Abstract
Ten strains of Geotrichum candidum were studied on a liquid cheese model medium for the production of sulfur compounds which contribute to the aroma of cheeses. The volatile components produced by each cultured strain were extracted by dynamic headspace extractions, separated and quantified by gas chromatography (GC), and identified by GC-mass spectrometry. It was shown that four strains of this microorganism produced significant quantities of S-methyl thioacetate, S-methyl thiopropionate, S-methyl thiobutanoate, S-methyl thioisobutanoate, S-methyl thioisovalerate, and S-methyl thiohexanoate. This is the first example of the production of these compounds by a fungus. In addition, dimethyldisulfide, dimethyltrisulfide, dimethylsulfide, and methanethiol, which are more commonly associated with the development of cheese flavor in bacterial cultures, were also produced by G. candidum in various yields, depending on the strain selected. The potential application of these strains in cultured microbial associations to produce modified cheeses with more desirable organoleptic properties is discussed.
Sulfur compounds are present in many foods (38) and beverages (9, 33) and are found commonly in a range of dairy products, including yogurt (21, 39) and ripened cheeses (8, 17). In the case of cheese, it has been shown that the sulfur flavors are comprised of a structurally diverse class of molecules which provide a whole range of characteristic aromatic notes (e.g., “cheesy” and “garlic”) in a particular cheese, as is evident from the analysis of cheddar (32, 46), Limburger (40), Camembert (13, 26), blue (16), and other mold-ripened varieties (12, 36). Additionally, the sensory properties of these sulfur compounds are pronounced at very low concentrations due to their low odor thresholds (7, 25).
The origin of many sulfur flavors in cheese is associated with the production of methanethiol (MTL) by bacterial cultures which are used in the preparation of cheese (20, 23, 30, 43). Numerous bacteria, such as lactobacilli, lactococci (11), and Brevibacterium linens in particular (6, 10, 11, 15), produce useful quantities of this compound. The direct metabolic pathway responsible for the generation of MTL involves the bacterial degradation of l-methionine, and significant effort has been made to isolate and characterize l-methionine-γ-demethiolase, which is the intracellular enzyme responsible for this bioconversion (6, 10, 15, 24). Other bacterial enzymes involved in l-methionine metabolism include cystathionine-γ-lyase and cystathionine-β-lyase, which are also implicated in the production of MTL, but their role in the development of cheese flavor remains tentative at present (11). Further bacterial metabolism of MTL leads to the generation of a range of sulfur compounds which contribute significantly to the aroma of cheese, including dimethyldisulfide (DMDS) (26, 36), dimethyltrisulfide (DMTS) (14), and some thioesters, such as S-methyl thioacetate (MTA) and S-methyl thiobutyrate (MTB) (4, 7, 27, 28, 46).
In association with bacteria, other microorganisms are commonly present in cheese-ripening cultures used in the dairy industry (19), including Geotrichum candidum, which develops very early during the ripening process (2, 18, 31). Although there was no direct evidence for the generation of MTL by this microorganism, a strain was identified recently which can produce DMDS (22). Based on the assumption that DMDS was likely to be formed from MTL by G. candidum, the aim of this investigation was to demonstrate that strains of this microorganism could indeed generate this thiol and, if this was the case, to assess the ability of the microorganism to produce other useful sulfur flavors from this precursor.
MATERIALS AND METHODS
Materials.
Dimethylsulfide (DMS), DMDS, and MTB were purchased from Sigma-Aldrich (St. Quentin Fallavier, France). MTA was obtained from Lancaster (Bischeim, France), and DMTS was obtained from Acros Organics (Noisy-le-Grand, France).
Growth and storage of G. candidum.
Ten strains of G. candidum, G1 to G10 (SKW Biosystems, la Ferté-sous-Jouarre, France), were cultured at 23°C in the absence of light on a liquid medium (potato dextrose; Difco, Detroit, Mich.) with shaking at 200 rpm for 30 h. Skim milk (25 ml; Difco) containing glycerol (5% [vol/vol]) was added to 100 ml of each culture for cryoprotection, and the samples were then transferred to 10-ml tubes and stored at −80°C. Prior to use of the samples as inocula, the concentration of the cell suspensions was determined by plate counting (chloramphenicol glucose agar; Biokar Diagnostics, Beauvais, France) with serial dilution (10−3 to 10−5).
Cell culturing on a model liquid cheese medium.
