Abstract
Background
The direct oral anticoagulants (DOACs), apixaban and rivaroxaban, have been studied for extended‐phase treatment of venous thromboembolism (VTE). Yet, scant evidence exists surrounding clinician practice and decision‐making regarding dose reduction.
Aims
Report clinician practice and characteristics surrounding dose reduction of DOACs for extended‐phase VTE treatment.
Methods
We conducted a 16‐question REDCap survey between July 14, 2021, and September 13, 2021, among ISTH 2021 Congress attendees and on Twitter. We explored factors associated with dose reduction using logistic regression. We used k‐means clustering to identify distinct groups of dose‐reduction decision‐making. Random forest analysis explored demographics with respect to identified groups.
Results
Among 171 respondents, most were attending academic physicians from North America. Clinicians who treated larger volumes of patients had higher odds of dose reduction. We identified five clusters that showed distinct patterns of behavior regarding dose reduction. Cluster 1 rarely dose reduces and likely prescribes rivaroxaban over apixaban; cluster 2 dose reduces frequently, does not consider age when dose‐reducing, is least likely to temporarily reescalate dosing, and prescribes apixaban and rivaroxaban equally; cluster 3 dose reduces <50% of the time, and temporarily reescalates dosing during increased VTE risk; cluster 4 dose reduces frequently, temporarily reescalates dosing, and is most likely to prescribe apixaban over rivaroxaban; and cluster 5 dose reduces most frequently, and takes the fewest risk factors into consideration when deciding to dose reduce.
Conclusions
Most clinicians elect to dose‐reduce DOACs for extended‐phase anticoagulation. The likelihood of a clinician to dose reduce increases with volume of patients treated. Clinician prescribing patterns cluster around VTE risk factors as well as reescalation during high‐risk periods.
Keywords: anticoagulant, apixaban, reduced‐dose, rivaroxaban, treatment, venous thromboembolism
Essentials.
Extended phase direct oral anticoagulants (DOACs) are used to prevent 2° venous thrombosis (VTE).
Clinicians variably elect reduced‐dose DOACs apixaban & rivaroxaban to prevent 2° VTE.
We surveyed 171 ISTH2021 & Twitter™ respondents about DOAC prescription to prevent 2° VTE.
Most respondents were academic & elect dose‐reduction‐especially if they treat many patients.
1. INTRODUCTION
Patients who experience deep vein thrombosis (DVT) and pulmonary embolism (PE) in the absence of an identifiable, transient, major provoking factor 1 are often advised to continue anticoagulation indefinitely. 2 , 3 , 4 Extended‐phase therapy with anticoagulation is continued for the prevention of recurrent thrombosis after the treatment phase (usually 3–6 months) is completed. Various direct oral anticoagulants (DOACs) have been studied for the prevention of recurrent thrombosis. 5 , 6 , 7 Two DOACs, apixaban and rivaroxaban, have evidence reporting the effectiveness and safety of reduced dose compared with treatment phase dose anticoagulation for extended‐phase therapy. 5 , 7 Meta‐analysis of these studies demonstrated that over 1 year, reduced‐dose DOAC therapy was as effective as treatment‐phase dose therapy to prevent recurrent venous thrombosis (VTE; relative risk 0.85; 95% confidence interval [CI] 0.51–1.42) and had a trend toward less bleeding (relative risk 0.74; 95% CI 0.52–1.05). 8 Subsequently, the US Food and Drug Administration approved the reduced‐dose regimens of apixaban and rivaroxaban for reducing the risk of recurrent DVT and PE following the treatment phase of 6–12 months. 9 , 10 However, concern exists surrounding the dose reduction of DOACs among certain patient populations thought to be at a higher than usual risk for recurrent thrombosis. These include patients with obesity, 11 , 12 cancer, 13 , 14 and antiphospholipid syndrome. 15 , 16 Yet, little is known regarding physician preferences in selecting anticoagulants 17 and how physicians elect to either continue treatment‐ or reduced‐dose anticoagulation for the prevention of recurrent VTE. Therefore, we conducted a study to better understand physician decision‐making regarding DOAC dose reduction for extended‐phase anticoagulation treatment.
