Abstract
We evaluated predictors of the Clinical Frailty Scale (CFS) scored by an interdisciplinary team (Home FIRsT) performing comprehensive geriatric assessment (CGA) in our Emergency Department (ED). This was a retrospective observational study (service evaluation) utilising ED-based CGA data routinely collected by Home FIRsT between January and October 2020. A linear regression model was computed to establish independent predictors of CFS. This was complemented by a classification and regression tree (CRT) to evaluate the main predictors. There were 799 Home FIRsT episodes, of which 740 were unique patients. The CFS was scored on 658 (89%) (median 4, range 1-8; mean age 81 years, 61% women). Independent predictors of higher CFS were older age (p<0.001), history of dementia (p<0.001), mobility (p≤0.007), disability (p<0.001), and higher acuity of illness (p=0.009). Disability and mobility were the main classifiers in the CRT. Results suggest appropriate CFS scoring informed by functional baseline.
Keywords: Clinical Frailty Scale, Disability, Emergency Department, Geriatric Assessment, Mobility
The Clinical Frailty Scale (CFS) is a frailty identification tool based on clinical judgement that considers geriatric dimensions including multimorbidity, degree of symptom control, mobility, physical activity, help with activities of daily living, dependency, and cognition[1]. The CFS has been used in many care settings across the world and found to be significantly associated with clinical outcomes[2]. When used in busy acute care settings, a concern is that the CFS scoring may be based on subjective ‘eyeballing’ rather than pre-illness functional baseline[3]. In its ‘Fit for Frailty Part 1’ document[4], the British Geriatrics Society warned that it is inappropriate to use the CFS without a formal clinical assessment because the CFS was designed to be used to measure severity of frailty after a comprehensive geriatric assessment (CGA). This point is also emphasised by existing CFS training modules[5,6].
An interdisciplinary team in our hospital conducts CGA in older people presenting to the Emergency Department (ED), from which a CFS score is routinely obtained[7]. Our aim was to conduct a service evaluation as to how the CFS was scored by this team, by investigating CFS predictors among routinely collected CGA variables. This was a retrospective observational study (service evaluation) utilising data routinely collected by Home FIRsT, a Frailty Intervention & Response Team embedded in the ED of St James’s Hospital, Dublin, Ireland, and comprised of an advanced nurse practitioner, clinical specialist physiotherapist, clinical specialist occupational therapist and senior medical social worker, all with specialist training and competencies in the care of the older person and use of geriatric assessment tools. The operational aspects of the Home FIRsT team have been detailed elsewhere[7]. All last attendances to Home FIRsT between 20th of January and 22nd of October 2020. As part of the ED-based CGA, Home FIRsT collected the following:
Pre-attendance status:
Sociodemographics: age; sex; living alone (yes/no); formal home care package (yes/no).
History of dementia and other past medical history (the latter was retrospectively derived as a modified Charlson Comorbidity Index (CCI)[8] that did not include the former); history of falls in the past year (yes/no).
Walking mobility: 1: independent without aid; 2: independent with stick/cane; 3: independent with frame; 4: assistance required; 5: unable to mobilise/wheelchair dependent.
Stairs mobility: 1: independent; 2: independent with aid; 3: assistance of 1; 4: assistance of 2; 5: fully dependent/stairlift.
Mobility for transfers: 1: independent; 2: independent with aid; 3: assistance of 1; 4: assistance of 2; 5: fully dependent/hoist.
Personal Activities of Daily Living (PADLs) (related to self-care such as feeding, dressing, toileting, washing, bathing)[9]: 1: independent; 2: some help required; 3: fully dependent.
Domestic Activities of Daily Living (DADLs) (a subset of instrumental activities of daily living concerned with managing the home situation such as preparing own meals, doing light housework, managing own money, shopping for personal items)[9]: 1: independent; 2: some help required; 3: fully dependent.
Related to attendance:
Delirium scored with the 4AT[10].
Manchester Triage Category (MTC)[11]: from 1 (very urgent) to 5 (non-urgent).
