society (EACS) Guidelines for the treatment of adult HIV-positive persons version 9.0. HIV Med. 2018;19(5):309–315. doi: 10.1111/hiv.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arribas JR, Pozniak AL, Gallant JE, Dejesus E, Gazzard B, Campo RE, Chen S-S, McColl D, Holmes CB, Enejosa J. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008;47:74–78. doi: 10.1097/QAI.0b013e31815acab8. [DOI] [PubMed] [Google Scholar]
- 18.Izzedine H., Hulot J.S., Vittecoq D., Gallant J.E., Staszewski S., Launay-Vacher V., Cheng A., Deray G. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol. Dial. Transplant. 2005;20(4):743–746. doi: 10.1093/ndt/gfh658. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann A.E., Pizzoferrato T., Bedford J., Morris A., Hoffman R., Braden G. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin. Infect. Dis. 2006;42(2):283–290. doi: 10.1086/499048. [DOI] [PubMed] [Google Scholar]
- 20.Focà E., Motta D., Borderi M., Gotti D., Albini L., Calabresi A., Izzo I., Bellagamba R., Narciso P., Sighinolfi L., Clò A., Gibellini D., Quiros-Roldan E., Brianese N., Cesana B.M., Re M.C., Torti C. Prospective evaluation of bone markers, parathormone and 1,25-(OH) vitamin D in HIV-positive patients after the initiation of tenofovir/emtricitabine with atazanavir/ritonavir or efavirenz. BMC Infect. Dis. 2012;12:38. doi: 10.1186/1471-2334-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Güerri-Fernández R., Lerma-Chippirraz E., Fernandez Marron A., García-Giralt N., Villar-García J., Soldado-Folgado J., González-Mena A., Trenchs-Rodríguez M., Guelar A., Díez-Pérez A., Brown T.T., Knobel H. Bone density, microarchitecture, and tissue quality after 1 year of treatment with tenofovir disoproxil fumarate. AIDS. 2018;32(7):913–920. doi: 10.1097/QAD.0000000000001780. [DOI] [PubMed] [Google Scholar]
- 22.Sax P.E., Wohl D., Yin M.T., Post F., DeJesus E., Saag M., Pozniak A., Thompson M., Podzamczer D., Molina J.M., Oka S., Koenig E., Trottier B., Andrade-Villanueva J., Crofoot G., Custodio J.M., Plummer A., Zhong L., Cao H., Martin H., Callebaut C., Cheng A.K., Fordyce M.W., McCallister S. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 23.Chen R., Scherzer R., Hsue P.Y., Jotwani V., Estrella M.M., Horberg M.A., Grunfeld C., Shlipak M.G. Association of tenofovir use with risk of incident heart failure in HIV-infected patients. J. Am. Heart Assoc. 2017;6(4):e005387. doi: 10.1161/JAHA.116.005387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández Lisón L.C., Vázquez Domínguez B., Rodríguez Gómez F.J., Hevia Alonso A., Pujol de la Llave E. Estudio de utilización de Tenofovir DF en el tratamiento antirretroviral de gran actividad. An. Med. Interna. 2006;23(12):573–576. doi: 10.4321/S0212-71992006001200004. [DOI] [PubMed] [Google Scholar]
- 25.Negredo E., Diez-Pérez A., Bonjoch A., Domingo P., Pérez-Álvarez N., Gutierrez M., Mateo G., Puig J., Echeverría P., Escrig R., Clotet B. Switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: changes in bone turnover markers and circulating sclerostin levels. J. Antimicrob. Chemother. 2015;70(7):2104–2107. doi: 10.1093/jac/dkv063. [DOI] [PubMed] [Google Scholar]
