Abstract
The cDNA that encodes an isoform of laccase from Trametes versicolor (LCCI), as well as a truncated version (LCCIa), was subcloned and expressed by using the yeast Pichia pastoris as the heterologous host. The amino acid sequence of LCCIa is identical to that of LCCI except that the final 11 amino acids at the C terminus of LCCI are replaced with a single cysteine residue. This modification was introduced for the purpose of improving the kinetics of electron transfer between an electrode and the copper-containing active site of laccase. The two laccases (LCCI and LCCIa) are compared in terms of their relative activity with two substrates that have different redox potentials. Results from electrochemical studies on solutions containing LCCI and LCCIa indicate that the redox potential of the active site of LCCIa is shifted to more negative values (411 mV versus normal hydrogen electrode voltage) than that found in other fungal laccases. In addition, replacing the 11 codons at the C terminus of the laccase gene with a single cysteine codon (i.e., LCCI→LCCIa) influences the rate of heterogeneous electron transfer between an electrode and the copper-containing active site (khet for LCCIa = 1.3 × 10−4 cm s−1). These results demonstrate for the first time that the rate of electron transfer between an oxidoreductase and an electrode can be enhanced by changes to the primary structure of a protein via site-directed mutagenesis.
The active site of an oxidoreductase seldom is found on the surface of the protein. Consequently, the electrochemistry of an oxidoreductase typically is plagued by poor kinetics of heterogeneous electron transfer. To address this problem, we used site-directed mutagenesis to alter the structure of an oxidoreductase with the aim of improving the kinetics of heterogeneous electron transfer between the active site of the modified protein and an electrode. The motivation for this work stems from our interest in using oxidoreductases as catalysts in biofuel cells (37, 38). Unlike conventional fuel cells, which use precious metals as catalysts (7), biofuel cells use enzymatic catalysts, either as they occur in microorganisms or as isolated proteins (39). The limited power output of biofuel cells thus far reported derives, in part, from the lack of biocatalysts that exhibit fast kinetics of heterogeneous electron transfer.
The problem of poor kinetics of heterogeneous electron transfer typically has been circumvented with either surface promoters or redox mediators, which facilitate the transfer of reducing equivalents between the active site of an oxidoreductase and an electrode surface (1, 11, 15, 16, 20, 44, 53). The use of surface promoters is problematic, however, because the ability of a surface promoter to facilitate heterogeneous electron transfer is not based on any criteria and therefore must be determined empirically. Mediated electron transfer also is problematic because it depends on criteria that limit the number of useful mediators from which to choose. These criteria include the following: (i) the mediator functions as a substrate of the oxidoreductase; (ii) both oxidized and reduced forms of the mediator are stable chemically and do not inhibit the biocatalytic reaction; and (iii) the mediator exhibits reversible electrochemical behavior. As a result, not all oxidoreductases are amenable to mediated electron transfer.
The difficulties raised by surface promoters and redox mediators could be avoided with oxidoreductases engineered specifically for heterogeneous electron transfer (i.e., engineered for an electrode substrate). Accordingly, we have initiated a program of research to examine site-directed modifications of an oxidoreductase in the context of electron transfer at a heterogeneous interface. Laccase was chosen as the protein with which to begin our studies for several reasons. First, laccase (polyphenol-oxidase [EC 1.10.3.2]) is a multicopper oxidase that couples the one-electron oxidation of four substrate molecules to the four-electron reduction of dioxygen to water (2, 27, 45, 49). Thus, laccase is a promising candidate for the biocatalytic reduction of dioxygen to water in electrochemical applications such as biofuel cells and biosensors (37–39). Second, several genes that encode different isoforms of laccase have been isolated and sequenced (8, 23–25, 31, 32, 35, 36, 50–52). The availability of these genes provides us with the means, via site-directed mutagenesis, to change systematically the structure of laccase. Third, the crystal structures of laccase (although an isoform of laccase different from that described in this work) and ascorbate oxidase (a similarly structured copper oxidase) are known (17, 30). These crystal structures function as topographical guides in selecting targets on the primary sequence of laccase for modification. Fourth, the active site of laccase has been characterized spectroscopically, which provides a spectroscopic basis for comparing modified laccases with their corresponding wild-type proteins (2–4, 41).
In this paper, we describe the subcloning and production of laccase (LCCI) and its truncated version (LCCIa) in the heterologous host Pichia pastoris. Other isoforms of laccase have been produced by using a heterologous host (e.g., Lcc1 from Trametes villosa in Aspergillus oryzae and Lcc1 from Trametes versicolor in P. pastoris) (24, 52). Prior to this report, however, a heterologous host has not been used to produce LCCI (or LCCIa). P. pastoris was selected as the heterologous host because this organism is known to secrete foreign protein in the presence of low levels of native proteins, most importantly, proteinases. The two laccases (LCCI and LCCIa) are compared in terms of their relative activity with two substrates that have different reduction potentials. In addition, we include results from electrochemical studies on solutions containing LCCI and LCCIa. Results from these studies indicate that the deletion at the C terminus of laccase (i.e., LCCI→LCCIa) influences both the reduction potential of laccase and the rate of heterogeneous electron transfer between an electrode and the copper-containing active site. These results demonstrate for the first time that the rate of electron transfer between an oxidoreductase and an electrode surface can be enhanced through changes to the primary structure of the protein via site-directed mutagenesis.
MATERIALS AND METHODS
Materials.
Commercial buffers and growth media of at least reagent grade were prepared in accordance with the manual supplied by Invitrogen. The substrates used with laccase, 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS) and N,N-diethyl-1,4-phenylenediamine sulfate (DEPDA), were purchased from Aldrich and used as received. Spectroscopic assays for enzyme activity and measurements of protein concentration were conducted with a Cary-17 UV-VIS spectrophotometer upgraded by On-Line Instrument Systems. Genes for laccase are indicated in lowercase, italic type (i.e., lccI). It is important to note that “1” and “I” are not equivalent labels and, therefore, lcc1 and lccI are different genes. The corresponding proteins are indicated in uppercase type (i.e., LCC1 from lcc1 and LCCI from lccI).
Vectors for subcloning and strains for expression.
The cDNA that codes for LCCI from T. versicolor 52J was obtained from Edgar Ong as a clone of lccI in the pBK-CMV vector (pBK117) (36). Expression vectors pPIC3.5K and pPIC9K and the strains of P. pastoris used for the heterologous production of laccase were purchased from Invitrogen. Three strains of P. pastoris were used: KM71 (arg4 his4 aox1::ARG4), GS115 (his4), and SMD1168 (his4 pep4). Plasmids and vectors were amplified by using a laboratory stock of Escherichia coli DH5α electrocompetent cells.
Construction of vectors for expression of lccI and lccIa by P. pastoris.
