Abstract
Background
The aim of this case-control study was to investigate the severity of the cerebellar cognitive affective syndrome (CCAS) in patients treated for pediatric posterior fossa tumors (PFT) and evaluate its diagnostic validity and predictive value for long-term effects.
Methods
Using neuropsychological test data from 56 patients with PFT (average age: 14 years), the severity of deficits in the CCAS core areas (executive functions, verbal functions, visuospatial abilities and emotions/behaviour) was examined. Neuropsychological and academic long-term outcomes of patients with CCAS were compared to two control groups of PFT patients (treated with either surgery or surgery followed by radio-/chemotherapy) without the syndrome. Risk factors associated with various deficits were considered.
Results
All but one PFT patient suffered from slight to severe impairments in at least one CCAS domain, while complete CCAS occurred in 35.7%. Seven years after tumor diagnosis CCAS patients performed worse in information processing, logical reasoning, verbal functions, visuospatial skills, and executive functioning and required more special educational support compared to the control groups. CCAS patients performed equally poor as patients treated with chemo-/radiotherapy in tasks measuring information processing speed. Risk factors were significantly associated with deficits in information processing speed but not CCAS emergence.
Conclusions
Deficits in the core CCAS domains are commonly found in PFT patients, but varying in severity, which suggests the syndrome to be continuous rather than dichotomous. However, the validity of CCAS diagnosis was low and unspecific. The exclusion of relevant functions typically impaired in PFT patients (eg, information processing) resulted in difficulties being overlooked.
Keywords: CCAS, cerebellar tumor, neuro-oncology, pediatric, rehabilitation
Key Points.
CCAS is continuous rather than dichotomous.
It excludes relevant functions typically impaired in PFT patients (eg, information processing), resulting in difficulties being overlooked.
CCAS is not an appropriate concept for pediatric patients with PFT.
Importance of the Study.
An increasing amount of research has been focusing on posterior fossa tumors (PFT) and their cognitive long-term effect. Although cerebellar cognitive affective syndrome (CCAS) is considered the best-known attempt to encapsulate posterior fossa repercussions, the scientific community disagrees about its validity. Recent research has confirmed one or more CCAS symptoms in children and adolescents with PFT. However, no standardized diagnostic criteria have been established and it is still unclear whether deficits in only one domain are sufficient for CCAS diagnosis. To establish more consistency among studies, the present article critically discusses the concept and applicability of CCAS in a large homogenous sample of pediatric PFT patients. By including special educational needs as an additional external criterion at the behavioral level, the diagnostic validity of CCAS should be evaluated.
Posterior fossa tumors (PFT) are the most common solid neoplasms in childhood.1 While improved treatment options have led to a considerable decrease in mortality rates, long-term survival has been associated with vast neuropsychological deficits,2–6 leading to negative impacts on everyday life, such as academic career, satisfactory social bonds, or even the ability to live independently.2,7 Although etiological studies on neuropsychological late sequelae have focused on risk factors, such as treatment type, hydrocephalus, young age at diagnosis, or tumor characteristics,3–5,7,8 the exact mechanisms underlying neuropsychological impairments remain unclear.
For decades, the cerebellum was predominantly associated with motor skills. The first to describe the involvement of the cerebellum in cognition and emotion were Schmahmann and Sherman in 1998.9 They found that adult patients with cerebellar lesions suffered from impairments in executive functioning, language, and visuospatial skills in addition to emotional/behavioral difficulties and introduced the term “Cerebellar Cognitive Affective Syndrome” (CCAS).9 In 2000, CCAS existence was also affirmed in children with cerebellar tumors.10 To date, the term CCAS has been used interchangeably with different symptom clusters, including the posterior fossa syndrome (PFS), cerebellar mutism (CM), and cerebellar mutism syndrome (CMS).11 Despite multiple similarities and overlaps between these syndromes, there are also differences in the symptoms which hint toward distinct pathophysiological mechanisms.12 The inconsistencies in cluster definitions have led to heterogeneity and problems with comparability in research, which emphasizes the need for a uniform nomenclature.11,13–15
In addition to discrepancies in syndrome definitions, diagnostic criteria also vary across studies sparking discordance among the scientific community, regarding the significance as well as the general existence of CCAS. Although many studies have recorded one or more CCAS symptom(s) in patients with posterior fossa lesions,3,16 it is argued that the cognitive dysfunction after infratentorial lesions is significantly less pronounced compared to impairment after cerebral cortical lesions.17 Furthermore, CCAS diagnosis varies across studies. For instance, it is still unclear whether children must present more than one abnormality in the main CCAS features for it to be diagnosed.5,10 If only one domain must be impaired, this might lead to an overdiagnosis in PFT patients. Moreover, many pediatric patients with PFT experience poor information processing speed, which secondarily can lead to decreased performance in other CCAS-related tasks, although these are then not primary signs.18 Lastly, most studies are conducted shortly after tumor treatment, while long-term studies on CCAS are missing.4,5,17
To date, etiological research on CCAS emergence is scarce, mainly focusing on pathobiological variables.5,19 The few studies on risk factors in patients with pediatric PFT mostly investigate individual neuropsychological domains like executive functioning or memory, not considering predefined symptom clusters7,20 or using CCAS synonymously with CM, CMS, and PFS.11 The few existing studies have suggested that lesion location and disruptions of the cerebrocerebellar circuits might be significant predictors for CCAS,5,13,19 with patients with lesions in the cerebellar posterior lobe and the vermis being at an especially high risk for CCAS.5
To improve the validity of diagnosis, external criteria associated with neuropsychological difficulties such as special educational needs (SEN) must be considered. Hence, recent research calls for studies with large homogenous CCAS samples to increase diagnostic validity and consistency among studies,5 and to be able to provide adequate treatment including appropriate rehabilitative support.