- 26.Vlot M.C., Grijsen M.L., Prins J.M., de Jongh R.T., de Jonge R., den Heijer M., Heijboer A.C. Effect of antiretroviral therapy on bone turnover and bone mineral density in men with primary HIV-1 infection. PLoS One. 2018;13(3):e0193679. doi: 10.1371/journal.pone.0193679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen T.A., Jensen D., Tolstrup M., Nielsen U.S., Erlandsen E.J., Birn H., Østergaard L., Langdahl B.L., Laursen A.L. Comparison of bone and renal effects in HIV-infected adults switching to abacavir or tenofovir based therapy in a randomized trial. PLoS One. 2012;7(3):e32445. doi: 10.1371/journal.pone.0032445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramalho J., Martins C.S.W., Galvão J., Furukawa L.N., Domingues W.V., Oliveira I.B., Dos Reis L.M., Pereira R.M.R., Nickolas T.L., Yin M.T., Eira M., Jorgetti V., Moyses R.M. Treatment of human immunodeficiency virus infection with tenofovir disoproxil fumarate-containing antiretrovirals maintains low bone formation rate, but increases osteoid volume on bone histomorphometry. J. Bone Miner. Res. 2019;34(9):1574–1584. doi: 10.1002/jbmr.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ofotokun I., Titanji K., Vunnava A., Roser-Page S., Vikulina T., Villinger F., Rogers K., Sheth A.N., Lahiri C.D., Lennox J.L., Weitzmann M.N. Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS. 2016;30(3):405–414. doi: 10.1097/QAD.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanis J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359(9321):1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 31.Lewiecki E.M. Osteoporosis: Clinical Evaluation. Endotext. Available from: http://www.ncbi.nlm.nih.gov/books/NBK279049/
- 32.De Laet C., Kanis J.A., Odén A., Johanson H., Johnell O., Delmas P., Eisman J.A., Kroger H., Fujiwara S., Garnero P., McCloskey E.V., Mellstrom D., Melton L.J., III, Meunier P.J., Pols H.A., Reeve J., Silman A., Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos. Int. 2005;16(11):1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 33.Amrein K., Scherkl M., Hoffmann M., Neuwersch-Sommeregger S., Köstenberger M., Tmava Berisha A., Martucci G., Pilz S., Malle O. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur. J. Clin. Nutr. 2020;74(11):1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aloia J.F., Feuerman M., Yeh J.K. Reference range for serum parathyroid hormone. Endocr. Pract. 2006;12(2):137–144. doi: 10.4158/EP.12.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Assesment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO. Technical report series. Ginebra. Suiza. 1994 [PubMed] [Google Scholar]
- 36.2019 ISCD Official Positions - Adult - International Society for Clinical Densitometry (ISCD). 2019. Available from: http://www.iscd.org/official-positions/2019-iscd-official-positions-adult/
- 37.Cook D.B., McLucas B.C., Montoya L.A., Brotski C.M., Das S., Miholits M., Sebata T.H. Multiplexing protein and gene level measurements on a single Luminex platform. Methods. 2019;158:27–32. doi: 10.1016/j.ymeth.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Revised Surveillance Case Definition for HIV Infection - United States. 2014. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6303a1.htm?s_cid=rr6303a1_e. [PubMed]
- 39.Gallant J.E., Daar E.S., Raffi F., Brinson C., Ruane P., DeJesus E., Johnson M., Clumeck N., Osiyemi O., Ward D., Morales-Ramirez J., Yan M., Abram M.E., Plummer A., Cheng A.K., Rhee M.S. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3(4):e158–e165. doi: 10.1016/S2352-3018(16)00024-2. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Lu X., Yang X., Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: Meta-analysis. Medicine (Baltimore) 2016;95(41):e5146. doi: 10.1097/MD.0000000000005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orkin C., Molina J-M., Negredo E., Arribas J.R., Gathe J., Eron J.J., Van Landuyt E., Lathouwers E., Hufkens V., Petrovic R., Vanveggel S., Opsomer M. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): A phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5(1):e23–e34. doi: 10.1016/S2352-3018(17)30179-0. [DOI] [PubMed] [Google Scholar]