Sterile culture medium was prepared with a salted lactic curd (Coulomiers type; SOREDAB, Le Boissière Ecole, France) which was ground in a blender (Waring Blender, Prolabo, France) with sterile Milli-Q water (300:200 [wt/wt]) for 2 min at maximum speed. The pH was adjusted to 4.86 ± 0.05 with sterile 2 N NaOH, and the medium was pasteurized in a water bath at 60°C for 90 min (Grant Instruments, Cambridge, United Kingdom). This moderate heat treatment was chosen to eliminate lactic flora present in the curd without the generation of compounds which could impart flavor. All manipulations were conducted under laminar flow with sterile glassware.
For each strain tested, a thawed concentrated suspension of cells (composed of spores and mycelia) was used to innoculate pasteurized lactic acid curd (500 ml). The volume of innoculum used varied according to the strain of G. candidum employed and was adjusted to provide a final cell concentration of 104 CFU/ml in the cheese-based growth medium. Each strain of G. candidum (G1 to -10) was cultivated in triplicate in the absence of light at 12°C for 21 days. After this incubation period, the strains of G. candidum were visible on the surface of the cheese-based medium as homogeneous flora with a cell density between 2.3 × 107 and 6.6 × 107 CFU/ml. The purity of each culture was confirmed by testing with respect to (i) total flora (plate count agar; Biokar Diagnostics), (ii) yeast and mold (chloramphenicol glucose agar; Biokar Diagnostics), and (iii) bacteria (brain heart infusion; Biokar Diagnostics). Only those cultures which were shown by plate testing to be free from microbial contamination were used for the assessment of sulfur flavor production by the individual Geotrichum strains. All samples prepared in these experiments were then frozen and stored in hermetically sealed glass flasks at −80°C. Prior to dynamic headspace analysis, each culture medium flask was thawed at 4°C and sterile Milli-Q water (2:1 [wt/wt]) was added. The mixture was homogenized over two 30-s intervals at 13,500 rpm (Ultra-Turax T25; Janke and Kundel, Strangen, Germany), and a 5-ml sample volume was transferred to an analytical glass tube for immediate analysis. Each triplicate sample of the strain cultures (G1 to G10) was analyzed by three independent measurements.
Dynamic headspace extraction and gas chromatography (GC)-mass spectrometry analysis of the cultures.
The volatile compounds in each culture sample were extracted with a dynamic headspace analyzer (purge and trap concentrator; model 7695A; Hewlett-Packard, Avondale, Pa.). Each sample tube was connected to the apparatus and heated at 60°C for 10 min and then purged with high-purity helium at a flow rate of 30 ml/min for 15 min. The volatiles were extracted by adsorption to a porous-polymer-adsorbent Tenax trap column (60/80 mesh; 0.25 g; 30 by 0.32 cm; Teckmar Inc., Cincinnati, Ohio) at room temperature. This column was heated at 225°C for 2 min to desorb the volatiles, which were directly transferred at 150°C to the head of a capillary column with cryofocusing at −150°C. Water was removed by condensation with an in-line moisture control system.
The condensed volatile compounds were analyzed by GC (model 6890; Hewlett-Packard) by heating the interface to 180°C for 1 min and automatic injection (splitless) onto a nonpolar capillary column (HP-5MS; 30.0 m by 0.25 mm; 0.25-μm film thickness) at a helium flow rate of 1.6 ml/min. The oven temperature was held at 5°C for 8 min and then programmed from 5 to 20°C at 3°C/min followed by a gradient of 10°C/min to a final temperature of 150°C. The GC column was also connected directly to a mass-sensitive detector (model 6890A quadrupole mass spectrometer; Hewlett-Packard). The electron impact energy was set at 70 eV, and data were collected in the range of 29 to 300 atomic mass units at a scan rate of 1.68 scans/s. Using these experimental procedures, the minimum detection limit for each sulfur compound investigated was 1 ppb.
General synthesis of S-methyl thioesters.