To insert the cDNA of laccase into pPIC3.5K, lccI was amplified in the presence of an upstream primer (5′-GTACGAATTCACCATGGGTCTGCAGCGA-3′) and a downstream primer (5′-TCGACCTAGGTCACTGGTTAGCCTCGCT-3′) that contain restriction sites for EcoRI and AvrII, respectively. A different downstream primer (5′-TATAATCCTAGGTCAGCAGCACAGGTCCGACCA-3′) was used with lccIa. The upstream primer contains a Kozak consensus sequence (underlined) to ensure proper initiation of translation when cloned. Similarly, the upstream primer (5′-GTACGAATTCATGGGTCTGCAGCGATTC-3′) and downstream primer (5′-TCGACCTAGGTCACTGGTTAGCCTCGCT-3′) were used to insert lccI into pPIC9K. It is important to note that the upstream primer used to insert lccI into pPIC9K contains only a restriction site for EcoRI since the Kozak consensus sequence is present in the α-secretion signal. lccIa was not inserted into pPIC9K.
Amplification of lccI and lccIa by using Pwo DNA polymerase was accomplished with the following protocol: (i) 94°C for 2 min (once) and 94°C for 15 s, (ii) 50°C for 30 s, (iii) 72°C for 2 min (10 cycles) and 94°C for 15 s, (iv) 50°C for 30 s, (v) 72°C for 3 min (20 cycles) and 72°C for 7 min (once). The products of PCR were purified from an 0.8% agarose gel and hydrolyzed with EcoRI and AvrII. Ligation of gene and vector was achieved by mixing purified PCR products with pPIC3.5K or pPIC9K vectors previously digested with EcoRI and AvrII (New England Biolabs) in a molar ratio of 1:3. Ligase (1.0 μl of T4 DNA ligase from Boehringer Mannheim) and 2 μl of 10× ligation buffer (supplied with enzyme, consisting of 660 mM Tris-HCl [pH 7.5], 50 mM MgCl2, 10 mM dithioerythritol, and 10 mM ATP) were added to the ligation mixture. The volume was adjusted to 20 μl with sterilized Milli-Q water and left overnight at 16°C. The ligation mixture was dialyzed subsequently against water for at least 1 h prior to introduction into electrocompetent cells of E. coli.
Transformation of P. pastoris and production of laccase.
Expression vectors pZH98 (pPIC3.5K plus lccI), pVS98 (pPIC9K plus lccI), and pNP2 (pPIC3.5K plus lccIa) were digested with SacI prior to introduction into electrocompetent cells of P. pastoris. Electrocompetent cells of P. pastoris were grown and transformed with pZH98, pVS98, or pNP2 as described in the manual supplied by Invitrogen (22). Subsequent to transformation, cells of P. pastoris were grown on minimal dextrose and regeneration dextrose buffered plates in the absence of histidine. After 5 days, transformants were transferred to yeast extract-peptone-dextrose (YPD) plates containing Geneticin (antibiotic G418) at concentrations between 0.25 and 4.0 mg ml−1. Transformants were found to survive G418 concentrations of ≤2 mg ml−1. The surviving transformants were transferred to methanol minimal (MM) plates supplemented with 0.2 mM ABTS and 0.1 mM CuSO4. Colonies that produced active laccase developed a green color due to the oxidation of ABTS to its colored radical.
Production of laccase by different transformants in liquid media.
Strains of P. pastoris transformed with pZH98 and pNP2 (or as controls, strains transformed with the parent vector, pPIC3.5K, which does not contain the gene for laccase) were examined for production of laccase in liquid medium. Ten to 15 transformants were cultivated at 30°C in liquid medium (i.e., 25 ml of glycerol minimal [GM] or buffered glycerol minimal [BGM] medium) until the optical density at 600 nm (OD600) of diluted samples corresponded to a value between 6.0 and 10.0 for undiluted culture. Note that an OD600 of 1.0 is the equivalent of 5 × 107 cells ml−1. The culture was centrifuged and the cell pellet was diluted with MM or buffered methanol minimal (BMM) medium to an OD600 of ∼1.0. Replacing glycerol medium with MM or BMM medium induces the production of laccase by P. pastoris. Transformants were cultivated at 30°C in MM or BMM medium supplemented with 0.1 mM CuSO4, and methanol (125 μl or 0.5% of the total volume of medium) was added daily to maintain the induced production of laccase.
Active laccase (LCCI) is produced faster in the GS115 strain of P. pastoris than in KM71 and SMD1168 strains. Slow production of LCCI laccase is expected for the KM71 strain of P. pastoris since it is a MutS transformant, i.e., it grows slowly in methanol medium. Although transformation of pZH98, after linearization with SacI, into SMD1168 strains of P. pastoris generates Mut+ transformants, they appear to grow at rates more similar to MutS transformants. The maximum activity of laccase obtained, per cell density, is nearly the same in all three strains of P. pastoris grown in buffered medium. The SMD1168 strain of P. pastoris is deficient of proteinase and, therefore, maintains a constant activity over longer periods of time in comparison to the KM71 and GS115 strains of P. pastoris, which secrete active proteinase.
The quantity of laccase produced by all three strains of P. pastoris (indicated by the amount of activity in 1 ml of culture) grown in BMM medium is 4- to 15-fold higher than the quantity of laccase produced by strains of P. pastoris grown in MM medium. The pH of MM medium decreases from 5 to 3 within the first day of growth of the yeast cells, whereas the pH of BMM medium remains at 5 throughout the growth cycle. Thus, it appears that low pH has a negative impact on the production of laccase by the yeast cells.
DNA sequencing of laccase cDNA.
The sequences of the laccase cDNA in pZH98, pVS98, and pNP2 were obtained by using a 5% Long Ranger gel on an ABI PRISM 377 DNA sequencer. Samples were sequenced with a Ready Reaction Kit containing AmpliTaq DNA polymerase FS and the Big Dye Terminator Chemistry. ABI PRISM Sequencing 2.1.1 software was used to analyze the raw sequence tracks.
Purification of laccase.
