Abstract
Opioid use disorder (OUD) and opioid-related deaths remain a significant public health crisis having reached epidemic status globally. OUDs are defined as chronic, relapsing conditions often characterized by compulsive drug seeking despite the deleterious consequences of drug taking. The use of nicotine-containing products has been linked to increased likelihood of prescription opioid misuse, and there exists a significant comorbidity between habitual nicotine use and opioid dependence. In rodent models, nicotine administration nearly doubles the amount of opioids taken in intravenous self-administration paradigms. Here, we examined the effect of acute systemic nicotine administration in male rats on responding for the synthetic opioid remifentanil (RMF) in a contextual punishment paradigm using either an exteroceptive punisher (foot-shock) or an interoceptive punisher (histamine). Nicotine administration, relative to saline, increased RMF intake in both unpunished and punished contexts, regardless of form of punishment, and resulted in significantly higher motivation to obtain RMF in the previously punished context, as measured by progressive ratio breakpoint. Additionally, regardless of context, nicotine-treated rats were slower to extinguish RMF responding following drug removal and displayed higher levels of cue-induced reinstatement than saline-treated controls. Furthermore, these data support that, compared to histamine adulteration, contingent foot-shock is a more potent form of punishment, as histamine punishment failed to support contextual discrimination between the unpunished and punished contexts. In contrast to RMF administration, augmentation of responding for an audiovisual cue by nicotine pretreatment was lost following contextual punishment. In conclusion, acute nicotine administration in adult male rats significantly enhances compulsive-like responding for RMF that persists despite contingent punishment of drug-directed responding.
Keywords: Opioid, Comorbidity, Polysubstance, Conditioning, Motivation, Histamine
1.0. Introduction
Nearly 16 million individuals meet diagnostic criteria for an opioid use disorder (OUD), and opioid-related overdoses contribute to approximately 120,000 deaths per year worldwide 1,2. Of note, it is estimated that approximately 83–95% of individuals currently enrolled in an opioid treatment program (OTP) are habitual users of nicotine, and concurrent use of nicotine significantly increases the likelihood of misusing prescription opioids 3–5. Furthermore, we have recently demonstrated in rodents that acute nicotine administration prior to opioid intravenous self-administration (IVSA) nearly doubles the amount of opioids taken 6. As such, it is imperative to elucidate mechanisms contributing to the development and maintenance of OUDs and the facilitation of opioid addiction liability by nicotine.
A critical component of the development and maintenance of substance use disorders (SUDs), including OUD, is the formation and recall of drug-associated memories, including learning about the discrete and contextual cues associated with opioid use. The reinforcing and aversive properties of drugs of abuse can become associated with various cues and contexts, and these associations later serve to coordinate motivated behaviors to either approach or avoid associated stimuli and even drugs themselves 7–10. There is ample evidence to demonstrate that nicotine administration incentivizes approach to cues associated with the positive, reinforcing properties of subsequently administered drugs 11–14 as well as limits avoidance of cues associated with the aversive consequences of drug administration 14–20. Given that SUDs are primarily characterized as chronic, relapsing conditions and that a major hurdle to sustained abstinence is the high likelihood of relapse when exposed to environmental cues previously associated with drug use 21, it follows that this high approach, low avoidance phenotype to drug-associated cues induced by nicotine treatment may exacerbate the liability for continued drug-seeking in the face of the aversive consequences of drug use.
Generally speaking, successful recovery from SUDs in humans is largely dependent on self-imposed abstinence, as opposed to forced extinction resulting from the complete removal of abused substances and associated contexts 22,23. The perceived imbalance between the adverse and reinforcing properties of continued drug use is often a significant contributor to self-imposed abstinence 24. Recently, many studies have successfully modeled this self-imposed abstinence through an adaptation of the ABA renewal paradigm, in which abstinence is achieved through explicit punishment of drug taking within a distinct context 10,25–28. Here, we employed a further adaptation of this model, in which we alternated the contexts daily rather than imposing abstinence through repeated presentations of the punished context at the end of the intravenous self-administration (IVSA) sessions. Specifically, we were interested in the degree to which nicotine administration would enhance remifentanil (RMF) IVSA in the face of contingent punishment and what effect, if any, nicotine would have on the ability to discriminate between unpunished and punished contexts. To this end, male rats were tested for RMF self-administration within two distinct contexts, one in which RMF IVSA was unpunished and a second in which RMF IVSA resulted in contingent punishment. Nicotine or saline was acutely administered prior to IVSA sessions. Additionally, we tested the efficacy of two different forms of punishment in supporting contextual discrimination between the unpunished and punished environments. In one experiment we used an exteroceptive punisher (foot-shock), and in the second we used an interoceptive punisher (histamine adulteration). Finally, because nicotine has been shown to enhance responding for audiovisual (AV) stimuli,29–31 we conducted a third experiment in nicotine- and saline-treated rats wherein we tested the effect of contingent foot-shock punishment on responding for saline infusions paired with the AV cue.
2.0. Methods
2.1. Animals and Housing
Sixty adult male Long-Evans rats (Envigo; Indianapolis, IN) weighing 250-275 g upon arrival were individually housed on a reverse light cycle in standard polycarbonate rat cages in a humidity- and temperature-controlled vivarium. Because the stage of estrus cycle has been shown to affect responsivity in foot-shock conditioning paradigms32, we chose to solely use males in this initial characterization of the effect of nicotine on compulsive-like responding for RMF. Following arrival to the facilities, rats were handled daily for three days and given at least one week to acclimate prior to surgical procedures. All procedures were approved by the University at Buffalo Institutional Animal Care and Use Committee and were carried out in accordance with all relevant guidelines and regulations, including applicable Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
2.2. Surgical Procedures
Rats were surgically implanted with chronically indwelling catheters in the right jugular vein under isoflurane anesthesia (1–3%). Briefly, a 15 cm catheter line (C30PU-RJV1402, Instech) was implanted into the right external jugular vein, and the opposite end was connected to an externalized vascular access button (VABR1B/22, Instech). Each access button was implanted just posterior to the scapular region on the dorsal side of the rat. Catheters were flushed with 0.1 ml of heparinized saline and enrofloxacin (Baytril, Bayer HealthCare LLC; Shawnee Mission, KS) before and after self-administration on test days. Catheter patency was verified at the conclusion of the study by flushing 0.1 ml of ketamine (10 mg/ml) and verifying the expression of ataxia.
2.3. Chemical Stimuli
Nicotine tartrate (NIDA DSP) was dissolved in sterile saline at a concentration of 0.4 mg/ml (freebase) and the pH was adjusted to ~7.4 with dilute NaOH. All nicotine injections were administered subcutaneously (s.c.) at a dose of 0.4 mg/kg. Remifentanil hydrochloride (RMF; NIDA DSP) was dissolved in sterile saline such that each intravenous infusion of RMF was delivered at a dose of 3.2 μg/kg. In Experiment 2, histamine dihydrochloride (Sigma Aldrich) was dissolved in RMF solutions such that each infusion of 3.2 μg/kg RMF resulted in simultaneous delivery of 1.0 - 4.0 mg/kg 33,34 of histamine.
2.4. Behavioral Procedures
2.4.1. Acquisition of intravenous remifentanil self-administration
Prior to beginning operant training, rats were assigned to receive either nicotine (0.4 mg/kg) or saline (1.0 ml/kg) in a weight-balanced fashion. For two days prior to starting training, rats were given a single noncontingent injection of their assigned stimulus in their home cages in order to acclimate the rats to the effects of nicotine and the injection procedures. Throughout all training and testing sessions, rats were administered their assigned treatment (nicotine or saline, s.c.), returned to home cages for 15 min, and then transported to the operant experiment room, where they immediately began the behavioral procedures.
Rats were trained in 2-h sessions to press a retractable lever in standard operant cages (Med Associates; St Albans, VT) for delivery of a drug reinforcer. Each operant box was equipped with a standard red house-light, a sound attenuating fan, two retractable levers, and two stimulus lights located directly above each lever. Behavior was shaped such that one press of the active lever resulted in retraction of both levers, illumination of the cue light, and intravenous delivery of RMF (3.2 μg/kg). The cue light remained illuminated during the infusion period (~ 3s). Presses of the inactive lever had no programed consequence. Lever assignments (active/inactive) were counter-balanced across rats. Once stable responding was established (5 days), rats were advanced to an FR-2 schedule of reinforcement (4 days). Upon completion of each 2-h session, the house-light was turned off, both levers were retracted, and rats were promptly returned to their home cages. A control group underwent identical acquisition training, however, infusions contained only sterile saline (0.9%) rather than RMF.
2.4.2. Contextual punishment of remifentanil self-administration with contingent foot-shock
Following acquisition of operant RMF self-administration, rats were trained such that, in one context, they were able to freely administer RMF on an FR-2 schedule with no consequence while, in a second context, RMF infusions were punished with a 1-s foot-shock on a RR-2 schedule. Contexts were distinguished by visual and tactile stimuli: grid vs. bar flooring, addition of black stripes to the cage walls, and alternating house light orientation from the back of the cage to the front. These 2-h self-administration sessions alternated daily between the punished and unpunished contexts. The intensity of the foot-shock was systematically increased across punished sessions (0.0 - 0.6 mA). Contextual conditioning occurred over 12 days, so that each rat was exposed to a total of six days in each of the alternating contexts. The order of first context presentation (i.e. punished or unpunished) was counter-balanced across all rats. Prior to contextual conditioning, rats were exposed to the contexts for 30 min in order to acclimate them to the new chamber orientation. No levers were presented during these acclimatization sessions and rats were not allowed to self-administer RMF during the 30-min session.
2.4.3. Contextual punishment of remifentanil self-administration with histamine adulteration
Contextual conditioning of histamine punishment was identical to that of foot-shock punishment with the exception that no foot-shock was delivered and RMF solutions were adulterated with histamine in the punished context. Briefly, rats were trained in alternating contexts, such that in one context they were free to administer RMF with no additional consequence, whereas in the alternate context RMF infusions contained histamine. Contextual presentations were alternated daily and the concentration of histamine was systematically increased across conditioning sessions (0.0 – 4.0 mg/kg/infusion). Conditioning took place across 12 days such that each rat was exposed to a total of six days in each of the alternating contexts. The order of context presentation was counter-balanced across all rats.