The respective acid chloride (75 mmol) was dissolved in dry dichloromethane (200 ml) at 0°C, and sodium thiomethoxide (1.5 molar equivalents) was added in small portions (approximately 0.5 g) with vigorous stirring over 30 min under dry nitrogen. Stirring was continued for 3 h at 0°C followed by 16 h at room temperature. The reaction mixture was filtered, and the organic phase was washed with ice-cold 5% aqueous citric acid (100 ml) and 5% aqueous NaHCO3 (100 ml). The organic layer was then dried over anhydrous Na2SO4 and filtered, and the solvent was removed in vacuo (10 mm Hg) at 0°C to obtain a colorless oil, which was purified further by three successive distillations under vacuum. S-Methyl thiopropionate (MTP): bp, 18 to 20°C (10 mm Hg); GC retention time, 2.42 min; 1H nuclear magnetic resonance (NMR) (270.1 MHz, CDCl3) δ (ppm) 1.13 (3H, t, CH3), 2.25 (3H, s, SCH3), 2.55 (q, 2H, CH2); 13C NMR (67.9 MHz, CDCl3) δ (ppm) 9.7 (CH3), 11.4 (SCH3), 37.2 (CH2), 200.7 (C⩵O). S-Methyl thioisobutanoate (MTIB): bp, 17 to 19°C (10 mm Hg); GC retention time, 2.19 min; 1H NMR (270.1 MHz, CDCl3) δ (ppm) 1.19 (6H, d, 2xCH3), 2.27 (3H, s, SCH3), 2.75 (m, 1H, CH). S-Methyl thioisovalerate (MTIV): bp, 24 to 26°C (10 mm Hg); GC retention time, 3.22 min; 1H NMR (270.1 MHz, CDCl3) δ (ppm) 0.94 (6H, d, 2xCH3), 2.16 (1H, m, CH), 2.28 (3H, s, SCH3), 2.42 (2H, d, CH2); 13C NMR (67.9 MHz, CDCl3) δ (ppm) 11.5 (SCH3), 22.3 (CH3), 26.4 (CH), 52.7 (CH2), 199.4 (C⩵O). S-Methyl thiohexanoate (MTH): bp, 46 to 48°C (10 mm Hg); GC retention time, 5.42 min; 1H NMR (270.1 MHz, CDCl3) δ (ppm) 0.95 (3H, t, CH3), 1.38 (4H, m, 2xCH2), 1.73 (2H, m, CH2), 2.26 (3H, s, SCH3), 2.52 (2H, t, CH2); 13C NMR (67.9 MHz, CDCl3) δ (ppm) 11.4 (CH3), 13.7 (SCH3), 22.2 (CH2), 25.2 (CH2), 31.0 (CH2), 43.6 (CH2), 199.9 (C⩵O).
Structural analysis and purity of S-methyl thioesters.
1H and 13C NMR spectroscopic analysis was performed on a Jeol EX270A multinuclear Fourier transform spectrometer. Samples were prepared in deuteriated chloroform, and the residual solvent peak was used as an internal reference. The purity of the distilled thioesters (>99.5%) was assessed by GC with a Hewlett-Packard 5890 GC (split) system with a BPX-5 capillary column (12 m by 0.3 mm; film thickness, 0.33 μm) connected to a flame ionization detector with helium as a carrier gas at a flow rate of 0.7 ml/min. Samples were injected with a split-splitless injector (split ratio, 1/100). For analysis, the injection temperature was set to 40°C and the detector was at 370°C. The oven temperature was programmed from 40 to 145°C at 10°C/min.
Identification and quantification of sulfur compounds produced by the cultures.
MTP, MTIB, MTIV, and MTH were synthesized and characterized as described above, and all other compounds investigated were purchased from commercial sources. The sulfur compounds produced in the cultures were identified by GC comparison with these pure references (retention time and mass spectra), and the concentration of products formed was determined with calibrated standards. Known amounts of reference compounds were added to a plain lactic curd at various final concentrations (0 [plain curd], 0.05, 0.10, 0.25, and 0.50 ppm), and for each of the five concentrations, triplicate curd samples were prepared. Each of these was submitted to headspace analysis three times. A linear regression between compound areas (obtained by integration of the mass-sensitive detector signal) and their known concentrations (in parts per million) was performed to accurately determine the concentration of sulfur compounds in each culture. Controls were also prepared containing no inocula and cultivated under exactly the same conditions as the inoculated media. In these samples, and in fresh noncultured media, DMDS and DMTS were present at very low concentrations (40 and 10 ppb, respectively), and these values were taken into account during the construction of calibration curves. Due to the high volatility of MTL, the concentration of this compound was extrapolated by using a calibration equation based on DMDS.
Data analysis.
The data set consisted of 10 variables (sulfur compounds) and 30 samples (10 strains of G. candidum cultured in triplicate), which were submitted to one-way analysis of variance by the general linear model procedure as implemented in the SAS software (42). With the exception of MTL, which has a low boiling point and leads to a relatively high coefficient of variation (40%), for each sulfur compound produced by a single strain of G. candidum, low coefficients of variation (15%) were obtained from analysis among the three replicate cultures. After the strains were cultured over a 3-week period, variations in cell density among the strains were less than 1 log unit, and these coefficients of variation (<30%) were taken into consideration during the statistical analysis. An assessment of significant differences in the production of individual sulfur compounds by each strain of G. candidum was performed according to the Student-Newman-Keuls multiple-comparison test (P ≤ 0.005). The mean concentrations of the individual sulfur compounds produced by each culture of G. candidum were categorized into four statistically significant groups, a, b, c, and d (where an intermediate grouping such as ab is not statistically significantly different from a or b).
