Abstract
Some transition metals, like manganese, iron, cobalt, nickel, copper and zinc, required for the biosynthesis of metalloenzymes and metalloproteins, are essential micronutrients for the growth and development of pathogenic microorganisms. Among the defenses put in place by the host organism, the so-called “nutritional immunity” consists of reducing the availability of micronutrients and thus “starving” the pathogen. In the case of metals, microorganisms can fight the nutritional immunity in different ways, i.e. by directly recruiting the metal ion or capturing an extracellular metalloprotein or also through the synthesis of specific metallophores which allow importing the metal in the form of a chelate complex. The best known and most studied metallophores are those directed to iron (siderophores), but analogous chelators are also expressed by microorganisms to capture other metals, such as zinc. An efficient zinc recruitment can also be achieved by means of specialized zinc-binding proteins. A deep knowledge of the properties, structure and action mechanisms of extracytoplasmic zinc chelators can be a powerful tool to find out new therapeutic strategies against the antibiotic and/or antifungal resistance. This review aims to collect the knowledge concerning zincophores (small molecules and proteins in charge of zinc acquisition) expressed by bacterial or fungal microorganisms that are pathogenic for the human body.
Keywords: Zincophores, metal binding proteins, zinc, nutritional immunity, bacteria, fungi, antimicrobial agents
1. INTRODUCTION
It is well known that the number of pathogenic microorganisms capable of adapting and resisting current therapeutic treatments has significantly increased over the last decades. There is currently only a limited number of effective drugs against bacterial and fungal infections and the threat of antimicrobial resistance (AMR) is a major concern in clinical practice. As a result of antimicrobial resistance, common treatments become inefficient and infections persist in the host organism, making pathogens extremely dangerous for the patient's subsistence and increasing the risk of spreading to other individuals. Not to mention that the mortality rate associated with infectious diseases dramatically increases in the case of immunocompromised individuals [1-5].
The first-row d-block metal ions, manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu) and zinc (Zn), are known to be essential for the physiology of all living organisms, functioning as cofactors in metalloproteins or structural components for enzymes. It has been estimated that approximately 30% of all proteins require metals for their biological function [6, 7]. Nonetheless, both a reduced and an excessive concentration of metal inside the cell are potentially toxic and fatal for its survival. From this perspective, one can easily understand that metal ions play a key role in the pathogen subsistence and as virulence factors [8]. Pathogenic microorganisms, like bacteria and fungi, can meet the physiological metals’ demand through the acquisition of micronutrients from the surrounding microenvironment during the colonization of a host organism. To prevent infections, the host restricts access to essential metal micronutrients, withholding and limiting these resources by means of an innate immune response termed “nutritional” immunity [9, 10]. This phenomenon is a primary defense line put in place by vertebrate organisms to limit the efficiency of microbial invaders. In fact, during inflammation, the concentrations of non-bound trace minerals, such as iron and zinc, proved to undergo a severe drop, which can eventually result in the occurrence of human diseases [11-15]. The host metal homeostasis perturbation is caused by the pathogen organism, which can rely on sophisticated systems to sequester metal micronutrients and overcome their scarce bioavailability. The mechanisms put in play by pathogens to obtain the necessary metal nutrients are various and include both active and passive transport through the pathogen cell membranes. In the former case, to make the metal recruitment more efficient, pathogens can synthesise proteins, peptides or other molecules with the specific function of metal scavenger, adapting to the host-mediated metal limitations [7, 16]. These so-called “metallophores” are here intended as specialised extracytoplasmic (extracellular or periplasmic) chelators acting as metal shuttles, capable of catching the metal ion from the host microenvironmental niche and transfer it to an appropriate target protein (most often a transmembrane transporter).
Although all forms of life require the strict control of transition metal ions homeostasis, in this work, we focus on zinc acquisition by bacterial and fungal human pathogens. We aim to summarize and describe the main systems involved in Zn2+ uptake from the periplasmic and/or extracellular space, with particular emphasis on the very first step of metal sequestration at the interface with the host organism. More precisely, we are interested in “zincophores”, usually defined as small molecules involved in zinc uptake in bacteria [17, 18], in analogy with siderophores. However, the term zincophore has also been employed for proteins that are able to chelate zinc in fungi [19, 20]. In both cases, the cellular zinc acquisition is facilitated by the interaction of the zincophore with a transmembrane transporter. Zinc-binding proteins in charge of zinc uptake, which deliver the metal ion to a target transporter, are well known also for bacterial systems and most of them are generally classified as substrate binding-proteins (SBPs) [21, 22]. According to the above definitions, we decide to extend the term zincophore to any specialised zinc chelator (small molecule, e.g. opine metallophores, peptide; independent protein, e.g. Pra1; transmembrane transporter-associated substrate binding-protein, e.g. ZnuA; or zinc metallochaperone, e.g. ZinT; more details on these systems are given below) involved in the zinc acquisition pathway, expressed or secreted by pathogens (e.g., bacteria and fungi) in the periplasm or in the extracellular environment and able to reversibly bind zinc ions and to deliver them to the transporter system (Fig. 1).
Fig. (1).
Schematic illustration of the defined zincophore systems. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The divalent Zn2+ ion has a high rate of ligand exchange, a reasonably high electron affinity, a flexibility of coordination geometry (mostly tetrahedral) and no redox activity. A zinc ion can organize the biological structure and stabilize the folding of a protein, as in zinc fingers. Regulation of the transcription and translation of the genetic message are also dependent on Zn2+, which is required by RNA and DNA polymerases and participates in ribosome activity. Its polarizing power makes the pKa of coordinated water molecules quite low, displaying Lewis acid properties and allowing a catalytic function when, for instance, it coordinates to three rather than four protein ligands. The large family of Zn2+-enzymes includes superoxide dismutases and metalloproteases involved in infectious events (e.g., ADAM metalloproteinases or deuterolysin [23-25]), and also human innate and adaptive immune responses are largely dependent on this metal ion [26]. The biological key role of Zn2+ is therefore well-documented, and a deep look into the bio-chemistry of pathogens’ primary zinc-chelating systems is fundamental in order to rationally deal with the discovery and design of new antimicrobial drugs. Although genome analyses have identified numerous homologues of each zincophore and import systems, and various genes encoding further putative zinc transporters in human pathogenic species [27], the vastness of the topic forces us to focus on the most relevant representative zincophores for each class of homologous proteins.
This review covers (i) bacterial ABC transporter related zincophores, i.e. the zinc-specific substrate binding proteins (SBPs) constituting the extramembrane component of the ABC import systems, (ii) their associated zinc-chaperones (when existing), which are a miscellaneous group of proteins able to bind and transfer zinc to the SBP-zincophore, (iii) the bacterial nicotianamine-like zincophores (also known as opine metallophores) and, analogously to bacterial microorganisms, (iv) the most important zincophores from fungal pathogens, comprising Pra1 and its orthologue Aspf2. The use of these zincophores as therapeutic targets against bacterial and fungal infections is finally discussed.
1.1. The Rules of Zinc Tug-of-War
To better understand the properties and the mechanisms of action of the above-mentioned zincophores, some preliminary considerations are necessary. First of all, we must distinguish between two environmental niches in which the metal-binding proteins can be found: the periplasm and the extracellular space. Gram-negative bacteria are characterized by an additional outer membrane which defines the periplasmic compartment (the space between the outer and the inner/cytoplasmic membranes). On the contrary, Gram- positive bacterial and eukaryotic cells (such as fungi) lack the outer membrane and the periplasm. The presence of the periplasm requires a series of import systems to let the metal pass through the outer membrane and make it caught by specialised proteins that transfer it to further cytoplasmic import systems. This function is usually delegated to diffusible soluble, highly specific, periplasmic metal-binding proteins or low molecular weight metal chelators. The secretion of metal-binding proteins in the Gram-positive bacteria and eukaryotic cells, instead, implies the release of these metallophores directly into the host environment, and it requires a further reassociation step with the cell. Alternatively, the metallophore can be a lipid-anchored protein located at the cell surface of Gram-positive bacteria (reaching in some cases up to 40% of Gram-positive surface lipoproteins [28]) and, with less extent, of Gram-negative bacteria (both at outer or inner membrane) [29].
This context imposes specific zinc-coordination features and, in particular, the necessity of kinetically labile but thermodynamically stable coordination bonds; in other words, the systems involved in the trafficking mechanisms must ensure adequate thermodynamic complex stability to prevent unwanted reactions or the premature release of the loaded ions, and at the same time, an easy and favourable metal transfer to the proper target must not be hindered. A further complication arises from the presence of different metal ions in the periplasmic and extracellular environments, which can act as competitors for the same binding sites. As a general rule, the competitiveness is inversely proportional to the metal bioavailability in the cell, and, in the first instance, it depends on the chelate binding affinities for a given metal ion. This concept comes from the Irving-Williams series, which elucidates the natural order of stability of metal complexes with divalent transition metal atoms (Mg2+, Ca2+ < Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+). In the case of flexible proteins and the unstructured portion of polypeptides, the ligand cannot rely on specific steric effects that can favour the interaction with a specific metal ion (such as allostery, protein-protein interaction mediated specificity, steric hindrance, access to the binding site, kinetics, etc.) and therefore the metal selection follows the absolute order of metal affinity, with copper and zinc predominating in the protein bound-form (low bioavailability) [30-32]. More details regarding these concepts are discussed below for each individual zincophore system.
2. ABC TRANSPORTER RELATED ZINCOPHORES: ZINC-SUBSTRATE BINDING PROTEINS AND THEIR ZINC-CHAPERONES
According to the classification first proposed by Berntsson et al. in 2010 [29] and later improved for novel classes of Substrate Binding-Proteins (SBPs) by Scheepers et al. in 2016 [33], we can distinguish seven different classes (“clusters”) of SBPs, including metal-binding proteins. Phylogenetical and structural analyses on a wide variety of known SBPs highlight that Zn2+-substrate-binding proteins share certain evolutionarily conserved structural characteristics, although they display different sizes, poor or no sequence homology and have multiple biological roles. Therefore, only one class (cluster A) is representative of Zn2+-related chelators, and currently, all of them are known as extra-membrane components of the ATP-Binding Cassette (ABC) transporters, with the function of withdrawing and delivering the cognate substrate to the transmembrane domains (TDMs) of the transport system. The ABC transporters are extremely important and often involved in the processes of metal homeostasis, being able to translocate the bound targets across the cell membranes in both directions (they can be classified as importers or exporters).
Cluster A consists, in general, of SBP-dependent ABC transporter proteins able to directly bind free Zn2+, Mn2+, Fe2+/3+ ions (cluster A-I) or to interact with metals in their protein-bound form, like siderophore, catecholate, heme (cluster A-II). The main common feature of the cluster A metallophores is the presence of a single rigid α-helical hinge region that links the two globular, pseudo symmetrical α/β domains typical of all the SBPs [22]. This α-helix linker makes the structure relatively rigid and seems to be responsible for the different mechanism of substrate delivery proposed for cluster A-I SBPs, which significantly differs from the common “Venus flytrap” identified in SBPs with the non-metal cognate substrate [29, 33-35]. This latter mechanism, in fact, resembles the carnivorous plant Venus flytrap (Dionaea muscipula) trapping action, and is indicative of large conformational changes induced by the substrate binding, which causes the α/β lobes closure and fold around the linker [36]. In the case of cluster A-I proteins, instead, structural comparisons between the apo- (metal-free) and holo- (metal-bound) forms show only minor changes and a slight domains motion upon metal exchange, about 4°-5° rigid-body rotation with respect to ~ 40° to 60° of other SBPs [37].
Understanding how exactly the metal ions are recruited and how these zincophores discriminate within the variegated pool of available metal micronutrients remains a challenge. Many studies underline the crucial contribution of the metal-binding sites specificity and of the consequent resulting coordination mode that can facilitate or block the conformational changes required to perform a specific function. An elegant example is provided by the pneumococcal surface adhesin A (PsaA) protein, which belongs to cluster A-I SBPs. Its structural studies revealed that “imperfect”, far-from-ideal metal coordination modes are preferable to favour the metal transport processes. This is also the reason why Zn2+ ion (coordinated to His67, His139, Asp280 and Glu205) deactivates PsaA, blocking the protein in its closed conformation and preventing the metal release after binding [34]. Similar results concern the Fe/Mn2+ chelator YfeA from Yersinia pestis, which is able to strongly bind Zn2+ in vitro but not in vivo, and whose PsaA-like “spring-hammer” mechanism of action has been recently elucidated [37-39]. Therefore, it is desirable that Zn2+-specific SBPs avoid strong interactions with the metal substrate and this can be achieved by means of different sets of donor atoms, possibly located at mobile, flexible regions of the protein, or close to them, that make the ideal coordination sphere enough distorted, or occupying some coordination positions with exchangeable solvent molecules, i.e. water.
Taking into account these observations, we can now introduce the further sub-classification that differentiates cluster A-I zincophores on the basis of (i) the donor atoms of the metal coordination sphere, (ii) the organism type, (iii) the presence of a flexible loop near the metal-binding site, (iv) the length of the loop, and (v) the presence of histidine residues in the loop [22, 29, 40, 41]. In the case of cluster A-I Zn2+ binding proteins, it is possible to summarize the following common characteristics: (1) they generally display a tetrahedral (3NImidazole, 1COO– or 1H2O) metal coordination mode, which means that Zn2+ is bound to 3 histidine residues and 1 carboxylate group from glutamic acid or aspartic acid or 1 exchangeable water molecule; (2) the flexible loop is always present, with only one exception represented by TroA protein, and it generally indicates specificity for Zn2+; (3) in Gram-negative bacteria, there is always a long His-rich loop while (4) in Gram-positive bacteria, the loop is usually shorter and may or may not contain His residues. The proper coordination mode and the presence of a flexible loop close to the binding site are therefore two indispensable features that ABC-transporter-related zincophores must display to perform their biological role and facilitate the interconversion between their metal -free and -loaded forms (Fig. 2). At the present day, only a relatively limited number of cluster A-I zincophores have been identified (Table 1). Unfortunately, neither their three-dimensional structures nor the mechanistic implications of their metal uptake function are always clear.
Fig. (2).