2. METHODS
This work was conducted by members the Venous ThromboEmbolism Network U.S. (VENUS) 18 Subcommittee on Venous thromboembolism Treatment and Anticoagulation Management. VENUS is a collaborative working group administered by the Hemostasis and Thrombosis Research Society and sponsored by the Anticoagulation Forum, National Blood Clot Alliance, and American Society of Hematology Foundation. Survey questions were generated during standing VENUS committee meetings and then iteratively refined by the members of the subcommittee via email consensus. The final survey (Appendix S1) included clinician demographic and practice characteristics, as well as clinical situations that were hypothesized based on VTE literature to potentially impact dose‐reduction behaviors.
A targeted population‐specific survey design was selected. A REDCap link was linked to the survey questionnaire. The survey was first deployed as part of the International Society of Thrombosis and Haemostasis (ISTH) 2021 meeting. 19 The ISTH includes more than 5000 clinicians and researchers in more than 100 countries. A link to the survey was embedded in the ISTH2021 Congress daily email newsletter, and posted during the meeting by investigators on the ISTH2021 Twitter feed. Second, an explanatory paragraph accompanied by an embedded link to the survey was disseminated by email to members of VENUS and the Hemostasis and Thrombosis Research Society, 20 a North American professional society dedicated to research, education, investigation, and advancement of work in hemostatic and thrombotic disorders. Last, VENUS members on Twitter created Twitter posts with a pithy explanation of the study and an embedded link to the REDCap survey. The survey was initiated on July 14, 2021, and closed on September 13, 2021. Study review was performed, and a waiver of signed informed consent was granted by the Intermountain Healthcare institutional review board. No financial support existed for the study.
Survey responses were described with descriptive statistics. Logistic regression was performed to assess demographics associated with electing dose reduction. In a sensitivity analysis, probit regression was used to identify demographics associated with frequency of dose reduction (rarely, sometimes, usually).
To understand whether identifiable cohorts of respondents approached dose reduction in a similar fashion, we used an unsupervised machine learning approach using clustering. We searched for the optimal number of clusters (2 ≤ k ≤ 10) with k‐means clustering by minimizing the silhouette score while maintaining sufficient cluster size (n ≥ 20). 21 Leveraging simulations by Dalmaiher et al., 22 we anticipate almost 100% accuracy for detecting the correct number of clusters when recruiting 200 participants. A visual inspection of the first two components of a principal component analysis was used to ensure sufficient distinctness between identified clusters. Dosing behaviors of the clusters were described with descriptive statistics, and frequencies were depicted with a heatmap.
We used a random forest approach to identify the importance of respondent demographics with respect to model accuracy in predicting DOAC dosing‐behavior groups. 23 Hyperparameters were optimized with a grid search and model performance was assessed with out‐of‐bag estimate of error. A randomly selected subset of samples are used to train the decision trees. After the decision trees are trained on selected samples, the samples left out, or out‐of‐bag, are then presented to the decision trees for prediction. The out‐of‐bag‐error rate is then computed as the number of errors that were predicted from the out‐of‐bag sample. All analyses were conducted using R version 4.0.3.
3. RESULTS
Among the 172 individuals that accessed the survey, 171 engaged in the survey and 170 responded to all questions. Most (86.5%) respondents self‐identified as attending physicians, 84.2% reported practicing in an academic setting, 67.8% were from North America, 50.3% had been practicing for fewer than 10 years, 38% spent greater than 80% of their clinical time in an ambulatory setting, and 24.6% prescribed more than 250 DOAC prescriptions annually. See Table 1 for additional demographics.
TABLE 1.
