Abstract
We describe the case of an 86-year-old man with an extensive cardiac history, including previous coronary artery bypass grafting, who experienced a delayed extracardiac hematoma, 350 mL in volume, after retrograde chronic total occlusion—percutaneous coronary intervention. The patient was successfully treated with resultant liquefaction of the hematoma. (Level of Difficulty: Advanced.)
Key Words: cardiac magnetic resonance, complication, computed tomography, echocardiography, percutaneous coronary intervention
Abbreviations and Acronyms: CABG, coronary artery bypass graft; CAD, coronary artery disease; CAP, coronary artery perforation; CTO, chronic total occlusion; LCx, left circumflex artery; LMS, left main stem; OM1, obtuse marginal artery; PCI, percutaneous coronary intervention; SVG, saphenous vein graft
Central Illustration
History of Presentation
An 86-year-old man with severely calcified 3-vessel coronary artery disease (CAD) after 3-vessel CABG performed in 1999 presented to our institution with worsening angina pectoris (Canadian Cardiovascular Society III–IV) despite maximally tolerated medical therapy. Four months previously the patient had undergone chronic total occlusion—percutaneous coronary intervention (CTO-PCI) to his ostial left main stem coronary artery without success. The patient had an exceptional functional baseline and was independent in his activities of daily living. Given his ongoing ischemic chest pain, the patient’s case was discussed at a multidisciplinary meeting, and a decision was made to reattempt an antegrade/retrograde approach via the occluded saphenous venous graft (SVG) to the obtuse marginal artery (OM1) to revascularize the left circumflex via the occluded left main stem in the hope of alleviating some of his anginal symptoms and returning the patient to his very active baseline (Video 1).
Learning Objectives
-
•
To demonstrate that patients presenting after coronary artery perforation who are poor surgical candidates can be successfully treated medically with close clinical monitoring and serial imaging.
-
•
To illustrate the role of multimodality imaging in the diagnosis and monitoring of extracardiac left atrial hematomas.
The patient was admitted electively the day of the procedure and received a loading dose of 600 mg of clopidogrel. An initial retrograde attempt was made via the occluded SVG to the OM1; the guidewire successfully reached the distal cap but subsequently progressed down the distal left circumflex artery (LCx) rather than toward the occlusion. A decision was then made to attempt an antegrade approach. The Gladius Mongo guidwire (Asahi Inc) successfully reached the distal LCx. The right coronary artery was then intubated, and bilateral injections were performed, which revealed the guidewire to be in the true lumen. Multiple predilatations were performed. We then proceeded to stent the LCx to the LMS with 3 drug-eluting using intravenous ultrasonographic guidance with a good angiographic result (Video 2).
After the procedure, the patient was asymptomatic without any hemodynamic compromise or arrhythmia. He was transferred to the coronary care unit for close monitoring. Four hours after the procedure, the patient became diaphoretic and began experiencing sudden-onset severe central chest pain (8/10) with associated nausea and vomiting. After this the patient became hypotensive, with a blood pressure of 70/40 mm Hg.
Medical History
The patient had a significant cardiac history, including previous CABG (left internal mammary artery to left anterior descending [patent], saphenous vein graft to OM1 [occluded], and SVG2 to right coronary artery [occluded]). The patient also had a history of atrial fibrillation and hyperlipidemia. The patient’s preprocedure echocardiogram displayed a preserved ejection fraction with no evidence of valvular heart disease.
Differential Diagnosis
The differential diagnosis included acute in-stent restenosis, coronary artery dissection, or coronary artery perforation (CAP) resulting in a loculated pericardial effusion.