To help improve the diagnostic validity of CCAS for clinical practice, we conducted a case-control study in a homogenous sample of patients with pediatric PFT. We determined the severity of deficits in the core CCAS features as well as in other neuropsychological areas. The need of educational support was considered as an external criterion to evaluate the syndrome’s impact on a behavioral level, seeking better rehabilitative care for patients at follow-up. We also aimed to assess risk factors for CCAS development.
Methods
The present investigation was part of a larger study protocol approved by the local ethics committee of the Medical University of Vienna according to the Declaration of Helsinki (EC: 1678/2018).
Patient Population
Patients diagnosed and treated consecutively at the Medical University of Vienna for a pediatric PFT between 2000 and 2015 were included in this study. Only those patients with histologically confirmed medulloblastomas, cerebellar pilocytic astrocytomas (WHO grade I), and posterior fossa ependymomas, who had already completed at least one comprehensive post-treatment neuropsychological examination, were considered (n = 87). Exclusion criteria were age at tumor diagnosis >18 years and being deceased (n = 8) or inaccessible due to psychological, social, geographical, or family-related reasons (n = 15). The remaining 64 patients were invited to a second neuropsychological investigation. Of those, 13% (n = 8) refused to participate in the study, and/or the retrospective data of the first neuropsychological examination was partly incomplete. Hence, 56 patients were included in the analyses. Socioeconomic and medical data were obtained from the clinical charts.
Procedure
Patients underwent comprehensive neuropsychological investigations on a regular basis, including routinely monitoring years after tumor treatment.21 This study analyzed the available neuropsychological data for slight and severe impairments of 56 patients treated for pediatric PFT in the core CCAS features verbal functions, executive functioning, visuospatial skills, and behavior/affect. If patients suffered from at least slight impairments (−1 standard deviation [SD] below standard norm) in all four domains at follow-up, they were assigned to the CCAS group (n = 20; 35.7%). The remaining patients were categorized into group A (children with tumor resection only; n = 18; 32.1%) and group B (tumor resection and radio-/chemotherapy; n = 18; 32.1%). Subsequently, the latest neuropsychological data of the three samples (CCAS, group A, group B) were contrasted against each other. Furthermore, patients with and without CCAS were compared regarding risk factors and the academic outcome.
Measures
All participants underwent at least two comprehensive standard-of-care neuropsychological investigations following distinct psychosocial oncology guidelines (Table 1).21 Parents completed behavioral ratings. All tests were applied in German language and compared to the age-appropriate healthy norm populations from the test manuals. Following the CCAS scale for adults,22 CCAS was diagnosed when patients scored 1 SD below the norm in all domains listed in Table 1 at follow-up.
Table 1.
Measures
| Investigated Neuropsychological Domains | Test Measures |
|---|---|
| CCAS | |
| Visuospatial skills | • Rey Complex Figure Test23 or • Block Design (Wechsler Scales)24–27 or • Analyze/Synthesize (Adaptive Intelligence Test 2.2)28 or • Triangles (Kaufman Assessment Battery for Children)29 |
| Verbal functions | • Similarities (Wechsler Scales)24–27 or • Vocabulary (Wechsler Scales)24–27 or • Finding Synonyms (Adaptive Intelligence Test 2.2)28 or • Abstraction of functions (Adaptive Intelligence Test 2.2)28 or • Vocabulary Test (Kaufman Assessment Battery for Children)29 |
| Executive functioning | • Digit Span (Wechsler Scales)24–27 • Repeating Letters Forwards divided by Repeating Letters Backwards (Adaptive Intelligence Test 2.2)28 • Trail Making Test (TMT-B)30 • Perservative Errors/Errors Total/Concept Completion (Wisconsin Card Sorting Test31; and its computer version32) |
| Affect/ behavior | • Strengths and Difficulties Questionnaire (SDQ)33 or • Quality of Life (revised questionnaire to assess Health-Related Quality of Life in children and adolescents)34,35 or • Behavioral abnormalities observed by the investigating neuropsychologists |
| Group comparisons | |
| Verbal memory | • Total Learning Efficiency in Verbal Learning and Memory Test (VLMT)36 |
| Logical reasoning | • Matrix Reasoning (Wechsler Scales)24–27 |
| Information processing speed | • Coding (Wechsler Scales)24–27 |
| Visuospatial skills | • Block Design (Wechsler Scales)24–27 or • Analyze/Synthesize (Adaptive Intelligence Test 2.2)28 or • Triangles (Kaufman Assessment Battery for Children)29 |
| Verbal functions | • Similarities (Wechsler Scales)24–27 or • Finding Synonyms (Adaptive Intelligence Test 2.2)28 • Vocabulary (Wechsler Scales)24–27 or • Abstraction of Functions (Adaptive Intelligence Test 2.2)28 or • Vocabulary (Kaufman Assessment Battery for Children)29 |
| Executive functioning | • Digit Span (Wechsler Scales)24–27 or • Repeating Letters Forwards divided by Repeating Letters Backwards (Adaptive Intelligence Test 2.2)28 |
Abbreviation: CCAS, cerebellar cognitive affective syndrome.