2.4.4. Progressive ratio responding for remifentanil in both the punished and unpunished contexts
Following contextual conditioning sessions, all rats were subjected to two progressive ratio (PR) test sessions: one in the unpunished context and one in the punished context. Here, the number of lever presses required to earn RMF infusions was systematically increased following each earned reinforcer35. Importantly, RMF infusions were not punished in this phase in order to examine the degree to which the context itself would serve to limit RMF IVSA. Each session was terminated either when the rat failed to complete the current ratio requirement within 1 h of earning their last RMF infusion or after a maximum session duration of 4 h. The order of the PR sessions (punished context vs. unpunished context) followed the same counter-balanced assignment as the conditioning sessions.
2.4.5. Operant responding during drug withdrawal
Following the last PR session, rats were tested for responding under extinction conditions. In these 2-h sessions, pressing the active (or inactive) lever had no programmed consequences. First, rats were tested for 10 days in the unpunished context and then once in the punished context. Rats continued to receive their assigned drug pretreatment (nicotine or saline) during these sessions. Three rats were removed due to the development of illness prior to completion of the extinction tests (n = 45).
2.4.6. Cue-induced reinstatement following contextual punishment of foot-shock
Following extinction tests, a subset of rats (n=19) from the foot-shock experiment were tested for responding in a 2-h cue-induced reinstatement test. Here, rats were tested either in the unpunished (n=10) or punished context (n=9) such that successful completion of FR-2 on the active lever resulted in illumination of the cue light but no RMF delivery. Rats continued to receive their assigned drug pretreatment (nicotine or saline) during these sessions.
2.4.7. Contextual punishment of responding for an AV reinforcer with contingent foot-shock punishment
Contextual punishment of AV responding with contingent foot-shock was identical to that of RMF punishment. Briefly, nicotine- and saline-treated rats responded on an FR-2 schedule for an IV infusion of saline paired with lever retraction and cue-light illumination. Contexts were alternated daily and the first presented context was counter-balanced across both groups. The intensity of foot-shock was increased across sessions in the punished context (0.0 - 0.6 mA). Conditioning lasted for 12 days, six sessions in either context.
2.4.8. Progressive ratio responding for an AV reinforcer in both the punished and unpunished contexts
Following conditioning, rats were given two PR sessions, one in the punished context and one in the unpunished context. All experimental conditions mirrored those of the RMF PR sessions with the exception that no RMF was delivered upon completion of the ratio, only saline and the AV cue.
2.5. Data Analyses
For the acquisition of RMF and saline self-administration, active and inactive lever presses as well as earned reinforcers were independently analyzed with mixed-measures ANOVAs with Drug administration (nicotine or saline) as a between-subjects factor and Session as a within-subjects factor.
During conditioning for both foot-shock and histamine punished experiments, active lever presses, infusions, and latencies were independently analyzed with mixed-measures ANOVAs with Drug as a between-subjects factor and Session and Context as a within-subjects factors. Breakpoints from the PR sessions were analyzed with Drug and form of Punishment (foot-shock or histamine) as between-subjects factor and Context as within-subjects factor. Breakpoints from the PR session in control rats were analyzed with Drug as between-subjects factor and Context as within-subjects factor.
Responding on the active and inactive levers during withdrawal sessions were analyzed with mixed-measures ANOVAs with Drug and Punishment as between-subjects factors and Session as a within-subjects factor.
Responding on the active and inactive levers during the reinstatement test was analyzed with ANOVAs with Drug and Context as between-subjects factors.
Significant main and interactive effects were further explored with Newman-Keuls post hoc tests where appropriate.
3.0. Results
3.1. Nicotine enhances the acquisition of remifentanil self-administration
Pretreatment with nicotine, relative to saline, produces significantly higher drug-directed responding resulting in significantly higher RMF intake across the 9-day acquisition phase (Fig 1). Analysis of active vs. inactive lever responding in a three-factor ANOVA revealed a significant Lever x Session x Drug interaction (F(8,368) = 2.81, P < 0.01). Post hoc analyses demonstrate that nicotine-treated rats significantly elevated their active lever presses above inactive lever presses on the fourth session, while saline-treated rats did not reliably do so until the fifth session. Furthermore, nicotine-treated rats, relative to saline-treated rats, responded more on the active lever starting on session six and continued to remain reliably elevated throughout the remaining sessions. There were no statistical differences in responding on the inactive lever between nicotine- and saline-treated rats. Consistent with these results, a two-factor ANOVA conducted on the number of earned infusions revealed a significant Session x Drug interaction (F(8,368) = 2.04, P < 0.05), indicating that nicotine-treated rats took more infusions of RMF, relative to saline-treated rats.
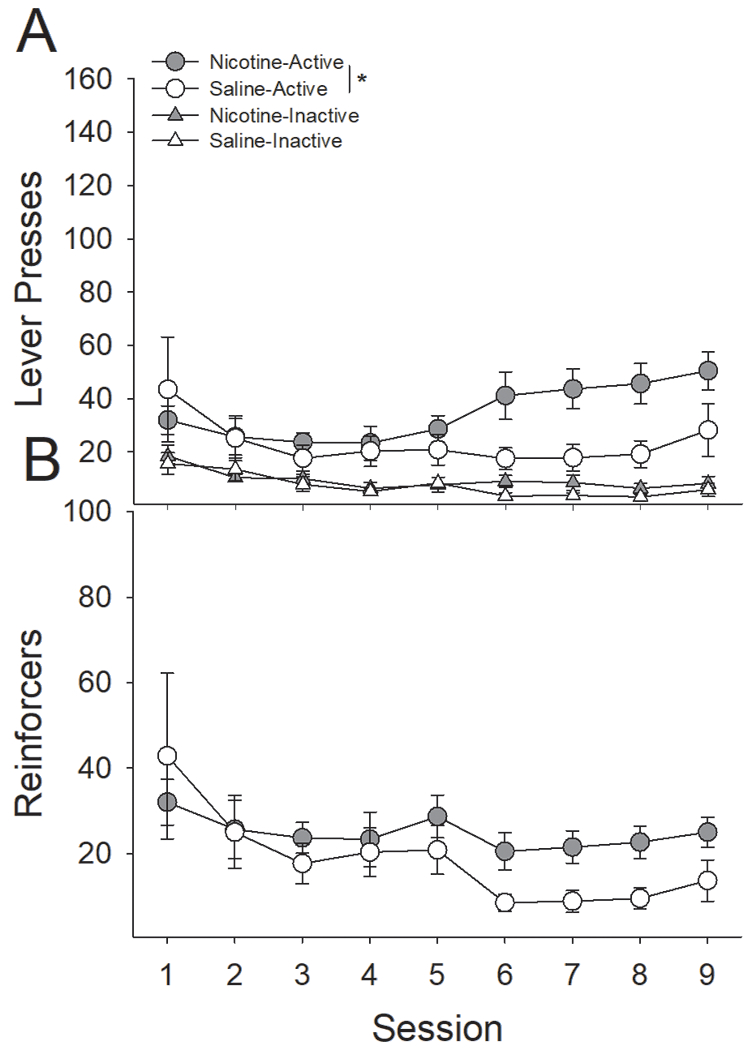
Figure 1:
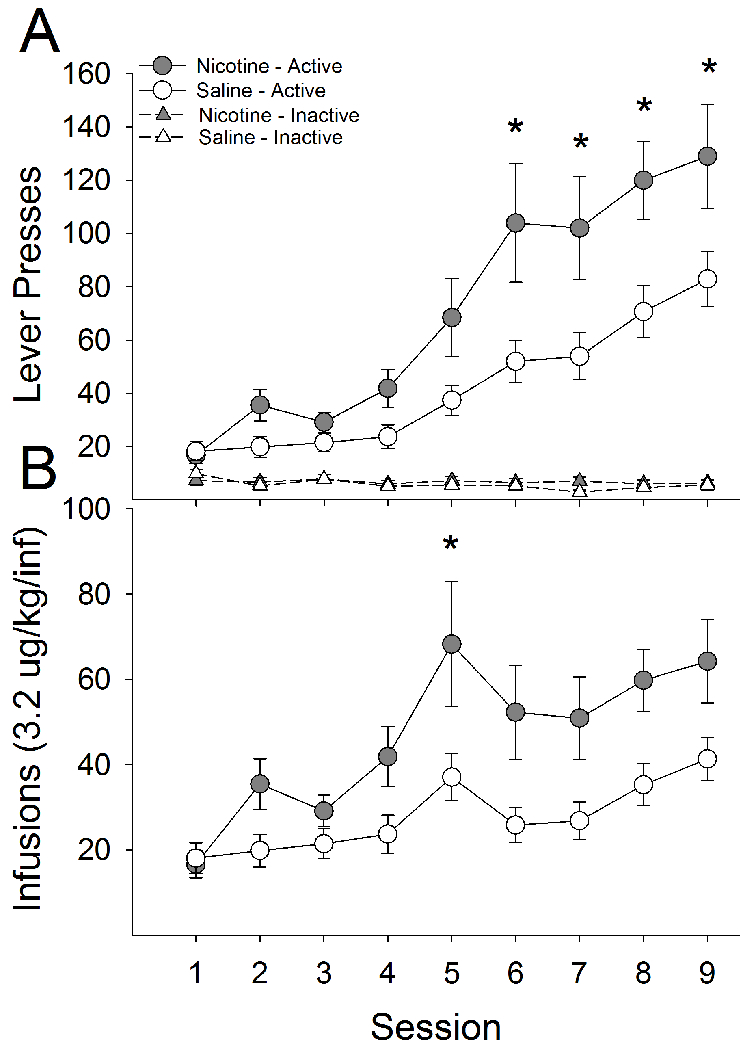
Pretreatment with nicotine significantly increases intravenous self-administration of remifentanil. Rats were trained in 2-h sessions for five days on an FR-1 schedule of reinforcement to press a retractable lever in standard operant chambers for IVSA of RMF before being moved to an FR-2 schedule for the remaining four days. (A) Rats treated with nicotine, relative to those treated with saline, made significantly more active lever presses on days 6-9 of training. There were no differences between groups on inactive lever presses. (B) As a result, nicotine pretreatment resulted in significantly more earned RMF infusions on day five of acquisition training. *s indicate significant group differences (P < 0.05).