Investigations
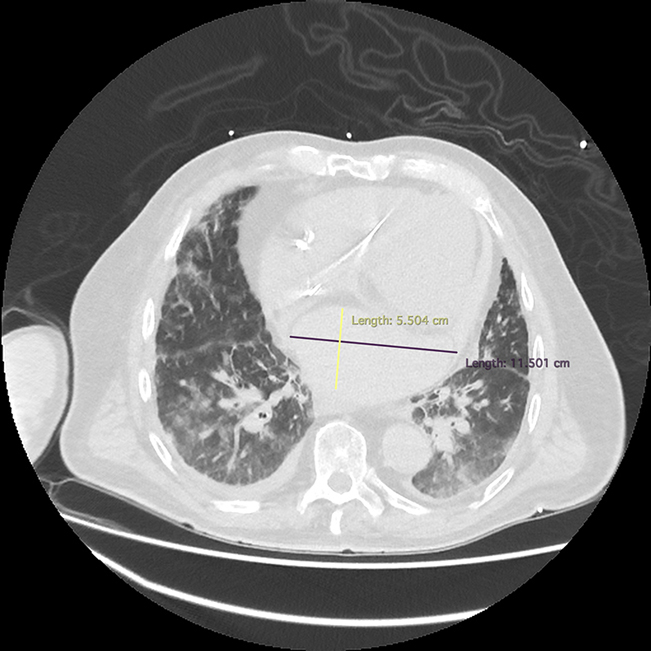
Initial investigations included an electrocardiogram that revealed atrial fibrillation with rapid ventricular rate and no acute ST-segment or T-wave changes. An immediate bedside echocardiogram revealed a hypoechoic likely extracardiac mass resulting in compression of the left atrium. Chest computed tomography revealed an extracardiac atrial hematoma, measuring 11.5 × 13.3 × 5.5 cm, approximately 350 mL in volume, compressing the left atrium, with associated pulmonary edema (Figure 1). Interval imaging of the loculated hematoma 12 hours after the procedure demonstrated stable size but persistent left ventricular systolic dysfunction secondary to the compressive effects of the hematoma (Figure 2).
Figure 1.
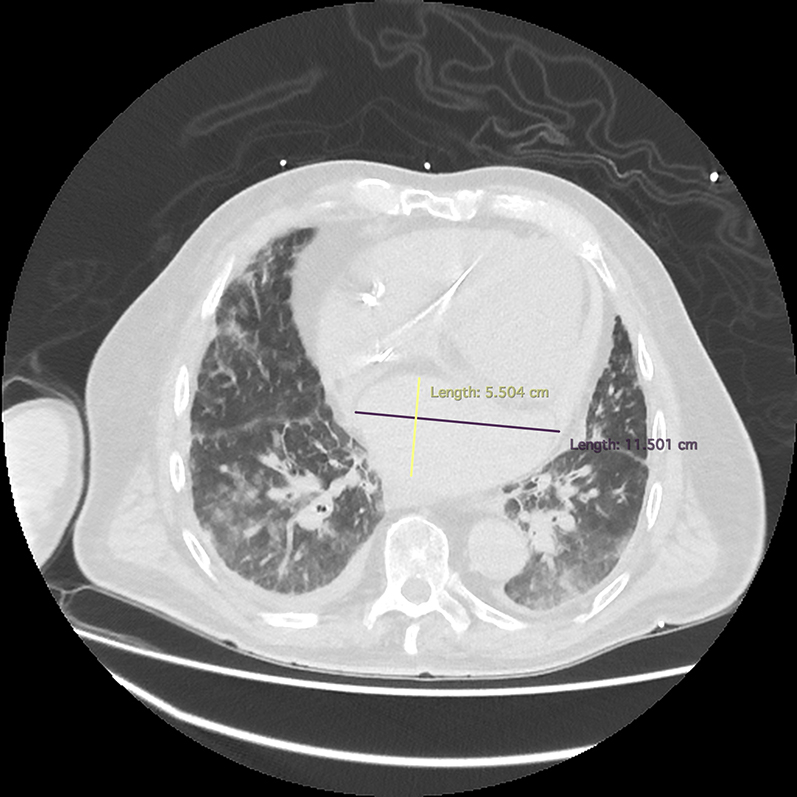
Cardiac CT Showing Left Atrial Hematoma
Chest computed tomography demonstrating an atrial hematoma measuring 11.5 × 13.3 × 5.5 cm, compressing the left atrium with associated extensive pulmonary congestion.
Figure 2.