SEN as an external criterion was defined by special curriculums, support classes, or class repeats.
Statistics
For the comparison of different test measures, the values were converted into percentile ranks (PR). An alpha level of .05 was used.
First, the neuropsychological data of the four CCAS domains were categorized into four groups: severe impairments (more than 2 SDs below the norm, PR ≤2), slight impairments (more than 1 SD below the norm, PR = 3-15), at risk for impairments (more than half a SD below the norm, PR = 16-24) and no impairments (within or above the norm, PR ≥25). Secondly, the latest and most complete neuropsychological data (Table 1) of the three investigated groups (CCAS, group A, group B) were compared using analysis of variance (ANOVA), and effect size r was calculated. Separate analyses for CCAS patients treated with surgery and CCAS patients treated with chemo-/radiotherapy can be retrieved from the Supplementary material (Figure A, Table A). The medical risk factors were categorized as follows: hydrocephalus (yes/no), treatment type (surgery/surgery and chemo-/radiotherapy), and tumor relapse (yes/no). Additionally, gender (male/female) was considered as an individual risk factor in these analyses. Chi-square tests were conducted. Time since diagnosis and age at diagnosis were analyzed using Mann-Whitney U tests and effect size r. Post hoc analyses were performed to investigate the association between deficits in information processing speed and the above-mentioned risk factors (Mann-Whitney U tests and Spearman rank correlations).
Results
Study Population
Table 2 lists the patient characteristics of all 56 participants. The three study cohorts were well balanced.
Table 2.
Patient Characteristics (n = 56)
| Total Sample | Group A | Group B | CCAS | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Gender | ||||||||
| Male | 31 | 55.4 | 10 | 55.6 | 11 | 61.1 | 10 | 50.0 |
| Female | 25 | 44.6 | 8 | 44.4 | 7 | 38.9 | 10 | 50.0 |
| Histology | ||||||||
| Medulloblastoma | 19 | 33.9 | 0 | 0 | 10 | 55.6 | 9 | 45.0 |
| Pilocytic astrocytoma | 28 | 50.0 | 18 | 100.0 | 1 | 5.6 | 9 | 45.0 |
| Posterior fossa ependymoma | 9 | 16.1 | 0 | 0 | 7 | 38.9 | 2 | 10.0 |
| Treatment | ||||||||
| Resection | 26 | 46.4 | 18 | 100.0 | 0 | 0 | 8 | 40.0 |
| + Chemotherapy | 4 | 7.1 | 0 | 0 | 0 | 0 | 4 | 20.0 |
| + Radiotherapy | 1 | 1.8 | 0 | 0 | 1 | 5.6 | 0 | 0 |
| + Chemo-/radiotherapy | 25 | 44.6 | 0 | 0 | 17 | 94.4 | 8 | 40.0 |
| Hydrocephalus | ||||||||
| Yes | 38 | 67.9 | 9 | 50.0 | 15 | 83.3 | 14 | 70.0 |
| No | 18 | 32.1 | 9 | 50.0 | 3 | 16.7 | 6 | 30.0 |
| CM | ||||||||
| Yes | 6 | 10.7 | 1 | 5.6 | 3 | 16.7 | 2 | 10.0 |
| No | 50 | 89.3 | 17 | 94.4 | 15 | 83.3 | 18 | 90.0 |
| Tumor relapse | ||||||||
| Yes | 10 | 17.9 | 0 | 0 | 5 | 27.8 | 5 | 25.0 |
| No | 46 | 82.1 | 18 | 100.0 | 13 | 72.2 | 15 | 75.0 |
| Total Sample | Group A | Group B | CCAS | |||||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| Age ata | ||||||||
| Tumor diagnosis | 7.23 (4.48) | 1-17 | 7.50 (3.90) | 2-15 | 8.06 (5.13) | 1-17 | 6.25 (4.32) | 1-16 |
| Neuropsychological examination 1 | 9.93 (4.40) | 4-21 | 9.72 (4.87) | 4-21 | 10.67 (4.79) | 4-20 | 9.45 (3.68) | 4-17 |
| Neuropsychological examination 2 | 14.71 (4.86) | 5-29 | 14.33 (5.22) | 7-27 | 14.67 (5.65) | 5-29 | 15.10 (3.91) | 7-23 |
| Time betweena | ||||||||
| Tumor diagnosis and neurocognitive test 1 | 2.52 (2.59) | 0-13 | 2.17 (3.29) | 0-13 | 2.33 (2.11) | 0-7 | 3.00 (2.29) | 0-8 |
| Tumor diagnosis and neurocognitive test 2 | 7.20 (4.30) | 1-19 | 6.72 (4.86) | 1-19 | 6.17 (3.45) | 1-13 | 8.55 (4.31) | 1-16 |
Abbreviations: CCAS, patients with cerebellar cognitive affective syndrome (n = 20); CM, cerebellar mutism; group A, patients with tumor resection only (n = 18); group B, patients with tumor resection and subsequent chemo-/radiotherapy (n = 18); SD, standard deviation.
aAge and time data are given in years.
Some patients (26.8%, n = 15) did not carry out all test measures. Missing data occurred in Coding (n = 2), Verbal Memory (n = 4), Matrix Reasoning (n = 1), Block Design (n = 3), Vocabulary (n = 2), Similarities (n = 2), and Digit Span (n = 2). Patients with and without missing data were compared (t tests). No significant differences were observed.