Major Diagnostic Categories (MCD) related to the present complaint[7]: MDC8 (musculoskeletal system and connective tissue); MDC5 (circulatory system); MDC6 (digestive system); MDC4 (respiratory system); MDC18 (systemic infections); MDC9 (skin, subcutaneous tissue and breast); MDC11 (kidney and urinary tract); MDC21 (injuries, poison and toxic effects of drugs); and MDC1 (nervous system). In addition, whether fall was a present complaint (yes/no).
All statistical analyses were carried out using IBM SPSS Statistics for Windows (Version 26.0. Armonk, NY: IBM Corp). Descriptives were given as mean with standard deviation (SD), median with interquartile range (IQR), or count with percentage (%). A stepwise linear regression model was computed to establish independent predictors of CFS. Multicollinearity was defined as a predictor having a Variance Inflation Factor (VIF)≥2. The regression model was repeated on a sample with multiple imputation of missing data. On the original sample, the statistical analysis was complemented by a classification and regression tree (CRT, exhaustive Chi-square automatic interaction detection method) to evaluate the main predictors of CFS. The level of statistical significance was set at p<0.05 throughout.
This study received service evaluation approval by the Tallaght University Hospital/St James’s Hospital Joint Research Ethics Committee (9/9/2020) and St James’s Hospital Research & Innovation Office (Ref: 6499).
During the study period, there were 799 Home FIRsT episodes, of which 740 were unique patients (last attendances). Among the latter, the CFS was scored on 658 patients (89%). The descriptors of the n=658 sample are summarised in Table 1. Table 2 shows the results of the stepwise linear regression model predicting CFS. With listwise deletion of cases with missing data, the model included 545 patients, adjusted R2=0.66. The predictors selected at the final (8th) step were older age (p<0.001), history of dementia (p<0.001), mobility for walking, transfers and stairs (p≤0.007) PADL and DADL (p<0.001), and higher acuity of illness (p=0.009). None of the included predictors had VIF≥2. The results of the model with multiple imputation of missing data (n=658) are in Table 3. The results of the CRT, with all pre-attendance and attendance-related factors in Table 1 entered as predictors, are shown in Figure 1.
Table 1.
Descriptors of the n=658 with Clinical Frailty Scale (CFS) information.
| Pre-attendance factors | Descriptive | Number of observations |
|---|---|---|
| Mean age, years (range; SD) | 81.0 (58-101; 7.0) | 658 |
| Female sex (%) | 61.1 | 658 |
| Living alone (%) | 29.8 | 651 |
| Formal home care package (%) | 6.2 | 658 |
| History of dementia (%) | 7.9 | 618 |
| Median CCI (range; IQR) | 1 (0-7; 3) | 621 |
| History of falls in the past year (%) | 54.0 | 631 |
| Median walking mobility score (range; IQR) | 1 (1-5; 1) | 642 |
| Median stairs mobility score (range; IQR) | 2 (1-5; 3) | 620 |
| Median transfers mobility score (range; IQR) | 1 (1-5; 1) | 628 |
| Median PADL score (range; IQR) | 1 (1-3; 1) | 636 |
| Median DADL score (range; IQR) | 2 (1-3; 2) | 630 |
| Factors related to attendance | ||
| Median 4AT score (range; IQR) | 0 (0-8; 0) | 618 |
| Median MTC score (range; IQR) | 3 (1-5; 0) | 646 |
| MDC8 (%) | 41.6 | 651 |
| MDC5 (%) | 16.1 | 651 |
| MDC6 (%) | 11.4 | 651 |
| MDC4 (%) | 6.6 | 651 |
| MDC18 (%) | 5.5 | 651 |
| MDC9 (%) | 4.6 | 651 |
| MDC11 (%) | 4.6 | 651 |
| MDC21 (%) | 3.4 | 651 |
| MDC1 (%) | 2.8 | 651 |
| Fall as a present complaint (%) | 24.1 | 651 |
| CFS distribution | ||
| Median CFS score (range; IQR) | 4 (1-8; 2) | 658 |
| CFS 1 (very fit) (%) | 0.3 | |
| CFS 2 (fit) (%) | 7.8 | |
| CFS 3 (managing well) (%) | 33.4 | |
| CFS 4 (very mildly frail) (%) | 28.0 | |
| CFS 5 (mildly frail) (%) | 19.5 | |
| CFS 6 (moderately frail) (%) | 8.2 | |
| CFS 7 (severely frail) (%) | 2.7 | |
| CFS 8 (very severely frail) (%) | 0.2 | |
| CFS 9 (terminally ill) (%) | 0.0 | |
SD: standard deviation; CCI: modified Charlson Comorbidity Index; IQR: interquartile range; PADL: personal activities of daily living; DADL: domestic activities of daily living; MTC: Manchester triage category; MDC8: major diagnostic category related to the musculoskeletal system and connective tissue; MDC5: circulatory system; MDC6: digestive system; MDC4: respiratory system; MDC18: systemic infections; MDC9: skin, subcutaneous tissue and breast; MDC11: kidney and urinary tract; MDC21: injuries, poison and toxic effects of drugs; MDC1: nervous system.