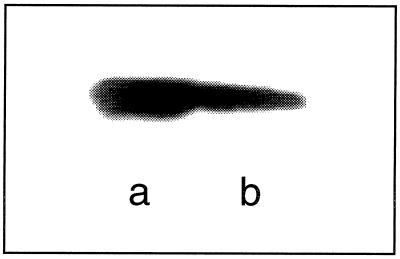
Liquid culture (200 ml) was centrifuged at 5,000 rpm (Tomy TX-160 centrifuge) for 10 min at room temperature. The supernatant containing either 1.6 (LCCI) or 1.2 (LCCIa) mg of protein ml−1 was dialyzed overnight against a polyethylene glycol (PEG) compound with a molecular weight of 15,000 to 20,000 to a volume of 10 ml. The dialysis tubing had a molecular weight cutoff of 10,000. The concentrate containing 4.5 (LCCI) or 3.1 (LCCIa) mg of protein ml−1 was purified with a BioCAD 700E perfusion chromatography workstation (PE Biosystems) equipped with an anion-exchange column (Poros HQ/M; 4.6 by 100 mm) and with the UV detector set at 280 nm. The HQ/M column initially was equilibrated with 2 column volumes of 20 mM Tris–bis-Tris methane buffer (pH 6). Samples (1 ml each for 10 runs) were injected, and the column was eluted with an NaCl gradient (0 to 500 mM in 20 column volumes) dissolved in the same buffer used for column equilibration. The rate of elution was 10 ml min−1, and 1-ml fractions were collected. Fractions that contained active laccase (i.e., those fractions that corresponded to a peak in the chromatogram that eluted at 2.5 to 3.0 min) were combined (10 runs, 5 ml each) and dialyzed to 7 (LCCI, 0.8 mg ml−1) or 5 (LCCIa, 0.5 mg ml−1) ml. The total concentration of protein in each step of the purification procedure was determined with a Bio-Rad kit by the method of Bradford (9). Pure samples of bovine serum albumin, Agaricus bisporus laccase (Sigma), and T. versicolor laccase (Wacker Consortium fur Elektrochemische Industrie GmbH) were used as calibration standards. Protein gels were 10% polyacrylamide, and electrophoresis was performed by the method of Laemmli except that sodium dodecyl sulfate (SDS) and 2-mercaptoethanol were omitted and the samples were not boiled (28). A pure sample of Coriolus hirsitus laccase (SynectiQ) with a molecular size of 60 kDa (see Fig. 1) was used as a calibration standard on protein gels of LCCI and LCCIa.
FIG. 1.
Nondenaturing PAGE of (a) C. hirsitus laccase (10 ng) and (b) LCCI (∼1 ng). Laccase activity was detected by incubating the gel in 5 mM solution of ABTS in 50 mM glycine-HCl buffer (pH 3.5). Similar results were obtained with LCCIa by using DEPDA as the substrate.
Assay of laccase activity.
Measurement of the activity of laccase was performed with ABTS (12) or DEPDA (42) as the substrate. Two assay mixtures were used. The first assay mixture contained 9.3 μmol of ABTS (4.65 mM) and 250 μl of liquid culture in 2 ml of 50 mM glycine-HCl buffer (pH 3). The second assay mixture contained 10 μmol of DEPDA (5 mM) and 250 μl of liquid culture in 2 ml of 100 mM citrate buffer (pH 3.5). The mixtures were held at 20°C and monitored at either 436 nm (ɛABTS = 2.93 × 104 M−1 cm−1) for 10 min or at 555 nm (ɛDEPDA = 48.4 M−1 cm−1) for 30 min. A similar methodology was used to assay the activity of solutions containing purified protein.
EPR spectroscopy.
The electron paramagnetic resonance (EPR) spectrum of LCCIa was collected on a Bruker ECS-106 CW EPR spectrometer equipped with a Bruker dual-mode cavity ER 4116DM and an Oxford ESR 900 cryostat with an ITC503 temperature controller. Experimental conditions were as follows: temperature, 12 K; microwave frequency, 9.67 GHz; microwave power, 1.01 mW; modulation amplitude, 10 G; modulation frequency, 9.67 GHz; center of field, 3,100 G; conversion time, 163 ms; time constant, 81.92 ms.
Cyclic voltammetry.
An EG&G potentiostat-galvanostat (model 263A) was used to obtain cyclic voltammograms of laccase, ABTS, and DEPDA. A single-compartment cell was purged with nitrogen gas for 10 min prior to each measurement. The counter and reference electrodes were platinum gauze (6.0 cm2) and saturated calomel electrode (SCE; 241 mV versus normal hydrogen electrode voltage [NHE]), respectively. The working electrode used with LCCI and LCCIa was a gold flag (4.0 cm2), which was cleaned with piranha solution (3:1 volumetric ratio of concentrated H2SO4–30% H2O2), followed by dilute aqua regia (5:3:1 volumetric ratio of H2O-HCl-HNO3). The clean gold electrode was coated subsequently with a monolayer of pyridine thiolate by immersing the electrode in a 10 mM aqueous solution of 4,4′-dipyridyl disulfide overnight. The working electrode used with ABTS and DEPDA was a disc of gold (3.14 by 10−2 cm2), which was cleaned and polished prior to use, first with 1-mm α-Al2O3 and then with 0.05-mm γ-Al2O3 (Micropolish II; Buehler). Electrochemical symbols are defined as follows: NHE, normal hydrogen electrode voltage (0 V); SCE, saturated calomel electrode (241 mV versus NHE); E0′, measured potential of a redox couple; Ep/2, potential of the anodic peak at 0.5ip; Ep,a and Ep,c, potential at peak current in the anodic (upper) or cathodic (lower) wave of the cyclic voltammogram, respectively; khet, rate constant for heterogeneous electron transfer; D, diffusion coefficient; v, rate at which the potential is changed during the cyclic voltammetry experiment in units of Vs−1; F, Faraday constant (9.6846 × 104 C equivalents−1).
RESULTS AND DISCUSSION
At present, only one laccase-type polyphenol oxidase isolated from bacteria has been described (18). In contrast, several genes and their corresponding laccases have been isolated due to the wide distribution of laccase in both plants and fungi. The gene (lccI) used in this work was isolated originally from T. versicolor and characterized by Ong et al. (35, 36). The lccI cDNA contains an open reading frame of 1,560 bp and encodes the LCCI isoform of laccase, which consists of 499 amino acid residues preceded by a native signal sequence that is 20 amino acids in length. Laccase is secreted by T. versicolor as a glycosylated protein with four copper atoms and two disulfide bridges. The histidinyl and cysteinyl residues that bind copper are conserved in all isoforms of laccase (14). Many of these residues are found near the C terminus of the protein and for LCCI include His-415, His-418, His-420, His-472, Cys-473, His-474, and His-478.
LCCI is nearly identical to isoform Lcc2 (i.e., 3 of 519 amino acid residues are different), a laccase isolated from T. villosa and characterized by Yaver et al. (52). The production of Lcc2 in a heterologous host, however, has not been reported previously. Lcc2 has a molecular mass of ∼65 kDa as determined by SDS-polyacrylamide gel electrophoresis (PAGE) and a UV-visible spectrum with a peak at 276 nm (protein) and a shoulder around 600 nm. When syringaldazine is used as the substrate, the optimal activity of Lcc2 is between pH 5.0 and 5.5 with a specific activity of 90 μmol min−1 mg of protein−1.