- 42.Stellbrink H.J., Orkin C., Arribas J.R., Compston J., Gerstoft J., Van Wijngaerden E., Lazzarin A., Rizzardini G., Sprenger H.G., Lambert J., Sture G., Leather D., Hughes S., Zucchi P., Pearce H. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin. Infect. Dis. 2010;51(8):963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 43.Han W.M., Wattanachanya L., Apornpong T., Jantrapakde J., Avihingsanon A., Kerr S.J., Teeratakulpisarn N., Jadwattanakul T., Chaiwatanarat T., Buranasupkajorn P., Ramautarsing R., Phanuphak N., Sunthornyothin S., Ruxrungtham K., Phanuphak P. Bone mineral density changes among people living with HIV who have started with TDF-containing regimen: A five-year prospective study. PLoS One. 2020;15(3):e0230368. doi: 10.1371/journal.pone.0230368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez-Barco E., Mallon P.W.G. What’s new in bone disease and fractures in HIV? Curr. Opin. HIV AIDS. 2021;16(3):186–191. doi: 10.1097/COH.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 45.DeJesus E., Haas B., Segal-Maurer S., Ramgopal M.N., Mills A., Margot N., Liu Y-P., Makadzange T., McCallister S. Superior efficacy and improved renal and bone safety after switching from a tenofovir disoproxil fumarate- to a tenofovir alafenamide-based regimen through 96 weeks of treatment. AIDS Res. Hum. Retroviruses. 2018;34(4):337–342. doi: 10.1089/aid.2017.0203. [DOI] [PubMed] [Google Scholar]
- 46.Seto W-K., Asahina Y., Brown T.T., Peng C-Y., Stanciu C., Abdurakhmanov D., Tabak F., Nguyen T.T., Chuang W-L., Inokuma T., Ikeda F., Santantonio T.A., Habersetzer F., Ramji A., Lau A.H., Suri V., Flaherty J.F., Wang H., Gaggar A., Subramanian G.M., Mukewar S., Brunetto M.R., Fung S., Chan H.L. Improved bone safety of tenofovir alafenamide compared to tenofovir disoproxil fumarate over 2 years in patients with chronic HBV infection. Clin. Gastroenterol. Hepatol. 2018:S1542-3565(18)30633-5. doi: 10.1016/j.cgh.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Guan W-M., Pan W., Yu W., Cao W., Lin Q., Zhang Z-Z., Song X-J., Li Y-L., Tian J-P., Xu Y., Li T.S., Hsieh E. Changes in trabecular bone score and bone mineral density in Chinese HIV-Infected individuals after one year of antiretroviral therapy. J. Orthop. Translat. 2021;29:72–77. doi: 10.1016/j.jot.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moran C.A., Weitzmann M.N., Ofotokun I., Neale Weitzmann M., Ofotokun I. Bone loss in HIV infection. Curr. Treat. Options Infect. Dis. 2017;9(1):52–67. doi: 10.1007/s40506-017-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad A.N., Ahmad S.N., Ahmad N. HIV Infection and Bone Abnormalities. Open Orthop. J. 2017;11:777–784. doi: 10.2174/1874325001711010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoy J.F., Grund B., Roediger M., Schwartz A.V., Shepherd J., Avihingsanon A., Badal-Faesen S., de Wit S., Jacoby S., La Rosa A., Pujari S., Schechter M., White D., Engen N.W., Ensrud K., Aagaard P.D., Carr A. Immediate Initiation of Antiretroviral Therapy for HIV Infection Accelerates Bone Loss Relative to Deferring Therapy: Findings from the START Bone Mineral Density Substudy, a Randomized Trial. J. Bone Miner. Res. 2017;32(9):1945–1955. doi: 10.1002/jbmr.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bloch M., Tong W.W., Hoy J., Baker D., Lee F.J., Richardson R., Carr A. Switch from tenofovir to raltegravir increases low bone mineral density and decreases markers of bone turnover over 48 weeks. HIV Med. 2014;15(6):373–380. doi: 10.1111/hiv.12123. [DOI] [PubMed] [Google Scholar]