Table 2.
Results of the stepwise linear regression model predicting CFS score in n=545 with complete data.
| B | 95% Confidence Interval for B | P | VIF | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Mobility for walking | 0.32 | 0.23 | 0.41 | <0.001 | 1.88 |
| DADL | 0.34 | 0.26 | 0.41 | <0.001 | 1.51 |
| PADL | 0.33 | 0.22 | 0.43 | <0.001 | 1.71 |
| Mobility for transfers | 0.34 | 0.22 | 0.45 | <0.001 | 1.44 |
| Age | 0.02 | 0.01 | 0.03 | <0.001 | 1.12 |
| History of dementia | 0.55 | 0.32 | 0.77 | <0.001 | 1.13 |
| Mobility for stairs | 0.07 | 0.02 | 0.11 | 0.007 | 1.50 |
| MTC score | -0.15 | -0.26 | -0.04 | 0.009 | 1.04 |
Table 3.
Results of the stepwise linear regression model predicting CFS score in n=658 after multiple imputation of missing data (adjusted R2=0.67).
| B | 95% Confidence Interval for B | P | VIF | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Mobility for walking | 0.39 | 0.31 | 0.47 | <0.001 | 1.84 |
| DADL | 0.33 | 0.26 | 0.40 | <0.001 | 1.52 |
| PADL | 0.38 | 0.29 | 0.47 | <0.001 | 1.74 |
| Mobility for transfers | 0.15 | 0.08 | 0.22 | <0.001 | 1.18 |
| Age | 0.02 | 0.01 | 0.02 | <0.001 | 1.14 |
| History of dementia | 0.48 | 0.26 | 0.70 | <0.001 | 1.17 |
| Mobility for stairs | 0.07 | 0.03 | 0.11 | 0.001 | 1.47 |
| MTC score | -0.17 | -0.28 | -0.07 | 0.001 | 1.04 |
B: unstandardised regression coefficient; VIF: variance inflation factor; DADL: domestic activities of daily living; PADL: personal activities of daily living; MTC: Manchester triage category.
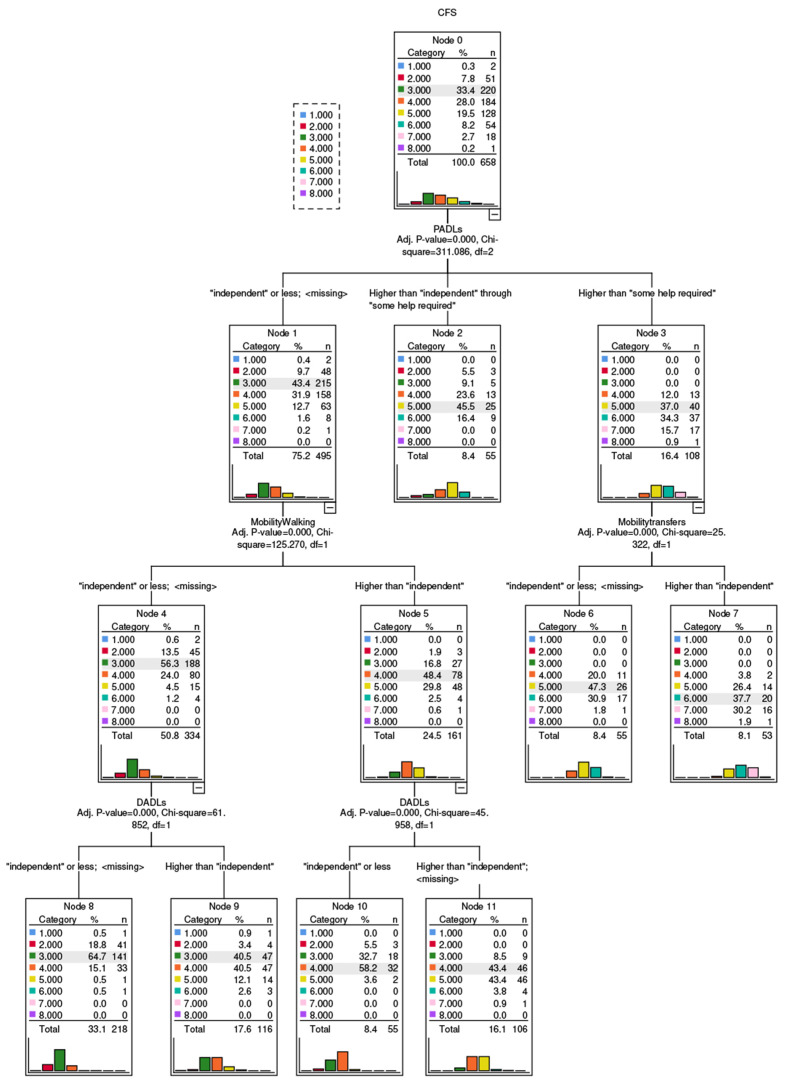
Figure 1.
Results of the classification and regression tree (method: exhaustive Chi-square automatic interaction detection method) to predict Clinical Frailty Scale (CFS) score. In each node, the predicted score is highlighted.
Patients with independent PADLs whose walking mobility and DADLs were independent (node 8, n=218) had a 65% probability of receiving a CFS score of 3. However, when patients had independent PADLs and walking mobility but some dependency in DADLs, they had an equal probability of receiving a CFS score of 3 or 4 (41% in each case, node 9, n=116). When patients had independent PADLs and DADLs but their walking mobility had some dependency, the probability of CFS=4 was 58% (node 10, n=55); however, when PADLs were independent but there was some dependency in walking mobility and DADLs, patients had an equal probability of receiving a CFS score of 4 or 5 (43% in each case, node 11, n=106). For those requiring some help for PADLs but not being fully PADL dependent, the most frequent CFS score was 5 (probability 46%, node 2, n=55). CFS score of 5 was also the most frequent in those fully dependent for PADLs but independent for transfers (probability 47%, node 6, n=55). Patients being fully dependent for PADLs and having some dependence for transfers had a 38% probability of being allocated a CFS of 6, and a 30% probability of receiving a CFS of 7 (node 7, n=53).