Based on our analysis of the crystal structures of laccase and ascorbate oxidase, we hypothesized that the rate of heterogeneous electron transfer might be increased if there was greater access to the type-1 copper site. We tested this hypothesis by reducing the number of amino acid residues between the last histidine that binds the type-1 copper ion in the active site of LCCI (His-478) and the C terminus (Gln-519). Thus, LCCIa is a truncated version of LCCI with a single cysteine residue replacing the 11 amino acids (i.e., PIYDGLSEANQ) at the C terminus of LCCI. Introduction of a cysteine residue at the C terminus of laccase provides a chemical target (i.e., thiol) for selective modification. The presence of activity in LCCIa is strong evidence that the disulfide bond between Cys-117 and Cys-205 is conserved. Which of the two cysteine residues at the C terminus of LCCIa forms a disulfide bond with Cys-85, however, cannot be determined with certainty. Alignment of the sequences of the LCCI and LCCIa with two proteins for which the crystal structures are available (laccase from Coprinus cinereus and ascorbate oxidase from Cucurbita pepo medullosa) is shown in Table 1.
TABLE 1.
Sequence alignment between LCCI, LCCIa, 1a65, and zAO
| Protein | Sequence alignmenta |
|---|---|
| LCCI | 471LHCHIDFHLD AGFAIVFAED VADVKAANPV PKAWSDLCPI YDGLSEANQ519 |
| LCCIa | 471LHCHIDFHLD AGFAIVFAED VADVKAANPV PKAWSDLCC509 |
| 1a65 | 450FHCHIEFHLM NGLAIVFAED MANTVDANNP PVEWAQLCEI YDDLPPEATS IQTTV504 |
| 1AOZ | 505FHCHIEPHLH MGMGVVFAEG VEKVGRIPTK ALACGGTAKS LINNPKNP552 |
| Cu-type | 31311 |
Residues in boldface type highlight the differences between LCCI and LCCIa. Underlined residues are ligands (coordinated or in close proximity) to type-1 and type-3 copper ions. Cysteine residues that are double-underlined are involved in formation of a disulfide bridge. 1a65 and zAO correspond to the crystal structures of laccase (Coprinus cinereus) and ascorbate oxidase (Cucurbita pepo medullosa), respectively, available through the Protein Data Bank (17, 30).
Our strategy to produce LCCI and LCCIa by using P. pastoris consisted of the following steps. The cDNA of lccI or lccIa, including putative native signal sequences, were subcloned into pPIC3.5K between the AOX1 promoter and the transcription termination signal. Similarly, the lccI cDNA, including the putative native signal sequence, was joined with the α-factor secretion signal of pPIC9K between the AOX1 promoter and the transcription termination signal. Based on the results of Jönsson et al. (24), we included both the α-factor secretion signal and the laccase signal sequence in our construct as a test to improve the level of secreted laccase. The result of this construct, however, was intracellular production of laccase. The resulting plasmids were linearized with SacI and transformed into electrocompetent cells of P. pastoris (strains KM71, GS115, and SMD1168) by electroporation. SacI cleaves the plasmids at the 5′-AOX1 promoter, which results in the integration of transformed DNA at the genomic AOX1 or his4 locus. Selection of transformants was based on their rate of growth on MM agar plates, which favors Mut+ (methanol utilization-positive) transformants in P. pastoris. If double crossover occurs at the alcohol oxidase locus, MutS transformants (which utilize methanol slowly) are generated. MutS transformants, however, grow slowly when methanol is the only carbon source, while Mut+ transformants grow much faster under the same conditions.
Initially, transformants were grown on YPD plates that contained different concentrations of the G418 antibiotic (0.25 to 4.0 mg ml−1). The highest concentrations of G418 antibiotic tolerated by the transformants in the three strains of P. pastoris were 0.5 to 2.0 mg ml−1 for GS115, 0.25 to 1.0 mg ml−1 for KM71, and 0.25 mg ml−1 for SMD1168. The G418 antibiotic is an analog of neomycin sulfate and is believed to inhibit protein synthesis in eukaryotic cells by binding to 80S ribosomes as well as other cellular components (6, 26). The concentrations of G418 antibiotic to which the transformants were tolerant (i.e., level of resistance) indicate that one copy of the laccase gene was integrated into the genome of P. pastoris (13, 22). Transformants that survived the G418 antibiotic subsequently were transferred to MM medium plates containing ABTS. ABTS functions as a substrate for laccase and, when oxidized, provides optical evidence for the presence and location (i.e., extracellular or intracellular) of active laccase. SMD1168 strains of P. pastoris containing the parent plasmids, pPIC3.5K and pPIC9K, were used as control colonies. The control colonies did not exhibit a reaction with ABTS either on agar plates or in liquid cultures. In contrast, all surviving transformants that contained pZH98 (i.e., those that produced LCCI) exhibited a positive reaction with ABTS. The surviving transformants that contained pNP2 (i.e., those that produced LCCIa), however, exhibited little or no reaction with ABTS due to the change in the reduction potential of the active site (vide infra). Instead, the presence of active LCCIa was indicated by a positive reaction with DEPDA, which is oxidized at a more negative potential than ABTS (i.e., 0.44 V versus NHE as compared to 0.68 V versus NHE).
The production of active laccase by P. pastoris transformed with pZH98 or pNP2 is extracellular, indicated by the presence of a green color (ABTS radical) in areas of the solid medium immediately surrounding but not occupied by colonies. This result confirms that the native secretion sequence of lccI and lccIa cDNAs is functional in P. pastoris. The absence of intracellular production of active laccase was confirmed by assaying a pellet of cells (transformed with either pZH98 or pNP2) that had been washed and ruptured with acid-washed glass beads (22). In studies reported by Jönsson et al. (24), the amount of laccase produced was higher when the native secretion signal was used in the construct instead of the α-factor secretion signal of pPIC9K. We joined lccI cDNA, including its native secretion signal, to the α-factor secretion signal of pPIC9K to test the effect of two secretion signals on the level of production of secreted laccase. The result of this construct, however, is the production of active laccase but not its secretion (indicated by the presence of a green color [ABTS radical] localized within the colonies). This result may be due to changes in conformation caused by an additional secretion sequence or the inappropriate processing of the fusion protein.
After subcloning, the lccI cDNA contained in recombinant plasmids pVS98 and pZH98 and lccIa cDNA contained in pNP2 were sequenced. DNA sequencing of lccI in pVS98 revealed only one deviation from the original sequence of lccI (36). This deviation (codon GAC instead of GCC) results in the substitution of Asp for Ala at position 297 in the protein. Although this substitution is not located near the binding sites of copper ions, it is likely that replacement of a hydrophobic amino acid with a negatively charged amino acid changes the local conformation of the protein. The sequences of lccI in pZH98 did not show any deviations from the original sequence. The sequence of lccIa in pNP2 did not deviate from the original sequence except for the modification purposefully introduced (i.e., TGC instead of CCC-ATC-TAC-GAC-GGG-CTG-AGC-GAG-GCT-AAC-CAG) to substitute a cysteine residue for the 11 amino acids (i.e., PIYDGLSEANQ) at the C terminus of LCCI. Both LCCI and LCCIa were produced by the SMD1168 strain of P. pastoris and purified under identical conditions. Isolation of LCCI and LCCIa was confirmed with a nondenaturing PAGE using a pure sample of C. hirsitus laccase (SynectiQ; 60 kDa) as a calibration standard (Fig. 1). The amounts of LCCI and LCCIa produced by the SMD1168 strain of P. pastoris and their corresponding activities in the presence of ABTS or DEPDA are summarized in Table 2.