RESULTS
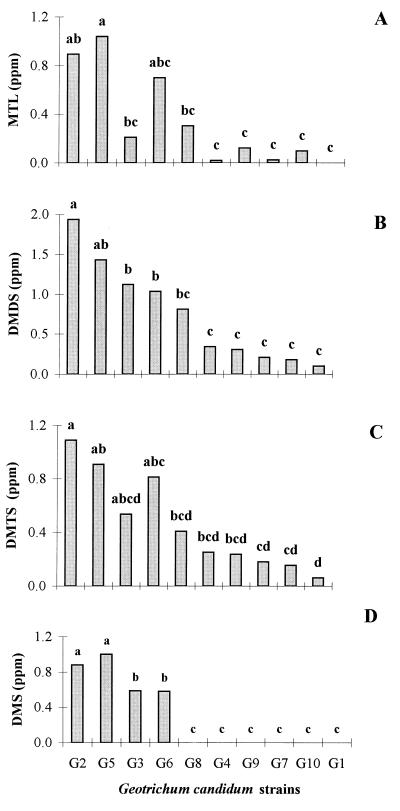
On the basis of their general utility in industrial cheese making, 10 strains of G. candidum (G1 to -10) were investigated for the production of MTL, sulfides, and thioesters. As determined by analysis of variance, significant differences were observed between these 10 strains (P ≤ 0.005) and the 10 sulfur compounds investigated (see Materials and Methods). Dynamic headspace analysis confirmed that DMDS was the major sulfur component present in all strain cultures tested (G1 to -10), at concentrations ranging from a minimum of 0.10 ppm (G1) to a maximum of 1.93 ppm (G2) as shown in Fig. 1B. With one exception (G1), it was shown that MTL was also generated by all cultures of G. candidum (Fig. 1A), suggesting that it was indeed the likely precursor of DMDS. The observation that strain G1, which did not generate significant levels of MTL, also produced the smallest quantities of DMDS provided further evidence to support this view. It was also clear that G. candidum produced DMTS (Fig. 1C), and as expected, the highest yields were obtained with those cultures (G2, G5, G3, and G6) which produced the greatest amounts of MTL and DMDS (Fig. 1A and B). As shown in Fig. 1D, data analysis with a typical Student-Newman-Keuls multiple-comparison test showed that four strains of Geotrichum could be categorized into groups a and b due to the statistically different mean concentrations of DMS generated. From the analysis of a correlation matrix based on the concentration of sulfur compounds present in the cultures (Table 1), there was a clear relationship between the concentration of MTL present and those of DMDS (r = 0.89) and DMTS (r = 0.95). Surprisingly, DMS was also detected in high concentrations (0.6 to 1.0 ppm) with cultures G2, G3, G5, and G6, as shown in Fig. 1D.
FIG. 1.
Production of MTL (A), DMDS (B), DMTS (C), and DMS (D) by 10 cultured strains of G. candidum (G1 to G10).
TABLE 1.
Correlation coefficient matrix among sulfur compound quantities produced by the 10 strains of G. candidum
| Compound | Correlation coefficienta
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MTL | DMS | MTA | DMDS | MTP | MTIB | MTB | MTIV | DMTS | MTH | |
| MTL | 1.00 | |||||||||
| DMS | 0.91 | 1.00 | ||||||||
| MTA | 0.75 | 0.69 | 1.00 | |||||||
| DMDS | 0.89 | 0.92 | 0.69 | 1.00 | ||||||
| MTP | 0.63 | 0.54 | 0.98 | 0.55 | 1.00 | |||||
| MTIB | 0.82 | 0.86 | 0.90 | 0.89 | 0.81 | 1.00 | ||||
| MTB | 0.50 | 0.41 | 0.93 | 0.41 | 0.98 | 0.70 | 1.00 | |||
| MTIV | 0.90 | 0.90 | 0.89 | 0.90 | 0.78 | 0.98 | 0.66 | 1.00 | ||
| DMTS | 0.95 | 0.93 | 0.81 | 0.97 | 0.69 | 0.92 | 0.56 | 0.95 | 1.00 | |
| MTH | 0.63 | 0.56 | 0.91 | 0.67 | 0.90 | 0.90 | 0.82 | 0.86 | 0.74 | 1.00 |
Boldface indicates the higher correlation coefficients (>0.90).