- 52.Brown T.T., Moser C., Currier J.S., Ribaudo H.J., Rothenberg J., Kelesidis T., Yang O., Dubé M.P., Murphy R.L., Stein J.H., McComsey G.A. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J. Infect. Dis. 2015;212(8):1241–1249. doi: 10.1093/infdis/jiv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McComsey G.A., Lupo S., Parks D., Poggio M.C., De Wet J., Kahl L.P., Angelis K., Wynne B., Vandermeulen K., Gartland M., Cupo M., Aboud M. Switch from tenofovir disoproxil fumarate combination to dolutegravir with rilpivirine improves parameters of bone health. AIDS. 2018;32(4):477–485. doi: 10.1097/QAD.0000000000001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conesa-Buendía F.M., Llamas-Granda P., Larrañaga-Vera A., Wilder T., Largo R., Herrero-Beaumont G., Cronstein B., Mediero A. Tenofovir causes bone loss via decreased bone formation and increased bone resorption, which can be counteracted by dipyridamole in mice. J. Bone Miner. Res. 2019;34(5):923–938. doi: 10.1002/jbmr.3665. [DOI] [PubMed] [Google Scholar]
- 55.Wheater G., Elshahaly M., Tuck S.P., Datta H.K., van Laar J.M. The clinical utility of bone marker measurements in osteoporosis. J. Transl. Med. 2013;11:201. doi: 10.1186/1479-5876-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skoumal M., Kolarz G., Haberhauer G., Woloszczuk W., Hawa G., Klingler A. Osteoprotegerin and the receptor activator of NF-kappa B ligand in the serum and synovial fluid. A comparison of patients with longstanding rheumatoid arthritis and osteoarthritis. Rheumatol. Int. 2005;26(1):63–69. doi: 10.1007/s00296-004-0579-1. [DOI] [PubMed] [Google Scholar]
- 57.Fakruddin J.M., Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J. Biol. Chem. 2003;278(48):48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 58.Seminari E., Castagna A., Soldarini A., Galli L., Fusetti G., Dorigatti F., Hasson H., Danise A., Guffanti M., Lazzarin A., Rubinacci A. Osteoprotegerin and bone turnover markers in heavily pretreated HIV-infected patients. HIV Med. 2005;6(3):145–150. doi: 10.1111/j.1468-1293.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 59.Hikita A., Yana I., Wakeyama H., Nakamura M., Kadono Y., Oshima Y., Nakamura K., Seiki M., Tanaka S. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappaB ligand. J. Biol. Chem. 2006;281(48):36846–36855. doi: 10.1074/jbc.M606656200. [DOI] [PubMed] [Google Scholar]
- 60.Kearns A.E., Khosla S., Kostenuik P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008;29(2):155–192. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moir S., Fauci A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009;9(4):235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hofbauer L.C., Dunstan C.R., Spelsberg T.C., Riggs B.L., Khosla S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem. Biophys. Res. Commun. 1998;250(3):776–781. doi: 10.1006/bbrc.1998.9394. [DOI] [PubMed] [Google Scholar]
- 63.Brown T.T., Ross A.C., Storer N., Labbato D., McComsey G.A. Bone turnover, osteoprotegerin/RANKL and inflammation with antiretroviral initiation: tenofovir versus non-tenofovir regimens. Antivir. Ther. 2011;16(7):1063–1072. doi: 10.3851/IMP1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feig J.L., Mediero A., Corciulo C., Liu H., Zhang J., Perez-Aso M., Picard L., Wilder T., Cronstein B. The antiviral drug tenofovir, an inhibitor of Pannexin-1-mediated ATP release, prevents liver and skin fibrosis by downregulating adenosine levels in the liver and skin. PLoS One. 2017;12(11):e0188135. doi: 10.1371/journal.pone.0188135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fredholm B.B., IJzerman A.P., Jacobson K.A., Linden J., Müller C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors- an update. Pharmacol. Rev. 2011;63(1):1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruane P.J., DeJesus E., Berger D., Markowitz M., Bredeek U.F., Callebaut C., Zhong L., Ramanathan S., Rhee M.S., Fordyce M.W., Yale K. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J. Acquir. Immune Defic. Syndr. 2013;63(4):449–455. doi: 10.1097/QAI.0b013e3182965d45. [DOI] [PubMed] [Google Scholar]