3.2. Nicotine continues to enhance remifentanil self-administration despite contextual punishment of drug-taking with contingent foot-shock
While contextual administration of contingent foot-shock punishment substantially reduced RMF intake in nicotine- and saline-treated rats, intake in nicotine-treated rats remained elevated relative to saline-treated rats (Fig 2). Importantly, nicotine pretreatment did not result in differential sensitivity to foot-shock punished drug seeking, as nicotine-treated rats reduced their RMF intake at a rate similar to that of saline-treated rats. A three-factor ANOVA conducted on the number of earned RMF infusions across sessions as a function of drug-pretreatment and contextual punishment revealed that nicotine-treated rats took significantly more RMF than saline-treated rats, regardless of context (main effect of Drug; F(1,25) = 7.21, P < 0.05). Administration of contingent foot-shock in the punished context significantly reduced drug-intake within that context, with no effect on intake in the unpunished context (Context x Session interaction; F(5,125) = 18.83, P < 0.0001). While nicotine-treated rats took larger overall amounts of RMF, they did decrease their RMF intake as a function of increasing foot-shock intensity at a rate largely similar to that of saline-treated rats yet continued to remain significantly elevated in the unpunished context (Context x Drug interaction; F(5,125) = 5.77, P < 0.05). In addition, we found a significant Session x Drug interaction (F(5,125) = 2.54, P < 0.05). In general, nicotine-treated rats took more RMF but were similarly impacted by escalating foot-shock intensity (Fig 2C & D). Nearly identical effects were observed when analyzing the number of active lever presses (Fig 3A & B: Drug; F(1,25) = 7.20, P < 0.05; Context x Drug; F(5,125) = 5.78, P < 0.05; Session x Drug; F(5,125) = 2.52, P < 0.05).
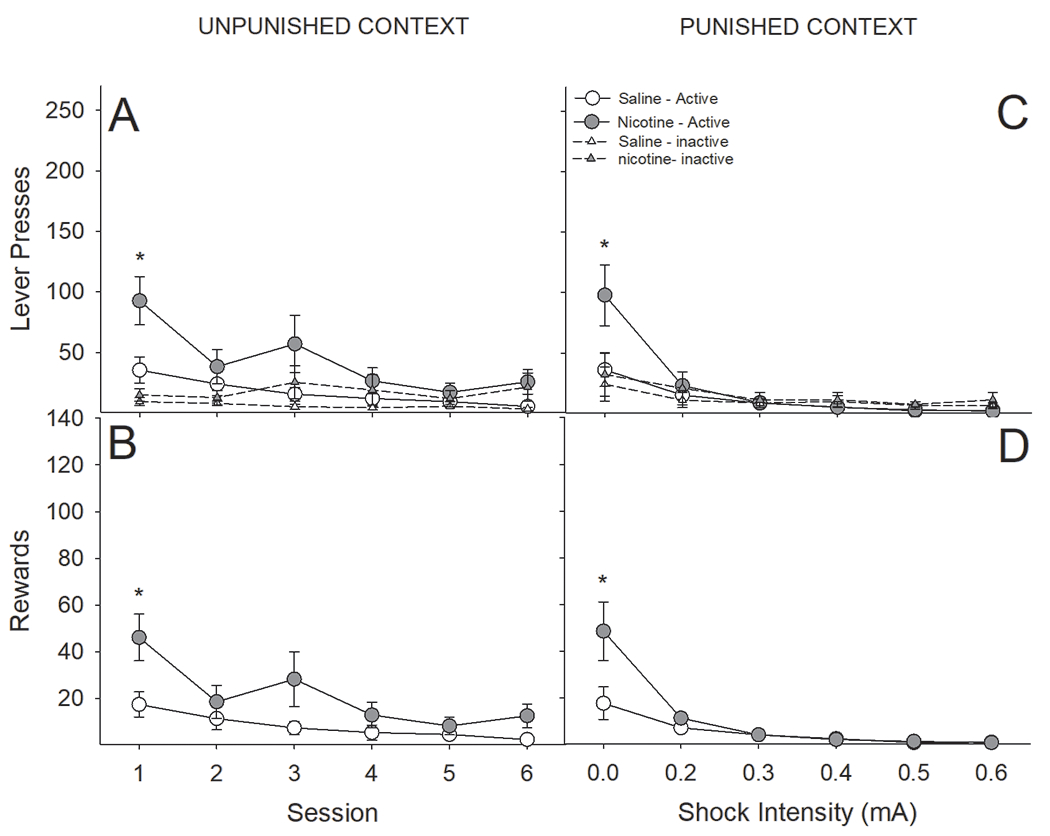
Figure 2:
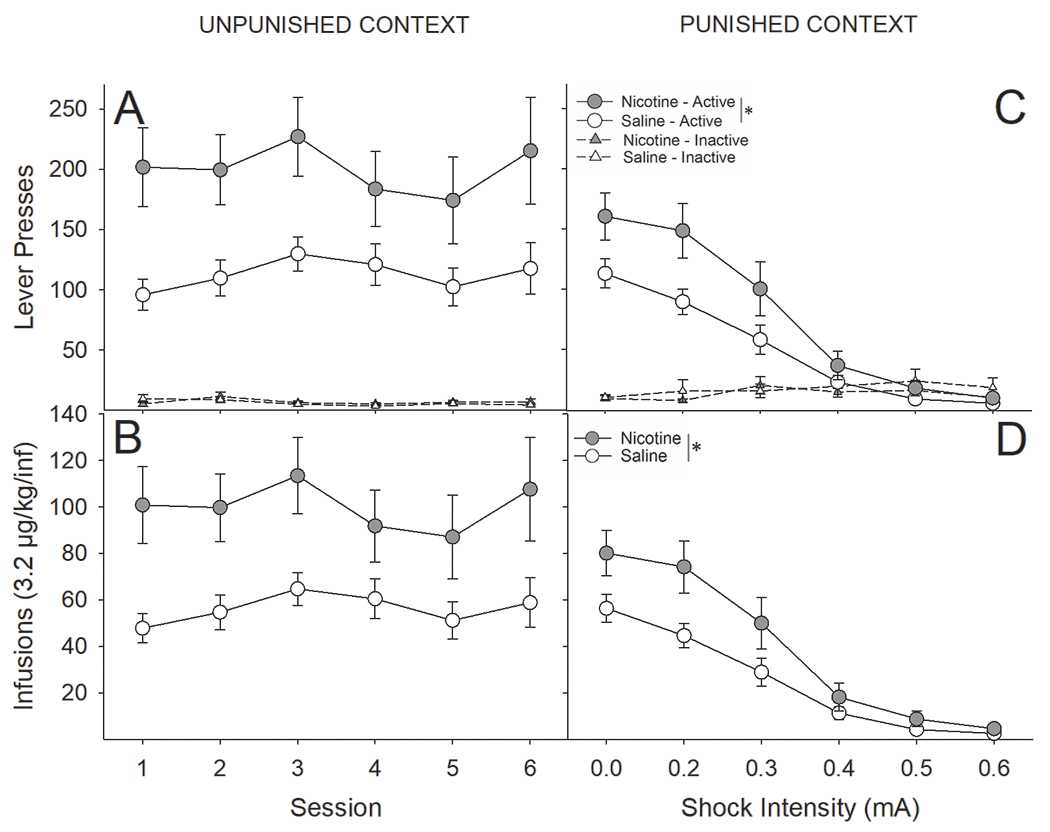
Despite administration of foot-shock punishment contingent on remifentanil infusions, nicotine-treated rats took significantly more remifentanil than saline-treated. (A) Nicotine-treated rats, compared to saline-treated rats, pressed significantly more on the active lever in the unpunished context, with no differences in presses on the inactive lever, resulting in (B) significantly more earned RMF infusions in the unpunished context. (C) Nicotine-treated rats, compared to saline-treated rats, pressed significantly more on the active lever in the punished context but reduced lever pressing as a function of escalating foot-shock intensity at a rate similar to that of saline-treated rats, ultimately resulting in (D) significantly more earned RMF infusions across the conditioning sessions. *s indicate significant main effect of nicotine (P < 0.05).
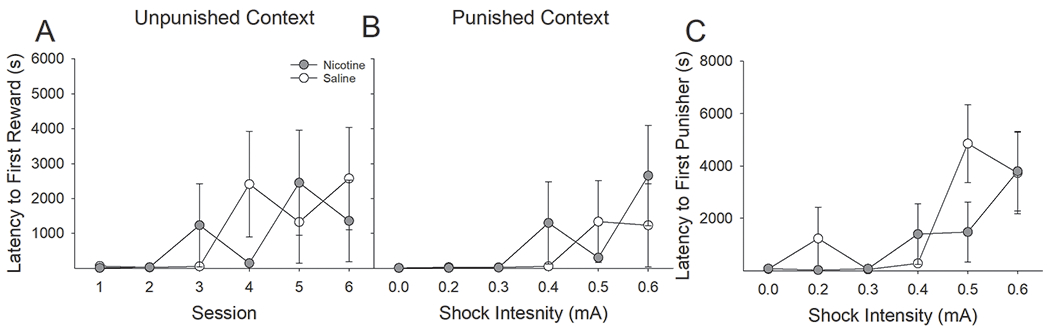
Figure 3:
Latency to self-administer remifentanil within the punished context increased as a function of foot-shock punishment but less so in nicotine-treated rats. (A) There was no difference in latency to first infusion in the unpunished context, and latency in the unpunished context was not affected by punishment in the alternate context. (B) Saline-treated rats reliably increased their latency to first RMF infusion following conditioning with 0.4 mA foot-shock, while nicotine-treated rats did not do so until the final conditioning session. Additionally, nicotine-treated rats were significantly faster than saline-treated rats to earn their first infusion during the final conditioning session (0.6 mA). (C) Similarly, compared to nicotine-treated rats, saline-treated rats reliably increased their latency to first earned punisher following conditioning with 0.4 mA foot-shock, while nicotine-treated rats did not do so until the final conditioning session. Nicotine-treated rats were significantly faster than saline-treated rats to earn their first punisher during final two conditioning sessions. *s indicate significant group differences (P < 0.05).