Demographics of survey participants
| Attribute | N (%) |
|---|---|
| Status | |
| Attending physician | 148 (86.5%) |
| Nurse practitioner/physician assistant/mid‐level provider | 10 (5.8%) |
| Trainee (student, resident, fellow) | 13 (7.6%) |
| Specialty | |
| Thrombosis | 75 (43.9%) |
| Other | 96 (56.1%) |
| Setting | |
| Academic | 144 (84.2%) |
| Nonacademic | 27 (15.8%) |
| Percent of clinical time is outpatient care | |
| <50 | 34 (19.9%) |
| 50–79 | 71 (41.5%) |
| ≥80 | 65 (38.0%) |
| Years in practice | |
| ≤10 | 86 (50.3%) |
| 11–25 | 69 (40.4%) |
| >25 | 16 (9.4%) |
| Number of patients where you are involved in DOAC prescriptions | |
| <50 | 16 (9.4%) |
| 51–250 | 51 (29.8%) |
| >250 | 42 (24.6%) |
| Institutional protocol to consider DOAC dose adjustment in place | |
| Yes | 14 (8.2%) |
| No | 93 (54.4%) |
| Don't know | 61 (35.7%) |
| Country | |
| North America | 116 (67.8%) |
| Europe | 31 (18.1%) |
| Other | 24 (14.0%) |
| US region, n = 98 | |
| East | 43 (43.9%) |
| Midwest | 34 (34.7%) |
| West | 21 (21.4%) |
Abbreviation: DOAC, direct oral coagulant.
Among all respondents, 82.5% reported that they dose‐reduce DOACs. Among those who dose reduce, 38% do so rarely (<25% of the time), 31.6% do so sometimes (25%–49% of the time), and 46.2% do so between 50% and 100% of the time. When asked to consider specific patient populations in which they refrain from dose reduction, 76.6% of participants responded that they refrain from dose reduction in patients with cancer, 66.7% in patients with antiphospholipid syndrome (for which vitamin K antagonists are recommended 15 ), 66.1% in patients who had prior VTE while receiving anticoagulation therapy, 64.9% in patients with recurrent VTE, and 53.8% in patients that are obese. When asked to consider the likelihood of dose reduction in certain clinical situations, 84.8% of respondents stated that they would preferentially elect dose reduction in patients with a history of bleeding, 77.2% in patients with a history of distal DVT, 74.9% in patients prescribed concomitant platelet therapy, and 44% in patients with unusual site thrombosis. Sixty‐two percent of respondents reported that they engage in temporary reescalation of DOAC dosing. When asked to consider clinical scenarios in which they elect to temporarily escalate DOAC dosing, 79% stated that they would do so in the setting of a new diagnosis of cancer, 75.8% in patients in the postoperative setting, 48.4% in patients requiring hospitalization, 45.3% in patients who have become bedbound or immobilized, and 21% in patients engaged in long‐haul travel. When asked whether they preferentially prescribe one DOAC over another, 46.8% of respondents preferentially prescribed apixaban, compared with 15.2% who preferentially prescribed rivaroxaban. Eighty‐two percent of respondents stated they dose reduce with each medication equally. Additional dosing behaviors are found in Table 2.
TABLE 2.
Dosing behaviors of survey participants
| Attribute | N (%) |
|---|---|
| Reduce dosage | |
| Yes | 141 (82.5%) |
| No | 30 (17.5%) |
| Frequency | |
| Usually (50%–100%) | 79 (46.2%) |
| Sometimes (25%–49% of the time) | 54 (31.6%) |
| Rarely (0%–24%) | 38 (22.2%) |
| Risk factors to NOT dose reduce | |
| Cancer | 131 (76.6%) |
| Antiphospholipid syndrome (if applicable to your practice) | 114 (66.7%) |
| Prior VTE event on therapy | 113 (66.1%) |
| Recurrent VTE | 111 (64.9%) |
| Obesity (BMI > 30) | 92 (53.8%) |
| Patient preference | 74 (43.3%) |
| Heritable thrombophilia | 60 (35.1%) |
| Estrogen‐based hormone therapy | 55 (32.2%) |
| Gestalt (just don't feel this is the right patient to decrease) | 50 (29.2%) |
| Bedbound or immobility or sedentary | 36 (21.1%) |