We conducted a retrospective evaluation as to how the CFS was scored by an interdisciplinary CGA team in our ED. Results suggest that the collection of CFS was feasible, with data for 89% of cases. The CRT suggested that measures of baseline disability (PADL, DADL) and mobility (walking, transfers) were the most important CFS classifiers, with the expected hierarchical effect[12,13]. In the presence of disability for self-care, CFS scores tended to be higher and further judged according to disability for transfers. On the other hand, when self-care was independent, CFS scores tended to be lower and further judged by walking disability first, and then by ability to manage domestic tasks.
The CRT classification had moderate accuracy, as evidenced by nodes where probabilities of different CFS scores were similar (e.g. equal probability of CFS 3 and 4 in node 9; or CFS 4 and 5 in node 11). Indeed, the multivariate linear regression analyses suggested that there were other predictors able to influence the classification, namely age, acuity of illness, and history of dementia. The latter would be expected as the specific assessment of dementia-related disability is mandated by the CFS (i.e. “the degree of frailty corresponds to the degree of dementia”, seen at the bottom right of the CFS scoring sheet).
Even though the consideration of chronological age is not a CFS scoring feature, our finding that higher age was an independent predictor of higher CFS score is consistent with the literature: of the 31 times the association between CFS score and age was examined, 77% found a significant relationship[2]. In addition, previous studies in the acute care setting have shown a direct association between acute illness severity and CFS score[14,15], which suggests that even with the best CFS training and clinical efforts to obtain a robust collateral history of the pre-illness function, at times high acuity of illness may inflate the clinical frailty judgement in the ED. Indeed, in older people who are not frail at baseline, an acute illness may suddenly reduce mobility and increase dependency, often triggering a ‘crisis’ resulting in ED presentation. A previous Home FIRsT evaluation showed that it was possible to avoid hospital admission in two thirds of ED presentations through the provision of CGA, acute interventions, and rapid follow-up supports[7].