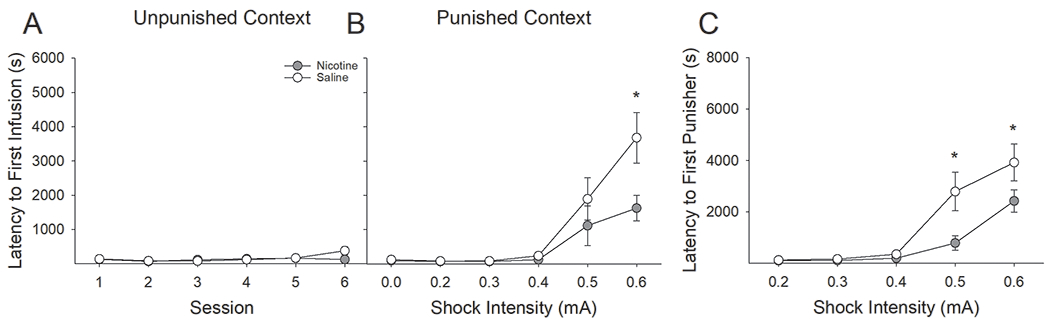
Next, we analyzed the latencies to the first earned infusion as a function of nicotine treatment and context (Fig 3A & B). This three factor ANOVA revealed a significant Context x Session x Drug interaction (F(5,125) = 2.30, P < 0.05). Consistent with the earned infusion data summarized above, we found no reliable change in the latency to earn the first infusion in the unpunished context, indicating that punishment in the opposite context did not significantly impact responding in the unpunished context; furthermore, there were no differential effects of nicotine treatment. Conversely, the latency to first infusion in the punished context increased with escalating shock intensity across sessions. Interestingly, this increase was reduced and delayed in nicotine-treated rats relative to saline-treated rats. Specifically, saline-treated rats significantly increased their latency to first infusion in the punished context on the session following conditioning with 0.4 mA foot-shock whereas nicotine-treated rats did not reliably do so until the final day of conditioning. Furthermore, the latency to first-earned infusion in the punished context was significantly lower in nicotine-treated rats, relative to saline treatment, on session six of conditioning, with a statistical trend on session 5 (P = 0.06). Similar analyses conducted on the latency to first earned punisher in the punished context revealed a significant Session x Drug interaction (F(4,100) = 3.18, P < 0.05). Here, we found that nicotine-treated rats were significantly quicker than saline-treated rats to earn their first punished RMF infusion on sessions five and six during conditioning with 0.5 and 0.6 mA, respectively (Fig 3C).
3.3. Nicotine continues to enhance remifentanil self-administration despite contextual punishment of drug taking with contingent histamine administration
Similar to foot-shock punishment of RMF intake, contingent histamine administration significantly lowered RMF self-administration (Fig 4) in the punished context, yet RMF intake in nicotine-treated rats remained elevated, relative to saline-treated rats, throughout the conditioning paradigm. Despite nicotine-treated rats taking more RMF than saline-treated rats, they were equally sensitive to the reduction in intake induced by increasing histamine concentration. A three-factor ANOVA conducted on the number of earned RMF infusions across sessions as a function of drug-pretreatment and context revealed that nicotine-treated rats self-administered more RMF, regardless of context, than saline-treated rats (Fig 3C & D; main effect of Drug; F(1,19) = 5.51, P < 0.05). Adulteration of the RMF solution with increasing concentrations of histamine across sessions resulted in a concentration-dependent decrease in RMF intake in the punished context, with no effect on intake in the unpunished context (Context x Session interaction; F(5,95) = 6.05, P < 0.0001). There were no interactive effects between Drug and Context, indicating that nicotine-treated rats were not differentially sensitive to increasing concentrations of histamine. Nearly identical effects were observed when analyzing the number of active lever presses (Fig 3A & B: Drug; F(1,19) = 5.48, P < 0.05; Context x Session; F(5,95) = 6.10, P < 0.0001).
Figure 4:
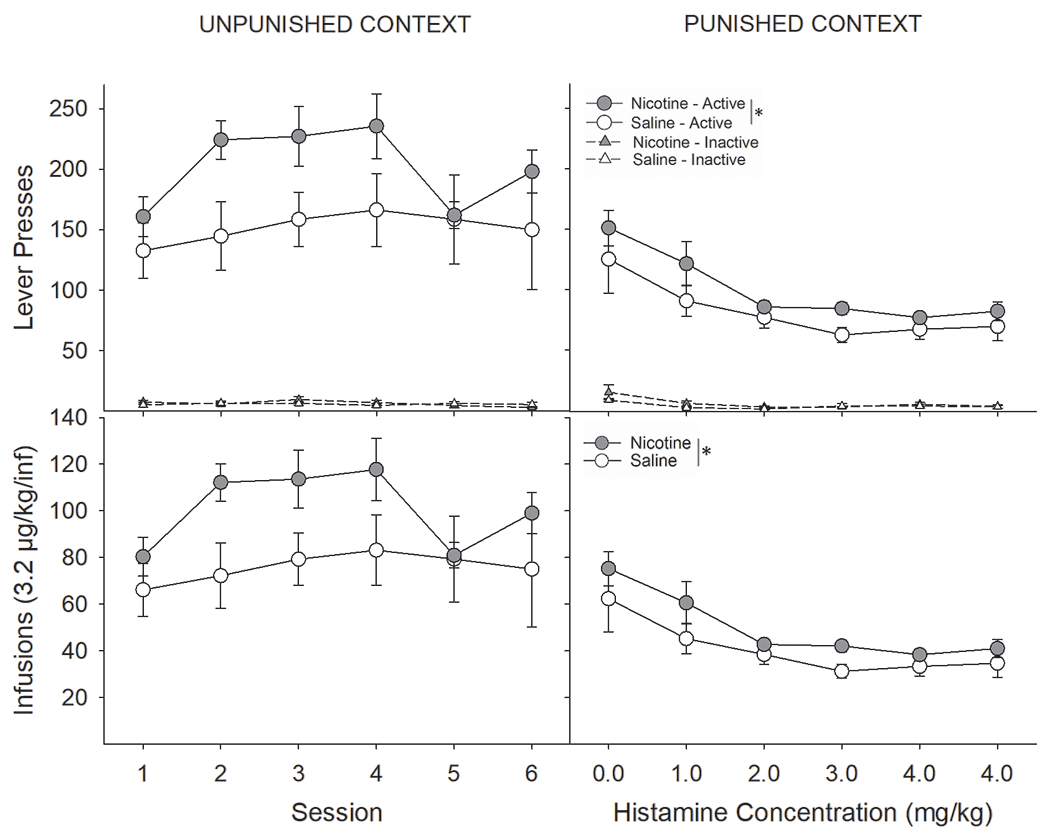
Similar to that seen with foot-shock punishment, punishment of remifentanil intake by histamine adulteration significantly lowered RMF self-administration, yet nicotine-treated rats continued to earn significantly more RMF. (A) Nicotine-treated rats pressed significantly more on the active lever than saline-treated rats in the unpunished context. There were no group differences in responding on the inactive lever. (B) Likewise, nicotine, relative to saline, increased RMF intake. (C) Adulteration of the RMF solution with increasing concentrations of histamine across sessions resulted in a concentration-dependent decrease in active lever presses in the punished context, yet nicotine-treated rats continued to press more on the active lever and (D) earned more RMF infusions across the six-day conditioning paradigm. *s indicate significant main effect of nicotine (P < 0.05).
Additionally, we analyzed the latencies to first earned infusion as a function of nicotine treatment and context (Fig 5). Here, we found main effects of Context (F(1,19) = 8.38, P < 0.01) and Session (F(5,95) = 4.10, P < 0.01). Importantly, unlike what was found with foot-shock punishment, regardless of nicotine treatment, rats punished with histamine did not systematically increase their latency to the first earned infusion, even in the punished context (Fig 5B). Thus, despite serving to significantly reduce RMF self-administration, histamine punishment did not support learning the contextual discrimination.
Figure 5:
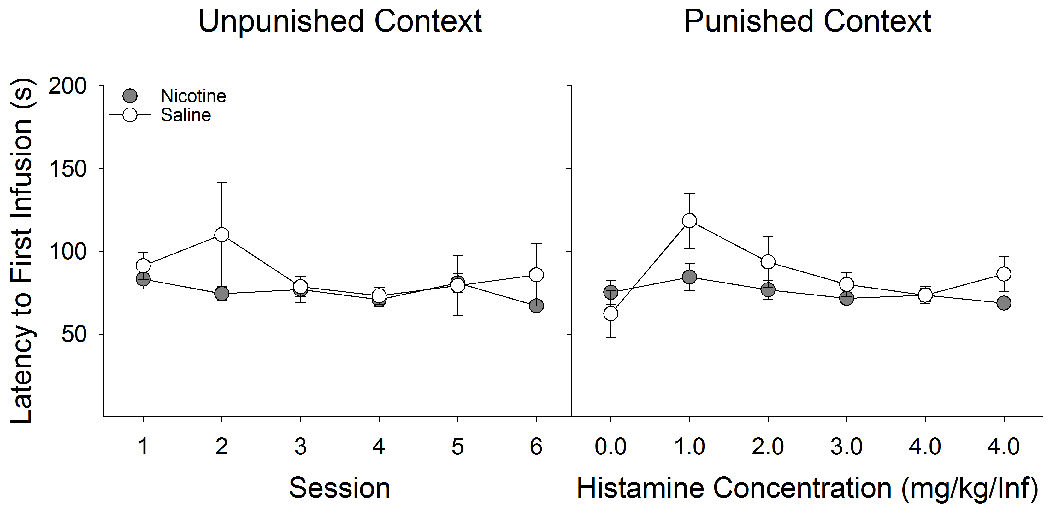
Regardless of drug pretreatment, rats punished with histamine did not systematically increase their latency to the first earned infusion, even within the punished context. (A) Latency to first earned RMF infusion did not differ between nicotine- and saline-treated rats in the unpunished context. (B) Likewise, latency to first infusion did not differ between nicotine- and saline-treated rats in the punished context. There was no effect of punishment history on the latency to earn an RMF infusion in either context.