Schematic representation of Zn2+ acquisition by means of ABC transporter related zincophores in Gram-negative (left) and Gram-positive (right) bacteria. The Substrate Binding Protein (SBP) contains two α/β domains, the rigid α-helical linker and the flexible loop facing the metal binding pocket. The flexible loop is long and rich in histidine and acidic residues in the case of G-negative bacteria (group II) and short with or without histidine residues in G-positive bacteria (group I). The additional SBP associated zincophores are different classes of proteins that may act as zinc-chaperone and deliver the Zn2+ ion to the SBP or help the SBP in the zinc recruitment process. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
Representative bacterial Zn2+ specific substrate-binding proteins of cluster A-I. A three-dimensional structure of the protein in the zinc bound form is available for the underlined species. Hypothetical zinc coordination mode for SBP lacking a 3D structure is written in italics.
| Protein | Coordination Mode | SBP Classification | Species | Refs. | |
|---|---|---|---|---|---|
| AdcAII | 3His, 1Glu | Ia | lipid-anchored | S. pneumoniae(G+), | [41] |
| 3His, 1Glu or H2O | S. agalactiae (G+) | ||||
| Lbp (or Lsp) |
3His, 1Glu | Ia | Lipid-anchored | S. pyogenes (G+) | [51] |
| Lmb | 3His, 1Glu | Ia | Lipid-anchored | S. agalactiae (G+) | [52] |
| AdcA |
3His, 1Glu or H2O (N-terminal domain); 3His (C-terminal domain) |
Ib | Lipid-anchored | S. pneumoniae (G+), S. agalactiae (G+), S. pyogenes (G+) | [53] |
| ZnuA | 3His, 1Glu | II | Soluble | E. coli (G-), S. enterica (G-) | [46, 54, 55] |
| 3His, 1H2O |
P. aeruginosa (G-), T. pallidum (G-), H. ducreyi (G-), H. influenzae (G-), Y. pestis (G-) |
||||
| TroA | 3His, 1Asp | III | Lipid-anchored | T. pallidum (G-), S. suis (G+) | [56, 57] |
As a matter of fact, the rigid nature of the long α-helix clamp connecting the two α/β domains disfavours large-scale domain rotations; on the contrary, small changes in helices and in the loop spatial conformation near the metal-binding site are observed between apo- and holo- forms in many Zn2+ specific SBPs, suggesting that metal uptake and release are likely mediated by the structural rearrangements of secondary elements, as also recently proposed for the SBP protein ZnuA of Escherichia coli (EcoZnuA) [42]. However, the role of the flexible region rich in acidic and histidine residues near the metal-binding site has not been established yet. It is supposed to facilitate zinc acquisition, to serve as a sensor under zinc-exceeding conditions, or to be recognised by the membrane permease component [43-45]. Some studies revealed that the His-rich loops of ZnuA homologues in E. coli and Salmonella enterica can bind Zn2+ through their His and Asp/Glu residues, providing secondary metal binding sites [43, 46]. Moreover, the loop can be involved in specific protein–protein interaction and potential Zn2+ acquisition from additional metallochaperones; the most interesting example concerns the formation of the protein binary complex between SenZnuA and the periplasmic zinc-binding protein SenZinT, where the N-terminal histidine-rich loop of ZnuA has been proved to play a critical role, embedding into a structural cavity of the partner protein [47].
In light of the above considerations, ABC transporter SBPs are not the only ABC related zincophores involved in the process of nutritional immunity. Very few other classes of periplasmic and/or extracellular zinc-binding proteins have been identified and characterized to date. One common feature of these systems is their activity as metallochaperones for the SBP component of the ABC zinc transporters. In many bacterial species, the SBP-zincophore can interact with a further “accessory” protein in order to overcome zinc starvation and increase the efficiency of zinc recruitment (as in the case of ZnuABC and ZinT). The most representative Zn2+-SBPs interact with a specific zinc-chaperone (Table 2), which is usually upregulated under zinc-limited conditions. The mechanisms by which the paired proteins interact are still uncertain and can differ for each system, in particular considering that the identified zinc-chaperones share very poor structural and sequence similarities. It is also important to mention that these “accessory-zincophores” are not indispensable for the proper function of ABC transporters in several bacteria, since their deletion in mutant strains affects only with minor extent the pathogen survival, and there are many cases of microorganisms expressing an ABC importer and not possessing the additional zinc-chaperone [48-50]. A more detailed discussion of the more relevant SBP-zincophores and their identified associated zinc-chaperones is given below. We selected at least one representative of each group of cluster A-I zincophores.
Table 2.
Summary of Zn2+ specific substrate-binding proteins (SBPs) and their associated accessory zinc-chaperone.
2.1. The Leading SBP-Zincophore of Gram-Negative Bacteria ZnuA
ZnuA is a periplasmic soluble protein typical of Gram-negative bacteria. It belongs to group II of cluster A-I and possesses a long N-terminal His-rich loop nearby its high-affinity primary Zn2+ binding site. The available crystal structures reveal that the binding residues and the surrounding environment are highly conserved among different bacterial species. The metal coordination occurs via (3His, 1Glu) in E. coli [46] and S. enterica [54] species, but can also involve a (3His, 1H2O) binding mode, as demonstrated for Synecocystis 6803 [44]. Similar coordination modes can be hypothesised for the other ZnuA homologues of different human pathogens; despite the lack of a solved 3D structure, the multiple sequence alignment of ZnuA proteins from different bacterial species (Scheme S1 (437.5KB, pdf) , Supplementary Information) allows to suggest that the (3His, 1H2O) binding mode is likely due to the absence of an oxygen-donor residue (Asp or Glu) proximal to His60 (as in the case of SynZnuA) [55]. In addition, upon Zn2+ complex formation, the conformational changes of the ZnuA structure mainly involve the flexible His-rich loop, which slightly shifts and possibly shields the metal substrate from the solvent, also inducing subtle rearrangements of the helices around the binding pocket and the flipping of one coordinated histidine [46]. Although the collected data are insufficient to clearly elucidate the exact mechanism by which the metal is delivered to ZnuB, according to the previous observations, one can suggest that the plasticity of the loop can facilitate the release of the zinc ion [42]. It is also worth noting that in the solved crystal structure of the Zn2+-SenZnuA complex, one histidine residue belonging to the flexible loop substitutes the conserved His60 (EcoZnuA numeration, Scheme S1 (437.5KB, pdf) , Supplementary Information) in the metal coordination. Moreover, the partially occupied Zn2+ binding conformation (0.7 occupancy) is characterized by a shift of Glu position, a change of its coordinated oxygen (from OE1 to OE2), and the binding of a water molecule as a fifth ligand [60]. Interestingly, the relatively long His-rich loop of ZnuA also provides several extra candidates as zinc ligands (His, Glu and Asp residues) in the proximity of the primary metal-binding site, potentially increasing the protein efficiency of zinc acquisition [21, 46, 61]. Several studies suggest that this unstructured loop may function as a metal scavenger or chaperone during zinc-limited conditions. The flexible loop can interact with the Zn2+ ion and shuttle the metal from the loop itself to the binding pocket (canonical binding site between the α/β domains). Moreover, it seems to play a crucial role in the interaction with ZinT (formerly known as YodA), a periplasmic protein primarily found in Gram-negative bacteria [46, 61].
ZinT is defined as a lipocalin/calycin-like protein because of its major β-barrel domain, which is structurally related to this protein family; additionally, it also contains a small helical domain. Lipocalins are extracellular proteins associated with several functions, including ligand binding, receptor binding and interaction with other macromolecules to form complexes.
Therefore, it is not surprising that ZinT may function as a zinc-chaperone for the ZnuABC system. ZinT is also involved in oxidative stress events [62], and its expression appears to be upregulated by strictinin isomers, which can display antimicrobial activity against E. coli [63]. Although different crystal structures of ZinT identify several possible zinc metal-binding residues (His167, His176, His178, Glu212, His216, His24, His26, His27 and His29) [64, 65], there is a general consensus that the three highly conserved His residues (His167, His176 and His178) facing the center of the calycin like-domain are the preferred zinc-binding sites with the well-known tetrahedral (3His, H2O) coordination mode [64]. Interestingly, the sequence portion between residues 24 and 29 (-HXHHXH-) is rich in histidines and is highly conserved in ZinT homologues from different bacterial species; moreover, it is highly flexible and it is reminiscent of the His-rich loop typical of zinc-specific SBPs. Thermodynamic studies reveal that this short N-terminal His-rich loop is a high-affinity zinc-binding site, displaying a (3 or 4His, H2O) coordination mode. This fragment is possibly in charge of zinc primarily sequestration from the surrounding environment [66]. The type of donors and the hypothesised mixture of tetrahedral and pyramidal complex geometries in the solution can be considered a “non-ideal” zinc coordination that can favour the further transfer of the metal ion to the canonical binding site of ZinT (3 His of the calycin-like domain) [65, 66]. As already mentioned, the Zn2+-ZinT complex from S. enterica can interact with ZnuA forming a binary complex where both proteins expose their binding pocket to the Zn2+ ion and where the His-rich loop of ZnuA enters the space delimited by the calycin and helical domains of ZinT. The proposed mechanism of metal uptake, therefore, involves the zinc transfer from ZinT to ZnuA [47]. The His-rich loop of ZnuA governs this interaction and exhibits higher metal affinity than the ZinT binding sites; this makes the flexible loop of ZnuA a hypothetical metal transfer intermediary between the two proteins. An example of such a mechanism has been described for Zn2+ transfer from the metallochaperone AztD to the SBP AztC of Paracoccus denitrificans.
2.2. The Streptococci Zincophores AdcA, AdcAII, Lsp and Lmb
AdcA and AdcAII are two lipid-anchored proteins able to deliver zinc to the ABC transporter AdcBC. This import system is an orthologue of ZnuABC from Gram-negative bacteria and it is in charge of Zn2+ acquisition in Streptococcus species. Both AdcA and AdcAII belong to the cluster A-I of substrate-binding proteins, however they do not show a totally redundant activity but, intriguingly, they seem to contribute additively to the in vivo virulence; nonetheless, they display significant genetic, structural and functional differences [67].
AdcA (whose 3D structure has not yet been obtained) is characterized by two main zinc-binding domains connected by a linker sequence. The N-terminal domain displays high homology with ZnuA protein, conferring to AdcA the typical features of cluster A-I zinc-specific binding proteins (group Ib); the C-terminal domain, instead, shares high sequence identity with the Zn2+ chaperone ZinT from Gram-negative organisms (Scheme S2 (437.5KB, pdf) , Supplementary Information), suggesting that AdcA C-terminal domain may play the same or similar biological role in the process of zinc homeostasis, functioning as metal ion scavenger under zinc limited conditions or as a facilitator of metal delivery to the AdcBC transporter [68]. The presence of this extended (~200 amino acid residues) C-terminal region is anomalous, but not totally out of context if one compares AdcA to the studied ZnuA-ZinT interaction in S. enterica. Interestingly, Isothermal Titration Calorimetry (ITC) data relative to AdcA revealed that its N-terminal (ZnuA-like) domain represents a more efficient zinc ligand with respect to the C-terminal (ZinT-like) region, about two orders of magnitude higher [58], thus mirroring the thermodynamic results obtained for ZnuA and ZinT [46, 61, 66]. The putative metal-binding residues in the ZnuA-like domain correspond to the three conserved histidines typical of cluster A-I. Assuming a tetrahedral geometry as the most likely, the fourth coordination position should be a water molecule or an acidic residue (according to previous considerations on ZnuA). In the case of the ZinT-like domain, the predicted zinc coordination mode is (3His, 1O) (carboxylic oxygen or a water molecule). Indeed, the three histidines in positions 167, 176 and 178 (ZinT nomenclature) are also conserved in AdcA (Fig. 3).
Fig. (3).
Simulated (Phyre2 [78]) three-dimensional structure of AdcA; green = ZnuA-like domain, pink = ZnuA-flexible loop, blue = ZinT- like domain. Zn2+ ions are represented as purple spheres. The proposed zinc binding mode is (3His, 1H2O). Protein picture was generated using CCP4mg [79]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In the solid-state structure of AdcAII in its Zn2+-bound form [41], the amino acid residues involved in the metal coordination sphere are the three characteristic conserved histidines of cluster A-I SBPs and one glutamic acid in position 286. The coordination geometry is tetrahedral and the binding pocket faces a 15-residues disordered loop. The classification proposed by Loisel et al. [41] inserts AdcAII into the Ia group, consisting of Gram-positive bacteria SBPs with a flexible loop lacking the typical abundance of His and charged residues (Glu and Asp). The structural differences between AdcA and AdcAII are evident and the singular characteristic of AdcAII flexible loop (deprived of the His-rich motif), which does not contribute to the metal-binding [41], opens the way to the search for an auxiliary system able to make up for the His-rich or ZinT- like region functionality. In S. pneumoniae, this role has been attributed to the pneumococcal histidine triad proteins (Pht), comprising PhtA, PhtB, PhtD, and PhtE. These surface proteins contain multiple (five to six) putative zinc-binding HXXHXH motifs, which have been proved to affect AdcAII pathogenicity, displaying only a partial functional redundancy both in vitro and in vivo [67]. In particular, the interaction between PhtD and AdcAII has been widely investigated, suggesting that (i) the AdcAII zinc uptake may occur through the directional transfer of a Zn2+ atom from PhtD, or (ii) it may be related to the simple increase of metal local concentration near the cell membrane, with Pht proteins acting as “zinc magnets” and consequently increasing the probability for AdcAII to encounter the metal [69, 70]. The three-dimensional structures of Zn2+ complexes with a 54-amino acid fragment of PhtA and a 137-amino acid segment of PhtD obtained by means of X-ray crystallography and NMR, respectively, show a (3His, 1Glu) coordination mode for both the systems [59, 71]. Despite the AdcAII main role in zinc import, Durmort et al. recently highlighted a correlation between AdcAII and the thickness of S. pneumoniae cellular capsule, which results in a higher virulence upon the protein deletion [72]. From this perspective, it is worth reporting that Lsp and Lmb, two homologues of AdcAII, display an additional function, being able to bind laminin and to participate in adhesion during infections [49], therefore we will briefly discuss these zinc-specific SBPs expressed by S. pyogenes and S. agalactiae.
Analogously to S. pneumonia, also in S. pyogenes and S. agalactiae, zinc acquisition is mediated by the ABC zinc importer AdcBC with its two associated lipoproteins AdcA and AdcAII [73, 74]. In S. pyogenes, the AdcAII homologous protein has been indifferently called Lsp or Lbp; while in S. agalactiae, it is known as Lmb. Their obtained 3D structure is in line with previous observations on group Ia SBPs, and the high similarity with SpnAdcAII is also confirmed by their metal-binding ability; both the formed Zn2+ complexes have a 1:1 ligand/metal ratio and the metal-binding site corresponds to three conserved histidines and one glutamic acid (3His, 1Glu) located at the interface between the two globular α/β domains. The flexible loop, typical of zinc-specific binding proteins, is present but does not contain the His-rich motif [75]. Their mechanism of metal binding and release has been only hypothesised by means of molecular dynamics simulation [76]. Despite the structural similarities and the genetic homology with SpnAdcAII, the laminin-binding ability has not been confirmed in the case of SpnAdcAII and the analysis of the surface charge distribution between SagLmb and SpnAdcAII revealed significant differences, attributable to the “extra” adhesion functionality [52, 77]. Noteworthy, this laminin-binding capacity proved to be zinc-mediated; a specific conformational structure is required to associate with laminin and Zn2+ coordination assures and maintains the proper correct protein fold [52].