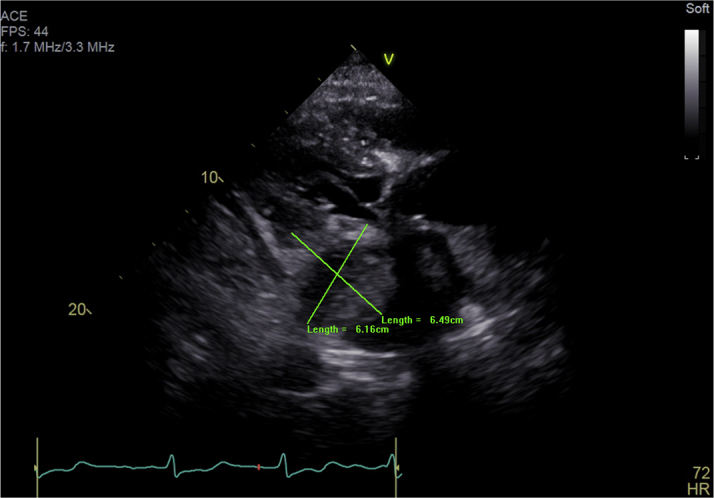
Transthoracic Echocardiogram Showing Left Atrial Hematoma
Transthoracic echocardiogram parasternal long-axis view demonstrating an atrial hematoma compressing the left atrium 12 hours after chronic total occlusion—percutaneous coronary intervention.
Management
After an initial bedside echocardiogram, it was clear that the patient’s hemodynamic instability was secondary to a mass compressing the left atrium. This mass was likely the result of hematoma formation from a previously undetected CAP. Initial fluid resuscitation with balanced crystalloid solution was successful in stabilizing the patients’ blood pressure to 100/60 mm Hg. After a discussion with the cardiothoracic and interventional radiology services, a decision was made to treat the patient conservatively, given the location of the effusion, which was not amenable to percutaneous drainage, as well as the patient’s earlier surgical history and comorbidity.
The patient remained in a stable condition overnight. However, 12 hours later he went into respiratory distress and had an increasing oxygen requirement. A chest x-ray at this point revealed worsening pulmonary edema, and the patient was given gentle intravenous diuresis with 40 mg of furosemide. He responded remarkably well, with an effective diuresis of 2.5 L of fluid over the subsequent 12 hours. His respiratory rate returned to normal, and he no longer required oxygen to maintain an SPO2 of 98%. At this point it was decided to commence a prophylactic dose of 4,500 U of tinzaparin to prevent clot formation in the compressed left atrium.
Subsequent serial echocardiograms displayed marked improvement in the left atrial hematoma and an ejection fraction returning to baseline. Baseline cardiac magnetic resonance was performed, and the patient was successfully discharged home from the hospital 9 days after the procedure (Figure 3).
Figure 3.
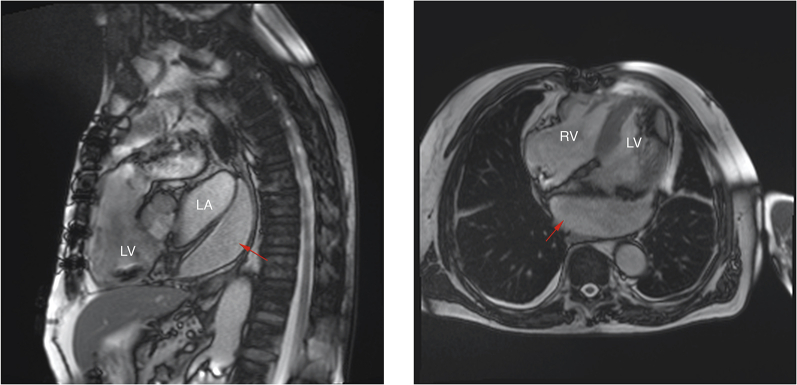
Cardiac Magnetic Resonance Showing Left Atrial Hematoma
Cardiac magnetic resonance demonstrating an extra cardiac hematoma (red arrows) compressing the left atrium. LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
Discussion
CAD and subsequent myocardial infarction / ischemic cardiomyopathy represents the leading cause of death worldwide.1 Increased efforts to tackle this global health burden has culminated in earlier identification and treatment of patients with CAD. Owing to associated advancements in equipment and operator experience, lesions once deemed unsuitable for PCI may now be successfully revascularized.