3.4. Despite a previous history of contextual punishment, nicotine enhances the motivation to obtain remifentanil regardless of form of punishment
The breakpoint in responding for RMF infusions was significantly higher in nicotine-treated rats, relative to saline-treated rats, regardless of context and form of punishment (Fig 6). A three-factor ANOVA conducted on the breakpoint in responding for RMF in both the punished and unpunished contexts as a function of nicotine treatment and form of punishment revealed that nicotine, relative to saline pretreatment, enhanced the motivation to obtain RMF (main effect of Drug; F(1,44) = 17.48, P < 0.001). Additionally, we found a Context x Punishment interaction (F(1,44) = 24.64, P < 0.0001). Post hoc analyses on this interaction showed that punishment with foot-shock resulted in significantly lower breakpoints in the punished context, relative to the unpunished context (Fig 6A), whereas there were no differences in the breakpoint between punished and unpunished contexts following conditioning with histamine punishment (Fig 6B).
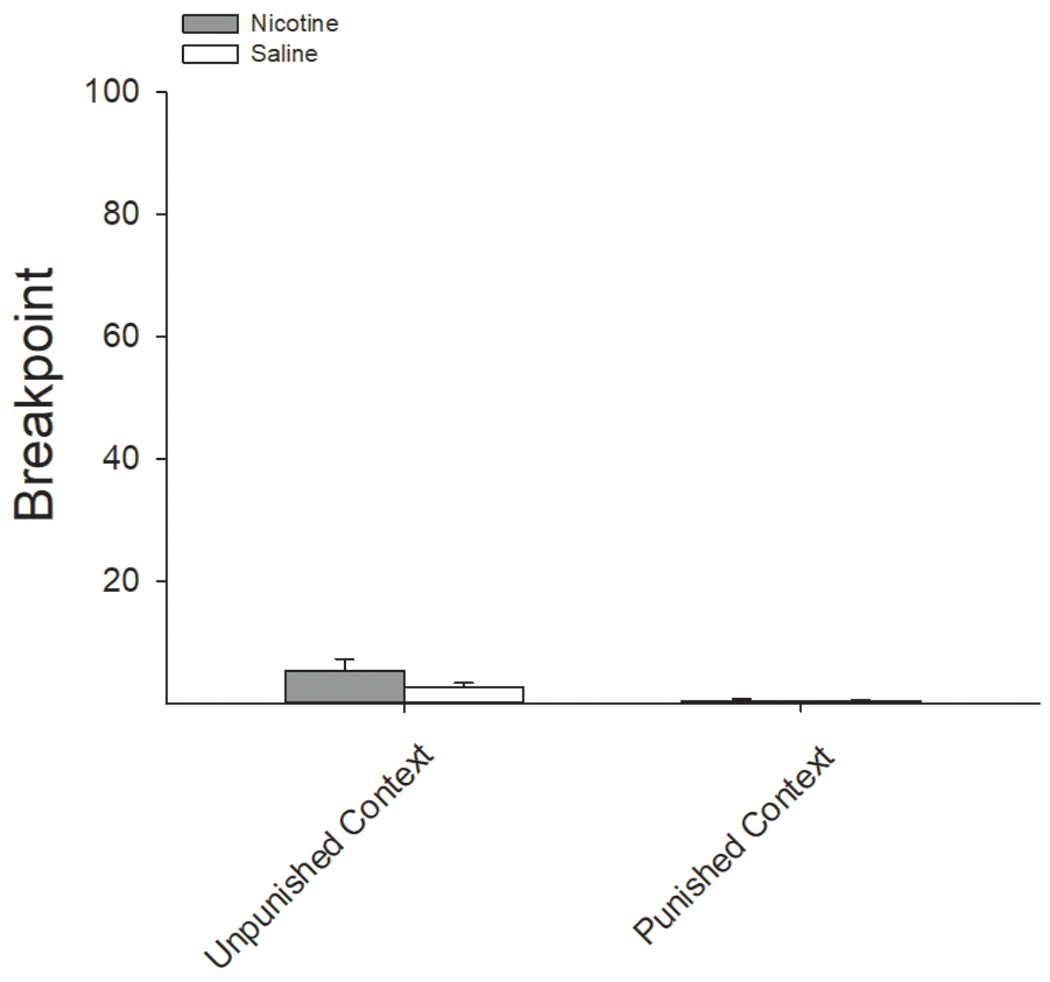
Figure 6:
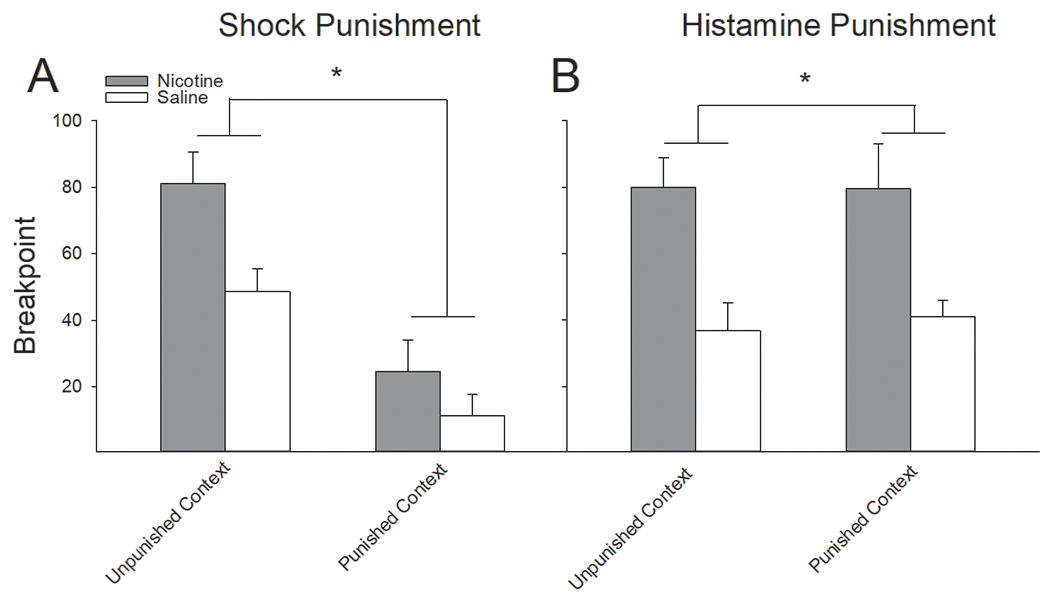
Nicotine administration significantly elevated the breakpoint in responding for RMF infusions, regardless of context or form of punishment. (A) Following punishment of RMF intake with foot-shock, the breakpoint for RMF administration was significantly higher in nicotine-treated rats than saline-treated rats in both the unpunished and punished contexts. (B) Similarly, nicotine produced significantly higher breakpoints in RMF responding following punishment of RMF intake with histamine adulteration. Regardless of drug pretreatment, histamine punishment of RMF did not affect the breakpoint for RMF in the punished context. *s indicate significant group differences (P < 0.05).
3.5. Treatment with nicotine enhances remifentanil seeking under extinction conditions in both punished and unpunished contexts
Following removal of RMF administration, responding on the active lever continued to be higher in nicotine-treated rats, relative to saline-treated rats, regardless of context. A four-factor ANOVA conducted on both active and inactive lever presses across the 10-session extinction phase in the unpunished context revealed a significant main effect of Punishment (F(1,41) = 6.86, P < 0.05) and a significant Lever x Session x Drug interaction (F(9,369) = 6.90, P < 0.0001). Post hoc analyses revealed that responding during extinction was higher following contextual punishment with histamine and that, regardless of form of punishment, nicotine-treated rats responded significantly more on the active lever during the first five sessions, relative to saline-treated rats (Fig 7A). There were no significant group differences in responding on the inactive lever.
Figure 7:
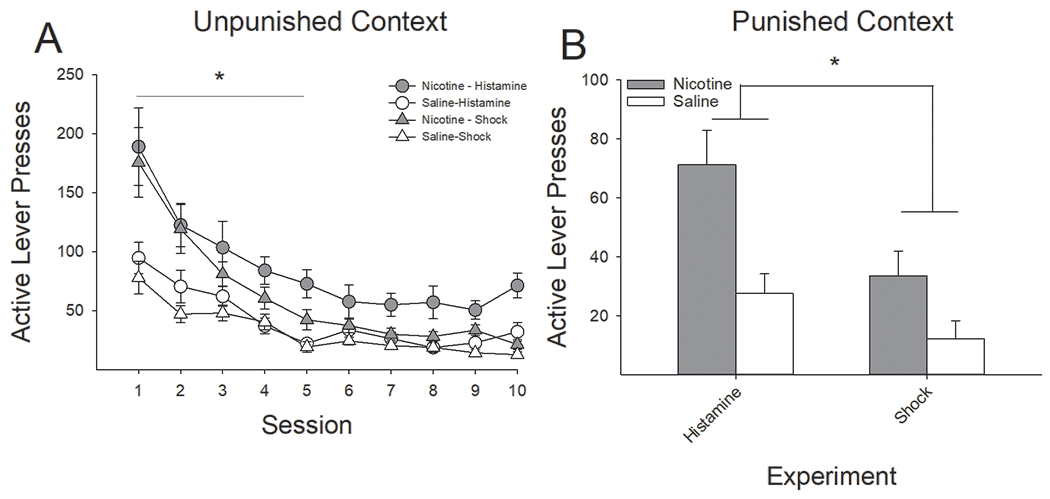
Nicotine-treated rats, relative to saline-treated controls, displayed significantly higher rates of remifentanil seeking in extinction, regardless of punisher or context. (A) Regardless of punishment type, nicotine-treated rats, compared to saline-treated rats, were slower to extinguish RMF seeking in the unpunished context, as evidenced by higher active lever presses under extinction conditions across the first five days. (B) Likewise, regardless of form of punishment, nicotine-treated rats pressed significantly more on the active lever than saline-treated rats within the punished context. *s indicate significant group differences (P < 0.05).
Similarly, a three-factor ANOVA conducted on the active and inactive lever presses in the histamine- and shock-punished contexts as a function of nicotine pretreatment revealed a significant main effect of Punishment (F(1,41) = 12.91, P < 0.001) and a significant Lever x Drug interaction (F(1,41) = 10.00, P < 0.01). Post hoc analyses of these effects revealed that responding was significantly higher in the histamine-punished rats relative to shock-punished rats and that, regardless of punishment type, nicotine-treated rats responded significantly more than saline-treated rats on the active lever (Fig 7B). There were no significant group differences in responding on the inactive lever.