TABLE 2.
Yield and activities of LCCI and LCCIa produced by P. pastoris SMD1168
| Enzyme and stepa | [Protein] (mg ml−1) | Total protein (mg) | Total activity (U)b | Sp act (mU mg−1) |
|---|---|---|---|---|
| LCCI | ||||
| Culture (200 ml) | 1.6 | 320 | 7.82 | 24 |
| Dialysis (10 ml) | 4.5 | 45 | 7.2 | 160 |
| Post-HPLC (50 ml) dialyzed to 7 ml | 0.8 | 5.6 | 3.68 (ABTS)c, 6.83 (DEPDA)c | 656 (ABTS)c, 1,220 (DEPDA)c |
| LCCIa | ||||
| Culture (200 ml) | 1.2 | 240 | 1.64 | 7 |
| Dialysis (10 ml) | 3.1 | 31 | 1.52 | 49 |
| Post-HPLC (50 ml) dialyzed to 5 ml | 0.5 | 2.5 | 0.54 | Inactive (ABTS)c, 216 (DEPDA)c |
HPLC, high-pressure liquid chromatography.
A unit of activity (U) is defined as the amount of enzyme, in milligrams, that will oxidize 10−6 mol of substrate per min with the corresponding reduction of dioxygen to water.
Substance in parentheses is the substrate used.
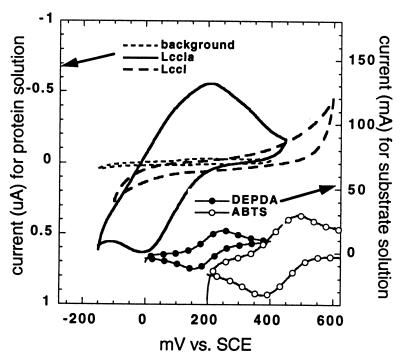
The inability of LCCIa to oxidize ABTS at a measurable rate suggests that the modification introduced at the C terminus has affected the potential of the active site. This effect is confirmed by the cyclic voltammogram of LCCIa shown in Fig. 2. LCCIa exhibits an electrochemical response with a quasireversible peak (Ep,a − Ep,c = 200 mV) centered at 411 mV versus NHE. Thus, the potential of the active site of LCCIa is more negative than the potential of ABTS (E0′ABTS = 681 mV versus NHE) but near that of DEPDA (E0DEPDA = 441 mV versus NHE). Potentiometric and redox titrations of laccase isolated from the Japanese lacquer tree (e.g., Rhus vernicifera) have shown that the reduction potentials of type-1 Cu (II) and type-3 Cu (II) at pH 7.5 are 434 and 483 mV versus NHE, respectively (41). In contrast, fungal laccases (e.g., Neurospora crassa, T. villosa, Rhizoctonia solani, and T. versicolor) have a range of reduction potentials (480 to 780 mV versus NHE) due to differences in the coordination environment of the copper ions (43). For example, replacing the methionine ligand at the axial position of type-1 copper with a noncoordinating phenylalanine residue stabilizes the reduced state of the type-1 copper, thus shifting the reduction potential to more positive values (19, 48). Similarly, a reduction potential of 710 mV versus NHE has been found in a laccase with a noncoordinating leucine residue at the axial position of type-1 copper (47). Thus, it appears that the primary sequence of a laccase with a reduction potential near 700 mV versus NHE requires a noncoordinating residue at the axial position of type-1 copper. The axial position of type-1 copper, however, is not the only position in the primary sequence of laccase that affects the reduction potential since other laccases with leucine residues at this position have been found to possess reduction potentials near 470 and 510 mV versus NHE (47).
FIG. 2.
Cyclic voltammograms (2.0 mV s−1) of solutions containing either 0.5 mg (8 μM) of LCCI or LCCIa ml−1 in 0.1 M phosphate buffer (pH 6.0). The cyclic voltammograms of both DEPDA (0.5 mM) and ABTS (0.5 mM) performed under the same conditions except at a higher scan rate (100 mV s−1) are included in the lower right corner to illustrate their potentials relative to that of the active site of LCCIa. Note that the current scale for laccase is in microamperes (left y-axis) and the current scale for substrates is in milliamperes (right y-axis).
Both LCCI and LCCIa have a noncoordinating phenylalanine residue at position 483 (a type-1 Cu binding site) and, therefore, are expected to have a reduction potential more positive than 700 mV versus NHE. The shift in the reduction potential of the active site of LCCIa is an unexpected consequence of a relatively distant modification to the C terminus. The EPR spectrum of LCCIa indicates that the active site of this protein is changed slightly from that observed for copper sites in other fungal laccases. The values for g∥ and A∥ of type-1 Cu (T1) are 2.2 and 90 × 10−4, respectively, and those for g∥ and A∥ of type-2 Cu (T2) are 2.26 and 179 × 10−4, respectively. These values are most similar to those reported for two other laccases: PoL isolated from Pleurotus ostreatus (for T1, g∥ = 2.179 and A∥ = 90 × 10−4; for T2, g∥ = 2.263 and A∥ = 176 × 10−4) (54) and an MtL triple mutant isolated from Myceliophthora thermophila (for T1, g∥ = 2.192 and A∥ = 90 × 10−4; for T2, g∥ = 2.247 and A∥ = 175 × 10−4, E0′ = 470 mV versus NHE) (47).
The distorted shape of the cyclic voltammogram of LCCIa reflects a case where the separation in potential between successive oxidations is less than 100(αn)−1 mV (i.e., individual waves are merged), and at least one of these oxidations is reversible on the time scale of the experiment. The ratio of the peak current of the anodic and cathodic waves in the cyclic voltammogram reflects the reversibility of the active site of LCCIa as a function of scan rate and ranges from 0.9:1.0 at 2 mV s−1 to 1.5:1.0 at 25 mV s−1. Equation 1 quantifies the behavior of a quasireversible system:
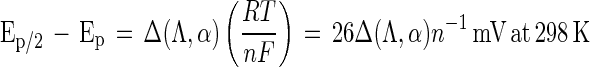 |
1 |
where Ep is the potential at which the anodic current is at a maximum in the cyclic voltammogram, Δ(Λ,α) is a function derived by Matsuda and Ayabe for electron transfer processes that are quasireversible, n is the number of electrons transferred during the oxidation and reduction of laccase (11, 15), and RT is 2.48 kJ/mol. When the values for Λ and α are 0.36 and 0.5 (vide infra), respectively, the function Δ(Λ,α) equals 3.0 and Ep/2 − Ep = 78n−1 mV at 298 K. The value for Ep/2 − Ep of the anodic wave in the cyclic voltammogram of LCCIa is a factor of 1.9 times greater than the value that corresponds to a quasireversible one-electron process (i.e., 148 versus 78 mV). Thus, the anodic wave in the cyclic voltammogram of LCCIa indicates that at least two of the Cu(I) ions in the active site of laccase are oxidized sequentially (Fig. 2). The difference in potential between Ep/2 and Ep for the cathodic wave is obtained in a similar manner (104 mV), indicating the reversible reduction of at least one Cu(II) ion in the active site of LCCIa.