| Male | 16 (9.4%) |
| Active smoking | 15 (8.8%) |
| ECOG Performance Status | 13 (7.6%) |
| Age | 12 (7%) |
| Other | 10 (5.8%) |
| Insurance coverage | 6 (3.5%) |
| Diagnosis for reduction | |
| History of bleeding | 145 (84.8%) |
| Distal DVT | 132 (77.2%) |
| Proximal DVT | 131 (76.6%) |
| Concurrent use of antiplatelet therapy | 128 (74.9%) |
| Pulmonary embolism | 123 (71.9%) |
| Unusual site thrombosis (cerebral vein thrombosis, splanchnic vein thrombosis) | 69 (40.4%) |
| Temporary reescalation | |
| No | 106 (62%) |
| Yes | 62 (36.3%) |
| Reason for temporary reescalation (n = 62) | |
| Cancer (if original etiology for VTE was not cancer) | 49 (79%) |
| Postsurgery | 47 (75.8%) |
| Hospitalization | 30 (48.4%) |
| Pregnancy or postpartum | 30 (48.4%) |
| Hormone use | 28 (45.2%) |
| Bedbound or immobility or sedentary | 27 (43.5%) |
| Long travel | 13 (21%) |
| Other | 1 (1.6%) |
| DOAC prescribed | |
| Apixaban | 80 (46.8%) |
| Prescribe apixaban and rivaroxaban equally | 65 (38%) |
| Rivaroxaban | 26 (15.2%) |
| Medication reduced | |
| Both | 140 (81.9%) |
| Apixaban | 12 (7%) |
| Neither | 12 (7%) |
| Rivaroxaban | 7 (4.1%) |
| More comfortable | |
| No | 150 (87.7%) |
| Yes | 21 (12.3%) |
| Which (n = 21) | |
| Apixaban | 17 (81%) |
| Rivaroxaban | 4 (19%) |
| Dosing frequency affects decision | |
| No | 141 (82.5%) |
| Yes | 30 (17.5%) |
Abbreviations: BMI, body mass index; DOAC, direct oral coagulant; DVT, deep vein thrombosis; VTE, venous thromboembolism.
Compared with respondents who treated fewer than 50 patients annually, respondents who treated greater than 250 patients annually had a 6.8‐fold (odds ratio [OR] 6.8; 95% CI 1.36–37.75; p = 0.02) greater odds and those who treated 151–250 patients annually had a 4.0‐fold (OR 4.0; 95% CI 1.01–16.33; p = 0.05) greater odds of dose‐reducing. Respondents who had been in practice greater than 25 years had a lower odds (OR 0.23; 95% CI 0.05–1.17; p = 0.07) of using dose reduction compared with those in practice fewer than 10 years. Other respondent characteristics were not found to have a significant association with dose reduction (Table 3). In the sensitivity analysis of dose‐reduction frequency, those who did not have a protocol had a lower odds (OR 0.23; 95% CI 0.06–0.74; p = 0.02) of dosing frequently, meanwhile those who were unsure if a protocol was in place had an even lower odds (OR 0.05; 95% CI 0.01–0.32; p = 0.002) of dosing frequently (Table 4).
TABLE 3.
Odds ratios for engaging in dose reduction; higher odds ratio indicates more likely to dose reduce
| Parameter | Odds ratio (95% confidence interval) |
|---|---|
| Status: attending physician | |
| Status: nurse practitioner/physician assistant/mid‐level | 0.43 (0.06–3.81) |
| Status: trainee | 0.44 (0.09–2.34) |
| Specialty: thrombosis | |
| Specialty: other | 0.57 (0.18–1.73) |
| Setting: academic | |
| Setting: nonacademic | 0.34 (0.09–1.20) |
| Outpatient time: <50% | |
| Outpatient time: 50%–79% | 0.89 (0.23–3.16) |
| Outpatient time: ≥80% | 3.03 (0.71–13.20) |
| Years in practice: ≤10 | |
| Years in practice: 11–25 | 0.64 (0.21–1.91) |
| Years in practice: >25 | 0.23 (0.05–1.17) |
| Number of patients: <50 | |
| Number of patients: 51–250 | 4.03 (1.01–16.33) |
| Number of patients: >250 | 6.80 (1.36–37.75) |
| International region: North America | |
| International region: Europe | 0.38 (0.04–2.59) |
| International fegion: other | 0.41 (0.04–3.05) |
| US region: East | |
| US region: Midwest | 1.39 (0.34–6.13) |
| US region: West | 1.74 (0.33–11.85) |
| US region: not US | 1.23 (0.19–11.31) |
| Protocol: yes | |
| Protocol: no | 0.00 (0.00–>500) |
| Protocol: don't know | 0.00 (0.00–>500) |
TABLE 4.