3.6. Nicotine-treated rats displayed equally high levels of cue-induced reinstatement, regardless of context
Following extinction training, nicotine-treated rats showed significantly higher cue-induced reinstatement of responding on the active lever, relative to saline-treated rats. A three-factor ANOVA conducted on the active and inactive lever presses in either the unpunished or punished context as a function of nicotine treatment revealed a Lever x Drug interaction (F(1,15) = 11.22, P < 0.01). Post hoc analysis showed that, regardless of context, nicotine-treated rats responded more on the active lever, as opposed to the inactive lever, relative to saline-treated rats (data not shown). A similar main effect of nicotine was found when examining the number of earned cue presentations (F(1,15) = 9.96, P < 0.01; Fig 8). We only tested rats from the foot-shock experiment because rats from the histamine experiment did not differ in their motivation to obtain RMF between the formerly unpunished and punished contexts.
Figure 8:
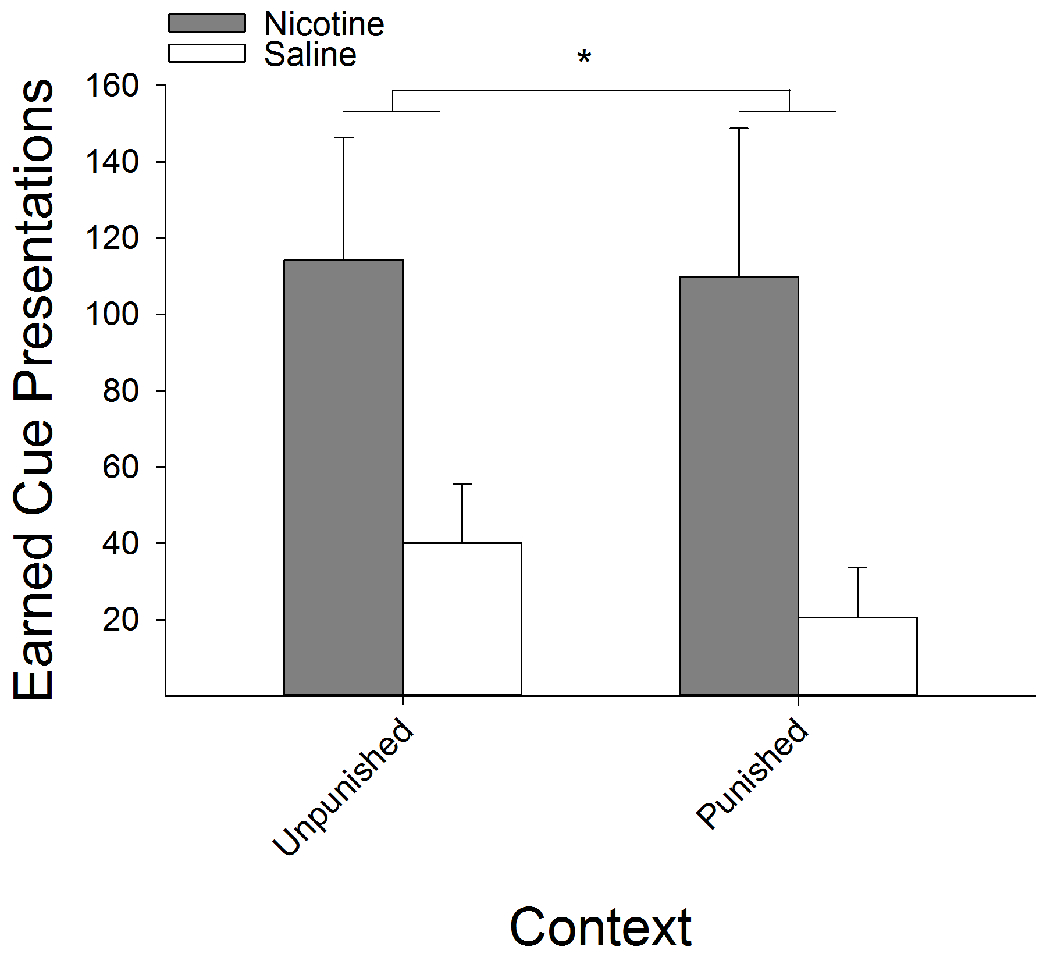
Nicotine-treatment, relative to saline, resulted in greater levels of cue-induced reinstatement. Additionally, following presentation of the RMF cue, nicotine treated rats relapsed to RMF seeking levels in the formerly foot-shock punished context comparable to the unpunished context. Regardless of context, nicotine-treated rats earned significantly more cue presentations, compared to saline-treated rats. *s indicate significant group differences (P < 0.05).
3.7. Nicotine enhances the acquisition of saline self-administration paired with an audiovisual cue
Pretreatment with nicotine, relative to saline, enhanced responding on the active lever with no effect on the inactive lever (Fig 9). Analysis of active vs. inactive lever responding across the 9-day acquisition period with a three-factor ANOVA revealed a significant Lever x Session x Drug interaction (F(8,80) = 2.80, P < 0.01). Group differences between saline- and nicotine-treated rats in responding on any given day failed to survive post-hoc correction. There were no statistical differences in responding on the inactive lever between nicotine- and saline-treated rats. Analyses of the number of reinforcers earned revealed a significant main effect of Session (F(8,80) = 3.89, P < 0.001) as earned reinforcers increased across sessions, however, there were no significant group differences between nicotine- and saline-treated rats on number of reinforcers earned across the 9-day acquisition phase.
Figure 9:
Nicotine-treatment, relative to saline, enhanced acquisition of responding for an AV reinforcer. (A) Nicotine-treated rats responded significantly more on the active lever compared to saline-treated rats. There were no effects of nicotine administration on responding on the inactive lever. (B) Nicotine-treated rats earned more AV reinforcers but this difference did not reach statistical significance within this 9-day period. *s indicate a significant main effect of nicotine.
3.8. Nicotine-induced differences in responding for an audiovisual cue are lost following contingent punishment with foot-shock
The observed increase in responding for an audiovisual cue induced by nicotine was lost following contingent punishment with footshock. Furthermore, both nicotine- and saline-treated rats were more likely to generalize between the two contexts as reinforcers earned decreased, and latency to respond increased, in both contexts (Fig 10). A three-factor ANOVA conducted on the number of earned reinforcers across sessions as a function of drug-pretreatment and contextual punishment revealed a significant Session x Drug interaction (F(5,50) = 5.19, P < 0.001). Post-hoc analyses revealed that nicotine-treated rats earned more reinforcers in the first session in both contexts, but these differences were lost following the first day of contingent foot-shock punishment (Fig 10C & D). We also found a significant main effect of context (F(1,50) = 5.57, P < 0.05) such that all rats earned more reinforcers in the unpunished context. Identical effects were observed when analyzing the number of active lever presses (Session x Drug; F(5,50) = 5.26, P < 0.001; Context; F(1,50) = 5.74, P < 0.05).
Figure 10:
The enhancement of responding for an AV reinforcer by nicotine administration was lost following contextual punishment with foot-shock. (A & B) Regardless of context, nicotine-treated rats, relative to saline-treated, pressed significantly more on the active lever on the first day of testing. Responding on the active lever decreased in both contexts across sessions (C & D) Likewise, nicotine-treated rats earned more AV reinforcers, regardless of context, on the first day of testing, but this difference was lost following administration of foot-shock punishment.
Next, we analyzed the latencies to the first earned infusion as a function of nicotine treatment and context (Fig 11A & B). This three factor ANOVA revealed a main effect of Session (F(5,50) = 4.30, P < 0.01) but no significant differences between nicotine- and saline-treated rats in latency to receive first infusion. Furthermore, there were no main or interactive effects of context demonstrating that, in contrast to Experiment 1, the increase in latency to earn a reinforcer increased in both the unpunished and punished contexts. Similar analyses on the latency to earn the first shock revealed a main effect of Session (F(4,40) = 5.26, P < 0.01) but, again, no main or interactive effects of nicotine administration (Fig 11C).
Figure 11:
Latency to earn an AV reinforcer increased in both the punished and unpunished contexts equally in nicotine- and saline-treated rats. (A) In contrast to observations from testing with RMF administration, the latency to earn an AV reinforcer increased across sessions in the unpunished context following punishment in the alternate context. (B) The latency to earn an AV reinforcer increased in the punished context equally in nicotine- and saline-treated rats. (C) Similarly, the latency to earn the first foot-shock increased equally in nicotine- and saline-treated rats.
3.9. Nicotine administration failed to enhance motivation for an audiovisual cue following contextual punishment conditioning
The breakpoint in responding for audiovisual reinforcers was similar in nicotine- and saline-treated rats regardless of context (Fig 12). A two-factor ANOVA conducted on the breakpoint in responding in both the punished and unpunished contexts as a function of nicotine treatment revealed a main effect of Context (F(1,10) = 13.99, P < 0.01), indicating that regardless of pretreatment condition, rats were less motivated to respond for the cue in the punished context. There were no main or interactive effects of nicotine administration.
Figure 12:
Nicotine failed to enhance motivation to earn an AV reinforcer following contingent foot-shock punishment. There were no differences in the breakpoint for responding for an AV reinforcer between nicotine- and saline-treated rats in either the punished or unpunished context.
4.0. Discussion
Prior nicotine use is often associated with the development of a number of subsequent substance use disorders 5,36–39. Although nicotine and alcohol interactions have been extensively studied, the role that nicotine may play in liability for OUD has been largely overlooked despite equally high rates of comorbidity 37,40,41. In a previous study, we demonstrated that nicotine administration prior to RMF and morphine IVSA sessions substantially increased the amount of drug consumed 6 relative to that rat’s own baseline intake. In the current study, we have replicated and extended those previous findings by demonstrating for the first time that nicotine enhances compulsive-like responding for RMF across multiple behavioral measures. More specifically, nicotine pretreatment enhanced RMF intake despite ongoing punishment and nicotine-treated rats, relative to saline-treated, remained more motivated for RMF regardless of prior punishment or extinction conditions, an effect that was not observed in rats responding for the AV cue alone. In addition, we compared the efficacy of an exteroceptive (foot-shock) and interoceptive (histamine) form of punishment and found that nicotine continued to enhance motivation for RMF, regardless of form of punishment. While both forms of punishment served to limit RMF intake as a function of increasing intensity, our data support that contingent foot-shock is a more potent form of punishment compared to histamine adulteration, as only foot-shock punishment supported the development of discrimination between the unpunished and punished contexts.