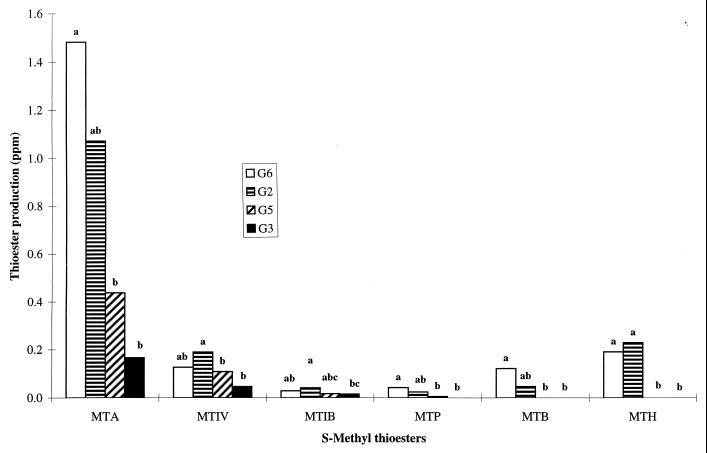
The four strains of G. candidum which proved to be most useful for the production of sulfides (G2, G3, G5, and G6) were also capable of generating six S-methyl thioesters, i.e., MTA, MTP, MTB, MTIB, MTIV, and MTH, as shown in Fig. 2. G2 and G6 in particular produced these compounds in relatively large quantities, but, with the notable exception of MTA (0.2 to 1.4 ppm), the thioesters were produced at 10- to 40-fold-lower concentrations than the sulfides (Fig. 1B, C, and D). It was also clear that the most suitable strains for the generation of thioesters (Fig. 2) were those which produced the highest concentrations of DMS (Fig. 1D), DMDS (Fig. 1B), and DMTS (Fig. 1C), and this dependence was presumably related to the requirement for MTL. Significantly, the type of thioester produced was specific to the strain of G. candidum used, with G2 and G6 generating a mixture of all six thioesters, including MTP, MTB, and MTH, whereas G3 and G5 produced only MTA, MTIB, and MTIV.
FIG. 2.
Production of MTA, MTP, MTB, MTIB, MTIV, and MTH by strains G6 (□), G2 (▤), G5 ( ), and G3 (■).
), and G3 (■).
DISCUSSION
Although G. candidum is known to produce esters (29), this is the first report of the generation of a whole range of specific sulfur compounds by this microorganism, including S-methyl thioesters (MTA, MTP, MTB, MTIB, MTIV, and MTH) and sulfides (DMS and DMTS). Prior to this investigation, it was believed that the generation of these flavor components (e.g., MTA and MTB) during cheese ripening was due solely to bacteria, despite the presence of yeasts or molds in the flora. Moreover, although sulfur compounds which result in cheesy notes have been identified by numerous groups (1, 5, 7, 8, 30, 36), there is limited information available on microorganisms responsible for the generation of these flavors and the cell metabolic pathways involved. It can be concluded from this study that G. candidum may well play an important role in the development of these cheese flavors. In the absence of any other contaminating microorganism, MTL was produced by nearly all strains tested, and it is reasonable to assume that the sulfur compounds identified were generated from this precursor. Although the involvement of other enzymes, such as cystathionine β- and γ-lyases (1, 5), or methylation of hydrogen sulfide (47) cannot be ruled out at present, it is most likely that MTL is produced by l-methionine-γ-demethiolase in this microorganism. It is also evident that the amount of MTL produced is dependent on the strain of G. candidum, and this may be due to a number of contributing factors, including the level of l-methionine-γ-demethiolase activity (45), the in vivo l-methionine available (44), and the degradation of l-methionine by aminotransferases (48) and/or amino acid oxidases (41, 44). Nevertheless, it is clear that even without a detailed knowledge of the intracellular metabolism of sulfur compounds in a given strain, a simple screen is sufficient to identify those cultures which (i) produce the highest levels of MTL (i.e., G2, G3, G5, G6, and G8 [Fig. 1A]); (ii) generate other specific classes of sulfur flavors, including sulfides (Fig. 1B, C, and D) and S-methyl thioesters (Fig. 2); and (iii) show specific variations in their propensities to produce sulfides (e.g., DMDS and DMTS are produced by all 10 strains whereas DMS is present only in cultures of G2, G3, G5, and G6) and thioesters (e.g., G2 and G6 produce all six thioesters, whereas G3 and G5 generate only MTA, MTIB, and MTIV).