A recent study from Moulin et al. on S. agalactiae suggests that this organism possesses a third zinc-substrate binding protein associated with the AdcBC importer, and therefore two overall redundant AdcAII homologues (the previously discussed SagLmb and the additional SagAdcAII) [49]. Noteworthy, the gene encoding SagLmb, SagAdcAII and SpyLsp is not situated in the same operon of the AdcB permease and the AdcC ATPase, but is rather cotranscribed along with a histidine triad protein, thus suggesting their primary role as zincophores rather than laminin-binding proteins [73, 74]. Further analyses also elucidated the interaction of SagLmb and SagAdcAII with two Pht proteins (ShtI and ShtII) (Fig. 4), as in the case of PhtD and AdcAII in S. pneumoniae [74].
Fig. (4).
Schematic representation of S. agalactiae zinc acquisition by means of Adc/Lmb importer system and the associate histidine triad proteins Sht and ShtII. Adapted from [74]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.3. The Curious Cases of TroA and AztC
TroA is the periplasmic Zn2+-binding component of an ABC transporter located in the cytoplasmic membrane of Treponella species (e.g. T. pallidum, the Gram-negative bacterium responsible for the sexually transmitted disease syphilis, and T. denticola) and in the cellular membrane of Streptococcus suis, which is instead a Gram-positive bacterium. TroA is classified as a type III of cluster A-I SBP; it is linked to the membrane lipid layer and, therefore, it is not able to freely diffuse into the surrounding space. TroA is the only zincophore among SBP-dependent ABC transporter proteins which does not contain a flexible loop close to its metal binding pocket. The particularity of TroA, besides the lack of the loop, is its absence of definite metal specificity: in vitro TroA binds divalent zinc, manganese and iron and in vivo its biosynthesis depends on Zn2+ and Mn2+ in T. pallidum or Mn2+ and Fe2+ in T. denticola, making its function as metal-specific transporter arguable [22, 80, 81]. Isothermal titration calorimetry highlighted that TpaTroA affinity for Zn2+ and Mn2+ is very similar and reaches nanomolar concentrations [45]. Mn2+ coordination, however, is driven by an entropic effect associated to the release of water molecules, while in Zn2+ binding the major contribution is given by the favourable change in enthalpy, which is in good agreement with the fact that Zn2+ is highly polarizable and can form bonds with increased covalent character [45].
Understanding the biological role of TroA and its mechanism of action can be easier when comparing different zinc import systems. In T. pallidum ZnuA and TroA represent two redundant proteins in charge of Zn2+ uptake and both are SBP-dependent ABC transporter proteins. ZnuA, as previously discussed, is metal-specific, binds Zn2+ with high affinity and displays at least two (three in TpaZnuA [45]) binding sites located at the canonical cleft between α/β domains and in the flexible His-rich loop. TpaTroA, lacking the flexible loop, has only one metal-binding site in the inter-domain pocket and does not show any detectable differences between its apo- and holo- forms, nor in the presence of Zn2+ or Mn2+ ions. Therefore, TpaTroA mechanism of action should necessarily differ not only from TpaZnuA, but also from most of the known cluster A-I SBPs, including Mn2+ specific binding proteins of cluster A-I group III (e.g. PsaA), where conformational changes usually occur, although their detailed associated mechanism is still under investigation [45, 57]. On the contrary, in S. suis, both Zn2+ and Mn2+ bindings induce almost the same conformational changes. The SsuTroA X-ray structure also revealed the presence of two flexible short regions which can have a biological function facilitating the metal transport mechanism or the interaction with other protein systems [57]. A hypothesis about how TroA can act as “multi-specific metal-binding protein” without being deactivated or inhibited, for instance, by Zn2+ ion - as in the case of PsaA -, as well as an explanation about the apparent inability to distinguish between Zn2+ and Mn2+ may be related to its putative collaboration with other zincophores.
In the case of T. pallidum, for instance, the highly specific ZnuA protein can very efficiently sequester Zn2+ from the periplasmic space and make TroA free to bind less competitive metals, like Mn2+ or Fe2+. The relatively high abundance of TroA within the cell also agrees with the putative function of binding other metals besides Zn2+ [45].
Although this review is exclusively dedicated to human pathogens, it is important to mention further zincophore systems, AztC and AztD from P. denitrificans species, whose homologues, nonetheless, have been identified in human pathogens like Klebsiella pneumonia and Enterobacter aerogenes [82]. AztC is the periplasmic component of the ABC zinc transporter AztABC and it is classified as group III of cluster A-I SBPs, together with TroA protein. It displays the highest structural similarity with TroA, showing a zinc coordination mode almost identical: 3His, 1Asp. Unusually, it possesses a relatively short flexible loop facing the metal binding pocket, which contains a few charged residues and only 2-3 histidines. This attribute is absent in TroA and in the other known group III SBPs, and represents an element of specificity for Zn2+ ion. However, in the case of AztC, the loop properties are rather unusual and locate halfway between the known cluster A-I groups; it possesses a short His-containing loop (group Ib), it belongs to Gram-negative organisms (group II), its structure and coordination mode is almost the same of TroA (group III) [82]. Analogously to ZnuA and AdcAII, AztC proved to interact with a zinc metallochaperone, AztD, to facilitate zinc acquisition under metal-depleted conditions. The study of AztC/AztD system provided detailed information on the specific role of its flexible loop, which is likely crucial for the interaction between the two proteins and facilitates the dislocation of a Zn2+ ion from AztD to AztC through its three His residues (H120, H122, H131), transiently binding the ion [40, 83].
2.4. Other SBP-zincophores
Several other zinc ABC importers and their orthologues have been identified in many bacteria by means of genome analyses (e.g., zrgABCDE in Vibrio cholera [27]) and biochemical characterizations. There are several examples of ABC transporters displaying controversial behaviour towards the Zn2+ ion, including the previously discussed proteins PsaA and TroA. In fact, PsaA is a Mn2+-specific binding protein which is also able to interact and bind zinc, although this metal has a detrimental effect on the protein functionality, while the TroABC transporter (where TroA is the associated SBP) exhibits a putative polyvalent metal transport activity. In a similar way, the MntABC is a further bacterial metal transporter capable of interacting with Zn2+ through its extra-membrane component (cluster A-I, group III SBP). Although Mn2+ is considered its physiological relevant metal substrate, in Neisseria gonorrhoeae, it appears to promote the transport of both Mn2+ and Zn2+ in vivo [84, 85].
Intriguingly, a previously classified Cobalt-Nickel ABC transporter CntABCDF from Staphylococcus aureus has been recently re-evaluated in its putative role of Zn2+ binding system by Grim et al. [86]. CntABCDF is a member of the Peptide, Opine and Nickel Uptake (PepT) transporter family, and its associated SBP is classified into cluster C. These are large size proteins characterized by an extra domain whose function has not been yet clarified but seems to take part in the coordination of the substrate oligopeptides [29, 85, 87]. The lipid-anchored SBP, CntA, contributes to S. aureus survival in zinc-limited conditions thanks to its capacity to transfer Zn2+ ions through the interaction with the bacterial nicotianamine-like metallophore staphylopine. The mechanism of staphylopine recognition and binding is common to many other non-metal-specific SBPs, as discussed in the introductive paragraph, and causes drastic domain motions and conformational changes [88]. Further details on the way of action of this zinc importer systems and the metallophore substrates are given in the next section.
It is also important to mention the existence of zinc-binding proteins involved in cellular detoxification, mostly belonging to Gram-negative bacteria and located in the periplasm. Although they are not specifically involved in the process of Zn2+ recruitment, they rather carry out a contrary function, interacting with exporter proteins and helping in delivering the metal outside the cell; they can be classified as periplasmic Zn2+ binding proteins as well. We briefly mention the two homologues ZraP and Spy [89, 90]. They are CpxP family homologues and ATP-independent molecular chaperones able to bind Zn2+ ions (ZraP is zinc specific and possesses two zinc-binding domains [91], while Spy displays lower metal binding affinity [89]) and to reduce the free metal concentration in the periplasm, preventing zinc-related cytotoxic phenomena.
3. NICOTIANAMINE-LIKE ZINCOPHORES
Staphylococcus aureus, Pseudomonas aeruginosa and Yersinia pestis are human pathogenic bacteria which, in a metal-deficient environment, are able to express specific opine-type metallophores, called staphylopine (StP), pseudopaline and yersinopine, respectively. These chelators are similar to nicotianamine and their biosynthesis proceeds in the following steps: 1) CntK, a histidine racemase (if present, as in S. aureus) converts L-His into D-His; 2) CntL, a nicotianamine synthase enzyme, attaches the aminobutyrate chain of S-adenosyl-L-methionine (SAM) to the histidine amino group, to form an intermediate called xNA (if contains D-His) or yNA (if contains L-His); 3) the opine dehydrogenase (ODH) enzyme, CntM, condenses the intermediate with an α-keto acid and, after reduction with NAD(P)H, the obtained opine-type metallophore exhibits stereochemistry (L, L) or (D, L) [92].
The opine dehydrogenases of S. aureus (SauODH), P. aeruginosa (PaeODH) and Y. pestis (YpeODH) show different substrate specificity: pyruvate for S. aureus and Y. pestis and α-ketoglutarate for P. aeruginosa, although they are structural homologues. SauODH, PaeODH and YpeODH have been investigated using both a steady-state kinetic method and X-ray crystallography [92], which revealed a dimeric structure, where the residues Arg-383, Asp-153 and His-242 play key roles in Schiff base formation and the selection of the α-keto acid substrate. All the structures were in an open conformation, but a closure is to be expected to build up the catalytic site.
A combination of bioinformatics, structural analysis, chemical synthesis and enzymatic studies has been employed to deeply explore the specificity of CntM opine dehydrogenases [93]. Following the S. aureus numbering, the amino acidic residue at position 33 was recognized as responsible for the NAD(P)H selectivity; the presence of an arginine decides the preferential binding to NADPH. On the other hand, the amino acidic residue at position 150 rules the selectivity towards α-ketoacids; an aspartate makes CntM selective towards pyruvate while alanine allows the use of both pyruvate and α-ketoglutarate. A further in silico analysis of the available genomes led Laffont et al. to identify a soil bacterium, Paenibacillus mucilaginosus, with all the characteristics required to synthesize a new metallophore belonging to the opaline family. It derived from D-His (like in the case of S. aureus) but reacted with NADPH and α-ketoglutarate (like P. aeruginosa). The new metallophore was named bacillopaline [93]. The synthetic pathways of these four zincophores are schematized in Fig. (5).
Fig. (5).
Biosynthetic pathway for the assembly of staphylopine, pseudopaline, yersinopine and bacillopaline. Adapted from [93].
3.1. Staphylopine
Staphylopine (StP) is a versatile metallophore, related to plant nicotianamine, expressed by Staphylococcus aureus, a Gram-positive bacterium. StP can bind various metals in vitro depending on the environmental conditions; its metal-affinity mirrors that of nicotianamine, with similar Kd values and the same affinity order: Cu2+ > Ni2+ > Co2+ > Zn2+ > Fe2+ [94]. The first extensive description of the cnt operon (cntKLMABCDFE), that encodes the functions required for the biosynthesis (CntKLM) and trafficking of StP, is due to Ghssein et al. [94]. The biosynthesis of StP is promoted by the combined enzymatic activity of CntK, CntL, and CntM using D-His as starting substrate for the fusion with a single α-aminobutyric acid moiety deriving from SAM. The acquisition of zinc is then realized with the importer CntABCDF which works in conjunction with StP, while the membrane transport protein CntE, belonging to the Major Facilitator Superfamily (MFS), is deputed to the export of the metallophore (Fig. 6). It is worthy of mention here that S. aureus is also capable of directly recruit Zn2+ ions via the ABC permeases of the AdcABC family, which are found in many other Gram-positive bacteria [86]. Adc is the first system employed by S. aureus to import Zn2+ ions; the Cnt system is induced only when the bacterium zinc demand cannot be satisfied by the Adc system alone [88, 95]. The capability of S. aureus to obtain the zinc required for its growth in a zinc-depleted environment is deeply impaired by the concomitant loss of both the Adc and Cnt systems. However, while the loss of AdcABC alone does not hamper the S. aureus ability to grow or cause disease in the presence of calprotectin (CP), the Cnt-StP system proved critical for S. aureus to face zinc starvation imposed by CP. The StP-based import system of S. aureus is the most important weapon of this bacterium in the tug-of-war with the host for zinc recruitment during infection [86].
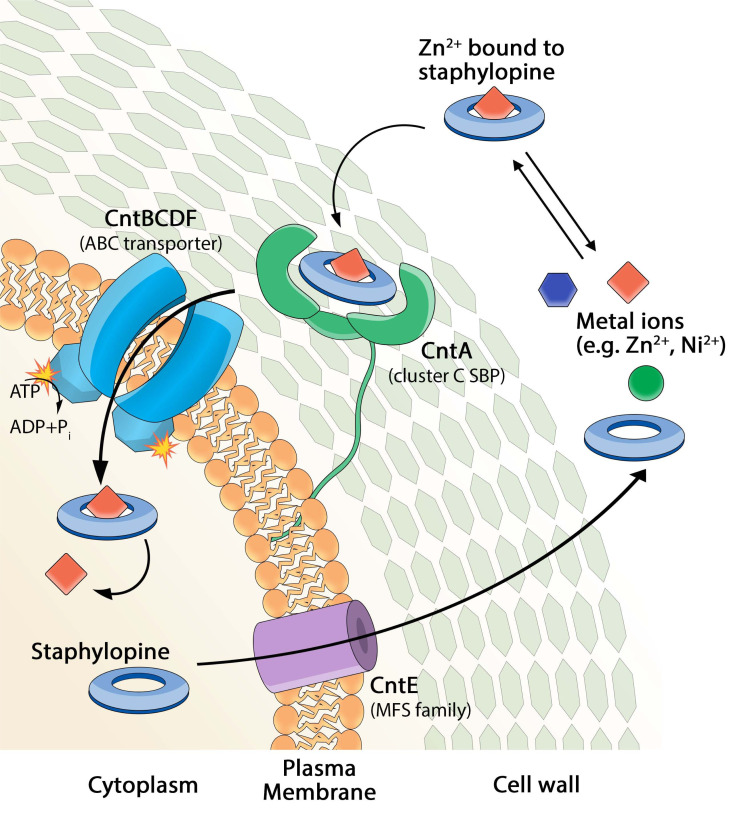
Fig. (6).