Incidence and Symptom Severity
Slight impairment (1 standard deviation below the norm).
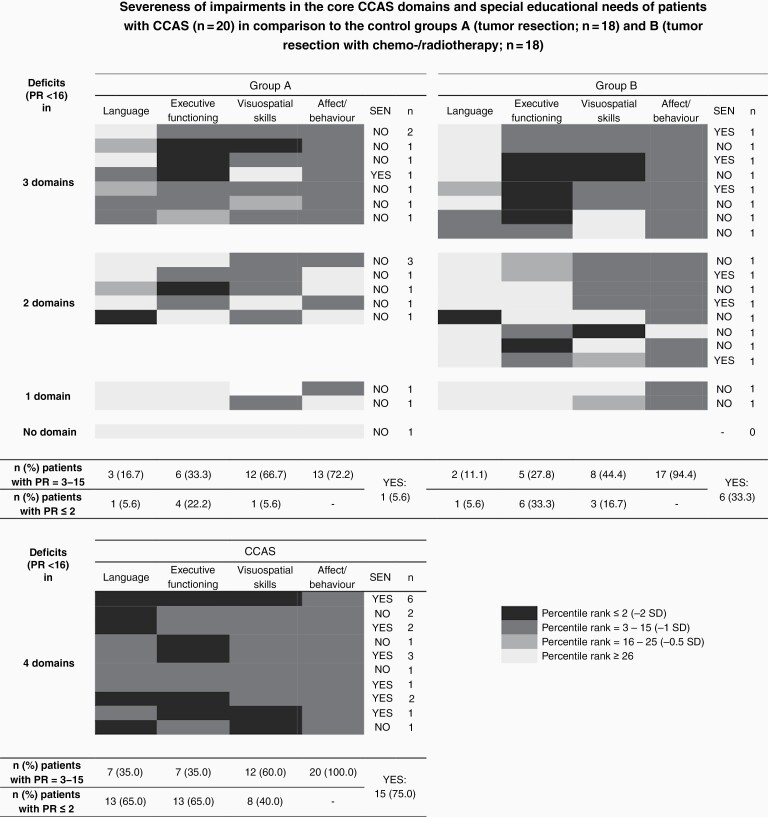
—As indicated in Figure 1, all patients with CCAS scored at least 1 SD below the norm in each of the four domains: visuospatial skills (60.0%), executive functioning (35.0%), language (35.0%), and affect/behavior (100.0%). In comparison, almost half the patients in the control groups (group A and B: 44.4%) showed slight impairments in at least three CCAS features. Deficits in two domains were indicated in a similar number of patients (group A: 38.9%; group B: 44.4%). The rest scored at least 1 SD below the norm in one domain. Only one group A patient showed no impairments at all.
Figure 1.
Demonstration of the number of patients impaired in CCAS features and their special educational needs as an external criterion. There are four categories of (non-)impairments, indicated with the colors black (−2 SD), dark gray (−1 SD), medium gray (−0.5 SD), and light gray. CCAS = patients scoring at least 1 SD below the norm in all four domains of the cerebellar cognitive affective syndrome (n = 20); group A = patients without CCAS, treated with tumor resection only (n = 18); group B = patients without CCAS, treated with tumor resection and subsequent chemo-/radiotherapy (n = 18). Abbreviations: CCAS, cerebellar cognitive affective syndrome; PR, percentile rank; SD, standard deviation; SEN, special educational needs.
In group A, 66.7% of patients had visuospatial difficulties and one-third (33.3%) were associated with executive dysfunction. In comparison, fewer group B patients showed impairments in visuospatial skills (44.4%), while executive dysfunction was equally common (27.8%). Emotional and behavioral alterations were indicated in 72.2% (group A) and 94.4% (group B) of cases. Verbal functions were only impaired in 16.7% (group A) and 11.1% (group B) of patients, respectively.
Severe impairments (2 standard deviations below the norm).
—Almost all patients (90.0%) suffering from CCAS scored more than 2 SDs below the norm in at least one domain. In contrast, this was only true for 27.8% of patients in group A and 44.4% of patients in group B. Similarly, 5.6% (group A), 11.1% (group B), and 50.0% (CCAS) of patients had severe impairments (−2 SD) in more than one domain.
Within the CCAS group, severe impairments most commonly affected verbal functions or executive functioning (65.0% of patients). Only 40.0% revealed such deficits in visuospatial skills. Executive dysfunction (−2 SD) was observed in 22.2% (group A) or 33.3% (group B) of patients in the control groups. In terms of verbal functions, the control groups rarely scored (5.6%) 2 SDs below the norm. More patients in group B showed visuospatial deficits (16.7%) compared to group A (5.6%).
Neurocognitive Long-term Outcome
Significant effects of CCAS on information processing speed (F(2,51) = 9.56, P < .001, r = .52), logical reasoning (F(2,43) = 3.50, P = .039, r = .37), visuospatial skills (F(2,50) = 10.65, P < .001, r = .55), verbal functions (similarities: F(2,51) = 22.24, P < .001, r = .68; vocabulary: F(2,51) = 16.74, P < .001, r = .62) and executive functioning (F(2,51) = 5.15, P = .009, r = .41) were observed. There was no significant effect on verbal memory.