Odds ratios for sensitivity analysis for engaging in dose reduction; higher odds ratio indicates more likely to dose reduce
| Parameter | Odds ratio (CI) | p value |
|---|---|---|
| Status: attending physician | ||
| Status: nurse practitioner/physician assistant/mid‐level | 0.558 (0.149–2.120) | 0.38 |
| Status: trainee | 1.045 (0.320–3.463) | 0.94 |
| Specialty: thrombosis | ||
| Specialty: other | 0.549 (0.270–1.100) | 0.09 |
| Setting: academic | ||
| Setting: nonacademic | 1.163 (0.457–3.020) | 0.75 |
| Outpatient time: <50% | ||
| Outpatient time: 50%–79% | 0.878 (0.381–2.000) | 0.76 |
| Outpatient time: ≥80% | 1.043 (0.440–2.452) | 0.92 |
| Years in practice: ≤10 | ||
| Years in practice: 11–25 | 1.423 (0.724–2.818) | 0.31 |
| Years in practice: >25 | 1.334 (0.438–4.324) | 0.62 |
| Number of patients: <50 | ||
| Number of patients: 51–250 | 1.710 (0.597–4.923) | 0.32 |
| Number of patients: >250 | 1.675 (0.545–5.132) | 0.36 |
| International region: North America | ||
| International region: Europe | 0.588 (0.173–1.930) | 0.39 |
| International region: other | 0.411 (0.111–1.459) | 0.17 |
| US region: East | ||
| US region: Midwest | 0.942 (0.391–2.281) | 0.89 |
| US region: West | 1.055 (0.374–3.024) | 0.92 |
| US region: Not US | 0.970 (0.317–3.034) | 0.96 |
| Protocol: yes | ||
| Protocol: no | 0.232 (0.063–0.740) | 0.02 |
| Protocol: don't know | 0.054 (0.008–0.317) | 0.002 |
Upon assessing for clustering of clinicians around dose‐reduction decision‐making, five clusters emerged that showed distinct patterns of behavior (Figures 1 and 2). The clusters were different as follows: cluster 1 rarely dose reduces and is the cluster most likely to prescribe rivaroxaban over apixaban; cluster 2 dose reduces with considerable frequency, does not consider age when dose‐reducing, is least likely to temporarily reescalate dosing during increased VTE risk, and prescribes apixaban and rivaroxaban equally; cluster 3 dose reduces <50% of the time, but temporarily reescalates dosing during increased VTE risk; cluster 4 dose reduces with considerable frequency, temporarily reescalates dosing during increased VTE risk, and is most likely to prescribe apixaban over rivaroxaban; and cluster 5 dose reduces with highest frequency, and takes the fewest risk factors into consideration to not dose reduce (Figure 3).
FIGURE 1.
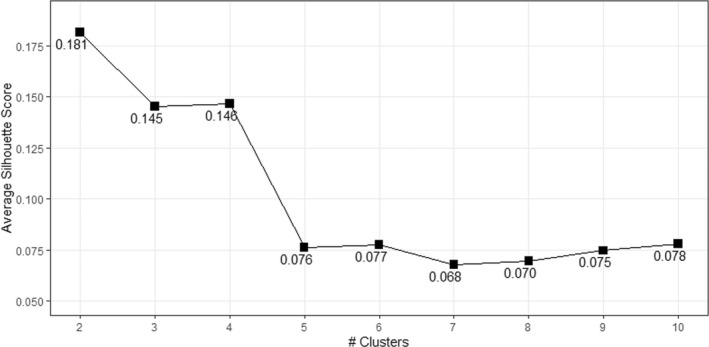
Silhouette scores for two to 10 clusters for direct oral coagulant dosing behaviors, with lower scores indicative of better separation between the clusters
FIGURE 2.