Model of staphylopine (StP) secretion and recovery of zinc ions. After Stp synthesis inside the cell, its export occurs by means of the MFS efflux pump CntE. The import of StP zinc complex is then mediated by the ABC import system CntABCDF. The complex dissociates in the cytoplasm and zinc is released [94]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The machinery of staphylopine synthesis and activity, encoded by the cntKLMABCDFE operon, was further enlightened by Fojcik et al. [96]; they found that this operon is preceded by a non-coding region (PcntK) and includes a promoter region (PcntA). The PcntK region contains two consecutive Fur and Zur boxes, a sRNA transcription start and a repetition; the PcntA region contains a Fur box overlapping a Zur box. The activity of PcntK is reduced by the repeated region and tightly controlled by Fur or Zur binding to their respective targets. The PcntA activity is cooperatively regulated by Fur and Zur binding to the Fur/Zur box. In addition, the StP-dependent metal acquisition in S. aureus proved to be subtly regulated by the two essential metals, iron and zinc [96].
As already described above, the cnt operon comprises an StP exporter, a membrane protein encoded by the cntE gene, and an StP metal-complex importer, encoded by the genes cntABCDF. In particular, CntA is an extracytoplasmic Substrate-Binding Protein (SBP) and plays a key role in StP/metal recognition. CntB and CntC are membrane proteins forming the channel required for StP/metal import. CntDF are two ATP-binding proteins that supply the energy necessary for transportation. In order to enlighten the molecular mechanisms ruling the recognition and transport of StP/metal complexes, Song et al. determined the crystal structures of the CntA/StP complexes with Co2+, Ni2+ and Zn2+ [88]. They also measured the affinity constants for the interactions of CntA with StP/Co2+, StP/Ni2+ and StP/Zn2+ complexes by ITC. CntA can distinguish among the three StP/metal complexes; it binds StP/Ni2+ and StP/Co2+ stronger than StP/Zn2+. Most likely, the nature of the metal ion recognized and transported by the Cnt system depends on the conditions of the environment where the bacteria grow.
S. aureus exports StP via the membrane CntE protein, and ΔcntE mutant exhibits a significant growth defect in a mouse subcutaneous-abscess model [97]. On the contrary, no defect was detected in the mutant ΔcntABCDF, lacking the StP importer [97]. Since the growth of the ΔcntE mutant was also compromised in zinc and iron-depleted medium, the StP accumulation in the bacterial cytoplasm along with its toxicity were hypothesized as the reasons for this effect [96]. Chen and Hooper [98] compared the growth of a ΔcntE ΔcntL double mutant with that of ΔcntE and ΔcntL single mutants and of their parental strain in a murine subcutaneous-abscess model, under conditions of metal deficiency. They also followed the StP concentrations in the cytoplasm and supernatant during the growth. In the absence of the exporter, StP was produced and accumulated inside the cell, but this did not cause a reduction in cntKLM expression under a metal shortage. On the other hand, in the absence of the gene cntL, the StP biosynthesis was blocked; no intracellular StP accumulation was observed and the growth defect of ΔcntE ΔcntL double mutant was less marked. To explain this behaviour, it was hypothesized that high concentrations of StP in the cytoplasm would result in metal ion sequestration thus impairing the cell metabolism. In fact, the growth defect for the ΔcntE mutant in a metal-depleted environment could be reversed by adding metals. However, the hypothesis was not supported by PCR experiments, since ΔcntE mutant and WT strain did not show any difference in the expression of their metal-regulated genes [98]. In order to better clarify the mechanism causing the growth defect of S. aureus ΔcntE mutant, a strain lacking the second zinc transporter AdcABC system was investigated under depleted metal conditions [95]; this mutant requires the Cnt transport system to stock up on zinc. A serious growth defect was confirmed for ΔcntE mutant, especially in the presence of CP, suggesting the inability of the cell to manage the zinc starvation. The addition of Zn2+ restored the growth for both the ΔcntE ΔadcA and ΔcntA ΔadcA mutants, while the addition of iron reversed the growth defect only of the former but not of the latter. The analysis of cellular metal content under various experimental conditions showed that the overall metal capacity of the cell is not stimulated by StP accumulation due to CntE loss. Instead, the accumulation of StP and its intermediates is toxic for the cell and this can be the main reason for the well-established growth defect when CntE is absent or blocked. These results make the StP efflux pump a promising drug target for new therapeutic strategies.
The SauODH enzyme catalyzes the condensation of pyruvate with the intermediate xNA to produce StP, the amount of which must be accurately regulated in order to maintain the correct metal levels in the cell but also to avoid any waste of StP or its toxic effect. The SauODH enzyme was recently structurally and functionally characterized by Hajjar et al. [99] who also first chemically synthesized the intermediate xNa. The crystal structure of SauODH substantially confirmed the previously reported results [92]. The metals were shown to play various and often opposite roles on the enzyme activity; copper and zinc, at low concentration, act as activators while they behave as strong inhibitors at high concentrations; manganese is always an activator; nickel and cobalt always behave as inhibitors. In the proposed model, the enzyme activation is ruled by both the metal concentration and its relative affinity towards the xNA intermediate and an inhibitory site on SauODH. SauODH is also somewhat inhibited by StP itself as a feedback mechanism to avoid overproduction of the valuable but toxic metallophore.
3.2. Pseudopaline
As already mentioned in the previous sections, the ZnuABC transporter is the main zinc recruiting system in many bacteria and its inactivation often causes a dramatic failure in cell growth. This is not the case with Pseudomonas aeruginosa, a Gram-negative human pathogen, which is able to proliferate in metal-depleted environments, in the presence of calprotectin, and also if znuABC is disrupted [100]. By genomic analysis of P. aeruginosa under zinc starvation, Mastropasqua et al. could identify the operon zrmABCD (also called cntOLMI) that encodes for an additional zinc import system [101]. This operon is stimulated by zinc deficiency and it is Zur-regulated; it contains the genes for an outer membrane transport protein (named ZrmA or CntO) and for a nicotianamine synthase (ZrmB or CntL). If one of the genes is inactivated together with the zinc transporter ZnuABC, the capability of P. aeruginosa to grow in a zinc-depleted environment and to collect Zn2+ is significantly impaired. These results suggested that P. aeruginosa can synthesize a zincophore which is transported outside the cell. This is a rather small molecule, structurally related to nicotianamine, and then named pseudopaline since it belongs to the opine family, like StP [102]. The expression of pseudopaline by P. aeruginosa proved to be very selective in response to zinc shortage.
As mentioned above, P. aeruginosa contains a four-gene cntOLMI operon homologue that is responsible for the synthesis of the metallophore StP in S. aureus. Besides the nicotianamine synthase (CntL) and the opine dehydrogenase (CntM) enzymes, which synthesize pseudopaline, the machinery comprises a TonB-dependent transporter (CntO), an inner membrane protein of the drug/metabolite transporter family (CntI) and an outer membrane efflux pump of the resistance-nodulation-division family (MexAB-OprM) (Fig. 7) [103]. The operon is regulated on the zinc level by the Zur repressor [102]. Pseudopaline is different from StP for the use of L-His in the place of D-His, and of α-ketoglutarate instead of pyruvate. A cntL mutant strain, incapable of synthesizing pseudopaline, is not able to provide nickel from minimal media, even when supplemented with additional nickel [102]. If a strong multi-target chelator (EDTA) is added to the medium, a situation that mimics the nutritional immunity conditions, for example, in airway mucus secretion or in the presence of calprotectin, the cntL mutant strain is also unable to import zinc, thus confirming the role of this operon to produce a zinc importer under metal shortage [102]. It is worthy of notice that ΔcntI mutants of P. aeruginosa, lacking the capability to export pseudopaline, showed more severe growth defects than mutants where pseudopaline synthesis or its metal-complex import were eliminated [103]. This observation suggests that the toxicity of pseudopaline is due to the chelation of intracellular metals [95].
Fig. (7).
Model of pseudopaline secretion and recovery of zinc ions. After pseudopaline synthesis inside the cell, its transfer across the inner membrane occurs by means of the DMT transporter CntI. The transport system in charge of its export from the periplasm to the extracellular space is the MexAB-OprM efflux pump. The import of pseudopaline zinc complex in the periplasm space is mediated by the TBDT transporter, CntO. The complex is later internalized by unknown system. The complex then dissociates and zinc is released [104]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The biosynthetic pathways leading to staphylopine and pseudopaline were reconstructed in vitro to confirm their potential substrates; the formed products were then checked by mass spectrometry [105]. The nicotianamine synthase and opine dehydrogenase enzymes of P. aeruginosa and S. aureus (PaeNAS/SauNAS and PaeODH/SauODH, respectively) were heterologously expressed in E. coli and purified. Both the affinities of Pae NAS for SAM and of PaeODH for NADPH were measured by fluorimetry and the found dissociation constants were in the micromolar range and lower than those previously reported for SauNAS and SauODH, respectively [94]. The inclusion of the imidazole side group of histidine in both staphylopine and pseudopaline was confirmed, although with opposite chirality. The α-ketoglutarate moiety incorporated in pseudopaline is bulkier than pyruvate used to synthesize staphylopine; it contains an additional carboxylic group which provides additional flexibility and higher affinity for metal ions.
3.3. Other Opine Zincophores
Also Y. pestis possesses a multiple zinc uptake system. Besides the ZnuABC importer, Y. pestis uses the siderophore yersinobactin to collect Zn2+ under conditions of metal restriction [106, 107]. In addition, the existence of a third mechanism has been reported. In fact, this pathogen can express opine dehydrogenases (named YpeCntM or YpeODH), which are able to condense pyruvate with yNA, using NADPH; the product is the nicotianamine-like metallophore called yersinopine. To date, no information is available on the interrelation between yersinopine production and Y. pestis virulence, and the description of yersinopine is limited to in vitro researches [92].
The existence of another opine zincophore has been hypothesized by Laffont et al. [93], who observed that P. mucilaginosus contains a histidine racemase homologue (like S. aureus) but, on the other hand, it is able to condense xNA with α-ketoglutarate (like P. aeruginosa). The resulting product should be a new type of opine metallophore named bacillopaline [93].
4. FUNGAL ZINCOPHORES
Adequate zinc acquisition is also critical for the survival and virulence of fungal pathogens, which just as in the case of bacterial invaders, have to overcome the nutritional immunity of the host, which is not a trivial task due to the sparse, even picomolar concentrations of free Zn2+. The tug of war between the fungus and its host, during which both are trying to control the availability of zinc, can, as in the case of bacteria, be regarded as a potential target for new antifungal therapies. In order to develop a highly specific anti-fungal medication, it is important to precisely understand and aim at the differences in human and fungal Zn2+ transport [18, 108]. One of the biggest obstacles in finding effective and drug-specific antifungal agents that do not cause serious side effects in patients comes from the fact that fungi share many basic metabolic pathways with their human hosts (both are eukaryotes), much more than with prokaryotic bacteria.
Unlike bacterial pathogens, fungi do not rely on ABC transporters for zinc transport, but they encode two independent classes of zinc transporters: (i) ZIPs (Zrt/Irt-like proteins), which transport zinc from outside the cell into the cytoplasm, and (ii) ZnTs (zinc transporters) which transport zinc from the cytoplasm out of the cell and from the cytoplasm into vesicles. Numerous fungal species secrete zinc-binding proteins – zincophores, one of the most exciting classes of molecules of fungal bioinorganic chemistry.
The best-described modes of Zn2+ acquisition are those of Candida albicans, Aspergillus fumigatus and the yeast Saccharomyces cerevisiae. S. cerevisiae encodes two membrane zinc importers, which transport Zn2+ into the cell: the high-affinity Zrt1 and the low-affinity Zrt2. Both of them are up-regulated at low Zn2+ concentration by the transcription factor Zap1; when the cellular concentration of Zn2+ becomes too high, the expression of Zrt1 and Zrt2 becomes down-regulated [109]. The orthologues of Zrt1 and Zrt2 in A. fumigatus are ZrfA and ZrfB. Their expression is up-regulated by ZafA (an orthologue of S. cerevisiae Zap1) when Zn2+ is sparsely available, and, under more basic conditions, down-regulated by PacC, which induces the expression of a third Zn2+ transporter, ZrfC [110, 111]. A similar expression pattern is observed in C. albicans; the expression of Zrt1 and Zrt2 zinc transporters is regulated by zinc availability [112], by Zap1 [113], and by pH via Rim101 [114]. Although modestly upregulated by acidic pH, Zrt2 remains functional in neutral and alkaline environment (just as the A. fumigatus orthologue ZrfB) [115]. However, it is noteworthy that at higher pH, the solubility of Zn2+ ions decreases, and most likely, this is the reason why the pH triggers the expression of a second, zincophore-based Zn2+ import system. Fungal zincophores are small proteins, able to sequester zinc from the host tissue by interacting either with the free soluble metal ion or with the metal coordinated to different ligands. The concept of fungal protein zincophores and the discovery of how they work is relatively new to the world of science. In 2012, Citiulo et al. explained the details of this mechanism in C. albicans; the zincophore is expressed under zinc limited conditions, and in neutral to basic pH, it is then released from the hyphal surface into the surrounding environment of the host, where it binds the Zn2+ ion and delivers it to the fungal pathogen via physical interactions with an appropriate zinc transporter (Fig. 8) [116].