Planned contrasts revealed that patients with CCAS are at an increased risk for impairments in the following domains: information processing speed (t(51) = 2.19, P = .033), logical reasoning (t(43) = 2.57, P = .014), visuospatial skills (t(49) = 5.11, P < .001), verbal functions (similarities: t(51) = 6.35, P < .001; vocabulary: t(51) = 5.75, P < .001) and executive functioning (t(51) = 2.72, P = .009).
No significant differences between treatment groups were observed in terms of logical reasoning, visuospatial skills, verbal functions (vocabulary), and executive functioning. However, group B performed significantly worse in tasks measuring information processing speed (t(51) = −3.82, P < .001) and verbal logical reasoning (t(51) = −2.03, P = .048). For more details, see Table 3.
Table 3.
Mean, Standard Deviations, and Group Comparisons (ANOVA) for the Main Study Variables (P, Effect Sizes)
| Variables | CCAS | Group A | Group B | Group Comparisons |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | P (r) | |
| Executive functioning | 26.89 (19.90) | 52.08 (28.56) | 38.74 (22.38) | .009* (.41) |
| Verbal functions | ||||
| Similarities | 24.05 (19.71) | 68.32 (21.06) | 53.94 (21.51) | .001** (.68) |
| Vocabulary | 18.00 (18.22) | 54.03 (23.16) | 50.26 (20.96) | .001** (.62) |
| Visuospatial skills | 14.94 (16.95) | 42.59 (23.90) | 47.19 (27.20) | .001** (.55) |
| Logical reasoning | 29.65 (23.41) | 49.92 (27.38) | 53.50 (32.15) | .039* (.37) |
| Information processing speed | 23.74 (19.73) | 55.61 (27.12) | 23.19 (27.99) | .001** (.52) |
| Verbal memory | 33.50 (29.98) | 53.59 (30.51) | 37.04 (32.41) | .139 (.28) |
Abbreviations: ANOVA, analysis of variance; CCAS, patients with cerebellar cognitive affective syndrome (n = 20); group A, patients without CCAS and tumor resection only (n = 18); group B, patients without CCAS, tumor resection and chemo-/radiotherapy (n = 18); r, effect size; SD, standard deviation.
Unless otherwise specified variables are metric and given in percentile ranks.
*P < .05,
** P < .01.
Educational Outcome
The analyses showed a significant association between suffering from CCAS and the need for educational support (χ 2(1) = 17.23, P < .001). The odds ratio indicated that the odds of needing educational support were 13.38 times higher if patients suffered from CCAS.
In total, 75.0% (n = 15) of patients with CCAS compared to 19.4% (n = 7) of patients without the syndrome needed educational support. As indicated in Figure 1, patients with surgery only (group A: 5.6%, n = 1) needed less educational support than patients with more intensive treatment (group B: 33.3%, n = 6). Only patients with deficits in two or more domains had SEN.
Risk Factors
Chi-square tests and Mann-Whitney U tests revealed no significant association between CCAS and any medical risk factors or gender. However, a trend indicates that CCAS is associated with more risk factors (Figure 2), including more intensive treatment (60.0% vs 50.0%) or relapses (25.0% vs 13.9%). In fact, post hoc analyses revealed that these two medical risk factors were associated with lower PR in tasks measuring information processing speed: treatment (Mean PROP: 48.28 ± 28.69, Mean PROP+RTx/CTx: 21.39 ± 24.61, P = .002, r = .44), and tumor relapse (Mean PRno relapse: 37.60 ± 30.14, Mean PRrelapse: 15.20 ± 18.41, P = .039, r = .29).
Figure 2.
The presence of risk factors in patients with and without CCAS. Abbreviations: CCAS, cerebellar cognitive affective syndrome; RTx/CTx, radio- and/or chemotherapy.
Postoperative transient mutism was observed in two patients with CCAS, one person in group A and three persons in group B. Due to the small number of patients, it was not considered as a risk factor in the statistical analyses.
Discussion
More than one third of patients treated for paediatric PFT showed impairments in executive functions, language, visuospatial skills and emotions/behaviour and were therefore diagnosed with CCAS. Compared to the control group, verbal and executive functioning were primarily affected in CCAS patients. This is consistent with the growing research on CM, after PFT surgery,10 as well as the findings of increased difficulties with expressive language like agrammatism and mild anomia as long-term consequences.8,9,11 With regard to executive dysfunction, working memory, and cognitive flexibility appear to be impaired in this patient group.20 Interestingly, children and adolescents with CCAS also had profound difficulties in tasks requiring abstract logical reasoning, which used to be considered a key executive feature of CCAS although now it is not typically regarded as an executive function by the neuropsychological community.9,12,15,37 Impairments in this domain might hence be explained by the strong association between executive functioning and logical reasoning38 or they could be a secondary consequence of problems with working memory, which is required in most tasks measuring logical reasoning (eg, matrix reasoning). In the present study, some patients without CCAS also suffered from executive dysfunction, while deficits in verbal functions were rare in the control groups, suggesting that verbal functions have the potential to become an essential discriminatory criterion for CCAS.