A more significant result of modifying the C terminus of LCCIa is that solutions containing this protein, in contrast to solutions containing LCCI, exhibit a cyclic voltammogram, which indicates that the active site in LCCIa is more accessible electrochemically. This result supports our original hypothesis, that is, reducing the number of amino acid residues between the last histidine residue that binds a copper ion in the active site of laccase (His-478) and the C terminus (Gln-519) lowers the barrier to heterogeneous electron transfer. Furthermore, this result suggests that an alternate sequence of amino acid residues between His-478 and the C terminus plausibly could transport electrons to the active site of laccase faster than the sequence currently present in LCCI.
Cyclic voltammograms of laccase adsorbed on the surface of either a graphite electrode (29, 46) or a gold electrode modified with β-mercaptoproprionate have been reported (21). The cyclic voltammogram of laccase isolated from Polyporous versicolor was indistinguishable from the background voltammogram (29). More recently, the cyclic voltammogram of this laccase was shown to exhibit broad peaks with a midpoint potential around 790 mV versus NHE after subtraction of the background voltammogram (46). The cyclic voltammogram of laccase isolated from R. vernicifera, when adsorbed onto a gold electrode modified with β-mercaptoproprionate, exhibits broad peaks with a midpoint potential at 330 mV versus NHE, the potential expected for a laccase isolated from the Chinese lacquer tree (21). Since the proteins were adsorbed on the surface of the electrodes in these studies, the diffusion coefficient of laccase (D) could not measured. Furthermore, the rate constant for heterogeneous electron transfer (khet) between laccase in solution and an electrode has not been reported previously.
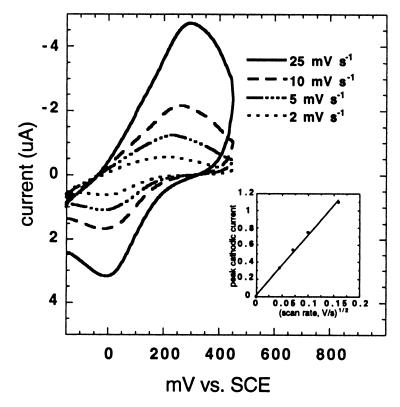
When laccase is dissolved in an electrolyte, both the diffusion coefficient and the rate constant for heterogeneous electron transfer can be determined from the corresponding cyclic voltammograms. The scan rate dependence of the cyclic voltammogram of LCCIa is shown in Fig. 3. The cathodic peak current (ip,c) in the cyclic voltammogram of LCCIa varies linearly with v1/2 (scan rate1/2) (Fig. 3, inset). Thus, the diffusion coefficient of laccase (D = 1.49 × 10−6 cm2 s−1) can be determined by using the Randles-Sevcik equation:
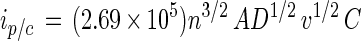 |
2 |
where ip/c is the value for peak cathodic current at a given scan rate, n is the number of electrons transferred, A is the area of the electrode measured in cm2, and C is the concentration of laccase in units of mol cm−3 (5).
FIG. 3.
Scan rate dependence of cyclic voltammograms of LCCIa. The linear dependence of cathodic peak current to (scan rate1/2) is shown in the inset. Anaerobic conditions were maintained by purging a single-compartment cell with nitrogen gas for 10 min prior to each measurement.
The value of the rate constant for heterogeneous electron transfer between an electrode and laccase is determined by using the method of Nicholson and equation 3:
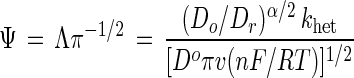 |
3 |
where Do and Dr are the diffusion coefficients of oxidized and reduced laccase, respectively, n is the number of electrons transferred in the rate-determining step, and α is the transfer coefficient and typically assigned the value of 0.5 (33, 34). Assuming that Do and Dr are equivalent, setting α at 0.5 and rearranging gives equation 4:
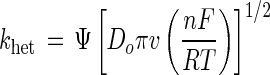 |
4 |
The value for the kinetic parameter Ψ is dependent on Ep,a − Ep,c, and thus for LCCIa, Ψ = 0.26 and khet = 1.3 × 10−4 cm s−1. For comparison, the rate of heterogeneous electron transfer to cytochrome c, an oxidoreductase with a heme group on the surface of the protein, is 6 × 10−3 cm s−1 (1). The rates of heterogeneous electron transfer for mediators such as K3Fe(CN)6 and ABTS are 4.4 × 10−2 and 4.5 × 10−3 cm s−1, respectively (38). The absence of any wave in the cyclic voltammogram of LCCI indicates that khet ≪ 1.3 × 10−4 cm s−1. It is unlikely that the diffusion coefficient of LCCI is markedly different from that of LCCIa due to the magnitude of the modification to the C terminus relative to the size of the protein.
In summary, vectors that contain either lccI cDNA from T. versicolor or a truncated version thereof, lccIa, were constructed. These vectors were used to express both genes (lccI and lccIa) in P. pastoris. Both the lccI and lccIa cDNA include a natural secretion sequence, and therefore their corresponding proteins are secreted. The alcohol oxidase promoter (AOX1 gene) controls the expression of lccI and lccIa in P. pastoris, which is activated by the addition of methanol to the growth medium. The proteinase-deficient strain of P. pastoris grown in buffered methanol medium produces the highest quantity of laccase. The main advantage of using P. pastoris to produce laccase is that the yeast cells secrete laccase in the presence of low levels of native proteinases, thus simplifying purification of the recombinant proteins.
Results from our electrochemical studies indicate that the barrier to heterogeneous electron transfer is reduced when the C terminus of LCCI is truncated. An additional consequence of truncating the C terminus of LCCI is a shift in the reduction potential of the active site to a more negative value. To the best of our knowledge, the reduction potential of the active site of LCCIa represents the most negative potential thus far reported for any fungal laccase. Studies are in progress to determine if other modifications to the C terminus of LCCI will increase further khet as well as spectroscopic studies of LCCIa to determine what structural changes are responsible for the shift in potential of the active site.
ACKNOWLEDGMENTS
This research was supported by grants awarded to G.T.R.P. from the Office of Naval Research, the California Space Institute, Sandia National Laboratory, and the National Science Foundation. H.-H.K. is a recipient of a KSAAP fellowship from the Bureau of Educational and Cultural Affairs of the USIA and thanks the Department of Energy for financial assistance through the GATE program. N.G.B. is a recipient of fellowships from the NSF (IGERT) and the Department of Energy (GATE).