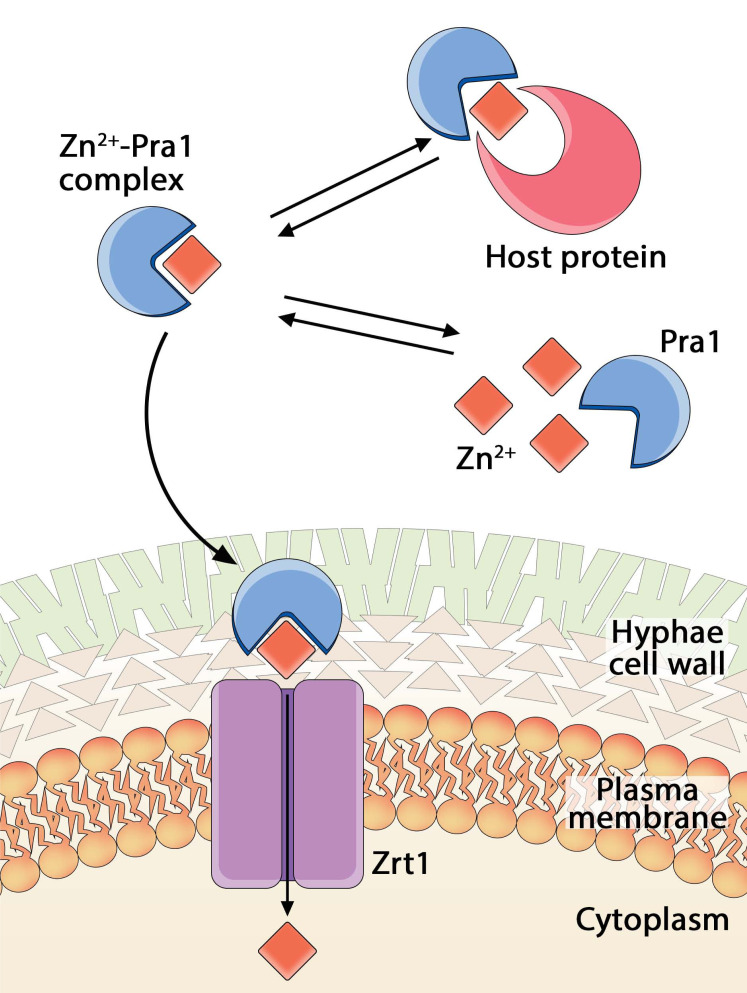
Fig. (8).
Schematic model of C. albicans zincophore-based Zn2+ acquisition [116]; after host invasion, Pra1 is expressed due to alkaline pH and low amount of soluble Zn2+ in the intracellular environment. It is secreted from the fungal cell surface, predominantly in the hyphal form and it binds host cellular zinc (either free cytosolic or bound to host protein). Pra1 returns to the fungal cell via physical interaction with Zrt1, a membrane transporter, to deliver the bound Zn2+ ions. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In the case of C. albicans, the 299 amino acid zincophore is named Pra1 (pH-regulated antigen 1); it captures zinc and returns to the cell via a synthenically expressed receptor, Zrt1 (A. fumigatus orthologues: Aspf2 and ZrfA, respectively) [116]. Both pra1 and zrt1 genes are encoded at the same locus and share the same promoter [117]. Deletion of the pra1 gene prevents C. albicans from using host zinc and this does not allow the fungus to grow and damage the surrounding cells [116]. It is quite noteworthy that although C. albicans and A. fumigatus are not related species, they possess a similar set of genes that are required for Zn2+ acquisition. Pra1 from C. albicans and Aspf2 from A. fumigatus share 43% of sequential identity upon sequence alignment, Pra1 from C. albicans and Pra1 from Blastomyces dermatitidis [118] share 37% of sequential identity (Scheme S3 (437.5KB, pdf) , Supplementary Information), and the zinc transporters Zrt1 (C. albicans) and ZrfC (A. fumigatus) share 48% identity. Both the zincophores and zinc transporters most likely share very similar Zn2+ binding motifs. Based on these considerations, one can suppose that both the fungal scavengers may bind zinc in a similar way [110].
Initial studies showed that Pra1 was able to bind zinc and copper ions, but did not interact with iron, manganese or calcium [116]. Further attempts to identify metal-binding sites in Pra1 were carried out on peptide models, which focused on potential Zn2+ binding sites, fulfilling three criteria: (i) contained multiple zinc-binding sites, including those with HXXXHXXGXXH and HXH motifs and other regions rich in histidines, cysteines and acidic residues; (ii) were located in unstructured regions of Pra1, as predicted by Phyre2 (a remote homology recognition technique, able to regularly generate reliable protein models [78]) and (iii) were evolutionarily conserved in the zincophores Pra1 of C. albicans and Aspf2 of A. fumigatus [19]. Combined NMR, potentiometric and mass spectrometric studies allowed pointing out the metal-binding sites and compare their stabilities, showing that the C-terminal region of Pra1 (SHQHTDSNPSATTDANSHCHTHADGEVHC, residues 271-299) has the highest affinity for Zn2+ and binds the metal ion by means of four imidazoles (from His288, His290, His292 and His298) (Fig. 9a) [19]. Most likely, this is also the region responsible for the interactions with the Zrt1 zinc transporter. To point out precise Zn2+ binding sites at the N-terminal, extracellular region of the Zrt1 transporter, a similar experimental approach was used. The His156, His161, His168 and Cys162 have been identified as Zn2+ binding residues in Zrt1 at physiological pH (Fig. 9b) [119]. Thermodynamic results also highlighted the possible favorable transfer of the Zn2+ ion from Pra1 to Zrt1, thus enabling an efficient transport of the metal ion from the zincophore to the transporter protein [119].
Fig. (9).
Proposed Zn2+ binding sites on C. albicans a) Pra1 zincophore and b) Zrt1 zinc transporter [19]. Structures are based on coordinates simulated by Phyre2 [78]. The figure was generated using CCP4mg [79]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Although our knowledge on the inorganic biochemistry of zincophores keeps expanding and is starting to be considered as a stepping stone towards finding new, specific antifungal therapeutics based on zincophore fragments connected with antifungal drugs, there are numerous issues that keep surprising us, such as the recent data which shows that the Pra1 zincophore may collaborate with Sap6, a secreted aspartyl protease involved in zinc scavenging. It was suggested that Sap6 may act upstream in the zincophore pathway, delivering the substrate to the Pra1/Zrt1 system [120]. In this context, Sap6 may function as a zinc-chaperone and may take the role of the above-mentioned bacterial SBP associated zincophores (e.g. ZinT), highlighting more similarities with bacteria organisms than might be expected. It is also worth mentioning that, from the functional point of view, the Pra1/Zrt1 zinc uptake system is analogous to bacterial zinc ABC transporters, involving a secreted zinc scavenger (e.g. ZnuA) and a transmembrane protein (e.g. ZnuB). Hence, the nutrient sequestration strategy from C. albicans appears to be well conserved in other pathogens [108, 121].
As far as fungal species are considered, Pra1 orthologues were identified in about 85% of the sequenced fungi [122]; however, some species, such as Candida glabrata, Histoplasma capsulatum, or Cryptococcus neoformans have lost the pra1 gene. It is hypothesised that this is due either to their adaptation to acidic environments, because Pra1-Zrt1 is non-functional at acidic pH [123], or to the fact that Pra1 could influence the pathogens’ interactions with host immunity, serving as a ligand for neutrophils and thus triggering their migration (both C. albicans Pra1 and A. fumigatus Aspf2 interact with several components of the complement cascade, such as factor H, plasminogen [124], C3 and C4b-binding protein [125]). Without the zincophore system, the fungus could benefit from avoiding attention from the immune system of the host.
CONCLUSION
The importance of transition metals as virulence factors is undoubtedly well recognised and accepted [9]. Metal availability depends on different physiological factors, such as pH, free oxygen concentration, metabolic activity, enzyme expression, imbalance of other free metal ions and the presence of competing chelators from the host organism. Understanding the dynamics behind metal acquisition processes is crucial to direct in vitro structural and functional studies and then provide rational guidelines for the design of new antimicrobial agents.
Zincophores represent the main targets in an antimicrobial scenario aimed at knocking out the pathogen through cellular starvation and inhibition of the metal import systems [126]. The amount of free zinc ions, in fact, must be strictly regulated both in the case of pathogens and humans. Overload and shortage of zinc can induce cellular apoptosis due to intrinsic cytotoxic effects (inhibition of enzymes through wrong metal coordination, formation of stable and irreversible protein complexes, disruption of the membrane potential, absence of crucial protein cofactors and consequent loss of enzymatic activity). Moreover, zincophore-targeted therapies may limit undesirable side effects for the host organism, since most eukaryotes, including humans, frequently exhibit different zinc acquisition pathways and totally lack most of the extracytosolic zinc-binding proteins here described for bacteria and fungi.
There are different possible zincophore-based strategies suitable for designing novel antimicrobial therapies: (i) zincophore-mediated drug delivery, (ii) cellular zinc overload through alteration of zincophore biosynthesis, (iii) disruption of zinc homeostasis by starvation due to competitive chelation or inhibition of zincophore activity. These approaches reflect the best known and most studied siderophore-based therapeutics [127].
One of the most promising (antimicrobial or diagnostic) potential use of zincophores is based on the Trojan Horse strategy, which has been widely explored in the case of siderophores. The success of this strategy lies in the elusion of the cell membrane-associated drug resistance and in an efficient and selective drug delivery. The general approach exploits the metallophore-mediated metal ion transport across the cell membrane. A conjugated system (Fig. 10), where the pharmacophore molecule is covalently bound to the metal-binding protein in its holo-form, can be recognised by the cognate membrane transporter by means of the metal-metallophore component. The complex is then internalized inside the cell together with the conjugated therapeutic agent. The zincophore-mediated highly selective transport can also be used for diagnostic purposes by substituting the antimicrobial compound with an imaging probe. The accumulation of the labeled compound inside the infected tissues can be highly specific, targeting only the pathogen and not affecting mammalian cells [128, 129]. The nicotianamine- like zincophores, such as staphylopine and pseudopaline, are promising target molecules for the design of conjugated-Trojan Horse systems which can be selectively acquired via their associated transporters (Fig. 10). In this context, a detailed knowledge of fungal Zn2+ uptake is also crucial to expand the limited arsenal of currently effective, highly specific treatments against fungal infections and open new therapeutic possibilities based on antifungal agents bound to the C-terminal fragment of the Pra1 zincophore, which would deliver the drug to the fungus in a selective way. The most common antifungal agents are those that (i) interfere with the functions of the cell membrane (polienols or pirridone derivatives), (ii) are inhibitors of ergosterol biosynthesis (azoles, triazoles, alliloamines and phenylomorfolines), (iii) are nucleic acid synthesis inhibitors (cytosine analogues), and (iv) are cell wall synthesis inhibitors (echinocandins) [130].
Fig. (10).
(Top) General design of potential Trojan Horse zincophore-based therapeutics. The conjugated system is constituted by four elements: (1) the zinc ion, (2) the zincophore molecule which is able to form a stable zinc complex, (3) a linker to covalently bind the zincophore to the pharmacophore, (4) the drug or imaging probe. (Bottom) Schematic representation of the Trojan Horse strategy. The transporter recognizes its substrate (the Zn2+-zincophore complex) and facilitates the transition of the conjugated pharmacophore across the membrane barrier. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
A cellular toxic effect of zinc by increasing the concentration of metal in the cytosol is also possible. Novel nicotianamine-like zincophores can be designed with this purpose or, alternatively, they can be exploited as cytotoxic compounds (in place of the excess of Zn2+). The staphylopine trafficking pathway is a promising drug target candidate, since the StP intracellular accumulation proved to be detrimental for the bacterial strain survival, even without disrupting the metal homeostasis, but rather primarily acting with a chelation-independent mechanism [95]. The biosynthesis of Stp in S. aureus is upregulated under low Zn2+ concentration and, together with the inhibition of the CntE efflux pump, it can result in a toxic overaccumulation of Stp inside the cell, offering a novel antimicrobial opportunity [95].
Impairing the cell zinc homeostasis represents another promising antimicrobial strategy with a high rate of selectivity and specificity. The administration of compounds able to mimic, undermine or inactivate the zincophores activity can result in less morbidity and in the attenuation of the pathogen virulence, until a most desirable microbicide property is achieved. For this purpose, one can rely on de novo designed molecules with unmodified characteristics and way of actions or on derivatives of already existing natural antimicrobial agents that commonly reside in the host organism and participate in the nutritional immunity process (e.g., peptidomimetics); some examples of human zinc chelating antimicrobial macromolecules which compete with pathogen zincophores include calprotectin, psoriasin, histatins, microplusin and calcitermin [131-133]. These systems, and their ad hoc synthesized derivatives, may exhibit antimicrobial activity by simply displaying higher metal binding affinity and thermodynamic stability, which results in the metal ions sequestration and reduction of their bioavailability. A further strategy implies the inhibition of metalloproteins through the coordination of the metal ion in the protein active site; the metal binding is thus aimed at preventing the proper function of the protein. Several reported metalloprotein inhibitors bear the hydroxamic acid as a pharmacophore functional group able to stably bind the target system through the Zn2+ ion [134]. One recent example of zincophore-targeted antimicrobial compounds regards two di-aryl pyrrole hydroxamic acids derivatives (RDS50 and RDS51) that have proved to interfere with the ZnuA activity in S. enterica serovar Thyphimurium by preventing the Zn2+ release [135].
Along with antimicrobial therapeutic applications, zincophores are also thought to be the most immunogenic components among the pathogenic metal transport systems, particularly in Gram-positive bacteria where they can be superficially exposed [136]. S. pneumoniae cell surface zincophores (e.g. histidine triad proteins, AdcA, AdcAII and their orthologues in other streptococci) are increasingly considered as promising novel vaccine candidate antigens [137-140], as well as TroA in T. pallidum and Chlamydia trachomati [141]. The metal-substrate binding protein PsaA has been already successfully employed for the production of immunizing agents [142] and its properties may contribute to the fight against SARS-CoV-2 [143]. In fact, it has been found that pneumococcal proteins, including PsaA, contain many sequence portions sharing high identity with various fragments of SARS-CoV-2 proteins. These pneumococcal proteins are known to contaminate polysaccharide-based pneumococcal vaccines; two epidemiological studies have suggested a protective effect of pneumococcal vaccination against SARS-CoV-2: a potential cross-reactivity between pneumococcal and SARS-CoV-2 proteins has been hypothesised [143]. Although in this case, PsaA effect is not directly dependent on the metal acquisition, the zinc-based antimicrobial strategies previously described can be basically applied also to fight viruses. In particular, SARS-CoV-2 has been proved to be susceptible to zinc overload, due to Zn2+ inhibition of SAR-Cov RNA polymerase. On the contrary, zinc deficiency could facilitate SARS-CoV-2 infection due to an increase in angiotensin-converting enzyme 2 (ACE2, a zinc metallopeptidase) activity that could facilitate the virus entry into target cells. Therefore, the use of zinc ionophores (therapeutic agents able to raise intracellular zinc concentrations) has been explored as a potential therapy [144]. Zinc-fingers have also been proposed to sequester Zn2+ from zinc metalloenzymes which are essential for SARS-CoV-2, thus deactivating the enzyme activity and increasing the intracellular free Zn2+ concentration [145]. It is also important to highlight that the onset of bacterial co-infections in patients affected by COVID-19 may represent a serious issue, and impairing zinc homeostasis may ultimately be an interesting and innovative strategy to ameliorate the progression of SARS-CoV-2 infections [146-148].