Our study has highlighted several problems regarding the diagnostic validity of CCAS. Firstly, although patients with the syndrome were more likely to display severe impairments (performing 2 SDs below the norm),8,9,15 deficit severity and its associations with everyday life varied, with some CCAS patients showing only slight deficits (1 SD below the norm) but still needing special educational support. Thus, only considering severe impairments for CCAS diagnosis, would lead to difficulties remaining unrecognized. This poses the question, whether CCAS might be a continuous rather than binary syndrome,19 requiring specific assessment methods to enable the detection of even fine difficulties, and hence avoid false-negative diagnoses. Secondly, the non-CCAS feature information processing speed was equally impaired in patients with and without the syndrome. This poses an important issue as information processing is well known to obstruct performance in different neuropsychological domains and can significantly impact everyday life.7 Therefore, CCAS diagnosis appears to omit important neuropsychological domains that are typically impaired in children and adolescents with PFT, leading to a skewed estimation of patients’ deficits, as well as inappropriate rehabilitative care.17 Corresponding screenings, such as the CCAS scale,22 are therefore inappropriate for this patient group. Thirdly and most importantly, the present results indicate that also patients without the syndrome performed below the norm. In fact, more than three quarters were at least slightly impaired in two or three CCAS features.2,4,9 Difficulties were predominantly found in tasks measuring executive functioning, visuospatial skills, and emotional or behavioral alterations. Regarding the controversy on the number of impaired domains necessary for diagnosis, the present results show that if deficits in only one domain were to be sufficient for CCAS diagnosis, the syndrome would be overdiagnosed in survivors of pediatric PFT. Hence, diagnostic validity would be low and render an insignificant association with everyday life participation, since only patients with neuropsychological deficits in two or more domains needed special educational support. Therefore, future research on CCAS should adopt a stricter classification, including slight deficits (1 SD below the norm) in all four domains, and comprehensive standard-of-care neuropsychological test measures (rather than screenings) to detect even minor impairments.
Risk factor analyses revealed that patients with radiotherapy demonstrated reduced information processing speed compared to patients with surgery only.5,7 However, in contrast to preceding studies the treatment groups without CCAS performed equally well in terms of logical reasoning, verbal functions, visuospatial and executive functioning. Furthermore, CCAS was also present in patients treated with surgery only, which is in line with previous findings,10 highlighting that treatment type alone cannot be regarded as crucial for the syndrome presence. Other risk factors, such as initial hydrocephalus, gender, tumor treatment, tumor relapse, time since diagnosis, and age at diagnosis were not associated with the emergence of CCAS. The fact that patients who are treated only surgically also develop neuropsychological deficits, suggests a more complex relationship. It must be assumed that a single risk factor cannot be considered sufficient to explain the occurrence of a syndrome as complex as CCAS.5 Furthermore, there might be an interaction effect between risk factors and the presence of neuropsychological symptoms. For instance, more aggressive tumors are not only treated more intensively and more prone to tumor relapse,1 but patients also spend more time in the hospital, which influences participation in daily life, which is crucial for health and child development.7 It can be hypothesized that these risk factors add up and lead to the presence of CCAS. Meanwhile, other factors such as socioeconomic background might contribute to the cognitive outcome.39 Such interactions must be analyzed with more comprehensive methods in future studies. On the other hand, while the risk factors investigated in the present study did not significantly predict CCAS emergence, treatment with chemo-/radiotherapy and tumor relapse were associated with deficits in information processing speed.5,18 It is hence recommendable for future research to include information processing when assessing CCAS risk factors and adverse treatment effects.
The improvement of the diagnostic validity of CCAS seems to be crucial considering that three quarters of the patients needed educational support. In comparison, it is four times more frequent than in patients without the syndrome. Cognitive functions, such as visuospatial skills, language, information processing, executive functions, and logical reasoning contribute to difficulties in school.2 Still, the present results suggest that the CCAS concept as classified in this study is inadequate for patients with childhood PFT. We advocate for comprehensive and regular routine evaluations of all children with PFT so that rehabilitative support can be assigned in time and impacts on the academic career can be alleviated.
Limitations
There are some limitations to this investigation. Firstly, partially retrospective data were considered which do not adhere to the current oncological guidelines on how to best assess neurocognitive and emotional difficulties in children with PFT.21,40 Therefore, some neuropsychological domains were not tested accurately enough in data sets that are fifteen years old or older. This was counteracted in the more recent data by using extensive neuropsychological test batteries, as well as continuous assessments, allowing more detailed statements on neuropsychological late sequelae. Secondly, socioeconomic data were not considered. Future studies should investigate interaction effects between medical and socioeconomic variables as risk factors for the emergence of late sequelae such as CCAS.
Conclusions
The present analyses show a high prevalence of CCAS in patients surviving pediatric PFTs associated with severe neuropsychological impairment and a worse academic outcome. The findings indicated that CCAS is a continuous rather than binary syndrome as deficits varied in intensity. Generally, long-term deficits in visuospatial skills, verbal functions, executive functioning, information processing speed, and logical reasoning were more pronounced in patients with CCAS compared to patients without the syndrome. However, impairments in information processing speed were also found in patients without CCAS but treated with chemo-/radiotherapy. This highlights the fact that solely assessing the core CCAS features is not sufficient, because the impairments of a substantial number of patients would not be detected if other domains such as information processing were ignored. Thus, for clinical practice, the CCAS concept as classified in this study is not a useful grading system to cover the variety of neuropsychological late effects of PFTs in childhood.3–5
Moreover, most PFT patients were at least slightly impaired in two or three CCAS domains. Hence, deficits in just one domain should not be enough to diagnose CCAS, as is usually done. Otherwise, almost every patient of our sample was to be diagnosed with the syndrome, rendering a low diagnostic validity. This suggests that for clinical practice the CCAS concept is not specific enough to describe our patients’ problems.