The production of the various classes of sulfur compounds by these strains inevitably involves a number of independent pathways which all rely on the availability of MTL. It is clear that DMDS and DMTS are formed in those cultures which produce high concentrations of this thiol, and based on the high correlation coefficient between the concentrations of MTL-DMDS (r = 0.89) and MTL-DMTS (r = 0.95) (Table 1), the oxidation of MTL to these higher polysulfides by G. candidum is likely to occur by a mechanism similar to that previously described in bacterial cultures (40). Moreover, the high correlation between the thioesters (r = 0.98 for MTP-MTB, MTA-MTP, and MTIB-MTIV [Table 1]) suggests that a common enzymatic system in G. candidum is responsible for the generation of these compounds, as in the case of bacteria (28). DMS has also been identified as a sulfur flavor in cheeses (26, 36), although there is only a limited understanding of the mechanism responsible for the production of this sulfide by microorganisms (18, 28). Based on the observation that cultures of G3 and G8 are able to produce DMDS and DMTS in similar concentrations and that DMS is produced by G3 but not by G8 (Fig. 1D), this compound may well be generated via a mechanism different from that of the polysulfides in this particular strain. In general, it is clear that sulfur metabolism in G. candidum involves significant intraspecies variation, and this may have important technological implications.
At present, the use of microbial associations, and their subsequent effects on the sensory properties of cheese, is still poorly understood (34, 35, 37). Previously, we showed that mixed cultures of Penicillium camemberti and specific strains of G. candidum produced Camemberts much more typical than those obtained with pure cultures of P. camemberti alone, due to the development of specific flavor notes (e.g., “cabbage” and “cowshed”) identified in the cheese by sensory analysis (35). Based on the results of this study of the generation of various sulfur compounds, it is possible that the presence of G. candidum in association with other microorganisms is responsible for the variations in flavor notes previously observed; e.g., S-methyl thioesters have recently been shown to produce cheesy notes at low odor thresholds (3). Therefore, the judicious use of suitable associations of various microorganisms and G. candidum in starter cultures could facilitate the modification of both the type and quantity of sulfur flavors generated and result in cheeses with more desirable organoleptic properties.
ACKNOWLEDGMENT
We are grateful to the EC Commission for the financial support of this work (grant PL 96 1196).
REFERENCES
- 1.Alting A C, Engels W J, Schalkwijk S, Exterkate F A. Purification and characterization of cystathionine β-lyase from Lactococcus lactis subsp. cremoris B78 and its possible role in flavor development in cheese. Appl Environ Microbiol. 1995;61:4037–4042. doi: 10.1128/aem.61.11.4037-4042.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartschi C, Berthier J, Valla G. Inventaire et évolution des flores fongiques de surface du reblochon de Savoie. Lait. 1994;74:105–114. [Google Scholar]
- 3.Berger C, Martin N, Collin S, Gijs L, Khan J A, Piraprez G, Spinnler H E, Vulfson E N. Combinatorial approach to flavour analysis. II. Olfactory investigation of a library of S-methyl thioesters and sensory evaluation of selected components. J Agric Food Chem. 1999;47:3274–3279. doi: 10.1021/jf990205v. [DOI] [PubMed] [Google Scholar]
- 4.Bloes-Breton S, Bergère J L. Production de composés soufrés volatils par des Micrococcaceae et des bactéries coryneformes d’origine fromagère. Lait. 1997;77:543–559. [Google Scholar]
- 5.Bruinenberg P G, deRoo G, Limsowtin G K. Purification and characterization of cystathionine γ-lyase from Lactococcus lactis subsp. cremoris SK11: possible role in flavor compound formation during cheese maturation. Appl Environ Microbiol. 1997;63:561–566. doi: 10.1128/aem.63.2.561-566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin J C, Law B A. Isolation and characterization of the L-methionine-γ-demethiolase from Brevibacterium linens NCDO 739. Sci Aliment. 1989;9:805–812. [Google Scholar]