Successful recovery from substance use disorders is largely dependent on volitional abstinence of drug taking, which often results from assessing the deleterious consequences of continued drug use 22,23. Here, we found that nicotine pretreatment significantly enhanced RMF self-administration (Fig 1) and that this increased intake in nicotine-treated rats, relative to saline-treated, persisted regardless of form of punishment (Figs 2 & 4). The number of active lever presses (Figs 2A & 4A) and resultant RMF intake (Figs 2B & 4B) was significantly higher following nicotine administration, relative to saline, in the unpunished context across both experiments. Likewise, active lever presses (Figs 2C & 4C) and RMF intake (Figs 2D & 4D) were significantly higher in nicotine-treated rats in the context punished with either foot-shock or histamine adulteration, despite administration of the punisher serving to limit RMF intake in an intensity-dependent fashion. There were no differences in responding on the inactive lever across any portion of either experiment.
Comparison of the latencies to first infusion and first earned foot-shock revealed that the latency to first RMF infusion in the unpunished context was unaffected by administration of punishment in context B and that latencies to first RMF infusion did not reliably differ between nicotine- and saline-treated rats in the unpunished context (Fig 3A). Conversely, administration of foot-shock significantly increased the latency to first RMF infusion and yet nicotine-treated rats were significantly faster to earn their first RMF infusion and their first foot-shock punisher in the punished context (Fig 3B). In contrast, we found no effect of histamine adulteration on the latency to earn RMF infusions in either the unpunished or punished contexts (Fig 5A & B, respectively), and there were no effects of nicotine administration on latency to earn RMF infusions. Examination of the breakpoint for RMF in the progressive ratio paradigms revealed that motivation for RMF was significantly higher in nicotine-treated rats, relative to saline-treated rats, regardless of context or form of punishment (Fig 6A & B). Similar to that revealed by the latency measures, we found no effect of context on the motivation to obtain RMF following punishment with histamine adulteration. Specifically, former punishment with foot-shock in context B resulted in significantly lower breakpoints compared to those observed in the unpunished context (Context A). In contrast, the motivation to obtain RMF following punishment with histamine adulteration did not differ between context A and B in either nicotine- or saline-treated rats. Considering that histamine adulteration of RMF also did not significantly affect latency to RMF infusion in context B in the histamine-adulteration experiment, these data support that foot-shock is a much more potent form of punishment than histamine adulteration. That is, despite both methods serving to significantly limit RMF intake during ongoing punishment, histamine adulteration failed to support the development of contextual discrimination within the present study.
We found that nicotine not only enhanced compulsive-like RMF IVSA but also significantly enhanced compulsive-like RMF seeking in the unpunished context that persisted under extinction conditions longer in nicotine-treated rats compared to saline-treated rats, regardless of punishment condition (Fig 7A). This enhancement of RMF seeking by nicotine was also clearly evident when tested under extinction conditions in the formerly punished context (Fig 7B), again regardless of form of previous punishment. Additionally, we tested a subset of rats in a cue-induced relapse test following prior contextual punishment with foot-shock. Here, completion of the FR-2 schedule resulted in the illumination of the RMF cue light but no delivery of RMF. Nicotine-treated rats, relative to saline-treated rats, displayed a higher propensity to relapse to drug-seeking behavior upon re-exposure to the RMF cue, regardless of context. When comparing the efficacy of cue-induced reinstatement, we found that addition of the RMF cue did not significantly increase RMF seeking in saline-treated rats above that of their last day of extinction conditioning but significantly enhanced RMF seeking in nicotine-treated rats. Furthermore, nicotine-treated rats displayed no difference in the cue-induced reinstatement between the unpunished and punished contexts (Fig 8). Therefore, the formerly punished context was sufficient to limit relapse to RMF seeking in saline-treated rats following re-exposure to the RMF cue, but not in nicotine-treated rats.
Because nicotine can enhance responding for nonpharmacological reinforcers, we examined these effects of nicotine in a group of rats trained to respond for presentation of an AV reinforcer. Consistent with previous reports29–31, we found that nicotine administration enhanced responding for an AV reinforcer alone, in the absence of RMF infusions (Fig 9). In contrast to what was observed in rats responding for RMF, the nicotine-induced augmentation of responding for an AV cue was lost following the first day of contingent foot-shock punishment (Fig 10). Furthermore, it appeared that the AV cue was devalued in both the punished and unpunished contexts, an effect that was not observed in rats responding for RMF infusions. Specifically, the number of earned AV reinforcers decreased across days in both contexts (Fig 10B & D). Likewise, the latency to earn an AV reinforcer increased in both contexts equally in both nicotine- and saline-treated rats (Fig 11A & B). Consistent with the notion that the AV cue was equally devalued, we found no effect of nicotine on motivation to earn AV reinforcers in a PR task (Fig 12), no differences in responding under extinction conditions (Fig S1), nor differences in reinstatement of AV cue responding (Fig S2). It has been demonstrated previously that the reinforcer enhancing effects of nicotine are modulated by the relative value of the reinforcer 30,42. Our data support and extend those previous findings such that the degree to which nicotine enhances a punishment-resistant phenotype is also modulated by the value of the reinforcer.
It is important to note that we did not find any evidence of differential sensitivity to the punishment itself in either experiment. Despite nicotine-treated rats demonstrating higher overall RMF intake compared with saline-treated controls, both nicotine- and saline-treated rats decreased their consumption of RMF at comparable rates as a function of increasing punishment intensity in both the foot-shock (Fig 2) and histamine (Fig 4) experiments. This is an important consideration given that nicotine has been demonstrated to have antinociceptive properties 43,44. The fact that both forms of punishment were equally as effective in nicotine- and saline-treated rats at reducing RMF administration from baseline as a function of increasing intensity demonstrates that the persistent differences in RMF intake following nicotine administration were unlikely due to any inoculating effect of nicotine on the aversive physiological effects of the punishers during their administration. Moreover, the lack of effect of nicotine administration, relative to saline, in enhancing foot-shock punished responding for an AV reinforcer further precludes the conclusion that the effects were entirely driven by any antinociceptive properties of nicotine. Rather, it appears more likely that the differences in RMF intake between nicotine- and saline-treated rats were due to an enhancement in the motivational efficacy of RMF induced by nicotine, a deficit in learning the contextual discrimination between the unpunished and punished environments when RMF was available, or perhaps a combination of the two. In support of the former, nicotine has been shown to enhance the motivational properties of subsequently administered substances across multiple classes of drugs of abuse 6,14,17,45–50, an effect demonstrated here with RMF. Nicotine administration not only increased total RMF intake but also increased the breakpoint for obtaining RMF infusions (Fig 6) and significantly delayed extinction of RMF seeking under extinction conditions (Fig 7). Support for the latter interpretation comes from studies demonstrating that nicotine pretreatment interferes with contextual conditioning with other commonly abused drugs 6,15,17,20. In the present study, the context associated with foot-shock punishment was sufficient to significantly limit RMF intake in the cue-induced reinstatement paradigm in saline-treated rats whereas nicotine-treated rats displayed relapse to RMF seeking identical to that seen in rats tested in the unpunished context (Fig 8). Furthermore, previous studies have demonstrated that contextual relapse to drug seeking following volitional abstinence is dependent on neural activity within the insular cortex 25,26,51, such that rats that were more likely to relapse to alcohol seeking within a punished context displayed more fos-like immunoreactivity within the insula 28. Previously, we have shown that nicotine delivered directly to the insular cortex is sufficient to interfere with insular-dependent contextual drug conditioning 6, suggesting that nicotinic modulation of insular function may impair the ability to withhold drug-directed responding in contexts previously associated with punishment. Given that nicotine generally enhances contextual fear conditioning with foot-shock 52–54, it follows that these effects are at least in part dependent on the availability of the reinforcing properties of RMF, a conclusion supported by the lack of effect of nicotine when rats were responding for the AV reinforcer alone. Consistent with this notion, nicotine pretreatment interferes with the ability to voluntarily suppress intake of a reinforcing saccharin solution following its pairing with commonly abused drugs but not following pairing with the purely emetic stimulus lithium chloride 17 that has no known reinforcing properties.
As a whole, these data support that nicotine administration substantially increases the expression of a number of compulsive-like behaviors directed towards obtaining opioids. This enhancement may arise from the augmentation of the motivational properties of RMF, such that the incentive to self-administer RMF outweighs the aversive consequences of the punishers, or from a reduced capacity to learn that a specific environmental context results in further deleterious consequences when a reinforcing drug is available. It is also possible that the enhanced resistance to punishment induced by nicotine may be due to the elevated RMF intake observed prior to punishment conditioning. Future studies should be conducted in which the presence of nicotine administration is systematically altered alongside the various phases of this experiment. Regardless of the underlying causes, our findings provide the initial characterization of a novel mechanism that may help to explain the extreme comorbidity of nicotine and opioid misuse. OUDs are primarily characterized by the compulsive use of opioids despite harmful or aversive consequences and a high rate of relapse to misuse despite efforts to remain abstinent. An extended history of drug taking results in an escalation of drug self-administration and ultimately produces insensitivity to punishment of drug-seeking for that particular drug 10,34,55. To the best of our knowledge, these are the first data to demonstrate that acute, noncontingent administration of nicotine is sufficient to engender a punishment-resistant phenotype in animals responding for opioids. As such, it is likely that nicotine use may be an antecedent risk factor for the development of OUDs. Nicotine has been shown to modulate the thalamacortical and corticostriatal circuits that are implicated in the maintenance of compulsive drug seeking 56. As such, future studies designed to dissect the relative contributions of nicotine on these circuits on the enhancement of compulsive drug taking are warranted.