In summary, CCAS is an inappropriate clinical concept for patients with childhood PFT. Nevertheless, the attempt to establish a uniform nomenclature and assessment for the neuropsychological late sequelae of patients with pediatric PFT is important. Bedside screenings cannot provide a picture that is differentiated enough. Instead, comprehensive standard-of-care neuropsychological investigations following guidelines21,40 that also consider domains such as information processing speed are preferable and recommended, as they allow for the detection of subtle deficits. We recommend that everyday life criteria such as the need of special educational support are considered as additional information in assessment to also identify problems at the behavioral level. Generally, the large differences in neurocognitive outcomes after PFT surgery and adjuvant therapy are difficult to explain. We assume that several factors play a role: Not only disease and treatment, but also environmental factors (income, educational degree, migration background, etc.) are relevant for rehabilitation. We appeal for research that puts more emphasis on the biopsychosocial model.
Supplementary Material
Contributor Information
Doris Hoffmann-Lamplmair, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria; Comprehensive Center for Pediatrics Vienna, Medical University of Vienna, Vienna, Austria.
Ulrike Leiss, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria; Comprehensive Center for Pediatrics Vienna, Medical University of Vienna, Vienna, Austria.
Andreas Peyrl, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria; Comprehensive Center for Pediatrics Vienna, Medical University of Vienna, Vienna, Austria.
Irene Slavc, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria; Comprehensive Center for Pediatrics Vienna, Medical University of Vienna, Vienna, Austria.
Thomas Czech, Comprehensive Center for Pediatrics Vienna, Medical University of Vienna, Vienna, Austria; Department of Neurosurgery, Medical University of Vienna, Vienna, Austria.
Alexandra Gram, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria; Comprehensive Center for Pediatrics Vienna, Medical University of Vienna, Vienna, Austria.
Thomas Pletschko, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria; Comprehensive Center for Pediatrics Vienna, Medical University of Vienna, Vienna, Austria.
Funding
This work was supported by the “Austrian Childhood Cancer Organization” (no grant number applicable) to Dr. Thomas Pletschko and the “Children’s Cancer Help Organisation for Vienna, Lower Austria and Burgenland” (no grant number applicable) to Dr. Thomas Pletschko.
Conflict of interest statement. There is nothing to report.
Authorship statement. Research design: D.H.L., A.G., A.P., U.L., I.S., T.C., and T.P. Data acquisition: D.H.L., A.G., A.P., and T.P. Data analyses: D.H.L., A.G., A.P., T.P. Data interpretation: D.H.L., A.G., A.P., U.L., I.S., T.C., and T.P. Manuscript drafting and revision: D.H.L., A.G., A.P., U.L., I.S., T.C., and T.P.
References
- 1. Rutkowski S, Trollmann R, Korinthenberg R, et al. Leitsymptome und Diagnostik der ZNS-Tumoren im Kindes- und Jugendalter. 2016. https://www.awmf.org/uploads/tx_szleitlinien/025-022l_S1_ZNS-Tumoren_Kinder_Jugendliche_2016-09-abgelaufen.pdf. Accessed November 4, 2021.
- 2. Stavinoha PL, Askins MA, Powell SK, Smiley NP, Robert RS. Neurocognitive and psychosocial outcomes in pediatric brain tumour survivors. Bioengineering. 2018;5(3):73. [Google Scholar]
- 3. Davis EE, Pitchford NJ, Jaspan T, McArthur D, Walker D. Development of cognitive and motor function following cerebellar tumour injury sustained in early childhood. Cortex. 2010;46(7):919–932. [DOI] [PubMed] [Google Scholar]
- 4. Cámara S, Fournier MC, Cordero P, et al. Neuropsychological profile in children with posterior fossa tumours with or without postoperative cerebellar mutism syndrome (CMS). Cerebellum. 2020;19(1):78–88. [DOI] [PubMed] [Google Scholar]
- 5. Dellatolas G, Câmara-Costa H. The role of cerebellum in the child neuropsychological functioning. Handb Clin Neurol. 2020;173:265–304. [DOI] [PubMed] [Google Scholar]
- 6. Carroll C, Watson P, Spoudeas HA, et al. Prevalence, associations, and predictors of apathy in adult survivors of infantile (<5 years of age) posterior fossa brain tumors. Neuro Oncol. 2013;15(4):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brinkman TM, Ness KK, Li Z, et al. Attainment of functional and social independence in adult survivors of pediatric CNS tumours: a report from the St Jude lifetime cohort study. J Clin Oncol. 2018;36(27):2762–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17):1760–1768. [DOI] [PubMed] [Google Scholar]
- 9. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. [DOI] [PubMed] [Google Scholar]
- 10. Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–1050. [DOI] [PubMed] [Google Scholar]
- 11. Gudrunardottir T, Morgan AT, Lux AL, et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst. 2016;32(7):1195–1203. [DOI] [PubMed] [Google Scholar]