We thank Xiaoting Li for technical assistance, Edgar Ong and Mark Brown for providing us with a sample of laccase cDNA (lccI) from T. versicolor, and Gunter Wich and Anton Candussio for a sample of laccase from T. versicolor.
REFERENCES
- 1.Allen P M, Hill H A O, Walton N J. Surface modifiers for the promotion of direct electrochemistry of cytochrome c. J Electroanal Chem. 1984;178:69. [Google Scholar]
- 2.Andreásson L-E, Branden R, Reinhammar B. Kinetic studies of Rhus vernicifera laccase. Evidence for multi-electron transfer and an oxygen intermediate in the reoxidation reaction. Biochim Biophys Acta. 1976;438:370–379. doi: 10.1016/0005-2744(76)90254-0. [DOI] [PubMed] [Google Scholar]
- 3.Andreásson L-E, Reinhammar B. Kinetic studies of Rhus vernicifera laccase: role of the metal centers in electron transfer. Biochim Biophys Acta. 1976;445:579–597. doi: 10.1016/0005-2744(76)90112-1. [DOI] [PubMed] [Google Scholar]
- 4.Andreásson L-E, Reinhammar B. The mechanism of electron transfer in laccase-catalyzed reactions. Biochim Biophys Acta. 1979;568:145–156. doi: 10.1016/0005-2744(79)90282-1. [DOI] [PubMed] [Google Scholar]
- 5.Bard A J, Faulkner L R. Electrochemical methods. New York, N.Y: John Wiley & Sons, Inc.; 1980. [Google Scholar]
- 6.Bar-Nun S, Shneyour Y, Beckmann J S. G-418, an elongation inhibitor of 80S ribosomes. Biochim Biophys Acta. 1983;741:123–127. doi: 10.1016/0167-4781(83)90018-0. [DOI] [PubMed] [Google Scholar]
- 7.Bergens S H, Gorman C B, Palmore G T R, Whitesides G M. A redox fuel cell that operates with methane as fuel at 120°C. Science. 1994;265:1418–1420. doi: 10.1126/science.265.5177.1418. [DOI] [PubMed] [Google Scholar]
- 8.Bourbonnais R, Paice M G, Reid I D, Lanthier P, Yaguchi M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol. 1995;61:1876–1880. doi: 10.1128/aem.61.5.1876-1880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Brett C M A, Brett A M O. Electrochemistry principles, methods, and applications. New York, N.Y: Oxford Science Publications; 1993. [Google Scholar]
- 11.Cass A E G, Davis G, Francis G D, Hill H A O, Aston W J, Higgins I J, Plotkin V, Scott L D L, Turner A P F. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal Chem. 1984;56:667–671. doi: 10.1021/ac00268a018. [DOI] [PubMed] [Google Scholar]
- 12.Childs R E, Bardsley W G. The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulphonic acid) as chromogen. Biochem J. 1975;145:93–103. doi: 10.1042/bj1450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clare J, Scorer C, Buckholz R, Romanos M. Expression of EGF and HIV envelope glycoprotein. In: Higgins D R, Cregg J M, editors. Pichia protocols. Totowa, N.J: Humana Press; 1998. p. 222. [DOI] [PubMed] [Google Scholar]
- 14.Cullen D. Recent advances on the molecular genetics of ligninolytic fungi. J Biotechnol. 1997;53:273–289. doi: 10.1016/s0168-1656(97)01684-2. [DOI] [PubMed] [Google Scholar]
- 15.Degani Y, Heller A. Direct electrical communication between chemically modified enzymes and metal electrodes. 1. Electron transfer from glucose oxidase to metal electrodes via electron relays, bound covalently to the enzyme. J Phys Chem. 1987;91:1285–1289. [Google Scholar]
- 16.Degani Y, Heller A. Direct electrochemical communication between chemically modified enzymes and metal electrodes. 2. Methods for bonding electron-transfer relays to glucose oxidase and d-amino acid oxidase. J Am Chem Soc. 1988;110:2615–2620. [Google Scholar]
- 17.Ducros V, Brzozowski A M, Wilson K S, Brown S H, Østergaard P, Schneider P, Yaver D S, Pedersen A H, Davies G J. Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat Struct Biol. 1998;5:310–316. doi: 10.1038/nsb0498-310. [DOI] [PubMed] [Google Scholar]
- 18.Givaudan A, Effosse A, Faure D, Potier P, Bouillant M-L, Bally R. Polyphenol oxidase in azospirillum-lipoferum isolated from rice rhizosphere—evidence for laccase activity in non-motile strains of Azospirillum-Lipoferum. FEMS Microbiol Lett. 1993;108:205–210. [Google Scholar]
- 19.Guckert J A, Lowery M D, Solomon E I. Electronic structure of the reduced blue copper active site—contributions to the reduction potentials and geometry. J Am Chem Soc. 1995;117:2814–2844. [Google Scholar]
- 20.Hill H A O. The development of bioelectrochemistry. Coord Chem Rev. 1996;151:115–123. [Google Scholar]
- 21.Hyung K H, Jun K Y, Hong H-G, Kim Y S, Shin W. Immobilization of laccase onto the gold electrode using β-mercaptopropionate. Bull Korean Chem Soc. 1997;18:564–566. [Google Scholar]
- 22.Invitrogen. Multi-copy Pichia expression kit manual, version C. Carlsbad, Calif: Invitrogen Corporation; 1998. [Google Scholar]
- 23.Jönsson L, Sjostrom K, Haggstrom I, Nyman P O. Characterization of a laccase gene from the white-rot fungus Trametes versicolor and structural features of basidiomycete laccases. Biochem Biophys Acta. 1995;1251:210–215. doi: 10.1016/0167-4838(95)00104-3. [DOI] [PubMed] [Google Scholar]
- 24.Jönsson L J, Saloheimo M, Penttilä M. Laccase from the white-rot fungus Trametes versicolor: cDNA cloning of lcc1 and expression in Pichia pastoris. Curr Genet. 1997;32:425–430. doi: 10.1007/s002940050298. [DOI] [PubMed] [Google Scholar]
- 25.Kojima Y, Tsukuda Y, Kawai Y, Tsukamoto A, Sugiura J, Sakaino M, Kita Y. Cloning, sequence analysis, and expression of ligninolytic phenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J Biol Chem. 1990;265:15224–15230. [PubMed] [Google Scholar]
- 26.Küng M, Stadelmann B, Brodbeck J, Bütikofer P. Addition of G418 and other aminoglycoside antibiotics to mammalian cells results in the release of GPI-anchored proteins. FEBS Lett. 1997;409:333–338. doi: 10.1016/s0014-5793(97)00452-3. [DOI] [PubMed] [Google Scholar]
- 27.Kuznertsov A M, Bogdanovskaya V A, Tarasevich M R, Gavrilova E F. The mechanism of cathode reduction of oxygen in a carbon carrier-laccase system. FEBS Lett. 1987;215:219–222. doi: 10.1016/0014-5793(87)80149-7. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lee C-W, Gray H B, Anson F C, Malmström B G. Catalysis of the reduction of dioxygen at graphite electrodes coated with fungal laccase A. J Electroanal Chem. 1984;172:289–300. [Google Scholar]
- 30.Messerschmidt A, Ladenstein R, Huber R, Bolognesi M, Avigliano L, Petruzzelli R, Rossi A, Finazzi-Agro A. Refined crystal structure of ascorbate oxidase at 1.9 Å resolution. J Mol Biol. 1992;224:179–205. doi: 10.1016/0022-2836(92)90583-6. [DOI] [PubMed] [Google Scholar]
- 31.Mikuni J, Morohoshi N. Cloning and sequencing of a second laccase gene from the white-rot fungus Coriolus versicolor. FEMS Microbiol Lett. 1977;155:79–84. doi: 10.1111/j.1574-6968.1997.tb12689.x. [DOI] [PubMed] [Google Scholar]
- 32.Mosbach R. Purification and some properties of laccase from Polyporus versicolor. Biochim Biophys Acta. 1963;73:204–212. doi: 10.1016/0006-3002(63)90304-4. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson R S. Theory and application of cyclic voltammetry for measurement of electrode-reaction kinetics. Anal Chem. 1965;37:1351–1355. [Google Scholar]
- 34.Nicholson R S, Shain I. Theory of stationary electrode polarography: single scan and cyclic methods applied to reversible, irreversible, and kinetics systems. Anal Chem. 1964;36:706–722. [Google Scholar]
- 35.Ong, E., B. Pollock, and M. Smith. 1996. GenBank accession no. U44430.