Limitations of this study include its single centre, retrospective observational design, and some missing data in predictor variables, which reflect the real-world nature of the study. Findings are not generalisable.
In conclusion, pre-illness disability and mobility were the most important geriatric dimensions considered by Home FIRsT for CFS scoring in the ED. This suggests appropriate CFS scoring principally informed by functional baseline. Robust CFS scoring education should be implemented when the CFS is used in acute care settings.
Funding
Roman Romero-Ortuno is funded by a Grant from Science Foundation Ireland under Grant number 18/FRL/6188. The funder had no role in the conduct of the research and/or preparation of the article; in study design; in the collection, analysis and interpretation of data; in writing of the report; or in the decision to submit the paper for publication.
Footnotes
Edited by: Yannis Dionyssiotis
References
- 1.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Church S, Rogers E, Rockwood K, Theou O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20(1):393. doi: 10.1186/s12877-020-01801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Caoimh R, Kennelly S, Ahern E, O'Keeffe S, Ortuno RR. Letter to the Editor:Covid-19 and the Challenges of Frailty Screening in Older Adults. J Frailty Aging. 2020;9(3):185–6. doi: 10.14283/jfa.2020.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BGS. Fit for Frailty Part 1:Consensus best practice guidance for the care of older people living in community and outpatient settings. 2018. [accessed 13 June 2021]. Available online: https://www.bgs.org.uk/sites/default/files/content/resources/files/2018-05-23/fff_full.pdf .
- 5.AIMS. Clinical Frailty Scale (CFS) Training Module. 2019. [accessed 13 June 2021]. Available online: https://rise.articulate.com/share/deb4rT02lvONbq4AfcMNRUudcd 6QMts3#/
- 6.CUH-NCHD. Clinical Frailty Scale Education Tool (April 2020) 2020. [accessed 13 June 2021]. Available online: https://ucc.cloud.panopto.eu/Panopto/Pages/Embed.aspx?id=ce9a4d94-fc1c-44ca-8a39-ab9600f624f3 .
- 7.O'Shaughnessy I, Romero-Ortuno R, Edge L, Dillon A, Flynn S, Briggs R, et al. Home FIRsT:interdisciplinary geriatric assessment and disposition outcomes in the Emergency Department. Eur J Intern Med. 2021;85:50–5. doi: 10.1016/j.ejim.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies:development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Legg LA, Drummond AE, Langhorne P. Occupational therapy for patients with problems in activities of daily living after stroke. Cochrane Database Syst Rev. 2006;(4):CD003585. doi: 10.1002/14651858.CD003585.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellelli G, Morandi A, Davis DH, Mazzola P, Turco R, Gentile S, et al. Validation of the 4AT, a new instrument for rapid delirium screening:a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496–502. doi: 10.1093/ageing/afu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke MW, Jinks S. Does the Manchester triage system detect the critically ill? J Accid Emerg Med. 1999;16(3):179–81. doi: 10.1136/emj.16.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunlop DD, Hughes SL, Manheim LM. Disability in activities of daily living:patterns of change and a hierarchy of disability. Am J Public Health. 1997;87(3):378–83. doi: 10.2105/ajph.87.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingston A, Collerton J, Davies K, Bond J, Robinson L, Jagger C. Losing the ability in activities of daily living in the oldest old:a hierarchic disability scale from the Newcastle 85+study. PLoS One. 2012;7(2):e31665. doi: 10.1371/journal.pone.0031665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subbe CP, Burford C, Le Jeune I, Masterton-Smith C, Ward D. Relationship between input and output in acute medicine - secondary analysis of the Society for Acute Medicine's benchmarking audit 2013 (SAMBA '13) Clin Med (Lond) 2015;15(1):15–9. doi: 10.7861/clinmedicine.15-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Ortuno R, Wallis S, Biram R, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults:An observational study. Eur J Intern Med. 2016;35:24–34. doi: 10.1016/j.ejim.2016.08.033. [DOI] [PubMed] [Google Scholar]