- 67.Calmy A., Fux C.A., Norris R., Vallier N., Delhumeau C., Samaras K., Hesse K., Hirschel B., Cooper D.A., Carr A. Low bone mineral density, renal dysfunction, and fracture risk in HIV infection: A cross-sectional study. J. Infect. Dis. 2009;200(11):1746–1754. doi: 10.1086/644785. [DOI] [PubMed] [Google Scholar]
- 68.McComsey G.A., Kitch D., Daar E.S., Tierney C., Jahed N.C., Tebas P., Myers L., Melbourne K., Ha B., Sax P.E. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J. Infect. Dis. 2011;203(12):1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fung S., Kwan P., Fabri M., Horban A., Pelemis M., Hann H-W., Gurel S., Caruntu F.A., Flaherty J.F., Massetto B., Kim K., Kitrinos K.M., Subramanian G.M., McHutchison J.G., Yee L.J., Elkhashab M., Berg T., Sporea I., Yurdaydin C., Husa P., Jablkowski M.S., Gane E. Tenofovir disoproxil fumarate (TDF) vs. emtricitabine (FTC)/TDF in lamivudine resistant hepatitis B: A 5-year randomised study. J. Hepatol. 2017;66(1):11–18. doi: 10.1016/j.jhep.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Mills A., Arribas J.R., Andrade-Villanueva J., DiPerri G., Van Lunzen J., Koenig E., Elion R., Cavassini M., Madruga J.V., Brunetta J., Shamblaw D., DeJesus E., Orkin C., Wohl D.A., Brar I., Stephens J.L., Girard P.M., Huhn G., Plummer A., Liu Y.P., Cheng A.K., McCallister S. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: A randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect. Dis. 2016;16(1):43–52. doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]
- 71.Cahn P., Madero J.S., Arribas J.R., Antinori A., Ortiz R., Clarke A.E., Hung C-C., Rockstroh J.K., Girard P-M., Sievers J., Man C., Currie A., Underwood M., Tenorio A.R., Pappa K., Wynne B., Fettiplace A., Gartland M., Aboud M., Smith K. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): Week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143–155. doi: 10.1016/S0140-6736(18)32462-0. [DOI] [PubMed] [Google Scholar]
- 72.Negredo E., Domingo P., Pérez-Álvarez N., Gutiérrez M., Mateo G., Puig J., Escrig R., Echeverría P., Bonjoch A., Clotet B. Improvement in bone mineral density after switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: Two-centre randomized pilot study (OsteoTDF study). J. Antimicrob. Chemother. 2014;69(12):3368–3371. doi: 10.1093/jac/dku300. [DOI] [PubMed] [Google Scholar]
- 73.Wohl D.A., Yazdanpanah Y., Baumgarten A., Clarke A., Thompson M.A., Brinson C., Hagins D., Ramgopal M.N., Antinori A., Wei X., Acosta R., Collins S.E., Brainard D., Martin H. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e355–e363. doi: 10.1016/S2352-3018(19)30077-3. [DOI] [PubMed] [Google Scholar]
- 74.Paccou J., Viget N., Drumez E., Cortet B., Robineau O. Prevalence and risk factors for low bone mineral density in antiretroviral therapy-naive HIV-infected young men. Med. Mal. Infect. 2018;48(7):442–448. doi: 10.1016/j.medmal.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Ceballos M.E., Carvajal C., Jaramillo J., Dominguez A., González G. Vitamin d and bone mineral density in HIV newly diagnosed therapy-naive patients without any secondary causes of osteoporosis. Calcif. Tissue Int. 2019;104(1):42–49. doi: 10.1007/s00223-018-0474-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.
Data Availability Statement
The authors confirm that the data supporting the results and findings of this study are available within the article.