Supplementary Material
Footnotes
Data Sharing
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Theisen K, Jacobs B, Macleod L, Davies B. The United States opioid epidemic: a review of the surgeon’s contribution to it and health policy initiatives. BJU Int. 2018;122(5):754–759. [DOI] [PubMed] [Google Scholar]
- 2.Chang HY, Kharrazi H, Bodycombe D, Weiner JP, Alexander GC. Healthcare costs and utilization associated with high-risk prescription opioid use: a retrospective cohort study. BMC Med. 2018;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon JH, Lane SD, Weaver MF. Opioid analgesics and nicotine: more than blowing smoke. Journal of pain & palliative care pharmacotherapy. 2015;29(3):281–289. [DOI] [PubMed] [Google Scholar]
- 4.Zale EL, Dorfman ML, Hooten WM, Warner DO, Zvolensky MJ, Ditre JW. Tobacco smoking, nicotine dependence, and patterns of prescription opioid misuse: Results from a nationally representative sample. Nicotine & Tobacco Research. 2014;17(9):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skurtveit S, Furu K, Selmer R, Handal M, Tverdal A. Nicotine dependence predicts repeated use of prescribed opioids. Prospective population-based cohort study. Ann Epidemiol. 2010;20(12):890–897. [DOI] [PubMed] [Google Scholar]
- 6.Loney GC, King CP, Meyer PJ. Systemic nicotine enhances opioid self-administration and modulates the formation of opioid-associated memories partly through actions within the insular cortex. Sci Rep. 2021;11(1):3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1507):3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHugh RK, Fitzmaurice GM, Carroll KM, et al. Assessing craving and its relationship to subsequent prescription opioid use among treatment-seeking prescription opioid dependent patients. Drug and alcohol dependence. 2014;145:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Progress in brain research. 2016;224:25–52. [DOI] [PubMed] [Google Scholar]
- 10.Krasnova IN, Marchant NJ, Ladenheim B, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(8):2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behavioral and cognitive neuroscience reviews. 2004;3(3):143–158. [DOI] [PubMed] [Google Scholar]
- 12.Maddux J- MN, Chaudhri N. Nicotine-induced enhancement of Pavlovian alcohol-seeking behavior in rats. Psychopharmacology. 2017;234(4):727–738. [DOI] [PubMed] [Google Scholar]
- 13.Stringfield SJ, Boettiger CA, Robinson DL. Nicotine-enhanced Pavlovian conditioned approach is resistant to omission of expected outcome. Behavioural brain research. 2018;343:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loney GC, Angelyn H, Cleary LM, Meyer PJ. Nicotine Produces a High-Approach, Low-Avoidance Phenotype in Response to Alcohol-Associated Cues in Male Rats. Alcoholism, clinical and experimental research. 2019;43(6):1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araki H, Kawakami KY, Jin C, et al. Nicotine attenuates place aversion induced by naloxone in single-dose, morphine-treated rats. Psychopharmacology. 2004;171(4):398–404. [DOI] [PubMed] [Google Scholar]
- 16.Ishida S, Kawasaki Y, Araki H, et al. alpha7 Nicotinic acetylcholine receptors in the central amygdaloid nucleus alter naloxone-induced withdrawal following a single exposure to morphine. Psychopharmacology. 2011;214(4):923–931. [DOI] [PubMed] [Google Scholar]
- 17.Loney GC, Meyer PJ. Nicotine pre-treatment reduces sensitivity to the interoceptive stimulus effects of commonly abused drugs as assessed with taste conditioning paradigms. Drug Alcohol Depend. 2019;194:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunin D, Smith BR, Amit Z. Nicotine and ethanol interaction on conditioned taste aversions induced by both drugs. Pharmacology, biochemistry, and behavior. 1999;62(2):215–221. [DOI] [PubMed] [Google Scholar]
- 19.Bienkowski P, Piasecki J, Koros E, Stefanski R, Kostowski W. Studies on the role of nicotinic acetylcholine receptors in the discriminative and aversive stimulus properties of ethanol in the rat. Eur Neuropsychopharmacol. 1998;8(2):79–87. [DOI] [PubMed] [Google Scholar]
- 20.Rinker JA, Hutchison MA, Chen SA, Thorsell A, Heilig M, Riley AL. Exposure to nicotine during periadolescence or early adulthood alters aversive and physiological effects induced by ethanol. Pharmacology, biochemistry, and behavior. 2011;99(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Annals of the New York Academy of Sciences. 1992;654:400–415. [DOI] [PubMed] [Google Scholar]
- 22.Marlatt GA. Do animal models provide a valid analogue for human drug lapse and relapse? Comment on Leri and Stewart (2002). Exp Clin Psychopharmacol. 2002;10(4):359–360; discussion 364-356. [DOI] [PubMed] [Google Scholar]
- 23.Peck JA, Ranaldi R. Drug abstinence: exploring animal models and behavioral treatment strategies. Psychopharmacology. 2014;231(10):2045–2058. [DOI] [PubMed] [Google Scholar]
- 24.Klingemann HK. The motivation for change from problem alcohol and heroin use. Br J Addict. 1991;86(6):727–744. [DOI] [PubMed] [Google Scholar]
- 25.Pelloux Y, Hoots JK, Cifani C, et al. Context-induced relapse to cocaine seeking after punishment-imposed abstinence is associated with activation of cortical and subcortical brain regions. Addict Biol. 2018;23(2):699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell EJ, Barker DJ, Nasser HM, Kaganovsky K, Dayas CV, Marchant NJ. Cue-induced food seeking after punishment is associated with increased Fos expression in the lateral hypothalamus and basolateral and medial amygdala. Behavioral neuroscience. 2017;131(2):155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y. Context-induced relapse to alcohol seeking after punishment in a rat model. Biol Psychiatry. 2013;73(3):256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell EJ, Flanagan JPM, Walker LC, Hill M, Marchant NJ, Lawrence AJ. Anterior Insular Cortex is Critical for the Propensity to Relapse Following Punishment-Imposed Abstinence of Alcohol Seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2019;39(6):1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhri N, Caggiula AR, Donny EC, et al. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology. 2007;190(3):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmatier MI, Matteson GL, Black JJ, et al. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007;89(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barret ST, Bevins RA. Nicotine enhances operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacology, biochemistry, and behavior. 2013;114–115:9–15. [PMC free article] [PubMed] [Google Scholar]
- 32.Manzano-Nieves G, Gaillard M, Gallo M, Bath KG. Early life stress impairs contextual threat expression in female, but not male, mice. Behavioral neuroscience. 2018;132(4):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holtz NA, Carroll ME. Cocaine self-administration punished by intravenous histamine in adolescent and adult rats. Behavioural pharmacology. 2015;26(4):393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gancarz-Kausch AM, Adank DN, Dietz DM. Prolonged withdrawal following cocaine self-administration increases resistance to punishment in a cocaine binge. Sci Rep. 2014;4:6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(8):1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCabe SE, West BT, McCabe VV. Associations Between Early Onset of E-cigarette Use and Cigarette Smoking and Other Substance Use Among US Adolescents: A National Study. Nicotine Tob Res. 2018;20(8):923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohut SJ. Interactions between nicotine and drugs of abuse: a review of preclinical findings. Am J Drug Alcohol Abuse. 2017;43(2):155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren M, Lotfipour S. Nicotine Gateway Effects on Adolescent Substance Use. West J Emerg Med. 2019;20(5):696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong SW, Lohrmann DK, Middlestadt SE, Lin HC. Is E-cigarette use a gateway to marijuana use? Longitudinal examinations of initiation, reinitiation, and persistence of e-cigarette and marijuana use. Drug Alcohol Depend. 2020;208:107868. [DOI] [PubMed] [Google Scholar]
- 40.Nahvi S, Richter K, Li X, Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addictive Behaviors. 2006;31(11):2127–2134. [DOI] [PubMed] [Google Scholar]
- 41.Chisolm MS, Fitzsimons H, Leoutsakos JM, et al. A comparison of cigarette smoking profiles in opioid-dependent pregnant patients receiving methadone or buprenorphine. Nicotine Tob Res. 2013;15(7):1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charntikov S, Pittenger ST, Swalve N, Barrett ST, Bevins RA. Conditioned enhancement of the nicotine reinforcer. Exp Clin Psychopharmacol. 2021;29(4):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahley TL, Berntson GG. Antinociceptive effects of central and systemic administrations of nicotine in the rat. Psychopharmacology. 1979;65(3):279–283. [DOI] [PubMed] [Google Scholar]
- 44.Tripathi HL, Martin BR, Aceto MD. Nicotine-induced antinociception in rats and mice: correlation with nicotine brain levels. Journal of Pharmacology and Experimental Therapeutics. 1982;221(1):91–96. [PubMed] [Google Scholar]
- 45.Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology. 2002;162(2):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortright JJ, Sampedro GR, Neugebauer NM, Vezina P. Previous exposure to nicotine enhances the incentive motivational effects of amphetamine via nicotine-associated contextual stimuli. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(10):2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. [DOI] [PubMed] [Google Scholar]
- 48.Nunes EJ, Bitner L, Hughley SM, et al. Cholinergic Receptor Blockade in the VTA Attenuates Cue-Induced Cocaine-Seeking and Reverses the Anxiogenic Effects of Forced Abstinence. Neuroscience. 2019;413:252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spiga R, Schmitz J, Day J, 2nd. Effects of nicotine on methadone self-administration in humans. Drug Alcohol Depend. 1998;50(2):157–165. [DOI] [PubMed] [Google Scholar]
- 50.Shams J, Sahraei H, Gholami A, et al. Effects of ultra-low doses of nicotine on the expression of morphine-induced conditioned place preference in mice. Behavioural pharmacology. 2006;17(7):629–635. [DOI] [PubMed] [Google Scholar]
- 51.Venniro M, Caprioli D, Zhang M, et al. The Anterior Insular Cortex-->Central Amygdala Glutamatergic Pathway Is Critical to Relapse after Contingency Management. Neuron. 2017;96(2):414–427 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80(2):147–157. [DOI] [PubMed] [Google Scholar]
- 53.Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behavioral neuroscience. 2003;117(6):1276–1282. [DOI] [PubMed] [Google Scholar]
- 54.Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2006;394(3):202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194(1):127–137. [DOI] [PubMed] [Google Scholar]
- 56.Bruijnzeel AW, Alexander JC, Perez PD, et al. Acute nicotine administration increases BOLD fMRI signal in brain regions involved in reward signaling and compulsive drug intake in rats. Int J Neuropsychopharmacol. 2014;18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