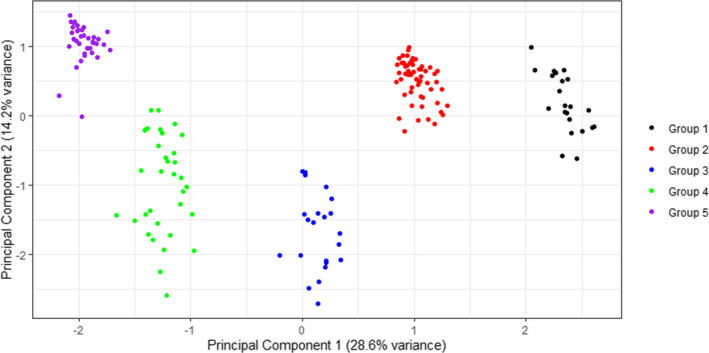
Principal component analysis demonstrates good separation between the five clusters. A summative description of each cluster is found in the Results.
FIGURE 3.
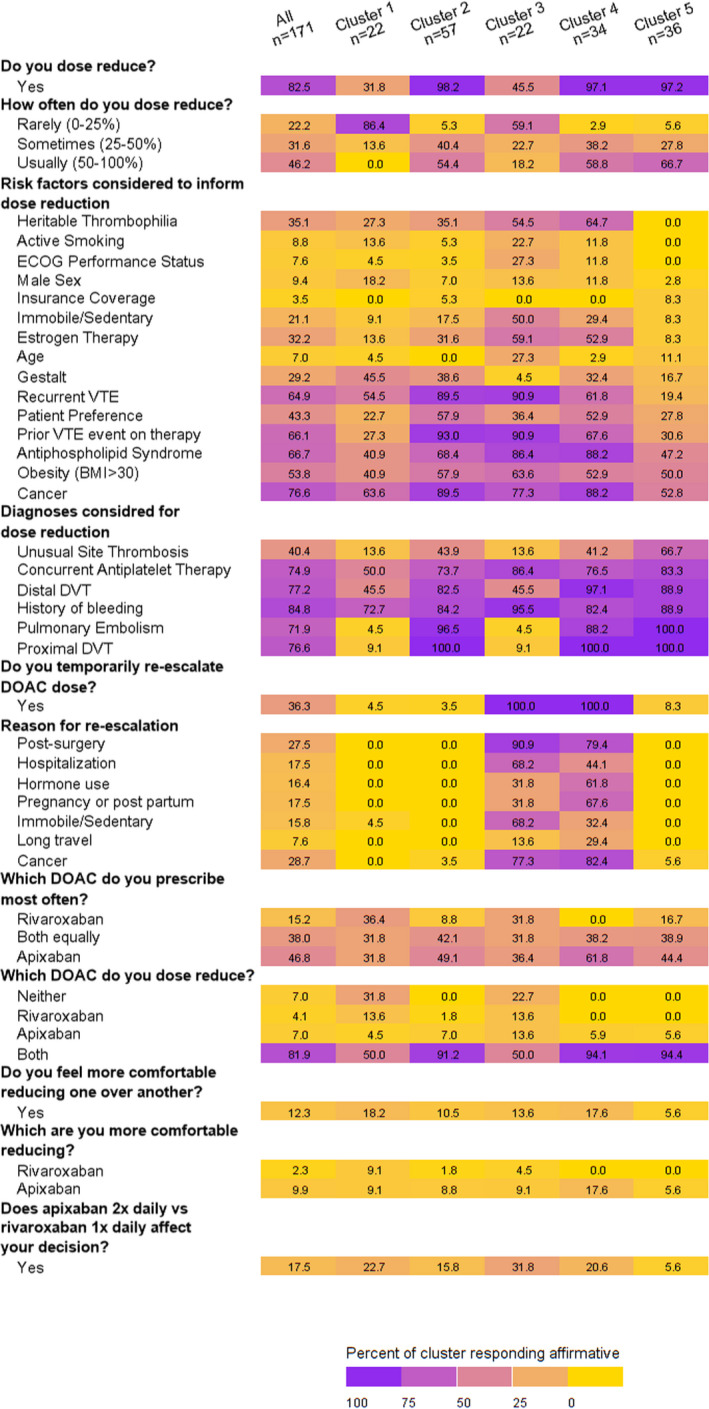
Dosing behaviors of five clusters and entire cohort, represented as percentage of respondents that belong to each group. A summative description each cluster is found in Results paragraph 3
The best performing random forest model had an out‐of‐bag error rate of 67.7%. Considering that there were five clusters, an error rate <80% is better than chance. The error rates, (90.9%, 95.5%, and 88.2%) were considerably high for clusters 1, 3, and 4, respectively. The model performed well when predicting clusters 2 and 5 with error rates 36.8% and 65.7%, respectively. The respondent demographics that were most informative in determining DOAC dosing behaviors based on the random forest analysis were number of patients treated, geographic region, and percentage of patients that were outpatient (Figure 4).
