Abstract
We describe the case of a young woman with a dual-chamber implantable cardioverter-defibrillator for long-QT syndrome who was referred to our emergency department (Cardiovascular Research Centre of Aalst, Belgium) because of an “arrhythmic storm” caused by atrial lead fracture. This case highlights the importance of the correct choice of both the device type and the pacing modality. (Level of Difficulty: Intermediate.)
Key Words: cardiac pacemaker, genetic disorders, ventricular tachycardia
Abbreviations and Acronyms: AAI, atrial-pacing atrial-sensing inhibited-response; DDD, dual-pacing dual-sensing dual-response; EGM, electrogram; ICD, implantable cardioverter-defibrillator; LQTS, long QT syndrome; VP, ventricular paced (beat); VVI, ventricular-pacing ventricular-sensing inhibited-response
Central Illustration
Introduction
Implantable cardioverter-defibrillators (ICDs) are indicated for the prevention of life-threatening arrhythmias, both in primary and secondary prevention, as well as in ischemic and nonischemic conditions.1,2 Although infections are still the most frequent and dreaded complications of cardiac implantable electronic devices, lead failure is an increasingly frequent condition that results from aging of the device recipients, with consequent prolongation of “lead life.”3,4 Because inserting dual-chamber devices theoretically doubles the possibility of both infective and mechanical problems and has only a relative benefit in improved arrhythmia recognition, the use of a single-chamber system may be preferred if atrial sensing or pacing is not needed.5 Also worth mentioning is the importance of optimal programmed stimulation in each patient, to achieve a specific goal (physiological activation vs fully paced rhythm).6
Learning Objectives
-
•
To make a differential diagnosis on the basis of endocavitary signals.
-
•
To choose the best device and pacing modality in patients with cardiomyopathy.
-
•
To think about uncommon causes of ventricular arrhythmias.
Here we describe a rare case of a young woman with a dual-chamber ICD for long-QT syndrome who was referred to our emergency department (Cardiovascular Research Centre of Aalst, Belgium) for an “arrhythmic storm” caused by atrial lead malfunction.
History of Presentation
A 41-year-old woman was referred to our institution’s emergency department after receiving numerous defibrillator shocks.
Past Medical History
A dual-chamber ICD was implanted in the past for secondary prevention after aborted sudden death in the context of long QT syndrome (LQTS), genetically confirmed by identification of a potassium voltage-gated channel subfamily Q member 1 (KCNQ1) mutation. In the following years, she experienced several ventricular arrhythmia episodes that were successfully treated by the ICD. Moreover, several reprogramming sessions were performed to adapt the pacing configuration to her specific characteristics, most notably chronotropic incompetence. In particular, the dual-pacing dual-sensing dual-response (DDD) stimulation with an atrial-pacing atrial-sensing inhibited-response (AAI)-to-ventricular-pacing ventricular-sensing inhibited-response (VVI) algorithm (AAI with VVI pacing backup) with a lower rate of 50 min-1 configuration was programmed to allow for physiological cardiac activation. As a consequence of episodes of vertigo and bradycardia for which vagal hypertonia was suspected, the lower rate was set to 65 min-1, and the AAI-to-VVI algorithm was switched off.
Differential Diagnosis
In a patient with confirmed LQTS and previous ventricular arrhythmias, the most probable diagnosis was appropriate shocks on ventricular arrhythmias.
Investigations
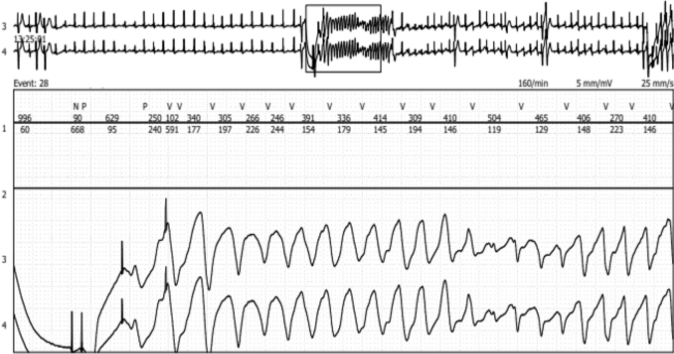
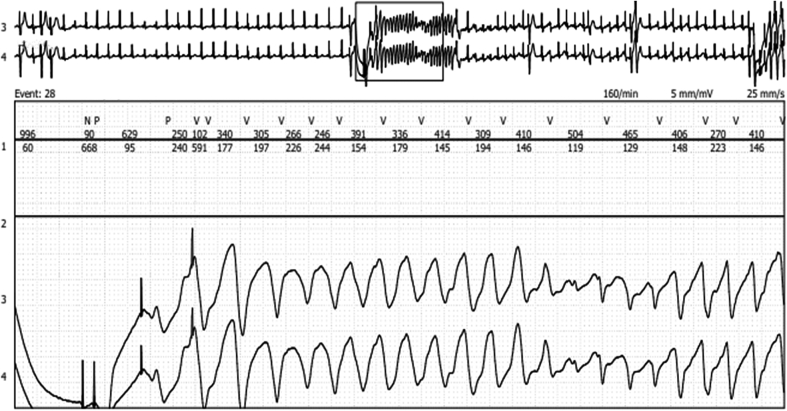
A quick ICD interrogation confirmed the presence of multiple ventricular arrhythmic episodes during the last 2 days, all successfully treated by the ICD. For management of the ongoing arrhythmic storm, the patient was admitted to the critical care unit for monitoring. During her subsequent hospitalization, the patient experienced numerous consecutive ventricular episodes, always correctly detected and appropriately treated by the ICD. Cardiac telemetry showed that unexpected ventricular paced (VP) beats during sinus rhythm preceded the ventricular arrhythmias (Figure 1).