- 12. Schmahmann JD. Pediatric post-operative cerebellar mutism syndrome, cerebellar cognitive affective syndrome, and posterior fossa syndrome: historical review and proposed resolution to guide future study. Childs Nerv Syst. 2020;36(6):1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Argyropoulos GPD, van Dun K, Adamaszek M, et al. The cerebellar cognitive affective/Schmemann syndrome: a task force paper. Cerebellum. 2020;19(1):102–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gudrunardottir T, Sehested A, Juhler M, Grill J, Schmiegelow K. Cerebellar mutism: definitions, classification and grading of symptoms. Childs Nerv Syst. 2011;27(9):1361–1363. [DOI] [PubMed] [Google Scholar]
- 15. Ahmadian N, van Baarsen K, van Zandvoort M, Robe PA. The cerebellar cognitive affective syndrome—a meta-analysis. Cerebellum. 2019;18(5):941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoche F, Daly MP, Chutake YK, et al. The cerebellar cognitive affective syndrome in ataxia-telangiectasia. Cerebellum. 2019;18(2):225–244. [DOI] [PubMed] [Google Scholar]
- 17. Omar D, Ryan T, Carson A, et al. Clinical and methodological confounders in assessing the cerebellar cognitive affective syndrome in adult patients with posterior fossa tumours. Br J Neurosurg. 2014;28(6):755–764. [DOI] [PubMed] [Google Scholar]
- 18. Wegenschimmel B, Leiss U, Veigl M, et al. Do we still need IQ-scores? Misleading interpretations of neurocognitive outcome in pediatric patients with medulloblastoma: a retrospective study. J Neurooncol. 2017;135(2):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albazron FM, Bruss J, Jones RM, et al. Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology. 2019;93(16):e1561–e1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffmann-Lamplmair D, Ritter I, Leiss U, Slavc I, Pletschko T. The assessment of executive functioning in pediatric patients with posterior fossa tumors: a recommendation to combine caregiver-based ratings and performance-based tests. Dev Neurorehabil. 2022;25(1):19–28. [DOI] [PubMed] [Google Scholar]
- 21. Schröder H, Lilienthal S, Schreiber-Gollwitzer B, et al. Psychosocial Care in Paediatric Oncology and Haematology. 2019. https://www.awmf.org/fileadmin/user_upload/Leitlinien/025_Ges_fuer_Paediatrische_Onkologie_und_Haematologie/025-002eng_S3_Psychosocial-Care-Paediatric-Oncology-Haematology__2020-02.pdf. Accessed November 4, 2021.
- 22. Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. 2018;141(1):248–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyers JE, Meyers KR, eds. Rey Complex Figure Test and Recognition Trial—RCFT: Supplemental Norms for Children and Adolescents. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 24. Petermann F, Petermann U, eds. Wechsler Intelligence Scale for Children WISC. German Adaption of WAIS-IV from D. Wechsler. 4th ed. Frankfurt: Pearson Assessment; 2007. [Google Scholar]
- 25. Petermann F, ed. Wechsler Adult Intelligence Scale WAIS. German Adaption from D. Wechsler. 4th ed. Frankfurt: Pearson Assessment; 2008. [Google Scholar]
- 26. Petermann F, ed. Wechsler Adult Intelligence Scale—Fourth Edition WAIS-IV. German Adaption from D. Wechsler. 2nd ed. Frankfurt: Pearson Assessment; 2012. [Google Scholar]
- 27. Petermann F, ed. Wechsler Intelligence Scale for Children—Fifth Edition WISC-V. German Adaption from D. Wechsler. 1st ed. Frankfurt: Pearson Assessment; 2017. [Google Scholar]
- 28. Kubinger KD, ed. Adaptives Intelligenz Diagnostikum—Version 2.2 AID 2. Göttingen: Beltz; 2009. [Google Scholar]
- 29. Kaufman A, Kaufman N, eds. K-ABC: Kaufman—Assessment Battery for Children. German Adaption from P. Melchers and U. Preuß. Frankfurt: Pearson; 2009. [Google Scholar]
- 30. Delis D, Kaplan E, Kramer J, eds. Delis-Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 31. Kongs S, Thompson L, Iverson G, Heaton RK, eds. The Wisconsin Card Sorting Test—64 (WCST 64). Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 32. Drühe-Wienholt C, Wienholt W, eds. CKV: Computergestütztes Kartensortierverfahren. Frankfurt: Swets; 1998. [Google Scholar]
- 33. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40(5):791–799. [PubMed] [Google Scholar]
- 34. Ravens-Sieberer U, Bullinger M. Assessing health related quality of life in chronically ill children with the German KINDL: first psychometric and content-analytical results. Qual Life Res. 1998a;7(5):399–407. [DOI] [PubMed] [Google Scholar]
- 35. Ravens-Sieberer U, Bullinger M. News from the KINDL-Questionnaire—a new version for adolescents. Qual Life Res. 1998b;7:653. [Google Scholar]
- 36. Helmstaedter C, Lendt M, Lux S, eds. Verbaler Lern- und Merkfähigkeitstest (VLMT). 1st ed. Weinheim: Beltz Test; 2001. [Google Scholar]
- 37. Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: four general conclusions. Curr Dir Psychol Sci. 2012;21(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brydges CR, Reid CL, Fox AM, Anderson M. A unitary executive function predicts intelligence in children. Intelligence. 2012;40(5):458–469. [Google Scholar]
- 39. Torres VA, Ashford JM, Wright J, et al. The impact of socioeconomic status (SES) on cognitive outcomes following radiotherapy for pediatric brain tumors: a prospective, longitudinal trial. Neuro Oncol. 2021;23(7):1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wiener L, Kazak AE, Noll R, Patenaude AF, Kupst MJ. Standards for psychosocial care for children with cancer and their families. Pediatr Blood Cancer. 2015;62(Suppl 5):419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.