FIGURE 4.
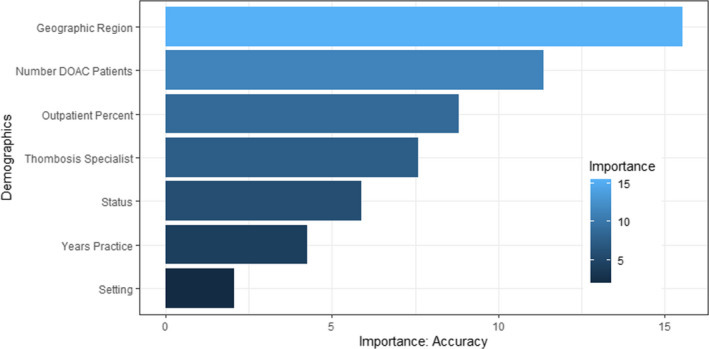
Importance of demographic features in random forest model accuracy to predict DOAC dosing behavior clusters. DOAC, direct oral coagulant
4. DISCUSSION
To our knowledge, this is the first study to report patterns surrounding the decision‐making process involved in dose reduction of rivaroxaban and apixaban for extended‐phase treatment of VTE. Our study demonstrates that most clinicians surveyed elect to dose reduce DOACs when prescribing extended‐phase anticoagulation therapy. Second, those who prescribe DOACs more frequently and those who have practiced for fewer than 10 years are more likely to dose reduce. Interestingly, we observed a clustering of respondents into five groups with specific behaviors surrounding dose reduction. We identified provider characteristics that help explain how these clusters are different from each other with a random forest model. We chose a random forest model for predicting clusters because the results (e.g., model performance, important variables) are easy to interpret. The random forest model was limited in its ability to associate and predict prescriber demographics within the identified clusters, which suggests that dose reduction behaviors are not readily characterized based on demographics alone.
Our study builds on previously reported prospective randomized trials that demonstrate the safety and efficacy of dose‐reduced apixaban and rivaroxaban 5 , 7 by providing important insight into how clinicians are translating these study findings into clinical practice. Understanding the clinician characteristics and practice behavior patterns permits greater insight into clinical practice, provides opportunities to intervene in a targeted fashion to enhance clinician knowledge surrounding evidence for dose reduction, as well as provides insight for futures studies. We assessed salient physician demographics that were characteristic of the clusters; however, the performance of the random forest model was not consistent across clusters, which hinders our ability to make any conclusions.
Strengths of our work include that to our knowledge, this is the first study of its kind to explore clinician practice patterns in the use of dose reduction of anticoagulants. Indeed, we were able to identify clusters of prescribing patterns in an international audience engaged in nonmalignant hematology and anticoagulation therapy. Limitations of our work include that most respondents self‐described as residing in an academic setting, and work from North America, as well as selection bias of those choosing to respond to the survey. Additionally, cluster size was small. These limitations may impact the generalizability of our findings. In future work, we intend to solicit dose‐reduction behaviors among other generalist and specialist physician groups.
In conclusion, we observed that the majority of respondents to the survey elect to dose reduce apixaban and rivaroxaban when prescribing extended‐phase anticoagulation therapy for the prevention of recurrent VTE. Further work is necessary to better understand all potential factors that affect this decision making and to inform future work to enhance optimal application of DOAC dose reduction.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept, design, drafting of manuscript: SCW, DG, KAM, LBK, RPR, KMS, MG, and MEE. Manuscript critical review and refinement: SCW, DG, KAM, LBK, RPR, KMS, MG, and MEE. Statistical analysis: DG, RPR, KMS, KAM, and SCW.