Figure 1.
Telemetry Trace
Induction of ventricular tachycardia from 2 ventricular paced beats.
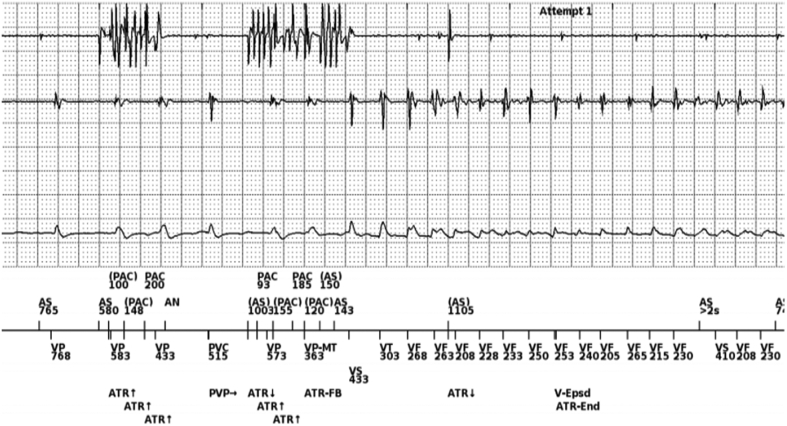
The ICD was ulteriorly interrogated in the electrophysiology unit. Surprisingly, it was observed that ventricular arrhythmias were always anticipated by episodes of noise on electrograms (EGMs) recorded from the atrial lead, probably resulting from atrial lead fracture. Episodes of noises were tracked and followed by double VP beats with delays of 363 milliseconds (165 beats/min) representing the maximal tracking rate. This sequence of “short-long-short intervals” induced ventricular arrhythmia (Figure 2). Device interrogation showed normal parameters of the right ventricular coil lead (sensing, 9.8 mV; pace impedance, 300 Ω; threshold, 1.6 V at 0.4 milliseconds; shock impedance, 62 Ω). Regarding the atrial lead, sensing of the A-wave and pacing threshold were normal (4 mV and 0.9 V at 0.4 milliseconds, respectively); pacing impedance, however, displayed an abrupt increase (>3,000 Ω). Fluoroscopy investigation ruled out macrofractures of any lead.
Figure 2.
Endocavitary Signals and Shock Electrogram at the Onset of the Ventricular Arrhythmia
On the atrial channel (first channel), it is possible to recognize 2 short episodes of noise misinterpreted as premature atrial contractions (PACs) because of undersensing (note also undersensing of the atrial signal between the 2 episodes of noise). On the ventricular channel (second channel), during the second episode of atrial noise, there are 2 ventricular paced (VP) beats with a delay of 363 milliseconds (VP-VP-MT) that represents the maximal ventricular paced tracking rate. This sequence of “short-long-short intervals” induced ventricular arrhythmia. AN = atrial noise; AS = atrial sense; ATR = atrial tachycardia response; FB = fallback; MT = maximum tracking rate; PVP = post ventricular atrial refractory period after premature ventricular complex; V-Epsd = ventricular episode; VF = ventricular Fibrillation; VT = ventricular Tachycardia.
Management
The therapeutic decision was made not to perform lead extraction because of the risk of dislodgment or lesion of the right ventricular coil lead and the perceived futility of the atrial pacing, thus postponing this intervention until generator replacement is needed. Rather, the ICD was reprogrammed to the VVI pacing modality during hospitalization. The patient was discharged the next day.