CTOs represent approximately 25% of CAD cases identified angiographically.2 This figure rises further in post-CABG patients. The angiographic and clinical complexity of these lesions has led to an array of new wire-crossing techniques to improve procedural success rates. A retrograde approach is being increasingly adopted to revascularize complex CTOs. These alternative techniques are having a significant effect on procedural success. The success rates of the retrograde approach in CTO-PCI varies between 75.3% and 85%.2, 3, 4 However, they have also affected the incidence and nature of complications. With the retrograde approach there is an increased risk of intraprocedural; and periprocedural complications such as CAP.5
CAP in CTOs occurs in approximately 4.1% to 5.5% of cases and carries a mortality rate of up to 16%.6,7 Several factors affect the management of CAP. CAPs that occur in patients who have previously undergone CABG represent particularly challenging cases because of preformed pericardial adhesions. Whereas these adhesions have previously been regarded as a protective factor against cardiac tamponade, they can often result in localized compression of cardiac chambers resulting from loculated pericardial effusions. There are several sites at which hematomas may form, including the myocardium, subpericardium, and intrapericardium, in addition to extracardiac manifestations.
There is no definitive standard of care for treating these patients, and each presentation must be taken on a case-by-case basis. This is the first case that we are aware of whereby an extracardiac hematoma compressing the left atrium was successfully managed conservatively. What was particularly interesting in our case was the balance between, and timeline of, fluid resuscitation versus diuresis as well as the need for anticoagulation.
After initial fluid resuscitation to restore and maintain left ventricular inflow volume, our patient became increasingly overloaded. The management of our patient’s worsening pulmonary edema had to be weighed against the need to maintain preload and cardiac output. Despite the large size of the hematoma and its ongoing compressive effects, our patient responded remarkably well to gentle diuresis. The hematoma resulted in functional mitral stenosis, putting our patient at increased risk of clot formation and stroke. After a risk-to-benefit discussion, a decision was made to give prophylactic tinzaparin. Clinically, this appeared to be the correct decision given, the subsequent improvement in hematoma size and stroke prevention.
Follow-up
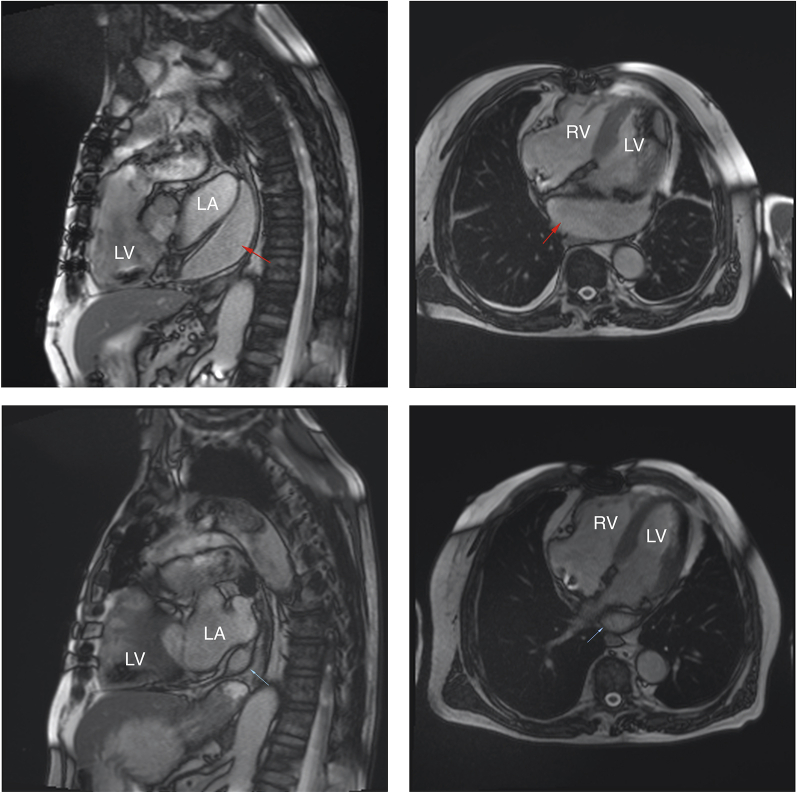
The patient was discharged home 9 days after CTO-PCI, with followup cardiac magnetic resonance 3 months later showing nearly complete resolution of the atrial hematoma, as demonstrated in Figure 4 (Videos 3 and 4).