RELATIONSHIP DISCLOSURE
SCW, DG, LBK, KMS, and MG report nothing to disclose. RPR discloses research funding to her institution from Janssen and BMS and serving as a consultant to Janssen, BMS, Dova, Inari, and Penumbra. KAM discloses research funding to her institution from Janssen. MEE discloses Institutional funding from SPARK/Genentec/Roche, Baxalta/Shire/Takeda, and Novo Nordisk.
Supporting information
Appendix S1
Groat D, Martin KA, Rosovsky RP, et al. Physician perceptions and use of reduced‐dose direct oral anticoagulants for extended phase venous thromboembolism treatment. Res Pract Thromb Haemost. 2022;6:e12740. doi: 10.1002/rth2.12740
Handling Editor: Pantep Angchaisuksiri
Contributor Information
Rachel P. Rosovsky, @RosovskyRachel.
Lisa Baumann Kreuziger, @Lbkreuziger.
Scott C. Woller, Email: scott.woller@imail.org, @SCWollerMD.
REFERENCES
- 1. Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14(7):1480‐1483. [DOI] [PubMed] [Google Scholar]
- 2. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and Expert panel report. Chest. 2021;160(6):e545‐e608. [DOI] [PubMed] [Google Scholar]
- 3. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Respir J. 2019;54(3):1901647. [DOI] [PubMed] [Google Scholar]
- 4. Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693‐4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699‐708. [DOI] [PubMed] [Google Scholar]
- 6. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709‐718. [DOI] [PubMed] [Google Scholar]
- 7. Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211‐1222. [DOI] [PubMed] [Google Scholar]
- 8. Vasanthamohan L, Boonyawat K, Chai‐Adisaksopha C, Crowther M. Reduced‐dose direct oral anticoagulants in the extended treatment of venous thromboembolism: a systematic review and meta‐analysis. J Thromb Haemost. 2018;16(7):1288‐1295. [DOI] [PubMed] [Google Scholar]
- 9. ELIQUIS package insert. 2021; https://packageinserts.bms.com/pi/pi_eliquis.pdf Accessed 15 December, 2020.
- 10. XARELTO package insert. 2021; package insert. Available at: http://www.janssenlabels.com/package‐insert/product‐monograph/prescribing‐information/XARELTO‐pi.pdf Accessed 3 October 2019, 2019.
- 11. Martin K, Beyer‐Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moll S, Carona DJ, Martin J. Direct oral anticoagulants in extremely obese patients: ok to use? Res Pract Thromb Haemost. 2019;3(2):152‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484‐3488. [DOI] [PubMed] [Google Scholar]
- 14. Mulder FI, Horvath‐Puho E, van Es N, et al. Venous thromboembolism in cancer patients: a population‐based cohort study. Blood. 2021;137(14):1959‐1969. [DOI] [PubMed] [Google Scholar]
- 15. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: Guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18(9):2126‐2137. [DOI] [PubMed] [Google Scholar]
- 16. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78(10):1296‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moyer GC, Bannow BS, Thornburg C, et al. Venous thromboembolism: a survey of oral anticoagulant preferences in the treatment of challenging patient populations. Clin Appl Thromb Hemost. 2018;24(9_suppl):209S‐216S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. U.S. VTN. 2021; https://www.htrs.org/HTRS/Research/Endorsed‐Studies/VENUS‐VTE‐Network Accessed 09/03/2021, 2021.
- 19. Haemostasis ISoTa. https://www.isth2021.org/ 2021.
- 20. Society HaTR. 2021; https://www.htrs.org/ Accessed 09/03/2021, 2021.
- 21. Hartigan JA, Wong MA. A K‐means clustering algorithm. J Roy Stat Soc: Ser C (Appl Stat). 1979;28(1):100‐108. [Google Scholar]
- 22. Dalmaijer E, Nord C, Astle D. Statistical power for cluster analysis. arXiv. 2020. doi: 10.48550/arXiv.2003.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breiman L. Random forests. Mach Learn. 2001;45:5‐32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