Discussion
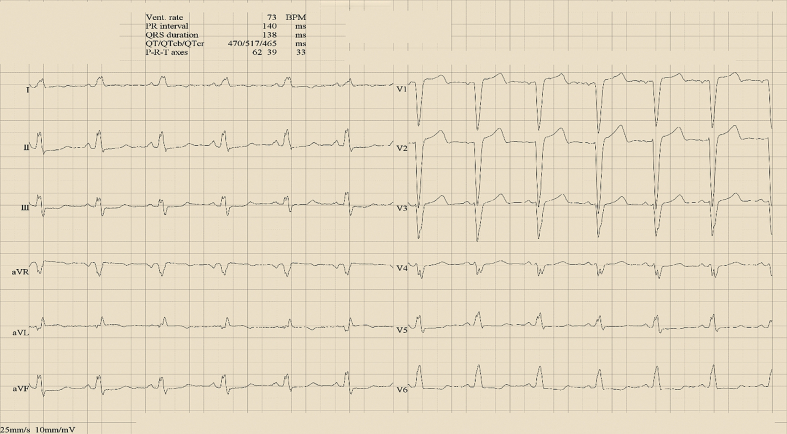
This case describes a very rare condition in which fracture of the atrial lead induced ventricular arrhythmias, that were—fortunately—always correctly treated by an ICD. The addition of an atrial lead in dual-chamber ICDs could theoretically reduce inappropriate shocks by improved detection capabilities, thereby allowing better identification of supraventricular arrhythmias. However, this potential benefit has not been assessed in randomized clinical trials. Moreover, a meta-analysis showed that the rates of inappropriate ICD shocks were not different between single- and dual-chamber devices.5 In addition, cardiac pacing was historically recommended as a potentially useful preventive strategy in LQTS because it was thought to prevent “pause-dependent” ventricular tachycardia induction.7 Although this is no longer a recommendation in contemporary guidelines, dual-chamber pacing is still commonly observed in patients with previously implanted ICDs. In terms of complication risk, the placement of an atrial lead requires a longer procedural time and is associated with higher rates of peri-implant complications and generator replacements.8 In the case detailed here, several factors created the perfect substrate for the development of ventricular arrhythmias. The sequence started with fracture of the atrial lead, followed by undersensing of the “extra waves” on the atrial EGM without switching from the DDD to the VVI configuration. The latter condition is caused by both undersensing and the shorter length of noise episodes. In addition, the programmed maximum tracking rate at 165 beats/min led to a coupled interval of 363 milliseconds. Finally, an arrhythmic substrate was present with a particularly long QTc interval of 517 milliseconds (Figure 3) that made the tissue especially susceptible to the induction of arrhythmias.
Figure 3.
Electrocardiogram Intervals
Note the prolonged QTc interval of 517 milliseconds during spontaneous sinus rhythm.
Follow-Up
During subsequent follow-up, also with home monitoring, the patient did not experience any problems, and no device malfunction was observed.
Conclusions
This case report highlights the importance of a correct choice of both the type of device and the pacing modality. In retrospect, in this case, from the beginning a single-chamber device should have been selected. In addition, different types of pacing modalities (ie, AAI, DDI, VVI) or different algorithms could have avoided such a complication. Finally, in patients with LQTS with historical insertion of a bradyarrhythmia-only dual-chamber pacing system, a risk exists for induction of potentially lethal ventricular arrhythmias after atrial lead failure.
Funding Support and Author Disclosures
Dr Fabbricatore has received support from a research grant provided by the Cardiopath PhD program at the University of Naples Federico II. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Theuns D.A.M.J., Smith T., Hunink M.G.M., et al. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or non-ischaemic heart disease: a systematic review and meta-analysis. Europace. 2010;12:1564–1570. doi: 10.1093/europace/euq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brignole M., Auricchio A., Baron-Esquivias G., et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2013;34:2281–2329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 3.Bongiorni M.G., Kennergren C., Butter C., et al. The European Lead Extraction ConTRolled (ELECTRa) study: a European Heart Rhythm Association (EHRA) registry of transvenous lead extraction outcomes. Eur Heart J. 2017;38:2995–3005. doi: 10.1093/eurheartj/ehx080. [DOI] [PubMed] [Google Scholar]
- 4.Diemberger I., Mazzotti A., Biffi M., et al. From lead management to implanted patient management: systematic review and meta-analysis of the last 15 years of experience in lead extraction. Expert Rev Med Devices. 2013;10:551–573. doi: 10.1586/17434440.2013.811837. [DOI] [PubMed] [Google Scholar]
- 5.Theuns D.A.M.J., Rivero-Ayerza M., Boersma E., Jordaens L. Prevention of inappropriate therapy in implantable defibrillators: a meta-analysis of clinical trials comparing single-chamber and dual-chamber arrhythmia discrimination algorithms. Int J Cardiol. 2008;125:352–357. doi: 10.1016/j.ijcard.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 6.Wilkoff B.L., Fauchier L., Stiles M.K., et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2016;13:e50–e86. doi: 10.1016/j.hrthm.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Viskin S. Cardiac pacing in the long QT syndrome: review of available data and practical recommendations. J Cardiovasc Electrophysiol. 2000;11(5):593–600. doi: 10.1111/j.1540-8167.2000.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 8.Defaye P., Boveda S., Klug D., et al. Dual- vs. single-chamber defibrillators for primary prevention of sudden cardiac death: long-term follow-up of the Défibrillateur Automatique Implantable-Prévention Primaire registry. Europace. 2017;19:1478–1484. doi: 10.1093/europace/euw230. [DOI] [PubMed] [Google Scholar]