In conclusion, a detailed in-depth comprehension of metal coordination and thermodynamic stability of the formed complexes is, therefore, crucial to evaluate the candidate drugs in terms of their ability to sequester metals or efficiently bind the protein–metal system and hamper the pathogen metal trafficking. Zincophore-targeted therapies have enabled to move forward in the design of novel promising antimicrobial agents and represent an outstanding opportunity to “checkmate” the pathogens.
ACKNOWLEDGEMENTS
Graphics and illustrations have been designed and prepared by the author Denise Bellotti. The financial support from the University of Ferrara (FAR 2020) and from the National Science Centre is gratefully acknowledged. This paper is based upon work from COST Action CA18202, NECTAR – Network for Equilibria and Chemical Thermodynamics Advanced Research, supported by COST (European Cooperation in Science and Technology).
LIST OF ABBREVIATIONS
- ABC
ATP-binding Cassette
- Ac
Acetyl
- ACE2
Angiotensin-converting Enzyme 2
- Am
Amide
- Afu
Aspergillus fumigatus
- AMR
Antimicrobial Resistance
- Bde
Blastomyces dermatitidis
- Cal
Candida albicans
- CP
Calprotectin
- DMT
Drug/metabolite Transporter
- Eco
Escherichia coli
- Hdu
Haemophilus ducreyi
- Hin
Haemophilus influenzae
- ITC
Isothermal Titration Calorimetry
- MFS
Major Facilitator Superfamily
- SBP
Substrate Binding Protein
- Pae
Pseudomonas aeruginosa
- PepT
Peptide, Opine and Nickel Uptake Transporter
- Pht
Histidine Triad Protein
- RND
Resistance-Nodulation-Division
- Sau
Staphylococcus aureus
- Sen
Salmonella enterica
- Stp
Staphylopine
- Syn
Synechocystis 6803
- TBDT
TonB-Dependent Transporter
- TMD
Transmembrane Domain
- Tpa
Treponella pallidum
- Ype
Yersinia pestis
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This paper has been supported by the University of Ferrara (FAR 2020) and the National Science Centre (UMO2017/26/A/ST5/00364 and 2017/26/A/ST5/ 00363). This paper is based upon work from COST Action CA18202, NECTAR – Network for Equilibria and Chemical Thermodynamics Advanced Research, supported by COST.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.Rello J., Kalwaje Eshwara V., Conway-Morris A., Lagunes L., Alves J., Alp E., Zhang Z., Mer M., Investigators T.S., TOTEM Study Investigators Perceived differences between intensivists and infectious diseases consultants facing antimicrobial resistance: A global cross-sectional survey. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38(7):1235–1240. doi: 10.1007/s10096-019-03530-1. [DOI] [PubMed] [Google Scholar]
- 2.Bloom D.E., Cadarette D. Infectious disease threats in the twenty-first century: Strengthening the global response. Front. Immunol. 2019;10:549. doi: 10.3389/fimmu.2019.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg J.A., Hohmann S.F., James B.D., Shah R.C., Hall J.B., Kress J.P., David M.Z. Hospital volume of immunosuppressed patients with sepsis and sepsis mortality. Ann. Am. Thorac. Soc. 2018;15(8):962–969. doi: 10.1513/AnnalsATS.201710-819OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European centre for disease prevention and control. Surveillance of antimicrobial resistance in europe. 2018. Available from: www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf.
- 5.Hancock R.E.W., Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 6.Porcheron G., Garénaux A., Proulx J., Sabri M., Dozois C.M. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: Correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front. Cell. Infect. Microbiol. 2013;3:90. doi: 10.3389/fcimb.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Z., Jacobsen F.E., Giedroc D.P. Coordination chemistry of bacterial metal transport and sensing. Chem. Rev. 2009;109(10):4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukada T., Yamasaki S., Nishida K., Murakami M., Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011;16(7):1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hood M.I., Skaar E.P. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012;10(8):525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg E.D. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. JAMA. 1975;231(1):39–41. doi: 10.1001/jama.1975.03240130021018. [DOI] [PubMed] [Google Scholar]
- 11.Djoko K.Y., Ong C.L., Walker M.J., McEwan A.G. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J. Biol. Chem. 2015;290(31):18954–18961. doi: 10.1074/jbc.R115.647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umair M., Alfadhel M. Genetic disorders associated with metal metabolism. Cells. 2019;8(12):1598. doi: 10.3390/cells8121598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grüngreiff K., Reinhold D., Wedemeyer H. The role of zinc in liver cirrhosis. Ann. Hepatol. 2016;15(1):7–16. doi: 10.5604/16652681.1184191. [DOI] [PubMed] [Google Scholar]
- 14.Abbaspour N., Hurrell R., Kelishadi R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014;19(2):164–174. [PMC free article] [PubMed] [Google Scholar]
- 15.Zalewski P.D., Truong-Tran A.Q., Grosser D., Jayaram L., Murgia C., Ruffin R.E. Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets. A review. Pharmacol. Ther. 2005;105(2):127–149. doi: 10.1016/j.pharmthera.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Capdevila D.A., Edmonds K.A., Giedroc D.P. Metallochaperones and metalloregulation in bacteria. Essays Biochem. 2017;61(2):177–200. doi: 10.1042/EBC20160076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morey J.R., Kehl-Fie T.E. Bioinformatic mapping of opine-like zincophore biosynthesis in bacteria. mSystems. 2020;5(4):e00554–e00520. doi: 10.1128/mSystems.00554-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wątły J., Potocki S., Rowińska-Żyrek M. Zinc homeostasis at the bacteria/host interface-from coordination chemistry to nutritional immunity. Chemistry. 2016;22(45):15992–16010. doi: 10.1002/chem.201602376. [DOI] [PubMed] [Google Scholar]
- 19.Łoboda D., Rowińska-Żyrek M. Zinc binding sites in pra1, a zincophore from Candida albicans. Dalton Trans. 2017;46(40):13695–13703. doi: 10.1039/C7DT01675A. [DOI] [PubMed] [Google Scholar]
- 20.Staats C.C., Kmetzsch L., Schrank A., Vainstein M.H. Fungal zinc metabolism and its connections to virulence. Front. Cell. Infect. Microbiol. 2013;3(65) doi: 10.3389/fcimb.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neupane D.P., Kumar S., Yukl E.T. Two ABC transporters and a periplasmic metallochaperone participate in zinc acquisition in paracoccus denitrificans. Biochemistry. 2019;58(2):126–136. doi: 10.1021/acs.biochem.8b00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yukl E.T. In: Encyclopedia of inorganic and bioinorganic chemistry. Scott R.A., editor. John Wiley & Sons, Ltd.; 2017. pp. 1–12. [Google Scholar]
- 23.Sigel H., Martin R.B. Coordinating properties of the amide bond. Stability and structure of metal ion complexes of peptides and related ligands. Chem. Rev. 1982;82(4):385–426. doi: 10.1021/cr00050a003. [DOI] [Google Scholar]
- 24.Kozłowski H., Bal W., Dyba M., Kowalik-Jankowska T. Specific structure–stability relations in metallopeptides. Coord. Chem. Rev. 1999;184(1):319–346. doi: 10.1016/S0010-8545(98)00261-6. [DOI] [Google Scholar]
- 25.Giebeler N., Zigrino P. A disintegrin and metalloprotease (adam): Historical overview of their functions. Toxins (Basel) 2016;8(4):122. doi: 10.3390/toxins8040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maret W. The metals in the biological periodic system of the elements: concepts and conjectures. Int. J. Mol. Sci. 2016;17(1):66. doi: 10.3390/ijms17010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikhaylina A., Ksibe A.Z., Scanlan D.J., Blindauer C.A. Bacterial zinc uptake regulator proteins and their regulons. Biochem. Soc. Trans. 2018;46(4):983–1001. doi: 10.1042/BST20170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchings M.I., Palmer T., Harrington D.J., Sutcliffe I.C. Lipoprotein biogenesis in Gram-positive bacteria: Knowing when to hold ‘em, knowing when to fold ’em. Trends Microbiol. 2009;17(1):13–21. doi: 10.1016/j.tim.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Berntsson R.P.A., Smits S.H.J., Schmitt L., Slotboom D-J., Poolman B. A structural classification of substrate-binding proteins. FEBS Lett. 2010;584(12):2606–2617. doi: 10.1016/j.febslet.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Waldron K.J., Robinson N.J. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 2009;7(1):25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 31.Braymer J.J., Giedroc D.P. Recent developments in copper and zinc homeostasis in bacterial pathogens. Curr. Opin. Chem. Biol. 2014;19:59–66. doi: 10.1016/j.cbpa.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finney L.A., O’Halloran T.V. Transition metal speciation in the cell: Insights from the chemistry of metal ion receptors. Science. 2003;300(5621):931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 33.Scheepers G.H., Lycklama A Nijeholt J.A., Poolman B. An updated structural classification of substrate-binding proteins. FEBS Lett. 2016;590(23):4393–4401. doi: 10.1002/1873-3468.12445. [DOI] [PubMed] [Google Scholar]
- 34.Couñago R.M., Ween M.P., Begg S.L., Bajaj M., Zuegg J., O’Mara M.L., Cooper M.A., McEwan A.G., Paton J.C., Kobe B., McDevitt C.A. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat. Chem. Biol. 2014;10(1):35–41. doi: 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y-H., Dorwart M.R., Hazlett K.R.O., Deka R.K., Norgard M.V., Radolf J.D., Hasemann C.A. The crystal structure of Zn(II)-free Treponema pallidum TroA, a periplasmic metal-binding protein, reveals a closed conformation. J. Bacteriol. 2002;184(8):2300–2304. doi: 10.1128/JB.184.8.2300-2304.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felder C.B., Graul R.C., Lee A.Y., Merkle H-P., Sadee W. The Venus flytrap of periplasmic binding proteins: an ancient protein module present in multiple drug receptors. AAPS PharmSci. 1999;1(2):E2. doi: 10.1208/ps010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radka C.D., Labiuk S.L., DeLucas L.J., Aller S.G. Structures of the substrate-binding protein YfeA in apo and zinc-reconstituted holo forms. Acta Crystallogr. D Struct. Biol. 2019;75(Pt 9):831–840. doi: 10.1107/S2059798319010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radka C.D., DeLucas L.J., Wilson L.S., Lawrenz M.B., Perry R.D., Aller S.G. Crystal structure of Yersinia pestis virulence factor YfeA reveals two polyspecific metal-binding sites. Acta Crystallogr. D Struct. Biol. 2017;73(Pt 7):557–572. doi: 10.1107/S2059798317006349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry R.D., Bobrov A.G., Fetherston J.D. The role of transition metal transporters for iron, zinc, manganese, and copper in the pathogenesis of Yersinia pestis. Metallomics. 2015;7(6):965–978. doi: 10.1039/C4MT00332B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neupane D.P., Avalos D., Fullam S., Roychowdhury H., Yukl E.T. Mechanisms of zinc binding to the solute-binding protein AztC and transfer from the metallochaperone AztD. J. Biol. Chem. 2017;292(42):17496–17505. doi: 10.1074/jbc.M117.804799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loisel E., Jacquamet L., Serre L., Bauvois C., Ferrer J.L., Vernet T., Di Guilmi A.M., Durmort C. AdcAII, a new pneumococcal Zn-binding protein homologous with ABC transporters: Biochemical and structural analysis. J. Mol. Biol. 2008;381(3):594–606. doi: 10.1016/j.jmb.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 42.Falconi M., Oteri F., Di Palma F., Pandey S., Battistoni A., Desideri A. Structural-dynamical investigation of the ZnuA histidine-rich loop: involvement in zinc management and transport. J. Comput. Aided Mol. Des. 2011;25(2):181–194. doi: 10.1007/s10822-010-9409-6. [DOI] [PubMed] [Google Scholar]
- 43.Wei B., Randich A.M., Bhattacharyya-Pakrasi M., Pakrasi H.B., Smith T.J. Possible regulatory role for the histidine-rich loop in the zinc transport protein, ZnuA. Biochemistry. 2007;46(30):8734–8743. doi: 10.1021/bi700763w. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee S., Wei B., Bhattacharyya-Pakrasi M., Pakrasi H.B., Smith T.J. Structural determinants of metal specificity in the zinc transport protein ZnuA from synechocystis 6803. J. Mol. Biol. 2003;333(5):1061–1069. doi: 10.1016/j.jmb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Desrosiers D.C., Sun Y.C., Zaidi A.A., Eggers C.H., Cox D.L., Radolf J.D. The general transition metal (Tro) and Zn2+ (Znu) transporters in Treponema pallidum: analysis of metal specificities and expression profiles. Mol. Microbiol. 2007;65(1):137–152. doi: 10.1111/j.1365-2958.2007.05771.x. [DOI] [PubMed] [Google Scholar]
- 46.Yatsunyk L.A., Easton J.A., Kim L.R., Sugarbaker S.A., Bennett B., Breece R.M., Vorontsov I.I., Tierney D.L., Crowder M.W., Rosenzweig A.C. Structure and metal binding properties of ZnuA, a periplasmic zinc transporter from Escherichia coli. J. Biol. Inorg. Chem. 2008;13(2):271–288. doi: 10.1007/s00775-007-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ilari A., Alaleona F., Tria G., Petrarca P., Battistoni A., Zamparelli C., Verzili D., Falconi M., Chiancone E. The Salmonella enterica ZinT structure, zinc affinity and interaction with the high-affinity uptake protein ZnuA provide insight into the management of periplasmic zinc. Biochim. Biophys. Acta. 2014;1840(1):535–544. doi: 10.1016/j.bbagen.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Petrarca P., Ammendola S., Pasquali P., Battistoni A. The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage. J. Bacteriol. 2010;192(6):1553–1564. doi: 10.1128/JB.01310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moulin P., Patron K., Cano C., Zorgani M.A., Camiade E., Borezée-Durant E., Rosenau A., Mereghetti L., Hiron A. The Adc/Lmb system mediates zinc acquisition in Streptococcus agalactiae and contributes to bacterial growth and survival. J. Bacteriol. 2016;198(24):3265–3277. doi: 10.1128/JB.00614-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rioux S., Neyt C., Di Paolo E., Turpin L., Charland N., Labbé S., Mortier M-C., Mitchell T.J., Feron C., Martin D., Poolman J.T. Transcriptional regulation, occurrence and putative role of the Pht family of Streptococcus pneumoniae. Microbiology (Reading) 2011;157(Pt 2):336–348. doi: 10.1099/mic.0.042184-0. [DOI] [PubMed] [Google Scholar]