- 7.Cuer A, Dauphin G, Kergomard A, Dumont J P, Adda J. Production of S-methylthioacetate by Brevibacterium linens. Appl Environ Microbiol. 1979;38:332–334. doi: 10.1128/aem.38.2.332-334.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuer A, Dauphin G, Kergomard A, Roger S, Dumont J P, Adda J. Flavour properties of some sulfur compounds isolated from cheeses. Lebensm Wiss Technol. 1979;12:258–261. [Google Scholar]
- 9.Dercksen A W, Meijering I, Axcell B. Rapid quantification of flavor-active sulfur compounds in beer. J Am Soc Brew Chem. 1992;50:93–101. [Google Scholar]
- 10.Dias B, Weimer B. Purification and characterization of l-methionine γ-lyase from Brevibacterium linens BL2. Appl Environ Microbiol. 1998;64:3327–3331. doi: 10.1128/aem.64.9.3327-3331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias B, Weimer B. Conversion of methionine to thiols by lactococci, lactobacilli, and brevibacteria. Appl Environ Microbiol. 1998;64:3320–3326. doi: 10.1128/aem.64.9.3320-3326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumont J P, Degas C, Adda J. L’arôme du Pont l’Evêque. Mise en évidence de constituants volatils quantitativement mineurs. Lait. 1976;553-554:177–180. [Google Scholar]
- 13.Dumont J P, Roger S, Adda J. L’arôme du Camembert: autres composés mineurs mis en évidence. Lait. 1976;559-560:595–599. [Google Scholar]
- 14.Engels W J M, Visser S. Isolation and comparative characterization of components that contribute to the flavour of different types of cheese. Neth Milk Dairy J. 1994;48:127–140. [Google Scholar]
- 15.Ferchichi M, Hemme D, Nardi M, Pamboukdjian N. Production of methanethiol from methionine by Brevibacterium linens CNRZ 918. J Gen Microbiol. 1985;131:715–723. doi: 10.1099/00221287-131-4-715. [DOI] [PubMed] [Google Scholar]
- 16.Gallois A, Langlois D. New results in the volatile odorous compounds of French cheese. Lait. 1990;70:89–105. [Google Scholar]
- 17.Grill H, Patton S, Cone J F. Aroma significance of sulfur compounds in surface-ripened cheese. J Dairy Sci. 1966;49:409–412. [Google Scholar]
- 18.Gueguen M, Delespaul G, Lenoir J. La flore fongique superficielle des fromages de St-Nectaire et de tome de Savoie. Rev Laitière Fr. 1974;325:1–11. [Google Scholar]
- 19.Gueguen M. Ph.D. thesis. Contribution à la connaissance de Geotrichum candidum et notamment de sa variabilité. Conséquences pour l’industrie fromagère. Caen, France: University of Caen; 1984. [Google Scholar]
- 20.Hemme D, Richard J. Utilization of L-methionine and production of methanethiol by bacteria isolated from raw milk Camembert cheese. Lait. 1986;66:135–142. [Google Scholar]
- 21.Imhof R, Bosset J O. Quantitative GC-MS analysis of volatile flavour compounds in pasteurized milk and fermented milk products applying a standard addition method. Lebensm Wiss Technol. 1994;27:265–269. [Google Scholar]
- 22.Jollivet N, Chataud J, Vayssier Y, Bensoussan M, Belin J M. Production of volatile compounds in model milk and cheese media by eight strains of Geotrichum candidum Link. J Dairy Res. 1994;61:241–248. [Google Scholar]
- 23.Kim S C, Olson N F. Production of methanethiol in milk fat-coated microcapsules containing Brevibacterium linens and methionine. J Dairy Res. 1989;56:799–811. [Google Scholar]
- 24.Kreis W, Hession C. Isolation and purification of L-methionine-α-deamino-γ-mercaptomethane-lyase (L-methioninase) from Clostridium sporogenes. Cancer Res. 1973;33:1862–1865. [PubMed] [Google Scholar]
- 25.Kubickova J, Grosch W. Quantification of potent odorants in Camembert cheese and calculation of their odour activity values. Int Dairy J. 1998;8:11–16. [Google Scholar]
- 26.Kubickova J, Grosch W. Evaluation of flavour compounds of Camembert cheese. Int Dairy J. 1998;8:17–23. [Google Scholar]
- 27.Lamberet G, Auberger B, Bergère J L. Aptitude of cheese bacteria for volatile S-methyl thioester synthesis. I. Effect of substrate and pH on their formation by Brevibacterium linens GC171. Appl Microbiol Biotechnol. 1997;47:279–283. [Google Scholar]