Figure 4.
Cardiac Magnetic Resonance Imaging at 1 Week and 3 Months Postcoronary Artery Perforation
Magnetic resonance imaging comparing the left atrial hematoma 1 week (red arrows)(top row) versus 3 months (blue arrows)(bottom row) after coronary artery perforation. Abbreviations as in Figure 3.
In conclusion, the medical and/or surgical management of CAP and its resulting complications remains unclear and highly dependent on the unique aspects of each clinical scenario. Through this case we demonstrate that patients presenting atypically after CAP who are poor surgical candidates may be conservatively treated medically with good effect, given close monitoring and serial imaging.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
James L. Januzzi, Jr., MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this article.
Appendix
Coronary Angiography Demonstrating the Occluded Left Main Stem Artery Before CTO PCI
Coronary Angiography Demonstrating Flow in LMS to LCx After CTO-PCI
Cardiac Magnetic Resonance of Left Atrial Hematoma Compressing the Left Atrium During Systole
Cardiac Magnetic Resonance Demonstrating Significant Resolution of Left Atrial Hematoma and Reinflation of Left Atrium 3 Months After CTO-PCI
References
- 1.Khan M., Hashim M., Mustafa H., et al. Global epidemiology of ischemic heart disease: results from the Global Burden of Disease study. Cureus. 2020;12:e9349. doi: 10.7759/cureus.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai T., Stanislawski M., Shunk K., et al. Contemporary incidence, management, and long-term outcomes of percutaneous coronary interventions for chronic coronary artery total occlusions: insights from the VA CART program. J Am Coll Cardiol Intv. 2017;10:866–875. doi: 10.1016/j.jcin.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 3.Galassi A., Sianos G., Werner G., et al. Retrograde recanalization of chronic total occlusions in Europe: procedural, in-hospital, and long-term outcomes from the multicenter ERCTO registry. J Am Coll Cardiol. 2015;65:2388–2400. doi: 10.1016/j.jacc.2015.03.566. [DOI] [PubMed] [Google Scholar]
- 4.Karmpaliotis D., Karastakis A., Alasawad K., et al. Outcomes with the use of the retrograde approach for coronary chronic total occlusion interventions in a contemporary multicenter US registry. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.115.003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel V., Brayton K., Tamayo A., et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. J Am Coll Cardiol Intv. 2013;6:128–136. doi: 10.1016/j.jcin.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Azzalini L., Poletti E., Ayoub M., et al. Coronary artery perforation during chronic total occlusion percutaneous coronary intervention: epidemiology, mechanisms, management, and outcomes. EuroIntervention. 2019;15:e804–e811. doi: 10.4244/EIJ-D-19-00282. [DOI] [PubMed] [Google Scholar]
- 7.Hendry C., Fraser D., Eichhofer J., et al. Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. EuroIntervention. 2012;7:79–86. doi: 10.4244/EIJV8I1A13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronary Angiography Demonstrating the Occluded Left Main Stem Artery Before CTO PCI
Coronary Angiography Demonstrating Flow in LMS to LCx After CTO-PCI
Cardiac Magnetic Resonance of Left Atrial Hematoma Compressing the Left Atrium During Systole
Cardiac Magnetic Resonance Demonstrating Significant Resolution of Left Atrial Hematoma and Reinflation of Left Atrium 3 Months After CTO-PCI