- 51.Linke C., Caradoc-Davies T.T., Young P.G., Proft T., Baker E.N. The laminin-binding protein Lbp from Streptococcus pyogenes is a zinc receptor. J. Bacteriol. 2009;191(18):5814–5823. doi: 10.1128/JB.00485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ragunathan P., Sridaran D., Weigel A., Shabayek S., Spellerberg B., Ponnuraj K. Metal binding is critical for the folding and function of laminin binding protein, Lmb of Streptococcus agalactiae. PLoS One. 2013;8(6):e67517. doi: 10.1371/journal.pone.0067517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao K., Li N., Wang H., Cao X., He J., Zhang B., He Q-Y., Zhang G., Sun X. Two zinc-binding domains in the transporter AdcA fromStreptococcus pyogenes facilitate high-affinity binding and fast transport of zinc. J. Biol. Chem. 2018;293(16):6075–6089. doi: 10.1074/jbc.M117.818997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ilari A., Alaleona F., Petrarca P., Battistoni A., Chiancone E. The X-ray structure of the zinc transporter ZnuA from Salmonella enterica discloses a unique triad of zinc-coordinating histidines. J. Mol. Biol. 2011;409(4):630–641. doi: 10.1016/j.jmb.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 55.Pederick V.G., Eijkelkamp B.A., Begg S.L., Ween M.P., McAllister L.J., Paton J.C., McDevitt C.A. ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Sci. Rep. 2015;5(1):13139. doi: 10.1038/srep13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y-H., Deka R.K., Norgard M.V., Radolf J.D., Hasemann C.A. Treponema pallidum TroA is a periplasmic zinc-binding protein with a helical backbone. Nat. Struct. Biol. 1999;6(7):628–633. doi: 10.1038/10677. [DOI] [PubMed] [Google Scholar]
- 57.Zheng B., Zhang Q., Gao J., Han H., Li M., Zhang J., Qi J., Yan J., Gao G.F. Insight into the interaction of metal ions with TroA from Streptococcus suis. PLoS One. 2011;6(5):e19510. doi: 10.1371/journal.pone.0019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plumptre C.D., Eijkelkamp B.A., Morey J.R., Behr F., Couñago R.M., Ogunniyi A.D., Kobe B., O’Mara M.L., Paton J.C., McDevitt C.A. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol. Microbiol. 2014;91(4):834–851. doi: 10.1111/mmi.12504. [DOI] [PubMed] [Google Scholar]
- 59.Bersch B., Bougault C., Roux L., Favier A., Vernet T., Durmort C. New insights into histidine triad proteins: solution structure of a Streptococcus pneumoniae PhtD domain and zinc transfer to AdcAII. PLoS One. 2013;8(11):e81168–e81168. doi: 10.1371/journal.pone.0081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Battistoni A., Ammendola S., Chiancone E., Ilari A. A novel antimicrobial approach based on the inhibition of zinc uptake in Salmonella enterica. Future Med. Chem. 2017;9(9):899–910. doi: 10.4155/fmc-2017-0042. [DOI] [PubMed] [Google Scholar]
- 61.Hecel A., Kola A., Valensin D., Kozlowski H., Rowinska-Zyrek M. Metal complexes of two specific regions of znua, a periplasmic zinc(ii) transporter from Escherichia coli. Inorg. Chem. 2020;59(3):1947–1958. doi: 10.1021/acs.inorgchem.9b03298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puškarova A., Ferianc P., Kormanec J., Homerova D., Farewell A., Nyström T. Regulation of yoda encoding a novel cadmium-induced protein in Escherichia coli. Microbiology (Reading) 2002;148(Pt 12):3801–3811. doi: 10.1099/00221287-148-12-3801. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y., Wang Y., Zhang H., Sun W., Li Z., Zhang F., Zhang H., Chen F., Zhang H., An J., He C. Antimicrobial mechanism of strictinin isomers extracted from the root of Rosa roxburghii Tratt (Ci Li Gen). J. Ethnopharmacol. 2020;250:112498. doi: 10.1016/j.jep.2019.112498. [DOI] [PubMed] [Google Scholar]
- 64.Chen J., Wang L., Shang F., Dong Y., Ha N-C., Nam K.H., Quan C., Xu Y. Crystal structure of E. coli ZinT with one zinc-binding mode and complexed with citrate. Biochem. Biophys. Res. Commun. 2018;500(2):139–144. doi: 10.1016/j.bbrc.2018.03.192. [DOI] [PubMed] [Google Scholar]
- 65.Colaço H.G., Santo P.E., Matias P.M., Bandeiras T.M., Vicente J.B. Roles of Escherichia coli ZinT in cobalt, mercury and cadmium resistance and structural insights into the metal binding mechanism. Metallomics. 2016;8(3):327–336. doi: 10.1039/C5MT00291E. [DOI] [PubMed] [Google Scholar]
- 66.Bellotti D., Rowińska-Żyrek M., Remelli M. Novel insights into the metal binding ability of ZinT periplasmic protein from Escherichia coli and Salmonella enterica. Dalton Trans. 2020;49(27):9393–9403. doi: 10.1039/D0DT01626H. [DOI] [PubMed] [Google Scholar]
- 67.Plumptre C.D., Hughes C.E., Harvey R.M., Eijkelkamp B.A., McDevitt C.A., Paton J.C. Overlapping functionality of the Pht proteins in zinc homeostasis of Streptococcus pneumoniae. Infect. Immun. 2014;82(10):4315–4324. doi: 10.1128/IAI.02155-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bayle L., Chimalapati S., Schoehn G., Brown J., Vernet T., Durmort C. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol. Microbiol. 2011;82(4):904–916. doi: 10.1111/j.1365-2958.2011.07862.x. [DOI] [PubMed] [Google Scholar]
- 69.Blindauer C.A. Advances in the molecular understanding of biological zinc transport. Chem. Commun. (Camb.) 2015;51(22):4544–4563. doi: 10.1039/C4CC10174J. [DOI] [PubMed] [Google Scholar]
- 70.Eijkelkamp B.A., Pederick V.G., Plumptre C.D., Harvey R.M., Hughes C.E., Paton J.C., McDevitt C.A. The first histidine triad motif of PhtD is critical for zinc homeostasis in Streptococcus pneumoniae. Infect. Immun. 2015;84(2):407–415. doi: 10.1128/IAI.01082-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riboldi-Tunnicliffe A., Isaacs N.W., Mitchell T.J. 1.2 Angstroms crystal structure of the S. pneumoniae PhtA histidine triad domain a novel zinc binding fold. FEBS Lett. 2005;579(24):5353–5360. doi: 10.1016/j.febslet.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 72.Durmort C., Ercoli G., Ramos-Sevillano E., Chimalapati S., Haigh R.D., De Ste Croix M., Gould K., Hinds J., Guerardel Y., Vernet T., Oggioni M., Brown J.S. Deletion of the zinc transporter lipoprotein AdcAII causes hyperencapsulation of Streptococcus pneumoniae associated with distinct alleles of the type I restriction-modification system. MBio. 2020;11(2):e00445–e00420. doi: 10.1128/mBio.00445-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weston B.F., Brenot A., Caparon M.G. The metal homeostasis protein, Lsp, of Streptococcus pyogenes is necessary for acquisition of zinc and virulence. Infect. Immun. 2009;77(7):2840–2848. doi: 10.1128/IAI.01299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moulin P., Rong V., Ribeiro E Silva A., Pederick V.G., Camiade E., Mereghetti L., McDevitt C.A., Hiron A. Defining the role of the Streptococcus agalactiae Sht-family proteins in zinc acquisition and complement evasion. J. Bacteriol. 2019;201(8):e00757–e00718. doi: 10.1128/JB.00757-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ragunathan P., Spellerberg B., Ponnuraj K. Structure of laminin-binding adhesin (Lmb) from Streptococcus agalactiae. Acta Crystallogr. D Biol. Crystallogr. 2009;65(12):1262–1269. doi: 10.1107/S0907444909038359. [DOI] [PubMed] [Google Scholar]
- 76.Sridharan U., Ragunathan P., Spellerberg B., Ponnuraj K. Molecular dynamics simulation of metal free structure of Lmb, a laminin-binding adhesin of Streptococcus agalactiae: metal removal and its structural implications. J. Biomol. Struct. Dyn. 2019;37(3):714–725. doi: 10.1080/07391102.2018.1438923. [DOI] [PubMed] [Google Scholar]
- 77.Brown L.R., Gunnell S.M., Cassella A.N., Keller L.E., Scherkenbach L.A., Mann B., Brown M.W., Hill R., Fitzkee N.C., Rosch J.W., Tuomanen E.I., Thornton J.A. AdcAII of Streptococcus pneumoniae affects Pneumococcal invasiveness. PLoS One. 2016;11(1):e0146785. doi: 10.1371/journal.pone.0146785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McNicholas S., Potterton E., Wilson K.S., Noble M.E.M. Presenting your structures: The CCP4mg molecular-graphics software. Acta Crystallogr. D Biol. Crystallogr. 2011;67(4):386–394. doi: 10.1107/S0907444911007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wichgers Schreur P.J., Rebel J.M.J., Smits M.A., van Putten J.P.M., Smith H.E. TroA of Streptococcus suis is required for manganese acquisition and full virulence. J. Bacteriol. 2011;193(19):5073–5080. doi: 10.1128/JB.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saraithong P., Goetting-Minesky M.P., Durbin P.M., Olson S.W., Gherardini F.C., Fenno J.C. Roles of TroA and TroR in metalloregulated growth and gene expression in Treponema denticola. J. Bacteriol. 2020;202(7):e00770–e00719. doi: 10.1128/JB.00770-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Handali M., Neupane D.P., Roychowdhury H., Yukl E.T. Transcriptional regulation, metal binding properties and structure of pden1597, an unusual zinc transport protein from Paracoccus denitrificans. J. Biol. Chem. 2015;290(19):11878–11889. doi: 10.1074/jbc.M115.645853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meni A., Yukl E.T. Structural features mediating zinc binding and transfer in the aztabcd zinc transporter system. Biomolecules. 2020;10(8):1156. doi: 10.3390/biom10081156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim K.H.L., Jones C.E., vanden Hoven R.N., Edwards J.L., Falsetta M.L., Apicella M.A., Jennings M.P., McEwan A.G. Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect. Immun. 2008;76(8):3569–3576. doi: 10.1128/IAI.01725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka K.J., Song S., Mason K., Pinkett H.W. Selective substrate uptake: The role of ATP-binding cassette (ABC) importers in pathogenesis. Biochim. Biophys. Acta Biomembr. 2018;1860(4):868–877. doi: 10.1016/j.bbamem.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grim K.P., San Francisco B., Radin J.N., Brazel E.B., Kelliher J.L., Párraga Solórzano P.K., Kim P.C., McDevitt C.A., Kehl-Fie T.E. The metallophore staphylopine enables Staphylococcus aureus to compete with the host for zinc and overcome nutritional immunity. MBio. 2017;8(5):e01281–e01217. doi: 10.1128/mBio.01281-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Remy L., Carrière M., Derré-Bobillot A., Martini C., Sanguinetti M., Borezée-Durant E. The Staphylococcus aureus Opp1 ABC transporter imports nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol. Microbiol. 2013;87(4):730–743. doi: 10.1111/mmi.12126. [DOI] [PubMed] [Google Scholar]
- 88.Song L., Zhang Y., Chen W., Gu T., Zhang S.Y., Ji Q. Mechanistic insights into staphylopine-mediated metal acquisition. Proc. Natl. Acad. Sci. USA. 2018;115(15):3942–3947. doi: 10.1073/pnas.1718382115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang D., Fierke C.A. The BaeSR regulon is involved in defense against zinc toxicity in E. coli. Metallomics. 2013;5(4):372–383. doi: 10.1039/c3mt20217h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Appia-Ayme C., Hall A., Patrick E., Rajadurai S., Clarke T.A., Rowley G. ZraP is a periplasmic molecular chaperone and a repressor of the zinc-responsive two-component regulator ZraSR. Biochem. J. 2012;442(1):85–93. doi: 10.1042/BJ20111639. [DOI] [PubMed] [Google Scholar]
- 91.Noll M., Petrukhin K., Lutsenko S. Identification of a novel transcription regulator from Proteus mirabilis, PMTR, revealed a possible role of YJAI protein in balancing zinc in Escherichia coli. J. Biol. Chem. 1998;273(33):21393–21401. doi: 10.1074/jbc.273.33.21393. [DOI] [PubMed] [Google Scholar]
- 92.McFarlane J.S., Davis C.L., Lamb A.L. Staphylopine, pseudopaline, and yersinopine dehydrogenases: a structural and kinetic analysis of a new functional class of opine dehydrogenase. J. Biol. Chem. 2018;293(21):8009–8019. doi: 10.1074/jbc.RA118.002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laffont C., Brutesco C., Hajjar C., Cullia G., Fanelli R., Ouerdane L., Cavelier F., Arnoux P. Simple rules govern the diversity of bacterial nicotianamine-like metallophores. Biochem. J. 2019;476(15):2221–2233. doi: 10.1042/BCJ20190384. [DOI] [PubMed] [Google Scholar]
- 94.Ghssein G., Brutesco C., Ouerdane L., Fojcik C., Izaute A., Wang S., Hajjar C., Lobinski R., Lemaire D., Richaud P., Voulhoux R., Espaillat A., Cava F., Pignol D., Borezée-Durant E., Arnoux P. Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science. 2016;352(6289):1105–1109. doi: 10.1126/science.aaf1018. [DOI] [PubMed] [Google Scholar]
- 95.Grim K.P., Radin J.N., Párraga Solórzano P.K., Morey J.R., Frye K.A., Ganio K., Neville S.L., McDevitt C.A., Kehl-Fie T.E. Intracellular accumulation of staphylopine can sensitize Staphylococcus aureus to host-imposed zinc starvation by chelation-independent toxicity. J. Bacteriol. 2020;202(9):e00014–20. doi: 10.1128/JB.00014-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fojcik C., Arnoux P., Ouerdane L., Aigle M., Alfonsi L., Borezée-Durant E. Independent and cooperative regulation of staphylopine biosynthesis and trafficking by Fur and Zur. Mol. Microbiol. 2018;108(2):159–177. doi: 10.1111/mmi.13927. [DOI] [PubMed] [Google Scholar]