- 36.Ong E, Pollock W B R, Smith M. Cloning and sequence analysis of two laccase complementary DNAs from the ligninolytic basidiomycete Trametes versicolor. Gene. 1997;196:113–119. doi: 10.1016/s0378-1119(97)00215-1. [DOI] [PubMed] [Google Scholar]
- 37.Palmore G T R, Bertschy H, Bergens S H, Whitesides G M. A methanol/dioxygen biofuel cell that uses NAD+-dependent dehydrogenases as catalysts: application of an electro-enzymatic method to regenerate nicotinamide adenine dinucleotide at low overpotentials. J Electroanal Chem. 1998;443:155–161. [Google Scholar]
- 38.Palmore G T R, Kim H-H. Electro-enzymatic reduction of dioxygen to water in the cathode compartment of a biofuel cell. J Electroanal Chem. 1999;464:110–117. [Google Scholar]
- 39.Palmore G T R, Whitesides G M. Microbial and enzymatic biofuel cells. In: Himmel M E, Baker J O, Overend R P, editors. Enzymatic conversion of biomass for fuels production. ACS Symposium Series. Washington, D.C.: American Chemical Society; 1994. pp. 271–290. [Google Scholar]
- 40.Reinhammar B. An EPR signal from the half-reduced type 3 copper pair in Rhus vernicifera laccase. J Inorg Biochem. 1981;15:27–39. [Google Scholar]
- 41.Reinhammar B R M. Oxidation-reduction potentials of the electron acceptors in laccase and stellacyanin. Biochim Biophys Acta. 1972;275:245–259. doi: 10.1016/0005-2728(72)90045-x. [DOI] [PubMed] [Google Scholar]
- 42.Rescigno A, Sanjust E, Montanari L, Sollai F, Soddu G, Rinaldi A C, Oliva S, Rinaldi A. Detection of laccase, peroxidase and polyphenol oxidase on a single polyacrylamide gel electrophoresis. Anal Lett. 1997;30:2211–2220. [Google Scholar]
- 43.Solomon E I, Machonkin T E, Sundaram U M. Spectroscopy of multi-copper oxidases. In: Messerschmidt A, editor. Multi-copper oxidases. River Edge, N.J: World Scientific; 1997. pp. 103–128. [Google Scholar]
- 44.Taniguchi I, Iseki M, Yamaguchi H, Yasykouchi K. Surface enhance Raman scattering study of horse heart cytochrome c at a silver electrode in the presence of bis(4-pyridyl)disulfide and purine. J Electroanal Chem. 1984;175:341. [Google Scholar]
- 45.Tarasevich M R, Yaropolov A I, Bogdanovskaya V A, Varfolomeev S D. Electrocatalysis of a cathodic reduction by laccase. J Electroanal Chem. 1979;104:393–403. [Google Scholar]
- 46.Thuesen M H, Farver O, Reinhammar B, Ulstrup J. Cyclic voltammetry and electrocatalysis of the blue copper oxidase polyporus versicolor laccase. Acta Chem Scand. 1998;52:555–562. [Google Scholar]
- 47.Xu F, Berka R M, Wahleithner J A, Nelson B A, Shuster J R, Brown S H, Palmer A E, Solomon E I. Site-directed mutations in fungal laccase: effect on redox potential, activity and pH profile. Biochem J. 1998;334:63–70. doi: 10.1042/bj3340063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu F, Palmer A E, Yaver D S, Berka R M, Gambetta G A, Brown S H, Solomon E I. Targeted mutation in a Trametes villosa laccase. J Biol Chem. 1999;274:12372–12375. doi: 10.1074/jbc.274.18.12372. [DOI] [PubMed] [Google Scholar]
- 49.Yaropolov A I, Kharybin A N, Emnéus J, Marko-Varga G, Gorton L. Electrochemical properties of some copper-containing oxidases. Bioelectrochem Bioenerg. 1996;40:49–57. [Google Scholar]
- 50.Yaver D S, Golightly E J. Cloning and characterization of three laccase genes from the white-rot basidiomycete Trametes villosa: genomic organization of the laccase gene family. Gene. 1996;181:95–102. doi: 10.1016/s0378-1119(96)00480-5. [DOI] [PubMed] [Google Scholar]
- 51.Yaver, D. S., F. Xu, H. Dalboge, P. Schneider, and D. A. Aaslyng. 4 January 1996. U.S. Patent WO 96/00290.
- 52.Yaver D S, Xu F, Golightly E J, Brown K M, Brown S H, Rey M W, Schneider P, Halkier T, Mondorf K, Dalboge H. Purification, characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa. Appl Environ Microbiol. 1996;62:834–841. doi: 10.1128/aem.62.3.834-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoneyama H, Kajiya Y. Achievement of direct electron transfer between glucose oxidase and an electrode by adsorption of hydroquinonesulfate on the enzyme. Sens Actuators B. 1993;13:65–67. [Google Scholar]
- 54.Youn H-D, Kim K-J, Maeng J-S, Han Y-H, Jeong I-B, Jeong G, Kang S-O, Hah Y C. Single electron transfer by an extracellular laccase from the white-rot fungus Pleurotus ostreatus. Microbiology. 1995;141:393–398. doi: 10.1099/13500872-141-2-393. [DOI] [PubMed] [Google Scholar]