- 28.Lamberet G, Auberger B, Bergere J L. Aptitude of cheese bacteria for volatile S-methyl thioester synthesis. II. Comparison of coryneform bacteria, Micrococcaceae and some lactic acid bacteria starters. Appl Microbiol Biotechnol. 1997;48:393–397. [Google Scholar]
- 29.Latrasse A, Dameron P, Hassani M, Staron T. Production d’un arôme fruité par Geotrichum candidum (Staron) Sci Aliment. 1987;7:637–645. [Google Scholar]
- 30.Law B A, Sharpe M E. Formation of methanethiol by bacteria isolated from raw milk and cheddar cheese. J Dairy Res. 1978;45:267–275. [Google Scholar]
- 31.Lecocq J, Gueguen M, Coiffier O. Importance de l’association Geotrichum candidum-Brevibacterium linens pour l’affinage de fromages à croûte lavée. Sci Aliment. 1996;16:317–327. [Google Scholar]
- 32.Manning D J. Sulfur compounds in relation to cheddar cheese flavour. J Dairy Res. 1974;41:81–87. [Google Scholar]
- 33.Mestres M, Busto O, Guasch J. Headspace-solid-phase microextraction analysis of volatile sulphides and disulphides in wine aroma. J Chromatogr. 1998;808:211–218. doi: 10.1016/s0021-9673(98)00100-9. [DOI] [PubMed] [Google Scholar]
- 34.Molimard P, Bouvier I, Issanchou S, Lesschaeve I, Vassal L, Spinnler H E. Cooperation between Penicillium camemberti and Geotrichum candidum: effect on taste and flavour qualities of Camembert type cheese. In: Etiévant P, Schreier P, editors. Bioflavour ’95. Paris, France: INRA; 1995. pp. 173–175. [Google Scholar]
- 35.Molimard P, Lesschaeve I, Issanchou S, Brousse M, Spinnler H E. Effect of the association of surface flora on the sensory properties of mould-ripened cheese. Lait. 1997;77:181–187. [Google Scholar]
- 36.Molimard P, Spinnler H E. Compounds involved in the flavor of surface mold-ripened cheeses: origins and properties. J Dairy Sci. 1996;79:169–184. [Google Scholar]
- 37.Mourgues R, Bergere J L, Vassal L. Possibilités d’améliorer les qualités organoleptiques des fromages de camembert grâce à l’utilisation de Geotrichum candidum. Technique Laitiére. 1983;978:11–15. [Google Scholar]
- 38.Mussinan C J, Keelan M E. Sulfur compounds in food: an overview. In: Mussinan C J, Keelan M E, editors. Sulfur compounds in foods. Washington, D.C.: American Chemical Society; 1994. pp. 1–6. [Google Scholar]
- 39.Ott A, Fay L B, Chaintreau A. Determination and origin of the aroma impact compounds of yogurt flavor. J Agric Food Chem. 1997;45:850–858. [Google Scholar]
- 40.Parliment T H, Kolor M G, Rizzo D J. Volatile components of Limburger cheese. J Agric Food Chem. 1982;30:1006–1008. [Google Scholar]
- 41.Reuiz-Herrera J, Starkey R L. Dissimilation of methionine by Achromobacter starkeyi. J Bacteriol. 1970;104:1286–1293. doi: 10.1128/jb.104.3.1286-1293.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.SAS Institute. SAS user’s guide. Statistics, version 6. Cary, N.C: SAS Institute, Inc.; 1990. [Google Scholar]
- 43.Sharpe M E, Law B A, Phillips B A, Pitcher D G. Methanethiol production by coryneform bacteria: strains from dairy and human skin sources and Brevibacterium linens. J Gen Microbiol. 1977;101:345–349. doi: 10.1099/00221287-101-2-345. [DOI] [PubMed] [Google Scholar]
- 44.Soda K. Microbial sulphur amino acids: an overview. Methods Enzymol. 1987;143:453–459. doi: 10.1016/0076-6879(87)43080-2. [DOI] [PubMed] [Google Scholar]
- 45.Soda K, Tanaka H, Esaki N. Multifunctional biocatalysis: methionine γ-lyase. Trends Biochem Sci. 1997;8:214–217. [Google Scholar]
- 46.Urbach G. Relations between cheese flavour and chemical composition. Int Dairy J. 1993;3:389–422. [Google Scholar]
- 47.Walker M D, Simpson W J. Production of volatile sulphur compounds by ale and lager brewing strains of Saccharomyces cerevisiae. Lett Appl Microbiol. 1993;16:40–43. [Google Scholar]
- 48.Yvon M, Thirouin S, Ruein L, Fromentier D, Gripon J C. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl Environ Microbiol. 1997;63:415–419. doi: 10.1128/aem.63.2.414-419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]