- 97.Ding Y., Fu Y., Lee J.C., Hooper D.C. Staphylococcus aureus NorD, a putative efflux pump coregulated with the Opp1 oligopeptide permease, contributes selectively to fitness in vivo. J. Bacteriol. 2012;194(23):6586–6593. doi: 10.1128/JB.01414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen C., Hooper D.C. Intracellular accumulation of staphylopine impairs the fitness of Staphylococcus aureus cntE mutant. FEBS Lett. 2019;593(11):1213–1222. doi: 10.1002/1873-3468.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hajjar C., Fanelli R., Laffont C., Brutesco C., Cullia G., Tribout M., Nurizzo D., Borezée-Durant E., Voulhoux R., Pignol D., Lavergne J., Cavelier F., Arnoux P. Control by metals of Staphylopine dehydrogenase activity during metallophore biosynthesis. J. Am. Chem. Soc. 2019;141(13):5555–5562. doi: 10.1021/jacs.9b01676. [DOI] [PubMed] [Google Scholar]
- 100.D’Orazio M., Mastropasqua M.C., Cerasi M., Pacello F., Consalvo A., Chirullo B., Mortensen B., Skaar E.P., Ciavardelli D., Pasquali P., Battistoni A. The capability of Pseudomonas aeruginosa to recruit zinc under conditions of limited metal availability is affected by inactivation of the ZnuABC transporter. Metallomics. 2015;7(6):1023–1035. doi: 10.1039/C5MT00017C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mastropasqua M.C., D’Orazio M., Cerasi M., Pacello F., Gismondi A., Canini A., Canuti L., Consalvo A., Ciavardelli D., Chirullo B., Pasquali P., Battistoni A. Growth of Pseudomonas aeruginosa in zinc poor environments is promoted by a nicotianamine-related metallophore. Mol. Microbiol. 2017;106(4):543–561. doi: 10.1111/mmi.13834. [DOI] [PubMed] [Google Scholar]
- 102.Lhospice S., Gomez N.O., Ouerdane L., Brutesco C., Ghssein G., Hajjar C., Liratni A., Wang S., Richaud P., Bleves S., Ball G., Borezée-Durant E., Lobinski R., Pignol D., Arnoux P., Voulhoux R. Pseudomonas aeruginosa zinc uptake in chelating environment is primarily mediated by the metallophore pseudopaline. Sci. Rep. 2017;7(1):17132. doi: 10.1038/s41598-017-16765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gomez N.O., Tetard A., Ouerdane L., Laffont C., Brutesco C., Ball G., Lobinski R., Denis Y., Plésiat P., Llanes C., Arnoux P., Voulhoux R. Involvement of the Pseudomonas aeruginosa mexab-oprm efflux pump in the secretion of the metallophore pseudopaline. Mol. Microbiol. 2021;115(1):84–98. doi: 10.1111/mmi.14600. [DOI] [PubMed] [Google Scholar]
- 104.Laffont C., Arnoux P. The ancient roots of nicotianamine: Diversity, role, regulation and evolution of nicotianamine-like metallophores. Metallomics. 2020;12(10):1480–1493. doi: 10.1039/D0MT00150C. [DOI] [PubMed] [Google Scholar]
- 105.McFarlane J.S., Lamb A.L. Biosynthesis of an opine metallophore by Pseudomonas aeruginosa. Biochemistry. 2017;56(45):5967–5971. doi: 10.1021/acs.biochem.7b00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bobrov A.G., Kirillina O., Fetherston J.D., Miller M.C., Burlison J.A., Perry R.D. The Yersinia pestis siderophore, Yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice. Mol. Microbiol. 2014;93(4):759–775. doi: 10.1111/mmi.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bobrov A.G., Kirillina O., Fosso M.Y., Fetherston J.D., Miller M.C., VanCleave T.T., Burlison J.A., Arnold W.K., Lawrenz M.B., Garneau-Tsodikova S., Perry R.D. Zinc transporters YbtX and ZnuABC are required for the virulence of Yersinia pestis in bubonic and pneumonic plague in mice. Metallomics. 2017;9(6):757–772. doi: 10.1039/C7MT00126F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walencik P.K., Wątły J., Rowinska-Zyrek M. Fungal zinc homeostasis -a tug of war between the pathogen and host. Curr. Med. Chem. 2016;23(32):3717–3729. doi: 10.2174/0929867323666160817163834. [DOI] [PubMed] [Google Scholar]
- 109.Soares L.W., Bailão A.M., Soares C.M.A., Bailão M.G.S. Zinc at the host–fungus interface: How to uptake the metal? J. Fungi. 2020;6(4):305. doi: 10.3390/jof6040305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson D. An evolutionary perspective on zinc uptake by human fungal pathogens. Metallomics. 2015;7(6):979–985. doi: 10.1039/C4MT00331D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amich J., Leal F., Calera J.A. Repression of the acid ZrfA/ZrfB zinc-uptake system of Aspergillus fumigatus mediated by PacC under neutral, zinc-limiting conditions. Int. Microbiol. 2009;12(1):39–47. [PubMed] [Google Scholar]
- 112.Crawford A.C., Lehtovirta-Morley L.E., Alamir O., Niemiec M.J., Alawfi B., Alsarraf M., Skrahina V., Costa A.C.B.P., Anderson A., Yellagunda S., Ballou E.R., Hube B., Urban C.F., Wilson D. Biphasic zinc compartmentalisation in a human fungal pathogen. PLoS Pathog. 2018;14(5):e1007013. doi: 10.1371/journal.ppat.1007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Finkel J.S., Xu W., Huang D., Hill E.M., Desai J.V., Woolford C.A., Nett J.E., Taff H., Norice C.T., Andes D.R., Lanni F., Mitchell A.P. Portrait of Candida albicans adherence regulators. PLoS Pathog. 2012;8(2):e1002525. doi: 10.1371/journal.ppat.1002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bensen E.S., Martin S.J., Li M., Berman J., Davis D.A. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol. Microbiol. 2004;54(5):1335–1351. doi: 10.1111/j.1365-2958.2004.04350.x. [DOI] [PubMed] [Google Scholar]
- 115.Crawford A., Wilson D. Essential metals at the host-pathogen interface: Nutritional immunity and micronutrient assimilation by human fungal pathogens. FEMS Yeast Res. 2015;15(7):fov071. doi: 10.1093/femsyr/fov071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Citiulo F., Jacobsen I.D., Miramón P., Schild L., Brunke S., Zipfel P., Brock M., Hube B., Wilson D. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog. 2012;8(6):e1002777. doi: 10.1371/journal.ppat.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mayer F.L., Wilson D., Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4(2):119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kujoth G.C., Sullivan T.D., Merkhofer R., Lee T-J., Wang H., Brandhorst T., Wüthrich M., Klein B.S. CRISPR/Cas9-mediated gene disruption reveals the importance of zinc metabolism for fitness of the dimorphic fungal pathogen Blastomyces dermatitidis. MBio. 2018;9(2):e00412–e00418. doi: 10.1128/mBio.00412-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Łoboda D., Rowińska-Żyrek M. Candida albicans zincophore and zinc transporter interactions with Zn(ii) and Ni(ii). Dalton Trans. 2018;47(8):2646–2654. doi: 10.1039/C7DT04403H. [DOI] [PubMed] [Google Scholar]
- 120.Kumar R., Breindel C., Saraswat D., Cullen P.J., Edgerton M. Candida albicans Sap6 amyloid regions function in cellular aggregation and zinc binding, and contribute to zinc acquisition. Sci. Rep. 2017;7(1):2908. doi: 10.1038/s41598-017-03082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patzer S.I., Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 1998;28(6):1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 122.Lehtovirta-Morley L.E., Alsarraf M., Wilson D. Pan-domain analysis of zip zinc transporters. Int. J. Mol. Sci. 2017;18(12):2631. doi: 10.3390/ijms18122631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilson D., Deepe G.S., Jr The intersection of host and fungus through the zinc lens. Curr. Opin. Microbiol. 2019;52:35–40. doi: 10.1016/j.mib.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dasari P., Shopova I.A., Stroe M., Wartenberg D., Martin-Dahse H., Beyersdorf N., Hortschansky P., Dietrich S., Cseresnyés Z., Figge M.T., Westermann M., Skerka C., Brakhage A.A., Zipfel P.F. Aspf2 from Aspergillus fumigatus recruits human immune regulators for immune evasion and cell damage. Front. Immunol. 2018;9:1635. doi: 10.3389/fimmu.2018.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luo S., Blom A.M., Rupp S., Hipler U-C., Hube B., Skerka C., Zipfel P.F. The pH-regulated antigen 1 of Candida albicans binds the human complement inhibitor C4b-binding protein and mediates fungal complement evasion. J. Biol. Chem. 2011;286(10):8021–8029. doi: 10.1074/jbc.M110.130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Couñago R.M., McDevitt C.A., Ween M.P., Kobe B. Prokaryotic substrate-binding proteins as targets for antimicrobial therapies. Curr. Drug Targets. 2012;13(11):1400–1410. doi: 10.2174/138945012803530170. [DOI] [PubMed] [Google Scholar]
- 127.Ribeiro M., Simões M. Advances in the antimicrobial and therapeutic potential of siderophores. Environ. Chem. Lett. 2019;17(4):1485–1494. doi: 10.1007/s10311-019-00887-9. [DOI] [Google Scholar]
- 128.Mislin G.L.A., Schalk I.J. Siderophore-dependent iron uptake systems as gates for antibiotic Trojan horse strategies against Pseudomonas aeruginosa. Metallomics. 2014;6(3):408–420. doi: 10.1039/C3MT00359K. [DOI] [PubMed] [Google Scholar]
- 129.Petrik M., Zhai C., Haas H., Decristoforo C. Siderophores for molecular imaging applications. Clin. Transl. Imaging. 2017;5(1):15–27. doi: 10.1007/s40336-016-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Denning D.W., Bromley M.J. Infectious disease. How to bolster the antifungal pipeline? Science. 2015;347(6229):1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- 131.Pearson R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963;85(22):3533–3539. doi: 10.1021/ja00905a001. [DOI] [Google Scholar]
- 132.Tay W.M., Hanafy A.I., Angerhofer A., Ming L-J. A plausible role of salivary copper in antimicrobial activity of histatin-5--metal binding and oxidative activity of its copper complex. Bioorg. Med. Chem. Lett. 2009;19(23):6709–6712. doi: 10.1016/j.bmcl.2009.09.119. [DOI] [PubMed] [Google Scholar]
- 133.Bellotti D., Toniolo M., Dudek D., Mikołajczyk A., Guerrini R., Matera-Witkiewicz A., Remelli M., Rowińska-Żyrek M. Bioinorganic chemistry of calcitermin -the picklock of its antimicrobial activity. Dalton Trans. 2019;48(36):13740–13752. doi: 10.1039/C9DT02869B. [DOI] [PubMed] [Google Scholar]
- 134.Chen A.Y., Adamek R.N., Dick B.L., Credille C.V., Morrison C.N., Cohen S.M. Targeting metalloenzymes for therapeutic intervention. Chem. Rev. 2019;119(2):1323–1455. doi: 10.1021/acs.chemrev.8b00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ilari A., Pescatori L., Di Santo R., Battistoni A., Ammendola S., Falconi M., Berlutti F., Valenti P., Chiancone E. Salmonella enterica serovar Typhimurium growth is inhibited by the concomitant binding of Zn(II) and a pyrrolyl-hydroxamate to ZnuA, the soluble component of the ZnuABC transporter. Biochim. Biophys. Acta. 2016;1860(3):534–541. doi: 10.1016/j.bbagen.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 136.Garmory H.S., Titball R.W. ATP-binding cassette transporters are targets for the development of antibacterial vaccines and therapies. Infect. Immun. 2004;72(12):6757–6763. doi: 10.1128/IAI.72.12.6757-6763.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khan M.N., Pichichero M.E. Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine. 2012;30(18):2900–2907. doi: 10.1016/j.vaccine.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kunitomo E., Terao Y., Okamoto S., Rikimaru T., Hamada S., Kawabata S. Molecular and biological characterization of histidine triad protein in group A Streptococci. Microbes Infect. 2008;10(4):414–423. doi: 10.1016/j.micinf.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Hughes M.J.G., Wilson R., Moore J.C., Lane J.D., Dobson R.J., Muckett P., Younes Z., Pribul P., Topping A., Feldman R.G., Santangelo J.D. Novel protein vaccine candidates against Group B Streptococcal infection identified using alkaline phosphatase fusions. FEMS Microbiol. Lett. 2003;222(2):263–271. doi: 10.1016/S0378-1097(03)00310-0. [DOI] [PubMed] [Google Scholar]
- 140.Brookes R.H., Ming M., Williams K., Hopfer R., Gurunathan S., Gallichan S., Tang M., Ochs M.M. Passive protection of mice against Streptococcus pneumoniae challenge by naturally occurring and vaccine-induced human anti-PhtD antibodies. Hum. Vaccin. Immunother. 2015;11(7):1836–1839. doi: 10.1080/21645515.2015.1039210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hokynar K., Korhonen S., Norja P., Paavonen J., Puolakkainen M. Antibody toChlamydia trachomatis proteins, troa and htra, as a biomarker for chlamydia trachomatis infection. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36(1):49–56. doi: 10.1007/s10096-016-2769-7. [DOI] [PubMed] [Google Scholar]
- 142.González-Miro M., Rodríguez-Noda L., Fariñas-Medina M., García-Rivera D., Vérez-Bencomo V., Rehm B.H.A. Self-assembled particulate PsaA as vaccine against Streptococcus pneumoniae infection. Heliyon. 2017;3(4):e00291. doi: 10.1016/j.heliyon.2017.e00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Root-Bernstein R. Possible cross-reactivity between sars-cov-2 proteins, crm197 and proteins in pneumococcal vaccines may protect against symptomatic sars-cov-2 disease and death. Vaccines (Basel) 2020;8(4):559. doi: 10.3390/vaccines8040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mayor-Ibarguren A., Busca-Arenzana C., Robles-Marhuenda Á. A hypothesis for the possible role of zinc in the immunological pathways related to covid-19 infection. Front. Immunol. 2020;11:1736–1736. doi: 10.3389/fimmu.2020.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Doboszewska U., Wlaź P., Nowak G., Młyniec K. Targeting zinc metalloenzymes in coronavirus disease 2019. Br. J. Pharmacol. 2020;177(21):4887–4898. doi: 10.1111/bph.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J., Tsatsakis A., Tinkov A.A. Zinc and respiratory tract infections: perspectives for COVID-19 (Review). Int. J. Mol. Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pormohammad A., Monych N.K., Turner R.J. Zinc and SARS-CoV-2: A molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int. J. Mol. Med. 2021;47(1):326–334. doi: 10.3892/ijmm.2020.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reinhold D., Brocke S. The differential roles of zinc in immune responses and their potential implications in antiviral immunity against SARS-CoV-2. Clin. Nutr. 2021;40(2):652–652. doi: 10.1016/j.clnu.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.